
1
Fifth stage
Pediatrics
Lec-
Dr. Athl
22/2/2017
Nephrotic Syndrome
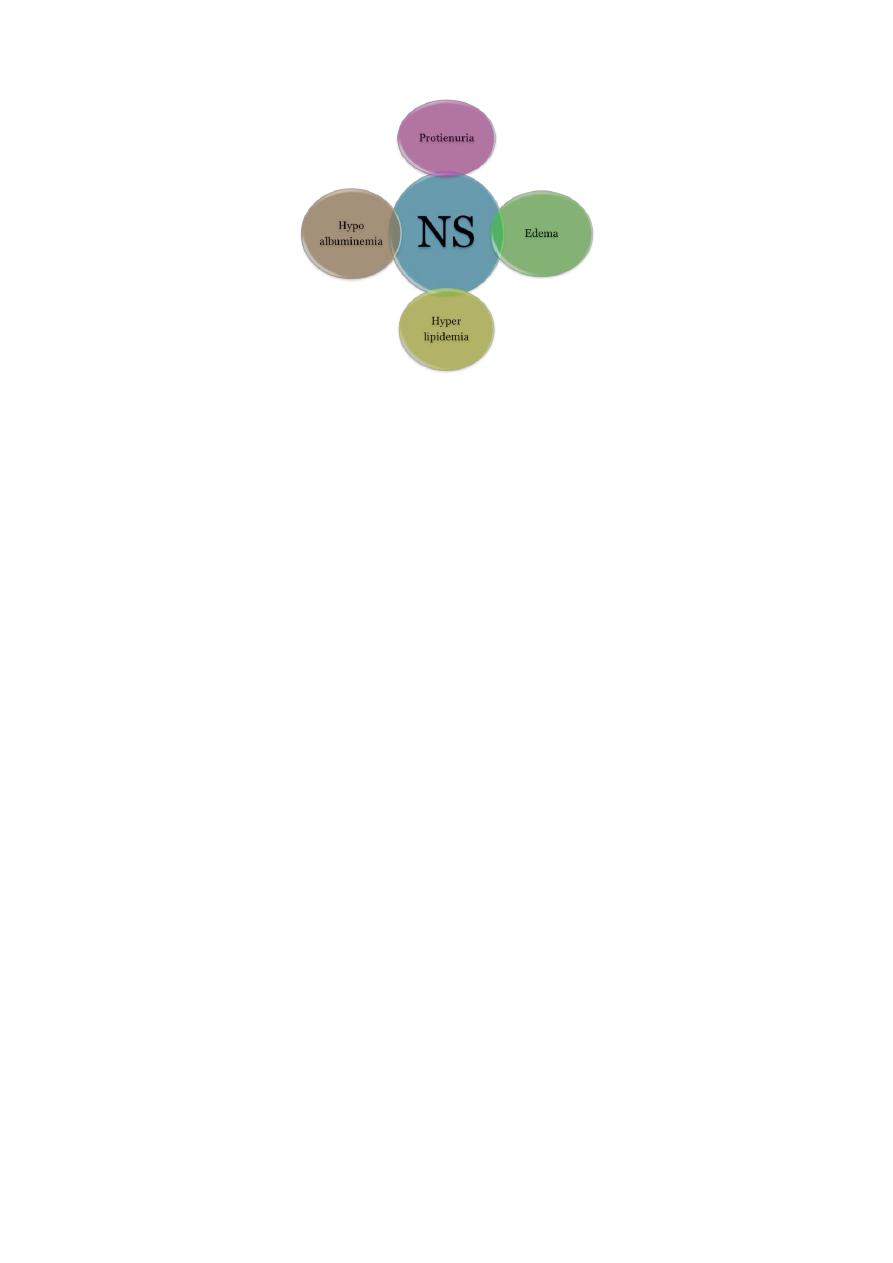
Nephrotic syndrome, is a manifestation of glomerular disease. Characterized by:
1) Heavy proteinuria (Nephrotic range proteinuria is defined protein excretion of >
40 mg/m2/hr or a first morning protein : creatinine ratio of >2-3 : 1).
2) Hypoalbuminemia(serum albumin <2.5 g/dL)
3) Edema.
4) Hyperlipidemia (serum cholesterol >250 mg/dL).
5) The annual incidence is 2-3 cases per 100,000 children per year.
Etiology :
1. Most children with nephrotic syndrome have a form of primary or idiopathic
nephrotic syndrome. Glomerular lesions associated with idiopathic nephrotic
syndrome include:
▫ minimal change disease (the most common).
▫ focal segmental glomerulosclerosis.
▫ membranoproliferative glomerulonephritis.
▫ membranous nephropathy.
▫ diffuse mesangial proliferation
2. Nephrotic syndrome may also be secondary to systemic diseases such as:
▫ Infections (hepatitis, HIV, malaria, toxoplasmosis)
▫ Drugs (gold, penicillamine, NSAI, Interferon)
▫ Immunologic or allergic disorders(bee sting, food allergens)
▫ Associated with malignant disease(Lymphoma & Leukemia)
3. A number of hereditary proteinuria syndromes are caused by mutations in genes
that encode critical protein components of the glomerular filtration apparatus such
as:
▫ Finnish-type congenital nephrotic syndrome
▫ Denys-Drash syndrome
2
Pathophysiology
:
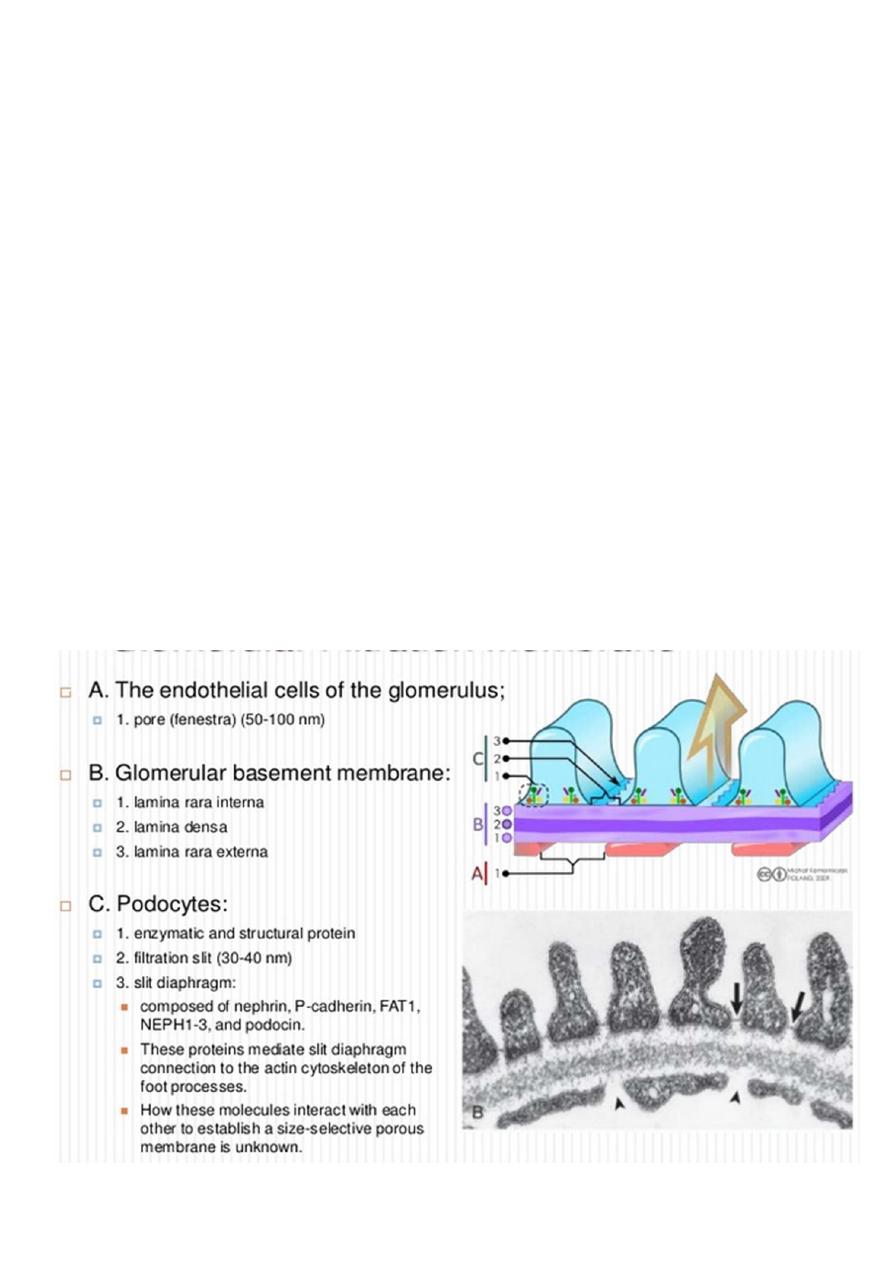
For understanding pathophysiology of NS should know the Normal Glomerular barrier &
Function.
One of the kidney’s most important functions is filtration of the blood by glomeruli,
allowing excretion of fluid and waste products, while retaining all blood cells and the
majority of blood proteins within the bloodstream.
Glomerular filter acts via two phenomenon size selectivity and charge selectivity.
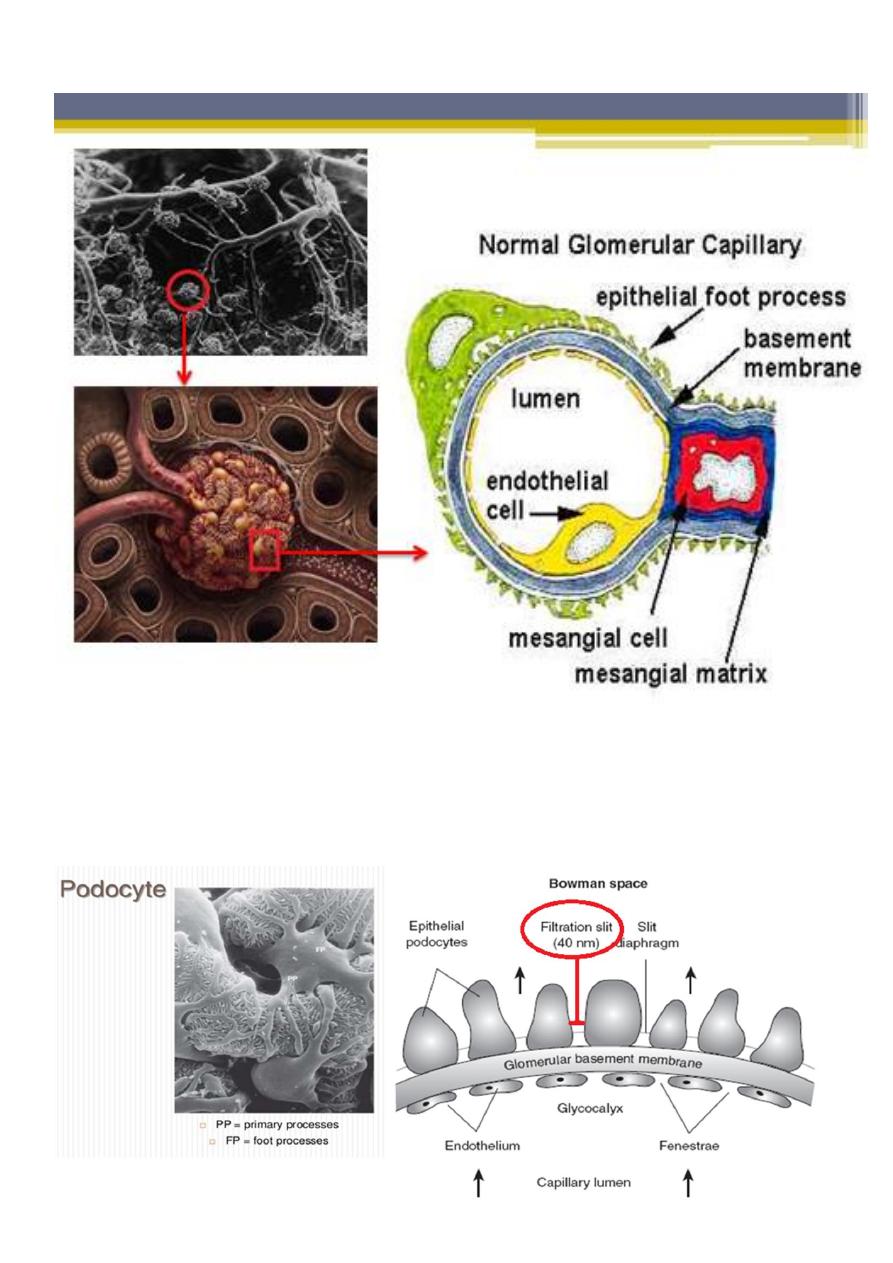
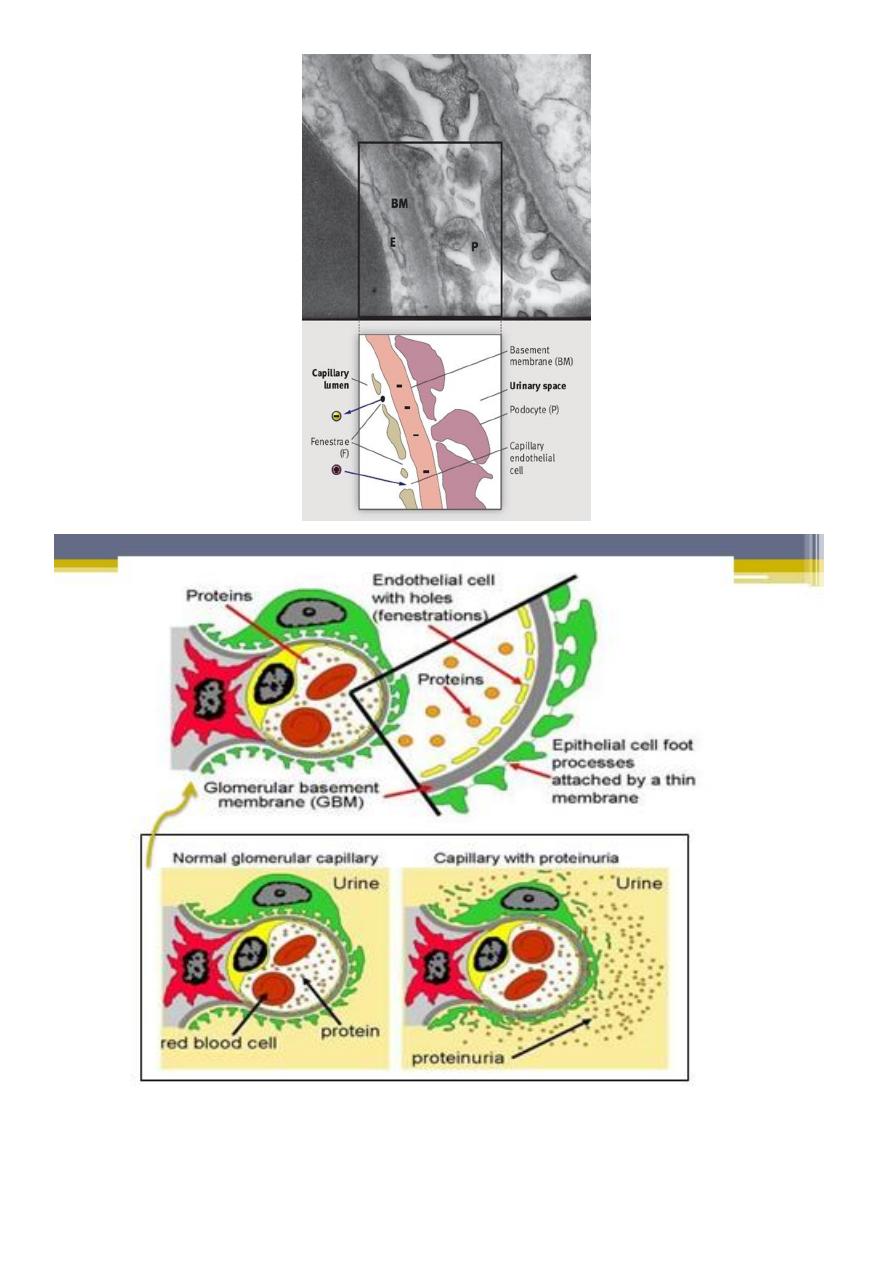
Each glomerulus is composed of numerous capillaries which have evolved to permit
ultrafiltration of the fluid that eventually forms urine. The capillary walls are
composed of :
▫ an inner endothelial cell cytoplasm, with pores known as ‘fenestrations’.
▫ GBM.
▫ external, epithelial cell (podocytes), the epithelial cells are large, with
specialized cytoplasmic extensions known as foot processes, which firmly
anchor them to the GBM. The adjacent foot processes do not come into
contact with each other and are separated by ~40 nm wide spaces known as
filtration slits. These slits are bridged by a continuous membrane-like structure
known as the slit diaphragm
3
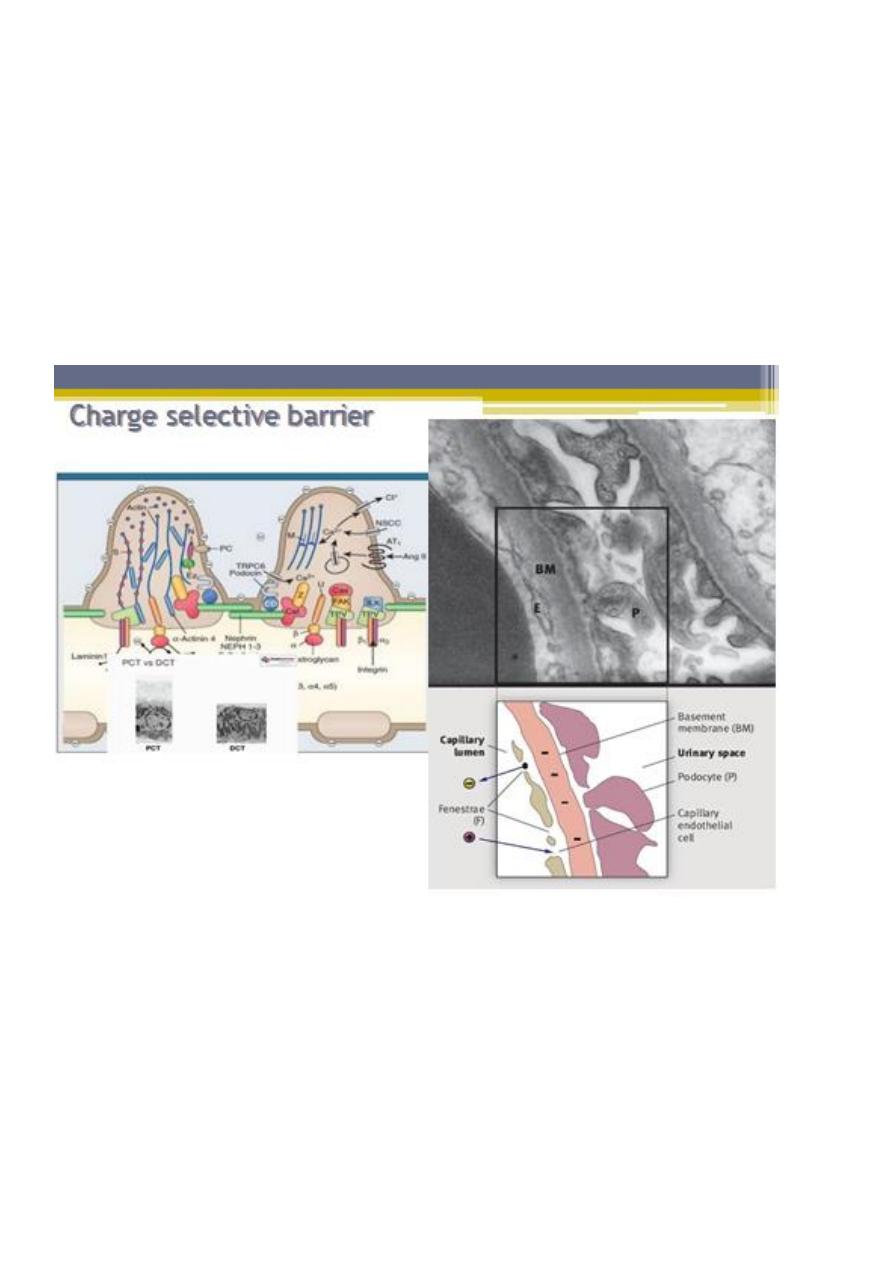
Size selectivity:
under normal conditions, molecules greater than 42 Å in diameter are unable to cross the
filtration barrier.
4
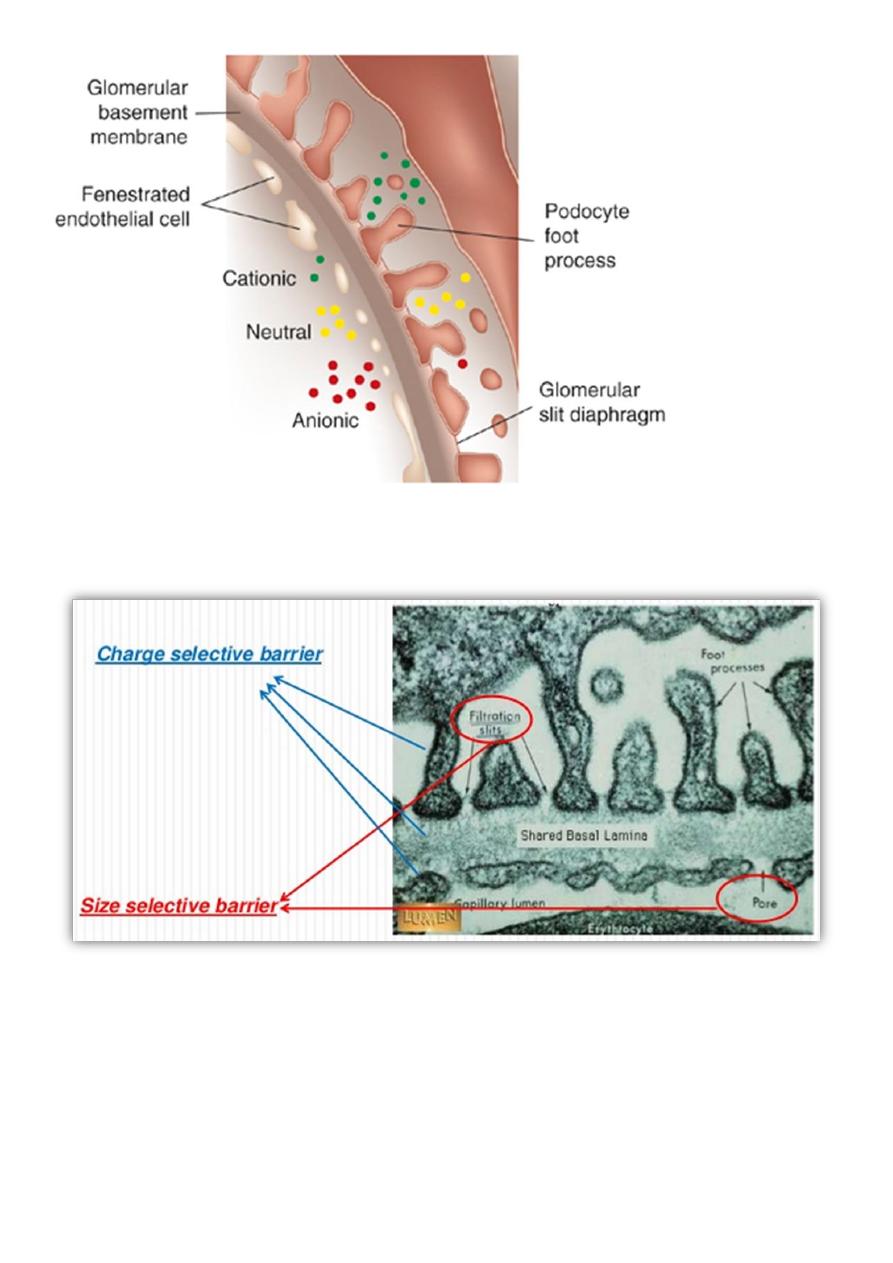
Charge selectivity:
• In addition to molecular size, molecular charge has also been shown to influence the
clearance of macromolecules from the glomerular capillary.
• Electrical charge is an important determining factor for the passage of protein
molecules through the glomerular filtration barrier. Several reports have indicated a
reduction in the negative charge of the GBM in nephrotic syndrome
• This has led to the concept that the passage of anionic molecules such as plasma
proteins is actively hindered by the anionic surface charge of the glomerular filter
• Negative charge is thought to retard filtration of anionic molecules (red) such as
albumin, facilitate filtration of cationic molecules (green), and have little impact on
neutral molecules (yellow).
5
Glomerular Filtration Membrane charge & size selective barrier :
6
Pathophysiology in NS:
1. Proteinuria:
The underlying abnormality in nephrotic syndrome is an increased
permeability of the glomerular capillary wall, which leads to massive
proteinuria.
In idiopathic NS found that it is associated with complex disturbances in the
immune system, especially T cell–mediated immunity which lead to :
Extensive effacement of podocyte foot processes (the hallmark of
idiopathic nephrotic syndrome) suggests a vital role for the podocyte,
which is normally prevent passage of large molecule as albumin.
loss of the glomerular basement membrane sialoproteins, which leads to
a loss of the normal negative charge responsible for the increase in
capillary wall permeability.(normally prevent passage of anionic
molecules as albumin)
In focal segmental glomerulosclerosis, a plasma factor, probably produced by a
subset of activated lymphocytes, may be responsible for the increase in
capillary wall permeability.
Changes to capillary endothelial cells, GBM, or podocytes, which
normally filter serum protein selectively by size and charge.
Glomerular Proteinuria develops when there is alteration in either
charge selectivity and size selectivity
7

8
2. Hypoalbuminemia:
Massive proteinuria that results leads to a decline in serum proteins, especially
albumin.
3. Edema:
Although the mechanism of edema formation in nephrotic syndrome is
incompletely understood, it seems likely that in most instances, massive
urinary protein loss leads to hypoalbuminemia, which causes a decrease in
plasma oncotic pressure and transudation of fluid from the intravascular
compartment to the interstitial space. The reduction in intravascular volume
decreases renal perfusion pressure, activating the renin-angiotensin-
aldosterone system, which stimulates tubular reabsorption of sodium. The
reduced intravascular volume also stimulates the release of antidiuretic
hormone, which enhances the reabsorption of water in the collecting duct.
This theory does not apply to all patients with nephrotic syndrome because
some patients actually have increased intravascular volume with diminished
plasma levels of renin and aldosterone. Therefore, other factors, including
primary renal avidity for sodium and water, may be involved in the formation
of edema in some patients with nephrotic syndrome.
4. Hyperlipidemia:
In the nephrotic state, serum lipid levels (cholesterol, triglycerides) are elevated for 2
reasons:
Hypoalbuminemia stimulates generalized hepatic protein synthesis, including
synthesis of lipoproteins. This is also why a number of coagulation factors are
increased, increasing the risk of thrombosis.
In addition, lipid catabolism is diminished as a result of reduced plasma levels
of lipoprotein lipase related to increased urinary losses of this enzyme.

9
Idiopathic Nephrotic Syndrome :
Approximately 90% of children with nephrotic syndrome have idiopathic nephrotic
syndrome. Idiopathic nephrotic syndrome is associated with primary glomerular disease
without evidence of a specific systemic cause.
Pathology :
Idiopathic nephrotic syndrome includes multiple histologic types:
1. minimal change disease: about 85% of total cases of nephrotic syndrome in
children, 95% are steroid responsive .
2. mesangial proliferation: 50% are steroid responsive .
3. focal segmental glomerulosclerosis: only 20% are steroid responsive, the
disease is often progressive, ultimately involving all glomeruli, and ultimately
leads to end-stage renal disease in most patients.
4. membranous nephropathy.
5. membranoproliferative glomerulonephritis
Clinical Manifestations :
1. The idiopathic nephrotic syndrome is more common in boys than in girls (2 : 1).
2. Most commonly appears between the ages of 2 and 6 yr. However, it has been
reported as early as 6 mo of age and throughout adulthood.
3. MCNS is present in 85-90% of patients <6 yr of age. In contrast, only 20-30% of
adolescents who present for the first time with nephrotic syndrome have MCNS. The
more common cause of idiopathic nephrotic syndrome in this older age group is
FSGS.
4. The initial episode of INS, as well as subsequent relapses, usually follows minor
infections and, uncommonly, reactions to insect bites, bee stings, or poison ivy.
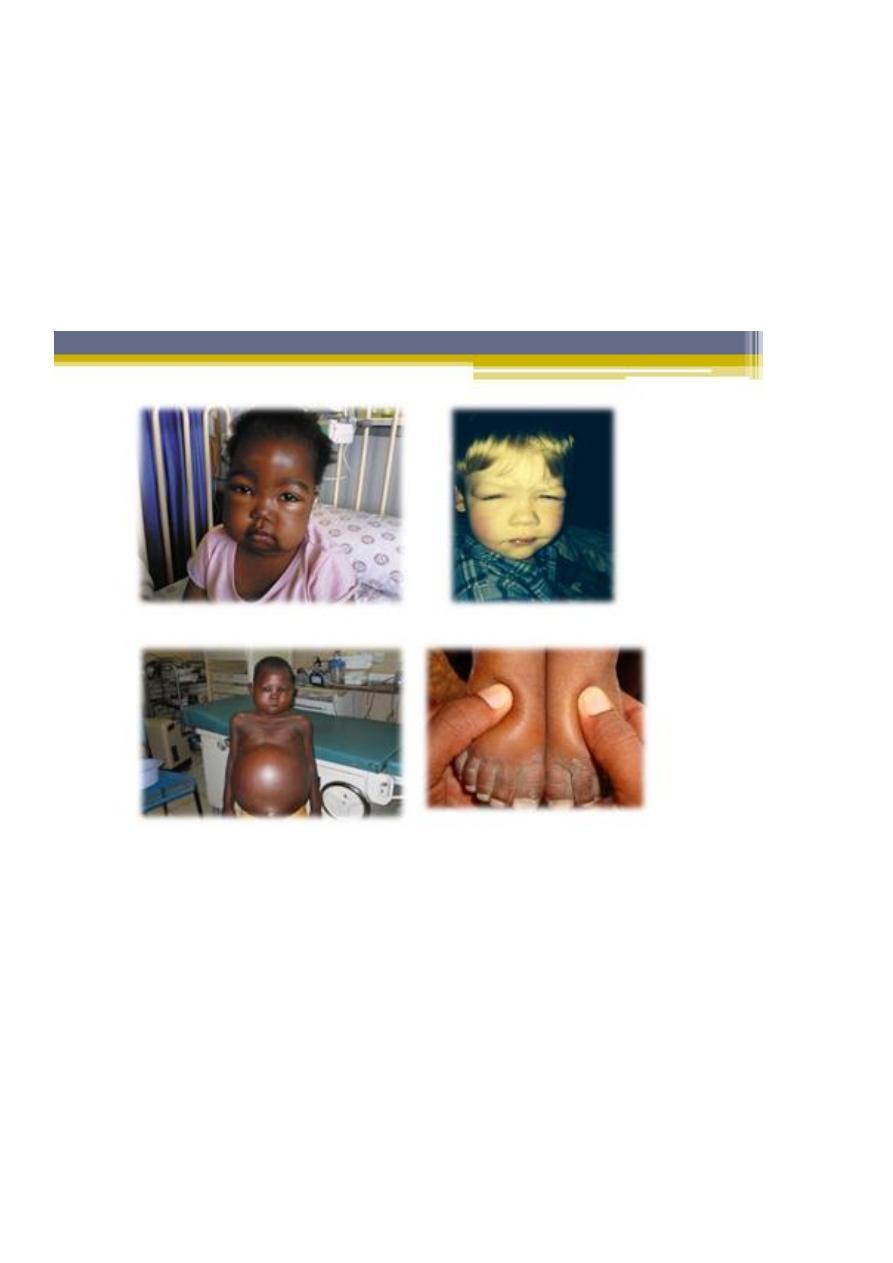
5. Children usually present with mild edema, which is initially noted around the eyes
and in the lower extremities.
6. Nephrotic syndrome can initially be misdiagnosed as an allergic disorder because of
the periorbital swelling that decreases throughout the day. With time, the edema
becomes generalized, with the development of ascites, pleural effusions, and genital
edema.
7. Anorexia, irritability, abdominal pain, and diarrhea are common. Important features
of minimal change idiopathic nephrotic syndrome are the absence of hypertension
and gross hematuria (previously termed nephritic features).
8. A diagnosis other than MCNS should be considered in:
10
▫ children <1 yr of age.
▫ a positive family history of nephrotic syndrome.
▫ presence of extrarenal findings (e.g., arthritis, rash, anemia).
▫ hypertension or pulmonary edema.
▫ acute or chronic renal insufficiency.
▫ gross hematuria.
Differential Diagnosis :
The differential diagnosis of the child with marked edema includes:
protein-losing enteropathy.
hepatic failure.
heart failure.
acute or chronic glomerulonephritis.
protein malnutrition.

11
Diagnosis :
1. Urinalysis :
Proteinuria: 3+ or 4+
Urinary protein excretion exceeds 40 mg/m2/hr.
A spot urine protein: creatinine ratio exceeds 2.0.
Microscopic hematuria is present in 20%.
Serum creatinine value is usually normal, but it may be abnormally
elevated if there is diminished renal perfusion from contraction of the
intravascular volume.
2. Serum albumin level is <2.5 g/dL.
3. Serum cholesterol and triglyceride levels are elevated.
4. Serum complement levels are normal.
5. Renal biopsy: if there is atypical feature( suggest other than MCNS):
Gross hematuria.
Hypertension.
Renal insufficiency.
Hypocomplementemia.
Age <1 yr or >8 yr.
Frequently relapsing nephrotic syndrome.
Steroid-dependent nephrotic syndrome.
Steroid-resistant nephrotic syndrome .
Treatment :
1. Children with their first episode of nephrotic syndrome and mild to moderate edema
may be managed as outpatients.
2. The pathophysiology and treatment of nephrotic syndrome must be carefully
reviewed with the family to enhance understanding of their child's disease. The
child's parents must be able to recognize the signs and symptoms of the
complications of the disease and its treatment and must be taught how to use and
interpret the results of urinary dipstick testing for protein.
3. Children with nephrotic syndrome should attend school and participate in physical
activities as tolerated.
4. Low sodium diet until remission.

12
5. Oral diuretics used with cautious if needed because the risk of thromboembolic
complication.
Indication for hospitalization:
1. Initial episode may need hospitalization for teaching of parents.
2. Children with severe symptomatic edema, including:
▫ Large pleural effusions.
▫ Ascites.
▫ Severe genital edema.
▫ Infection (e.g., severe cellulitis, peritonitis)
3. Compromised renal function
4. Significant hypertension
5. Significant electrolyte abnormalities
Treatment in hospital:
1. Sodium restriction.
2. Fluid restriction may be necessary if the child is hyponatremic.
3. A swollen scrotum may be elevated with pillows to enhance fluid removal by gravity.
4. Diuresis may be augmented by the administration of loop diuretics (furosemide),
orally or intravenously, with caution.
5. IV administration of 25% albumin (0.5-1.0 g albumin/kg), as a slow infusion followed
by furosemide (1-2 mg/kg/dose IV) is sometimes necessary when a patient has
significant generalized edema with evidence of intravascular volume depletion (e.g.,
hemoconcentration). Such therapy mandates close monitoring of volume status,
blood pressure, serum electrolyte balance, and renal function. Symptomatic volume
overload, with hypertension, heart failure, and pulmonary edema is a potential
complication of parenteral albumin therapy, particularly when administered as rapid
infusions.
6. Children with typical feature suggestive MCNS, prednisone is started without need
for biopsy.
7. prednisone should be administered at a dose of 60 mg/m2/day or 2 mg/kg/day
(maximum daily dose, 60 mg) in a single daily dose for 4-6 consecutive wk.
8. An initial 6-wk course of daily steroid treatment leads to a significantly lower relapse
rate than previously recommended shorter courses of daily therapy.
9. About 80-90% of children respond to steroid therapy (clinical remission, diuresis, and
urine trace or negative for protein for 3 consecutive days) within 3 wk. The vast
majority of children who respond to prednisone therapy do so within the first 5 wk of
treatment.

13
After the initial 6-wk course, the prednisone dose should be tapered to
40 mg/m2/day given every other day as a single daily dose for at least 4 wk.
The alternate-day dose is then slowly tapered and discontinued over the next 1-2 mo.
There is evidence that both an increased dose of steroids and a prolonged duration
of therapy are important factors in reducing the risk of relapse, but the frequency of
SE is higher.
Children who continue to have proteinuria (2+ or greater) after 8 wk of steroid
therapy are considered steroid resistant, and a diagnostic renal biopsy should be
performed.
Relapse :
• Many children with nephrotic syndrome experience at least 1 relapse (3-4+
proteinuria plus edema). Although relapse rates decrease after treated with longer
initial steroid courses.
• Relapses should be treated with 60 mg/m2/day (80 mg daily max) in a single am dose
until the child enters remission (urine trace or negative for protein for 3 consecutive
days). The prednisone dose is then changed to alternate-day dosing as noted with
initial therapy, and gradually tapered over 1-2month.
• Patients who respond well to prednisone therapy but relapse ≥4 times in a 12-mo
period are termed frequent relapsers.
• A subset of patients relapse while on alternate-day steroid therapy or within 28 days
of completing a successful course of prednisone therapy. Such patients are termed
steroid dependent.
• Children who fail to respond to prednisone therapy within 8 wk of therapy are
termed steroid resistant. Steroid-resistant nephrotic syndrome is usually caused by
FSGS (80%), MCNS, or mesangial proliferative glomerulonephritis.
Alternative therapies :
Steroid-dependent patients, frequent relapsers, and steroid-resistant patients are
candidates for alternative therapies, particularly if the child has severe corticosteroid
toxicity (cushingoid appearance, hypertension, cataracts, and/or growth failure).
Cyclophosphamide
Cyclosporine or tacrolimus
Mycophenolate

14
Levamisole
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II blockers may be
helpful as adjunct therapy to reduce proteinuria in steroid-resistant patients.
Complications :
1. Infection: is a major complication of nephrotic syndrome. Children in relapse have
increased susceptibility to bacterial infections because of:
▫ urinary losses of immunoglobulins and properdin factor B.
▫ defective cell-mediated immunity.
▫ their immunosuppressive therapy.
▫ Malnutrition.
▫ edema or ascites acting as a potential culture medium.
▫ Spontaneous bacterial peritonitis is a common infection. Although
Streptococcus pneumoniae is the most common organism causing peritonitis,
gram-negative bacteria such as Escherichia coli may also be encountered.
Because fever & physical finding may be minimal in presence of steroid
therapy, the patient's caregivers should be counseled to seek medical
attention if the child appears ill, has a fever, or complains of persistent
abdominal pain. A high index of suspicion for bacterial peritonitis, prompt
evaluation (including cultures of blood and peritoneal fluid), and early
initiation of antibiotic therapy are critical.
▫ Sepsis.
▫ Pneumonia.
▫ Cellulitis.
▫ Urinary tract infections.
2. Thromboembolic events:
▫ The incidence of this complication in children is 2-5%.
▫ Both arterial and venous thromboses may be seen, including renal vein
thrombosis, pulmonary embolus, sagittal sinus thrombosis, and thrombosis of
indwelling arterial and venous catheters.
▫ The risk of thrombosis is related to:
Increased prothrombotic factors (fibrinogen, thrombocytosis,
hemoconcentration, relative immobilization).

15
Decreased fibrinolytic factors (urinary losses of antithrombin III, proteins
C and S).
▫ Prophylactic anticoagulation is not recommended in children unless a previous
thromboembolic event has occurred.
▫ To minimize the risk of thromboembolic complications, aggressive use of
diuretics and the use of indwelling catheters should be avoided if possible.
Prognosis :
Steroid-responsive nephrotic syndrome:
Most children with steroid-responsive nephrotic syndrome have repeated
relapses, which generally decrease in frequency as the child grows older.
Although there is no proven way to predict an individual child's course,
children who respond rapidly to steroids and those who have no relapses
during the first 6 mo after diagnosis are likely to follow an infrequently
relapsing course.
It is important to indicate to the family that the child with steroid-responsive
nephrotic syndrome is unlikely to develop chronic kidney disease, that the
disease is rarely hereditary, and that the child (in the absence of prolonged
cyclophosphamide therapy) will remain fertile.
To minimize the psychologic effects of the condition and its therapy, children
with idiopathic nephritic syndrome should not be considered chronically ill and
should participate in all age-appropriate childhood activities and maintain an
unrestricted diet when in remission.
Steroid-resistant nephrotic syndrome:
Children with steroid-resistant nephrotic syndrome, most often caused by
FSGS, generally have a much poorer prognosis.
These children develop progressive renal insufficiency, ultimately leading to
end-stage renal disease requiring dialysis or kidney transplantation.
Recurrent nephrotic syndrome develops in 30-50% of transplant recipients
with FSGS.
16
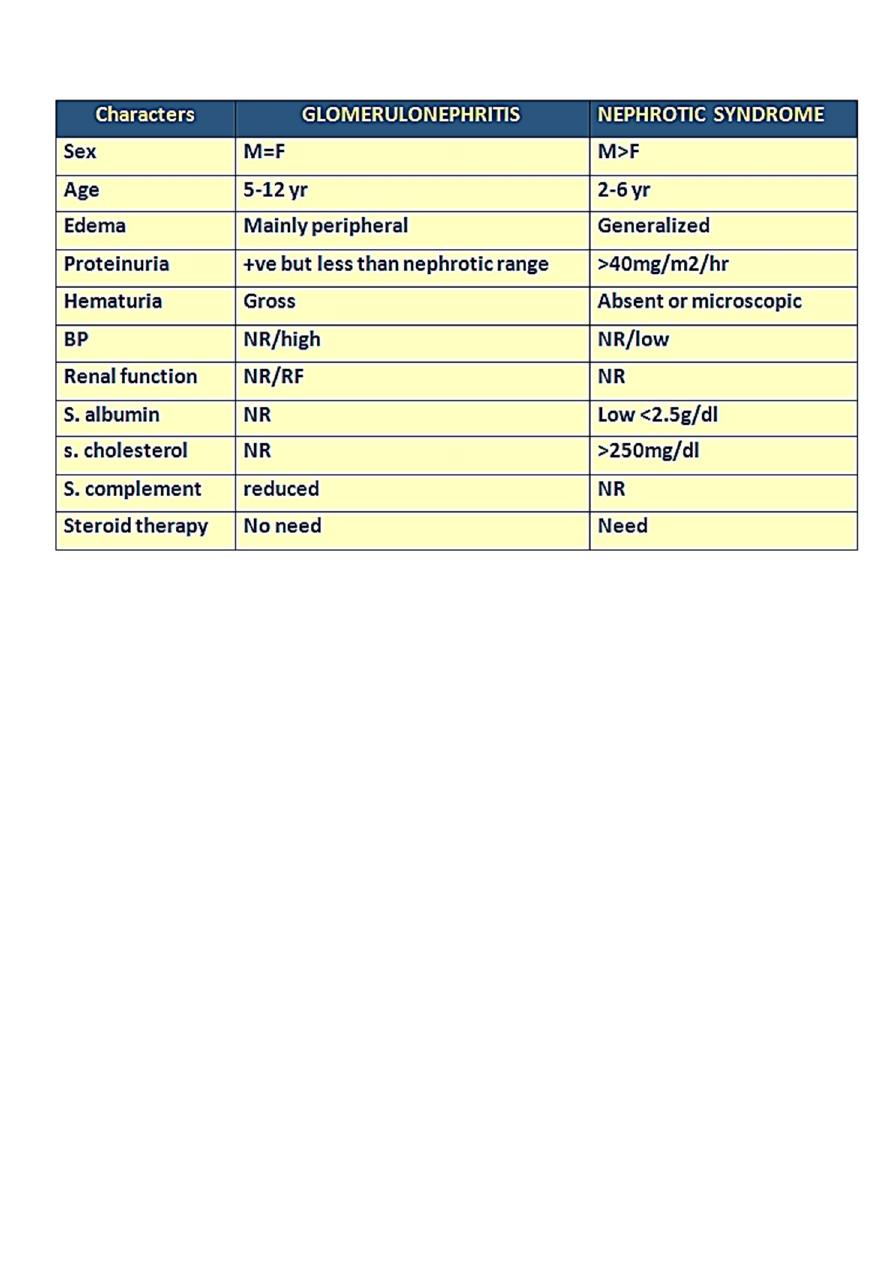
The difference between APGN & NS :
