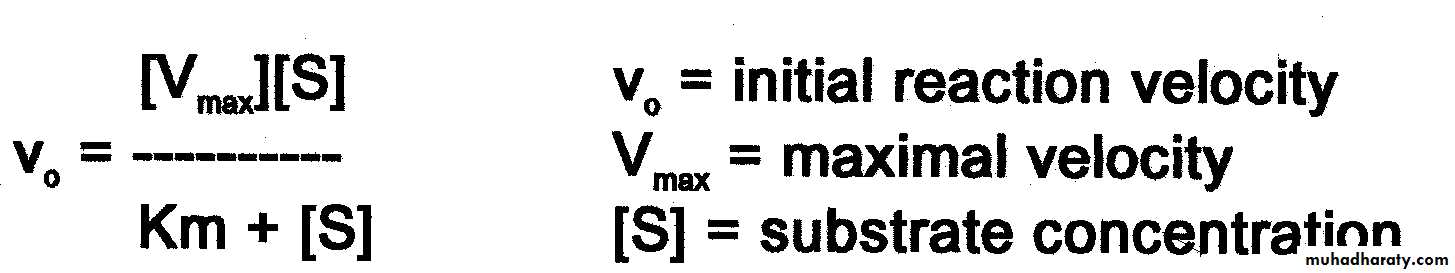د.جنان كيمياء حياتية2 lec: 1
عدد الاوراق( 7 ) م\2\موصل 18\10\2017Enzymes
DefinitionNomenclature
Classification of enzymes
Factors affecting enzyme activity.
Application of enzyme inhibition.
Isoenzymes.
Enzyme in the Diagnosis of Pathology
Definition
Enzyme : It is a protein, catalyst, synthesized in all living cells that regulate a biochemical reaction without being changed.Characteristics
They are high catalytic rate.They catalyze reaction without being changed.
They are very specific .
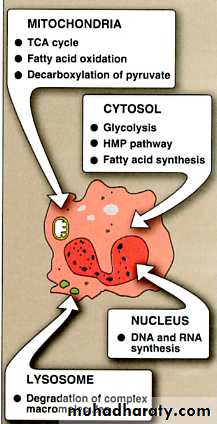
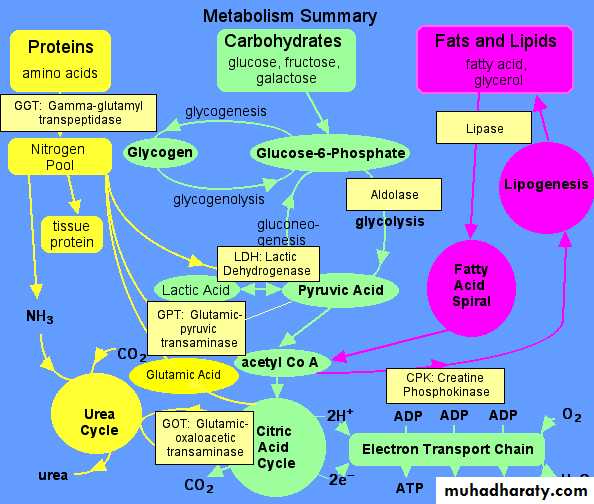
Enzyme distribution
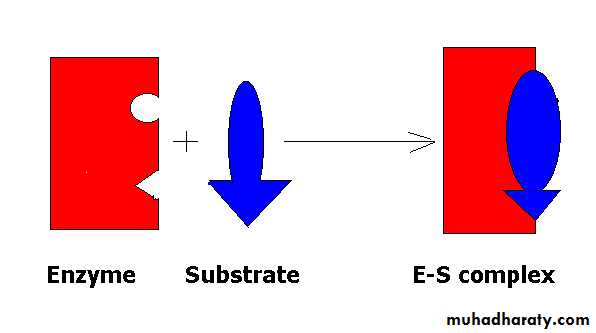
Substrate
The molecule being utilized and/ormodified by a particular enzyme at its active site
Cofactor
Definition: A non-protein unit ,its presence is important in many enzymes.Types:
1-Inorganic metals: Mn ,Zn ,Fe ,Cu.
2-Organic Complex ( Coenzyme ) .
Cofactors
Metal-activated enzymes:- active in the presence of metal ions as K+,Mg+or Ca++.
- Example: Kinase uses Mg++-ATP.
Metalloenzyme:
- Firmly bound metal ion in the active site as Iron , copper,Zn & Co.
- Examples:1-Carbonic AnhydraseZn.
2- Cytochrome oxidaseFe2+.
COENZYMES
Many enzymes require for their action on substrate,
specific ,heat stable ,low M. Wt . and organic substance called .coenzymes
Enzyme which requires a coenzyme for its catalytic action
is called apoenzyme and complete catalytic unit which
contain enzyme and its coenzyme is called holoenzyme.
Coenzyme it self may covalantly or non covalantly bound to enzyme and when coe. is covalent linked to its enzyme it will be then called PROSTHETIC GROUP.
. Majority of enzyme in the body required coe. in their action
Apoenzyme + Cofactor
Holoenzyme
Apoenzyme: inactive protein part.
Cofactor: Non protein part.
Holoenzyme: Active enzyme .
(Nomenclature)
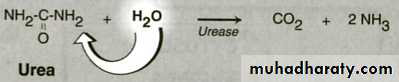
Unsystematic nomenclature:1- Enzyme is named by adding (ase) to the name of the substrate e.g. (Urease ).
2-Some other enzymes as (Trypsin ,pepsin ) are known by their historic names.
one enzyme has > one name or
many enzymes have the same name.
Systematic Nomenclature
Adopted by (IUB).According to the type of reaction which is catalyzed.
It divided the enzymes into 6 classes.
Classification of enzymes
Class no I OxidoredoctaseClass no II Transferase
Class no III Hydrolases
Class no IV Lyases
Class no V Isomerases cis and Trans
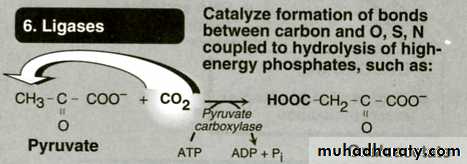
Class no VI Ligases
Class 1: Oxido-Reductase:
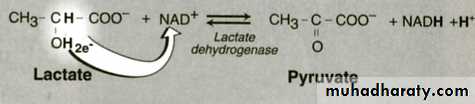
Catalyses Oxidation ,reduction reactions as:Dehydrogenase ,Oxidase ,Hydroxylase ,Peroxidase.
Usually they require coenzymes as : (NAD+,NADP+,FAD,FMN ).
Class 2:Transferase
Catalyze transfer of functional group between donor & acceptor molecule as methyl , formyl ,carboxyl ,nitrogenous, phosphorus & sulfur containing groups.Class 3:Hydrolases
Catalyze hydrolytic reaction by adding H2Ocleavage of bond between C & others as : C-O , C-N & C-S.Class 4 :Lyases
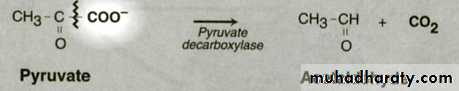
Catalyze non-hydrolytic reactionExamples: Decarboxylase ,.
Class 5 :Isomerase
Catalyze transfer of groups within a molecule (rearrange).Class 6:Ligase
Catalyze bond formation coupled to ATP-hydrolysis joining 2 molecules.Enzyme Specificity
The most significant properties in the enzyme catalytic reaction is the ability of the enzyme in catalyze one specific reaction and no other that is a characteristic of enzyme and when these enzyme is absent the respective reaction will not occur and this behavior is called specificity of enzyme and this behavior is usually appear in the following TWO properties.I-optical specificity
The enzyme has an absolute specificity in particular optical region of the substrate. Almost all human enzyme are being specific for an optical part of substrate . ex: enzyme acting on CHO. Metabolism (sugar breakdown )are usually specific for D-sugar not act on L-sugar or other enzyme acting on amino acid metabolism are usually acting on L- amino acid (not D-amino acid ) with exception of D- amino acid oxidase in the kidney .II- Selective group:
In this properties enzyme is usually affective on specific chemical group that is present in the structure of substrate. ex:glycosidase,glycosidase catalyze hydrolysiso f glycosidic bond between sugar and alcohol are highly specific for sugar portion not specific for alcohol.
Trypsin and pepsin act on peptide bond.
Some enzymes have a higher degree of specificity ex: amino peptidase act on amino group , carboxypeptidase act on carboxy end of peptide bond .
Chymotrypsin will act on peptide bond on which carboxy terminal end of peptide bond is being contributed to an aromatic a.a. Which may be phenyl alanen , tyrosine and tryptophane split of a.a one at atime from the carboxy or amino terminal end of polypeptide chain respectively.
Tyrosine
Tyrosine
CH NH2COOH
CH NH2COOH
CH2
CH2
-HO
-HO
Enzyme velocity (V)
It is moles of product (P) appearing or substrate (S) disappearing per unit of time.Mole / liter /sec.
Enzyme units
International unit:a mount of enzyme that converts one micromole (μm) of substrate per minute at 25 Co under the optimal conditions of the measurement.
Katal: amount of enzyme that converts one mole of substrate to product/sec.
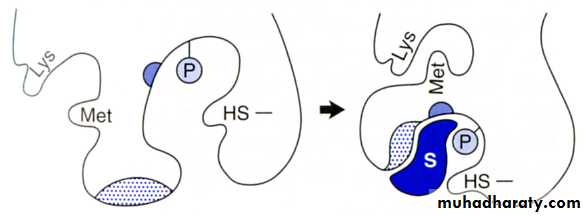
Catalytic Site:
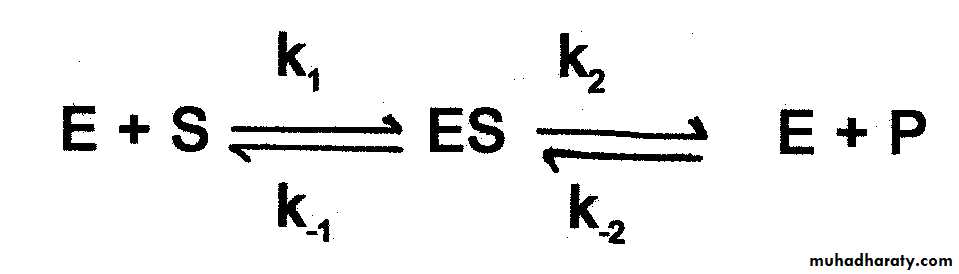
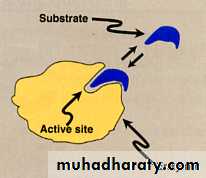
The large size of the enzyme molecule in comparison with substrate size that a small part or limited number of amino acids in the enzyme molecule is being responsible for the catalytic reaction these size is called CATALYTIC SITE or ACTIVE SITE or ACTIVE CENTER of the enzyme. There are two theory or mode or type to explain the interaction between the substrate and enzyme.(Active site)
Active site: is an important structural feature to recognize and to bind substrates.It is very specific.
Type I
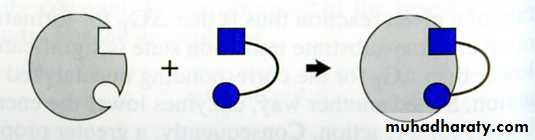
The lock & key (Fisher) model:- Enzyme fits substrate as a lock & key .
- Its rigid type.
Type II
Induced fit (Koshland model ):-the substrate induces conformational changes in the active site rearrangement of the A.A Enzyme fits substrate exactly.
This type discovered by Koshland in which there is a source of flexibility in substrate – enzyme binding in which certain physical changes take place in the enzyme that include arrangement of certain a.as both to the substrate binding site and at catalytic site. These changes are called (conformational changes) and the site in which these changes take place are called Allosteric site being important for the enzyme catalytic reaction. This type is more flexible than the lock and key type and it has wide application in explaining the mechanism of the majority of enzyme – catalytic reaction.
Catalytic efficiency
Most enzyme-catalyzed reactions are highly efficient, proceeding from 103 to 108 times faster than uncatalyzed reactions.Factors affecting Enz. Activity
1.Enzyme concentration.2.Temperature.
3.PH
4.Substrate concentration.
5. Inhibitars
6. Activators
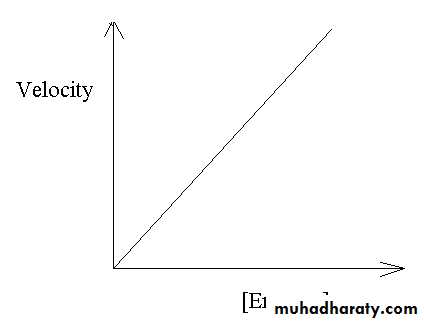
Enzyme concentration:
The rate of the reaction is directly proportional to [ enzyme ].
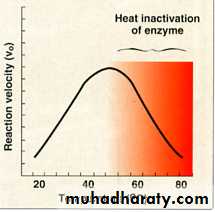
Temperature
The rate of the reaction increases with the temperature increasing until reaching the ( Maximal velocity ) at the (Optimal temperature).Increasing of the temperature after the optimal temperature decreasing in the reaction velocity.
The velocity decreases due to (enzyme denaturation ).
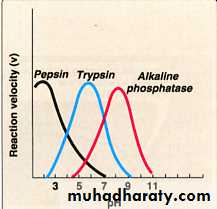
Effect of PH
Each enzyme has its own (Optimal PH ).Any change in the PH decreasing in the reaction velocity due to change in the ionization of the active site A.A.
This ionization inactivation of the active site decrease in enzyme activity.
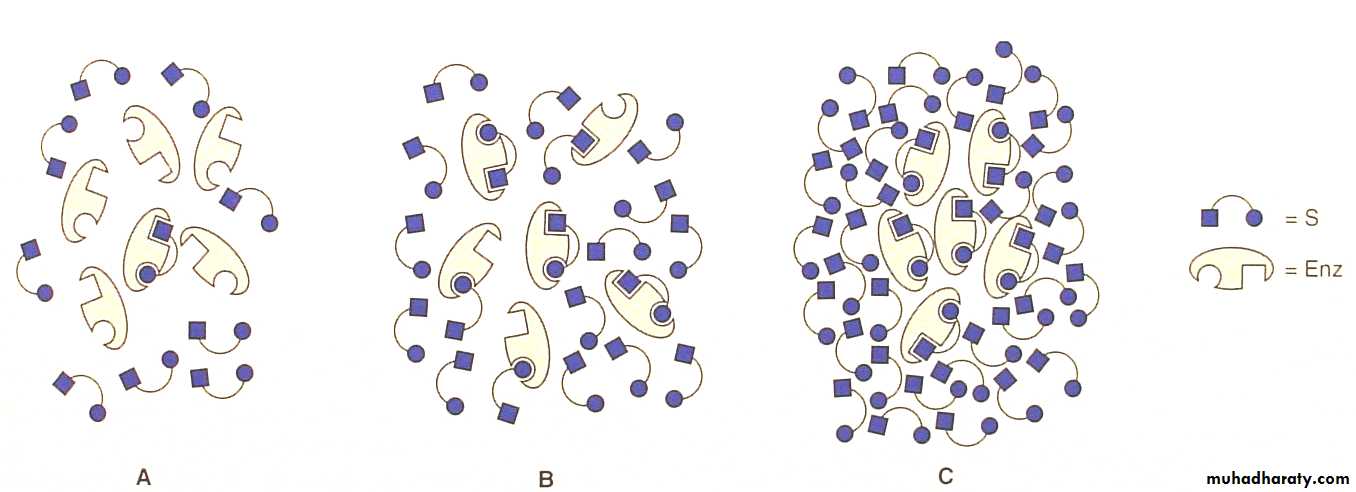
Substrate concentration
Rate of the enzyme increases rapidly constant.1-low [S] active sites are not saturatedrapid reaction .
2-High [S] Saturated active sites slow reaction.
Substrate concentration
The rate or velocity of a reaction (v) is the number of substrate molecules converted to product per unit time and is usually expressed as μmoles product formed per minute.The rate of an enzyme-catalyzed reaction increases with substrate concentration until a maximal velocity (Vmax) is reached.
. Low [S] B. 50% [S] or Km C. High, saturating [S]
AKm
The Michaelis menten constant .
The quantitative relationship between substrate concentration and Vmax. For different enzymes, it is defined as that substrate conc. at which a given enzyme give one – half it maximum velocity . In many cases the Km is an inverse measure of the affinity of the enzyme for its substrate : the lower the Km the higher the affinity .
Vmax [S]
V0= ----------------
Km+[ S]
:Characteristics of Km
The Michaelis constant is characteristic of an enzymeand a particular substrate, and reflects the affinity of
the enzyme for that substrate.
Km does not vary with the concentration of enzyme.
A numerically small (low) Km reflects a high affinity of
the enzyme for substrate because a low
concentration of substrate is needed to half-saturate
the enzyme.
Large Km:
A numerically large (high) Km reflects a low affinity of enzyme for substrate because a high concentration of, substrate is needed to half-saturate the enzyme.The rate of the reaction is directly proportional to the enzyme concentration at all substrate concentrations.
When [S] is much less than Km, the velocity of the reaction is proportional to the substrate concentration.