
Jabir Ibn Hayyan Medical College
Biochemistry
for 1
st
class students
Lecture 4
Oxygen Transport Proteins
April 27, 2017
DR. MONA ABDEL RIDHA Al-BARQAAWI

Lecture 4
Oxygen transport proteins

It is Important to know that:
• Ligand (L): binding molecule to a protein
• Binding site: site of a protein that a ligand binds
• Mostly they are specific
• Proteins are flexible: changing in conformation
• induced fit: ligand result in a conformational change on protein’s
binding site so it fits the binding site more tightly.
• Interactions may be regulated.
• Enzyme has a catalytic or active site (binding site)-substrate
(ligand)
Reversible binding of Ligand to Protein & Oxygen Transport
• O
2
Essential for cellular respiration
• O
2
is poorly soluble in plasma
• Impossible to transport by simple diffusion
• Only metals: iron, copper
• Free iron dangerous Reactive oxygen(OH)
Damage of DNA & other macromolecules
• So, O
2
is transported using two proteins (hemoglobin and myoglobin)
That contains essential prosthetic group
(heme: contains Fe atom)
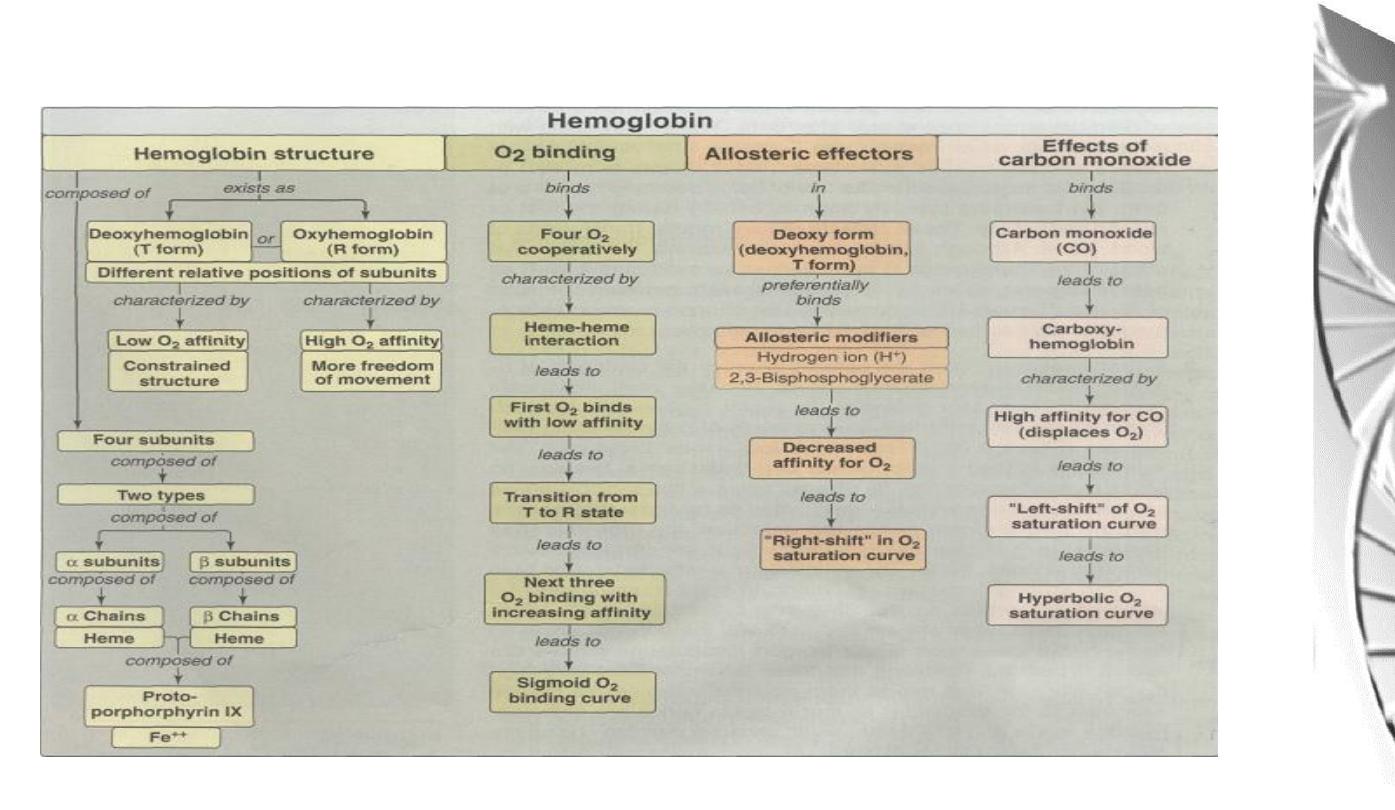
Myoglobin and Hemoglobin
Hemoglobin
Myoglobin
• Found in RBCs
• Function to transport O
2
from the lungs to
the capillaries of the tissues.
• Four polypeptide chains (4 subunits)
• In HbA 2α (146 residue each)
and 2β subunits (141 residue each).
• 3D structure of α & β is very similar
• In heart and skeletal muscle
• Function both as a reservoir for
O
2
and to transport it with in the
muscle cells.
• Single polypeptide chain (153
residue).
• 3D structure is similar to both α & β
The polypeptide chain of myoglobin is structurally similar to the individual
subunit of polypeptide of hemoglobin molecule.
This homology makes myoglobin a useful model for interpreting some of
the more complex properties of hemoglobin.
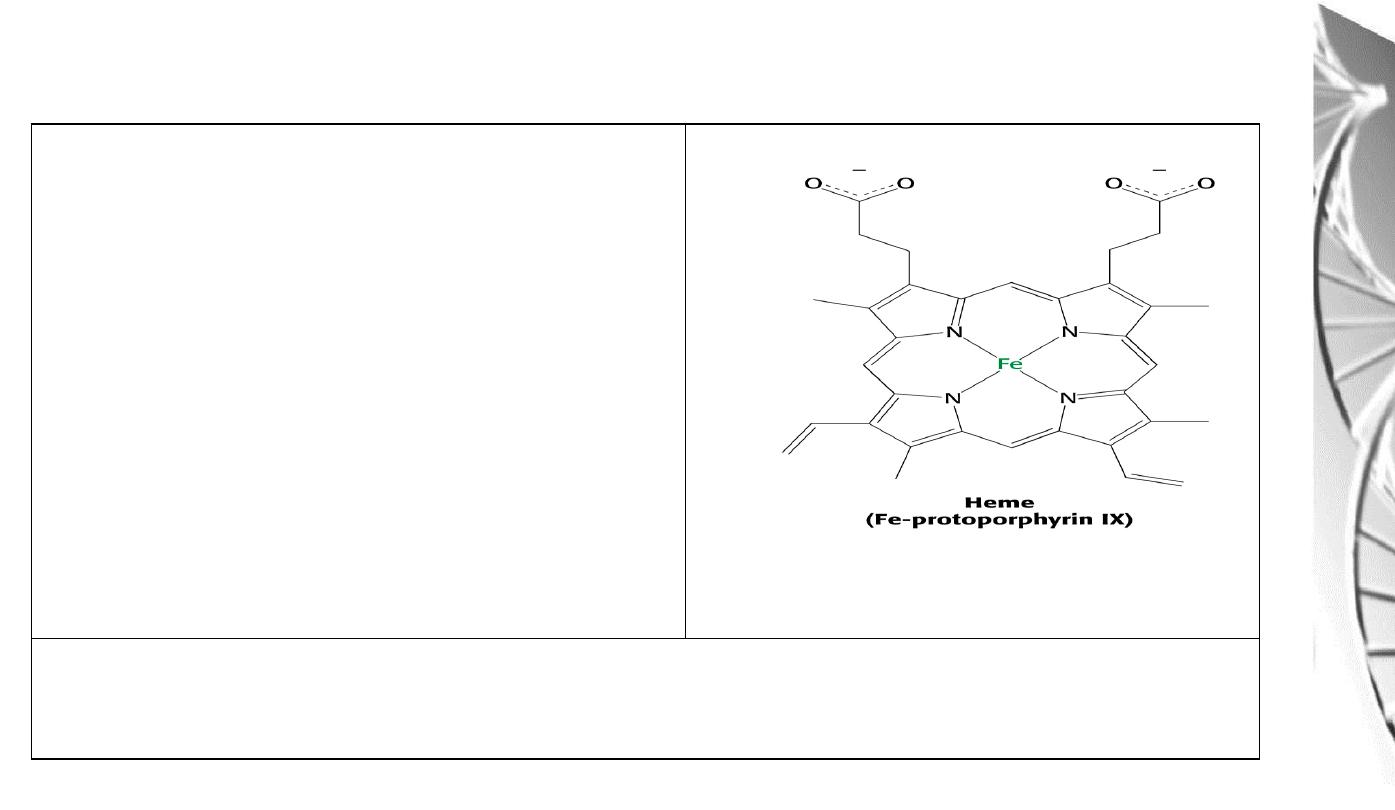
Heme
Consists of:
1) An organic part, protoporphyrin, made
up of 4 pyrrole rings, linked by methylene
bridges (tetrapyrrole ring) and with 4
methyl, 2 vinyl and 2 propionyl side
chains.
2) An atom of Fe, which binds to the 4 N
atoms of the protoporphyrin ring.
The Fe can form 2 additional bonds, one on either side of the plane. The
ferrous
(+2) state binds the oxygen.
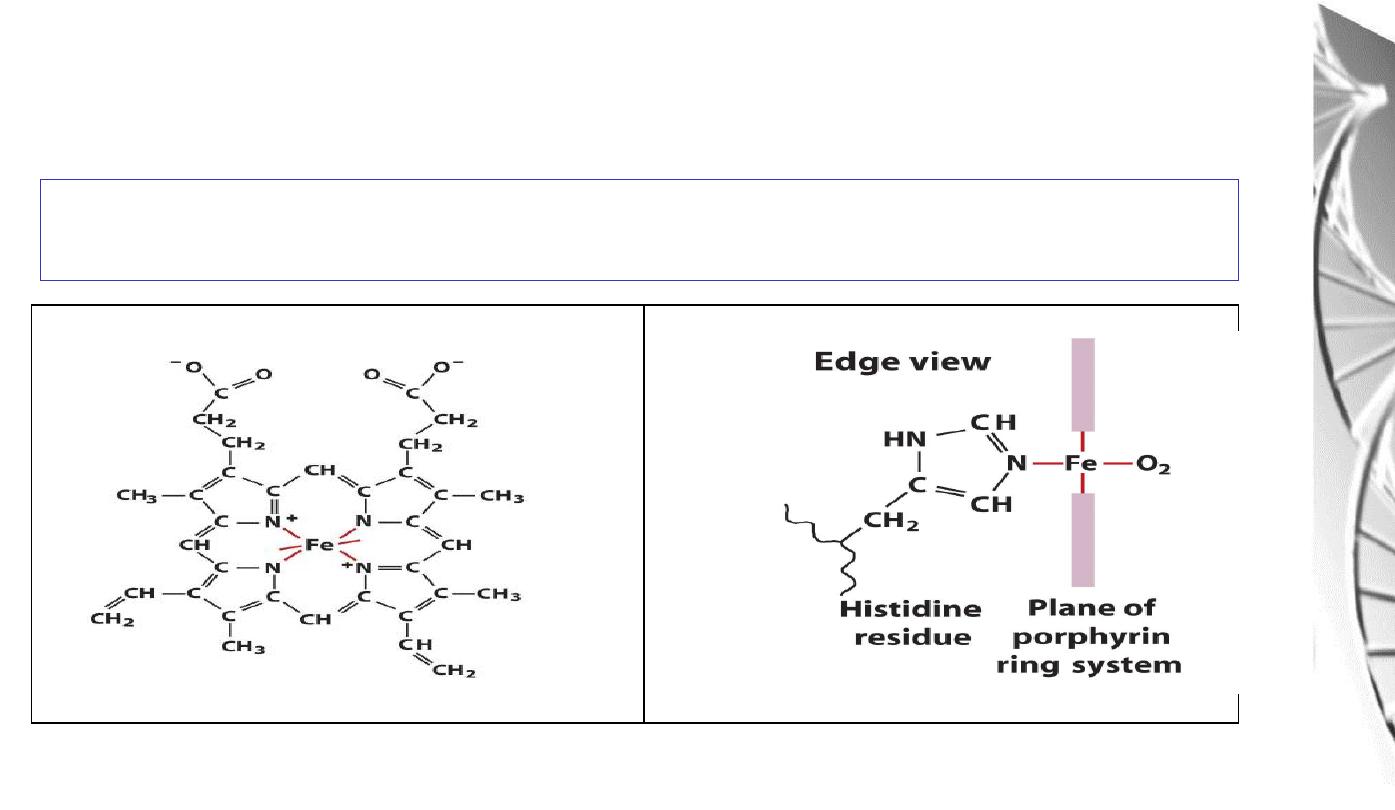
Fe binds to 4 N atoms of the protoporphyrin ring & two additional bonds one to
N of proximal histidine of F helix, another is to O
2
Fe binding to
Histidine
and Oxygen

Myoglobin Structure
• Compact molecule, approx. 80% of polypeptide chain folded into eight
stretches of α-helix, labeled A, B,C, D, E, F, G, and H.
• Internal residues are non-polar, except for two His residues which are involved
in O
2
-binding
• The heme is largely hidden, but the propionate side-chains (-ve charge) are on
the surface
• Histidine F8 (proximal His) is directly linked to Fe
• O
2
is bound directly only to Fe in heme, on the opposite side to His F8
• Histidine E7 (distal His) does not directly interact with the heme group, but
helps stabilize the binding of Oxygen to the ferrous ion.
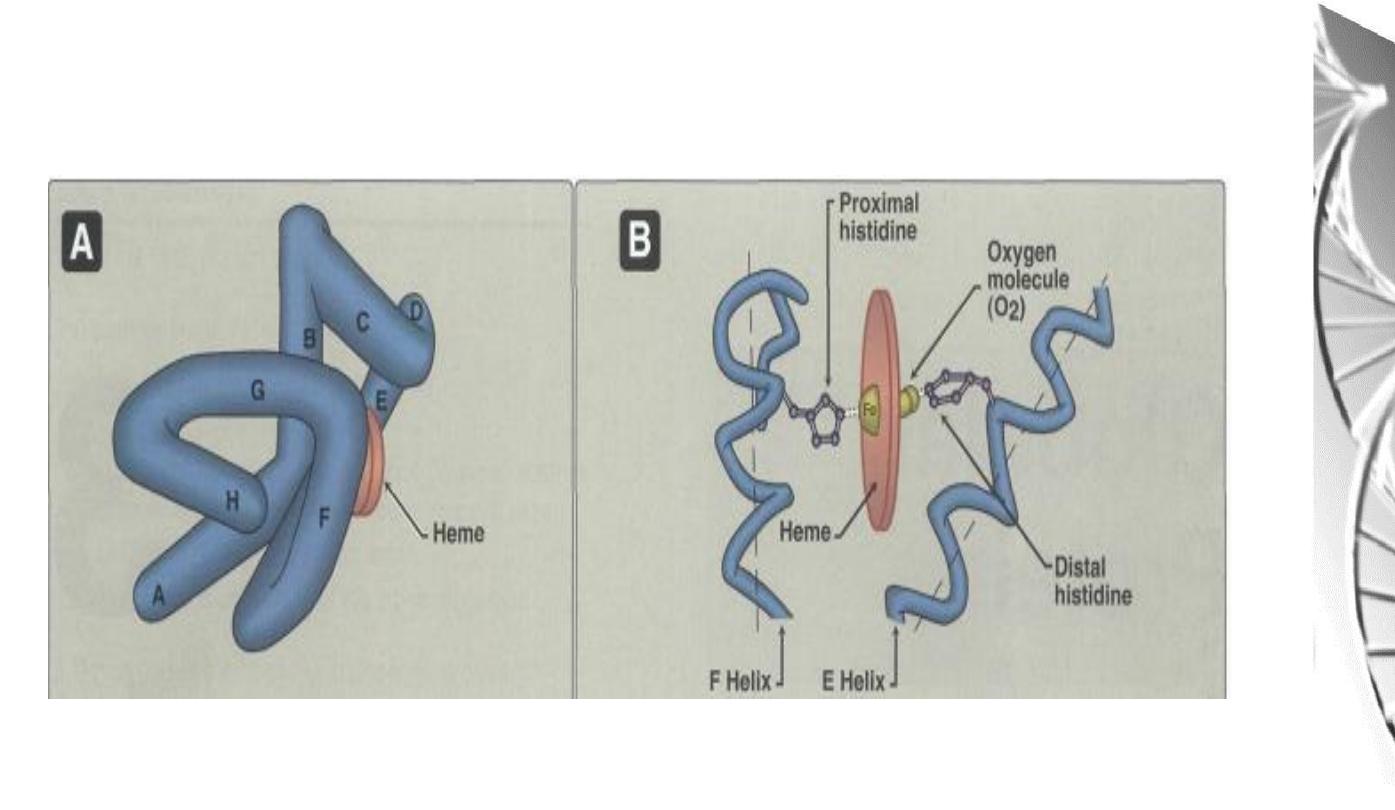
Oxygen binding site of Myoglobin
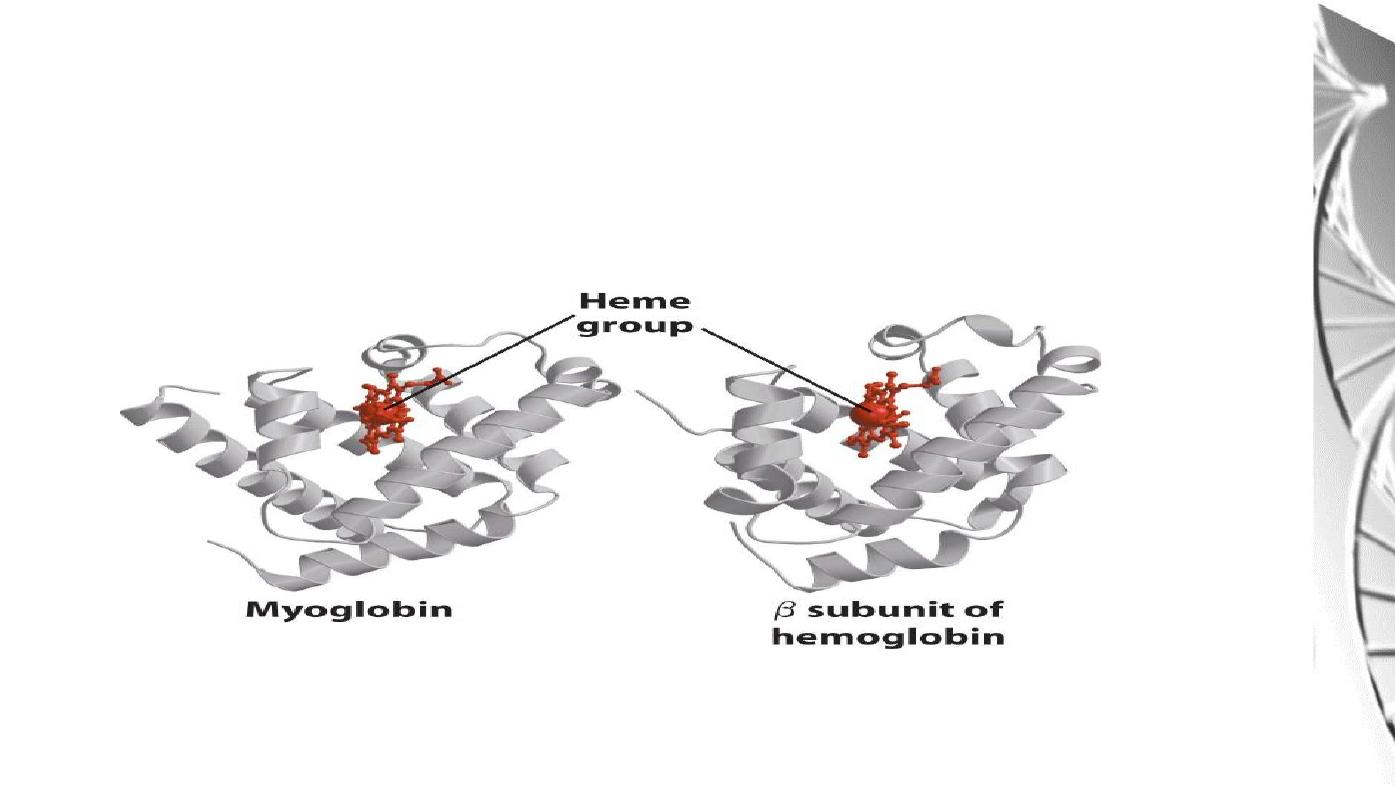
Myoglobin molecule is similar to hemoglobin
subunits

Hemoglobin
Hemoglobin (Hb) subunits and myoglobin are similar in their helical structure
and in heme binding pocket. However, the tetrameric hemoglobin molecule is
structurally and functionally more complex than myoglobin.
Hb can carry 4 O
2
from lungs to the cells of the body.
It also can transport H
+
and CO
2
from the tissues to the lung.
The oxygen-binding properties of Hb are regulated by interaction with
allosteric effectors.
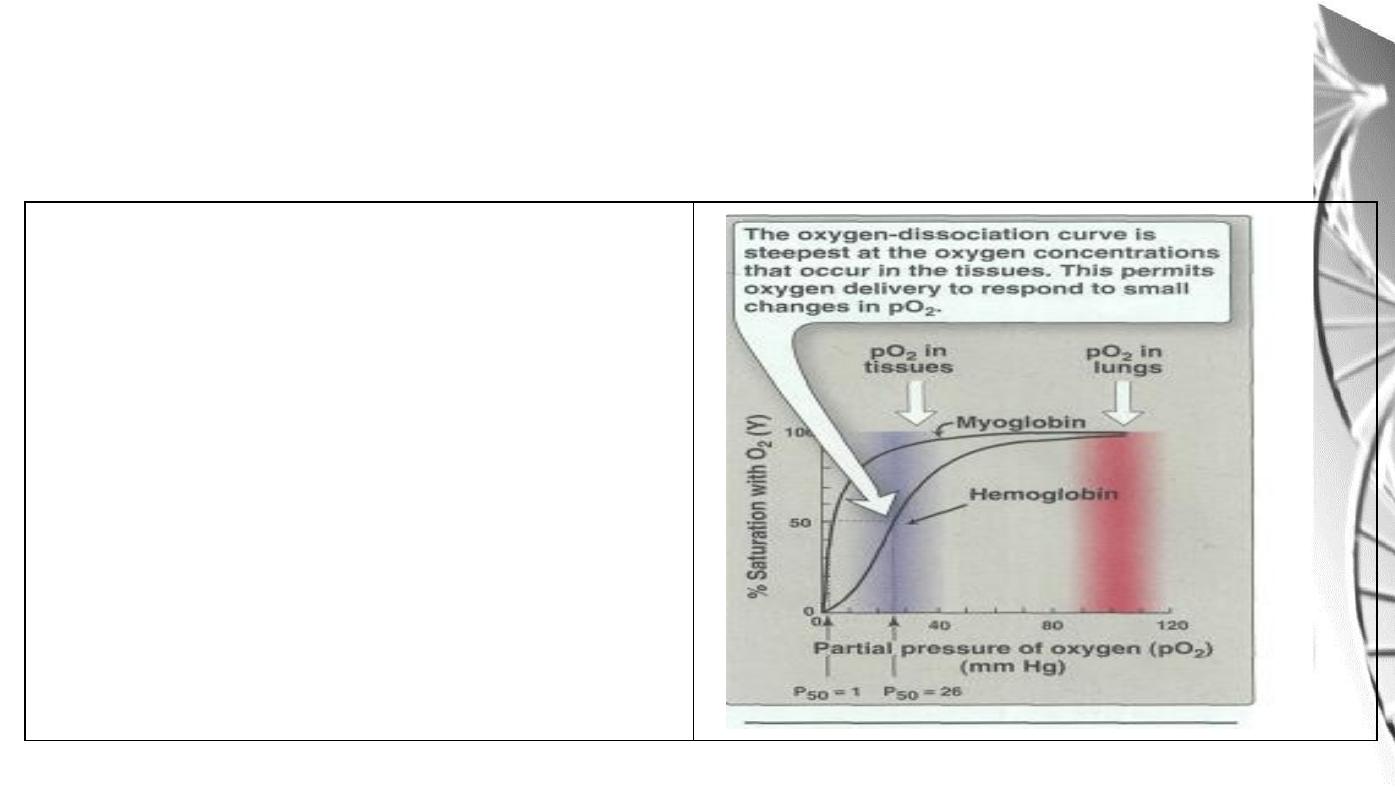
Oxygen Dissociation Curves for
myoglobin and hemoglobin
The degree of saturation(Y) of O
2
binding
sites on all myoglobin or hemoglobin
molecules can vary between zero (all sites
are empty) and 100% (all sites are full)
A plot of Y measured at different partial
pressures
of
O
2
is
called
Oxygen
Dissociation Curve.

Oxygen Dissociation Curves for
myoglobin and hemoglobin
important differences
• Myoglobin has a higher oxygen affinity than does hemoglobin.
• The partial pressure of oxygen needed to achieve half-saturation of the
binding sites (P
50
) is approximately 1 mm Hg for myoglobin and 26 mm Hg
for hemoglobin.
• The higher the oxygen affinity (that is the more tightly oxygen binds), the
lower the P
50
• a hyperbolic relationship between Y and pO
2
for myoglobin.
• A sigmoidal relationship between Y and pO
2
for Hb (cooperative).
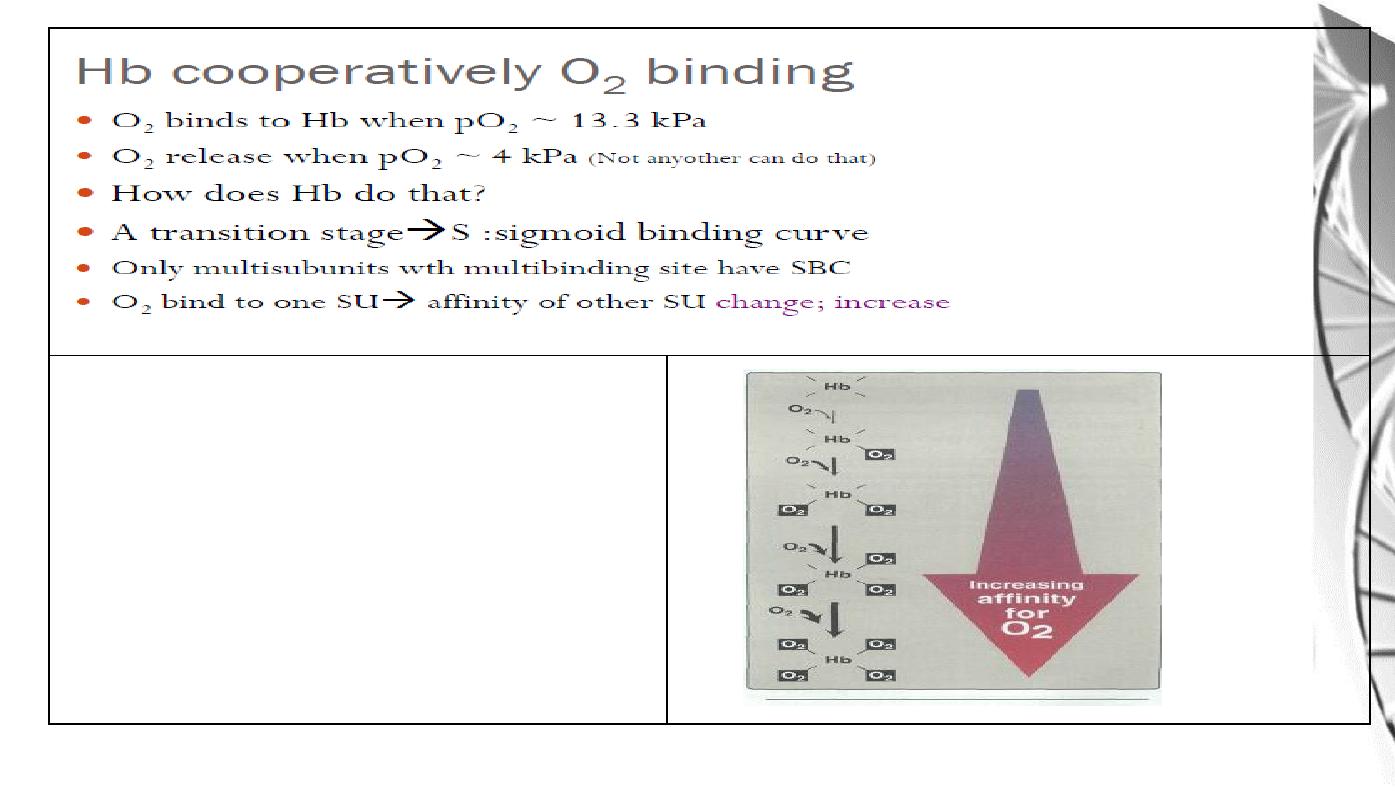
The affinity of hemoglobin for the last
oxygen bound is approximately 300
times greater than its affinity for the first
oxygen bound.
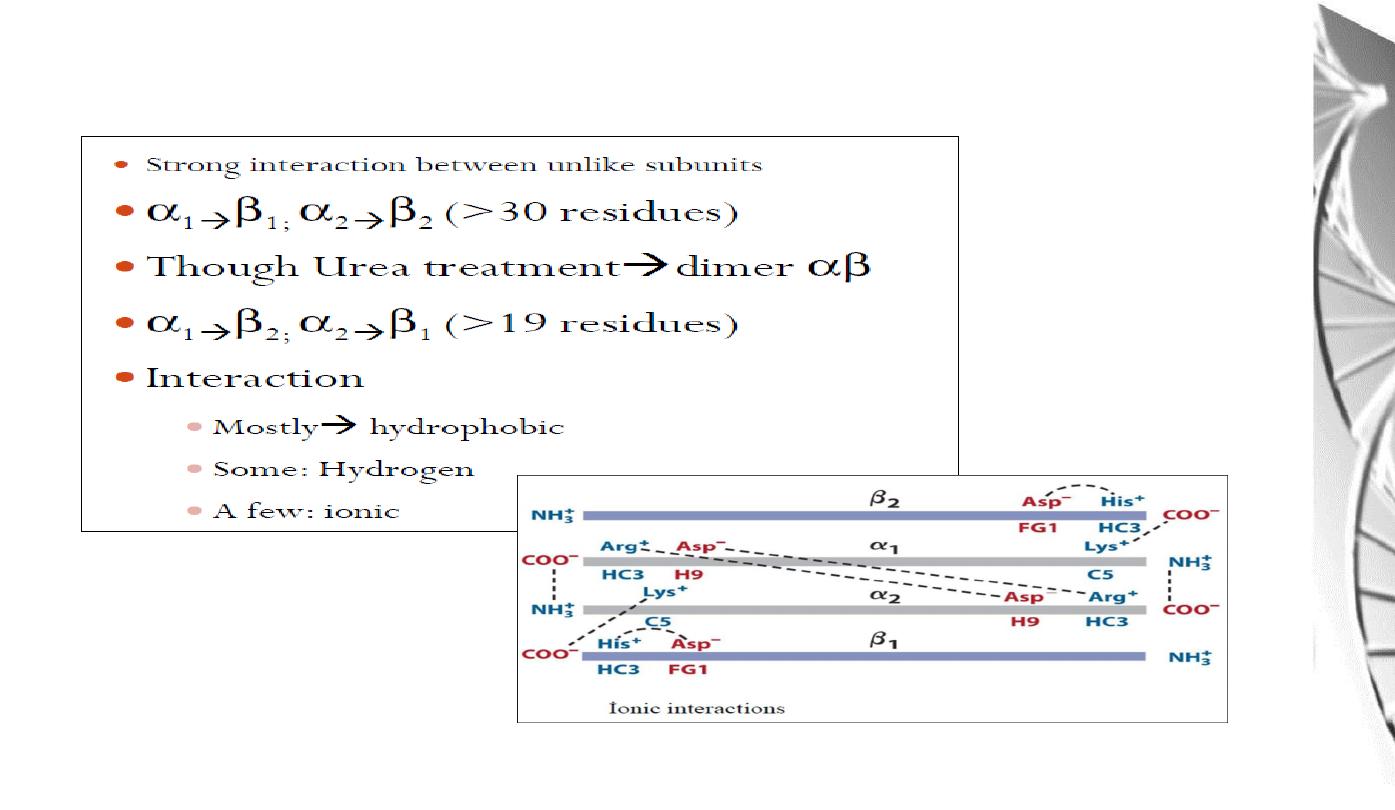
Interaction between α and β sub-uints of hemoglobin
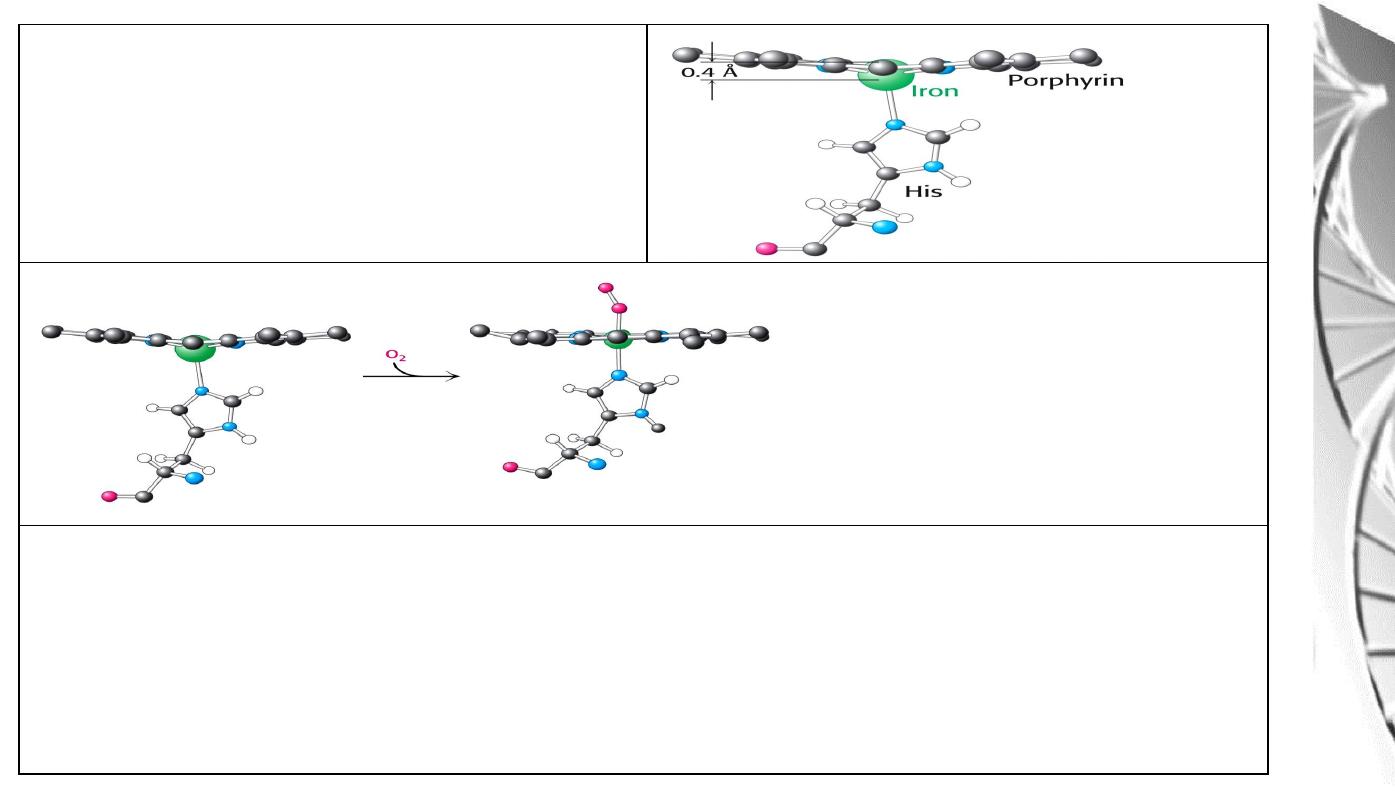
Position of Fe in myoglobin.
The Fe lies slightly out of the plane of
the porphyrin ring, towards His F8.
The movement of the iron atom during oxygenation brings the iron-associated His residue
towards the porphyrin ring. The associated movement of the His-containing a helix alters
the interface between the alpha beta pairs and initiates other structural changes involving
other subunits.
O
2
-binding to Fe pulls the iron
into the plane of the ring with
associated movement of His F8.
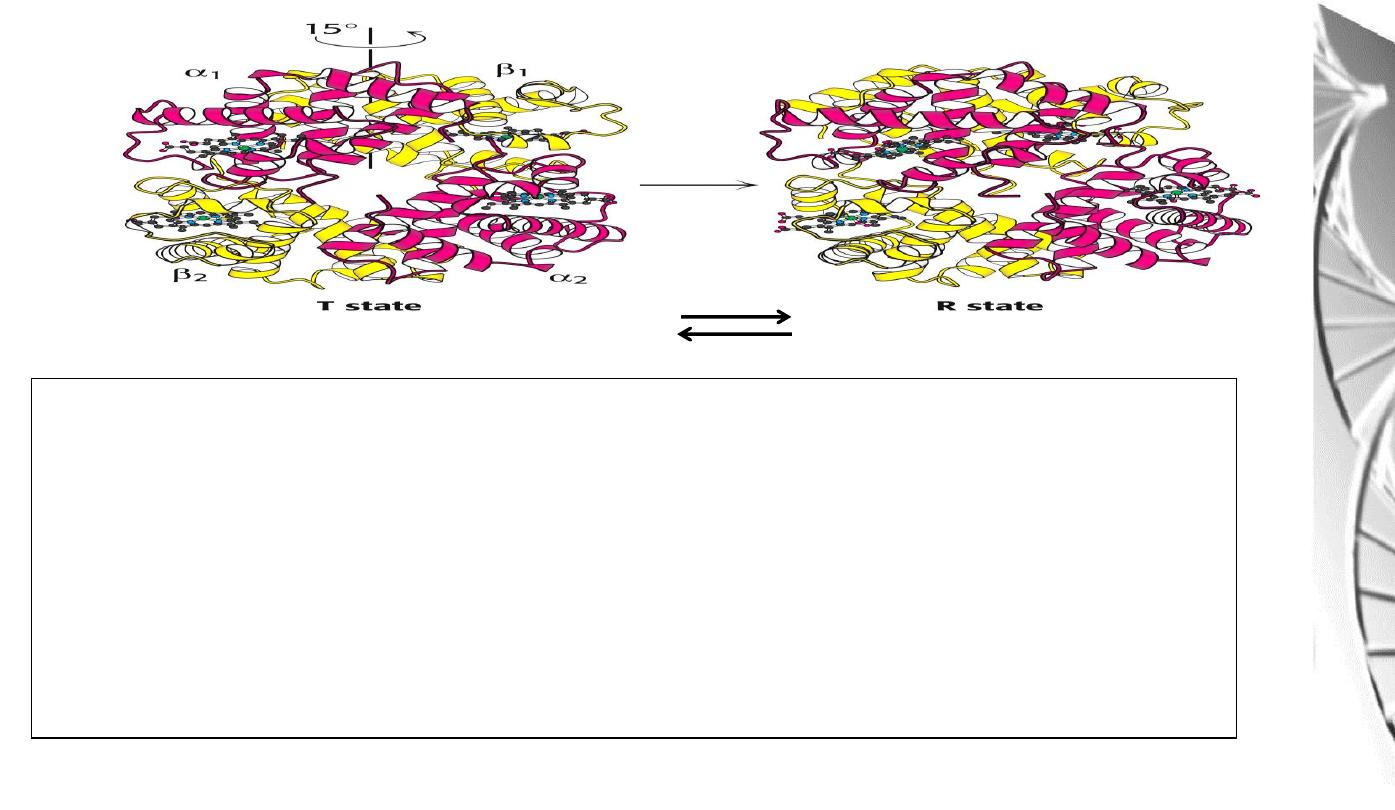
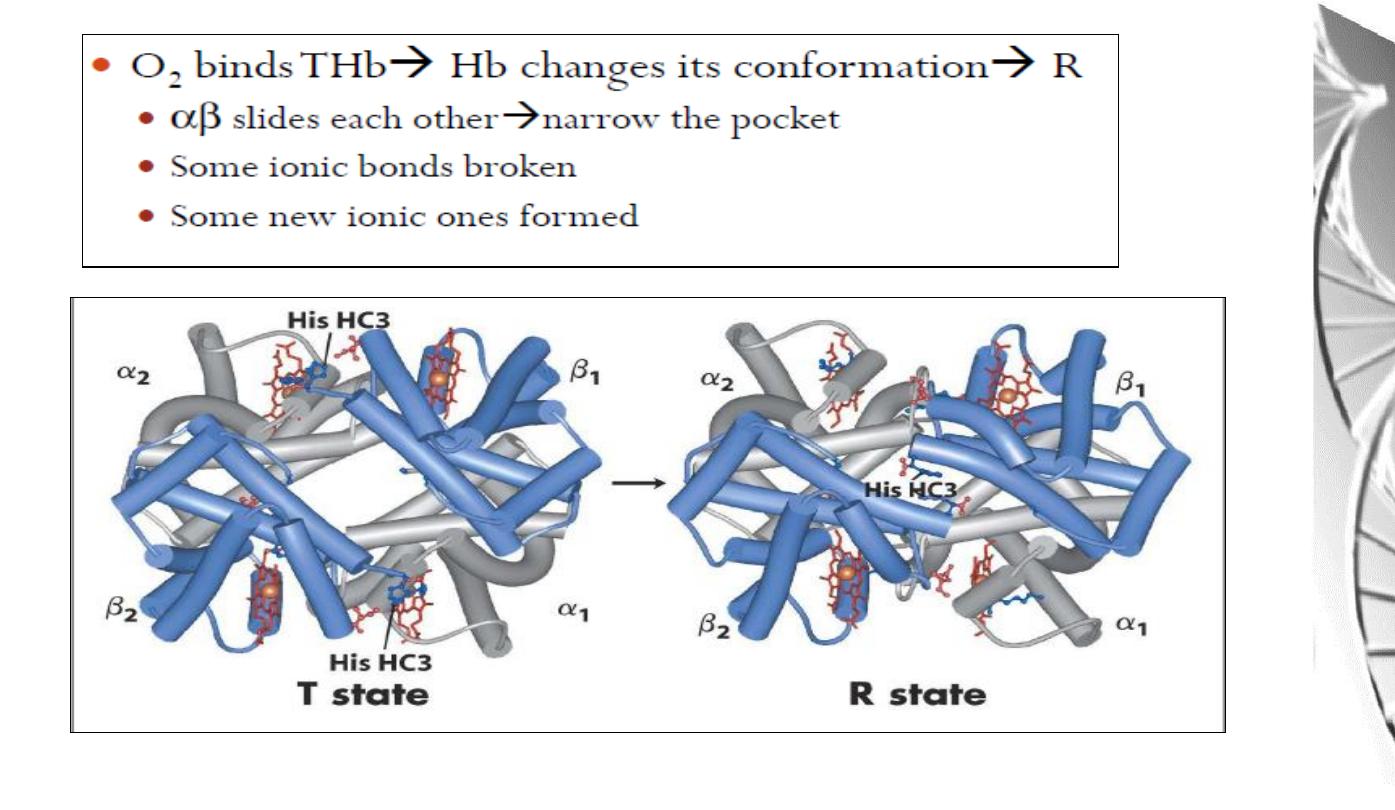
Transition from T to R state in haemoglobin
On binding oxygen, one pair of αβ
-subunits shifts with respect to the other by a
rotation of 15 degrees.
Binding of oxygen to one subunit ‘switches’ other subunits to a conformation
which favours oxygen binding - leading to ‘cooperative’ binding of oxygen.
Deoxy
Oxy

Allosteric effects
• The ability o f hemoglobin to reversibly bind oxygen is affected by
the following parameters:
• pH
• pO2
• pCO2
• the availability o f 2,3-bisphosphoglycerate .
These are collectively called allosteric ("other site") effectors,
because their interaction at one site on the hemoglobin molecule
affects the binding of oxygen to heme groups at other locations on the
molecule.
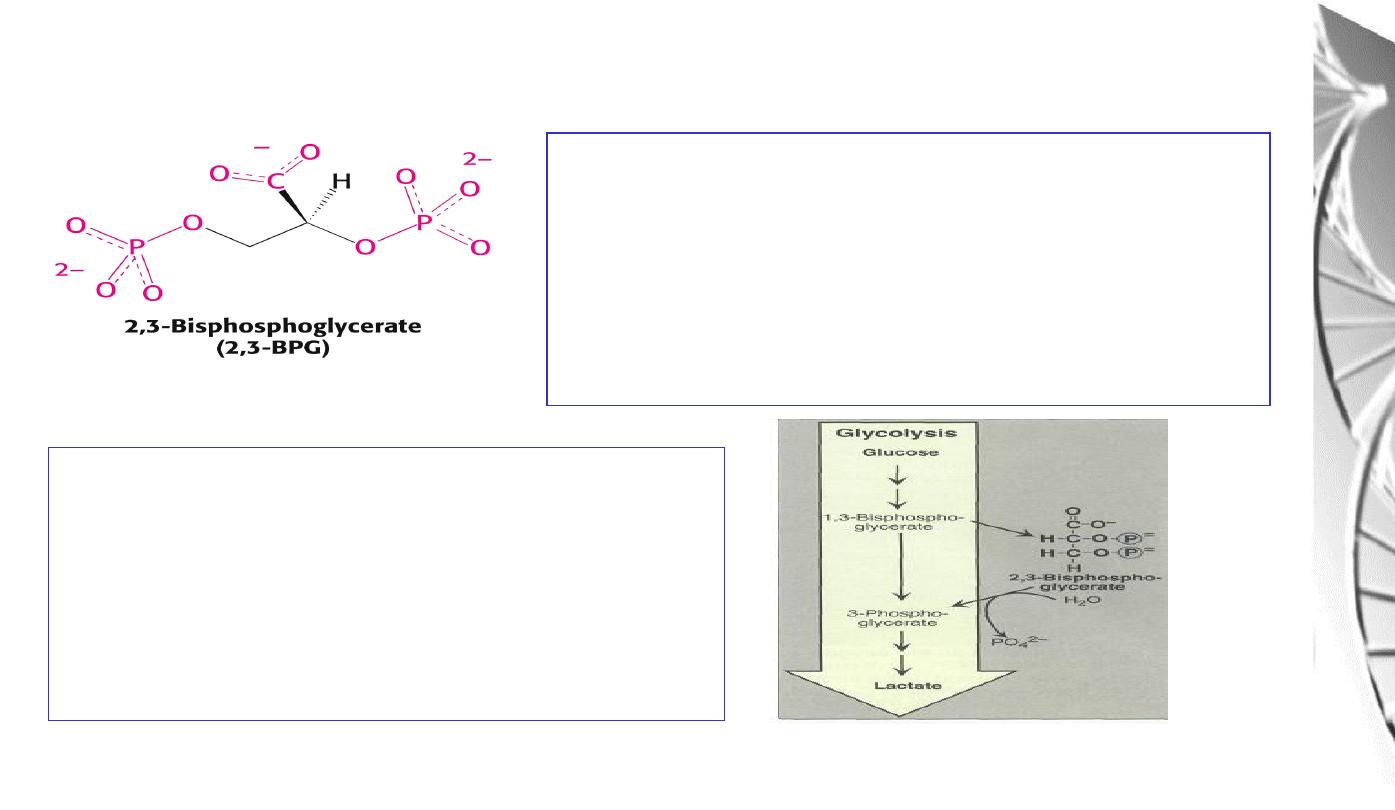
Regulation of oxygen binding
The highly anionic 2,3-BPG is present in red
blood cells at ~ 2 mM. It binds to haemoglobin
(one molecule per tetramer) and decreases the
affinity for O
2
, promoting release in the tissues.
The physiological adaptation to high altitude
involves increased tissue concentrations of
BPG, leading to more efficient O2 release to
compensate for the reduced O2 tension.
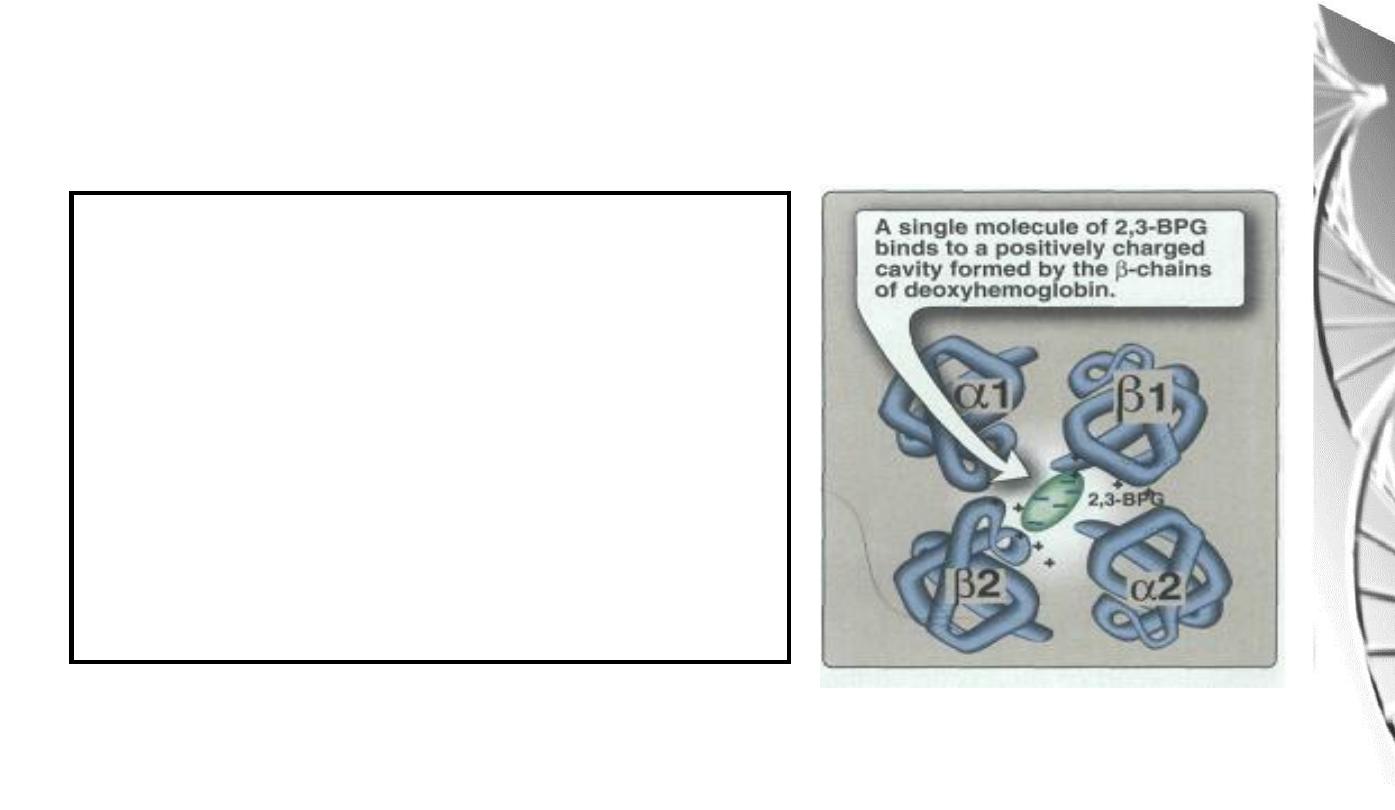
2,3-BPG binds in the central cavity of
the tetramer, interacting with three
positively charged groups (2 His, 1 Lys)
on each beta chain. The oxygenated
haemoglobin has a smaller central gap
and excludes 2,3-BPG.
Binding of 2,3-BPG to deoxyhaemoglobin
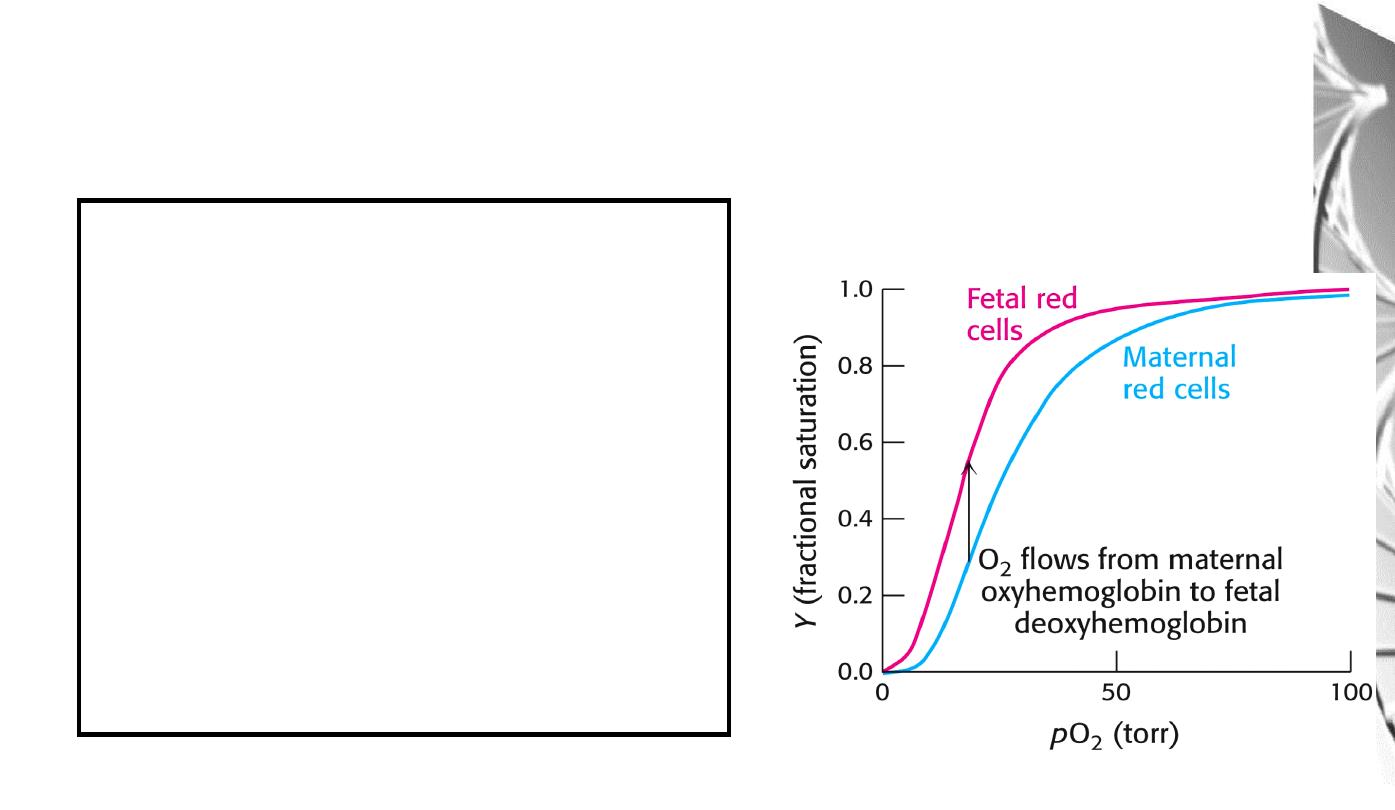
Differential oxygen affinity
of foetal and maternal red blood cells
Foetal haemoglobin contains a variant of
the β chain, called ɤ, which has a His
Ser
substitution in the 2,3-BPG-binding site.
The foetal haemoglobin thus has a reduced
affinity for 2,3-BPG, resulting in an
enhanced O
2
-binding affinity that allows
transfer of O
2
from the maternal to the
foetal red blood cells
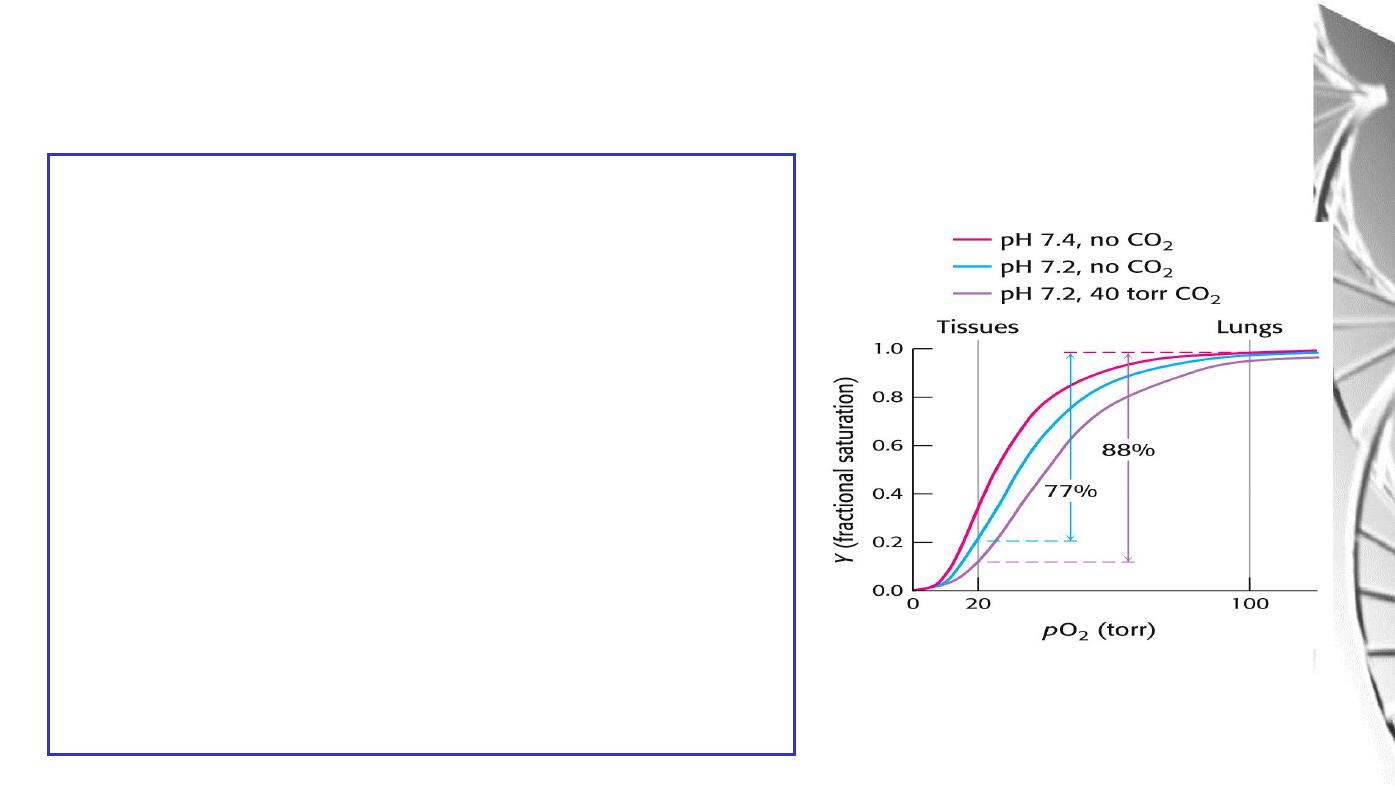
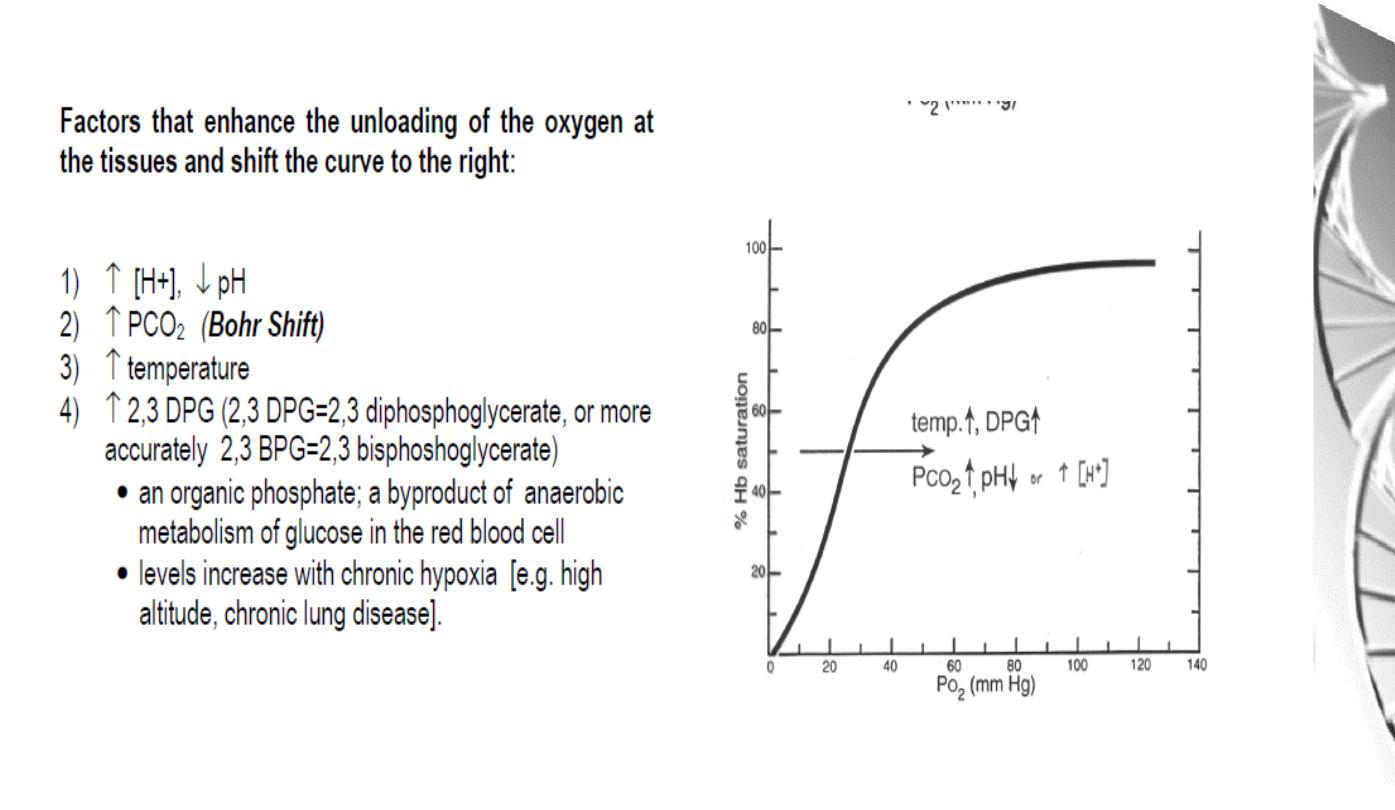
The Bohr effect
H
+
ions and CO
2
promote the release of O
2
Rapidly
metabolising
tissues,
such
as
contracting muscle, have a high need for O
2
and
generate large amounts of H
+
and CO
2
. Both of
these species interact with haemoglobin to
promote O
2
-release.
The O
2
affinity decreases as the pH decreases
from the pH 7.4 found in the lungs.
Increased CO
2
concentrations also lead to a
decrease in O
2
-affinity.
This regulation of O
2
-affinity by pH and CO
2
is
called the
Bohr effect
after its discoverer,
Christian Bohr (1904).
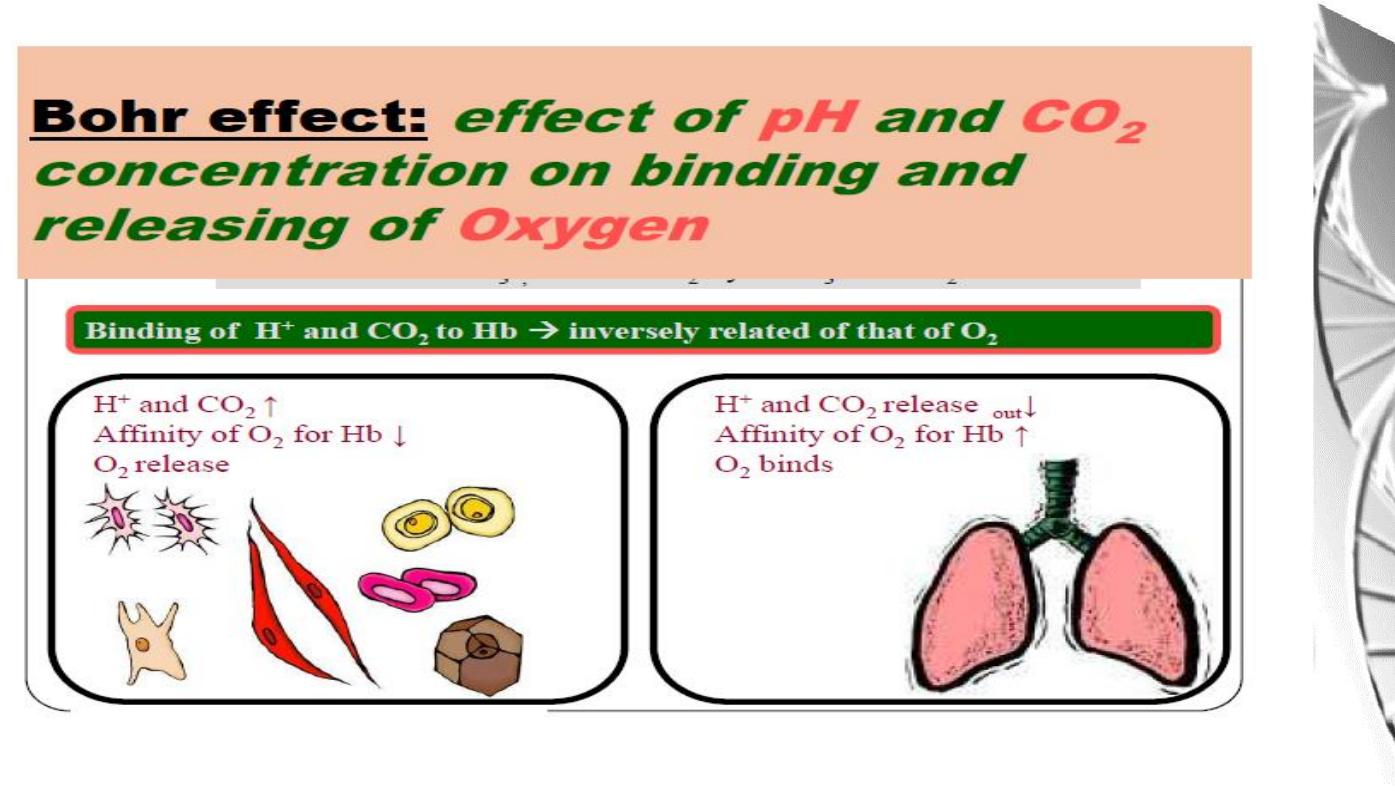
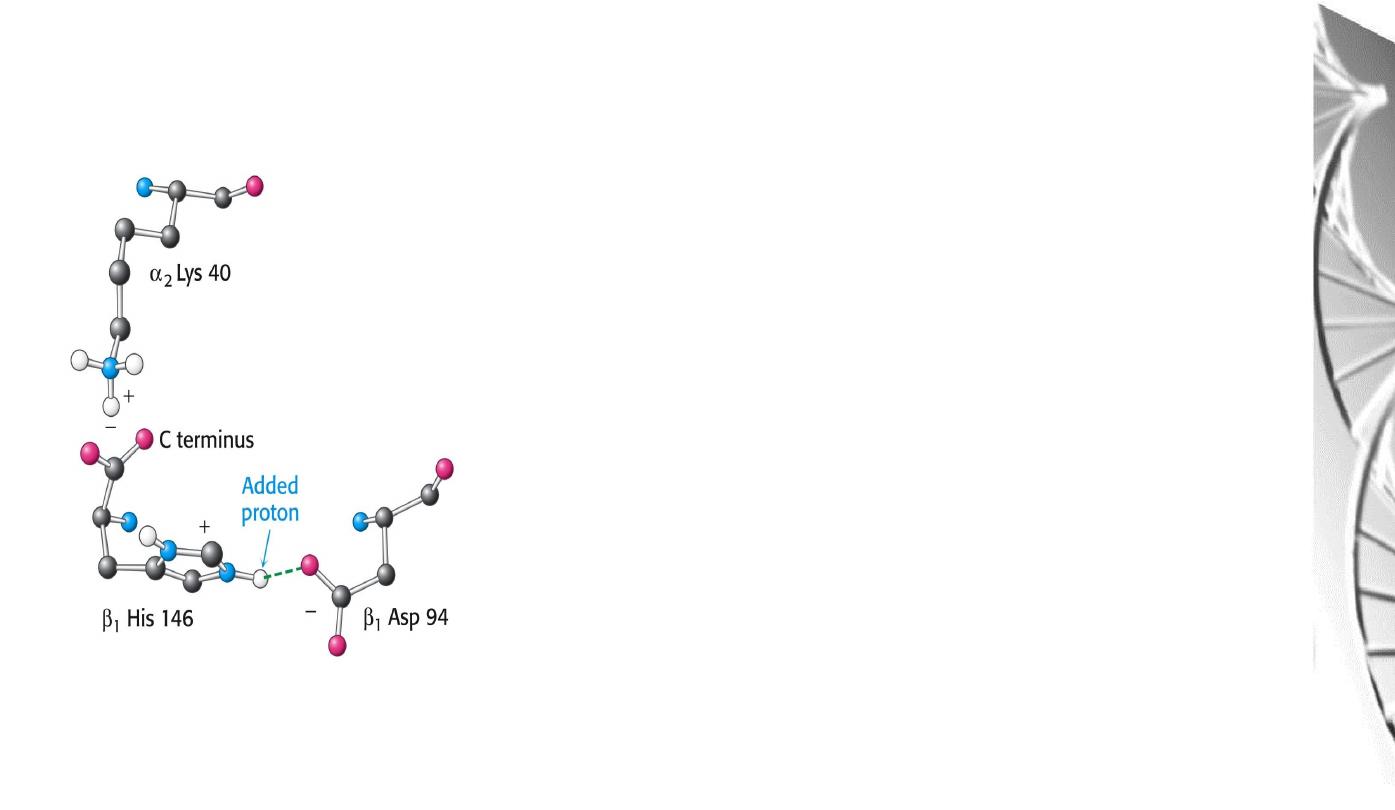
Chemical Basis of the Bohr effect
In deoxyhaemoglobin, three amino acid residues
form two salt bridges that stabilise the T state
(the low oxygen affinity state).
The formation of one of these depends on the
presence of a proton on His b146.
Lowering of the pH by metabolic activity favours
the proton-addition and formation of the salt
bridge with Aspb94, thus stabilising the T
structure and increasing the tendency for O
2
to be
released.
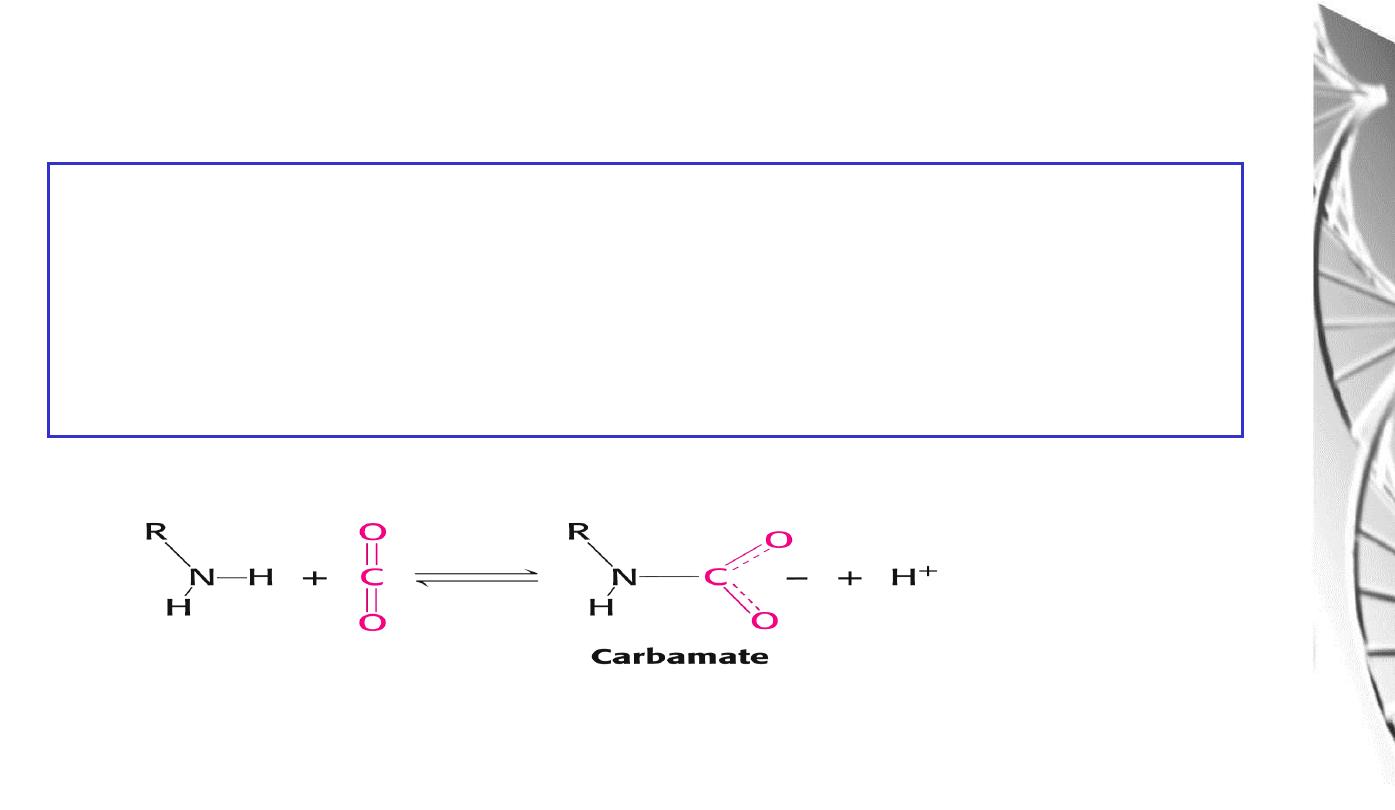
The CO
2
reacts with the terminal aNH
2
groups to form carbamates, which are
negatively charged. The N-termini lie at the interface between the ab dimers,
and the negatively charged carbamates participate in salt-bridge interactions that
favour the T state, stabilising the deoxy-form and favouring the release of O
2
The effect of CO
2
on deoxyhaemoglobin
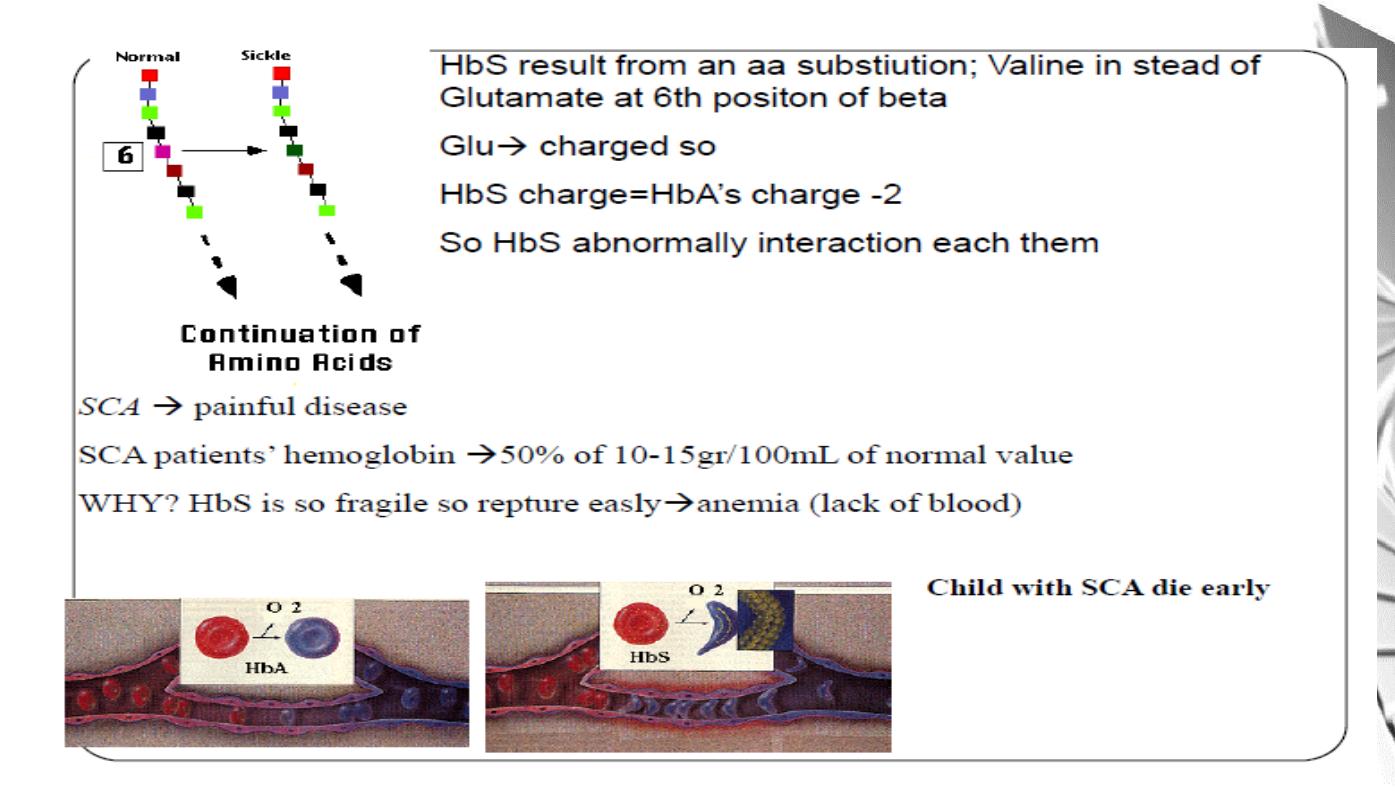
Carbon monoxide and haem iron
Carbon monoxide is a poison because it combines with
ferromyoglobin
and
ferrohaemoglobin
and
blocks
oxygen
transport.
Note
that
HisE7
sterically
inhibits
binding
of
CO,
and
lowers
its
affinity
for
the
haem
Fe.
This
is
then
sufficiently
low
that
endogenous
levels
of
CO
can
be
tolerated.
High
levels
of
CO
from
poorly
ventilated
gas
fires are highly toxic.
HisF8
N-Fe-O
O
(Plane of porphyrin ring)
(Plane of porphyrin ring)
HisF8
N-Fe-C
O
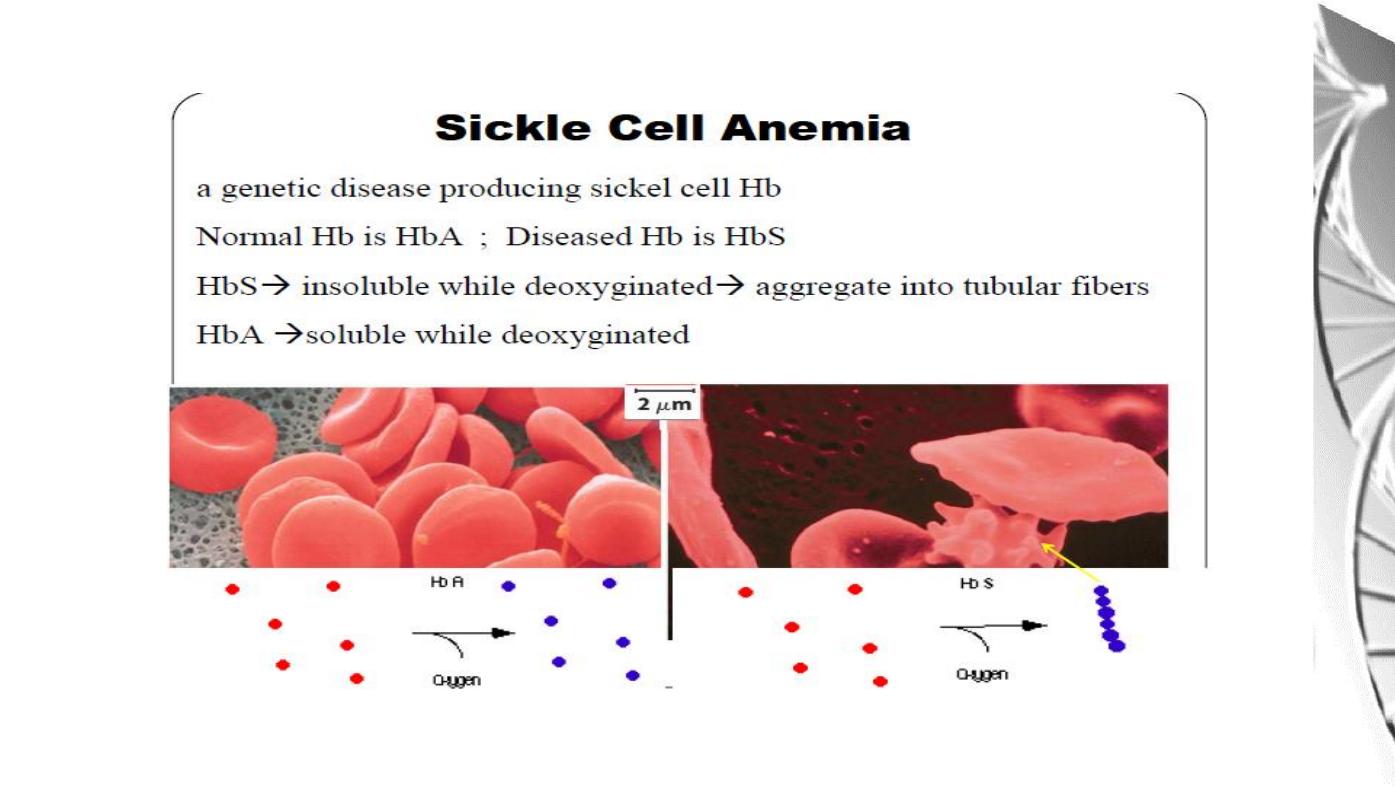
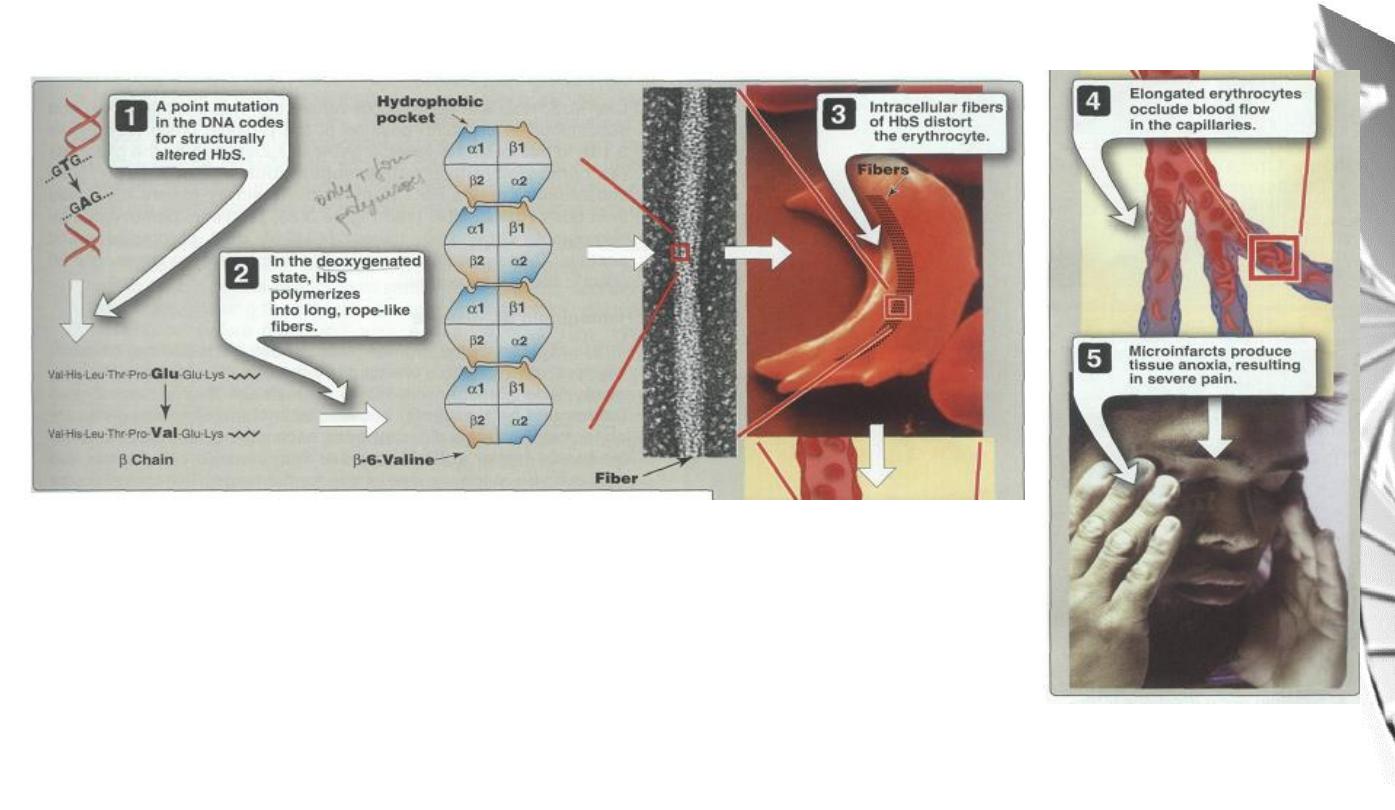
MGD 2016/ DR. Al-BARQAAWI
Summary
