The Body Fluid Compartments: Extracellular and Intracellular Fluids; Interstitial Fluid and Edema
The maintenance of a relatively constant volume and a stable composition of the body fluids is essential for homeostasis.
Fluid Intake and Output Are Balanced During Steady-State Conditions
The relative constancy of the body fluids is remarkable because there is continuous exchange of fluid and solutes with the external environment as well as within the different compartments of the body.
Daily Intake of Water
Water is added to the body by two major sources:
(1) it is ingested in the form of liquids or water in the food, which together normally add about 2100 ml/day to the body fluids.
(2) it is synthesized in the body as a result of oxidation of carbohydrates, adding about 200 ml/day. This provides a total water intake of about 2300 ml/day.
Daily Loss of Body Water
Insensible Water Loss. Some of the water losses cannot be precisely regulated. For example, there is a continuous loss of water by evaporation from the respiratory tract and diffusion through the skin, which together account for about 700 ml/day of water loss under normal conditions. This is termed insensible water loss because we are not consciously aware of it.The insensible water loss through the skin occurs independently of sweating, the average water loss by diffusion through the skin is about 300 to 400 ml/day.
Fluid Loss in Sweat.
The amount of water lost by sweating is highly variable, depending on physical activity and environmental temperature. The volume of sweat normally is about 100 ml/day, but in very hot weather or during heavy exercise, water loss in sweat occasionally increases to 1 to 2 L/hour. This would rapidly deplete the body fluids if intake were not also increased by activating the thirst mechanism.
Water Loss in Feces.
Only a small amount of water (100 ml/day) normally is lost in the feces. This can increase to several liters a day in people with severe diarrhea.
Water Loss by the Kidneys.
The remaining water loss from the body occurs in the urine excreted by the kidneys. There are multiple mechanisms that control the rate of urine excretion. In fact, the most important means by which the body maintains a balance between water intake and output, as well as a balance between intake
and output of most electrolytes in the body, is by controlling the rates at which the kidneys excrete these substances. For example, urine volume can be as low
as 0.5 L/day in a dehydrated person or as high as 20 L/day in a person who has been drinking tremendous amounts of water. This variability of intake is also true for most of the electrolytes of the body, such as sodium, chloride, and potassium. In some people, sodium intake may be as low as 20 mEq/day, whereas in others, sodium intake may be as high as 300 to 500 mEq/day.The kidneys are faced with the task of adjusting the excretion rate of water and electrolytes to match precisely the intake of these substances, as well as compensating for excessive losses of fluids and electrolytes that occur in certain disease states.
Body Fluid Compartments
The total body fluid is distributed mainly between two compartments:
the extracellular fluid and the intracellular fluid.
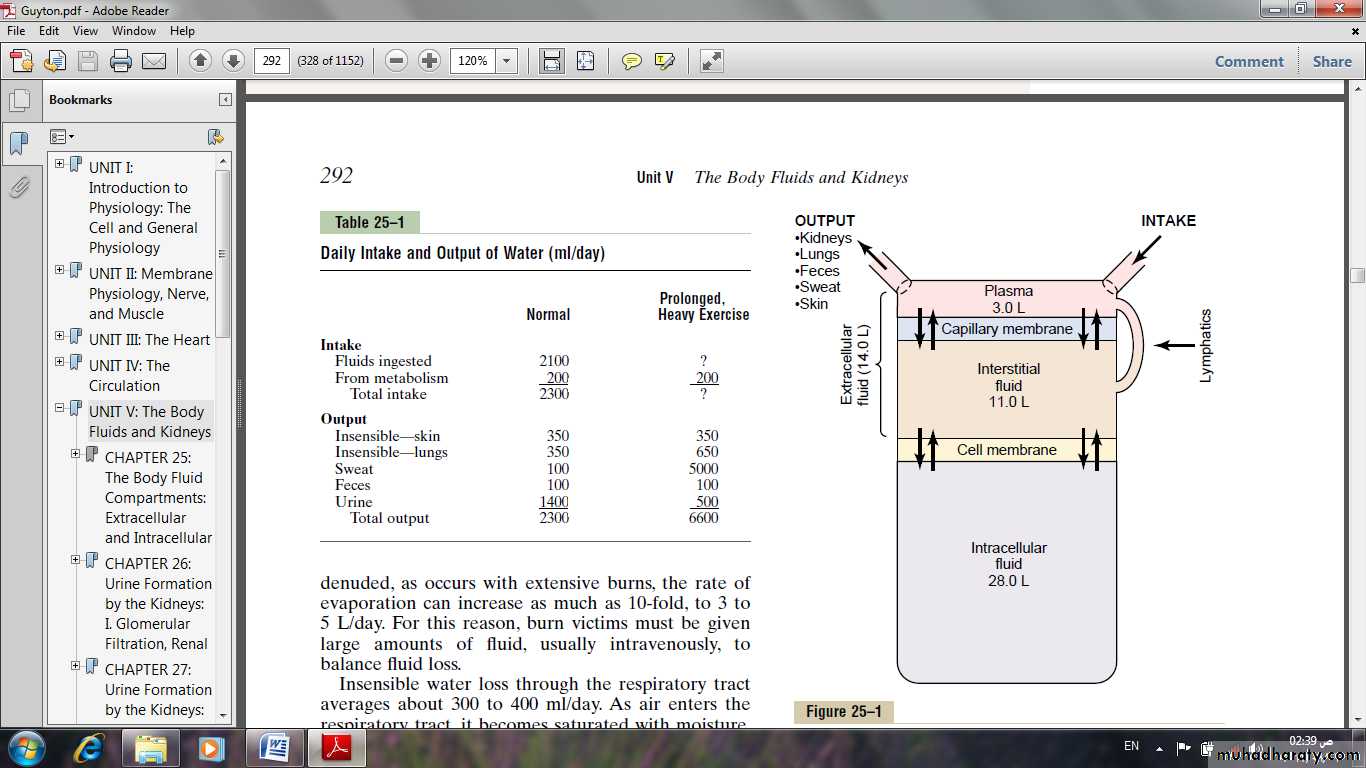
Fig: Summary of body fluid regulation, including the major body fluid compartments and the membranes that separate these compartments. The values shown are for an average 70-kilogram person
The extracellular fluid is divided into the interstitial fluid and the blood plasma.In the average 70-kilogram adult human, the total body water is about 60 per cent of the body weight, or about 42 liters.This percentage can change, depending on age, gender, and degree of obesity.
Intracellular Fluid Compartment
About 28 of the 42 liters of fluid in the body are inside the 75 trillion cells and are collectively called the intracellular fluid. Thus, the intracellular fluid constitutes about 40 per cent of the total body weight in an “average” person.
the intracellular fluid of all the different cells together is considered to be one large fluid compartment.
Extracellular Fluid Compartment
All the fluids outside the cells are collectively called the extracellular fluid. Together these fluids account for about 20 per cent of the body weight, or about 14 liters in a normal 70-kilogram adult. The two largest compartments
of the extracellular fluid are:
The interstitial fluid: which makes up more than three fourths of the extracellular fluid.
The plasma: which makes up almost one fourth of the extracellular fluid, or about 3 liters. The plasma is the non cellular part of the blood; it exchanges substances continuously with the interstitial fluid through the pores of the capillary membranes. These pores are highly permeable to almost all solutes in the extracellular fluid except the proteins. Therefore, the extracellular fluids are constantly mixing, so that the plasma and interstitial fluids have about the same composition except for proteins, which have a higher concentration in the plasma.
Blood Volume
Blood contains both extracellular fluid (the fluid in plasma) and intracellular fluid (the fluid in the red blood cells). However, blood is considered to be a separate fluid compartment because it is contained in a chamber of its own, the circulatory system. The blood volume is especially important in the control of cardiovascular dynamics. The average blood volume of adults is about 7 per
cent of body weight, or about 5 liters. About 60 per cent of the blood is plasma and 40 per cent is red blood cells, but these percentages can vary considerably in different people, depending on gender, weight, and other factors.
Constituents of Extracellular and Intracellular Fluids:
Ionic Composition of Plasma and Interstitial Fluid Is Similar
Because the plasma and interstitial fluid are separated only by highly permeable capillary membranes, their ionic composition is similar. The most important difference between these two compartments is the higher concentration of protein in the plasma; because the capillaries have a low permeability to the plasma proteins, only small amounts of proteins are leaked into the interstitial spaces in most tissues. Because of the Donnan effect, the concentration of positively charged ions (cations) is slightly greater (about 2 per cent) in the plasma than in the interstitial fluid. The plasma proteins have a net negative charge and, therefore, tend to bind cations, such as sodium and potassium ions, thus holding extra amounts of these cations in the plasma along with the plasma proteins. Conversely, negatively charged ions (anions) tend to have a slightly higher concentration in the interstitial fluid compared with the plasma, because the negative charges of the plasma proteins repel the negatively charged anions. For practical purposes, however, the concentration of ions in interstitial fluid and in the plasma is considered to be
about equal; the extracellular fluid, including the plasma and the interstitial fluid, contains large amounts of sodium and chloride ions, reasonably large amounts of bicarbonate ions, but only small quantities of potassium, calcium, magnesium, phosphate, and organic acid ions. The composition of extracellular fluid is carefully regulated by various mechanisms, but especially by the kidneys. This allows the cells to remain continually bathed in a fluid that contains the proper concentration of electrolytes and nutrients for optimal cell function.
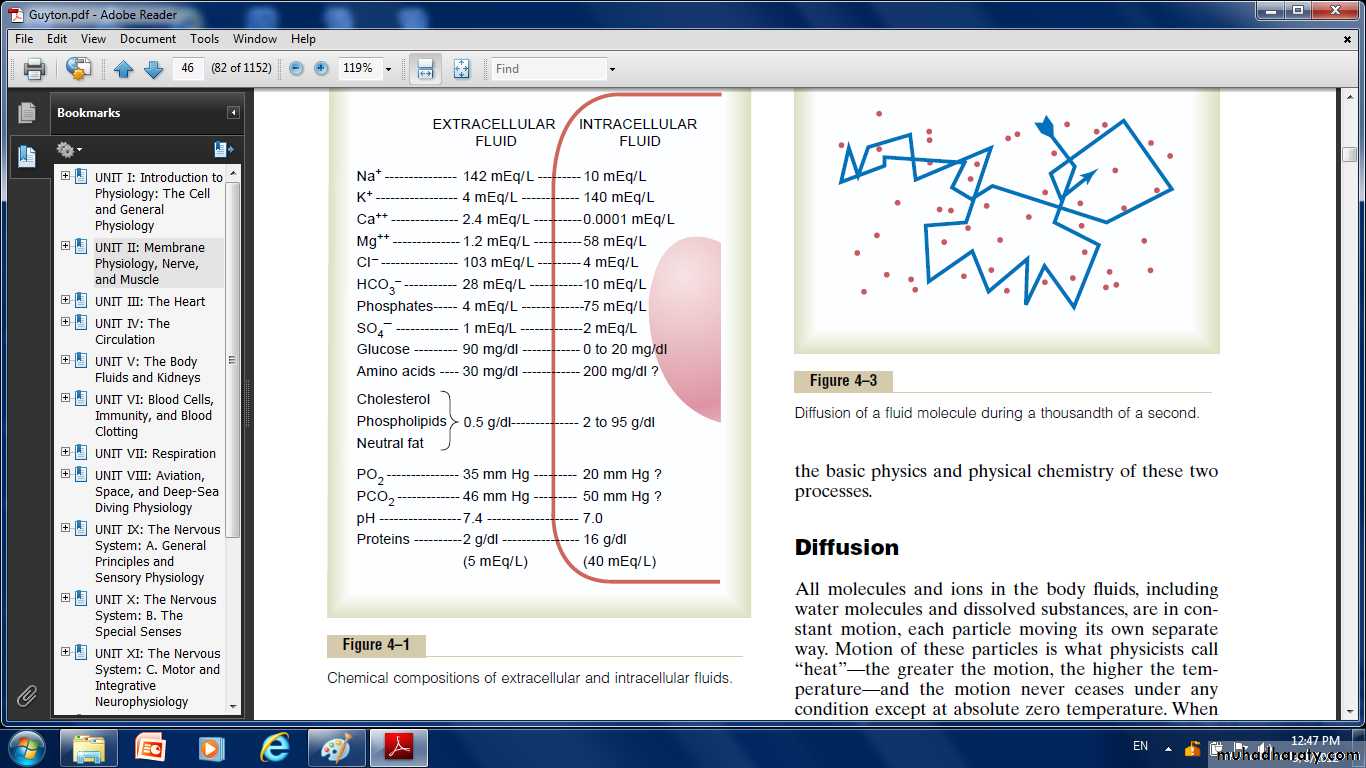
Fig: Chemical compositions of extracellular and intracellular fluids
Important Constituents of the Intracellular FluidThe intracellular fluid is separated from the extracellular fluid by a cell membrane that is highly permeable to water but not to most of the electrolytes in the body. In contrast to the extracellular fluid, the intracellular
fluid contains only small quantities of sodium and chloride ions and almost no calcium ions. Instead, it contains large amounts of potassium and phosphate
ions plus moderate quantities of magnesium and sulfate ions, all of which have low concentrations in the extracellular fluid. Also, cells contain large amounts of protein, almost four times as much as in the plasma.
Regulation of Fluid Exchange and Osmotic Equilibrium Between Intracellular and Extracellular Fluid:
The relative amounts of extracellular fluid distributed between the plasma and interstitial spaces are determined mainly by the balance of hydrostatic and colloid osmotic forces across the capillary membranes. The distribution of fluid between intracellular and extracellular compartments, in contrast, is determined mainly by the osmotic effect of the smaller solutes— especially sodium, chloride, and other electrolytes—acting across the cell membrane.
The reason for this is that the cell membranes are highly permeable to water but relatively impermeable to even small ions such as sodium and chloride. Therefore, water moves across the cell membrane rapidly, so that the intracellular fluid remains isotonic with the extracellular fluid.
Basic Principles of Osmosis and Osmotic Pressure
Osmosis is the net diffusion of water across a selectively permeable membrane from a region of high water concentration to one that has a lower water concentration. When a solute is added to pure water, this reduces the concentration of water in the mixture. Thus, the higher the solute concentration in a solution, the lower the water concentration. Further, water diffuses from a region of low solute concentration (high water concentration)
to one with a high solute concentration (low water concentration). Because cell membranes are relatively impermeable to most solutes but highly permeable to water (i.e., selectively permeable), whenever there is a higher
concentration of solute on one side of the cell membrane, water diffuses across the membrane toward the region of higher solute concentration. Thus, if a solute such as sodium chloride is added to the extracellular fluid, water rapidly diffuses from the cells through the cell membranes into the extracellular fluid until the water concentration on both sides of the membrane becomes equal. Conversely, if a solute such as sodium chloride is removed from the extracellular fluid, water diffuses from the extracellular fluid through the cell membranes and into the cells. The rate of diffusion of water is called the rate of osmosis.
The osmotic pressure of a solution is directly proportional to the concentration of osmotically active particles in that solution. This is true regardless of whether the solute is a large molecule or a small molecule. For example, one molecule of albumin with a molecular weight of 70,000 has the same osmotic effect as one molecule of glucose with a molecular weight of 180. One molecule of sodium chloride, however, has two osmotically active particles, Na+ and Cl–, and therefore has twice the osmotic effect of either an albumin molecule or a glucose molecule.
Osmolarity of the Body Fluids.
the approximate osmolarity of the various osmotically active substances in plasma, interstitial fluid, and intracellular fluid. Note that about 80 per cent of the total osmolarity of the interstitial fluid and plasma is due to sodium and chloride ions, whereas for intracellular fluid, almost half the osmolarity is due to potassium ions, and the remainder is divided among many other intracellular substances, the total osmolarity of each of the three compartments is about 300 mOsm/L, with the plasma being about 1 mOsm/L greater than that of the interstitial and intracellular fluids.The slight differencebetween plasma and interstitial fluid is caused by the osmotic effects of the plasma proteins, which maintain about 20 mm Hg greater pressure in the capillaries than in the surrounding interstitial spaces.
Isotonic, Hypotonic, and Hypertonic Fluids.
If a cell is placed in a solution of impermeant solutes having an osmolarity of 282 mOsm/L, the cells will not shrink or swell because the water concentration
in the intracellular and extracellular fluids is equal and the solutes cannot enter or leave the cell. Such a solution is said to be isotonic because it neither shrinks
nor swells the cells. Examples of isotonic solutions include a 0.9 per cent solution of sodium chloride or a 5 per cent glucose solution. These solutions are important in clinical medicine because they can be infused into the blood without the danger of upsetting osmotic equilibrium between the intracellular and extracellular fluids.
If a cell is placed into a hypotonic solution that has a lower concentration of impermeant solutes (less than 282 mOsm/L), water will diffuse into the cell, causing it to swell; water will continue to diffuse into the cell, diluting the intracellular fluid while also concentrating the extracellular fluid until both solutions have about the same osmolarity. Solutions of sodium chloride with a concentration of less than 0.9 per cent are hypotonic and cause cells to swell.
If a cell is placed in a hypertonic solution having a higher concentration of impermeant solutes, water will flow out of the cell into the extracellular fluid, concentrating the intracellular fluid and diluting the extracellular fluid. In this case, the cell will shrink until the two concentrations become equal. Sodium chloride solutions of greater than 0.9 per cent are hypertonic.
Edema: Excess Fluid in the Tissues
Edema refers to the presence of excess fluid in the body tissues. In most instances, edema occurs mainly in the extracellular fluid compartment, but it can involve intracellular fluid as well.
Intracellular Edema
Two conditions are especially prone to cause intracellular swelling:
(1) depression of the metabolic systems of the tissues.
(2) lack of adequate nutrition to the cells.
For example, when blood flow to a tissue is decreased, the delivery of oxygen and nutrients is reduced. If the blood flow becomes too low to maintain normal tissue metabolism, the cell membrane ionic pumps become depressed. When this occurs, sodium ions that normally leak into the interior of the cell can no longer be pumped out of the cells, and the excess sodium ions inside the cells cause osmosis of water into the cells. Sometimes this can increase intracellular volume of a tissue area—even of an entire ischemic leg, for example—to two to three times normal. When this occurs, it is usually a prelude to death of the tissue.
Intracellular edema can also occur in inflamed tissues. Inflammation usually has a direct effect on the cell membranes to increase their permeability, allowing sodium and other ions to diffuse into the interior of the cell, with subsequent osmosis of water into the cells.
Extracellular Edema
Extracellular fluid edema occurs when there is excess fluid accumulation in the extracellular spaces. There are two general causes of extracellular edema:
Abnormal leakage of fluid from the plasma to the interstitial spaces across the capillaries.
(2) Failure of the lymphatic to return fluid from the interstitium back into the blood. The most common clinical cause of interstitial fluid accumulation is excessive capillary fluid filtration.
Fluids in the “Potential Spaces” of the Body
Perhaps the best way to describe a “potential space” is to list some examples: pleural cavity, Pericardial cavity, peritoneal cavity, and synovial cavities, including both the joint cavities and the bursae. Virtually all these potential spaces have surfaces that almost touch each other, with only a thin layer of fluid in between, and the surfaces slide over each other. To facilitate the sliding, a viscous proteinaceous fluid lubricates the surfaces.
Edema Fluid in the Potential Spaces Is Called “Effusion.”When edema occurs in the subcutaneous tissues adjacent to the potential space, edema fluid usually collects in the potential space as well, and this fluid is called effusion. Thus, lymph blockage or any of the multiple abnormalities that can cause excessive capillary filtration can cause effusion in the same way that interstitial edema is caused. The abdominal cavity is especially prone to collect effusion fluid, and in this instance, the effusion is called ascites. In serious cases, 20 liters or more of ascitic fluid can accumulate. The other potential spaces, such as the pleural cavity, pericardial cavity, and joint spaces, can become seriously swollen when there is generalized edema. Also, injury or local infection in any one of the cavities often blocks the lymph drainage, causing isolated swelling in the cavity.
Isosmotic , Hyperosmotic, and Hypo-osmotic Fluids.
The terms isotonic, hypotonic, and hypertonic refer to whether solutions will cause a change in cell volume. The tonicity of solutions depends on the concentration of impermeant solutes. Some solutes, however, can permeate the cell membrane. Solutions with an osmolarity the same as the cell are called iso-osmotic, regardless of whether the solute can penetrate the cell membrane. The terms hyperosmotic and hypo-osmotic refer to solutions that have a higher or lower osmolarity, respectively, compared with the normal extracellular
fluid, without regard for whether the solute permeates the cell membrane. Highly permeating substances, such as urea, can cause transient shifts in fluid volume between the intracellular and extracellular fluids, but given enough time, the concentrations of these substances eventually become equal in the two compartments and have little effect on intracellular volume under steady-state conditions.
Effect of Adding Saline Solution to the Extracellular Fluid
If an isotonic saline solution is added to the extracellular fluid compartment, the osmolarity of the extracellular fluid does not change; therefore, no osmosis
occurs through the cell membranes. The only effect is an increase in extracellular fluid volume (Figure 25–6A).The sodium and chloride largely remain in the extracellular fluid because the cell membrane behaves as though it were virtually impermeable to the sodium chloride. If a hypertonic solution is added to the extracellular fluid, the extracellular osmolarity increases and causes osmosis of water out of the cells into the extracellular compartment (see Figure 25–6B).Again, almost all the added sodium chloride remains in the extracellular compartment, and fluid diffuses from the cells into the extracellular space to achieve osmotic equilibrium. The net effect is an increase in extracellular volume (greater than the volume of fluid added), a decrease in
intracellular volume, and a rise in osmolarity in both compartments.
If a hypotonic solution is added to the extracellular fluid, the osmolarity of the extracellular fluid decreases and some of the extracellular water diffuses into the cells until the intracellular and extracellular compartments have the same osmolarity (see Figure 25–6C). Both the intracellular and the extracellular volumes are increased by the addition of hypotonic fluid, although the intracellular volume increases to a greater extent.
Glucose and Other Solutions Administered for Nutritive Purposes
Many types of solutions are administered intravenously to provide nutrition to people who cannot otherwise take adequate amounts of nutrition. Glucose solutions are widely used, and amino acid and homogenized fat solutions are used to a lesser extent. When these solutions are administered, their concentrations of osmotically active substances are usually adjusted nearly to
isotonicity, or they are given slowly enough that they do not upset the osmotic equilibrium of the body fluids. After the glucose or other nutrients are metabolized, an excess of water often remains, especially if additional
fluid is ingested. Ordinarily, the kidneys excrete this in the form of a very dilute urine. The net result, therefore, is the addition of only nutrients to the body.
Fig: Chemical compositions of extracellular and intracellular fluids
The extracellular fluid contains a large amount of sodium but only a small amount of potassium. Exactly the opposite is true of the intracellular fluid. Also, the extracellular fluid contains a large amountof chloride ions, whereas the intracellular fluid contains very little. But the concentrations of phosphates and proteins in the intracellular fluid are considerably greater than those in the extracellular fluid.These differences are extremely important to the life of the cell.
Extracellular Fluid—The “Internal Environment”
About 60 per cent of the adult human body is fluid, mainly a water solution of ions and other substances. Although most of this fluid is inside the cells and is called intracellular fluid, about one third is in the spaces outside the cells and is called extracellular fluid. This extracellular fluid is in constant motion throughout the body. It is transported rapidly in the circulating blood and then mixed between the blood and the tissue fluids by diffusion through the capillary walls.
In the extracellular fluid are the ions and nutrients needed by the cells to maintain cell life. Thus, all cells live in essentially the same environment—the extracellular fluid. For this reason, the extracellular fluid is also called the internal environment of the body. Cells are capable of living, growing, and performing their special functions as long as the proper concentrations of oxygen, glucose, different ions, amino acids, fatty substances, and other constituents are available in this internal environment.
Differences Between Extracellular and Intracellular Fluids.
The extracellular fluid contains large amounts of sodium, chloride, and bicarbonate ions plus nutrients for the cells, such as oxygen, glucose, fatty acids, and amino acids. It also contains carbon dioxide that is being transported from the cells to the lungs to be excreted, plus other cellular waste products that are being transported to the kidneys for excretion.
The intracellular fluid differs significantly from the extracellular fluid; specifically, it contains large amounts of potassium, magnesium, and phosphate ions.