
Chapter 1 - 1
Materials Science
Chapter 1: Introduction

Chapter 1 - 2
Key Objectives
1. List six different property classifications of materials that
determine their applicability.
2. (a) Cite the four components that are involved in the design,
production, and utilization of materials.
(b) Briefly describe the interrelationships between these
components.
3. Cite three criteria that are important in the materials selection
process.
4. List the three primary classifications of solid materials, and then
cite the distinctive chemical feature of each.

Chapter 1 -
MATERIALS
• Materials may be defined as substance of
which something is composed or made.
• We obtain materials from earth crust and
atmosphere.
• Examples :
Silicon and Iron constitute 27.72 and 5.00
percentage of weight of earths crust
respectively.
Nitrogen and Oxygen constitute 78.08 and
20.95 percentage of dry air by volume
respectively.
3

Chapter 1 - 4
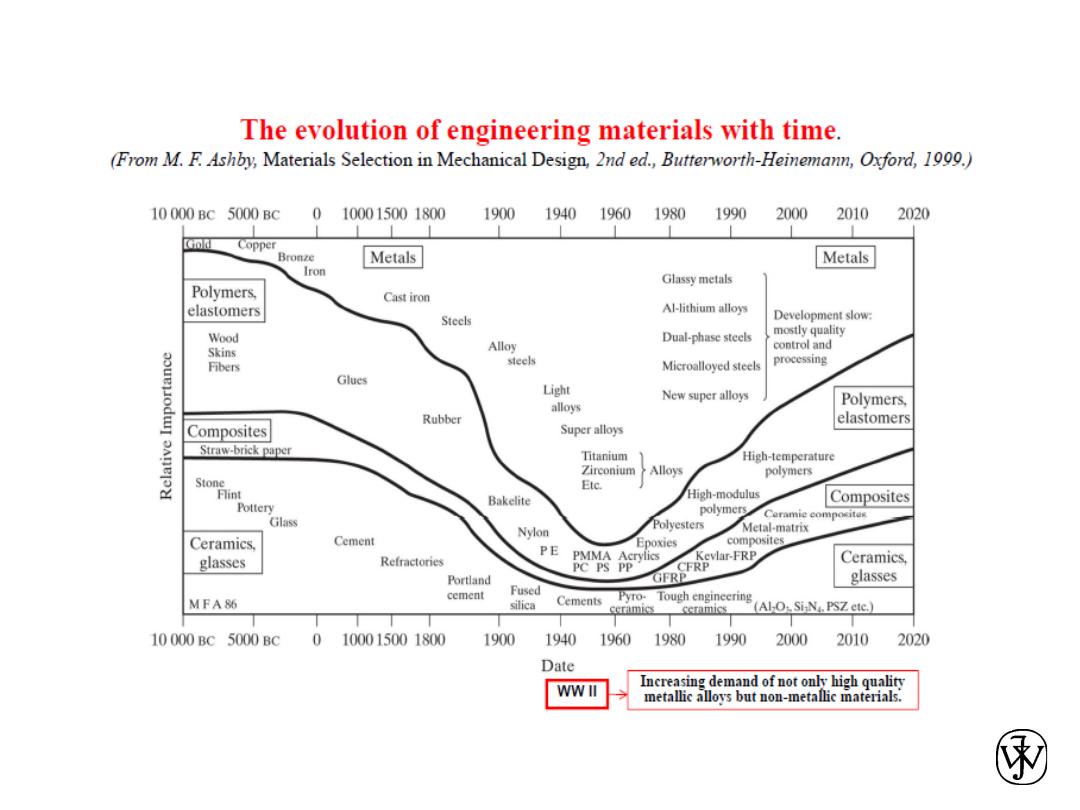
1.1 Historical Perspective
• Materials are probably more deep-seated in
our culture than most of us realize.
• Transportation, housing, clothing,
communication, recreation, and food
production—virtually every segment of our
everyday lives is influenced to one degree or
another by materials.

Chapter 1 - 5
1.1 Historical Perspective
• The earliest humans had access to only a
very limited number of materials, those that
occur naturally: stone, wood, clay, skins, and
so on.

Chapter 1 - 6
1.1 Historical Perspective
• With time they discovered techniques for
producing materials that had properties
superior to those of the natural ones;; these
new materials included pottery and various
metals.
• Furthermore,it was discovered that the
properties of a material could be altered by
heat treatments and by the addition of other
substances.

Chapter 1 - 7
1.1 Historical Perspective
• Materials drive our society
– Stone Age: Natural materials (2.5M BC)
– Bronze Age: Processed materials (3500 BC)
– Iron Age (1000 BC)
– Now?
• Silicon Age?
• Polymer Age?
• Bio-technology, Nano-technology, etc

Chapter 1 - 8
1.1 Historical Perspective
Chapter 1 - 9
1.1 Historical Perspective
Chapter 1 - 10
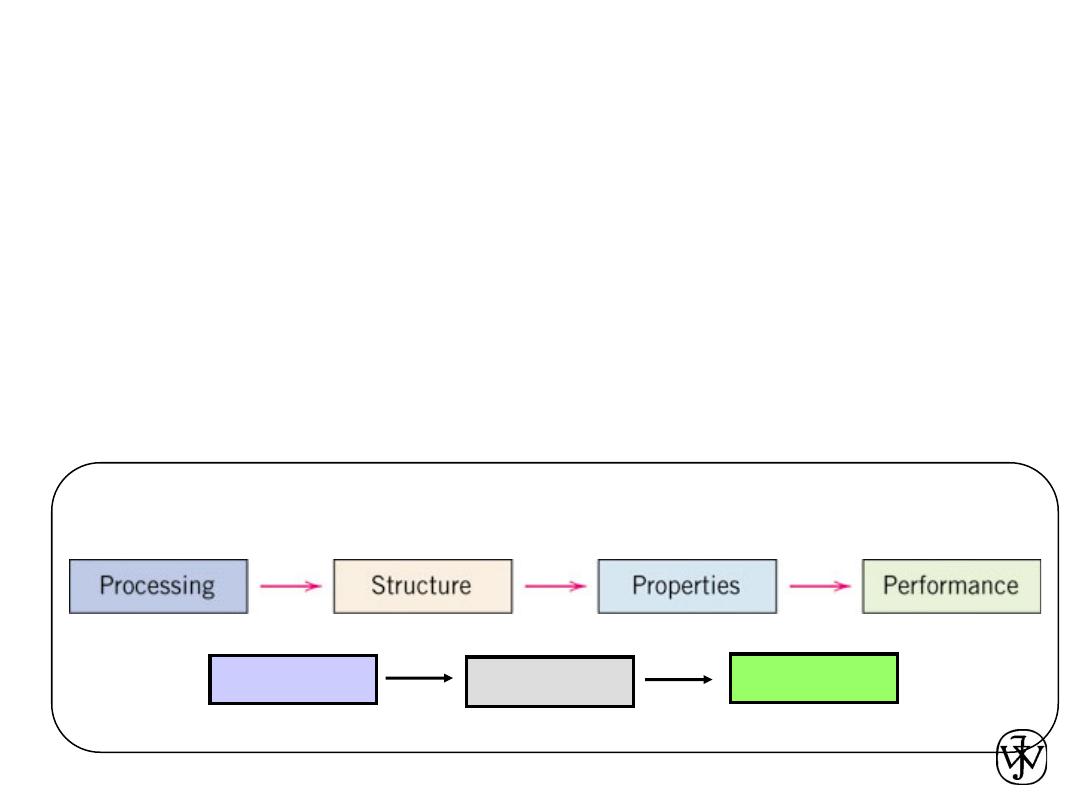
1.2 Materials Science & Materials Engineering
• Materials Science:
– involves the investigation of the
relationship between the
structures and properties of materials
• Materials Engineering:
– on the basis of the structure-property correlations,
designing
(or engineering) the structure to produce a target property
Materials Science and Engineering
Design
Production
Application
Chapter 1 - 11
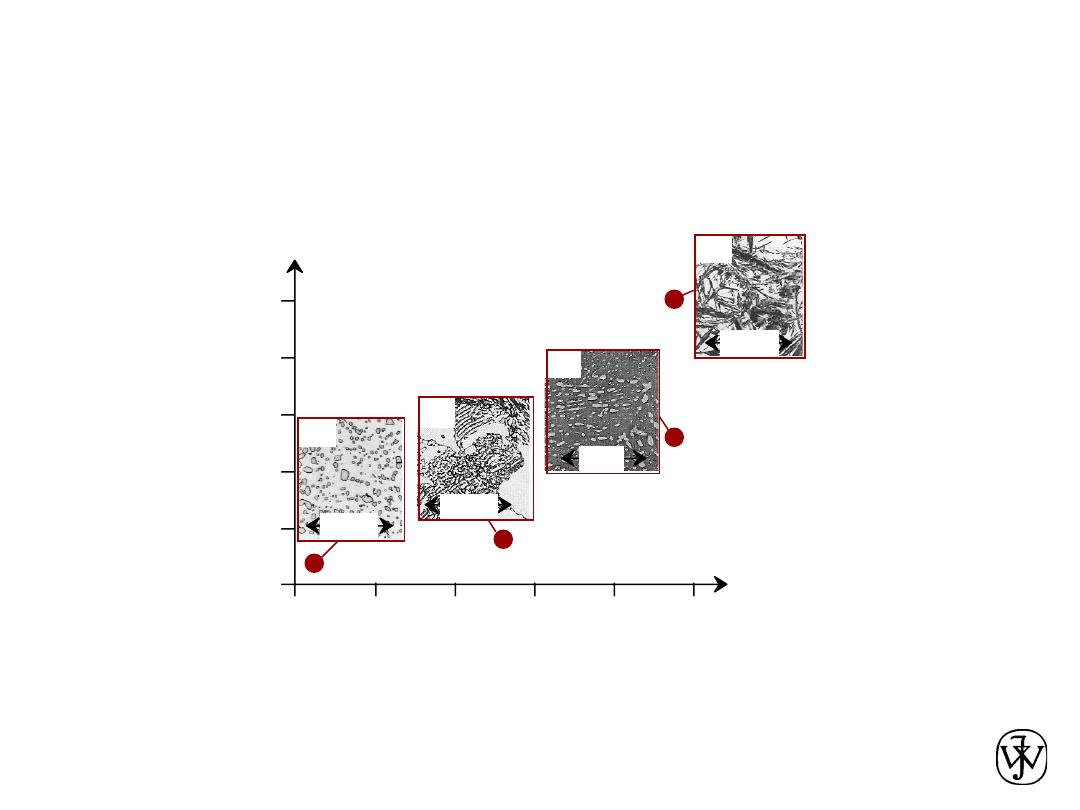
ex: hardness vs structure of steel
•
Properties
depend on
structure
Data obtained from Figs. 10.30(a)
and 10.32 with 4 wt% C composition,
and from Fig. 11.14 and associated
discussion, Callister 7e.
Micrographs adapted from (a) Fig.
10.19;; (b) Fig. 9.30;;(c) Fig. 10.33;;
and (d) Fig. 10.21, Callister 7e.
ex: structure vs cooling rate of steel
•
Processing
can change
structure
H
ar
dn
ess
(B
H
N
)
Cooling Rate (ºC/s)
100
200
300
400
500
600
0.01 0.1
1
10
100 1000
(d)
30
µm
(c)
4
µm
(b)
30
µm
(a)
30
µm
Structure-Property Relationship – Example #1

Chapter 1 - 12
Structure-Property Relationship – Example #2
• A: Single crystal – transparent
• B: Polycrystal – translucent
• C: Porous polycrystal - opaque
A
B
C
•
Three thin disks (A, B, and C) of aluminum oxide (alumina, Al
2
O
3
) –
same material
but
different microstructures
, and hence,
different optical
properties
(light-transmittance characteristics).
Chapter 1 - 13
1.
Pick
Application
Determine required
Properties
2.
Properties
Identify candidate
Material(s)
3.
Material
Identify required
Processing
Processing: changes structure and overall shape
ex: casting, sintering, vapor deposition, doping
forming, joining, annealing.
Properties: mechanical, electrical, thermal,
magnetic, optical, deteriorative.
Material: structure, composition.
The Materials Selection Process

Chapter 1 - 14
1.3 Structure
• Structure of a material relates to the arrangement of its internal
components.
– Subatomic structure
: electrons within the individual atoms
and interactions with their nuclei (Ch. 2)
– Atomic level
: structure encompasses the organization of
atoms or molecules relative to one another (Ch. 3)
– Microscopic level
: large groups of atoms that are normally
agglomerated together (Ch. 4)
– Macroscopic level
: structural elements that may be viewed
with the naked eye

Chapter 1 - 15
1.4 Classification of Properties
• Property: the kind and magnitude of response to a specific
imposed stimulus
• Properties of solid materials:
– Mechanical
: relate deformation to an applied load or force;;
elastic modulus, strength, etc (Ch. 6, 7, 8, etc)
– Electrical
: electrical conductivity (Ch. 18)
– Thermal
: heat capacity and thermal conductivity (Ch. 19)
– Magnetic
: response of a material to the application of a
magnetic field (Ch. 20)
– Optical
: response to electromagnetic or light radiation;; index
of refraction, reflectivity (Ch. 21)
– Deteriorative
: the chemical reactivity of materials (Ch. 17)
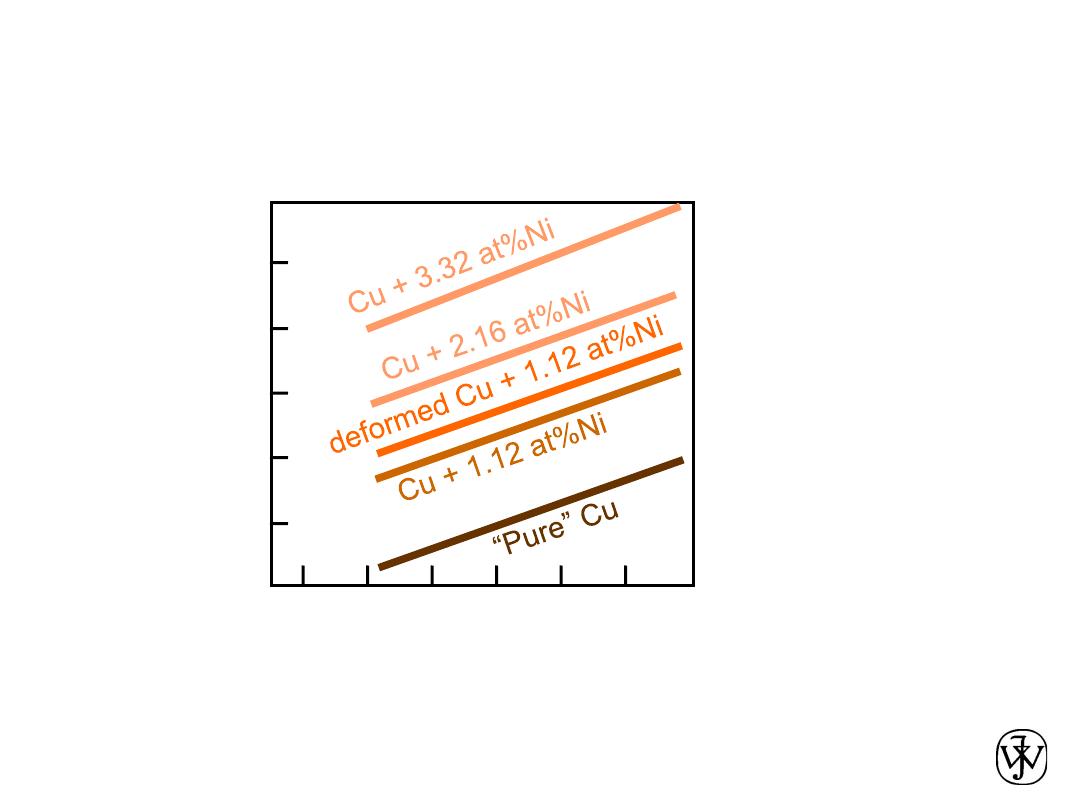
Chapter 1 - 16
ELECTRICAL
• Electrical Resistivity of Copper:
• Adding “
impurity
” atoms to Cu increases
resistivity
.
•
Deforming
Cu increases
resistivity
.
Adapted from Fig. 18.8, Callister 7e.
(Fig. 18.8 adapted from: J.O. Linde,
Ann Physik 5, 219 (1932);; and
C.A. Wert and R.M. Thomson,
Physics of Solids, 2nd edition,
McGraw-Hill Company, New York,
1970.)
T (°C)
-200
-100
0
1
2
3
4
5
6
R
esi
st
ivi
ty
, r
(1
0
-8
Oh
m
-m)
0
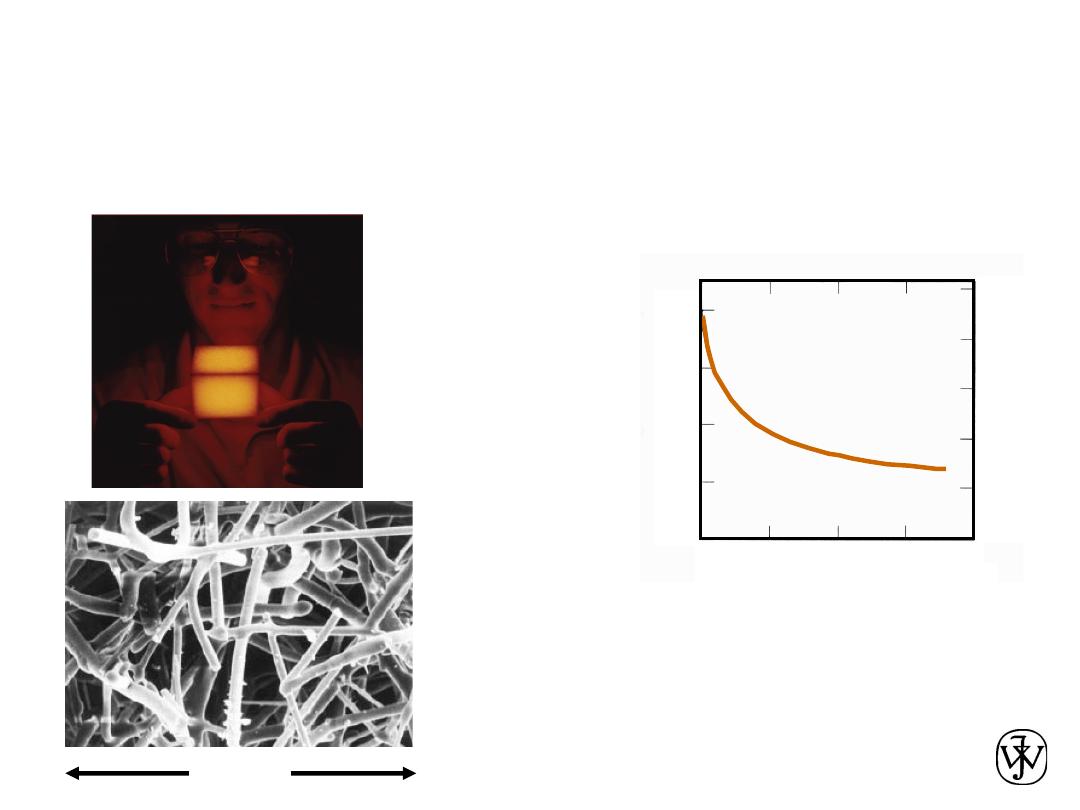
Chapter 1 - 17
THERMAL
• Space Shuttle Tiles:
--Silica fiber insulation
offers low
heat conduction
.
•
Thermal Conductivity
of Copper:
--It decreases when
you add zinc!
Adapted from
Fig. 19.4W, Callister
6e. (Courtesy of
Lockheed Aerospace
Ceramics Systems,
Sunnyvale, CA)
(Note: "W" denotes fig.
is on CD-ROM.)
Adapted from Fig. 19.4, Callister 7e.
(Fig. 19.4 is adapted from Metals Handbook:
Properties and Selection: Nonferrous alloys and
Pure Metals, Vol. 2, 9th ed., H. Baker,
(Managing Editor), American Society for Metals,
1979, p. 315.)
Composition (wt% Zinc)
T
he
rm
al
C
on
du
ct
ivi
ty
(W/
m
-K)
400
300
200
100
0
0
10
20
30
40
100
µm
Adapted from chapter-
opening photograph,
Chapter 19, Callister 7e.
(Courtesy of Lockheed
Missiles and Space
Company, Inc.)
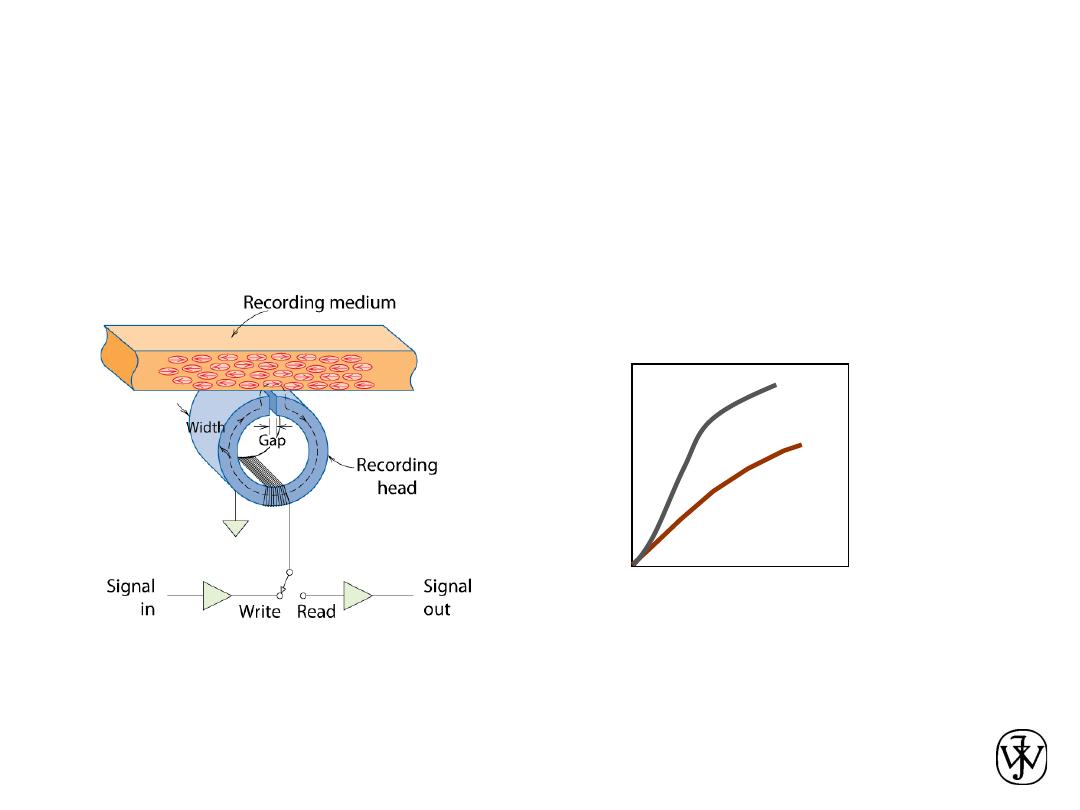
Chapter 1 - 18
MAGNETIC
•
Magnetic Permeability
vs. Composition:
--Adding 3 atomic % Si
makes Fe a better
recording medium!
Adapted from C.R. Barrett, W.D. Nix, and
A.S. Tetelman, The Principles of
Engineering Materials, Fig. 1-7(a), p. 9,
1973. Electronically reproduced
by permission of Pearson Education, Inc.,
Upper Saddle River, New Jersey.
Fig. 20.23, Callister 7e.
(Fig. 20.23 is from J.U. Lemke, MRS Bulletin,
Vol. XV, No. 3, p. 31, 1990.)
•
Magnetic Storage
:
--Recording medium
is magnetized by
recording head.
Magnetic Field
M
ag
ne
tiza
tio
n
Fe+3%Si
Fe
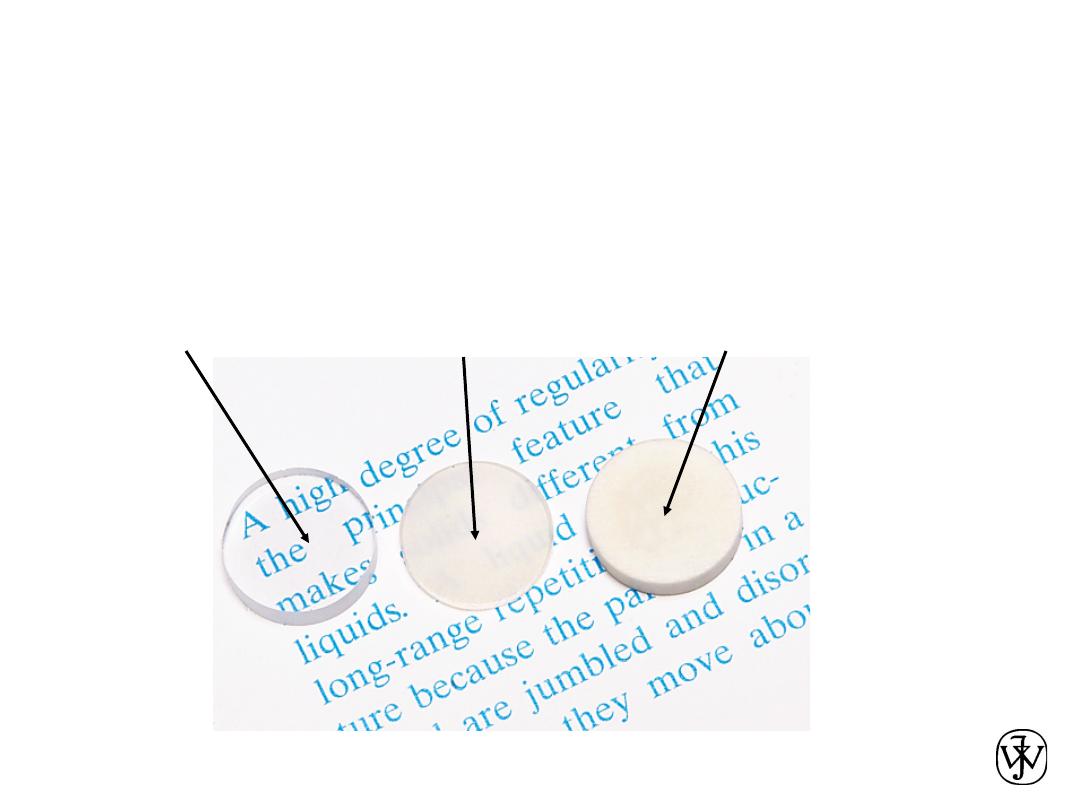
Chapter 1 - 19
•
Transmittance
:
--Aluminum oxide may be transparent, translucent, or
opaque depending on the material structure.
Adapted from Fig. 1.2,
Callister 7e.
(Specimen preparation,
P.A. Lessing;; photo by S.
Tanner.)
single crystal
polycrystal:
low porosity
polycrystal:
high porosity
OPTICAL
Chapter 1 - 20
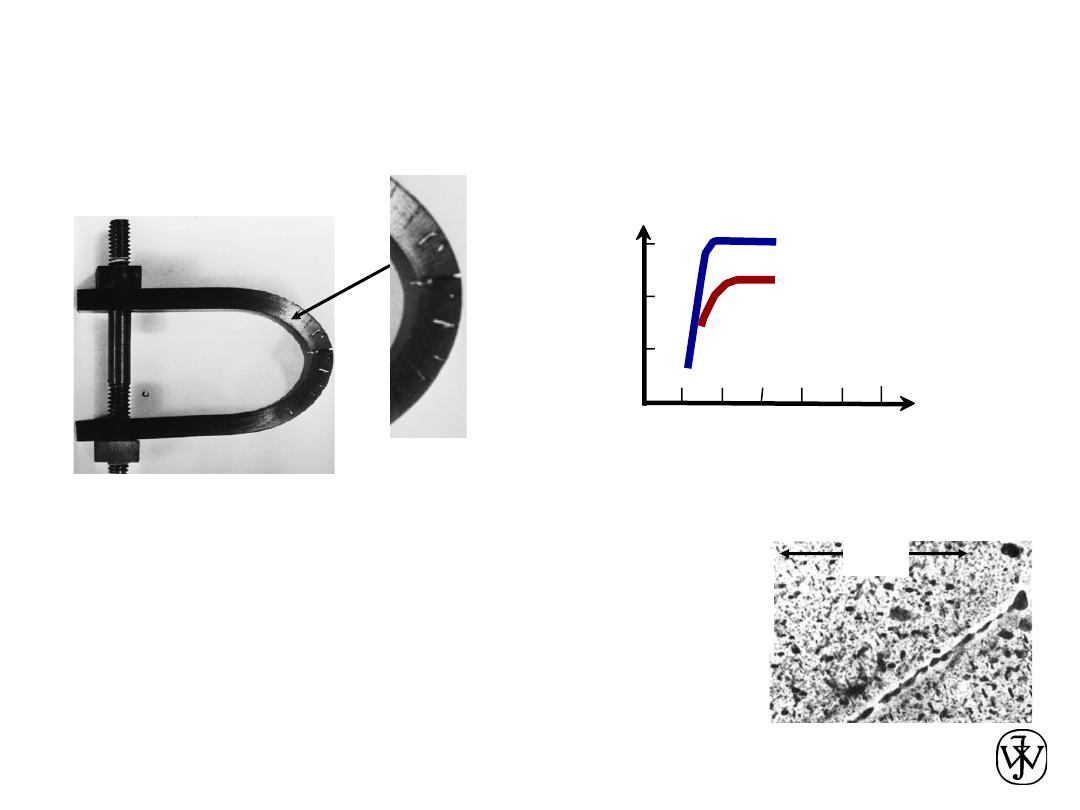
DETERIORATIVE
• Stress & Saltwater...
--causes cracks!
Adapted from chapter-opening photograph,
Chapter 17, Callister 7e.
(from Marine Corrosion, Causes, and
Prevention, John Wiley and Sons, Inc., 1975.)
4
µm
--material:
7150-T651 Al "alloy"
(Zn,Cu,Mg,Zr)
Adapted from Fig. 11.26,
Callister 7e. (Fig. 11.26 provided courtesy of G.H.
Narayanan and A.G. Miller, Boeing Commercial
Airplane Company.)
• Heat treatment:
slows
crack speed in salt water!
Adapted from Fig. 11.20(b), R.W. Hertzberg, "Deformation and
Fracture Mechanics of Engineering Materials" (4th ed.), p. 505, John
Wiley and Sons, 1996. (Original source: Markus O. Speidel, Brown
Boveri Co.)
“held at
160ºC for 1 hr
before testing”
increasing load
cr
a
ck
sp
e
e
d
(
m
/s)
“as-is”
10
-10
10
-8
Alloy 7178 tested in
saturated aqueous NaCl
solution at 23ºC

Chapter 1 - 21
1.5 Types of (Solid) Materials
•
Three basic classifications based on chemical makeup and atomic
structure:
Metals
,
Ceramics
, and
Polymers
.
•
In addition to these basic three: composites, advanced materials, etc.
•
Metals
:
– Metallic elements: Fe, Al, Cu, Ti, Au, Ni, etc.
– Metallic bonding
– Strong, ductile
– high thermal & electrical conductivity
– opaque, reflective.

Chapter 1 - 22
•
Ceramics
:
– compounds of metallic & non-metallic elements (oxides, carbides,
nitrides, sulfides)
– Al
2
O
3
, SiO
2
, SiC, Si
3
N
4
, traditional ceramics (porcelain), cement,
glass, etc
– Ionic bonding (refractory)
– Brittle, glassy, elastic
– non-conducting (insulators)

Chapter 1 - 23
•
Polymers
:
– Polyethylene, nylon, poly(vinyl chloride) (PVC), etc
– Covalent bonding à sharing of electrons
– Soft, ductile, low strength, low density
– thermal & electrical insulators
– Optically translucent or transparent.

Chapter 1 - 24
•
Composites:
– Composed of two (or more) of the basic materials
– Ceramic-fiber reinforced metal-matrix composites (MMC), carbon-
fiber-reinforced polymer (CFRP), etc
– Combination of properties

Chapter 1 - 25
1.6 Advanced Materials
• Advanced materials: materials for so-called
high-tech
applications
– Electronic equipment
– computers
– Spacecraft
– Aircraft
– Military rocketry
– Semiconductors
– Biomaterials
– Smart materials
– Nano-engineered materials

Chapter 1 - 26
Example – Smart Materials
• These materials are able to sense changes in their
environments and then respond to these changes in
predetermined manners.
• Sensors, actuators, etc.
• Actuators made of smart materials change shape,
position, natural frequency, or mechanical
characteristics in response to changes in
temperature, electric fields, and/or magnetic fields.

Chapter 1 - 27
Example – Smart Materials
•
Shape memory alloys
(SMA):
– Metals that, after having been deformed, revert back to their
original shapes when temperature is changes (Ch. 10.9)
•
Piezoelectric ceramics
(PZT):
– Expand and contract in response to an applied electric field (or
voltage)
– Or, they generate an electric field when their dimensions are
altered (Ch. 18.25)
•
Magnetostrictive materials
:
– Similar to PZT except that they are responsive to magnetic fields
•
Electrorheological and magnetorheological fluids
:
– Liquids that experience dramatic changes in viscosity upon the
application of electric and magnetic fields
Chapter 1 - 28
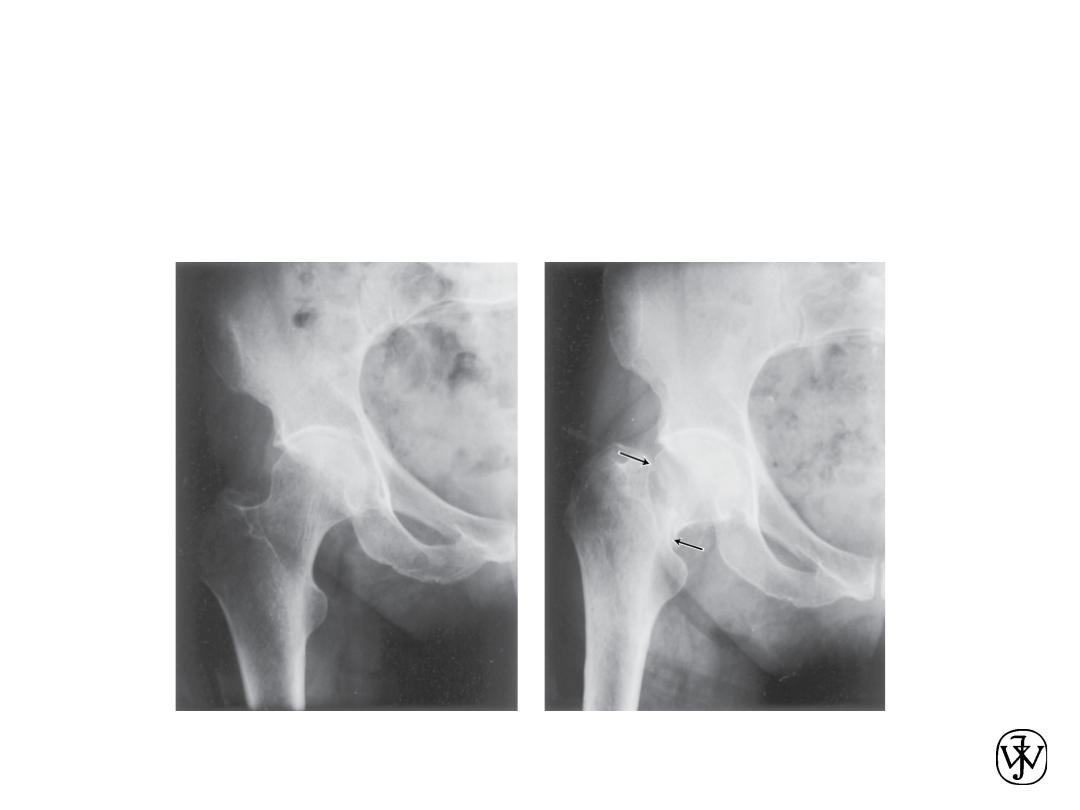
Example – Hip Implant
• With age or certain illnesses joints deteriorate.
Particularly those with large loads (such as hip).
Adapted from Fig. 22.25, Callister 7e.
Chapter 1 - 29
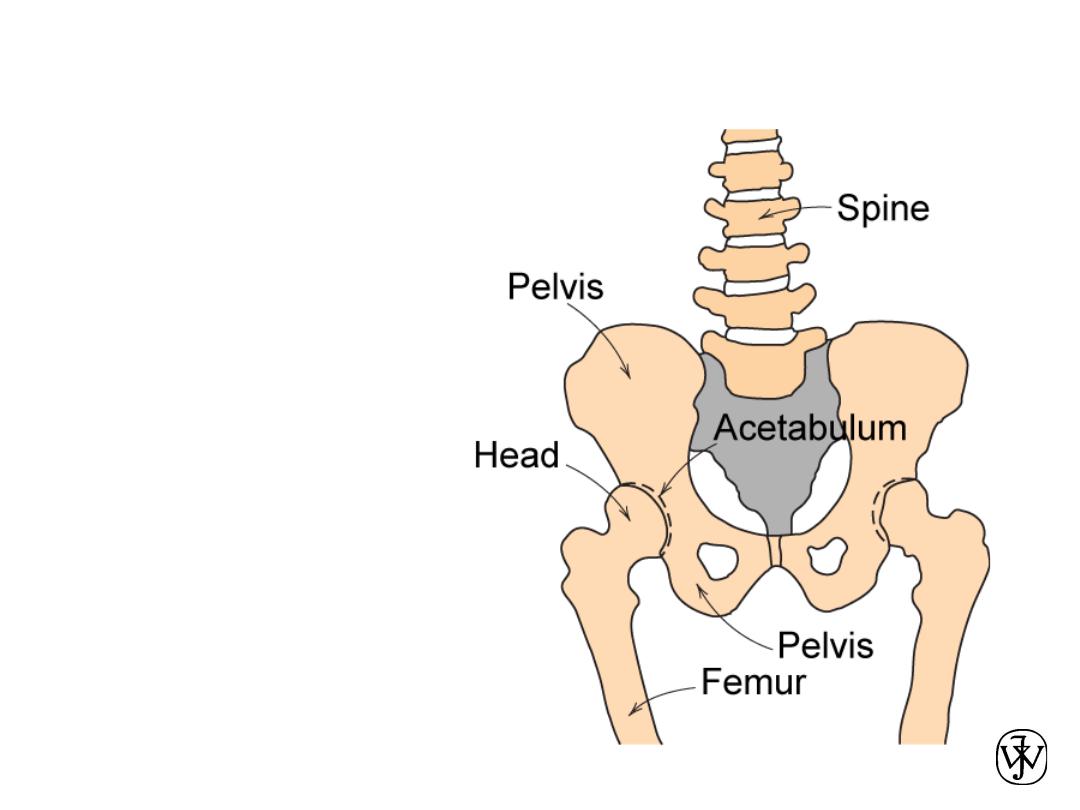
Example – Hip Implant
• Requirements
– mechanical
strength (many
cycles)
– good lubricity
– biocompatibility
Adapted from Fig. 22.24, Callister 7e.
Chapter 1 - 30
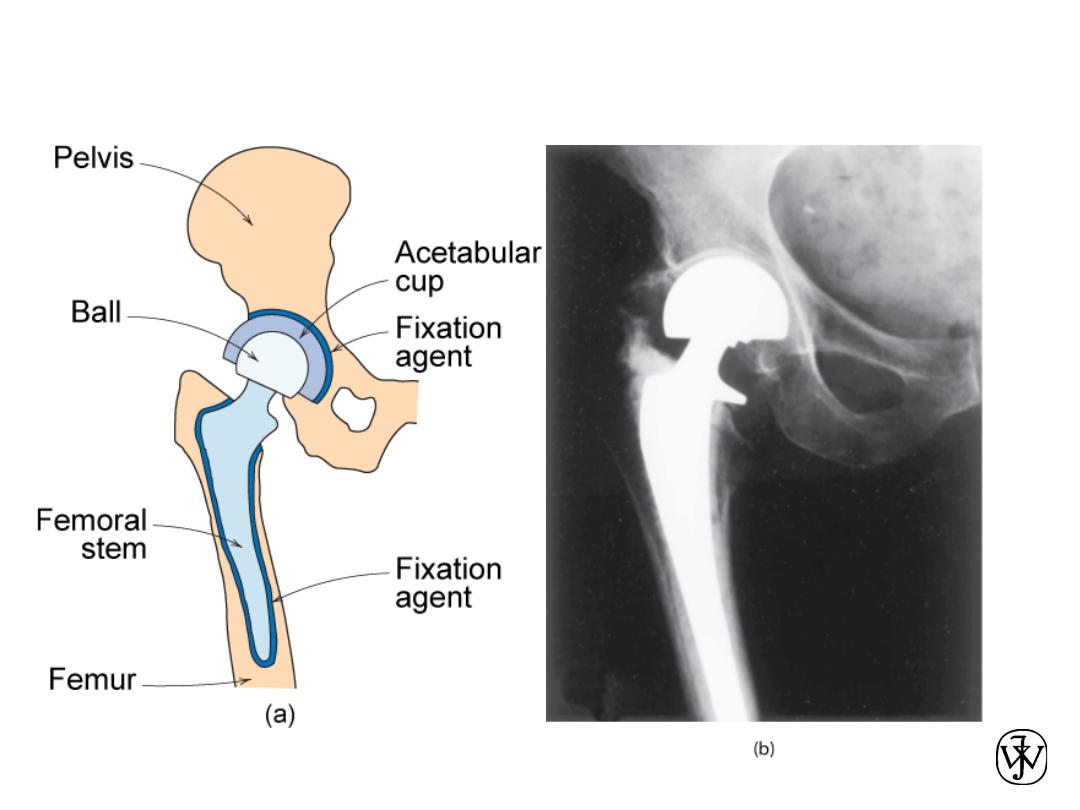
Example – Hip Implant
Adapted from Fig. 22.26, Callister 7e.
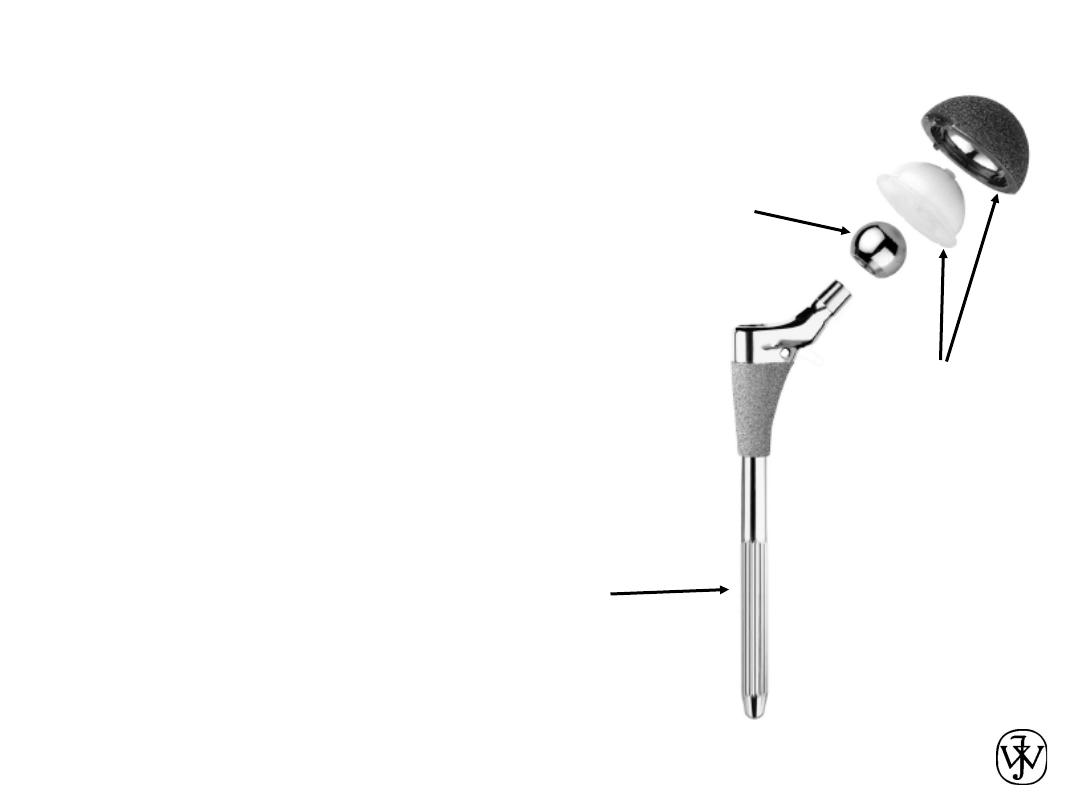
Chapter 1 - 31
Hip Implant
• Key problems to overcome
– fixation agent to hold
acetabular cup
– cup lubrication material
– femoral stem – fixing agent
(“glue”)
– must avoid any debris in cup
Adapted from chapter-opening photograph,
Chapter 22, Callister 7e.
Femoral
Stem
Ball
Acetabular
Cup and Liner

Chapter 1 -
Example : How to choose the right
material?
32

Chapter 1 -
Example : How to choose the right
material?
• One common item that presents some
interesting material property requirements is
the container for carbonated beverages.
• The material used for this application must
satisfy the following constraints:
• (1) provide a barrier to the passage
• of carbon dioxide, which is under pressure in
• the container;;
• (2) be nontoxic, unreactive with thebeverage,
and, preferably be recyclable;;
33

Chapter 1 -
• (3) be relatively strong, and capable of
surviving a drop from a height of several feet
when containing the beverage;;
• (4) be inexpensive and the cost to fabricate
the final shape should be relatively low;;
• (5) if optically transparent, retain its optical
clarity;; and
• (6) capable of being produced having
different colors and/or able to be adorned
with decorative labels.
34

Chapter 1 -
• All three of the basic material types—metal
(aluminum), ceramic (glass), and polymer
(polyester plastic)
• All of these materials are nontoxic, and
unreactive with beverages.
35

Chapter 1 - 36
• Use the right material for the job.
• Understand the relation between
properties
,
structure
, and
processing
.
• Recognize new design opportunities offered
by materials selection.
Course Goals:
SUMMARY
