
Jabir Ibn Hayyan Medical College
Biochemistry
for 1
st
class students
Lecture2
Protein Structure
March 30, 2017
DR. MONA ABDEL RIDHA Al-BARQAAWI
Lectures 2: References
• Marks’ Basic Medical Biochemistry Chapter 7
• Medical Biochemistry Chapters 2, 5
• Lippincott’s Illustrated Reviews: Biochemistry Chapters 2, 3
2

Lecture 2
Protein Structure
3
Peptides & Proteins
• In peptides and proteins, the primary amino group of one amino acid
is linked to the carboxyl group of the next amino acid, forming an
amide (peptide) bond. During the formation of a peptide bond, a
molecule of water is eliminated. (as shown in last lecture).
• The amino acid units on a peptide chain are referred to as amino acid
residues.
• A peptide chain may consists 2-50 amino acids.
4
Peptides & Proteins
• A peptide consisting of three amino acid residues is called a tri-peptide, e.g.
Glutathione (GSH), a tri-peptide with the sequence γ-glutamyl cysteinyl glycine.
This peptide has an important role in antioxidant defenses.
• Angiotensin, a peptide hormone that affects blood pressure, is another example
of a peptide with physiological importance, which has the following sequence of
amino acids.
Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu
• By convention, the amino terminus (N-terminus) is taken as the first residue,
and the sequence of amino acids is written from left to right.
5

Protein Structure
• Proteins contain between 50 and 2000 amino acid residues.
• The mean molecular mass of an amino acid residue is about 110 dalton units (Da).
Therefore the molecular mass of most proteins is between 5.5 and 220 kDa
• Proteins do not exist as linear polypeptides but rather fold to adopt a unique 3-
dimensional structure. The shape of the protein is important for defining the role
that protein plays.
• The linear sequence of the linked amino acids contains the information necessary
to generate a protein molecule with a unique three-dimensional shape.
6
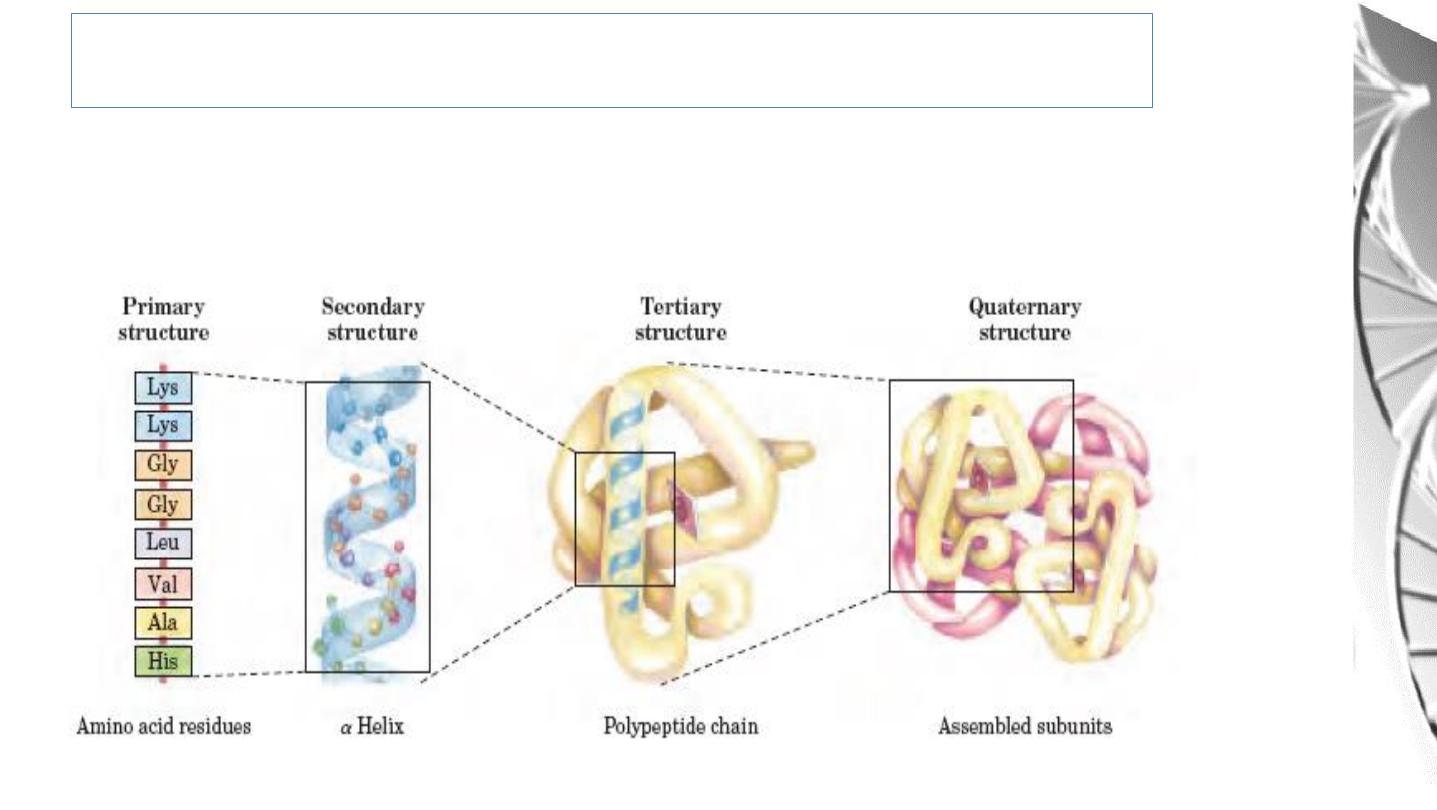
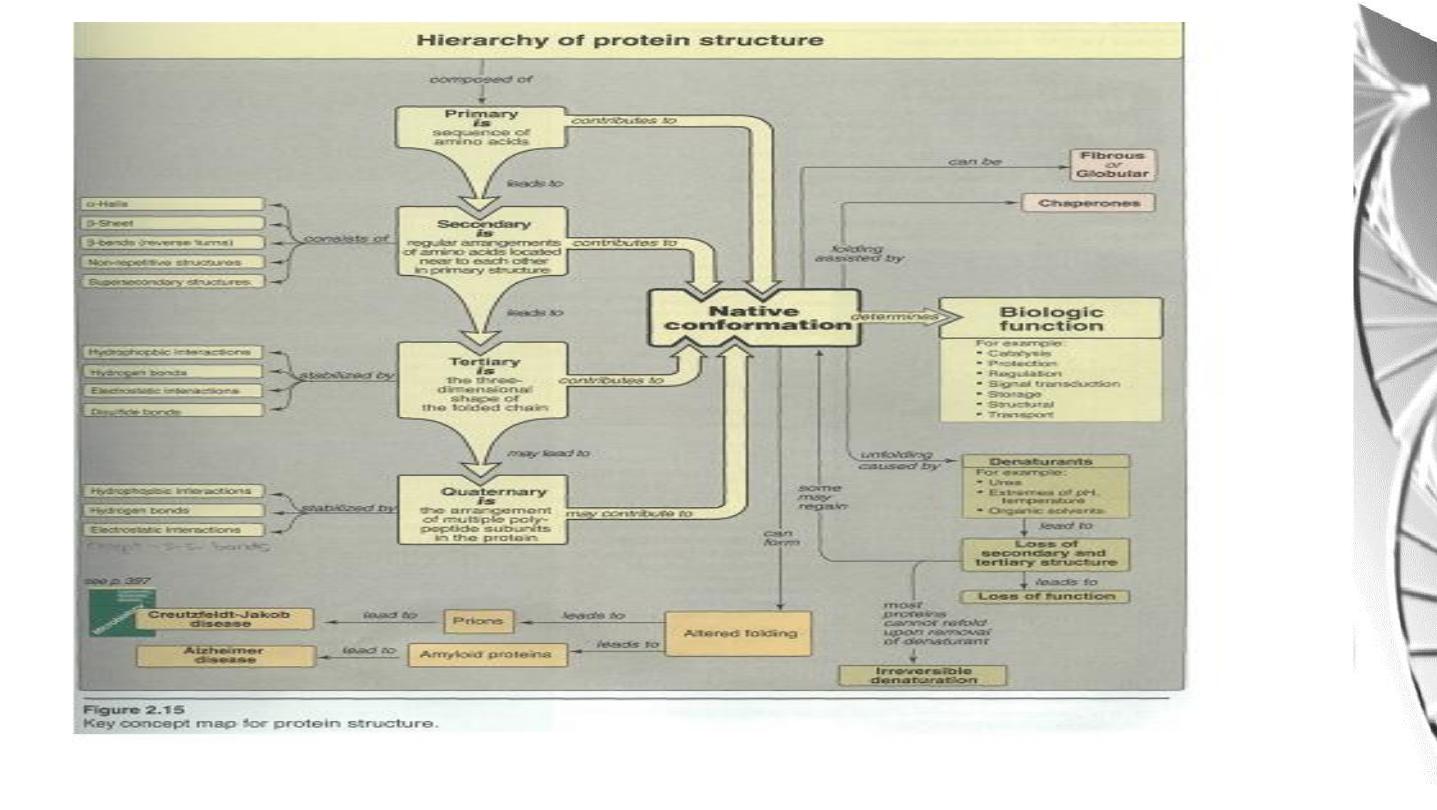
Levels of protein structure
The complexity of protein structure is best analyzed by considering the molecule in
terms of four organizational levels, namely , primary, secondary , tertiary , and
quaternary.
7
Primary Structure Of Proteins
• The sequence of amino acids in a protein is called the primary structure of the
protein.
• Understanding the primary structure of proteins is important because many
genetic diseases result in proteins with abnormal amino acid sequences, which
cause improper folding and loss or impairment of normal function.
• The primary structures of the normal and the mutated proteins are known, this
information may be used to diagnose or study the disease.
8
Primary Structure Of Proteins
• The bonds responsible for the stabilization of primary structure is only the
peptide bonds.
• Peptide bonds are not broken by heating or high concentrations of urea.
• Prolonged exposure to a strong acid or base at elevated temperatures is
required to hydrolyze these bonds.
9
Secondary Structure of Proteins
• The polypeptide backbone forms regular arrangements of amino acids that are
located near to each other in the linear sequence.
• These arrangements are termed the secondary structure of the polypeptide .
• The α-helix, β-sheet , and β-bend are examples of secondary structures.
• Collagen helix , another example o f secondary structure , will discussed in the
future.
• The Alpha Helix Is a Coiled Structure Stabilized by Intra- chain Hydrogen
Bonds.
10
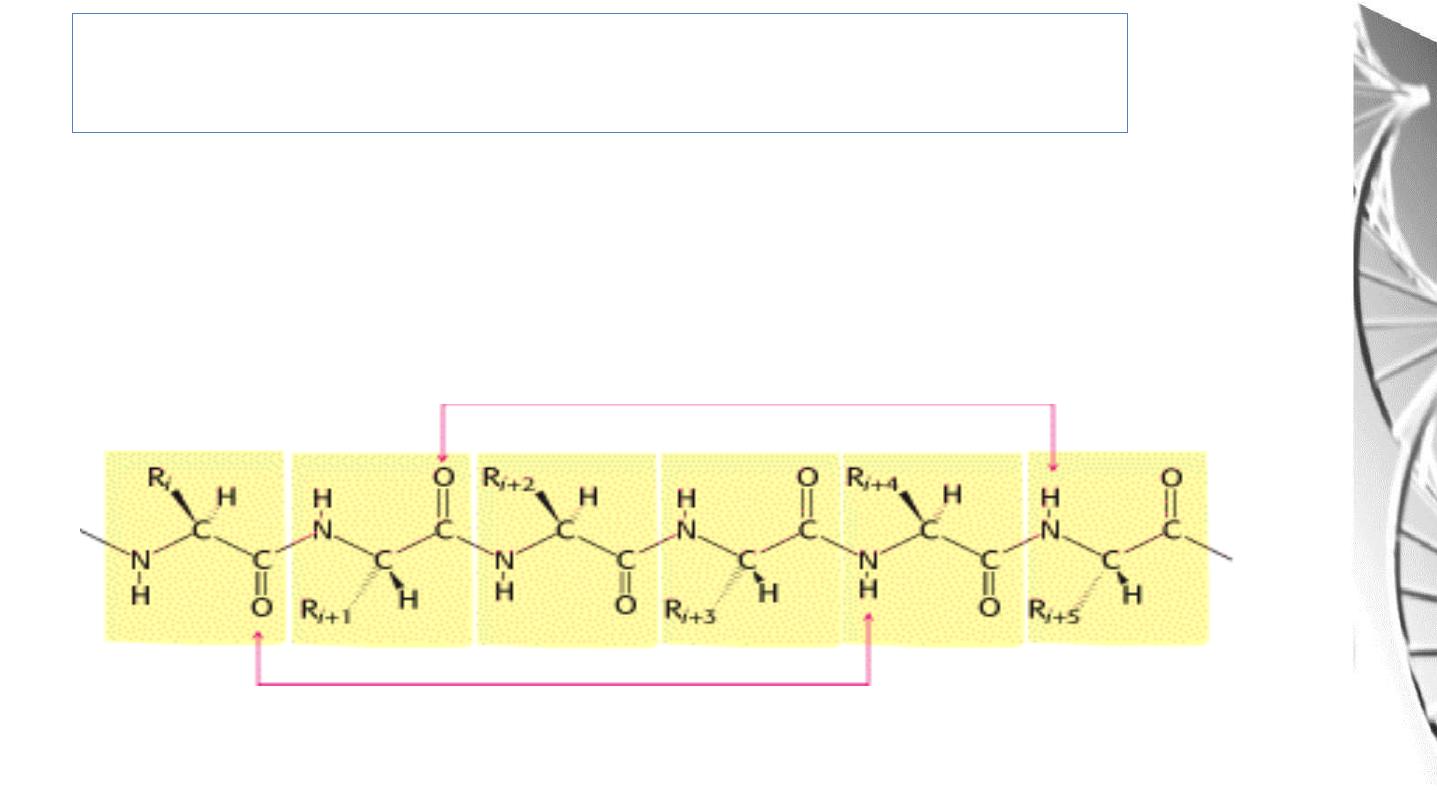
Secondary structure: α- Helix
• Essentially all α - helices found in proteins are right handed.
• In the α - helix, the CO group of residue n forms a hydrogen bond with the NH
group of residue n+ 4
11
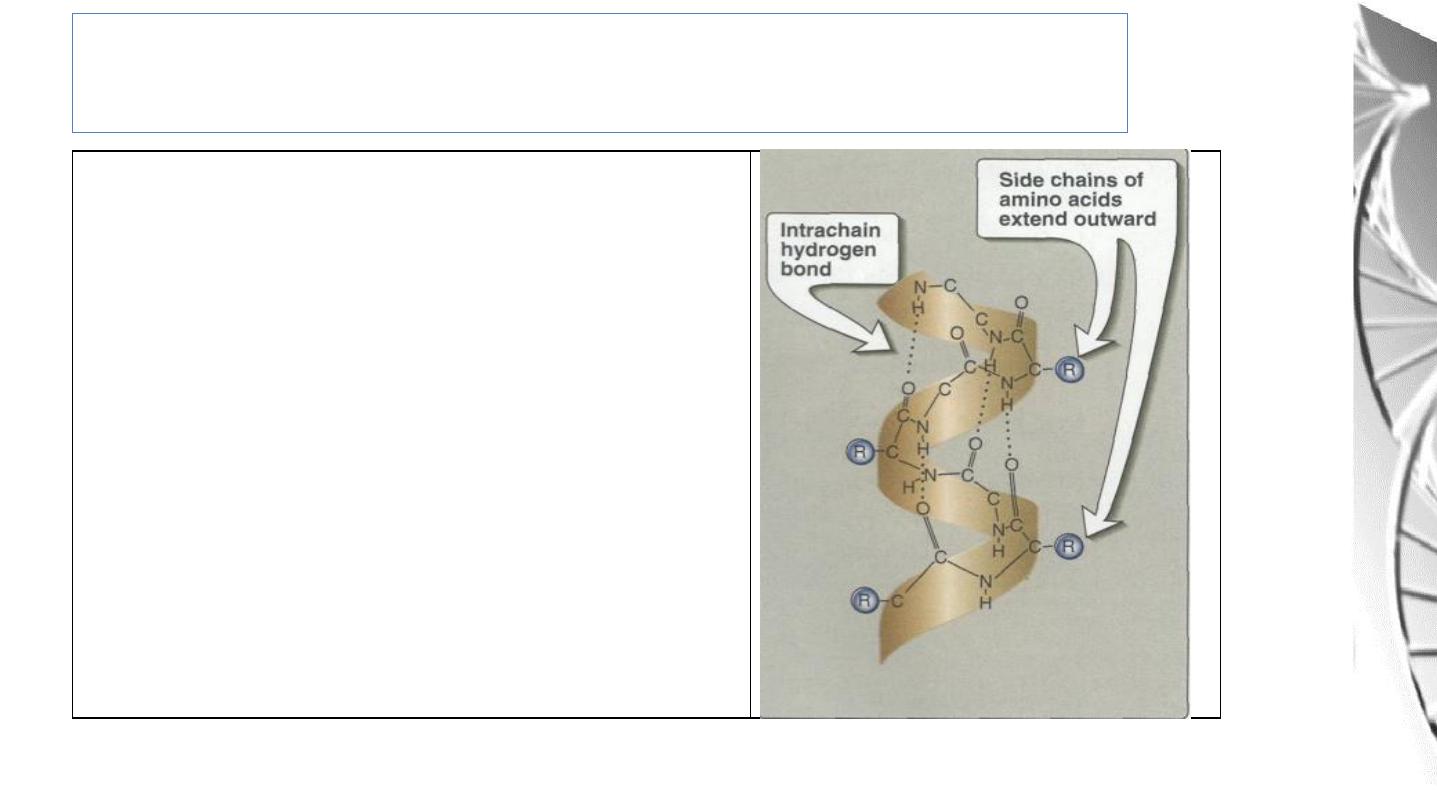
Each turn o f an α-helix contains 3.6
amino acids, and has a 0.54nm pitch.
Thus , amino acid residues spaced three
or four apart in the primary sequence are
spatially close together when folded in
the α-helix.
Amino acids per turn of α- Helix
12
• Proline.
• Large numbers of charged amino acid.
• Large numbers of amino acids with bulky side chains, such as tryptophan,
or amino acids, such as valine or isoleucine, that branch at the β-carbon.
Amin o acids that disrupt an α-helix
13
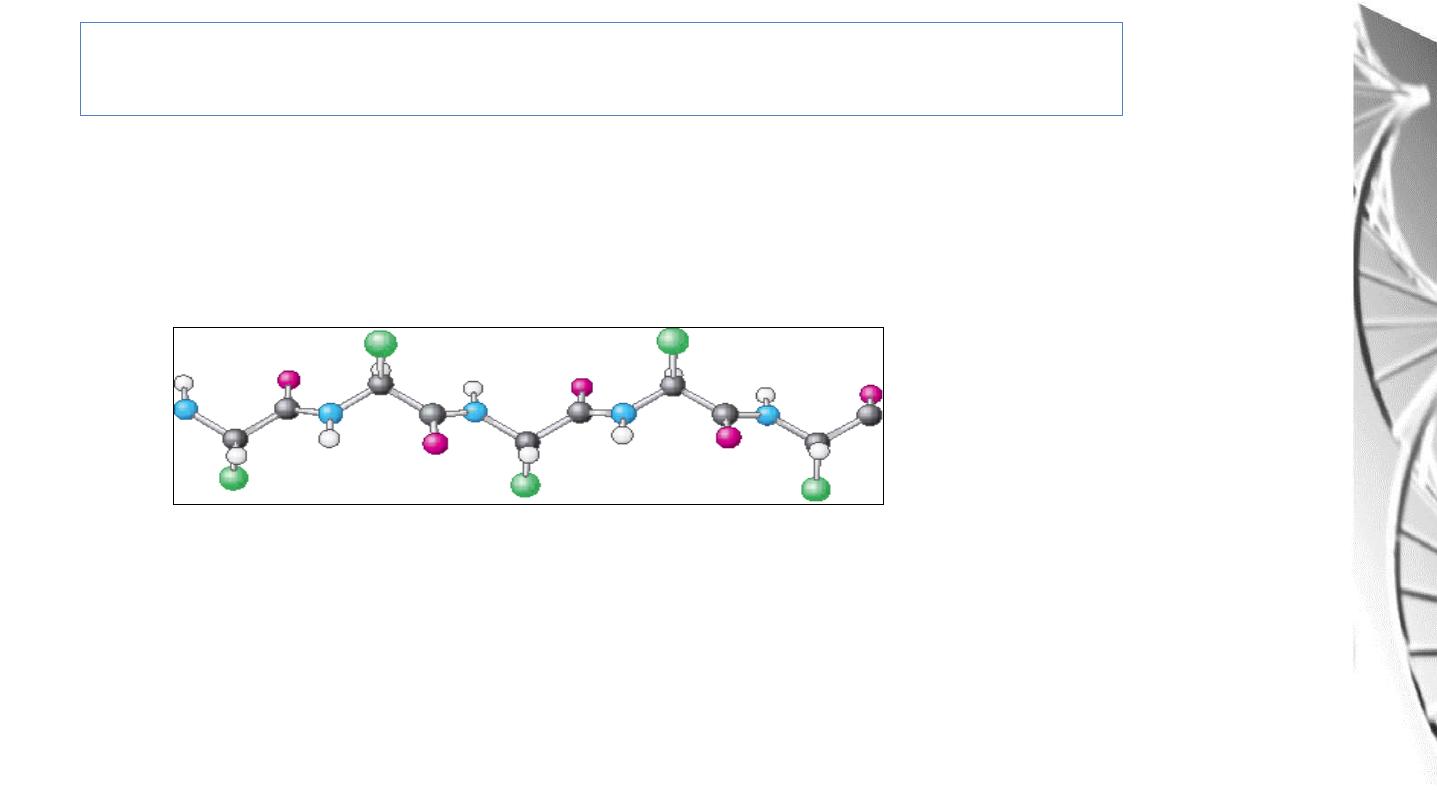
Secondary Structure: β- Sheet
β-Sheet is an Extended conformation in which
the side chains (green)
are alternately above and below the plane of the strand.
β Sheet:
1. Parallel
2. Antiparallel
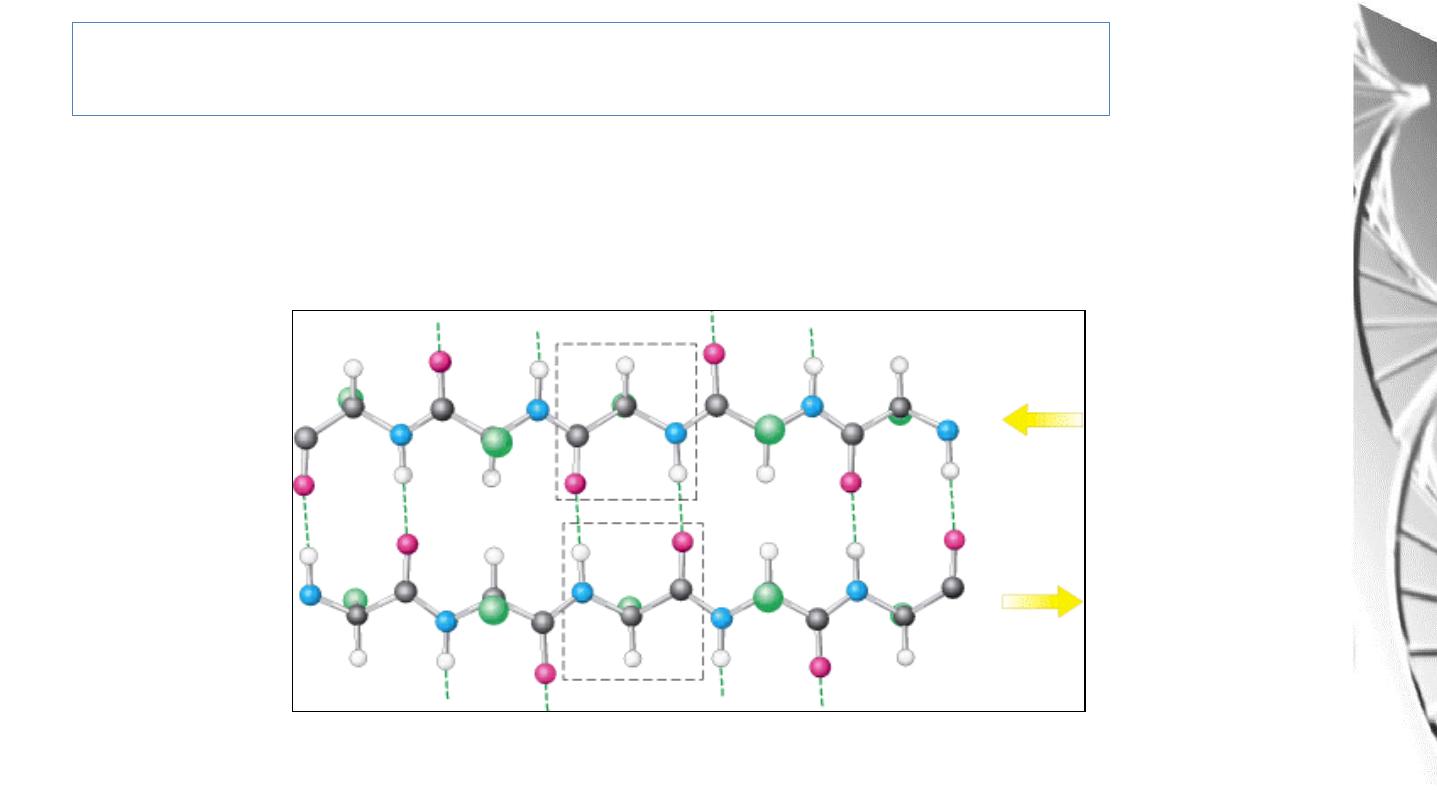
Secondary structure: antiparallel β Sheet
Adjacent β strands run in opposite directions. Hydrogen bonds between NH and
CO groups connect each amino acid to a single amino acid on an adjacent strand,
stabilizing the structure.
15
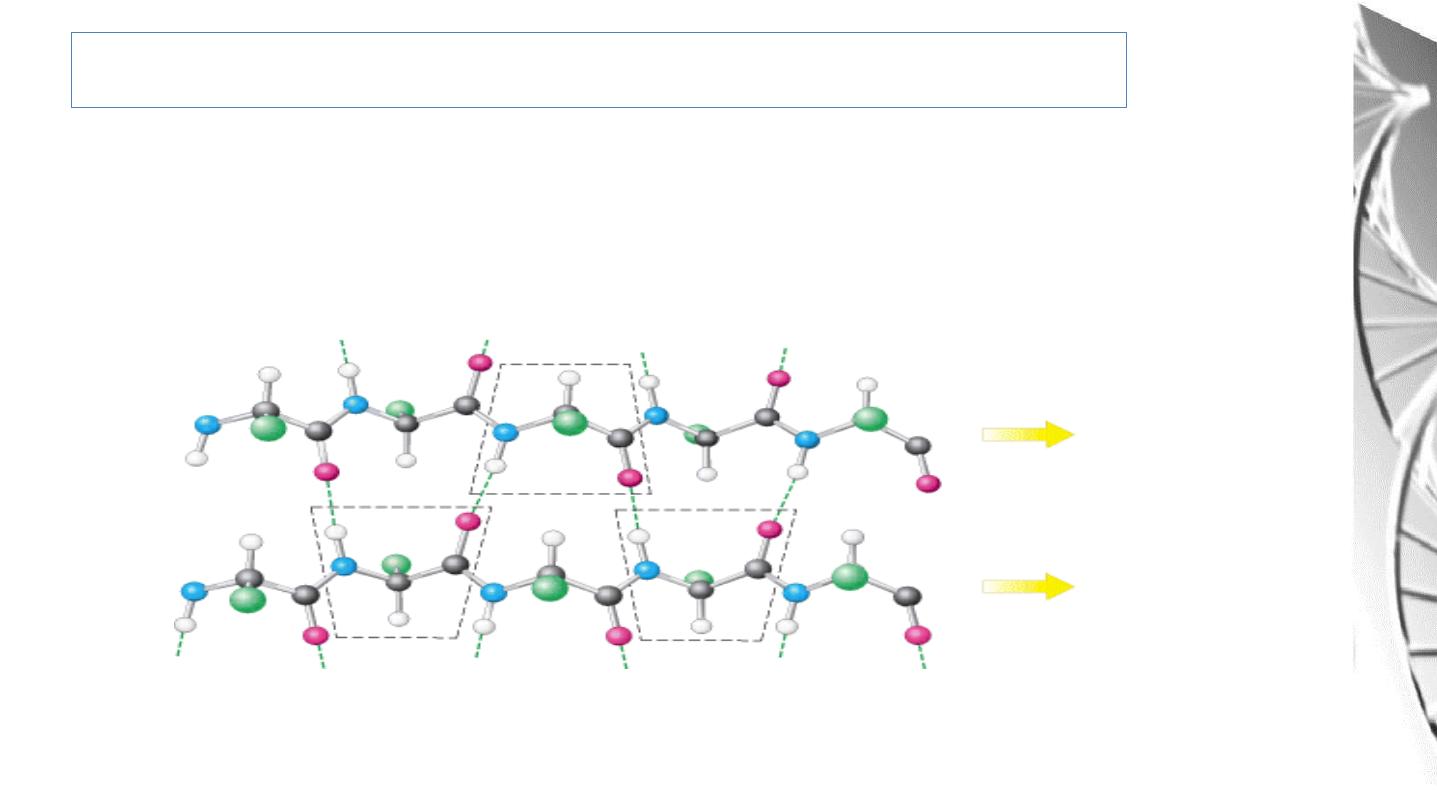
Secondary Structure: Parallel β-Sheet
Adjacent β-strands run in the same direction. Hydrogen bonds connect each
amino acid on one strand with two different amino acids on the adjacent strand.
16
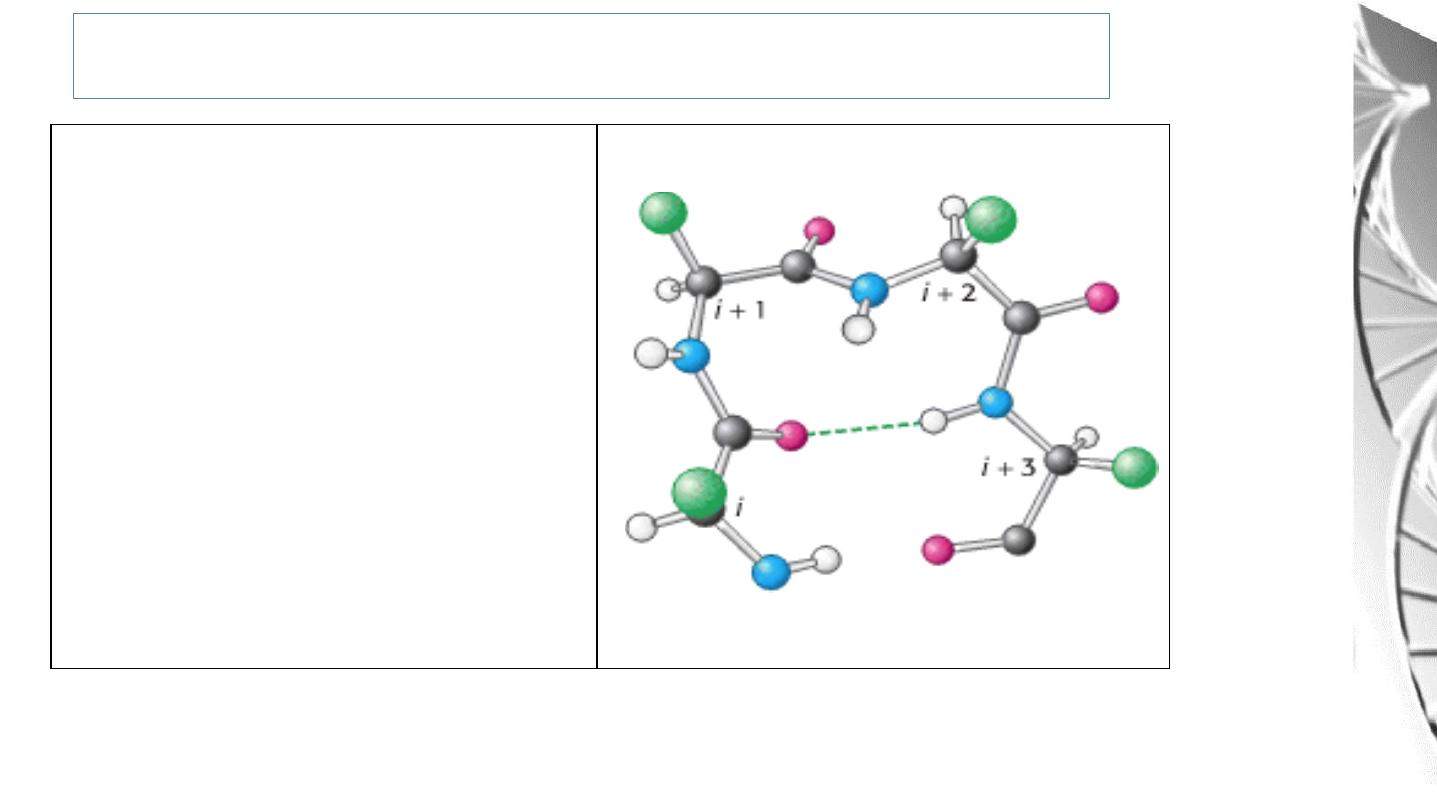
Secondary Structure: Bends or Turns
The CO group of residue i of
the
polypeptide
chain
is
hydrogen
bonded
to
the NH group of residue i + 3
to stabilize the turn
17
Tertiary Structure
• The overall 3-dimensional structure of a protein is referred to as the tertiary
structure. This involves folding up of the secondary structures so that amino
acids far apart in the primary sequence may interact.
• Larger proteins (~200 amino acids or greater) tend to have distinct domains.
These are regions of the polypeptide that have distinct structures and often serve
particular roles (e.g. ligand binding, interaction with other proteins etc.)
18
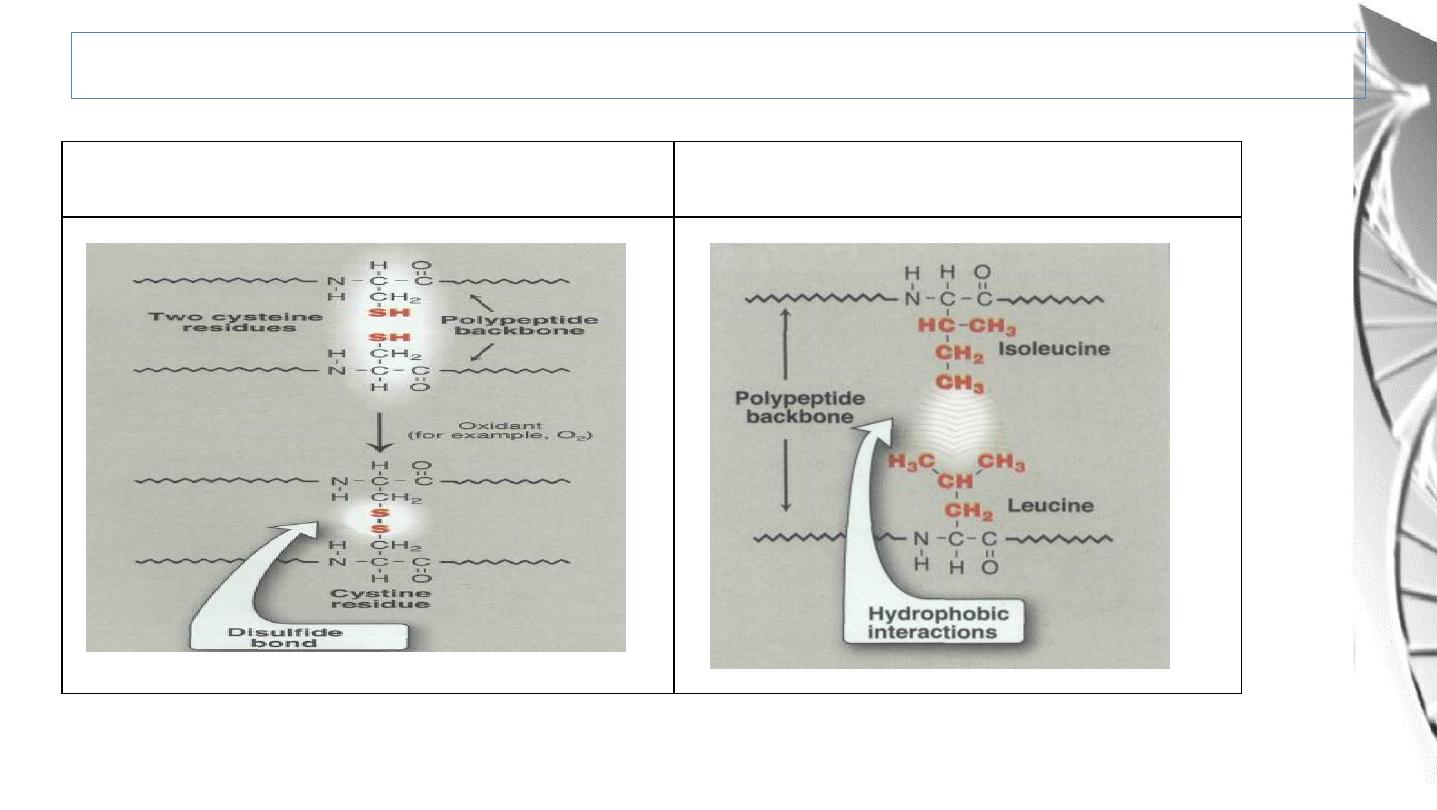
Hydrophobic
interactions
Covalent (disulphide) bonds
Tertiary Structure: Bonds Involved
19
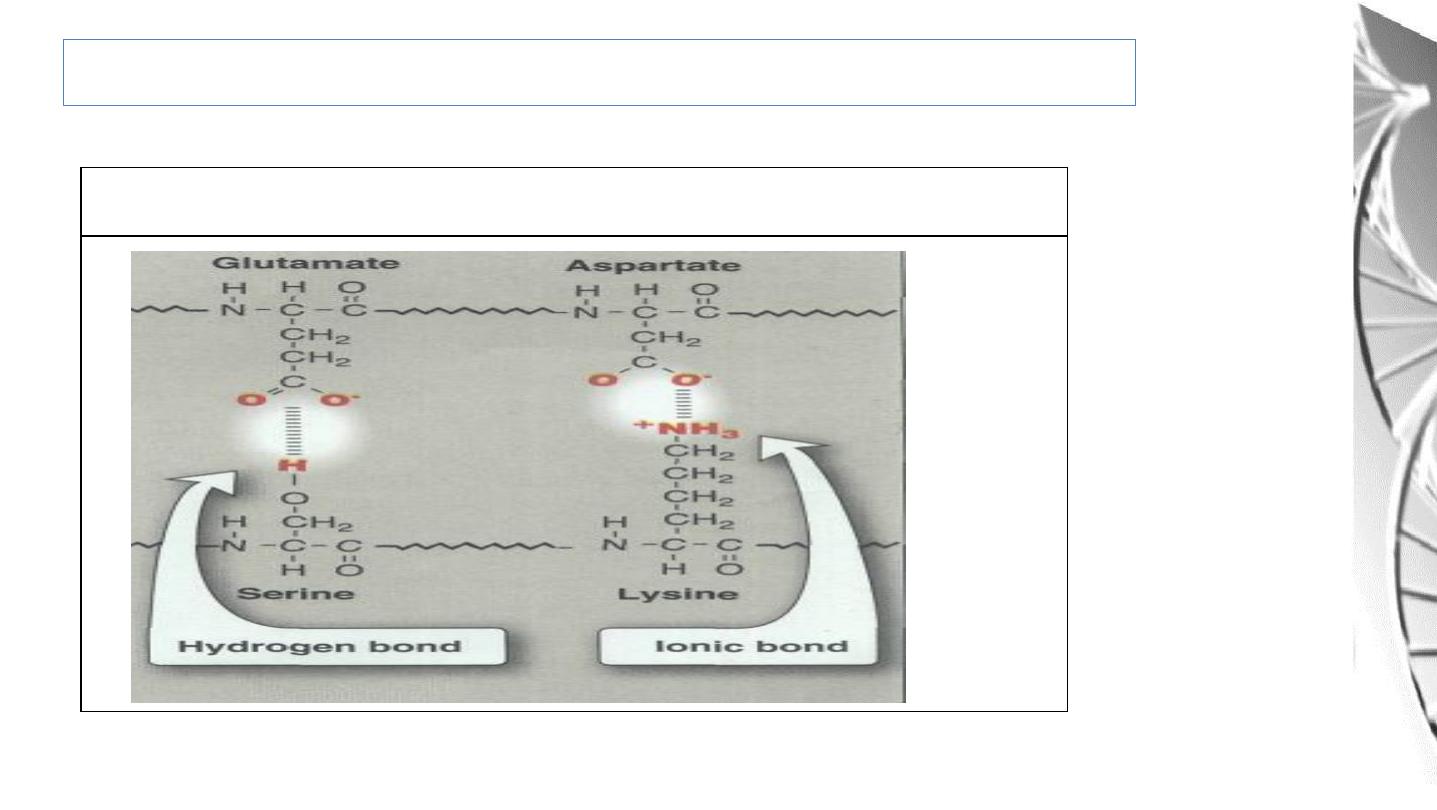
Ionic interactions, Hydrogen bonds &Van der Waals interaction
Tertiary Structure: Bonds Involved
20
Protein Folding
• Interactions between the side chains o f amino acids determine how a long
polypeptide chain folds into the complex three-dimensional shape o f the
functional protein.
• Protein folding, occurs within the cell in seconds to minutes.
• The information needed for correct protein folding is contained in the primary
structure of the polypeptide.
21
Role o f chaperones in protein folding
• Why most proteins when denatured (see below) do not take up again their native
conformations under favorable environmental conditions?
• One answer to this problem is that a protein begins to fold in stages during it s
synthesis, rather than waiting for synthesis of the entire chain to be totally
completed.
• In addition, a specialized group of proteins, named "chaperones," are required for
the proper folding of many species of proteins.
22
Quaternary Structure
• Many proteins consist of more than 1 polypeptide chain. The polypeptide
chains may be identical (homomeric proteins) or different (heteromeric
proteins).
• The arrangement of these subunits in such proteins is referred to as the
quaternary structure.
• The same types of bonds, that involved in tertiary structure, are involved in
Quaternary structure.
• Proteins can be categorised into 2 major groups depending of their higher
order structure: globular or fibrous.
• Most enzymes and regulatory proteins inside a cell tend to be globular
proteins whereas fibrous proteins tend to provide structure, support and
protection.
23
Protein Denaturation
• The loss of protein structure sufficient to cause the loss of function is known as
denaturation.
• Denaturation is brought about by breaking the bonds that hold that maintain the
protein’s tertiary and secondary structure.
• Denaturing agents include heat, organic solvents, mechanical mixing, strong
acids or bases , detergents, and ions of heavy metals such as lead and mercury.
24
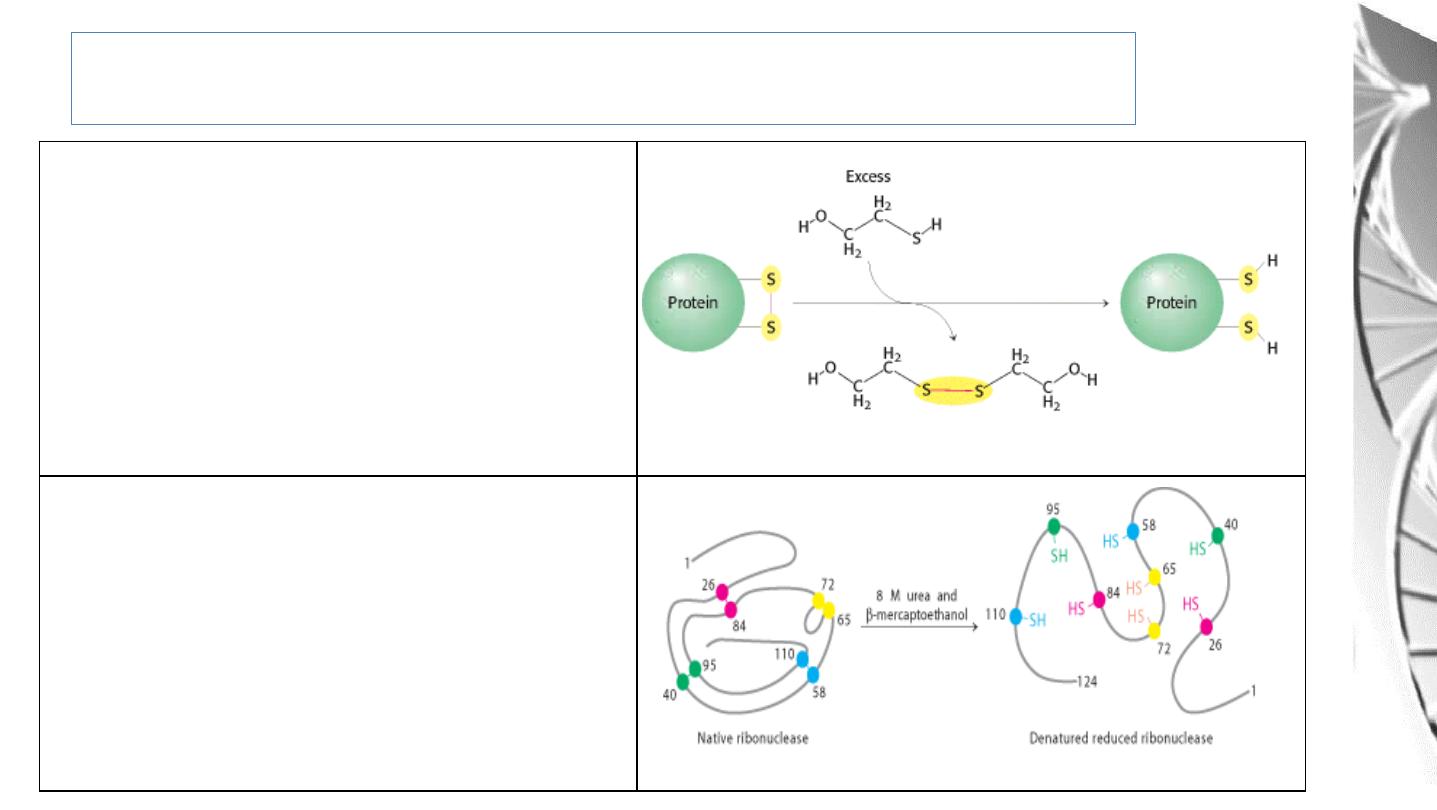
Protein Denaturation
Role
of
β-Mercapto-ethanol
in
Reducing Disulfide Bonds.
Note that, as the disulfides are reduced,
the β-mercapto-ethanol is oxidized and
forms dimers
Reduction
and
Denaturation
of
Ribonuclease
25
26
