RUMINAL FLUID EXAMINATIONS
Dr. Eva A AjajMsc. Clinical pathology
Indication of Ruminal fluid examination
-Diagnosis of ruminal diseases-Evaluation of ruminal fluid before use in therapeutic transfusion
Methods of collection:
-Needle puncture of the rumen
-Oral or nasal passage of stomach tubeGeneral precaution for rumen fluid examination:
-Samples should be evaluated directly after collection to minimize effects of cooling and air exposure on protozoal activity.-Transportation of ruminal fluid for long distance must be done in double jacket container.
-Estimation of chloride and ammonia conc. can be delayed up to 9 hrs. in room temp. and up to 24 hrs. in refrigerator.
Examination of ruminal fluid
Physical characters:
-Color
-Consistency
-Odor
-Sedimentation activity test
Chemical characters:
-pH
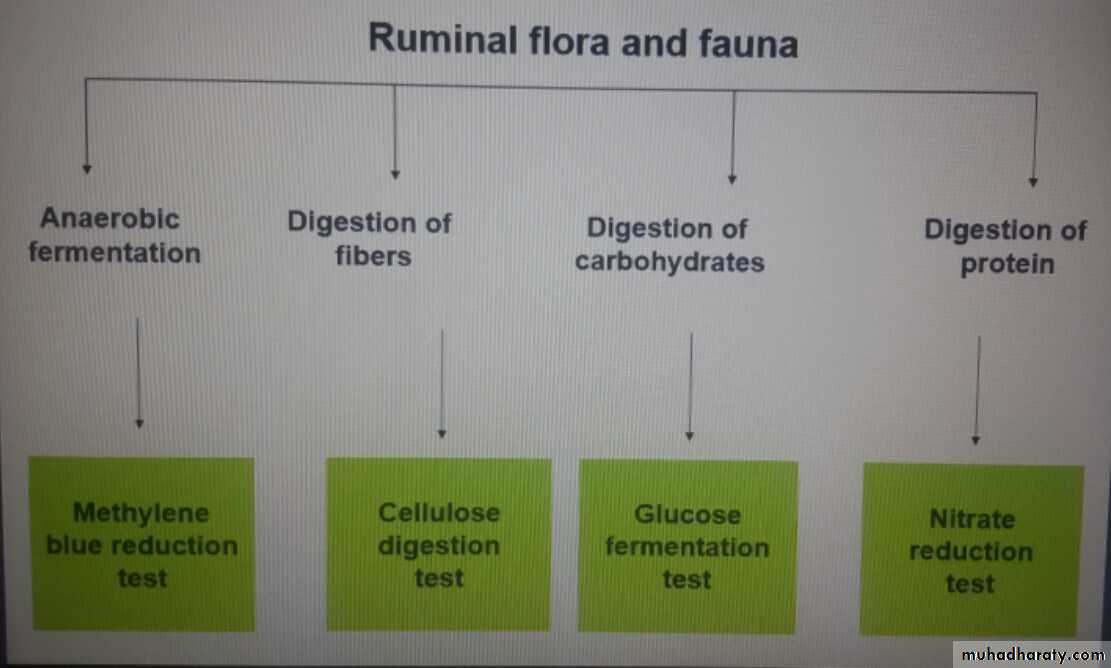
-Cellulose digestion test
-Glucose fermentation test
-Nitrate reduction test
-Rumen fluid chloride
Microscopical exam:
-Quantitative exam.
-Qualitative exam
Physical characters
-ColorNormal : Olive to brownish green (hay ration) Deeper green color (green ration) Yellowish brown color (grain or silage ration)
Abnormal: Milky grey (grain overfeeding) Darker greenish (ruminal stasis/decomposition) Grey with clots of milk (calves with abomasal reflux)
Physical characters
-ConsistencyNormal: Slightly viscous
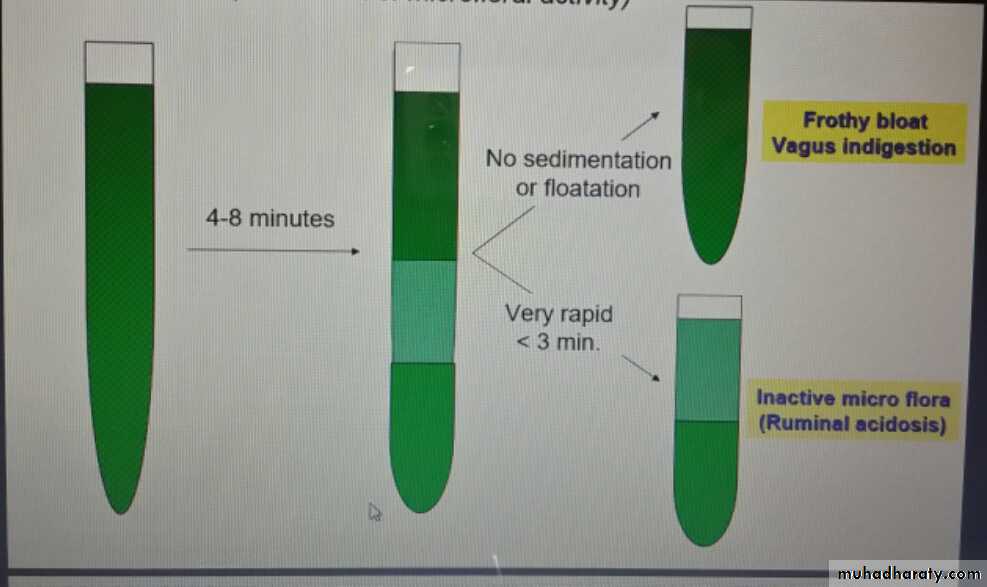
Abnormal: Watery ( Inactive bacteria or protozoa) Excess frothy (Frothy bloat/ vagus indigestion)
-Odor
Normal: Aromatic odor
Abnormal: Ammonia smell (Urea poisoning) Moldy rotting (protein putrefaction) Sour odor (excess lactic acid/grain overfeeding)
-Sedimentation activity test (Evaluation of microfloral activity)
Chemical characters:
-ruminal fluid pHNormal: ranged between 5.5 –6.5 (grain feeders)
and 6 –7 (green fodders)
Abnormal:
Elevated pH (Rumen alkalosis)
-Simple indigestion
-Urea indigestion
-Putrefaction of ruminal content
Lowered pH (Rumen acidosis)
-Grain overfeeding
-chronic ruminal acidosis
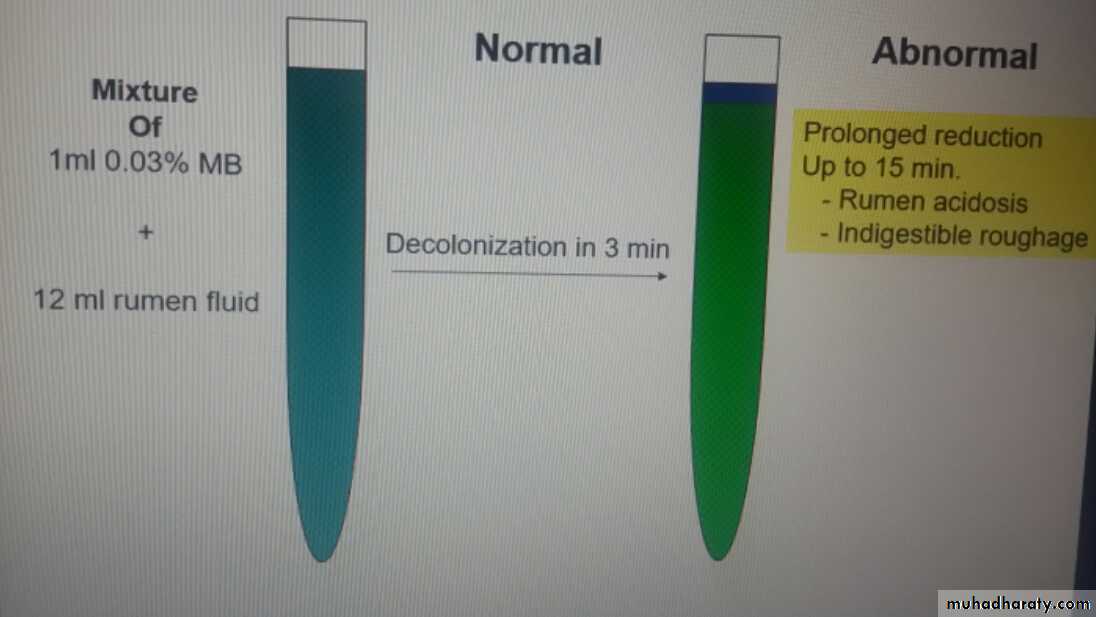
-Methylene blue reduction test
-Cellulose digestion test
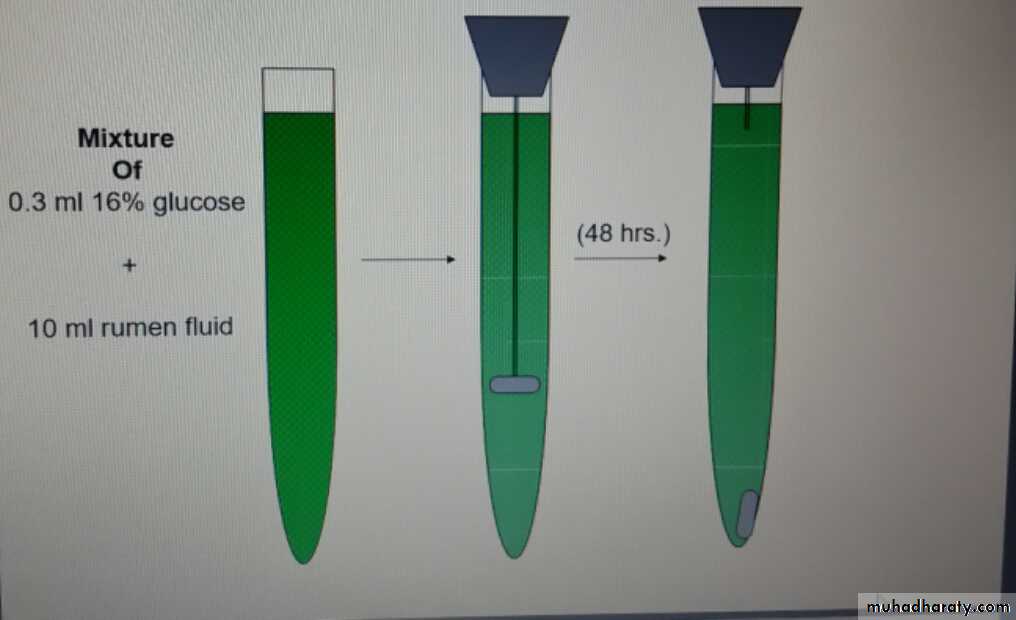
-Glucose fermentation test
0.5 ml of 16% glucose + 10 ml. of rumen fluidPlace the mixture in a fermentation saccharometer
Keep the saccharometerat 39˚C
Read the results after 30 –60 min.
The test measure indirectly the ability of ruminal flora to ferment glucose through measuring the volume of formed gas
Normal microflora (1-2 ml gas production / 1hour)
Inactive microflra (little or no gas formation)
-Nitrate reduction test
It is provide an idea on activity of microbes that synthesize nitrogen compounds.
10 ml of sieved ruminal fluid is placed into each of 3 test tubes and 0.2, 0.5, 0.7 ml of 0.025% potassium nitrate solution is added to 3 tubes.
Put the 3 tubes in water both at 39˚c. Every 5 minutes, one drop from each tube is placed in the small ceramic plate.
To each drop is added 2 drops of reagent 1 and 2 drops of reagent2.
Observe the change of color.
Sample that contain nitrates are colored red.
Rumen fluid of cattle fed a mixed ration will not change in color after 5-10 minutes in tube 1 and 20 minutes in tube II, and 30 minutes in tube III.
Reduction is more rapid when cattle are green fodder or have ruminal decomposition or bloat.
Reduction is more slower when a deficient ration is fed or when the animal lacks the appetite
1 Reagent I: 2 ml of sulphanilic acid in 30% acetic acid to make 200 ml.
2 Reagent II: 0.6 ml alpha-naphthylamine + 16 ml concentration acetic acid + 140 ml D.W
-Rumen fluid chloride
-Measured in a supernatant of a centrifuged sample-Measured by chloride meter.
-Normal level is ; 30 mEq/l
-Elevated level :
Abomasal disease.
* Abomasal reflux.
* Obstruction of intestinal flow
Microscopical examination
Qualitative method
-Prepare a fresh film.
-Examine by low power.
-The activity of the fauna is judged as follow:
Motility Activity
-Highly motile and very crowded +++
-Motile and crowded ++
-Sluggish motility and low numbers +-No or sporadic alive fauna 0
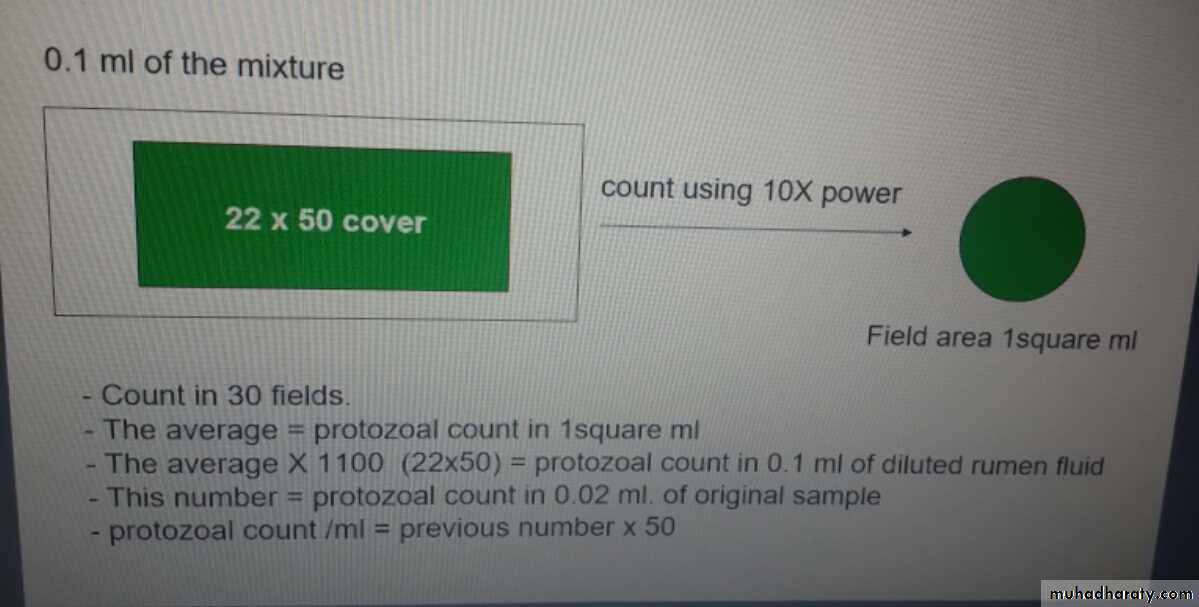
Quantitative method-Mix 1 ml of strained rumen fluid with 15 ml of saline and 5 ml of lugol’siodine.
-Spread 0.1 ml of the mixture on glass slide under
0.1 ml of the mixture
Examination of the Bacteria
Bacteria of the fore stomachs are vital for ruminants. Their concentration varies between 10^7 and 10^12 per ml of fluid rumen contents or per gram of solid contents. Standard bacteriological techniques such as direct counting and culture for identification, differentiation and colony counts have so far found no application in the clinical examination of rumen fluid.
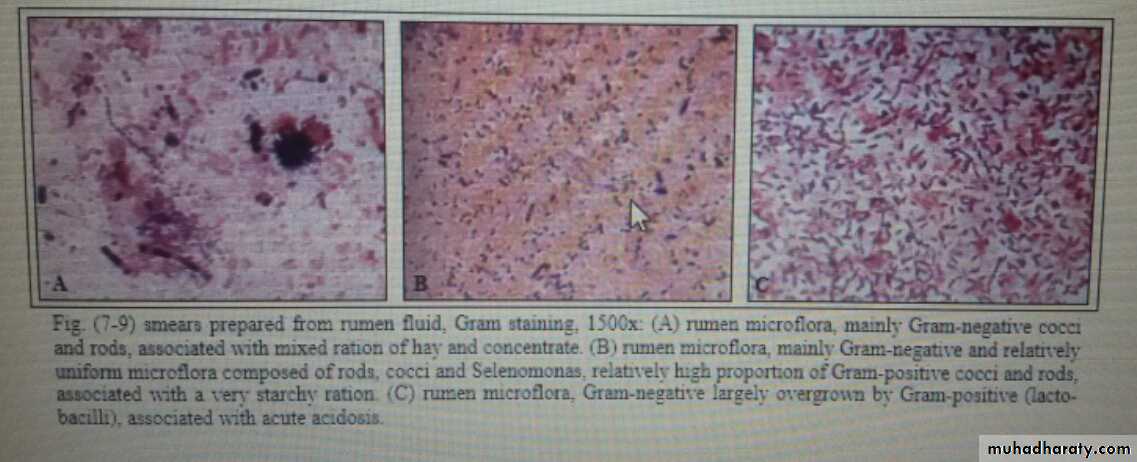
Microscopical observation of the fore stomach microflora is useful for diagnosis of rumen acidosis. Air-dried smear of rumen fluid is stained by Gram's method, or other staining methods as nigrosine, Congo red, modification of Giemsa stain can be applied to fresh or fixed specimens Criteria used for interpretation of the smear are:
Presence or absence of morphologically distinguishable bacterial species characteristic of a normal rumen flora, which called "leading bacteria".
The multiplicity or uniformity forms.
The ratio of Gram-positive to Gram-negative bacteria.A diet rich in starch produces a more uniform picture, with Gram-negative cocci, short and long rods, and a relatively high proportion of Gram-positive cocci and rods
A digestive disorder involving ruminal inactivity or decomposition may be suspected when the leading bacteria associated with a particular ration are absent, when there is unusual uniformity in the microflora, or when there are bacteria that are not normally present