
1
Lecture 5
Dr. Nihal Ezzat
Introduction to Chromatography
Chromatography is a set of techniques that separate the molecules,
components in a mixture
based on their partitioning between a stationary
and a mobile phase.
Chromatographic columns are part of the
instrumentation that is used in chromatography
Definition
of
Chromatography
A method for separation of chemical compounds by partition between
two media one is stationary and the other is mobile.The stationary may be
liquid or solid while the mobile may be gas or liquid.
The substance is distributed between the two phases.
Different molecules are distributed to different degree.
There are three types of chromatography based on the mechanism of
separation which are:
1- Adsorption as in ion exchange.
2- Partition as in paper and thin layer.
3- Permiation as in gel filtration.
Aplications:
Different chromatography used for : identification, isolation, purification,
quantitation of individual compounds and in some cases for molecular
weight detrmination by comparing as in gel filtration chromatography.
1-Ion exhange chromatography
A technique for separation of different molecules according to the
charge.
Ion exchange columns are used to separate ions and molecules that can be
easily ionized. Separation of the ions depends on the ion's affinity for the
stationary phase, which creates an ion exchange system. Only positively

2
or negatively charged complexes can interact with their respective cation
or anion exchangers.
Separation depends on the relative balance between the affinity of the
compounds for the adsorbent and their solubility in the solvent.
Two types of ion exchange: 1- Cation exchangers(Having negative
charge) as in Carboxy methyl(CM), phospho, sulphopropyl…., and
2- Anion exhangers(Having posative charge) as in amino ethyl(AE),
DEAE, …….(NH
2
+
).
The conditions of separation can be varified by:
1- The pH.
2- Ionic strength.
Ion exchange chromatography is perhaps the most commonly
employed chromatographic method for separating the proteins,
polypeptides, nucleic acids, and other charged molecules. The
principle underlying ion exchange chromatography is the
reversible exchange of the charged molecules (ions) present in
the solution with those electrostatically bound to the insoluble
support
medium.
The
molecules
in
ion
exchange
chromatography, therefore, get separated based on their charge.
2-Partition chromatograghy:
Two phases are used, the phase that is bound to the inert support is
called stationary phase . The other is mobile (Eluent). The substances are
separeted by differential soubility between the two liquid phases.
R
f
value( Retention factor) = Distance moved by the solute / distance
moved by the solvent.
(Paper and Thin layer chromatography(TLC)).
3- permiation chromatograghy (Gel filtration)
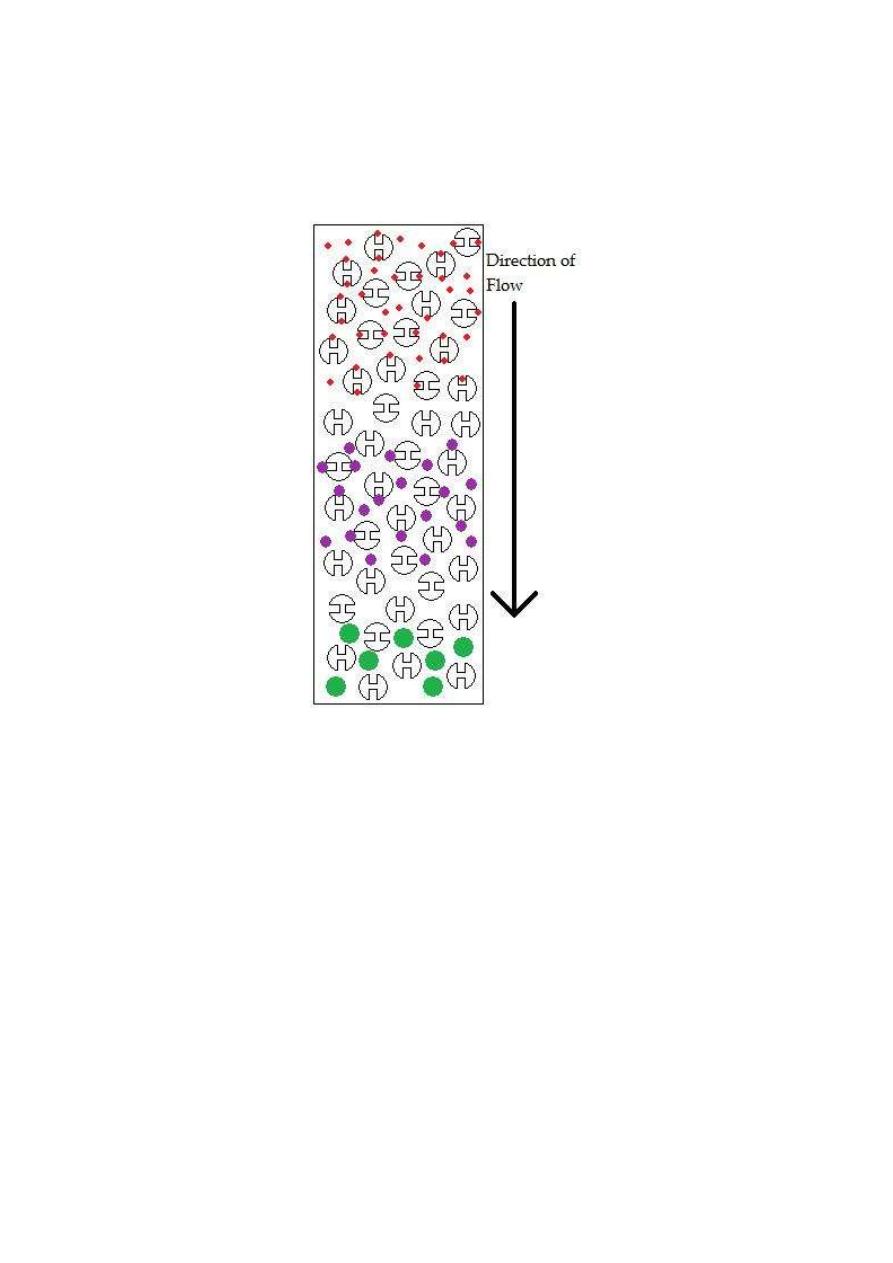
3
A technique for separatin of different macro-molecules according to
their size and molecular weights where a large molecular sieves such as
sephadex (cross-link polydextrans)are used.
Figure 1: Schematic of a size exclusion column. The larger particles will
elute first because they are too big to fit inside the pores. The smallest
particles will elute last because they fit very well inside the pores. This
figure was created with Microsoft Paint.
Modifications of
Chromatography
1- HPLC….2- GC…..3- Affinity chrom. 4- Reverse phase.
Types of
Chromatography

4
1-Thin Layer Chromatography (
TLC
).
Thin layer chromatography, abbreviated as TLC, is an analytical tool
that is frequently used in chemistry laboratories to study the purity of
organic compounds or to separate and analyze the components of
complex mixtures. TLC is a solid-liquid form of chromatography i.e. the
stationary phase is a solid while the mobile phase is a liquid. The
stationary phase is prepared by coating a very thin layer of a polar
adsorbent on a rectangular solid support. The solid support can be a glass,
plastic, or a metal plate such as Aluminum. Silica (SiO
2
·xH
2
O) or
alumina (Al
2
O
3
·xH
2
O) are the most commonly used adsorbents and are
coated as uniformly thin layers on the solid supports.
The sample, either liquid or dissolved in a volatile solvent, is deposited
as a spot on the stationary phase. One edge of the plate is then vertically
placed in a solvent reservoir and the solvent moves up the plate by
capillary action. The separated spots are then visualized with ultraviolet
light or by a suitable reaction procedure.
After a separation is complete, individual compounds appear as spots
separated vertically. Each spot has a retention factor (Rf) which is equal
to the distance migrated over the total distance covered by the solvent.
The Rf formula is: Retention Factor(Rf)
Rf=distance traveled by sample
distance traveled by solvent
The Rf value can be used to identify compounds due to their uniqueness
to each compound. When comparing two different compounds under the
same conditions, the compound with the larger Rf value is less polar
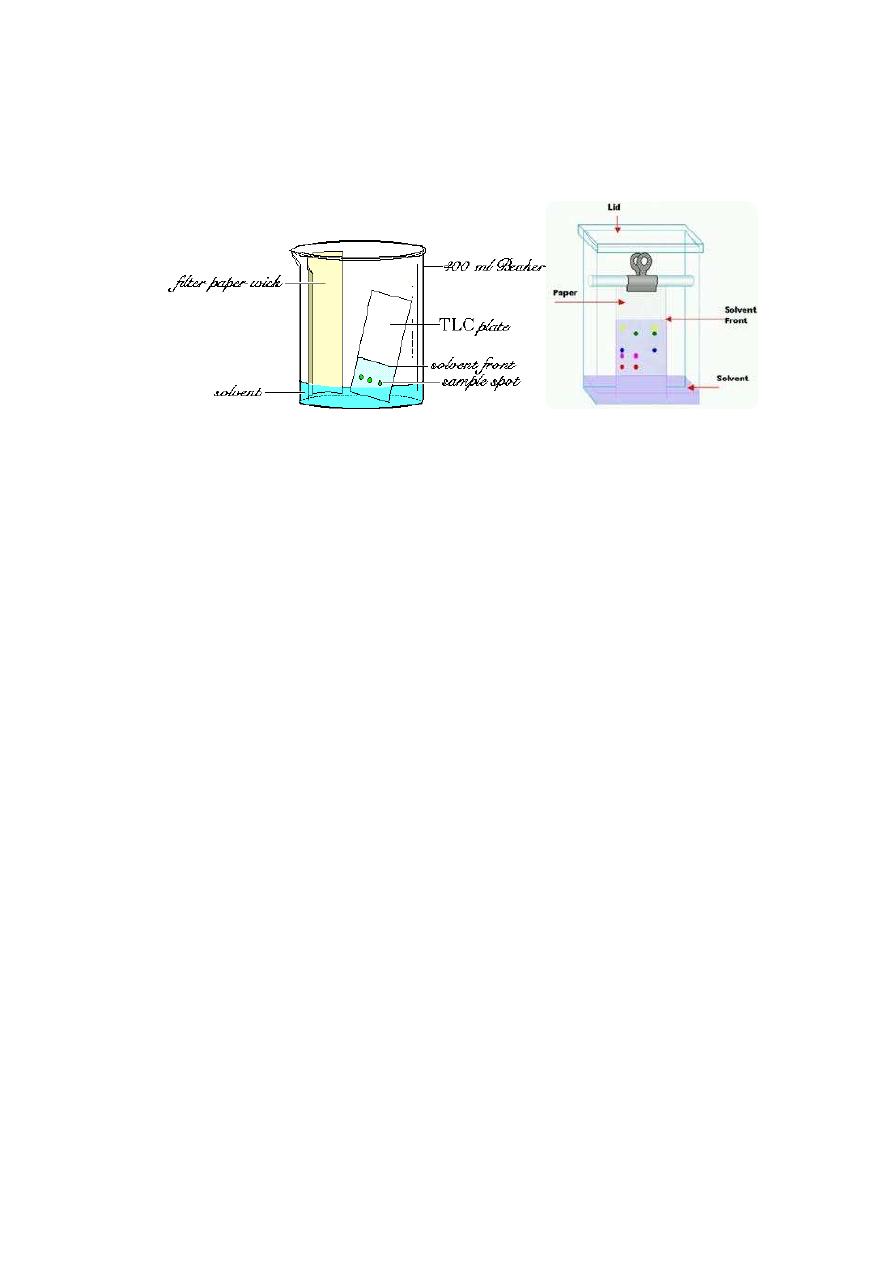
5
because it does not stick to the stationary phase as long as the polar
compound, which would have a lower Rf value.
Paper and Thin layer chromatography(TLC).
Advatages of TLC over paper
1- Greater resolving power. 2- Greater speed of separation. 3-
Relatively wide choice of adsorbents. These factors due to very
fine particles size and high surface to volume ratios of the
inorganic salts used in TLC.
4-The fibrous nature of cellulose in paper chrom. produces capillary
diffusion which increases spot size. The inorganic salt used in TLC do
not have a fibrous structure so capillary diffusion is eliminated
resulting in a smaller spots .
5- Furthermore, the very high surface area of the adsorbent also increases
the flow rate or (Retention Factor)
of the solvent. Because of the relatively inert nature of the inorganic
adsorbents.
6- detecting reagents such as sulfuric acid, can be sprayed onto the plates,
giving sensitive and simplified detecting system.
2-High Performance LiquidChromatographicColumns(HPLC)

6
High performance liquid chromatography
(HPLC) is aslo known as
. HPLC uses a liquid moblie phase. The same basic
principals
from
gas
chromatography
are
applied
to
liquid
chromatography.
The most common HPLC columns are made from stainless steel, but they
can be also made out of thick glass, polymers such as
polyetherethelketone, a combination of stainless steel and glass, or a
combination of stainless steel and polymers. Typical HPLC analytical
columns are between 3 and 25 cm long and have a diameter of 1 to 5 mm.
The columns are usually straight unlike GC columns. Particles that pack
the columns have a typical diameter between 3 to 5 µm. Liquid
chromatographic columns will increase in efficiency when the diameter
of the packed particles inside the column decreases.
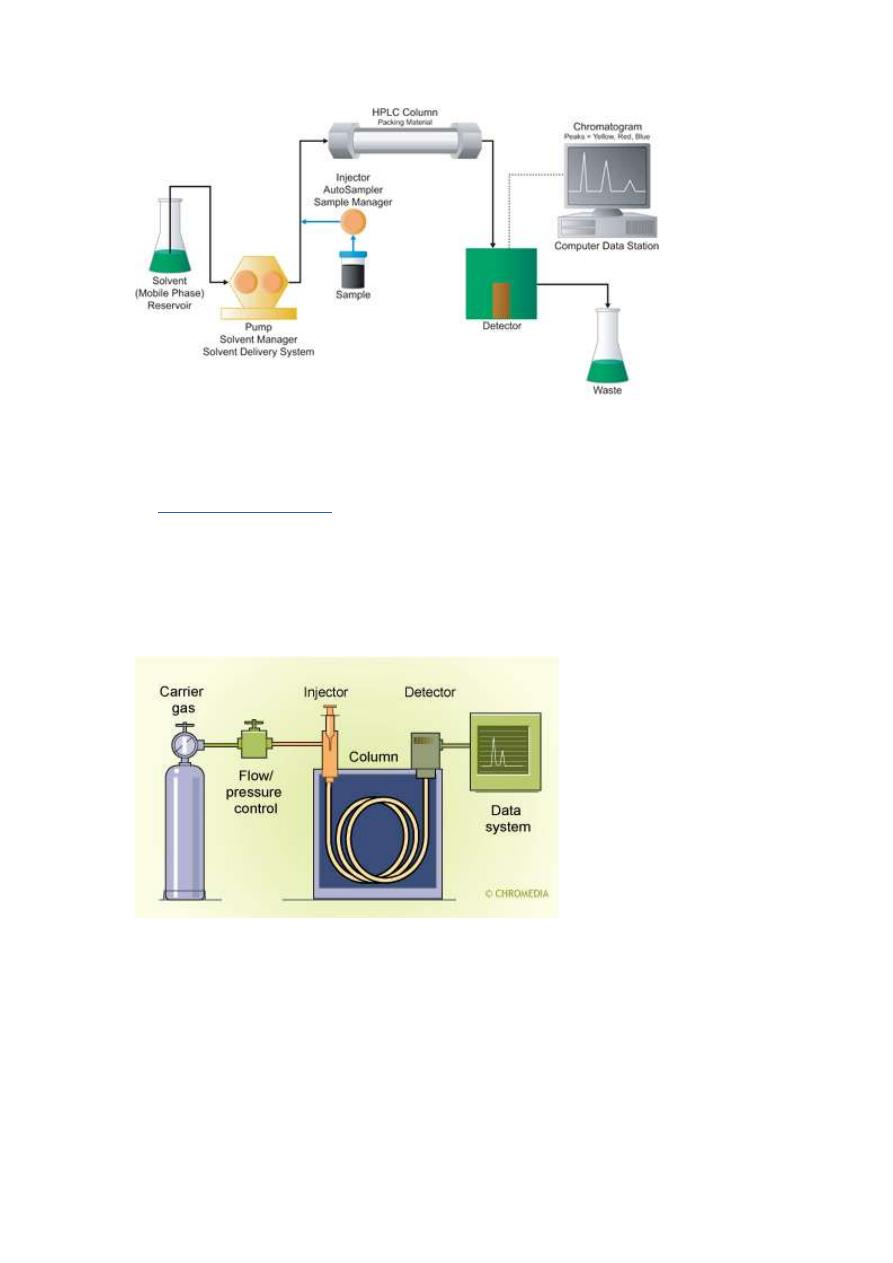
7
3-Gas Chromatographic Columns(GC).
the mobile phase is a gas. Gas chromatographic
columns are usually between 1 and 100 meters long. The liquid stationary
phase is bonded or adsorbed onto the surface of an open tubular
(capillary) column, or onto a packed solid support inside the column.
4- Gel filtration chromatography.
Gel filtration chromatography is also known as gel permeation,
molecular sieve, and size exclusion chromatography. The molecules are
separated based on their size. The column matrix is made up of small
spherical porous beads. The smaller molecules can enter the pores

8
present in the beads while the molecules larger than the maximum pore
size of the bead are completely excluded. The access to the pores is
determined by both the shape and the molecular weight of the molecules;
the separation, therefore, is based on the ability of the molecules to enter
the porous beads.
Gel filtration chromatography separates the molecules based on
their size; it is therefore also known as size exclusion
chromatography.
.

9
Lecture 6
Dr. Nihal Ezzat
Electrophoresis
It is a technique based on movement of charge molecules in an electric
field.
Mobility of charge molecule = Applied voltage X Net charge
/friction of the molecule
Aplication:
1- Purity of the molecule
2- Identification of the molecule
3- Comparative molecular weight determination
4- Separation of a protein.
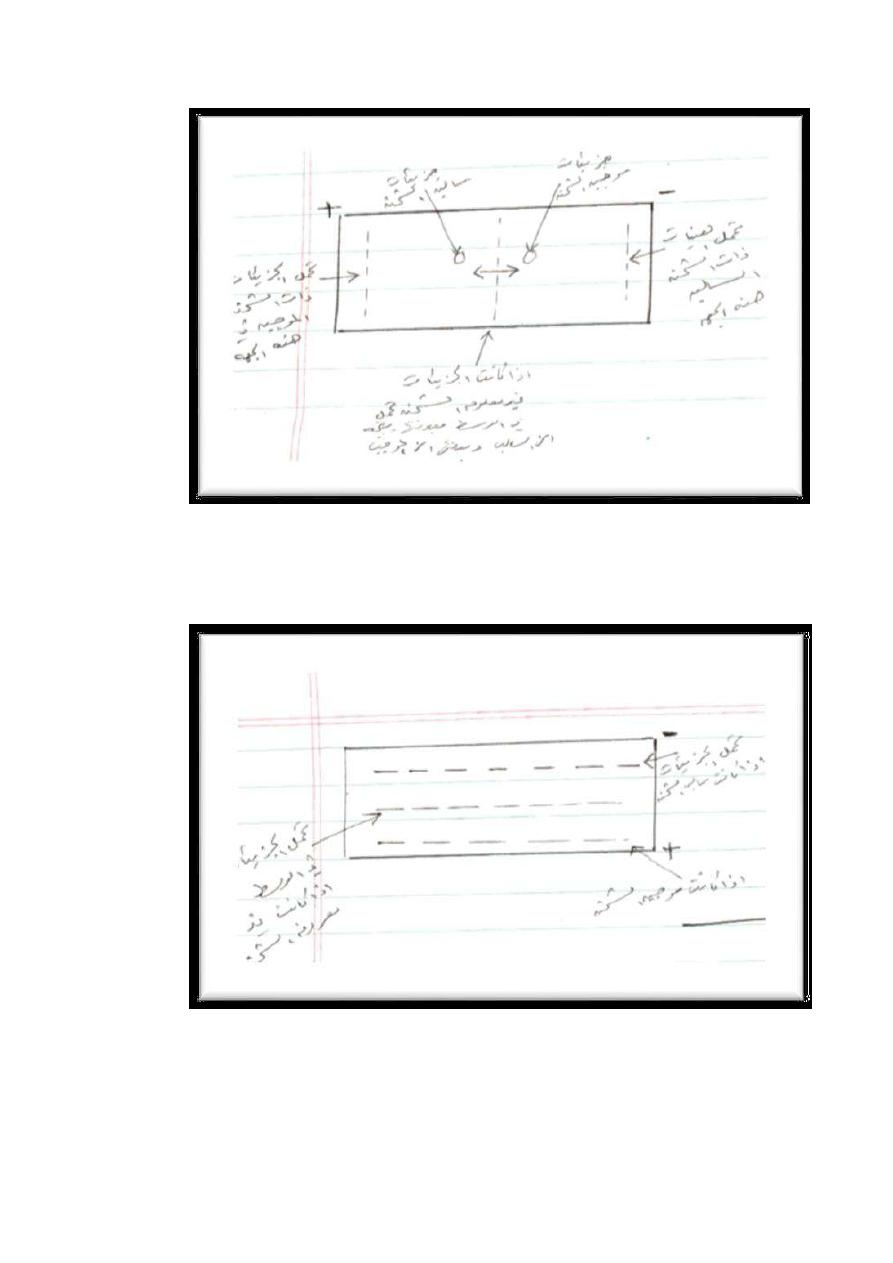
Basic Principle of Electrophoresis
Electrophoresis is the process of moving charged molecule in
solution by applying an electric field across the mixture.
Principle of Separation
(1) According to charge:
When charged molecules are placed in an electric field, they migrate
toward either the positive (anode) or negative (cathode) pole according to
their charge.
(2) According to size:
(1-Small , 2-Large).
Types of electrophoretic techniques
1-
2-
Cellulose acetate and cellulose nitrate electrophoresis
3-
Gel electrophoresis (Agaroes - polyacrylamide PAGE).
4-
Sodium dodecyl sulphate (SDS).
1- Paper
electrophoretic
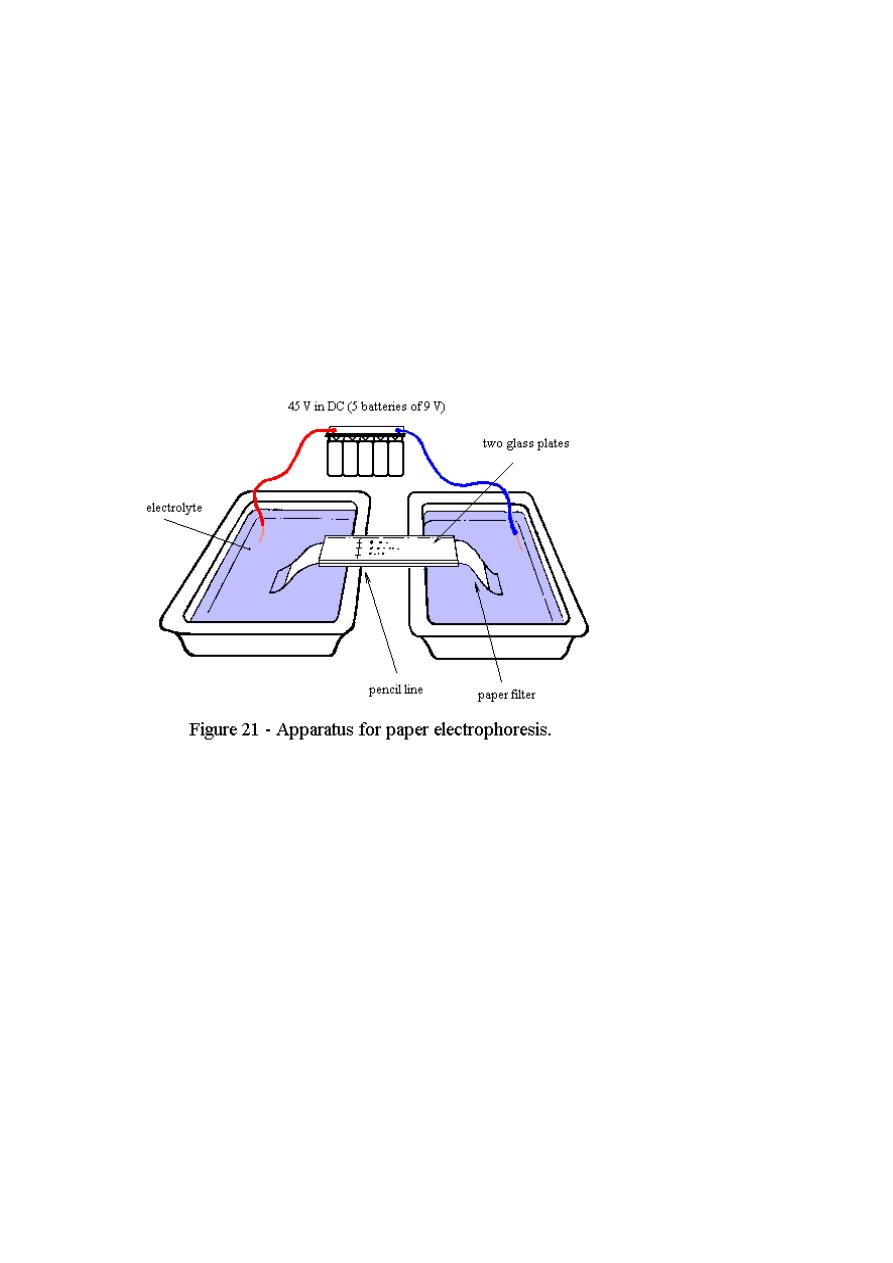
11
An electrode apparatus consists of a high-voltage supply, electrodes,
buffer, and a support for the buffer such as filter paper, cellulose acetate
strips, polyacrylamide gel, or a capillary tube. Open capillary tubes are
used for many types of samples and the other supports are usually used
for biological samples such as protein mixtures or DNA fragments. After
a separation is completed the support is stained to visualize the separated
components.
11

12
2-
Gel electrophoresis: is a method for separation and analysis of
) and their fragments, based on
and
macromolecules (
their size and charge.
It is used in clinical chemistry to separate proteins by charge and /or size
(
agarose,
essentially
size
independent)
and
in
-
2
to separate a mixed population of
and
fragments by length, to estimate the size of
and
Nucleic acid
by charge.
te
fragments or to separa
and
to move the
molecules are separated by applying an
or other
negatively charged molecules through a matrix of
substances. Shorter molecules move faster and migrate farther than longer
ones because shorter molecules migrate more easily through the pores of
the gel. This phenomenon is called sieving.
Agarose gel for DNA and RNA.
Applications of
Electrophoresis
1- Estimation of the size of DNA molecules following restriction enzyme
2-Analysis
of
products,
e.g.
in
3-Separation of restricted genomic DNA prior to
, or of RNA prior to
