
1
Fifth stage
Gynecology
Lec
Dr.Ahmad Jasim
D/A/2017
THE MANAGEMENT OF GESTATIONAL TROPHOBLASTIC
NEOPLASIA
DEFINITION
GTD is a spectrum of neoplastic disorders that arise from placental trophoblastic tissue after
abnormal fertilization.
Malignant GTD almost always following complete molar pregnancy, but can occur after any
gestation. e.g. abortion, ectopic, or term pregnancy. The maternal tumor arises from fetal not
maternal tissue.
HISTOLOGICAL TYPES OF GTD
1. Nonmalignant GTD:
a. Complete hydatidiform mole.
b. partial mole.
2. Malignant GTD which are
a.Persistent /invasive GTD
b. Choriocarcinoma
c. Placental site trophoblastic tumor
GESTATIONAL TROPHOBLASTIC NEOPLASIA (GTN)
1. Complete vesicular mole
2. Partial vesicular mole
3. Invasive mole
4. Placental-site trophoblastic tumor
5. Choriocarcinoma
EPIDEMIOLOGY
There is geographical variations in the incidence. Hydatidiform mole complicates 1-2 of
1000 pregnancies. Choriocarcioma occurs in 1 in every 30000 pregnancy
Malignant GTD is the fourth common gynecological malignancy in Iraq after carcinomas of
the ovary, endometrium and cervix.
2
Incidence
:
1:2000 pregnancies in United States and Europe, but 10 times more in Asia.
Predisposing factors include : Race , deficiency of protein or carotene
The incidence is higher toward the beginning and more toward the end of the
childbearing period.
It is 10 times more in women over 45 years old.
However, this may under represent the true incidence of the disease because of
problems with reporting, particularly with regard to partial moles.
Persistent GTN may develop after a :
1. Molar pregnancy,
2. Non-molar pregnancy or
3. Live birth.
The incidence after a live birth is estimated at 1/50 000.
Background
Hydatidiform mole can be subdivided into complete and partial mole based on:
1. Genetic
2. Histopathological features.
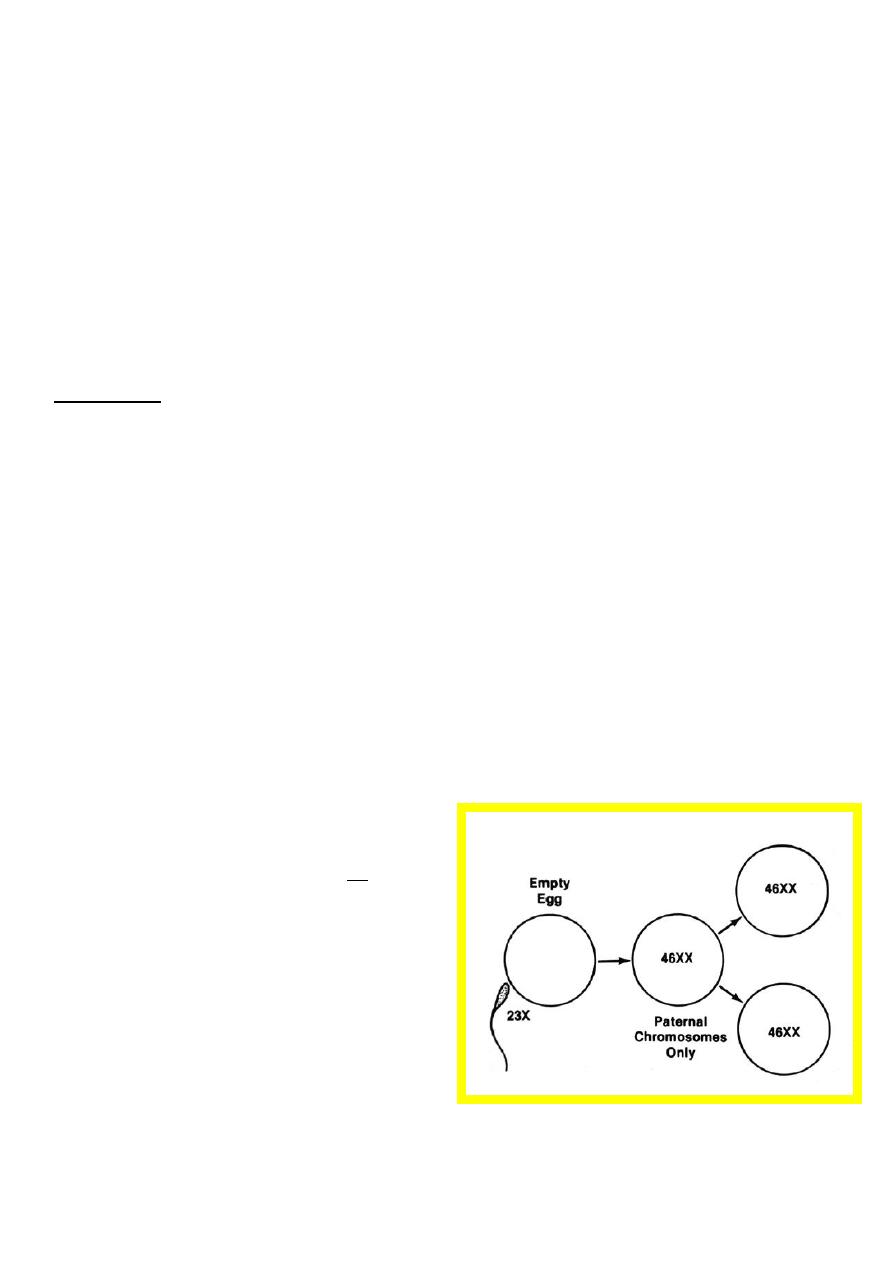
Complete moles
Complete moles are diploid and
androgenetic in origin, with no
evidence of fetal tissue.
Usually arise as a consequence of
duplication of the haploid sperm
following fertilization of an ‘empty’
ovum.
Some complete moles arise after
dispermic fertilization of an ‘empty’
ovum.
3
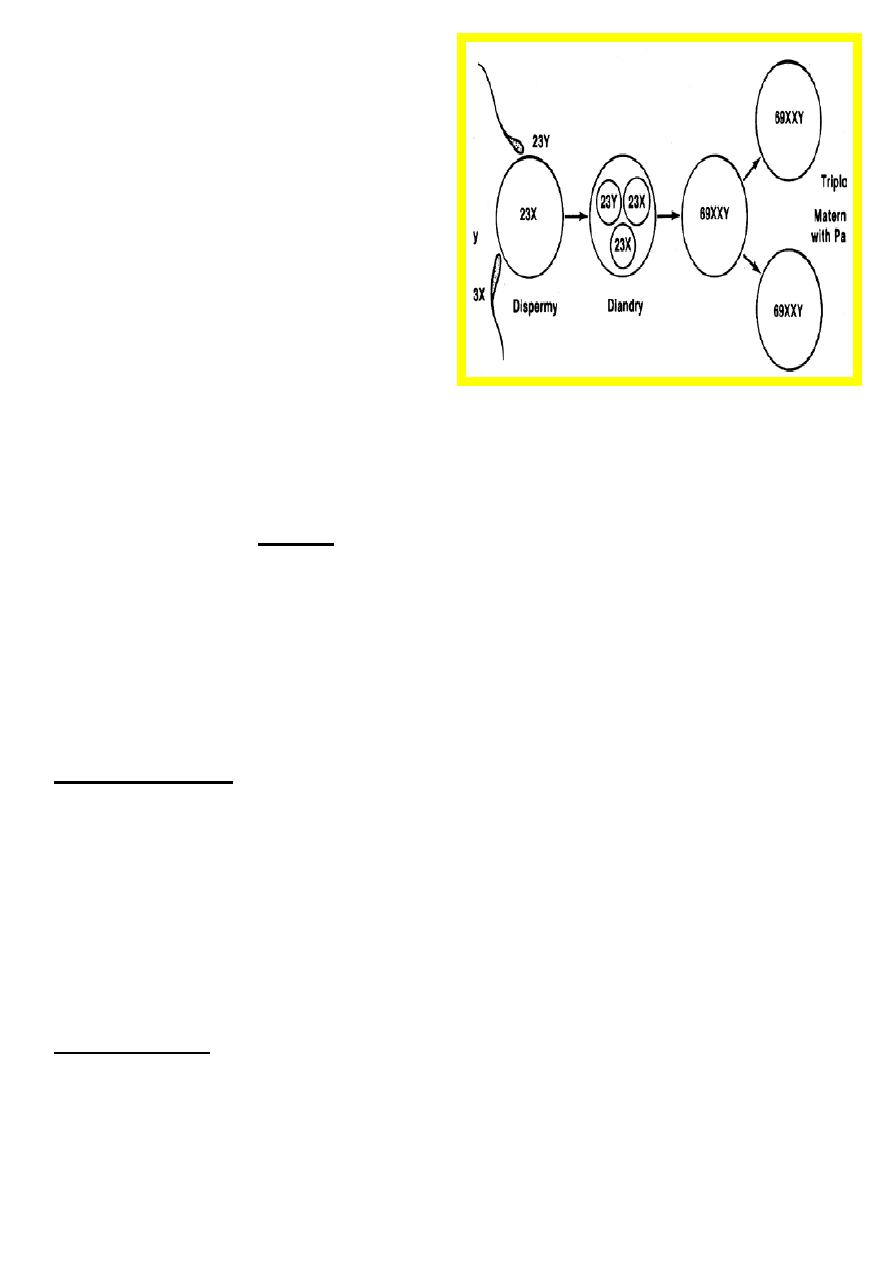
Partial moles
Are triploid in origin with two sets of
paternal haploid genes and one set of
maternal haploid genes.
They occur, in almost all cases,
following dispermic fertilization of an
ovum.
There is usually evidence of a fetus or
fetal red blood cells.
The widespread use of ultrasound has led to earlier diagnosis of pregnancy and has
changed the pattern of molar pregnancy.
The majority of women
present
with :symptoms of early pregnancy failure,
while presentation with:
1. Hyperemesis,
2. Early severe pre-eclampsia and
3. Hyperthyroidism
is very rare
.
RISK FACTORS
1. Nulliparity
2. Extremes of maternal age( under 20 and over 35)
3. The presence of enlarged theca lutien ovarian cysts.
4. Abnormally elevated beta –hCG concentrations during pregnancy.
5. History of previous GTD (double the risk).
PATHOLOGY
In complete mole characterized by the lack of a fetus, trophoblastic hyperplasia, edematous
chorionic villi, and a loss of normal villous blood vessels.
Partial mole may contain some normal appearing chorionic villi and fetal tissue. The
hydropic changes are focal and less prominent with little hyperplasia and no atypia of the
surrounding trophoblast.
4
Choriocarcinoma
:
The lesion appears as sheets of anaplastic cytotrophoblasts and syncytiotrophblasts frequent
mitoses, and multinucleated giant cells without chorionic villi.
CLINICAL FEATURES
: Complete and partial mole present with:
1. bleeding in early pregnancy.
2. Hyperemesis gravidarum.
3. Uterine size greater than expected for gestational age and absent fetal heart sounds.
4. Early onset pre-eclampsia(< 24 weeks of gestation)
5. Thyrotoxicosis
6. Excessively elevated serum B-hCG(>100,000 mIU/mL)
CLINICAL FEATURES
OF MALIGNANT GTD
1. Persistent vaginal bleeding after evacuation of a mole or after any pregnancy.
2. Secondary postpartum hemorrhage.
3. Symptoms of metastasis to the lung, brain, liver, and vagina.
4. With all these features there is always elevated serum hCG level.
RISK FACTORS
for developing malignant GTD after any pregnancy
Maternal age over 35 years.
Increasing parity.
Prolonged use of oral contraception( over 5 years).
Maternal type A blood group.
History of prior GTD.
Diagnosis of gestational trophoblastic neoplasia
Early complete molar pregnancies are commonly associated with the ultrasound
diagnosis of delayed miscarriage or anembryonic pregnancy.
Complete moles may be associated with suggestive ultrasonographic changes in the
placenta.
5
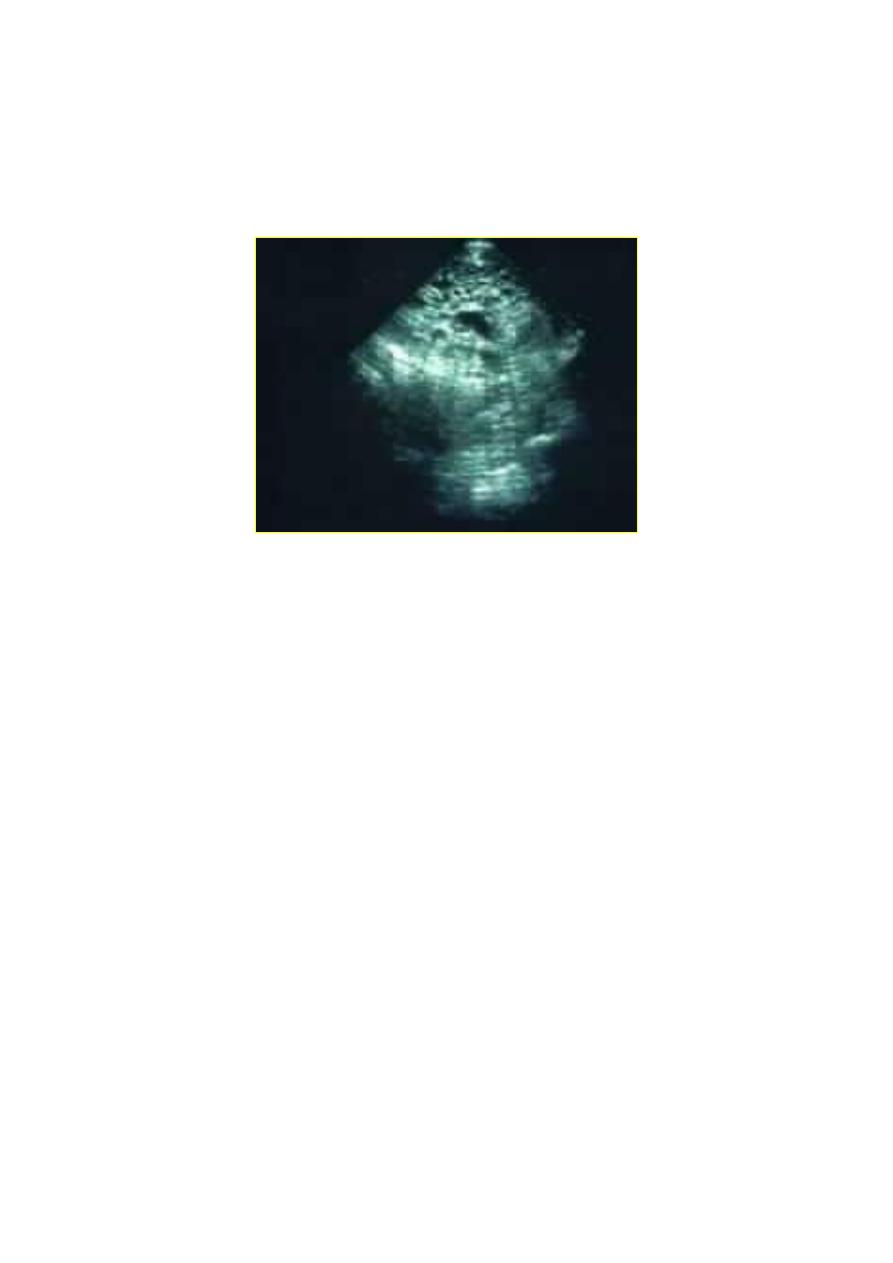
Ultrasonography
in Complete moles reveals:
1. The characteristic intrauterine " snow storm" appearance,
2. No identifiable foetus,
3. Bilateral ovarian cysts may be detected.
A real-time ultrasound of a hydatidiform mole.
The dark circles of varying sizes at the top center are the edematous villi.
It has limited value in detecting partial molar pregnancies.
The increasing use of ultrasound in early pregnancy has probably led to the earlier
diagnosis of molar pregnancy.
However, the majority of histologically proven complete moles are associated with an
ultrasound diagnosis of delayed miscarriage or anembryonic pregnancy.
The ultrasound features of a complete mole are reliable.
But the ultrasound diagnosis of a partial molar pregnancy is more complex.
The finding of multiple soft markers, including both :
1. cystic spaces in the placenta and
2. a ratio of transverse to anterior-posterior dimension of the gestation sac of greater than
1.5 is required for the reliable diagnosis of a partial molar pregnancy.
6
Estimation of human chorionic gonadotrophin (hCG) levels may be of value in
diagnosing molar pregnancies.
When there is diagnostic doubt about the possibility of a combined molar pregnancy with
a viable fetus then ultrasound examination should be repeated before intervention.
In case of Twins, when there is one viable fetus and the other pregnancy is molar
The pregnancy should be allowed to proceed if the mother wishes, following
appropriate counselling.
The probability of achieving a viable baby is 40% and there is a risk of complications
such as pulmonary embolism and pre-eclampsia.
Evacuation of molar pregnancies
Surgical evacuation of molar pregnancies is advisable.
Routine repeat evacuation after the diagnosis of a molar pregnancy is not warranted.
Suction curettage
Is the method of choice of evacuation for complete molar pregnancies.
Because of the lack of fetal parts a suction catheter, up to a maximum of 12 mm, is
usually sufficient to evacuate all complete molar pregnancies.
Medical termination of complete molar pregnancies
Including cervical preparation prior to suction evacuation, should be avoided where
possible.
Routine use of oxytocic agents : There is theoretical concern, because of the potential
to embolize and disseminate trophoblastic tissue through the venous system.
The contraction of the myometrium may force tissue into the venous spaces at the site
of the placental bed. The dissemination of this tissue may lead to the profound
deterioration in the woman, with embolic and metastatic disease occurring in the lung.
It is recognized that significant haemorrhage may occur as a consequence of
evacuating a large uterine cavity,
It is recommended, where possible, that oxytocic infusions are only commenced once
evacuation has been completed.
7
If the woman is experiencing significant haemorrhage prior to evacuation and some
degree of control is required ,then use of oxytocic infusions will be necessary
according to the clinical condition.
It is suggested that prostaglandin analogues should be reserved for cases where
oxytocin is ineffective.
Because evacuation of a large molar pregnancy is a rare event, advice and help from
an experienced colleague should be sought where appropriate.
In partial molar pregnancies where the size of the fetal parts deters the use of suction
curettage, medical terminationcan be used.
Partial molar pregnancies : These women may be at an increased risk of requiring
treatment for persistent trophoblastic neoplasia, although the proportion of women with
partial mole needing chemotherapy is low (0.5%).
Histological examination
of products of conception
All products of conception obtained after evacuation (medical or surgical) should
undergo histological examination.
In view of the difficulty in making a diagnosis of a molar pregnancy before
evacuation, the histological assessment of material obtained from the medical or
surgical management of incomplete abortions is recommended in order to exclude
trophoblastic neoplasia.
Because persistent trophoblastic neoplasia may develop after any pregnancy it is
recommended that all products of conception obtained after repeat evacuation should
undergo histological examination.
Products of conception from therapeutic terminations of pregnancy should be
examined if there is no evidence of fetal tissue.
The management
of women with gynecological symptoms after evacuation of a
molar pregnancy
In cases where there are persisting symptoms, such as vaginal bleeding, after initial
evacuation, consultation with the screening centre should be sought before surgical
intervention.
There is no clinical indication for the routine use of a second uterine evacuation
in the management of molar pregnancies.
Uterine evacuation may be recommended, in selected cases, by the screening centre as
part of the management of persistent trophoblastic neoplasia.

8
Persistent GTN after a non molar pregnancy
Women with persistent abnormal vaginal bleeding after a non molar pregnancy should
undergo a pregnancy test to exclude persistent GTN.
Persistent GTN can occur after nonmolar pregnancies.
Vaginal bleeding is a common presenting symptom but symptoms from metastatic
disease, such as dyspnoea or abnormal neurology, can occur.
Persistent GTN should be considered in any woman developing
acute respiratory or neurological symptoms after any pregnancy.
The prognosis for women with GTN after non-molar pregnancies may be worse
(21% mortality after a live birth, 6% after a non-molar miscarriage) and in part due to
the delay in diagnosis .
Registration of women with molar pregnancy
Registration of any molar pregnancy is essential. Women with the following molar
pregnancies should be registered and require follow up for 6–24 months as determined by the
screening centre:
1. Complete Hydatidiform mole
2. Partial Hydatidiform mole
3. Twin pregnancy with complete or partial hydatidiform mole
4. Choriocarcinoma
5. Placental site trophoblastic tumour.
Treatment of persistent GTN
Women with persistent GTN should be treated at a specialist centre with appropriate
chemotherapy.
The need for chemotherapy following a complete mole is 15% and
0.5 % after a partial mole.
Women with evidence of persistent GTN should undergo assessment of their disease
followed by chemotherapy.
Disease risk is scored according to the FIGO staging for GTN.
Women scoring six or less (low risk) receive intramuscular methotrexate on alternate
days, followed by six rest days, with each course consisting of four injections.
Women who develop resistance to methotrexate are treated with a combination of
intravenous dactinomycin and etoposide.
Women scoring seven or more (high risk) receive combination chemotherapy.

9
Placental site trophoblastic tumour
Placental site trophoblastic tumour is now recognized as a variant of gestational
trophoblastic neoplasia.
Surgery and multi-agent chemotherapy play major roles in the clinical management of
this tumour.
-------------------------------------------------
Future pregnancy
Women should be advised not to conceive until the hCG level has been normal for six
months.
The risk of a further molar pregnancy is low (1/55) and More than 98% of women
who become pregnant following a molar pregnancy will not :
1. Have a further mole or
2. Be at increased risk of obstetric complications.
If a further molar pregnancy does occur, in 68–80% of cases it will be of the
same histological type.
After the conclusion of any further pregnancy (at any gestation) further urine or blood
samples for hCG estimation are requested to exclude disease recurrence.
Women who undergo chemotherapy are advised not to conceive for one year after
completion of treatment.
Contraception and hormone replacement therapy
The combined oral contraceptive pill and hormone replacement therapy are safe to use after
hCG levels have reverted to normal.
The combined pills, if taken while hCG levels are raised, may increase the need for
treatment.
However, it can be used safely after the hCG levels have returned to normal.
The small potential risk of using emergency hormonal contraception, in women with
raised hCG levels, is outweighed by the potential risk of pregnancy to the woman.
