DR.EVA A AJAJ
Serology & IMMUNOLOGY• Some bacteria and viruses can not be identified by biochemical properties, but can be typed by the serological means. Also, it has been found that the diagnosis or detection of antibodies is more easier than the agent that produce the disease.
Serology & IMMUNOLOGY
There are 5 types of antibodies (immunoglobulins) as follows:
IgM. It has large molecular weight, and appear following initial exposure to anti-gens.IgG. It has small molecular weight, and comprise 80-85% of immunoglobulins. It is the predominant immunoglobulin in the serum, protecting against bacteria and viruses, and neutralize bacterial toxins.
IgA. It is approximately comprise 15% of immunoglobulins, selectively transported across mucous membranes, and it is the principal immunoglobulin in the body secretions (secretary antibody). It is prevent attachment of disease-producing agents to mucous membranes surface.
IgE. It is present in a lower concentration in the serum, and associated with allergic reactions.
IgD. It is immature antibody, function as an antigen receptor on B cells.
Applications of serological tests:
Determination of different serotypes of the microorganisms or their antigenic structures. Diagnosis of some bacterial and viral diseases.
Blood groupings and medico-legal tests.
(1) Rapid slide method
Agglutination Tests
It is a rapid qualitative test used for the rapid diagnosis and as field test for:
(a) determination of the antigenic structure of bacteria: for typing different serotypes of
Salmonella, E. coli, Pasteurella multocida, etc.,
Materials required: Slide agglutination test
Fat free glass slides.
Pure colonies of suspected strain.
Diagnostic antisera.
Sterile saline.
Technique:
Make a suspension of the smooth colonies in saline on one end of the slide, and on the
other end make a control from suspension only.
Add 2 drops of corresponding antisera to the suspension.
Read the result within one minute after continuous shaking.
(b) whole blood agglutination test: for identification of blood group and some poultry diseases.
Materials required:
• Agglutination plate.
• One drop of fresh blood.
• Stained antigen.
Result:
Clumping and agglutination within one minute is a positive result.
Suspension with regular turbidity is a negative result.
Rose-Bengal plate agglutination test
For Brucella abortus (colored antigen)
It is a quantitative test determine the level of antibodies in serum.
Materials required:Seven agglutination tubes.
Sterile pipettes (1 ml and 10 ml).
Known standard antigen suspension.
Serum from diseased animal.
Normal or buffered sterile saline.
(2) Tube agglutination method "slow method"
Technique:
• 0.8 ml of phenol-saline is placed in the first tube and 0.5 ml in each succeeding tube.• 0.2 ml of serum under test in transferred to the first tube and mixed thoroughly with the phenol-saline already there.
• 0.5 ml of the mixture is carried over to the second tube from which, after mixing, 0.5 ml is transferred to the third tube, and so on continue until the last tube from which, after mixing, 0.5 ml of the serum dilution is discarded. This process of doubling dilutions results in 0.5ml of dilutions 1:5, 1:10, 1:20, and so on.
• Add to each tube 0.5 ml of antigen at the recommended dilution and the contents of the tube are thoroughly mixed, thus giving final serum dilutions of 1:10. 1:20, etc.
• The tubes are then incubated at 37˚c for 20 hours ± 1 hours before the results are read.
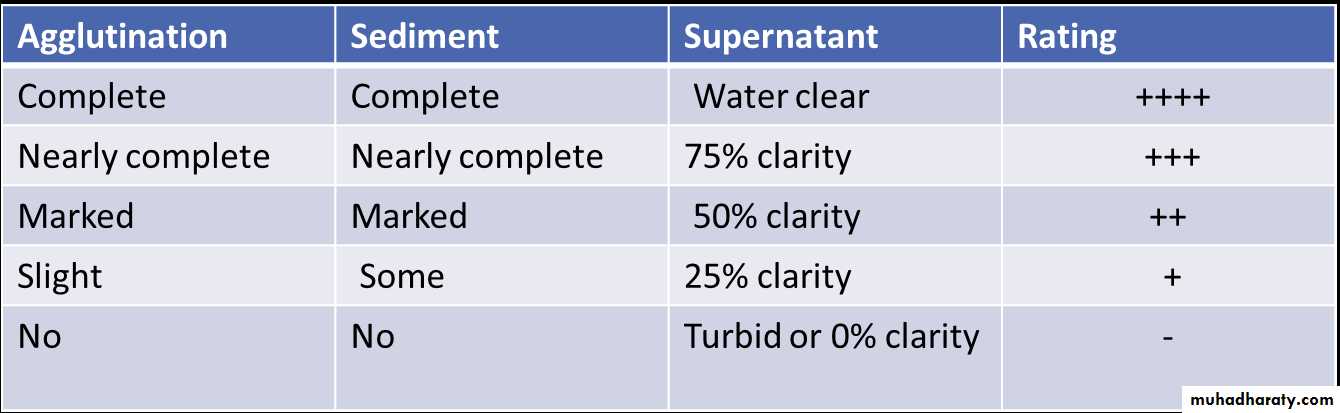
• The result are interpreted as follows
It is an important test in the diagnosis of viruses, because it provides a simple and rapid method by which virus can be detected in inoculated eggs, tissue culture or infected material.
Materials required:
Hemagglutination plate or agglutination tubes.
Pipettes 1 ml and 10 ml, and water bath 37ºc.
Suspected material containing the virus.
1% fowl RBCs suspension.
PBS 0.01 µ, pH 7.2.
Hemagglutination Test (HA):
Separate plasma from RBC by centrifuge cirated cells. Prepare a 0.5% suspension of RBC in PBS,
Prepare twofold serial dilution (1:10 to 1:640)
of virus suspension in PBS as follows:
Technique:
Dilutions
PBS(ml)
Virus(ml)
1:10
3.6
0.4
1:20
0.4
1:40
0.41:80
0.41:160
0.41:320
0.41:640
0.4Mix, transfer 0.4 ml
Mix, transfer 0.4 mlMix, transfer 0.4 ml
Mix, transfer 0.4 mlMix, transfer 0.4 ml
Mix, transfer 0.4 ml
Mix, discard 0.4 ml
• Add 0.4 ml of 0.5% RBC to all tubes.
• Control tube contain 0.4 ml of PBS plus 0.4ml of RBC.• Shake tubes and place them in a 37ºc water bath or at the appropriate temperature for the virus concerned until control cells have completely settled.
Result:
After lifting in room temperature for half to
one hour, read the result as (Fig 10-4):
• Positive: agglutination and clumping of RBC.
• Negative: precipitation of RBC.
Hemagglutination Inhibition Test (HI)
It is depends on the fact that the virus when mixed with its specific antibodies loses their power to agglutinate RBCs.HI-test is used for diagnosis of a number of viral diseases, and for antibody estimation.
Materials required:
• Hemagglutination plate and pipette.
• Standard concentration of known virus.
Technique:
• Make twofold serial dilutions of antiserum in PBS, beginning at 1:10.
• Distribute the serum dilution in 0.2 ml amount in small test tubes.
• Titrate the antigen as in the HA test.
• Determine the dilution which contains 4 units per 0.2 ml. for example: if the HA titer of the virus is 1:80, this represents 1HA unit, thus a dilution of 1:10 would contain 8HA units in 0.4 ml or 4HA units in 0.2 ml.
Include the following controls:
1. Serum alone 1:10 dilution, 0.4 ml.
2. Antigen alone, 4 units, 0.4 ml.3. Known positive serum, 1:10, 0.4 ml.
4. Known negative serum, 1:10, 0.4 ml.
5. As a check on dilution error or variations in red blood cells from one batch to another, run a back-titration on the 4
units per 0.2 ml dilution:
a. Add 0.4 ml PBS to each of 5 test
tubes.
b. To the first tube, add 0.4 ml of the virus dilution (in the case above,1:10) containing 8HA units. Mix and transfer 0.4 ml to the second tube.Continue and discard 0.4 ml from the last tube.
c. Incubate at room temperature for 30-
60 minutes.
d. Add 0.4 ml of 0.5ºc RBC to all tubes, shake, place all tubes at the appropriate temperature until control cell have completely settled.
e. Read the serum titer. Thus is the highest dilution of serum inhibiting haemagglutination by the virus and is expressed as the reciprocal of that serum dilution.
Result: Leave at 22˚c for half to one hour. Add 1% fowl RBCs suspension. Let at 22˚c for half to one hour. Read the result as follows:o Positive: no agglutination of RBC.o Negative: clumping and agglutination of RBC.
It is a microscopic test, used for diagnosis of different serotypes of Leptospira, and to determine the serum titer in animals suffering from Leptospirosis.
Materials used:
Dark-field illumination microscope.
Porcelain or plastic plates with depressions.
Well grown cultures of Leptospira.
Dilutions of patient's serum.
Sterile saline.
Result:
After incubation, put 1-2 drops of the serum antigen mixture on slide.
Read the result under dark-field illumination:
o Positive: clumping and agglutination of
Leptospira.
o Negative: lysis and reduction of the live
Leptospira.
Agglutination-Lysis Test
It is used when the antigen is in a colloid state (e.g. toxin or bacterial extract).
It is very useful serological test for identifying antigenic substances of all kinds.
There are different forms for application of the precipitation test:
• Flocculation Test "Ring form": It is used for:
Diagnosis of some infectious diseases, as Ascoli test in anthrax.
Classification of some microorganisms
Typing of different types of meat and blood spots.
Materials required:
Precipitation tubes with its rack.
Extract from lesions or microorganisms.
Specific antisera.
Result:
Apply controls for the extract and antisera, and read the results within 30 minutes to 1 hour:
Cloudy ring at the junction of extract and antisera: positive.
Clear fluid: negative.
Precipitation Test:
It is used for the diagnosis of anthrax. By means of a suitable anti-anthrax serum, the
presence of precipitinogens in the animal tissuescan be demonstrated.
Procedure:
Place 0.5 ml of anti-anthrax serum in a tube.
0.5 ml of a saline extract of the anthrax spleen, or other organ, this can be made by mincing the organ.
Mixed with normal saline.
Placed in a both of boiling water for few minutes, and filtered.
A distinct whitish precipitate forms at the junction of the two liquids.
Ascoli's test:
(2) Agar Gel Diffusion Test:
Gel of agar has been used to demonstrate the presence of diffusible antigen, by allowing antigen and antibody to diffuse towards each other. If the antigen and antibody are specific to each other, precipitation lines will be formed. The gel diffusion test is used for diagnosis of some viral diseases as cattle plague, mucosal disease
Agar gel immunodiffusion: (A) reaction of identify, the well on the left contained antiserum and the wells on the right homologous antigen. (B) reaction of partial identity showing spur formation
Material required:
Wasserman's tubes. Known standard antigen suspension.
Patient's serum (heated at 55˚c for 30 minutes to destroy the complement).
Complement1 (fresh serum of guinea pigs).
Procedure:
Mix the materials2, and incubate in water bath at 37˚c for 30 minutes.
Sensitized sheep erythrocytes 5% suspension (as indicator for the fixation of the complement).
Mix, and incubate in the water bath for 30-60minutes, and read the result :
o Positive: no hemolysis.
o Negative: hemolysis.
Complement Fixation Test:
ELISA used for the immunodiagnosis of infectious diseases to measure either antigen(direct, capture or sandwich ELISA), or antibody (indirect or competitive ELISA).
(1) Direct ELISA:
The antibody specific for the antigen to be detected is adsorbed to the surface of the wells of the microtiter plate.
A sample containing unidentified antigen is then added to each well3.
A second antibody specific for the antigen is then added if both the antibody adsorbed to the wall of the well and the antibody known to be specific for the antigen have reacted with antigen a "sandwich" will be formed, with the antigen between two antibody molecules. This reaction is visible only because the second added antibody is linked
to an enzyme, such as horseradish peroxidase or alkaline phosphatase unbound enzyme linked antibody is washed from the well.
The enzyme's substrate is added to it. Enzymatic activity is indicated by a color change that can be visually detected.
The test will be positive if the antigen has reacted with adsorbed antibodies in the first step. If the test antigen was not specific for the antibody adsorbed to the wall of the well the test will be negative because the unbound antigen will have been washed away.
Enzyme Linked Immunosorbent assay(ELISA)
A known antigen from the laboratory, rather than the antibody, is added to the shallow wells on the plate.
The antiserum is added to the well. If the serum contains antibody specific to the antigen, the antibody will bind to the
adsorbed antigen. All unreacted antiserum is washed from the well.
The conjugate (Anti-HISG) is then allowed to react with the antigen-antibody complex.
The anti-HISG, which has been linked with an enzyme, reacts with the antibodies that are bound to the antigens in the well.
Finally, all unbound anti-HISG is rinsed away and the correct substrate for the enzyme is added. A colored enzymatic reaction occurs in the wells in which bound antigen has combined with antibody in the serum sample
(2) Indirect ELISA:
Immunofluorescence, like ELISA, uses a labelled immunoglobulin for detecting anti-gen or antibody. The label employed is a fluorochrome, usually fluorescein isothiocyanate (FITC) or rhodamine isothiocyanate covalently attached to antibody molecules. When examined by ultra-violet light, fluorescein emits a characteristic green color and rhodamine a red color.
The procedure requires a special fluorescence microscope with mercury vapour light source.
There are many different methods of using fluorescent-labelled antibodies in microbiology and these include: direct, indirect and sandwich methods.
Immunofluorescence
It is frequently used for the detection of antibodies in serum. It is usually more sensitive than the direct test because more labelled antibody attaches per antigenic site.
Conjugated antibody is added directly to a smear, tissue section or monolayer.
Fix on a slide.
After incubation, unbound antibody is removed by washing.
The slide is examined in a fluorescence microscope and where labelled antibody binds to
antigen bright fluorescence is evident.
Indirect immunofluorescence: