LIPID
LIPIDSThe lipids are a large and heterogeneous group of substances of biological origin that are easily dissolved in organic solvents such as methanol, acetone, chloroform, and benzene. By contrast, they are either insoluble or only poorly soluble in water.
Their low water solubility is due to a lack of polarizing atoms such as O, N, S, and P.
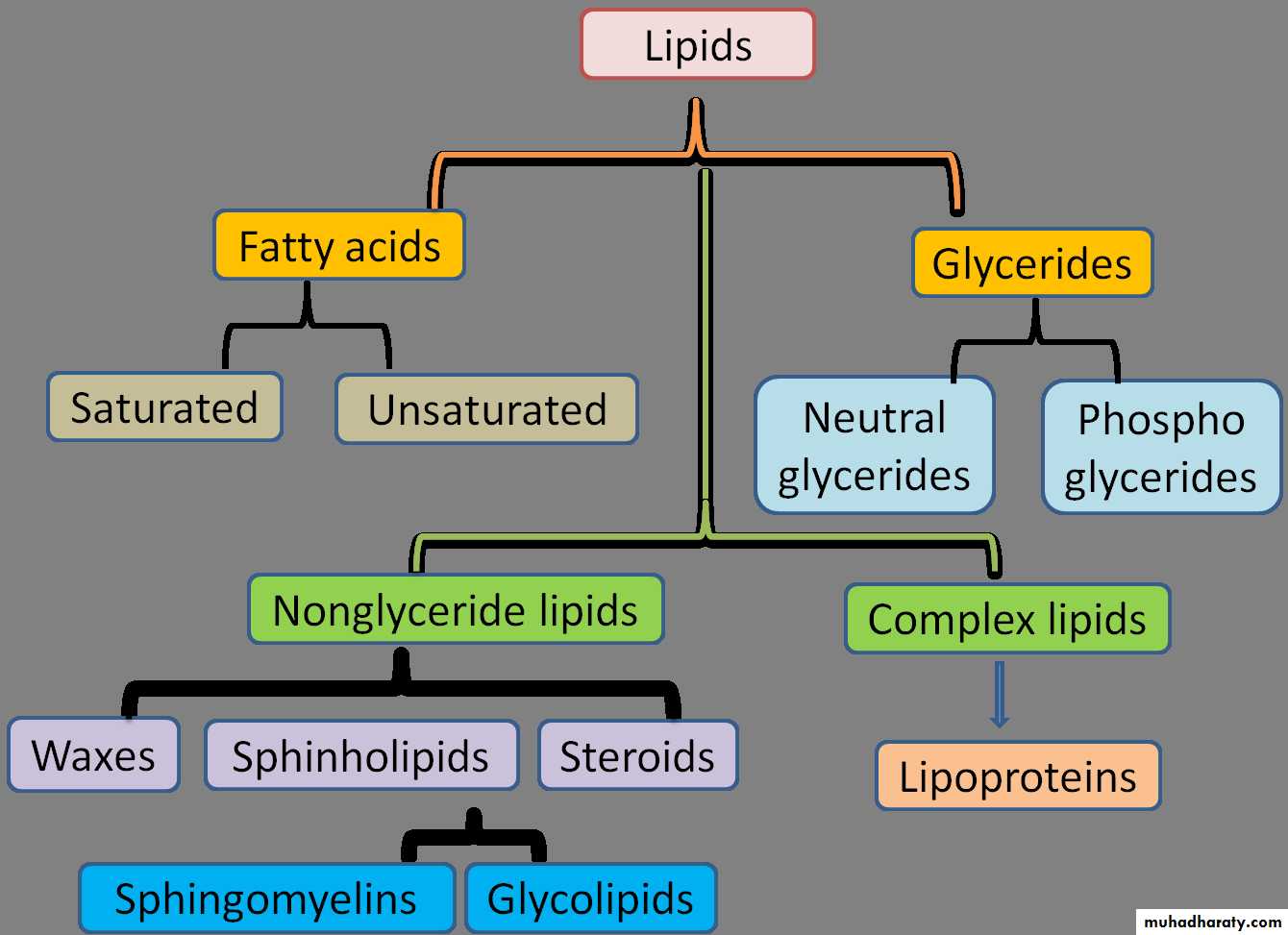
Lipids can be classified into substances that are either hydrolyzable— i.e., able to undergo hydrolytic cleavage—or nonhydrolyzable.
Hydrolyzable lipids: The simple esters include
The fats (triacylglycerol; one glycerol + three acyl residues)
the waxes (one fatty alcohol + one acyl residue)
the sterol esters (one sterol + one acyl residue).
The phospholipids are esters with more complex structures. Their characteristic component is a phosphate residue. The phospholipids include the phosphatidic acids (one glycerol + two acyl residues + one phosphate) the phosphatides (1 glycerol+ 2 acyl residues + 1 phosphate +amino alcohol).
In the sphingolipids, glycerol and one acyl residue are replaced by sphingosine. Particularly important in this group are the sugar-containing glycolipids (one sphingosine + one fatty acid + sugar).
The cerebrosides (one sphingosine + one fatty acid + one sugar) gangliosides (one sphingosine + one fatty acid + several different sugars) .
The components of the hydrolyzable lipids are linked to one another by ester bonds. They are easily broken down either enzymatically or chemically.
Non-hydrolyzable lipids:
The hydrocarbons include the alkanes and carotenoids. The lipid alcohols are also not hydrolyzable. They include long-chained alkanols and cyclic sterols such as cholesterol, and steroids such as estradiol and testosterone. The most important acids among the lipids are fatty acids. The eicosanoids also belong to this group.
Biological roles :
Lipids are an important source of energy in the diet. In quantitative terms, they represent the principal energy reserve in animals. Neutral fats in particular are stored in specialized cells, known as adipocytes. Fatty acids are released from these again as needed, and these are then oxidized in the mitochondria to form water and carbon dioxide, with oxygen being consumed. This process also gives rise to reduced coenzymes, which are used for ATP production in the respiratory chain .
2. Nutrients.
Amphipathic lipids are used by cells to build membranes .Typical membrane lipids include phospholipids, glycolipids, and cholesterol. Fats are only weakly amphiphilic and are therefore not suitable as membrane components.3. Insulation.
Lipids are excellent insulators. In the higher animals, neutral fats are found in the subcutaneous tissue and around various organs, where they serve as mechanical and thermal insulators. As the principal constituent of cell membranes, lipids also insulate cells from their environment mechanically and electrically. The impermeability of lipid membranes to ions allows the formation of the membrane potential .4. Special tasks.
Some lipids have adopted special roles in the body. Steroids, eicosanoids, and some metabolites of phospholipids have signaling functions. They serve as hormones, mediators, and second messengers . Other lipids form anchors to attach proteins to membranes . The lipids also produce cofactors for enzymatic reactions—e. g., vitamin K . The carotenoid retinal, a light-sensitive lipid, is of central importance in the process of vision. Several lipids are not formed independently in the human body. These substances, as essential fatty acids and fat-soluble vitamins, are indispensable components of nutrition Fatty acids and fats.Carboxylic acids :
The naturally occurring fatty acids are carboxylic acids with unbranched hydrocarbon chains of 4–24 carbon atoms. They are present in all organisms as components of fats and membrane lipids. In these compounds, they are esterified with alcohols (glycerol, sphingosine, or cholesterol). However, fatty acids are also found in small amounts in unesterified form. In this case, they are known as free fatty acids (FFAs). As free fatty acids have strongly amphipathic properties, they are usually present in protein-bound forms. The table lists the full series of aliphatic carboxylic acids that are found in plants and animals. In higher plants and animals, unbranched, longchain fatty acids with either 16 or 18 carbon atoms are the most common— e. g., palmitic and stearic acid. The number of carbon atoms in the longer, natural fatty acids is always even. This is because they are biosynthesized from C2 building blocks .Some fatty acids contain one or more isolated double bonds, and are therefore “unsaturated.” Common unsaturated fatty acids include oleic acid and linoleic acid. Of the two possible cis–trans isomers.), usually only the cis forms are found in natural lipids. Branched fatty acids only occur in bacteria. A shorthand notation with several numbers is used for precise characterization of the structure of fatty acids—e g., 18:2;9,12 for linoleic acid. The first figure stands for the number of C atoms, while the second gives the number of double bonds. The positions of the double bonds follow after the semicolon. As usual, numbering starts at the carbon with the highest oxidation state (i. e., the carboxyl group corresponds to C-1). Greek letters are also commonly used (α = C-2; β = C-3; ω = the last carbon, ω-3 = the third last carbon).
Essential fatty acids are fatty acids that have to be supplied in the diet. Without exception, these are all polyunsaturated fatty acids: the C20 fatty acid arachidonic acid (20:4;5,8,11,14) and the two C18 acids linoleic acid (18:2;9,12) and linolenic acid (18:3;9,12,15). The animal organism requires arachidonic acid to synthesize eicosanoids. As the organism is capable of elongating fatty acids by adding C2 units, but is not able to introduce double bonds into the end sections of fatty acids (after C-9), arachidonic acid has to be supplied with the diet. Linoleic and linolenic acid can be converted into arachidonic acid by elongation, and they can therefore replace arachidonic acid in the diet.
Structure of fats
Fats are esters of the trivalent alcohol glycerol with three fatty acids.When a single fatty acid is esterified with glycerol, the product is referred to as a monoacylglycerol (fatty acid residue = acyl residue). Formally, esterification with additional fatty acids leads to diacylglycerol and ultimately to triacylglycerol, the actual fat (formerly termed “triglyceride”). As triacylglycerols are uncharged, they are also referred to as neutral fats.
”The three acyl residues of a fat molecule may differ in terms of their chain length and the number of double bonds they contain. A chiral center can arise at the middle C atom of a triacylglycerol if the two external fatty acids are different. The monoacylglycerols and diacylglycerols shown here are also chiral compounds. Nutritional fats contain palmitic, stearic, oleic acid, and linoleic acid particularly often. Unsaturated fatty acids are usually found at the central C atom of glycerol. The length of the fatty acid residues and the number of their double bonds affect the melting point of the fats. The shorter the fatty acid residues and the more double bonds they contain, the lower their melting points.
Phospholipids and glycolipids
Fats are esters of glycerol with three fatty acids .Within the cell, they mainly occur as fat droplets. In the blood, they are transported in the hydrophobic interior of lipoproteins.Phospholipids
Are the main constituents of biological membranes. Their common feature is a phosphate residue that is esterified with the hydroxyl group at C-3 of glycerol. Due to this residue, phospholipids have at least one negative charge at a neutral pH. Phosphatidates (anions of the phosphatidic acids), the simplest phospholipids, are phosphate esters of diacylglycerol. They are important intermediates in the biosynthesis of fats and phospholipids .Phosphatidates can also be released from phospholipids by phospholipases. The other phospholipids can be derived from phosphatidates (residue = phosphatidyl). Their phosphate residues are esterified with the hydroxyl group of an amino alcohol(choline, ethanolamine, or serine) or with the cyclohexane derivative inositol. Phosphatidylcholine is shown here as an example of this type of compound. When two phosphatidyl residues are linked with one glycerol, the result is cardiolipina phospholipid that is characteristic of the inner mitochondrial membrane. Lysophospholipids arise from phospholipids by enzymatic cleavage of an acyl residue. The hemolytic effect of bee and snake venoms is due in part to this reaction. Phosphatidyl choline (lecithin) is the most abundant phospholipid in membranes. Phosphatidy lethanolamine (cephalin) has an ethanolamine residue instead of choline, and phosphatidyl serine has a serine residue. In
phosphatidylinositol, phosphatidate is esterified with the sugar like cyclic polyalcohol inositol. A doubly phosphorylated derivative of this phospholipid, phosphatidylinositol 4,5-bisphosphate, is a special component of membranes, which, by enzymatic cleavage, can give rise to two second messengers, diacylglycerol (DAG) and inositol 1,4,5 trisphosphate . Some phospholipids carry additional charges, in addition to the negative charge at the phosphate residue. In phosphatidyl choline and phosphatidyl ethanolamine, the N-atom of the amino alcohol is positively charged. As a whole, these two phosphatides therefore appear to be neutral. In contrast, phosphatidylserine—with one additional positive charge and one additional negative charge in the serine residue—and phosphatidylinositol (with no additional charge) have a negative net charge, due to the phosphate residue.
Sphingolipids
Sphingolipids which are found in large quantities in the membranes of nerve cells in the brain and in neural tissues, have a slightly different structure from the other membrane lipids. In sphingolipids, sphingosine, an amino alcohol with an unsaturated alkyl side chain, replaces glycerol and one of the acyl residues.When sphingosine forms an amide bond to a fatty acid, the compound is called ceramide .This is the precursor of the sphingolipids. Sphingomyelin the most important sphingolipid has an additional phosphate residue with a choline group attached to it on the sphingosine, in addition to the fatty acid.Glycolipids:
Glycolipids are present in all tissues on the outer surface of the plasma membrane. They consist of sphingosine, a fatty acid, and an oligosaccharide residue, which can sometimes be quite large. The phosphate residue typical of phospholipids is absent. Galactosylceramide and glucosylceramide (known as cerebroside) are simple representatives of this group. Cerebrosides in which the sugar is esterified with sulfuric acid are known as sulfatides. Gangliosides are the most complex glycolipids. They constitute a large family of membrane lipids with receptor functions. A characteristic component of many gangliosides is N-acetylneuraminic acid .Steroid structure
Steroid building blocks : Common to all of the steroids is a molecular core structure consisting of four saturated rings, known as gonane. At the end of the steroid core, many steroids also carry a side chain, as seen in cholestane, the basic component of the sterols (steroid alcohols).
Sterols
Sterols are steroid alcohols. They have a β-positioned hydroxyl group at C-3 and one or more double bonds in ring B and in the side chain. There are no further oxygen functions, as in the carbonyl and carboxyl groups. The most important sterol in animals is cholesterol. Plants and microorganisms have a wide variety of closely related sterols instead of cholesterol—e. g., ergosterol, β-sitosterol, and stigmasterol.Cholesterol is present in all animal tissues, and particularly in neural tissue. It is a major constituent of cellular membranes, in which it regulates fluidity .The storage and transport forms of cholesterol are its esters with fatty acids. In lipoproteins, cholesterol and its fatty acid esters are associated with other lipids .
Cholesterol is a constituent of the bile and is therefore found in many gallstones. Cholesterol-rich lipoproteins of the LDL type are particularly important in the development of arteriosclerosis, in which the arterial walls are altered in connection with an excess plasma cholesterol level. In terms of dietary physiology, it is important that plant foodstuffs are low in cholesterol. By contrast, animal foods can contain large amounts of cholesterol—particularly butter, egg yolk, meat, liver, and brain.
Bile acids :
Bile acids are synthesized from cholesterol in the liver. Their structures can therefore be derived from that of cholesterol.Characteristic for the bile acids is a side chain shortened by three C atoms in which the last carbon atom is oxidized to a carboxyl group. The double bond in ring B is reduced. Bile acids keep bile cholesterol in a soluble state as micelles and promote the digestion of lipids in the intestine .Cholic acid and chenodeoxycholic acid are primary bile acids that are formed by the liver. Their dehydroxylation at C-7 by microorganisms from the intestinal flora gives rise to the secondary bile acids lithocholic acid and deoxycholic acid.Steroid hormones
The conversion of cholesterol to steroid hormones is of minor importance quantitatively, but of major importance in terms of physiology. The steroid hormones are a group of lipophilic signal substances that regulate metabolism, growth, and reproduction.Humans have six steroid hormones:
progesterone, cortisol, aldosterone, testosterone, estradiol, and calcitriol. With the exception of calcitriol, these steroids have either no side chain or only a short side one consisting of two carbons.Steroid hormones with signaling functions also occur in plants.