CARBOHYDRATES
carbohydrates are the most abundant biomolecules on Earth. Each year, photosynthesis converts more than 100 billion metric tons of CO2 and H2O into cellulose and other plant products. Certain carbohydrates (sugar and starch) are a dietary staple in most parts of the world, and the oxidation of carbohydrates is the central energy-yielding pathway in most nonphotosynthetic cells. Insoluble carbohydrate polymers serve as structuraland protective elements in the cell walls of bacteria and plants and in the connective tissues of animals. Other carbohydrate polymers lubricate skeletal joints and participate in recognition and adhesion between cells. Carbohydrates are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis.
Many carbohydrates have the empirical formula (CH2O)n; some also contain nitrogen, phosphorus, or sulfur. There are three major size classes of carbohydrates: monosaccharides, oligosaccharides, and polysaccharides.
Monosaccharides, or simple sugars, consist of a single polyhydroxy aldehyde or ketone unit. The most abundant monosaccharide in nature is the six-carbon sugar D-glucose, sometimes referred to as dextrose. Monosaccharides of more than four carbons tend to have cyclic structures.
Oligosaccharides consist of short chains of monosaccharide units, or residues, joined by characteristic linkages called glycosidic bonds. The most abundant are the disaccharides, with two monosaccharide units. Typical is sucrose (cane sugar), which consists of the six-carbon sugars D-glucose and D-fructose. All common monosaccharides and disaccharides have names ending with the suffix “-ose.”
The polysaccharides are sugar polymers containing more than 20 or so monosaccharide units, and some have hundreds or thousands of units. Some polysaccharides, such as cellulose, are linear chains; others, such as glycogen, are branched. Both glycogen and cellulose consist of recurring units of D-glucose, but they differ in the type of glycosidic linkage and consequently have strikingly different properties and biological roles.
Monosaccharides
The simplest of the carbohydrates, the monosaccharides, are either aldehydes or ketones with two or more hydroxyl groups; the six-carbon monosaccharides glucose and fructose have five hydroxyl groups. Many of the carbon atoms to which hydroxyl groups are attached are chiral centers, which give rise to the many sugar stereoisomers found in nature
The Two Families of Monosaccharides Are Aldoses and Ketoses
Monosaccharides are colorless, crystalline solids that are freely soluble in water but insoluble in nonpolar solvents. Most have a sweet taste. In the open-chain form, one of the carbon atoms is double-bonded to an oxygen atom to form a carbonyl group; each of the other carbon atoms has a hydroxyl group. If the carbonyl group is at an end of the carbon chain (that is, in an aldehyde group) the monosaccharide is an aldose; if the carbonyl group is at any other position (in a ketone group) the monosaccharide is a ketose. The simplest monosaccharides are the two three-carbon trioses: glyceraldehyde, an aldotriose, and dihydroxyacetone, a ketotriose.Monosaccharides with four, five, six, and seven carbon atoms in their back bones are called, respectively, tetroses, pentoses, hexoses, and heptoses. There are aldoses and ketoses of each of these chain lengths aldotetroses and ketotetroses, aldopentoses and ketopentoses, and so on. The hexoses, which include the aldohexose D-glucose and the ketohexose D-fructose are the most common monosaccharides in nature.
The aldopentoses D-ribose and 2-deoxy-D-ribose are components of nucleotides and nucleic acids.
Monosaccharides Have Asymmetric Centers
All the monosaccharides except dihydroxyacetone contain one or more asymmetric (chiral) carbon atoms and thus occur in optically active isomeric forms .The simplest aldose, glyceraldehyde, contains one chiral center (the middle carbon atom) and therefore has two different optical isomers, or enantiomers.Of the 16 possible aldohexoses, eight are D forms and eight are L. Most of the hexoses of living organisms are D isomers.
The above figures show the structures of the D stereoisomers of all the aldoses and ketoses having three to six carbon atoms. The carbons of a sugar are numbered beginning at the end of the chain nearest the carbonyl group. Each of the eight D-aldohexoses, which differ in the stereochemistry at C-2, C-3, or C-4, has its own name: D-glucose, D-galactose, D-mannose, and so forth .The four- and five-carbon ketoses are designated by inserting “ul” into the name of a corresponding aldose; for example, D-ribulose is the ketopentose corresponding to the aldopentose D-ribose. The ketohexoses are named otherwise: for example, fructose and sorbose.
Two sugars that differ only in the configuration around one carbon atom are called epimers; D-glucose and D-mannose, which differ only in the stereochemistry at C-2, are epimers.
Disaccharides
Disaccharides (such as maltose, lactose, and sucrose) consist of two monosaccharides joined covalently by an O-glycosidic bond, which is formed when a hydroxyl group of one sugar reacts with the anomeric carbon of the other.This reaction represents the formation of an acetal from a hemiacetal
Glycosidic bonds are readily hydrolyzed by acid but resist cleavage by base. Thus disaccharides can be hydrolyzed to yield their free monosaccharide components by boiling with dilute acid.The disaccharide lactose which yields D-galactose and D-glucose on hydrolysis, occurs naturally only in milk.
The anomeric carbon of the glucose residue is available for oxidation, and thus lactose is a reducing disaccharide.
Sucrose (table sugar) is a disaccharide of glucose and fructose. It is formed by plants but not by animals. In contrast to maltose and lactose, sucrose contains no free anomeric carbon atom; the anomeric carbons of both monosaccharide units are involved in the glycosidic bond.
Sucrose is therefore a nonreducing sugar. Sucrose is a major intermediate product of photosynthesis; in many plants it is the principal form in which sugar is transported from the leaves to other parts of the plant body.
Trehalose, a disaccharide of D-glucose that, like sucrose, is a nonreducing sugar—is a major constituent of the circulating fluid of insects, serving as an energy-storage compound.
Polysaccharides
Most carbohydrates found in nature occur as polysaccharides, polymers of medium to high molecular weight. Polysaccharides differ from each other in the identity of their recurring monosaccharide units, in the length of their chains, in the types of bonds linking the units, and in the degree of branching. Homopolysaccharides contain only a single type of monomer; heteropolysaccharides contain two or more different kinds .Some homopolysaccharides serve as storage forms of monosaccharides that are used as fuels; starch and glycogen are homopolysaccharides of this type. Other homopolysaccharides (cellulose and chitin, for example) serve as structural elements in plant cell walls and animal exoskeletons. Heteropolysaccharides provide extracellular support for organisms of all kingdoms. For example, the rigid layer of the bacterial cell envelope (the peptidoglycan) is composed in part of a heteropolysaccharide built from two alternating monosaccharide units.
For polysaccharide synthesis there is no template; rather, the program for polysaccharide synthesis is intrinsic to the enzymes that catalyze the polymerization of the monomeric units, and there is no specific stopping point in the synthetic process.
Some Homopolysaccharides Are Stored Forms of Fuel
The most important storage polysaccharides are starch in plant cells and glycogen in animal cells. Both polysaccharides occur intracellularly as large clusters or granules. Starch and glycogen molecules are heavily hydrated, because they have many exposed hydroxyl groups available to hydrogen-bond with water. Most plant cells have the ability to form starch, but it is especially abundant in tubers, such as potatoes, and in seeds. Starch contains two types of glucose polymer, amylose and amylopectin.
Amylopectin
The former consists of long, unbranched chains of D-glucose residues connected by (α1-4) linkages. Such chains vary in molecular weight from a few thousand to more than a million.Amylopectin also has a high molecular weight (up to 100 million) but unlike amylose is highly branched. The glycosidic linkages joining successive glucose
residues in amylopectin chains are (α1-4); the branch points (occurring every 24 to 30 residues) are (α1-6) linkages.
Glycogen is the main storage polysaccharide of animal cells. Like amylopectin, glycogen is a polymer of (α1-4)-linked subunits of glucose, with (α1-6)-linked
branches, but glycogen is more extensively branched (on average, every 8 to 12 residues) and more compact than starch. Glycogen is especially abundant in the liver, where it may constitute as much as 7% of the wet weight; it is also present in skeletal muscle. In hepatocytes glycogen is found in large granules,
which are themselves clusters of smaller granules composed of single, highly branched glycogen molecules with an average molecular weight of several million. Such glycogen granules also contain, in tightly bound form, the enzymes responsible for the synthesis and degradation of glycogen. Because each branch in glycogen ends with a nonreducing sugar unit, a glycogen molecule has as many nonreducing ends as it has branches, but only one reducing end. When glycogen is used as an energy source, glucose units are removed one at a time from the nonreducing ends. Degradative enzymes that act only at nonreducing ends can work simultaneously on the many branches, speeding the conversion of the polymer to monosaccharides.
Why not store glucose in its monomeric form?
It has been calculated that hepatocytes store glycogen equivalent to a glucose concentration of 0.4 M. The actual concentration of glycogen, which is insoluble and contributes little to the osmolarity of the cytosol, is about 0.01 µM. If the cytosol contained 0.4 M glucose, the osmolarity would be threateningly elevated, leading to osmotic entry of water that might rupture the cell.
Furthermore, with an intracellular glucose concentration of 0.4 M and an external concentration of about 5 mM (the concentration in the blood of a mammal), the free-energy change for glucose uptake into cells against this very high concentration gradient would be prohibitively large.
Dextrans are bacterial and yeast polysaccharides made up of (α1-6)-linked poly-D-glucose; all have (α1-3) branches, and some also have (α1-2) or (α1-4) branches. Dental plaque, formed by bacteria growing on the surface of teeth, is rich in dextrans. Synthetic dextrans are used in several commercial products (for example, Sephadex) that serve in the fractionation of proteins by size-exclusion chromatography.
The dextrans in these products are chemically cross-linked to form insoluble materials of various porosities, admitting macromolecules of various sizes.
Some Homopolysaccharides Serve Structural Roles
Cellulose, a fibrous, tough, water-insoluble substance, is found in the cell walls of plants, particularly in stalks, stems, trunks, and all the woody portions of the plant body. Cellulose constitutes much of the mass of wood, and cotton is almost pure cellulose. Like amylose and the main chains of amylopectin and glycogen, the cellulose molecule is a linear, unbranched homopolysaccharide,
consisting of 10,000 to 15,000 D-glucose units. But there is a very important difference: in cellulose the glucose residues have the β configuration
Where as in amylose, amylopectin, and glycogen the glucose is in the α configuration. The glucose residues in cellulose are linked by (β1-4) glycosidic bonds, in contrast to the (α1-4) bonds of amylose, starch, and glycogen.
This difference gives cellulose and amylose very different structures and physical properties. Glycogen and starch ingested in the diet are hydrolyzed
by α-amylases, enzymes in saliva and intestinal secretions that break (α1-4) glycosidic bonds between glucose units. Most animals cannot use cellulose as a fuel source, because they lack an enzyme to hydrolyze the (α1-4) linkages. Termites readily digest cellulose
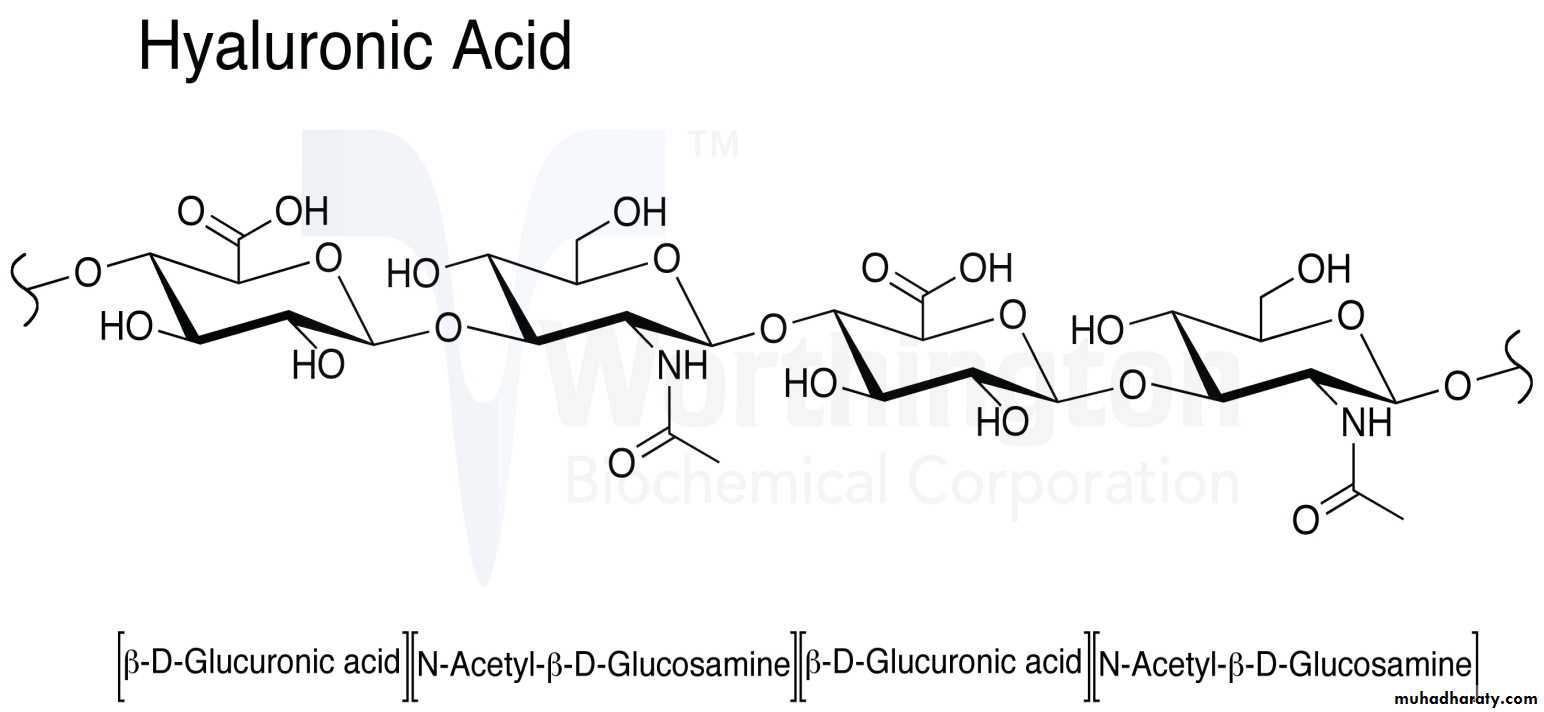
Hyaluronic acid :
Hyaluronic acid is an anionic, HYPERLINK "https://en.wikipedia.org/wiki/Sulfation" \o "Sulfation" nonsulfated HYPERLINK "https://en.wikipedia.org/wiki/Glycosaminoglycan" \o "Glycosaminoglycan" glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. Hyaluronic acid is a polymer of disaccharides, themselves composed of D-glucuronic acid and D-N-acetylglucosamine, linked via alternating β-1,4 and β-1,3 HYPERLINK "https://en.wikipedia.org/wiki/Glycosidic_bond" \o "Glycosidic bond" glycosidic bonds. Hyaluronic acid is an important component of articular cartilage, where it is present as a coat around each cell ( HYPERLINK "https://en.wikipedia.org/wiki/Chondrocyte" \o "Chondrocyte" chondrocyte)