
1
Oxygen Therapy
Oxygen was discovered independently by the Swedish apothecary Karl W.Scheele, in
1772, and by the English amateur chemist Joseph Priestly,in August 1774.
Priestley first liberated oxygen by intensely heating 'mercurius calcinatus' (mercuric
oxide) placed over liquid mercury in a closed vessel. He called this new gas
"dephlogisticated air, "oxygenated."
Joseph Priestley and Carl Wilhelm Scheele both independently discovered oxygen,
but Priestly is usually given credit for the discovery.
Priestley called the gas produced in his experiments 'dephlogisticated air' and
Scheele called his 'fire air'.
The name oxygen was created by Antoine Lavoisier who incorrectly believed that
oxygen was necessary to form all acids.
The Element Oxygen
• Atomic Number: 8
• Atomic Weight: 15.9994
• Melting Point: 54.36 K (-218.79°C or -361.82°F)
• Boiling Point: 90.20 K (-182.95°C or -297.31°F)
• Density: 0.001429 grams per cubic centimeter
• Phase at Room Temperature: Gas
• Element Classification: Non-metal
• Period Number: 2
• Group Number: 16 Group Name: Chalcoge
Oxygen is a drug
Colorless, odorless, tasteless gas, makes up 21% of room air .It is NOT flammable but
does support combustion.
• should be regarded as a drug .
• Has a Drug Identification Number (DIN)
• Oxygen must be prescribed in all situations (except for the immediate
management of critical illness).
• Oxygen should be prescribed to achieve a target saturation (Sp02), which
should be written on the drug chart .
2
Basic Concepts of Oxygen
• Composition of Room Air Nitrogen 78.08% ~78% Oxygen 20.946% ~21% Trace
gases ~1%
• Normal PO2 in arterial blood (PaO2) ≥ 95mmHg: decrease with age.
• PO2 in mitochondria ≥ 18 mmHg required to generate high energy phosphate
bonds e.x ATP
• At rest the average adult male consumes about 225-250 ml of O2/min.
• This can increase up to 10 folds during exercise.
• There’s very small O2 reserve that can be consumed within 4-6 minutes of
cessation of spontaneous ventilation.
Oxygen content of blood
The theoretical maximum oxygen carrying capacity is 1.39 ml O2/g Hb, but direct
measurement gives a capacity of 1.34 ml O2/g Hb.1.34 is also known as Hüfner’s
constant.
The oxygen content of blood is the volume of oxygen carried in each 100 ml blood.
It is calculated by: (O2 carried by Hb) + (O2 in solution) = (1.34 x Hb x SpO2 x 0.01) +
(0.023 x PaO2)
Basic Concepts of Oxygen
Oxygen Cascade:
Inspired = 150 mmHg at Sea Level
↓ Alveolar PO
2
= 103
↓ Arterial=100
↓ Capillary= 51
↓ Mitochondrial= 1-10
(FiO
2
expressed as 0.21-1.0 or 21- 100%)

3
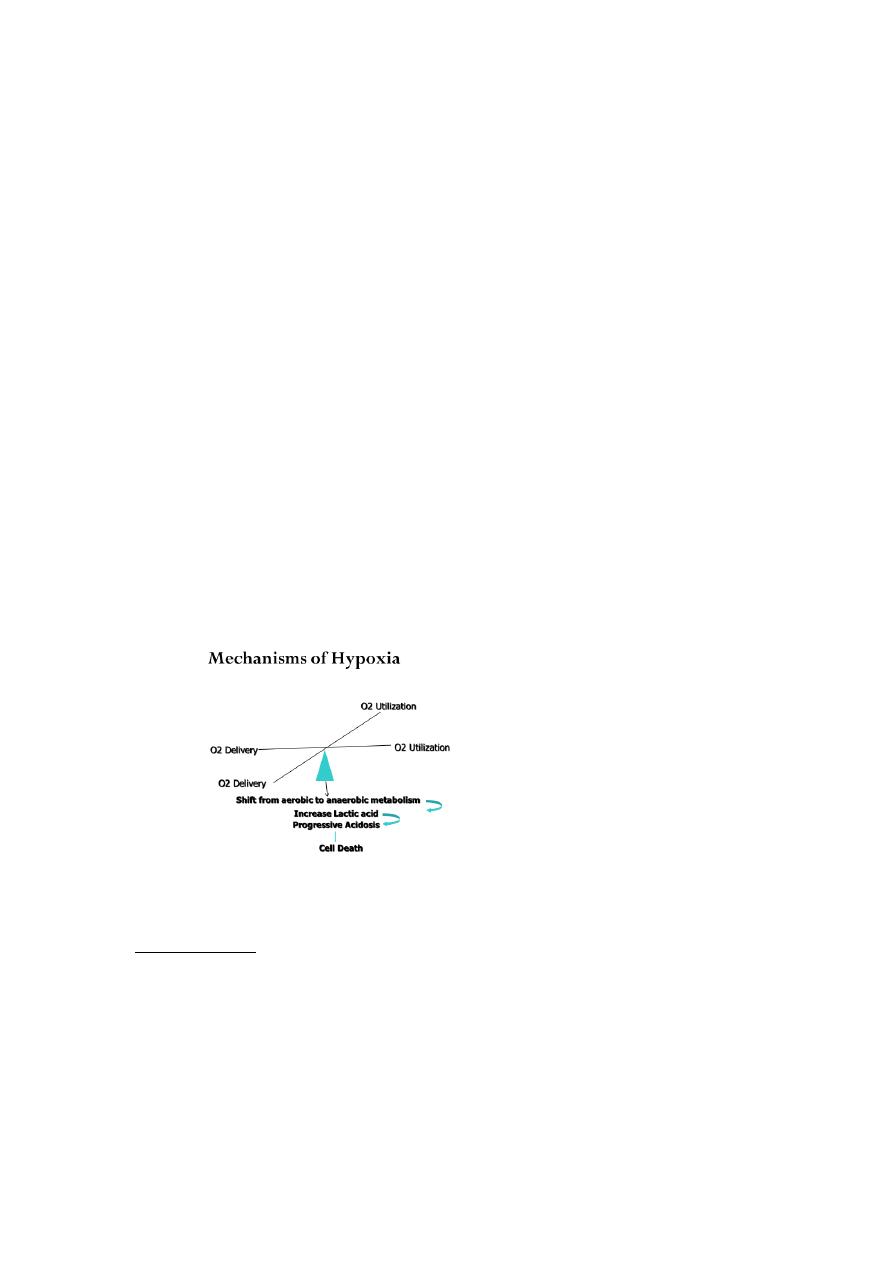
Clinical Conditions With Increased Risk of Hypoxia
1. Myocardial infarction
2. Acute pulmonary disorders
3. Sepsis
4. Drug overdose
5. Liver failure
6. Head trauma
7. CHF
8. Hypovolemic shock
9. Blunt chest trauma
10. Acute neuromuscular disease
11. Acute abdomen (splinting)
12. Acute pancreatitis
13. Spinal cord injury
Indications for Oxygen Therapy
1. Tachypnea
2. Cyanosis
3. Restlessness
4. Disorientation
5. Cardiac arrhythmias
6. Slow bounding pulse
7. Tachycardia
8. Hypertension
9. Dyspnea
10. Coma
11. Labored breathing (use of accessory muscles, nasal flaring)
12. Lethargy
13. Tremors/seizure activity
“Generally speaking”, a patient who is breathing less than 12 and more than 24 times
a minute needs oxygen of some kind
Oxygen therapy To ensure safe and effective treatment
Oxygen is required for the functioning and survival of all body tissues and deprivation
for more than a few minutes is fatal.
In immediately life threatening situations oxygen should be administered.
Hypoxaemia. Acute hypotension. Breathing inadequacy. Trauma. Acute illness. CO
poisoning. Severe anaemia. During the peri-operative period.

4
Oxygen therapy Humidification Is recommended if more than 4 litres/min is delivered.
Helps prevent drying of mucous membranes.
Helps prevent the formation of tenacious sputum.
Oxygen concentrations will be affected with all delivery systems if not fitted correctly
or tubing becomes kinked and ports obstructed.
The oxyhaemoglobin dissociation curve showing the relation between partial
pressure of oxygen and haemoglobin saturation
Methods of Oxygen Delivery
Most common methods of oxygen delivery include
1. Nasal Cannula
2. Venturi Mask
3. 100% Non-Rebreather Mask
4. Mechanical Ventilation
1. Nasal Cannula
Comfortable, convenient, mouth breathing will not effect % of O2 delivered
Liters/min = %
• 2 l/m = 24-28%
• 3 l/m = 28-30%
• 4 l/m = 32-36%
• 5 l/m = 36-40%
• 6 l/m = 40-44%
Cannot administer > 6 liters/minute (44%)

5
Provides limited oxygen concentration
Used when patients cannot tolerate mask
Prongs and other uses
Concentration of 24 to 44%
Flow rate set between 1 to 6 liters
For every liter per minute of flow delivered, the oxygen concentration the
patient inhales increases by 4%
2. Venturi Mask
FiO2 Delivery
• Blue 24% Yellow 28%
• White 31% Green 35%
• Pink 40%
Concerns
Tight seal is a must Interferes with eating/drinking
Condensation collection
Provides precise concentrations of oxygen
Entrainment valve to adjust oxygen delivery
Mostly used in the hospital setting for COPD patients
6
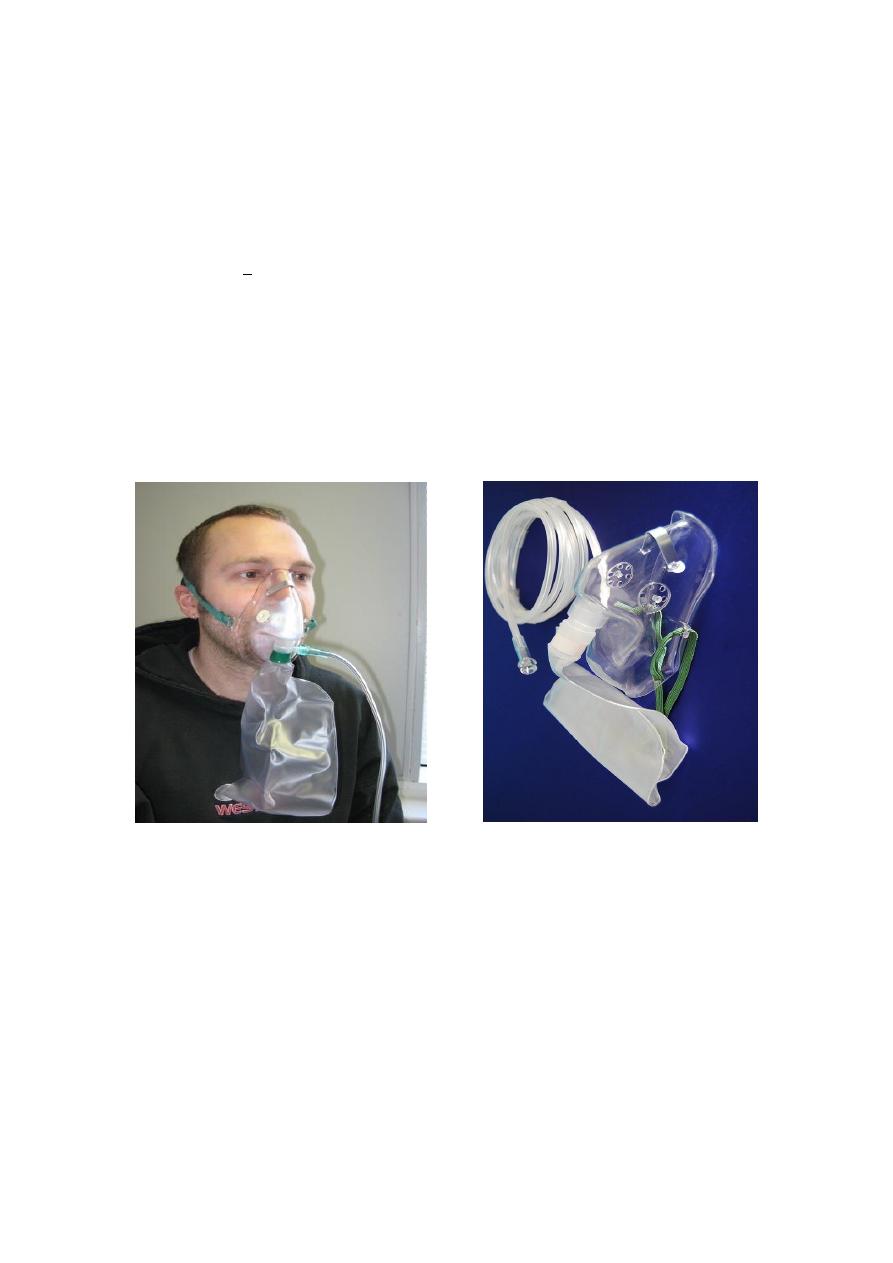
3. 100% Non-Rebreather
Delivery percentages
• 6 l/min = 55 – 60 %
• 8 l/min = 60 – 80 %
• 10 l/min = 80 – 90 %
• >12 l/min = 90 + %
Benefit: Has a one way expiratory valve that prevents re-breathing expired
gases
Concern
May lead to O2 toxicity
100% Non-Rebreather Mask partial rebreather Mask
4. Mechanical Ventilation
Allows administration of 100% oxygen
Controls breathing pattern for patients who are unable to maintain adequate
ventilation
Is a temporary support that “buys time” for correcting the primary pathologic
process

7
Indications for Mechanical Ventilation
1. Mechanical Failure
2. Ventilatory Failure
3. Oxygenation Failure
4. General Anesthesia
5. Post-Cardiac Arrest
Two categories of ventilators
1. Negative pressure ventilators
• Iron lung
• Cuirass ventilator
2. Positive pressure ventilators
Two categories
• Volume-cycled (volume-preset)
• Pressure-cycled (pressure-preset)
Mechanical Ventilation PEEP
• Description
Maintains a preset positive airway pressure at the end of expiration
Increases PaO2 so that FiO2 can be decreased
Increases DO2 (amt of delivered O2 to tissue)
Maximizes pulmonary compliance
Minimized pulmonary shunting
• Indications
1. PaO2 < 60 on FiO2 > 60% by recruiting dysfunctional alveoli
2. Increases intrapulmonary pressure after cardiac surgery to decrease
intrathoracic bleeding (research does not support this idea)
• Advantages
1. Improves PaO2 and SaO2 while allowing FiO2 to be decreased
2. Decreases the work of breathing
3. Keeps airways from closing at end expiration (esp. in pts with surfactant
deficiency)
• Disadvantages
1. Increased functional residual capacity (increases risk for barotrauma)
8
2. Can cause increased dead space and increased ICP
3. In pts with increased ICP, must assure CO2 elimination
4. Contraindicated: hypovolemia, drug induced low cardiac output,
unilateral lung disease, COPD
Mechanical Ventilation CPAP
• Description
Constant positive pressure is applied throughout the respiratory cycle
to keep alveoli open
• Indications
1. To wean without having to remove the ventilator and having to connect
to additional equipment
2. Mechanical Ventilation CPAP
• Advantages
Takes advantage of the ventilator alarm systems providing
psychological security of the ventilator being there
• Disadvantages
Patient may sense resistance as he breathes through the ventilator
tubing

9
Mechanical Ventilation Complications
1. Respiratory arrest from disconnection
2. Respiratory infection (VAP)
3. Acid-base imbalances
4. Oxygen toxicity
5. Pneumothorax
6. GI bleeding
7. Barotrauma
8. Decreased cardiac output
Ventilator Weaning
• Vital Capacity at least 10 – 15 ml/kg
• Tidal Volume > 5 ml/kg
• Resting minute volume > 10 L per minute
• ABG’s adequate on < 40% FiO2
• Stable vital signs
• Intact airway protective reflexes (strong cough)
• Absence of dyspnea, neuromuscular fatigue, pain, diaphoresis, restlessness,
use of accessory muscles
Primary Acid-base Disorders:
1. Respiratory alkalosis - A primary disorder where the first change is a lowering of
PaCO
2
, resulting in an elevated pH. Compensation (bringing the pH back down
toward normal) is a secondary lowering of bicarbonate (HCO
3
) by the kidneys; this
reduction in HCO
3
-
is not metabolic acidosis, since it is not a primary process.
Primary Event
Compensatory Event
HCO
3
-
↓HCO
3
-
↑ pH ~ ------- ↑ pH ~ --------
↓ PaCO
2
↓
PaCO
2
:

10
2. Respiratory acidosis - A primary disorder where the first change is an elevation of
PaCO
2
, resulting in decreased pH. Compensation (bringing pH back up toward
normal) is a secondary retention of bicarbonate by the kidneys; this elevation of
HCO
3
-
is not metabolic alkalosis since it is not a primary process.
Primary Event Compensatory Event
HCO
3
-
↑
HCO
3
-
↓ pH ~ ---------
↓ pH ~ ---------
↑PaCO
2
↑
PaCO
2
3. Metabolic acidosis - A primary acid-base disorder where the first change is a
lowering of HCO
3
-
, resulting in decreased pH. Compensation (bringing pH back up
toward normal) is a secondary hyperventilation; this lowering of PaCO
2
is not
respiratory alkalosis since it is not a primary process.
Primary Event
Compensatory Event
↓ HCO
3
-
↓HCO
3
-
↓ pH ~ ------------ ↓ pH ~ ------------
PaCO
2
↓
PaCO
2
4. Metabolic alkalosis - A primary acid-base disorder where the first change is an
elevation of HCO
3
-
, resulting in increased pH. Compensation is a secondary
hypoventilation (increased PaCO
2
), which is not respiratory acidosis since it is not
a primary process. Compensation for metabolic alkalosis (attempting to bring pH
back down toward normal) is less predictable than for the other three acid-base
disorders.
Primary Event Compensatory Event
↑
HCO
3
-
↑HCO
3
-
↑ pH ~------------ ↑ pH ~ ---------
PaCO
2
↑PaCO
2

11
Some Clinical Causes
METABOLIC ACIDOSIS
↓HCO
3
-
& ↓
pH
a. Increased anion gap
• lactic acidosis; ketoacidosis; drug poisonings
(e.g., aspirin, ethylene glycol, methanol)
b. Normal anion gap
• diarrhea; some kidney problems (e.g., renal
tubular acidosis, interstitial nephritis)
METABOLIC ALKALOSIS
↑ HCO
3
-
& ↑
pH
a. Chloride responsive (responds to NaCl or KCl therapy): contraction
alkalosis, diuretics, corticosteroids, gastric suctioning, vomiting
b. Chloride resistant: any hyperaldosterone state (e.g., Cushing’s
syndrome, Bartter’s syndrome, severe K
+
depletion)
RESPIRATORY ACIDOSIS
↑PaCO
2
& ↓
pH
a. Central nervous system depression (e.g., drug overdose)
b. Chest
bellows
dysfunction
(e.g.,
Guillain-Barré
syndrome,
myasthenia gravis)
c. Disease of lungs and/or upper airway (e.g., chronic obstructive lung
disease, severe asthma attack, severe pulmonary edema)
RESPIRATORY ALKALOSIS ↓PaCO
2
&
↑
pH
a. Hypoxemia (includes altitude)
b. Anxiety
c. Sepsis
d. Any acute pulmonary insult (e.g., pneumonia, mild asthma attack, early
pulmonary edema, pulmonary embolism)
