
Chapter 2 - 1
Chapter 2: Atomic Structure and
Interatomic Bonding

Chapter 2 - 2
Chapter 2: Atomic Structure &
Interatomic Bonding
Geckos, harmless tropical lizards, are extremely
fascinating and extraordinary animals. They
have very sticky feet that cling to virtually any surface. This
characteristic makes it possible for
them to rapidly run up vertical walls and along the
undersides of horizontal surfaces. In fact, a
gecko can support its body mass with a single toe! The
secret to this remarkable ability is the presence
of an extremely large number of microscopically small
hairs on each of their toe pads. When
these hairs come in contact with a surface, weak forces of
attraction (i.e., van der Waals forces)
are established between hair molecules and molecules on
the surface.

Chapter 2 - 3
ISSUES TO ADDRESS...
• What promotes bonding?
• What types of bonds are there?
• What properties are inferred from bonding?
Chapter 2: Atomic Structure &
Interatomic Bonding

Chapter 2 - 4
Chapter 2: Atomic Structure &
Interatomic Bonding
Learning Objectives
1. Name the two atomic models cited, and note
the differences between them.
2. Describe the important quantum-mechanical
principle that relates to electron energies.
3. bonding energy.
4. ionic, covalent, metallic, hydrogen, and van der Waals
bonds.

Chapter 2 - 5
WHY STUDY Atomic Structure and
Interatomic Bonding?
An important reason to have an understanding of interatomic
bonding in solids is that, in some instances,
the type of bond allows us to explain a material’s
properties.
For example, consider carbon, which may
exist as both graphite and diamond. Whereas graphite
is relatively soft and has a “greasy” feel to it, diamond
is the hardest known material. This dramatic disparity
in properties is directly attributable to a type of interatomic
bonding found in graphite that does not exist
in diamond

Chapter 2 - 6
Some of the important properties of solid materials depend on
geometrical atomic arrangements, and also the interactions that
exist among constituent atoms or molecules.
• atomic structure,
• Electron configurations in atoms
• the periodic table
• the various types of primary and secondary interatomic
bonds

Chapter 2 -
Wavelike behavior of electrons
• We often use dots to represent individual electrons and lines for
pairs of electrons in a covalent bond.
• However, the electrons have a wavelike behavior such that we
can only give the probability of an electron being in a particular
volume.
• So a more accurate representation of an electron is as a cloud.
7

Chapter 2 - 8
A set of principles and laws that govern systems of
atomic and subatomic entities that came to be known
as quantum mechanics.
An understanding of the behavior of electrons in atoms
and
crystalline
solids
necessarily
involves
the
discussion of quantum-mechanical concepts.

Chapter 2 - 9
An important quantum-mechanical principle stipulates that
the energies of electrons are quantized;; that is, electrons are
permitted to have only specific values of energy.
An electron may change energy, but in doing so it must make
a quantum jump either to an allowed higher energy (with
absorption of energy) or to a lower energy (with emission of
energy).
Often, it is convenient to think of these allowed
electron energies as being associated with energy levels or
states. These states do not vary continuously with energy;; that
is, adjacent states are separated by finite energies.

Chapter 2 - 10
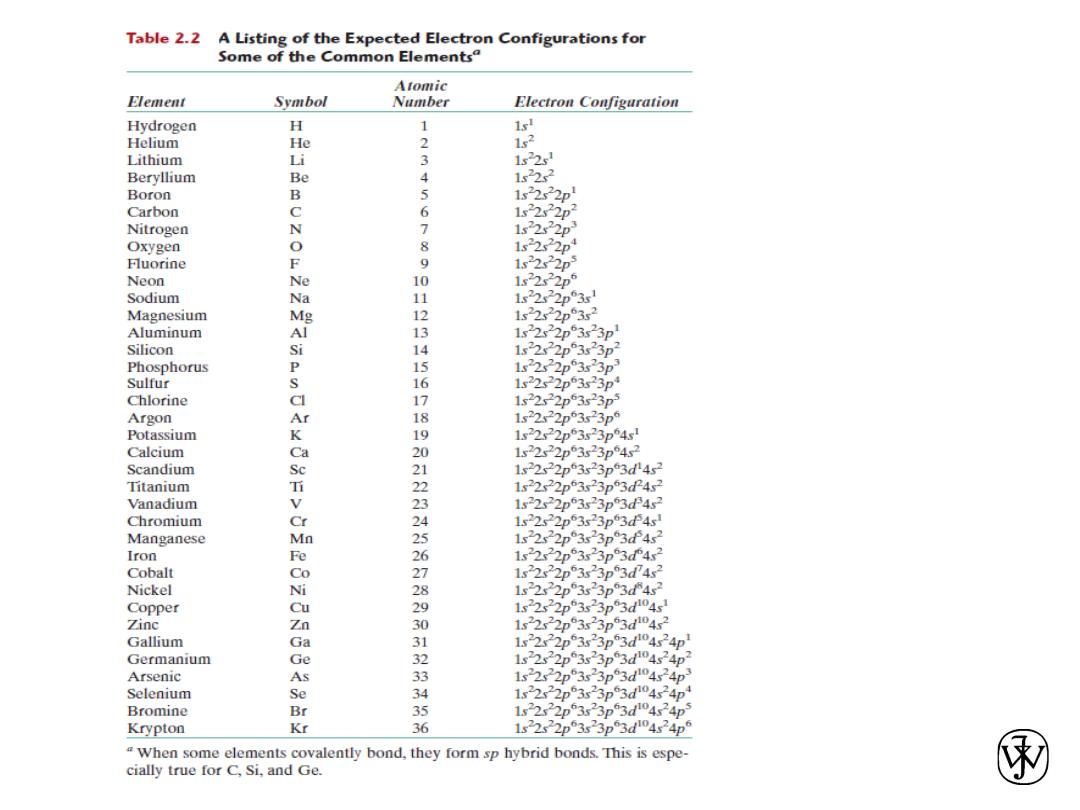
Atomic Structure
• Each chemical element is characterized by the number
of protons in the nucleus, or the atomic number (Z).
(for an electrically neutral atom also equals to number of
electrons)
• The atomic mass (A) of a specific atom may be
expressed as the sum of the masses of protons and
neutrons within the nucleus.
• Atoms of some elements have two or more different
atomic masses, which are called isotopes.
• The atomic weight of an element corresponds to the
weighted average of the atomic masses of the atom’s
naturally occurring isotopes.
• The atomic mass unit (amu) may be used for
computations of atomic weight.

Chapter 2 - 11
Atomic Structure
• atom –
electrons
– 9.11 x 10
-31
kg
protons
neutrons
• Charge magnitude of electrons and protons
• 1.60 x 10
-19
C
• atomic number
= # of protons in nucleus of atom
= # of electrons of neutral species
• A [=]
atomic mass unit
= amu = 1/12 mass of
12
C
Atomic wt
= wt of 6.022 x 10
23
molecules or atoms
1 amu/atom = 1g/mol
C 12.011
H 1.008 etc.
}
1.67 x 10
-27
kg

Chapter 2 - 12
number of neutrons = N
number of protons = Z
A= Z + N
(
2.1)
AVAGADRO’S NUMBER = 6.022 x 10
23
= N
A
ATOMIC OR MOLECULAR WEIGHT =
N
A
x WEIGHT PER ATOM.

Chapter 2 - 13
Atomic Structure
• Valence electrons determine all of the
following properties
1) Chemical
2) Electrical
3) Thermal
4) Optical
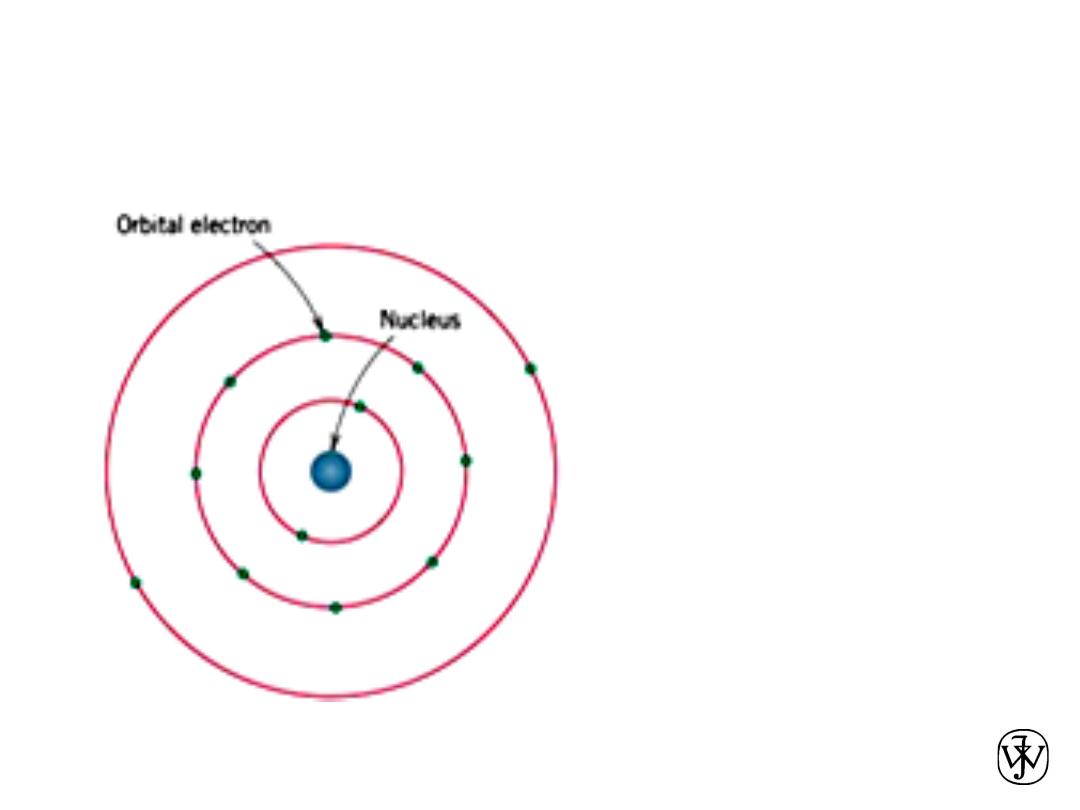
Chapter 2 -
BOHR ATOM
14
Bohr atomic
model, in which
electrons are assumed
to revolve around the
atomic nucleus
in discrete orbitals, and
the position of any
particular electron is
more or
less well defined in
terms of its orbital.
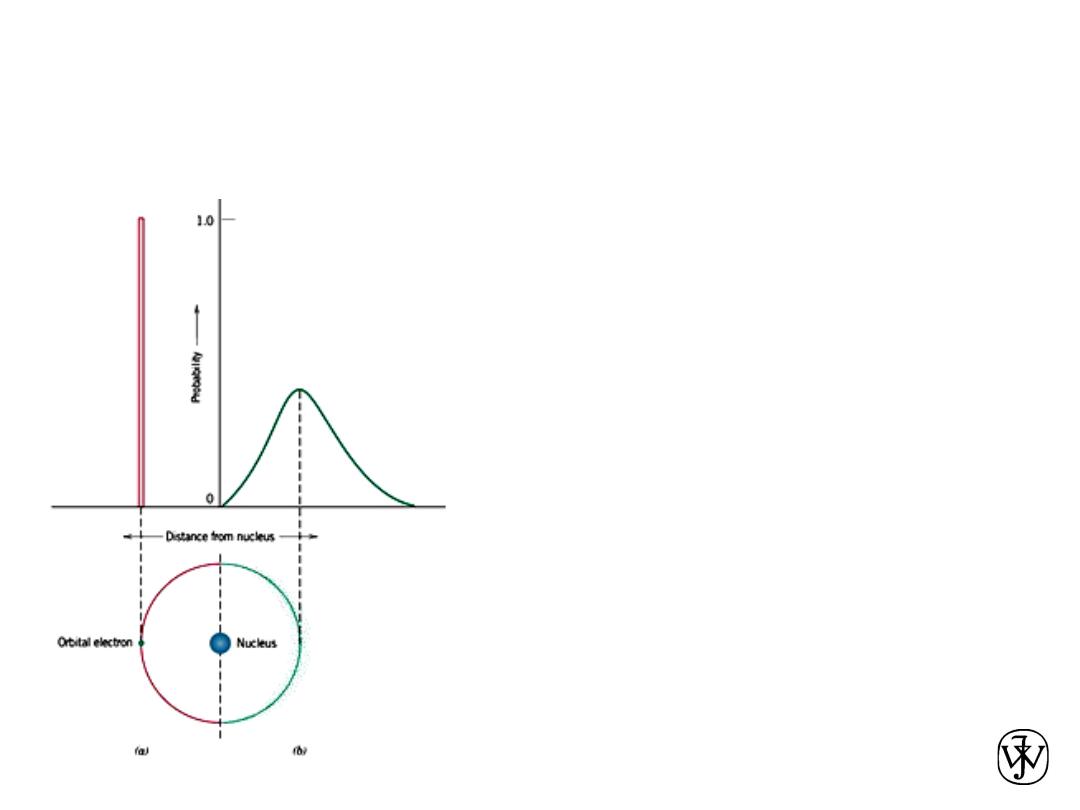
Chapter 2 -
WAVE MECHANICAL MODEL OF
ATOM
15
This Bohr model was eventually found to
have some significant limitations
because of its inability to explain several
phenomena involving electrons. A
resolution was reached with a
wave-
mechanical model,
in which the electron is
considered to exhibit both wave-like and
particle-like characteristics. With this
model, an electron is no longer treated as a
particle moving in a discrete orbital;
rather, position is considered to be the
probability of an electron’s being at
various locations around the nucleus. In other
words, position is described by a
probability distribution or electron cloud.

Chapter 2 - 16
Electronic structure of isolated atoms
• The characteristics below stem from their wavelike nature.
– electrons are in orbitals
– each orbital is at a discrete energy level determined by its quantum
numbers
– the letter designations below were given to bands observed in optical
emission and absorption, but not understood at the time.
Quantum Number
Designation
n = principal (energy level-shell)
1,2,3,4,5,6,7 (K, L, M, N, O,…)
l = angular (sub shell, shape)
s, p, d, f (n of them to max of 4)
m
l
= magnetic
- l to + l by integers, including 0
m
s
= spin
½, -½
v
Dynamic periodic table
v
Atomic orbitals
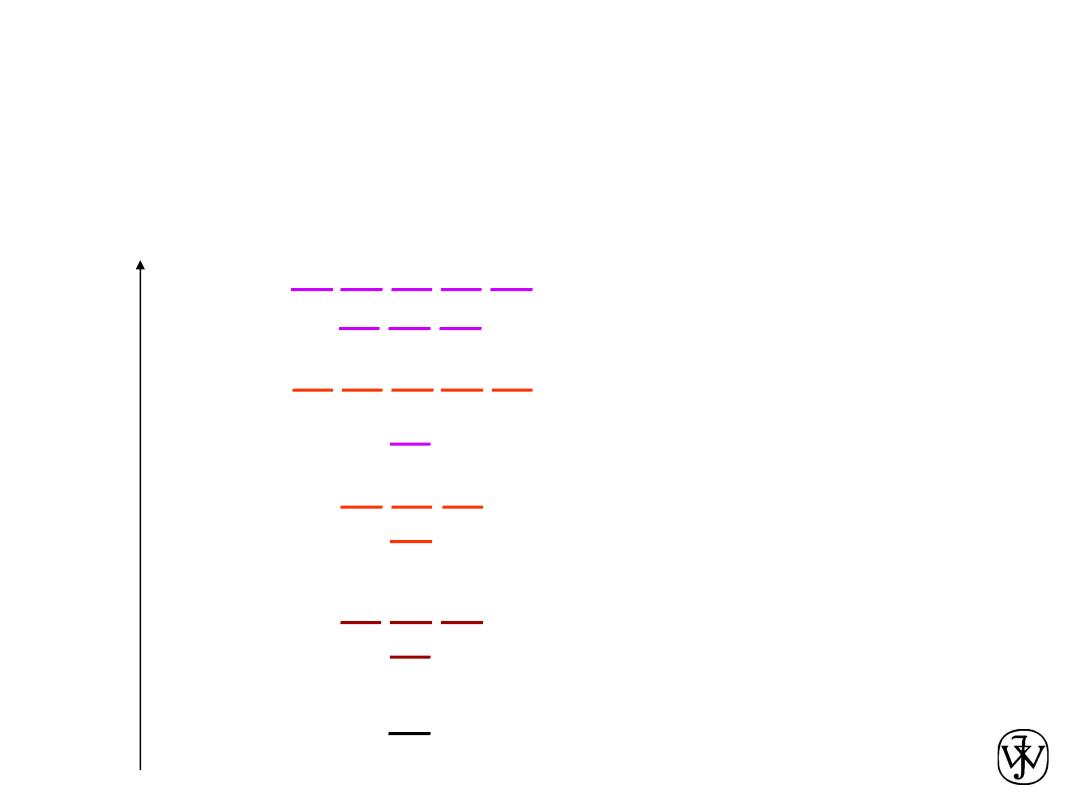
Chapter 2 - 17
Electron energy states of isolated atoms
1s
2s
2p
K-shell n = 1
L-shell n = 2
3s
3p
M-shell n = 3
3d
4s
4p
4d
Energy
N-shell n = 4
Electrons have discrete energy states. They occupy the lowest
possible energy levels, unless excited by an external source of energy,
e.g. thermal energy or absorption of photons (in light).
Two electrons of
opposite spin can
be in each level.
Chapter 2 - 18
The electron configuration is stable only for the noble gases. Except
for noble gases, the outer shell is not completely filled and so one or
more electrons may be lost or gained to form an ion,
or shared in a covalent bond.
Ground-state energy levels of some elements
Electron configuration
(stable)
...
...
1s
2
2s
2
2p
6
3s
2
3p
6
(stable)
...
1s
2
2s
2
2p
6
3s
2
3p
6
3d
10
4s
2
4p
6
(stable)
Atomic #
18
...
36
Element
1s
1
1
Hydrogen
1s
2
2
Helium
1s
2
2s
1
3
Lithium
1s
2
2s
2
4
Beryllium
1s
2
2s
2
2p
1
5
Boron
1s
2
2s
2
2p
2
6
Carbon
...
1s
2
2s
2
2p
6
(stable)
10
Neon
1s
2
2s
2
2p
6
3s
1
11
Sodium
1s
2
2s
2
2p
6
3s
2
12
Magnesium
1s
2
2s
2
2p
6
3s
2
3p
1
13
Aluminum
...
Argon
...
Krypton
Chapter 2 - 19
Electron Configurations
• Valence electrons
– those in unfilled shells
• Filled shells more stable
• Valence electrons are most available for
bonding and tend to control the chemical
properties
– example: C (atomic number = 6)
1s
2
2s
2
2p
2
valence electrons
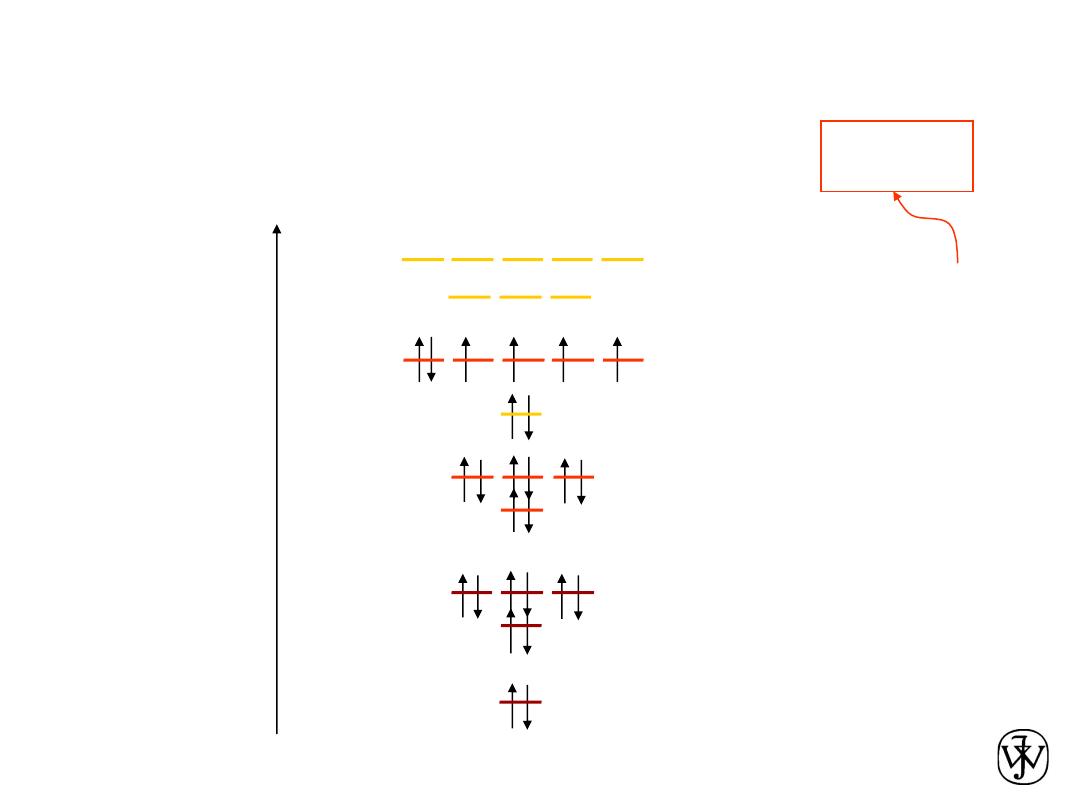
Chapter 2 - 20
Electronic Configurations
ex: Fe - atomic #
=
26
valence
electrons
Adapted from Fig. 2.4,
Callister & Rethwisch 8e.
1s
2s
2p
K-shell n = 1
L-shell n = 2
3s
3p
M-shell n = 3
3d
4s
4p
4d
Energy
N-shell n = 4
1s
2
2s
2
2p
6
3s
2
3p
6
3d
6
4s
2

Chapter 2 - 21
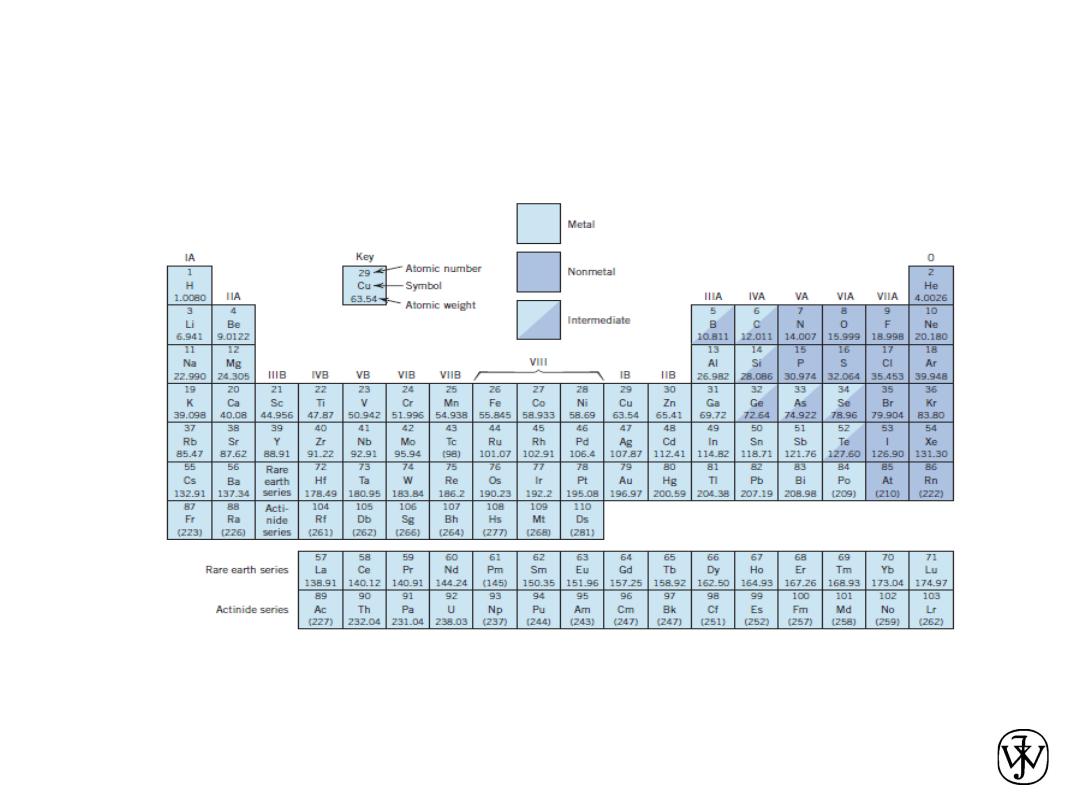
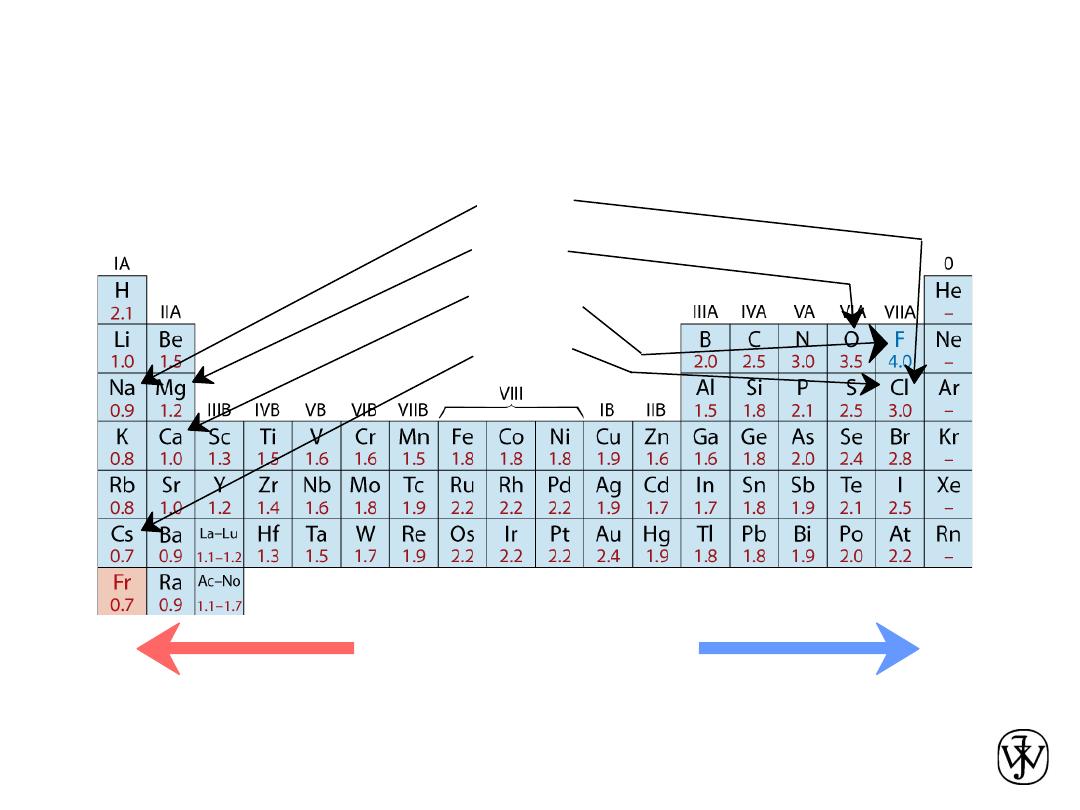
The Periodic Table
All the elements have been classified according to electron
configuration in the periodic table.
The elements are situated, with increasing atomic number, in
seven horizontal rows called periods.
The arrangement is such that all elements arrayed in a given
column or group have similar valence electron structures, as
well as chemical and physical properties.
These properties change gradually, moving horizontally
across each period and vertically down each column.
Chapter 2 - 22
The Periodic Table
Chapter 2 - 23
The Periodic Table
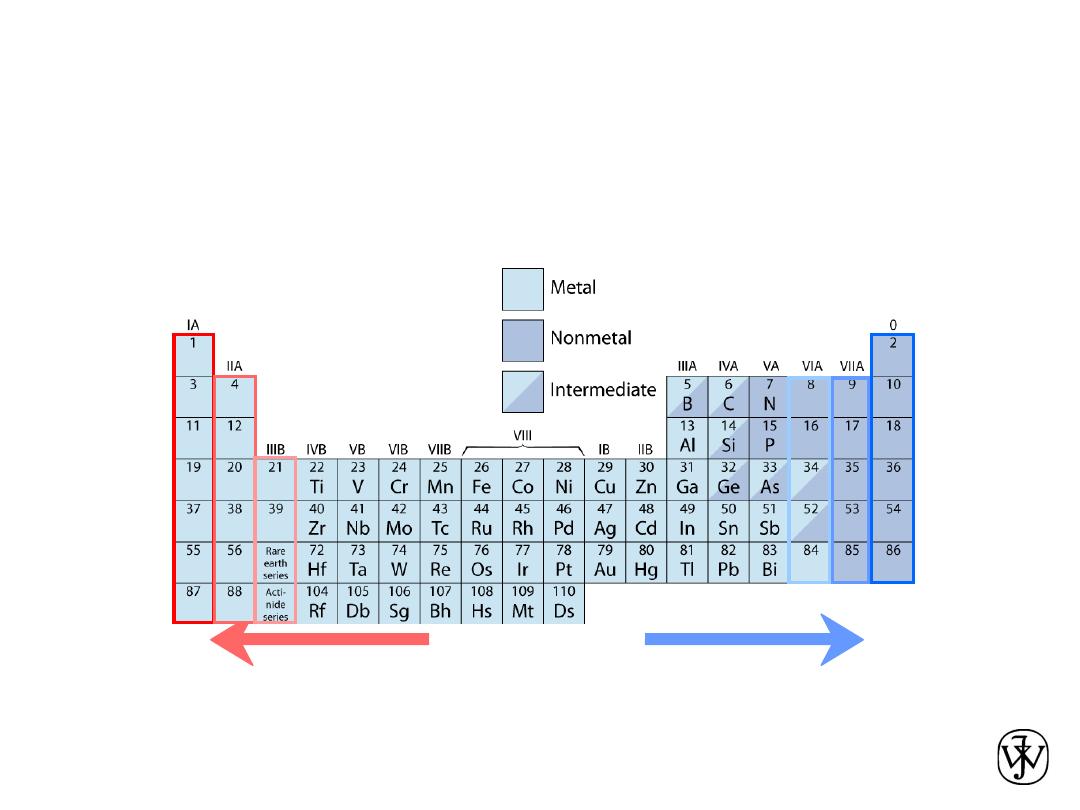
• Columns:
Similar
Valence
Structure
Adapted from
Fig. 2.6,
Callister &
Rethwisch 8e.
Electropositive elements:
Readily give up electrons
to become + ions.
Electronegative elements:
Readily acquire electrons
to become - ions.
gi
ve
u
p
1e
-
gi
ve
u
p
2e
-
gi
ve
u
p
3e
-
in
er
t g
ase
s
acce
pt
1
e
-
acce
pt
2
e
-
O
Se
Te
Po At
I
Br
He
Ne
Ar
Kr
Xe
Rn
F
Cl
S
Li
Be
H
Na
Mg
Ba
Cs
Ra
Fr
Ca
K
Sc
Sr
Rb
Y

Chapter 2 -
• electropositive elements,indicating that
they are capable of giving up their few
valence electrons to become positively
charged ions.
• the elements situated on the right-hand
side of the table are electronegative;;
that is, they readily accept electrons to
form negatively charged ions, or
sometimes they share electrons with
other atoms.
24
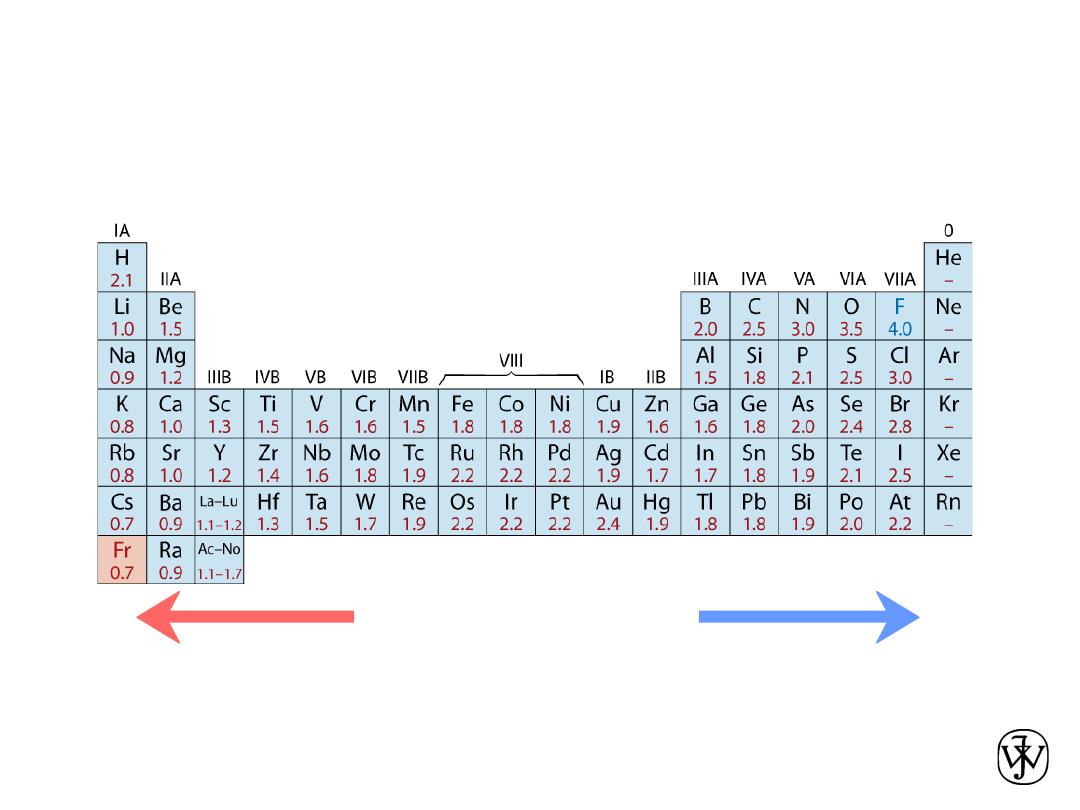
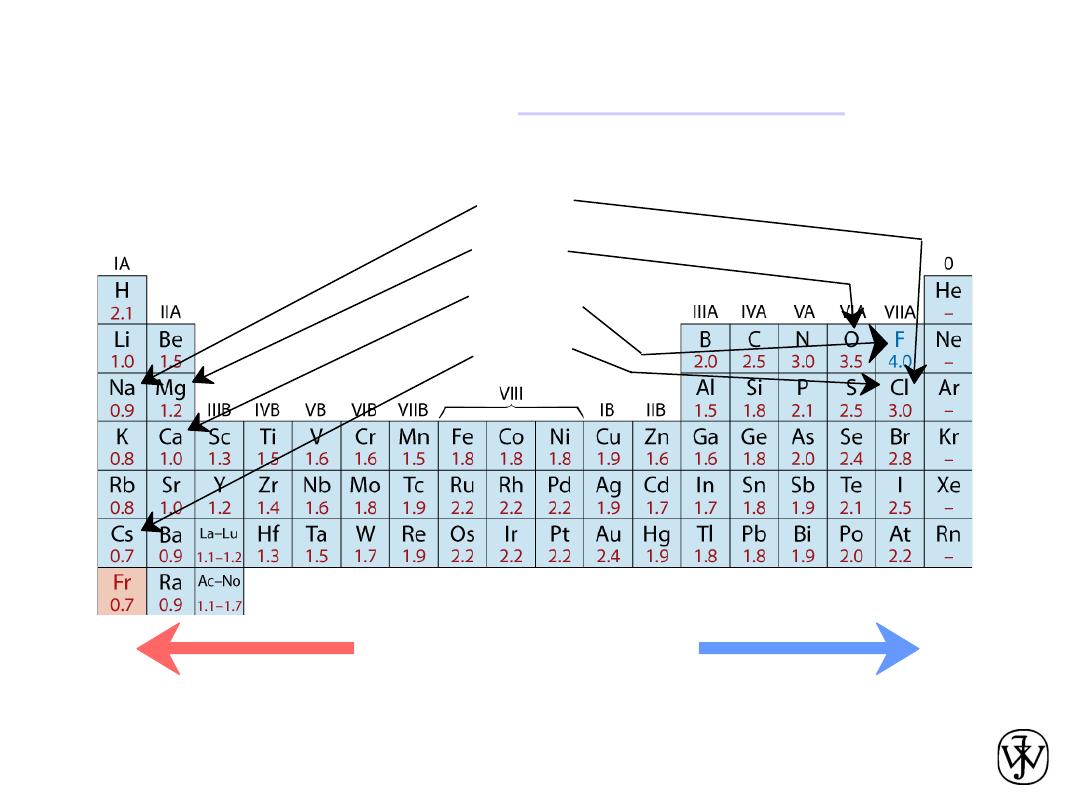
Chapter 2 - 25
• Ranges from
0.7
to
4.0
,
Smaller electronegativity
Larger electronegativity
• Large values: tendency to acquire electrons.
Adapted from Fig. 2.7, Callister & Rethwisch 8e. (Fig. 2.7 is adapted from Linus Pauling, The Nature of the
Chemical Bond, 3rd edition, Copyright 1939 and 1940, 3rd edition. Copyright 1960 by Cornell University.
Electronegativity
Chapter 2 - 26
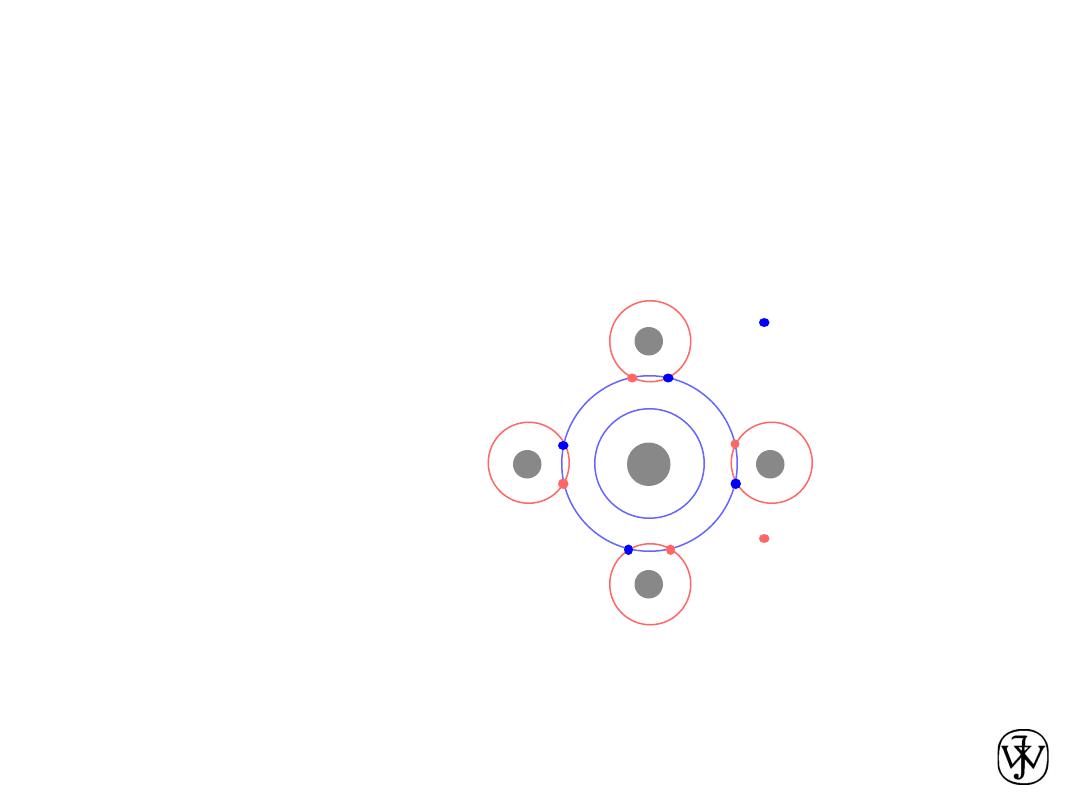
Ionic bond –
metal
+ nonmetal
donates accepts
electrons electrons
Dissimilar electronegativities
ex:
Mg
O
Mg
1s
2
2s
2
2p
6
3s
2
O
1s
2
2s
2
2p
4
[Ne] 3s
2
Mg
2+
1s
2
2s
2
2p
6
O
2-
1s
2
2s
2
2p
6
[Ne]
[Ne]
Chapter 2 -
Electrons in different shells
27
Chapter 2 - 28

Chapter 2 -
Atomic Bonding in Solids
• An understanding of many of the
physical properties of materials is
predicated on a knowledge of the
interatomic forces that bind the atoms
together.
• Perhaps
the
principles
of
atomic
bonding
are
best
illustrated
by
considering the interaction between two
isolated atoms as they are brought into
close
proximity
from
an
infinite
separation.
29

Chapter 2 -
Atomic Bonding in Solids
• At large distances, the interactions are
negligible, but as the atoms approach,
each exerts forces on the other. These
forces are of two types, attractive and
repulsive, and the magnitude of each is
a
function
of
the
separation
or
interatomic distance.
30
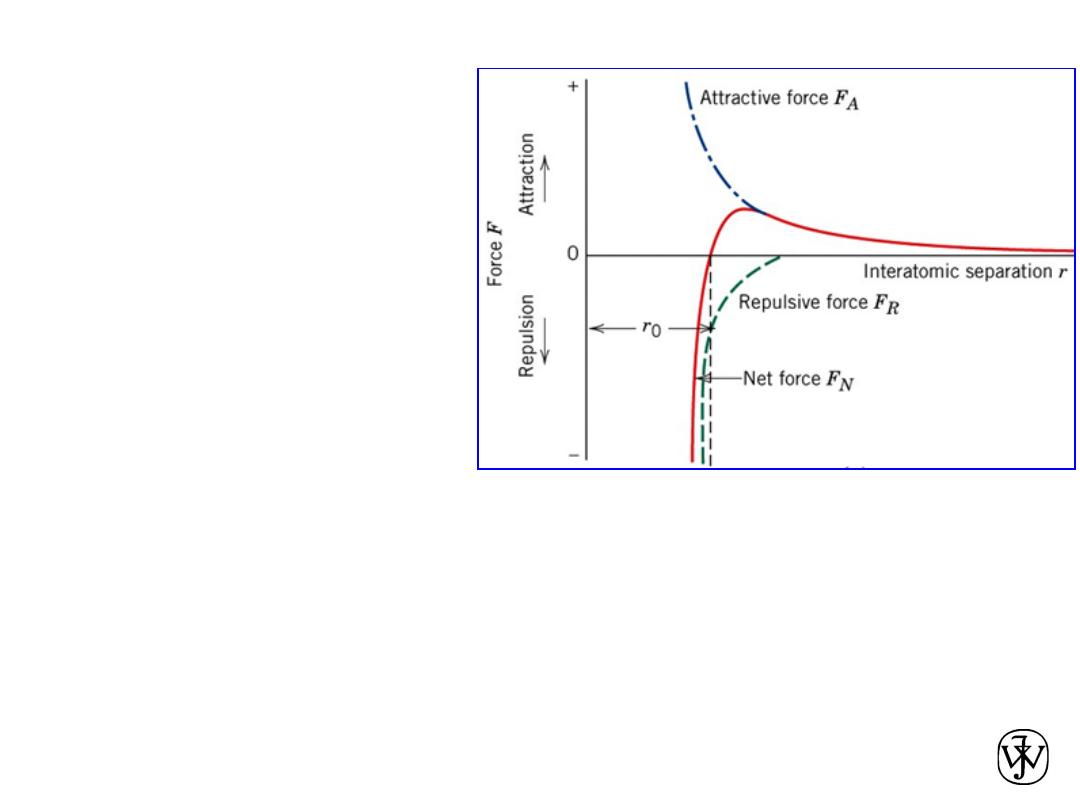
Chapter 2 -
Bonding in solids
• As two atoms approach one
another, they at first
experience an attraction.
• They repel one another when
they are brought very close.
• r
0
is the equilibrium distance.
• The type of bonding in a solid
depends on the behavior of the
atoms' outer “valence”
electrons.
• Metallic: outer electrons shared in a cloud or sea.
• Ionic
– Cations have given up one or more electrons
– Anions have gained one or more electrons
• Covalent: atoms share outer electrons
• Mixed ionic and covalent
• Van der Waals: electrostatic due to non-uniform charge distribution. Weak
Chapter 2 -
Typical ionic bond:
metal
+ nonmetal
Mg 1s
2
2s
2
2p
6
3s
2
O
1s
2
2s
2
2p
4
(Ne + 3s
2
)
(Ne – 2p
2
)
Mg
2+
1s
2
2s
2
2p
6
O
2-
1s
2
2s
2
2p
6
(Ne)
(Ne)
cation
anion
donates
electrons
accepts
electrons
• The greater the difference in electronegativity, the greater the tendency to
form an ionic bond.
• Consider magnesium and oxygen with electronegativities of 1.31 and 3.44.
• Here’s what happens when Mg and O come near one another:
electron(s)
+
-
Coulombic
Attraction
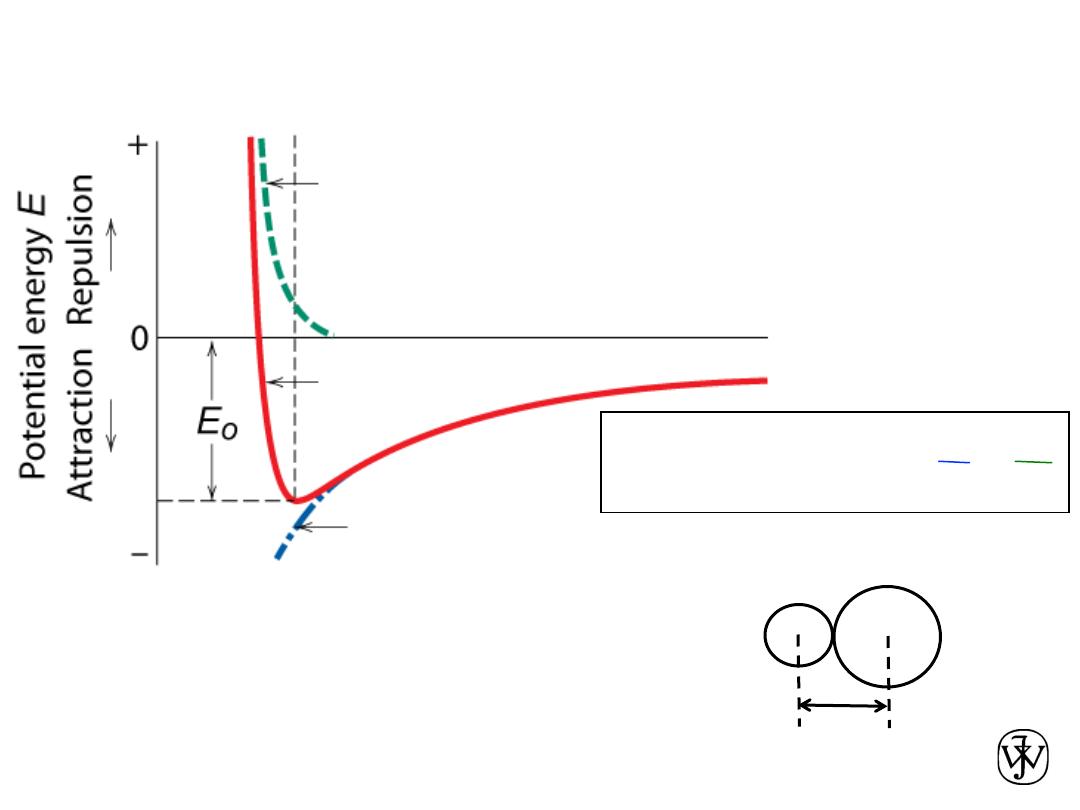
Chapter 2 - 33
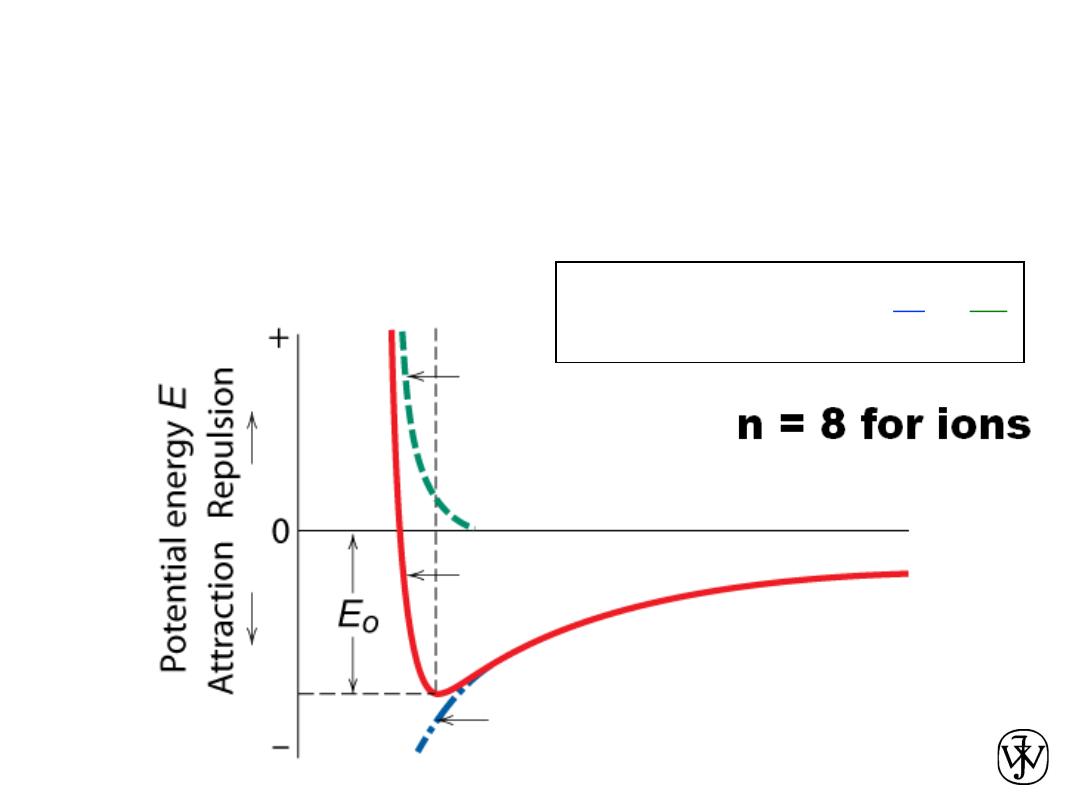
Ionic bonding between one cation (+)
and one anion (-)
Stable at minimum energy E
0
for radius r
0
.
Attractive energy E
A
Net energy E
N
Repulsive energy E
R
Interatomic separation r
r
0
r
A
n
r
B
E
N
=
E
A
+
E
R
=
+
-
r
0
Force = dE/dr = 0 at r
0
Chapter 2 - 34
• Predominant bonding in Ceramics
Adapted from Fig. 2.7, Callister & Rethwisch 4e. (Fig. 2.7 is adapted from Linus Pauling, The Nature of the
Chemical Bond, 3rd edition, Copyright 1939 and 1940, 3rd edition. Copyright 1960 by Cornell University.
Examples:
Ionic Bonding
Give up electrons
Acquire electrons
NaCl
MgO
CaF2
CsCl
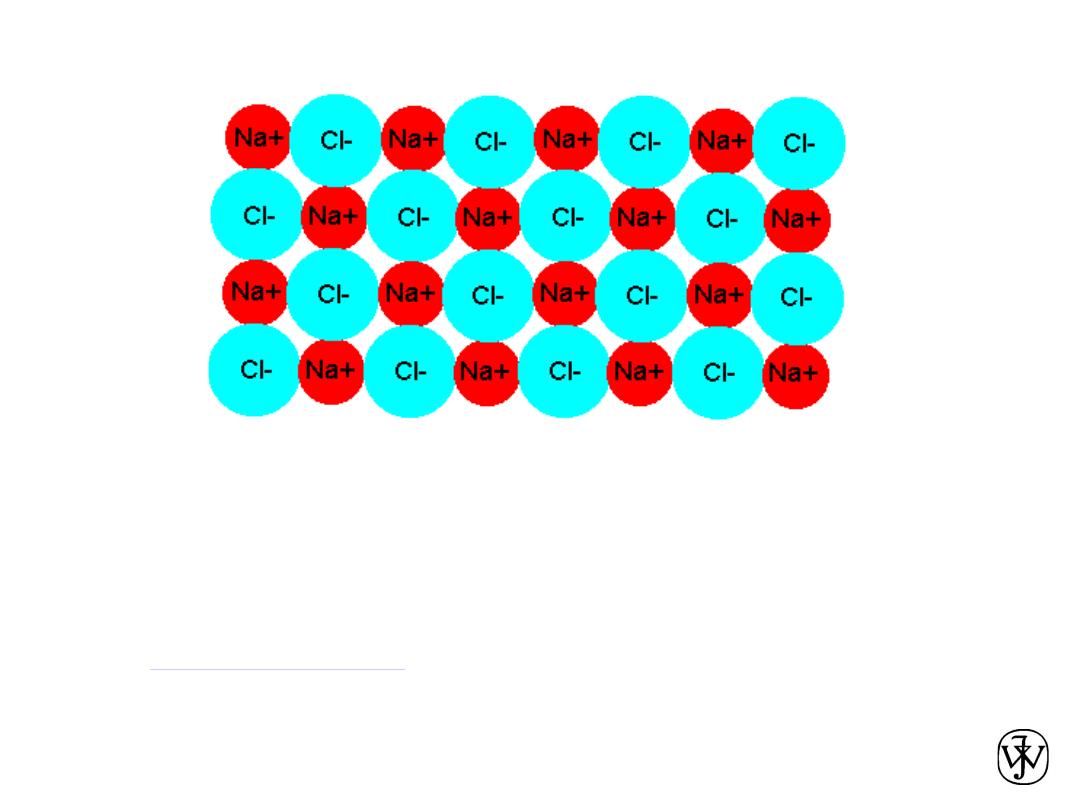
Chapter 2 -
Ionic bonding in a crystal
• In a crystal, a cation (+ charge) is attracted not only by the nearest
anions, but to a lesser extent by those farther away.
• Similarly, it is repelled by all other cations.
• The sum of the energy due to all attractions and repulsions is known
as the
Madelung energy
. This is approximately 60% greater than the
energy of attraction for isolated ions the same distance apart as in
the lattice.
35
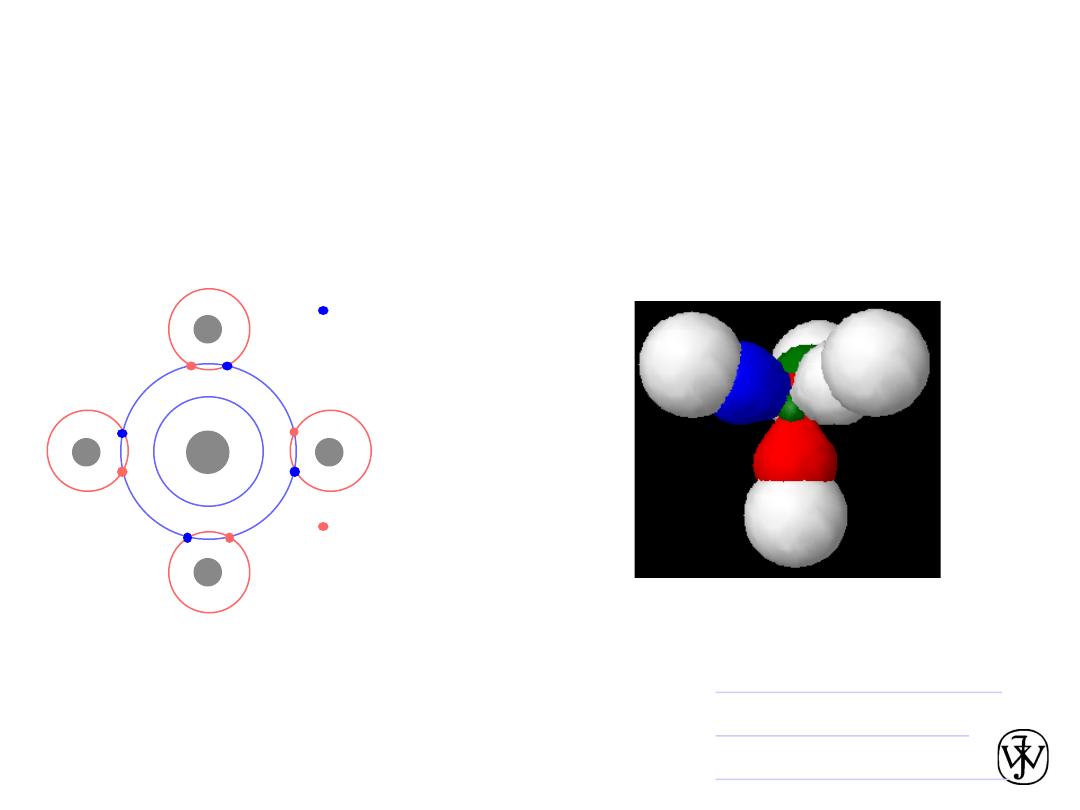
Chapter 2 -
Covalent Chemical Bonds
• Atoms with almost the same electronegativity share electrons
leading to hybrid electronic structures.
• The bonds are very directional, unlike ionic bonds.
• Example:
shared electrons
from carbon atom
shared electrons
from hydrogen
atoms
H
H
H
H
C
CH4
v
Methane orbitals
•
Hybrid orbitals
v
Covalent orbitals

Chapter 2 -
Mixed Ionic-Covalent Bonding
• Ionic-Covalent Mixed Bonding
• Approximate fraction ionic character
»
where X
A
& X
B
are the two Pauling electronegativities.
÷
÷
ø
ö
ç
ç
è
æ
-
-
-
4
2
1
)
X
X
(
B
A
e
Example: MgO. Using the 1960 values in the text,
X
Mg
= 1.2 and X
O
= 3.5,
the equation above predicts that the bond between Mg and O has
about 73% ionic character and 27% covalent.
Using the revised values given on Wikipedia,
X
Mg
= 1.31 and X
O
= 3.44,
the equation above predicts that the bond between Mg and O has
about 68% ionic character and 32% covalent.
For homework problems use the values in the text.
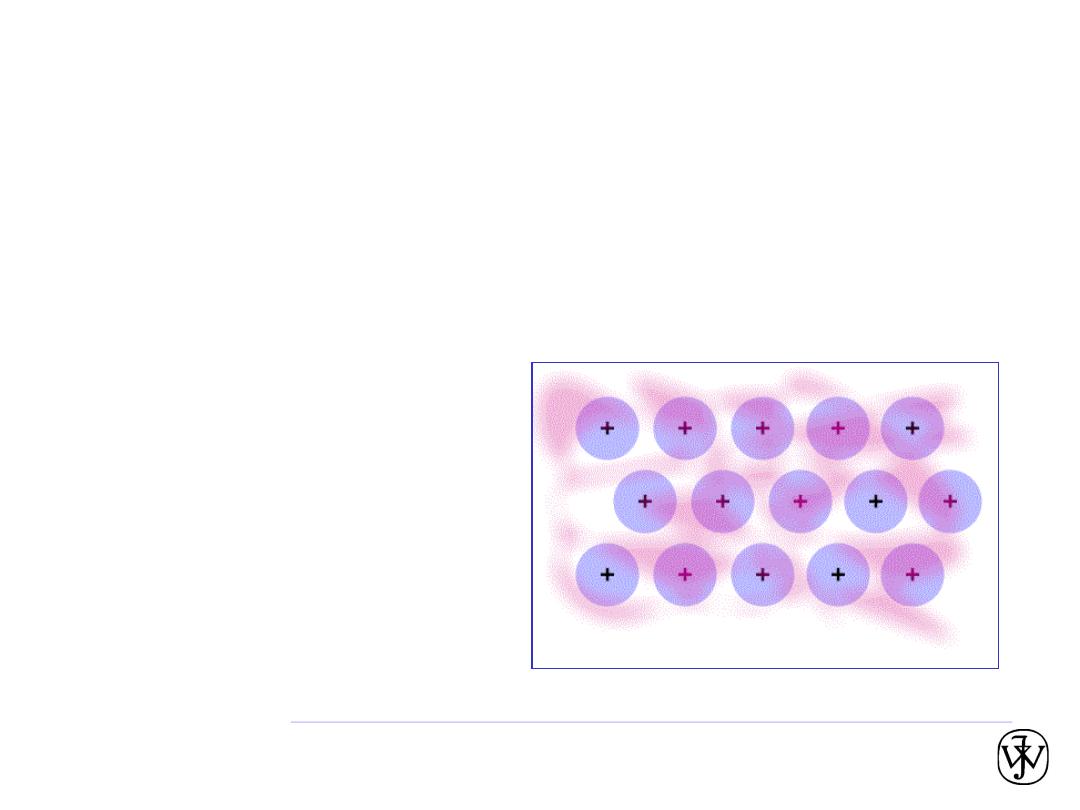
Chapter 2 -
Metallic bonding
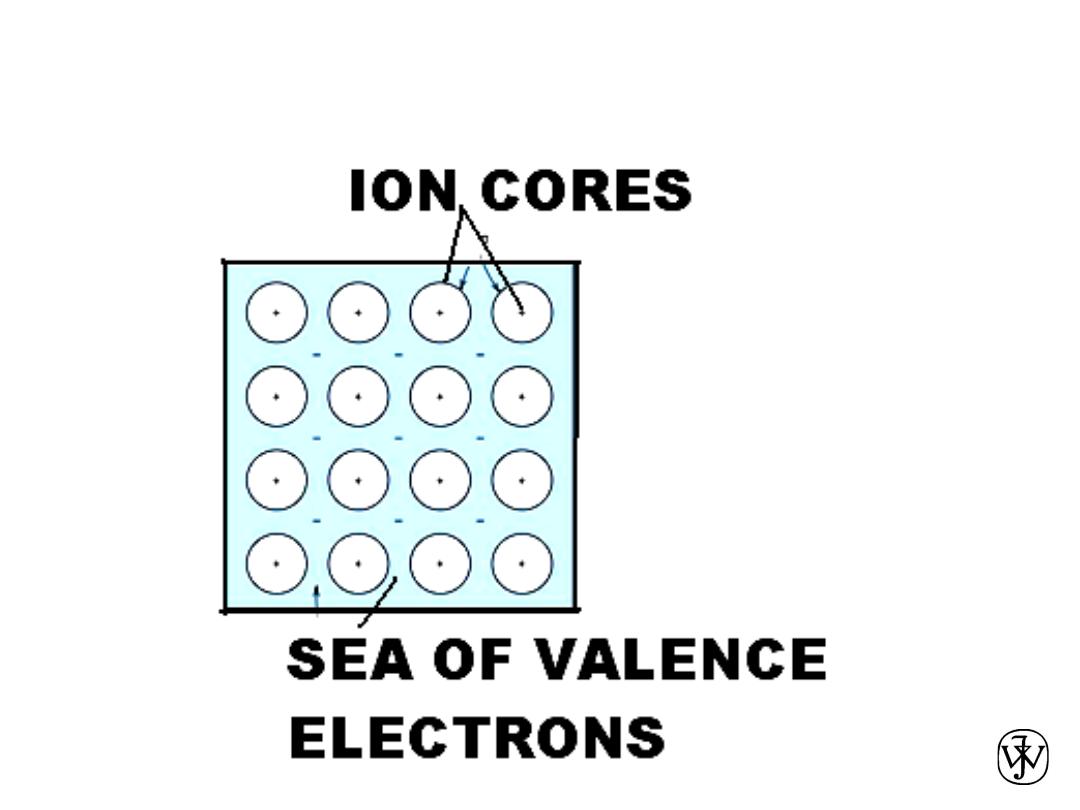
• Occurs with atoms that easily give up electrons.
• In a solid, these “conduction” electrons form a cloud or sea.
• No two electrons can have exactly the same quantum number, and
so they have a range of energies. Each “exists” throughout the solid.
• The attraction between the positively charged metal ions and the
electron cloud is what causes metallic bonding.
• Non directional.
• These “conduction” electrons
carry electric current and heat.
• Mixtures of metals sometimes
form intermetallic compounds.
• Animation (in full-screen
projection mode):
38
Another animation:
http://mypchem.com/myp9/myp9c/myp9c_swf/metal_vib.htm
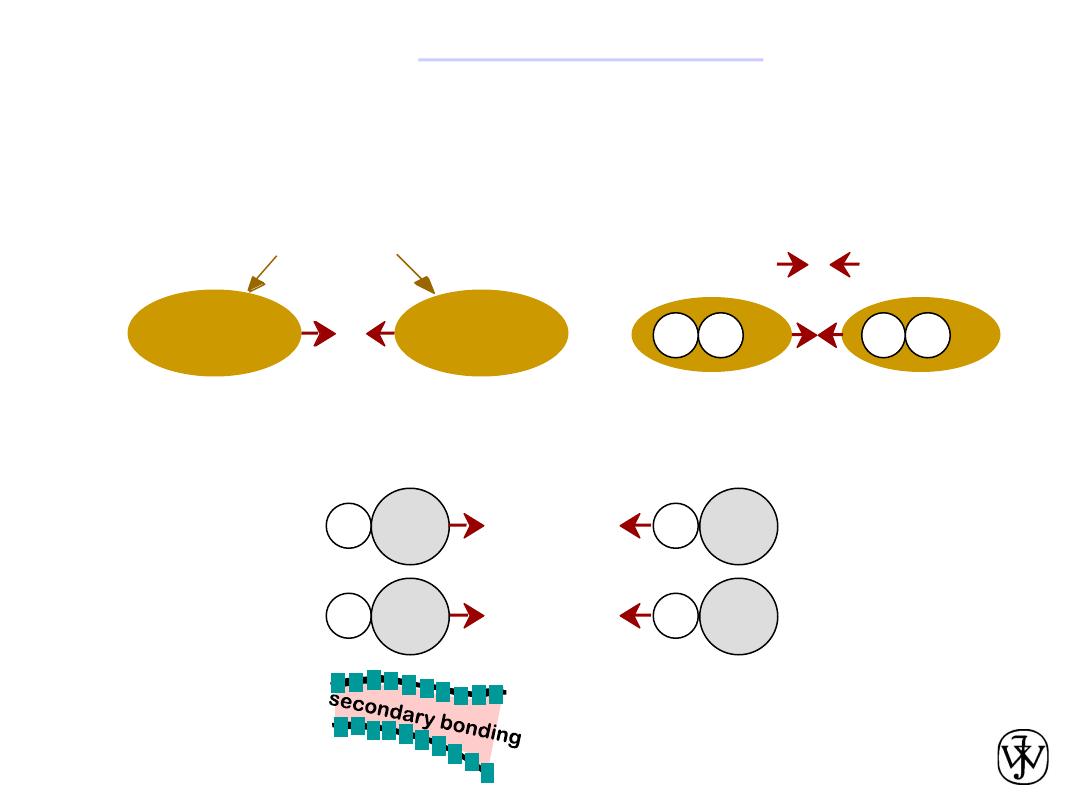
Chapter 2 -
Arises from interaction between electric
dipoles
Secondary (
van der Waals
) bonds
• Dipoles fluctuating rapidly and interacting
asymmetric electron
clouds
+
-
+
-
H
H
H
H
H2
H2
e.g. liquid H
2
• Permanent
dipoles
-general case:
-ex: liquid HCl
-ex: polymer
H Cl
H Cl
+
-
+
-
Within an organic molecule the bonding is
mostly covalent, while between molecules
the bonding is mostly van der Waals.

Chapter 2 -
Hydrogen bonds
• Between hydrogen atoms and the nearby negative end of a
molecular dipole, to strongly electronegative atoms such as O or N.
• Partly covalent and partly electrostatic.
• Much stronger than van der Waals bonds.
• Determines the unusual properties of water liquid and solid.
• Also occurs with other molecules, and even between parts of
complex molecules such as proteins.
40
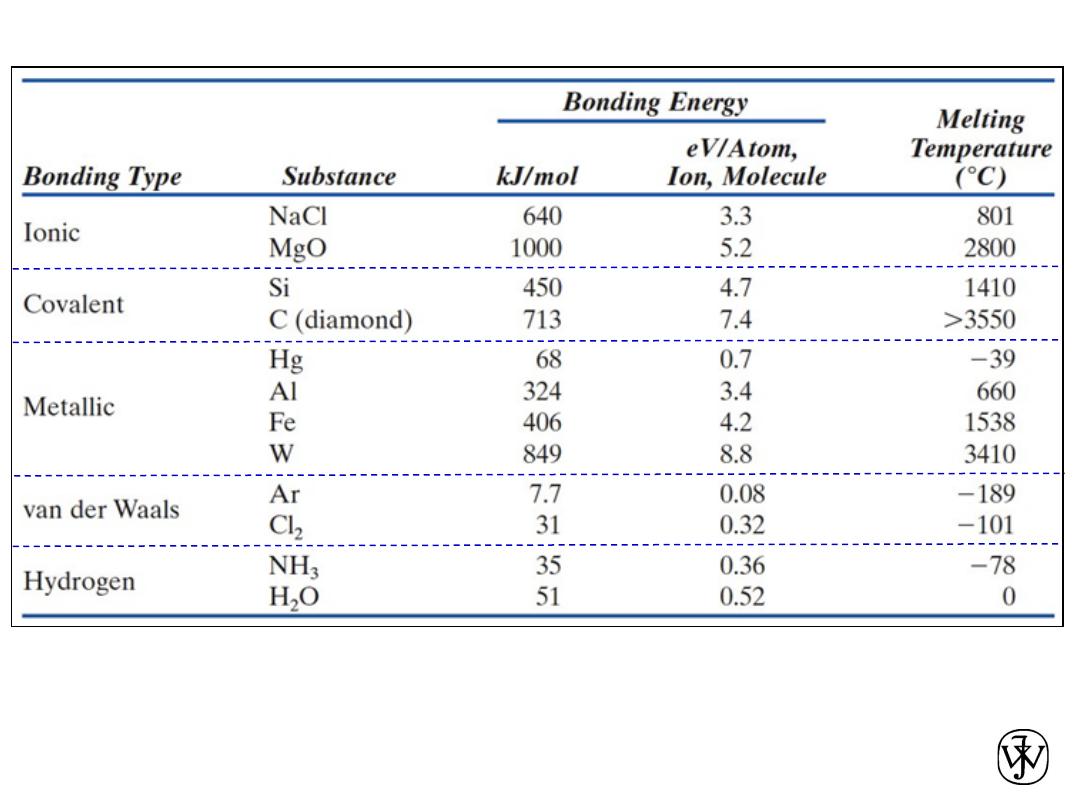
Chapter 2 -
Table 2.3.
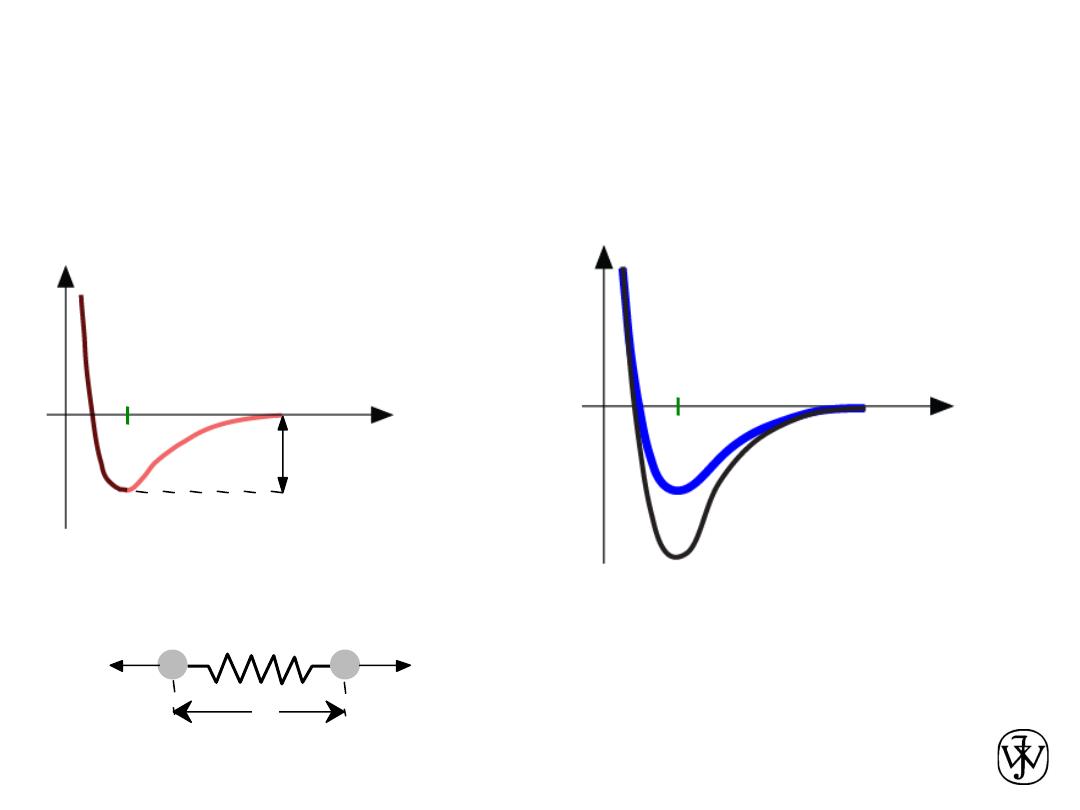
Chapter 2 - 42
Interaction energy E
versus atomic separation r
Melting Temperature, T
m
T
m
is larger when E
o
is larger
Properties From Bonding: Melting point
Atomic separation r
r
r
o
r
Energy
larger T
m
smaller T
m
E
o
“
bond energy”
Energy
r
o
r
unstretched bond length
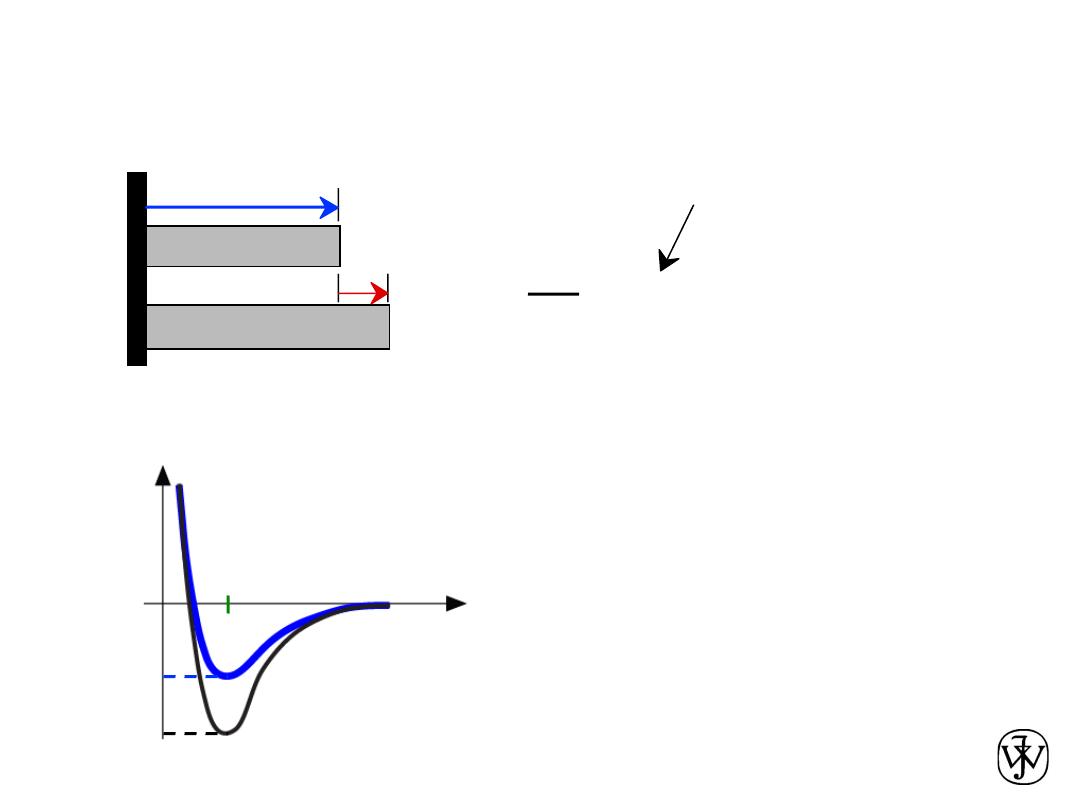
Chapter 2 - 43
Coefficient of thermal expansion,
a
a is larger when E
o
is smaller
Properties From Bonding: Thermal expansion
= a (
T
2
-
T
1
)
DL
Lo
coeff. thermal expansion
DL
length,
Lo
unheated, T1
heated, T2
r
o
r
smaller
a
larger
a
Energy
E
o
E
o
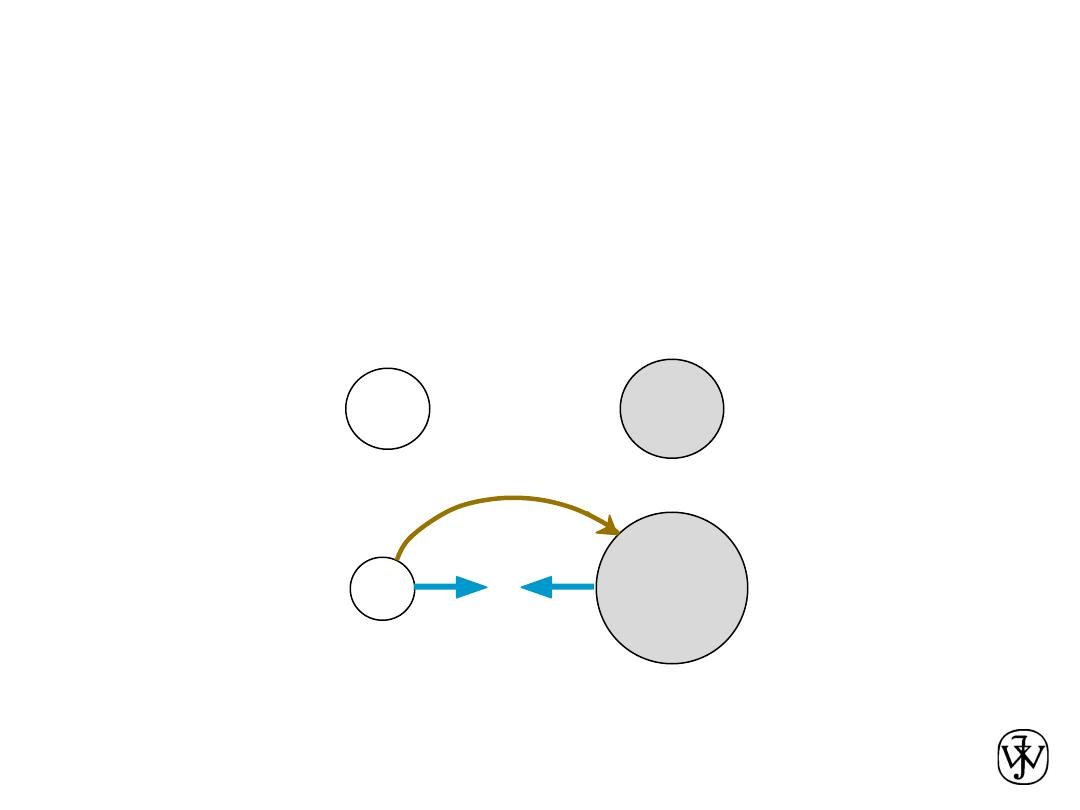
Chapter 2 - 44
• Occurs between + and - ions.
• Requires
electron transfer.
• Large difference in electronegativity required.
• Example: NaCl
Ionic Bonding
Na (metal)
unstable
Cl (nonmetal)
unstable
electron
+
-
Coulombic
Attraction
Na (cation)
stable
Cl (anion)
stable
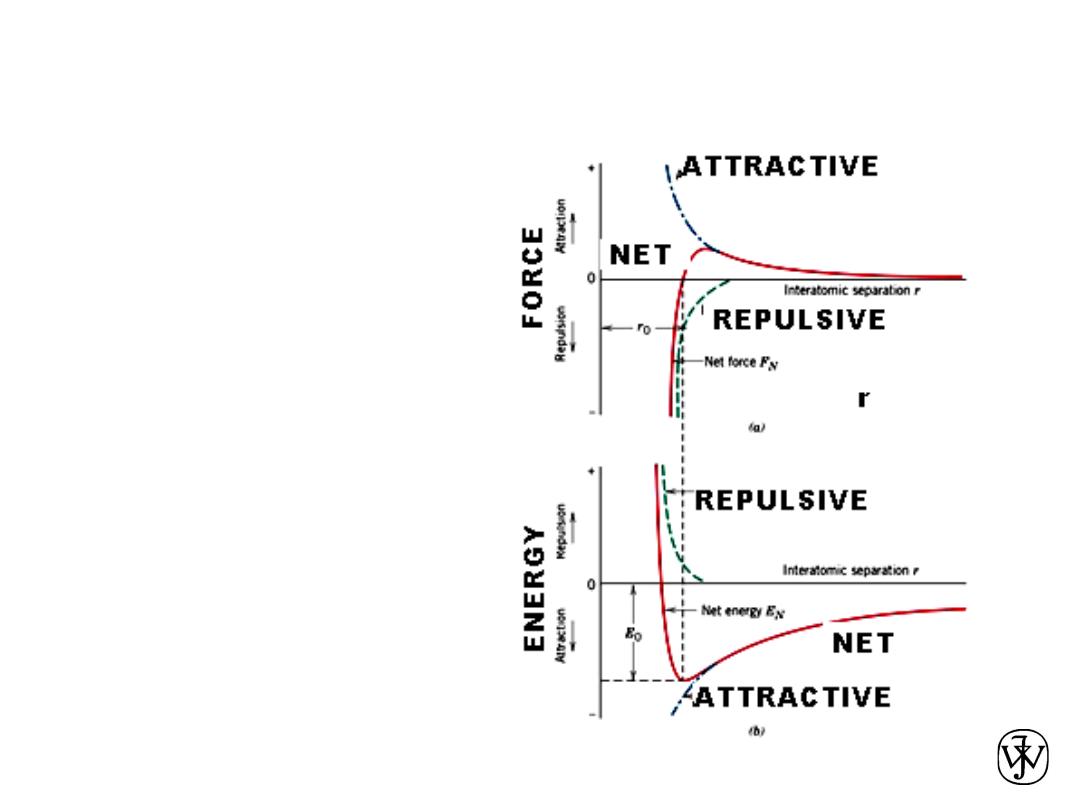
Chapter 2 -
FORCES AND ENERGIES
45
(a) The
dependence of repulsive,
attractive, and net forces
on interatomic
separation for two
isolated atoms.
(b) The dependence of
repulsive, attractive, and
net potential energies on
interatomic separation
for two isolated atoms.
Chapter 2 - 46
Attractive force F
A
Repulsive force F
R
Net force F
N
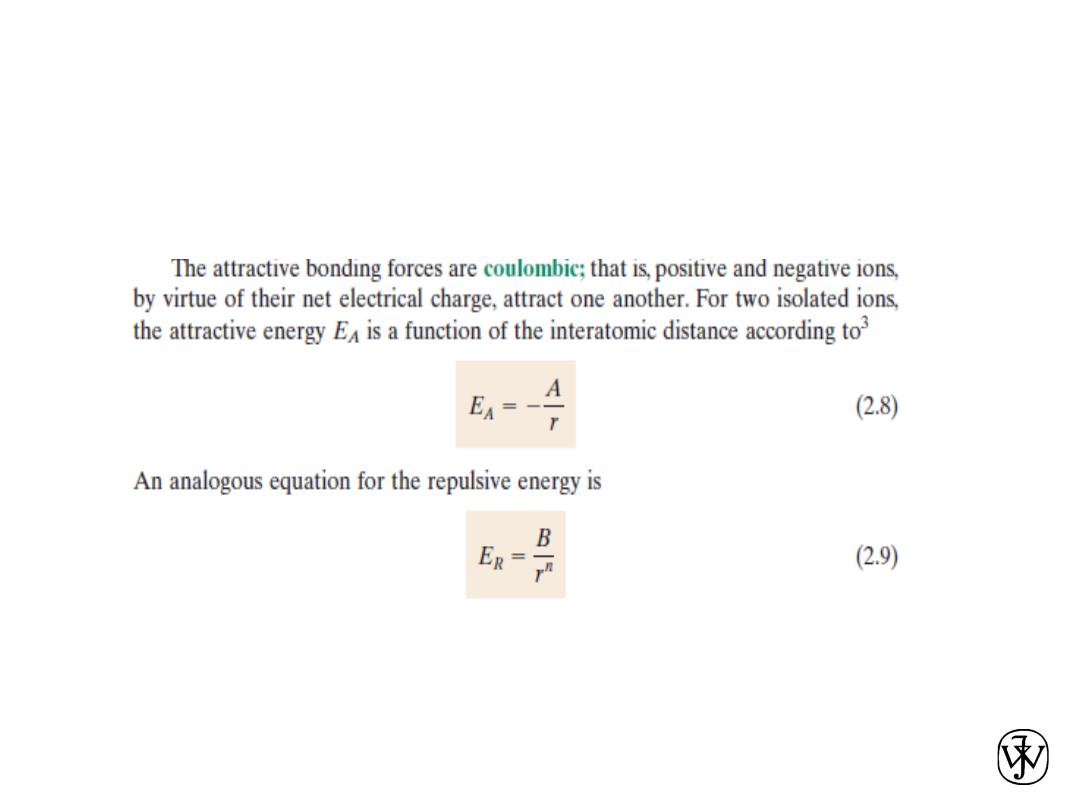
Chapter 2 - 47

Chapter 2 -
Bonding Forces and Energies
48
2.13 Calculate the force of attraction
between a K
+
and an O
2-
ion the
centers of which are separated by a
distance of r0 =1.5 nm.
The attractive force between two ions
FA is just the derivative with respect to
the interatomic separation of the
attractive energy expression, Equation
2.8, which is just
Solution
Chapter 2 - 49
F
A
=
dE
A
dr
=
d
-
A
r
æ
è
ç
ö
ø
÷
dr
=
A
r
2
The constant A in this expression is
defined in footnote 3. Since the valences
of the K+ and
O2- ions
(Z1 and Z2) are +1 and -2, respectively,
Z1 = 1 and Z2 = 2, then
Chapter 2 - 50
F
A
=
(Z
1
e) (Z
2
e)
4pe
0
r
2
=
(1)(2)
(
1.602 ´ 10
-19
C
)
2
(4)(p) (8.85 ´ 10
-12
F/m) (1.5 ´ 10
-9
m)
2
=2.05
´
10^(-10 ) N

Chapter 2 -
IONIC FORCE / P 31 FOOT-NOTE
51
F= (Z1 *Z2 * e^2)/(4*π*ε
0
*r^2);;
e= 1.602 *10^(-19) COULOMBS ;;
ε
0
= 8.85 * 10^(-12 )
Z1, Z2 = VALENCIES OF IONS
Chapter 2 - 52
Ionic Bonding
• Energy – minimum energy most stable
– Energy balance of
attractive
and
repulsive
terms
Attractive energy E
A
Net energy E
N
Repulsive energy E
R
Interatomic separation r
r
A
n
r
B
E
N
=
E
A
+
E
R
=
+
-
Adapted from Fig. 2.8(b),
Callister & Rethwisch 8e.
Chapter 2 - 53
• Predominant bonding in
Ceramics
Adapted from Fig. 2.7, Callister & Rethwisch 8e. (Fig. 2.7 is adapted from Linus Pauling, The Nature of the
Chemical Bond, 3rd edition, Copyright 1939 and 1940, 3rd edition. Copyright 1960 by Cornell University.
Examples: Ionic Bonding
Give up electrons
Acquire electrons
NaCl
MgO
CaF2
CsCl
Chapter 2 - 54
C: has 4 valence e
-
,
needs 4 more
H: has 1 valence e
-
,
needs 1 more
Electronegativities
are comparable.
Adapted from Fig. 2.10, Callister & Rethwisch 8e.
Covalent Bonding
• similar
electronegativity
\ share electrons
• bonds determined by valence – s & p orbitals
dominate bonding
• Example: CH
4
shared electrons
from carbon atom
shared electrons
from hydrogen
atoms
H
H
H
H
C
CH4

Chapter 2 - 55
Primary Bonding
• Metallic Bond
-- delocalized as electron cloud
• Ionic-Covalent Mixed Bonding
% ionic character
=
where X
A
& X
B
are Pauling electronegativities
%)
100
(
x
1
- e
-
(X
A
-X
B
)
2
4
æ
è
ç
ç
ç
ö
ø
÷
÷
÷
ionic
73.4%
(100%)
x
e
1
character
ionic
%
4
)
2
.
1
5
.
3
(
2
=
÷÷
÷
ø
ö
çç
ç
è
æ
-
=
-
-
Ex: MgO
X
Mg
= 1.2
X
O
= 3.5
Chapter 2 -
METALLIC BONDING
56
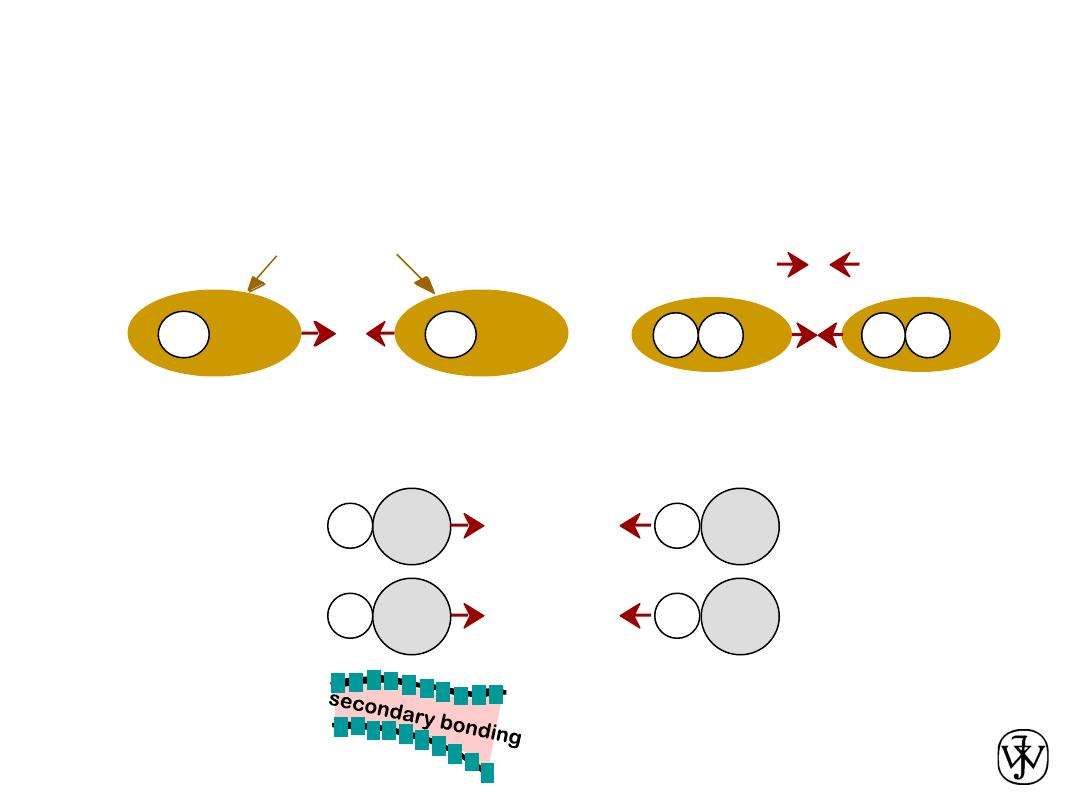
Chapter 2 - 57
Arises from interaction between
dipoles
• Permanent
dipoles
-molecule induced
• Fluctuating
dipoles
-general case:
-ex: liquid HCl
-ex: polymer
Adapted from Fig. 2.13,
Callister & Rethwisch 8e.
Adapted from Fig. 2.15,
Callister & Rethwisch 8e.
SECONDARY BONDING
asymmetric electron
clouds
+
-
+
-
secondary
bonding
H
H
H
H
H2
H2
secondary
bonding
ex: liquid H2
H Cl
H Cl
secondary
bonding
secondary
bonding
+
-
+
-
secondary bonding

Chapter 2 - 58
Type
Ionic
Covalent
Metallic
Secondary
Bond Energy
Large!
Variable
large-Diamond
small-Bismuth
Variable
large-Tungsten
small-Mercury
smallest
Comments
Nondirectional (
ceramics
)
Directional
(
semiconductors
,
ceramics
polymer
chains)
Nondirectional (
metals
)
Directional
inter-chain (
polymer
)
inter-molecular
Summary: Bonding
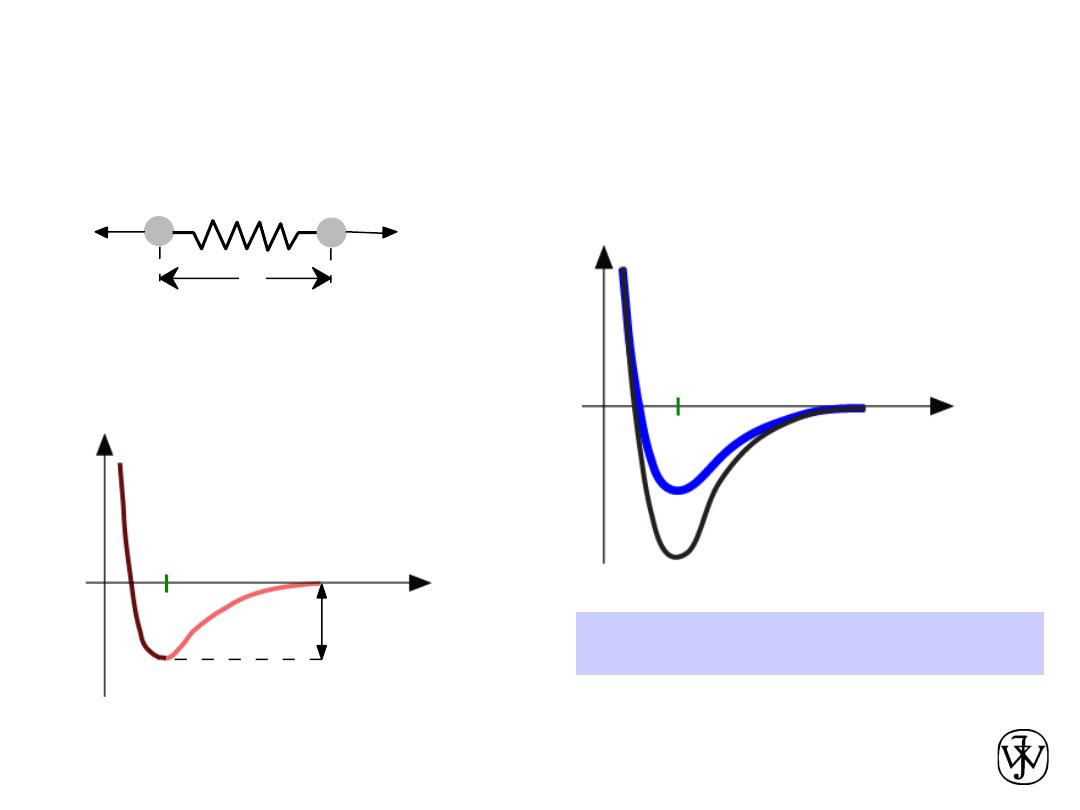
Chapter 2 - 59
•
Bond length
, r
•
Bond energy
, E
o
•
Melting Temperature
, T
m
T
m
is larger if E
o
is larger.
Properties From Bonding: T
m
r
o
r
Energy
r
larger T
m
smaller T
m
E
o
=
“bond energy”
Energy
r
o
r
unstretched length
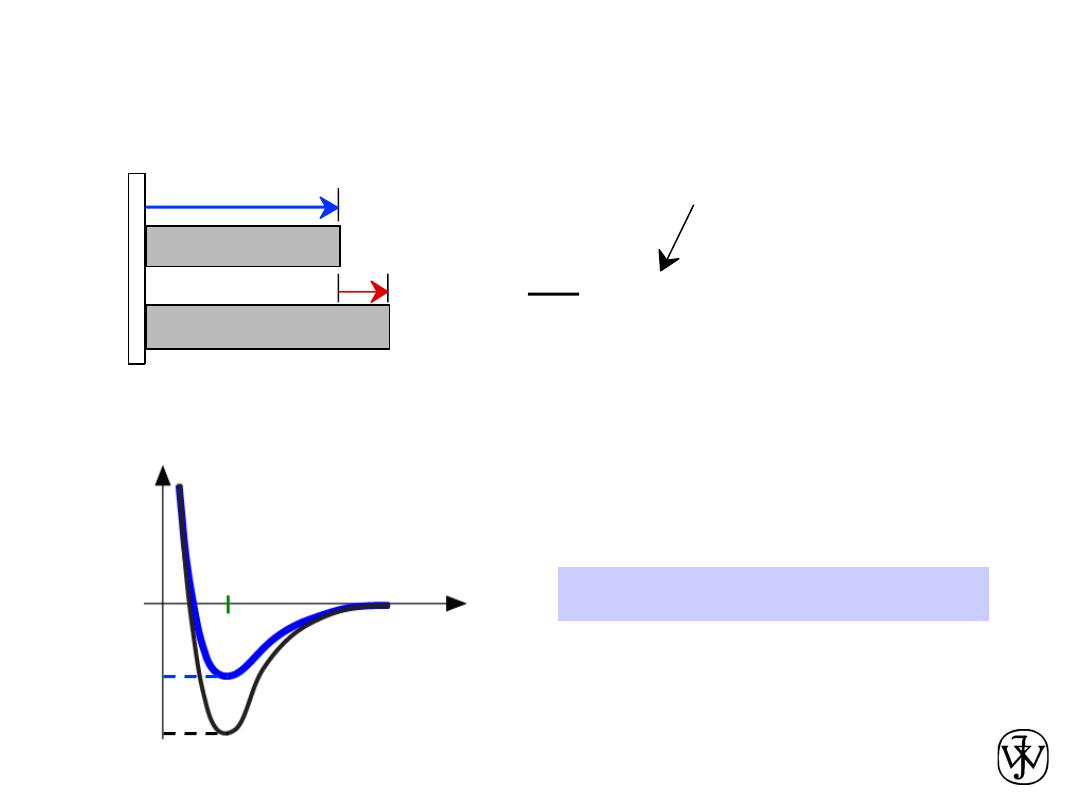
Chapter 2 - 60
•
Coefficient of thermal expansion
,
a
•
a ~ symmetric at r
o
a is larger if E
o
is smaller.
Properties From Bonding :
a
= a (
T
2
-
T
1
)
DL
Lo
coeff. thermal expansion
DL
length,
L
o
unheated, T1
heated, T2
r
o
r
smaller
a
larger
a
Energy
unstretched length
E
o
E
o
Chapter 2 - 61
Ceramics
(Ionic & covalent bonding):
Large bond energy
large T
m
large E
small
a
Metals
(Metallic bonding):
Variable bond energy
moderate T
m
moderate E
moderate
a
Summary: Primary Bonds
Polymers
(Covalent & Secondary):
Directional Properties
Secondary bonding dominates
small T
m
small E
large
a
