
Study Designs in Epidemiologic
Research
Thomas Songer, PhD
Basic Epidemiology
South Asian Cardiovascular
Research Methodology Workshop

Types of primary studies
• Descriptive studies
– describe occurrence of outcome
• Analytic studies
– describe
association
between
exposure and outcome
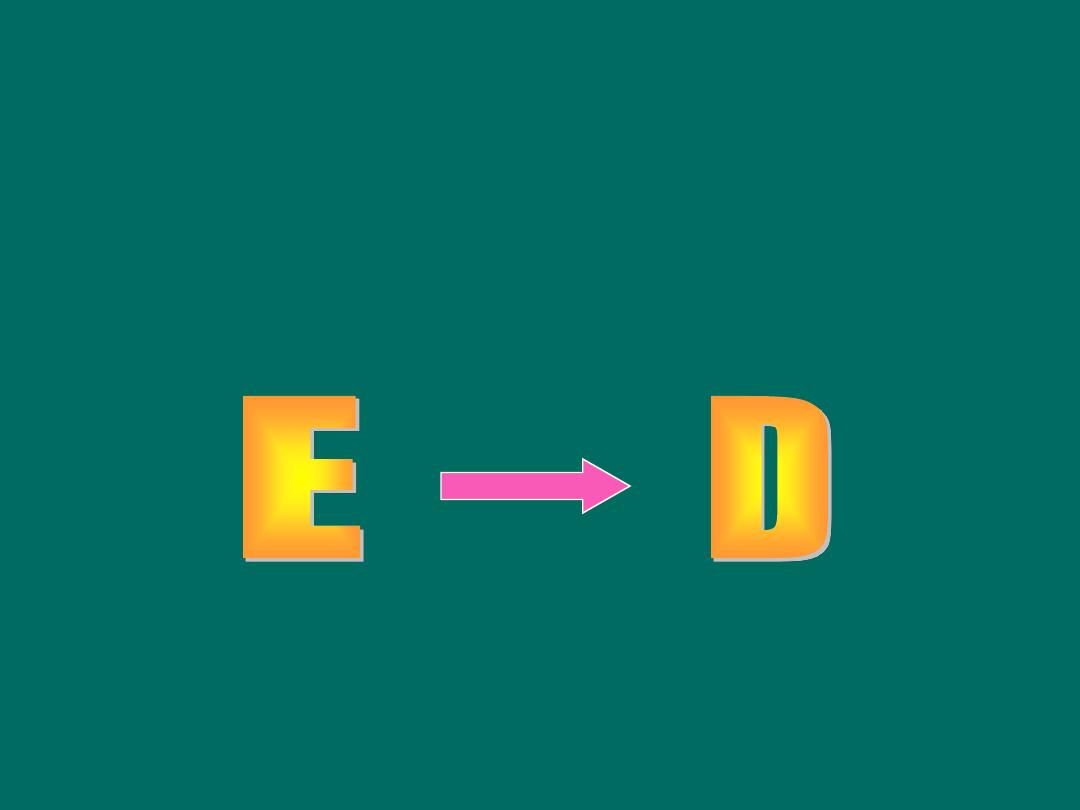
Basic Question in Analytic Epidemiology
• Are exposure and disease linked?
Exposure
Disease

Basic Questions in Analytic Epidemiology
• Look to link exposure and disease
–What is the exposure?
–Who are the exposed?
–What are the potential health effects?
–What approach will you take to study
the relationship between exposure and
effect?
Wijngaarden

Basic Research Study
Designs and their
Application to Epidemiology

Big Picture
• To prevent and control disease
• In a coordinated plan, look to
–identify hypotheses on what is related
to disease and may be causing it
–formally test these hypotheses
• Study designs direct how the
investigation is conducted

What designs exist to
identify and investigate
factors in disease?
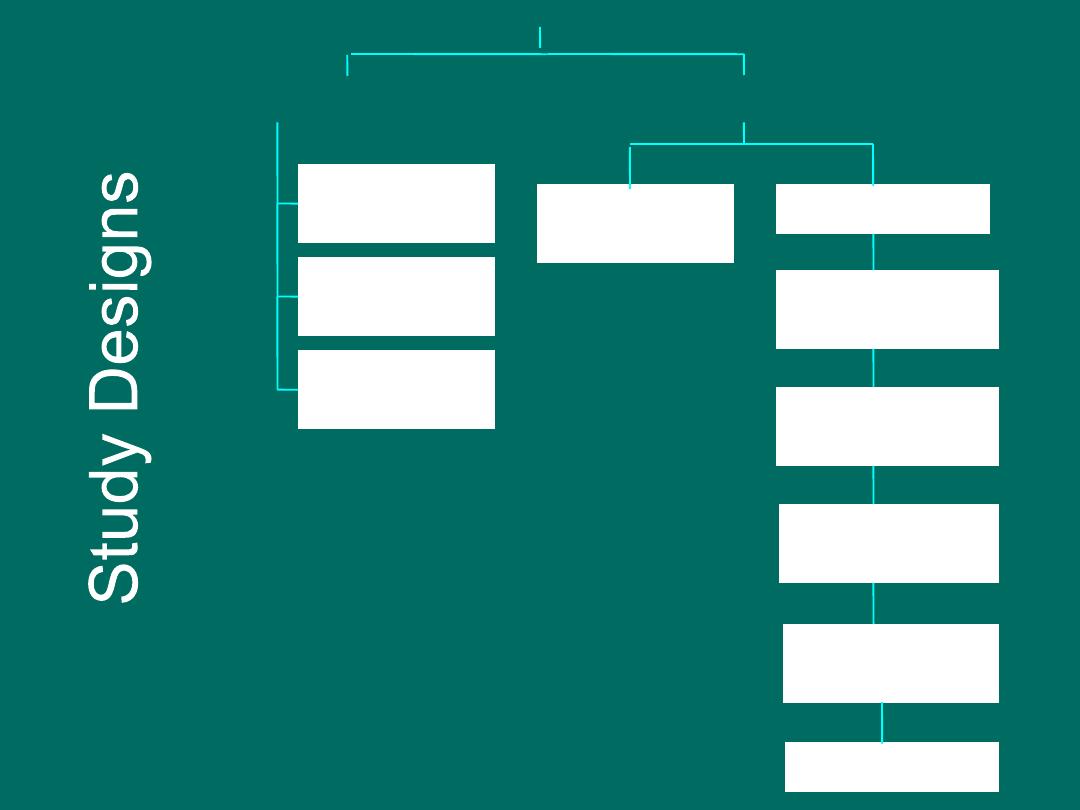
Case report
Case series
Descriptive
Epidemiology
Descriptive
RCT
Before-After
study
Cross-sectional
study
Case-Crossover
study
Case-Control
study
Cohort study
Analytic
Ecologic study
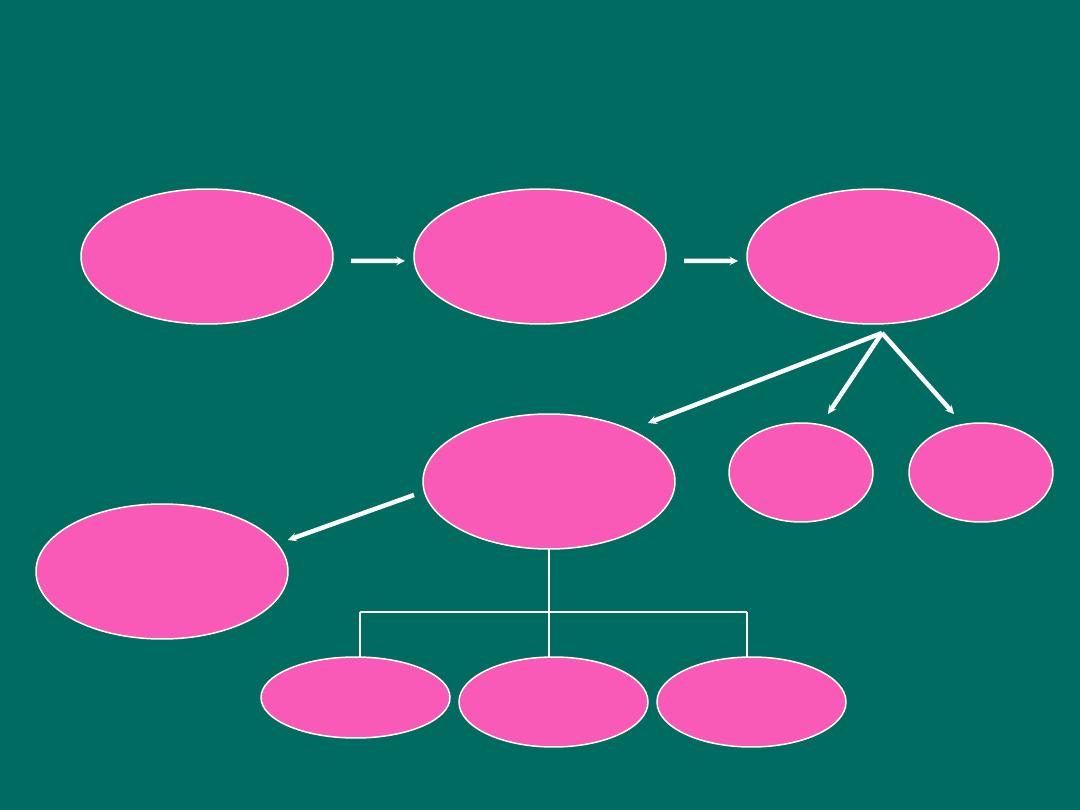
Study Design Sequence
Case reports
Case series
Descriptive
epidemiology
Analytic
epidemiology
Clinical
trials
Animal
study
Lab
study
Cohort
Case-
control
Cross-
sectional
Hypothesis formation
Hypothesis testing
Descriptive Studies
Case-control Studies
Cohort Studies
Develop
hypothesis
Investigate it’s
relationship to
outcomes
Define it’s meaning
with exposures
Clinical trials
Test link
experimentally
Incr
ea
sing
Know
ledge
of
Disea
se/Exposur
e

Descriptive Studies

Case Reports
• Detailed presentation of a single case or
handful of cases
• Generally report a new or unique finding
• e.g. previous undescribed disease
• e.g. unexpected link between diseases
• e.g. unexpected new therapeutic effect
• e.g. adverse events

Case Series
• Experience of a group of patients with a
similar diagnosis
• Assesses prevalent disease
• Cases may be identified from a single or
multiple sources
• Generally report on new/unique
condition
• May be only realistic design for rare
disorders

Case Series
• Advantages
• Useful for hypothesis generation
• Informative for very rare disease with few
established risk factors
• Characterizes averages for disorder
• Disadvantages
• Cannot study cause and effect
relationships
• Cannot assess disease frequency
Case Report
Case Series
Descriptive
Epidemiology Study
One case of unusual
injury finding
Multiple cases of
injury finding
Population-based
cases with denominator

Analytical Studies

Study Designs -
Analytic Epidemiology
• Experimental Studies
– Randomized controlled clinical trials
– Community trials
• Observational Studies
– Group data
• Ecologic
– Individual data
• Cross-sectional
• Cohort
• Case-control
• Case-crossover
Cross-sectional studies
• An “observational” design that surveys
exposures and disease status at a single point
in time (a cross-section of the population)
time
Study only exists at this point in time
Cross-sectional Design
time
Study only exists at this point in time
Study
population
No Disease
Disease
factor present
factor absent
factor present
factor absent

Cross-sectional Studies
• Often used to study conditions that are
relatively frequent with long duration of
expression (nonfatal, chronic conditions)
• It measures prevalence, not incidence of
disease
• Example: community surveys
• Not suitable for studying rare or highly fatal
diseases or a disease with short duration of
expression

Cross-sectional studies
• Disadvantages
• Weakest observational design,
(it measures prevalence, not incidence of
disease). Prevalent cases are survivors
• The temporal sequence of exposure and
effect may be difficult or impossible to
determine
• Usually don’t know when disease occurred
• Rare events a problem. Quickly emerging
diseases a problem

Epidemiologic Study Designs
• Case-Control Studies
–an “observational” design comparing
exposures in disease cases vs. healthy
controls from same population
–exposure data collected
retrospectively
–most feasible design where disease
outcomes are rare
Case-Control Studies
Cases: Disease
Controls: No disease
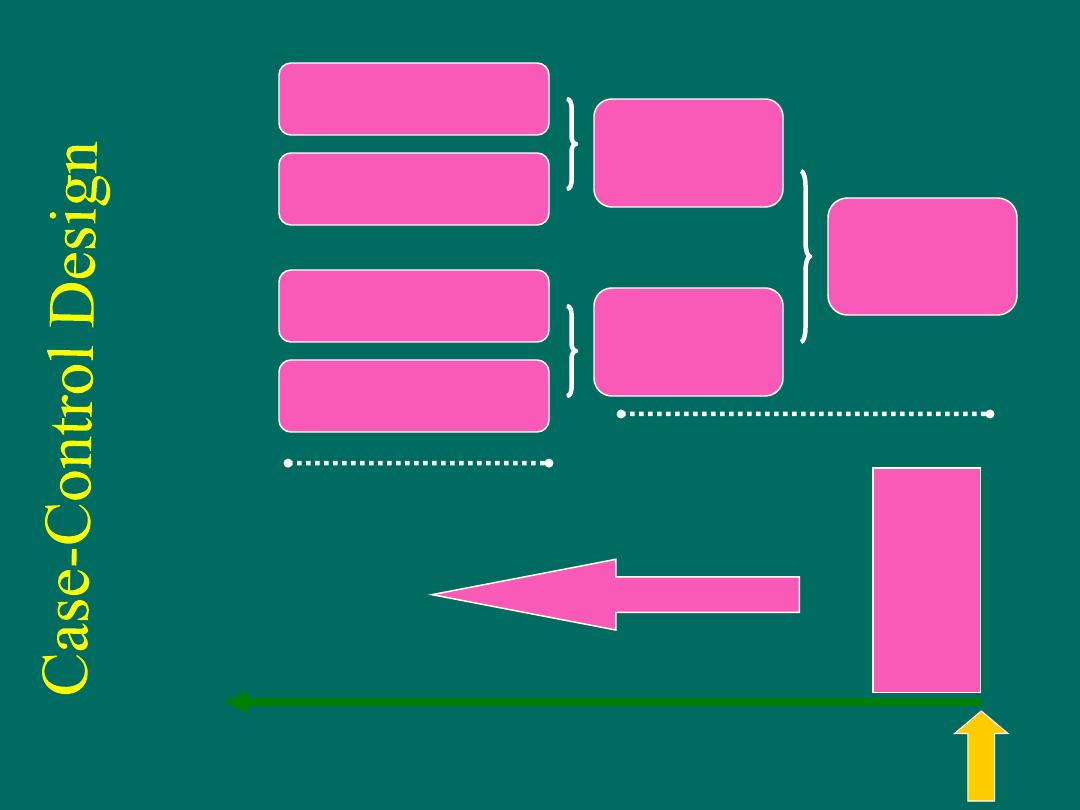
Study
population
Cases
(disease)
Controls
(no disease)
factor present
factor absent
factor present
factor absent
present
past
time
Study begins here

Case-Control Study
• Strengths
– Less expensive and time consuming
– Efficient for studying rare diseases
• Limitations
– Inappropriate when disease outcome for a specific
exposure is not known at start of study
– Exposure measurements taken after disease
occurrence
– Disease status can influence selection of subjects

Case-Crossover
• Each participant is a case acting as their own
control
– Accounts for effect of potential confounders (e.g.
matches on age, sex, genetic susceptibility)
• Exposure status immediately before
event/outcome compared with exposure
status @ some time prior to event
• Acute exposures and outcomes (e.g. anger &
MI; driving while using cell phone & injury)
• Recall of prior exposures
Hypothesis Testing: Case-Crossover Studies
• Study of “triggers” within an individual
• ”Case" and "control" component, but
information of both components will come
from the same individual
• ”Case component" = hazard period which is
the time period right before the disease or
event onset
• ”Control component" = control period which
is a specified time interval other than the
hazard period

Epidemiologic Study Designs
• Cohort Studies
– an “observational” design comparing
individuals with a known risk factor or
exposure with others without the risk
factor or exposure
– looking for a difference in the risk
(incidence) of a disease over time
– best observational design
– data usually collected prospectively (some
retrospective)
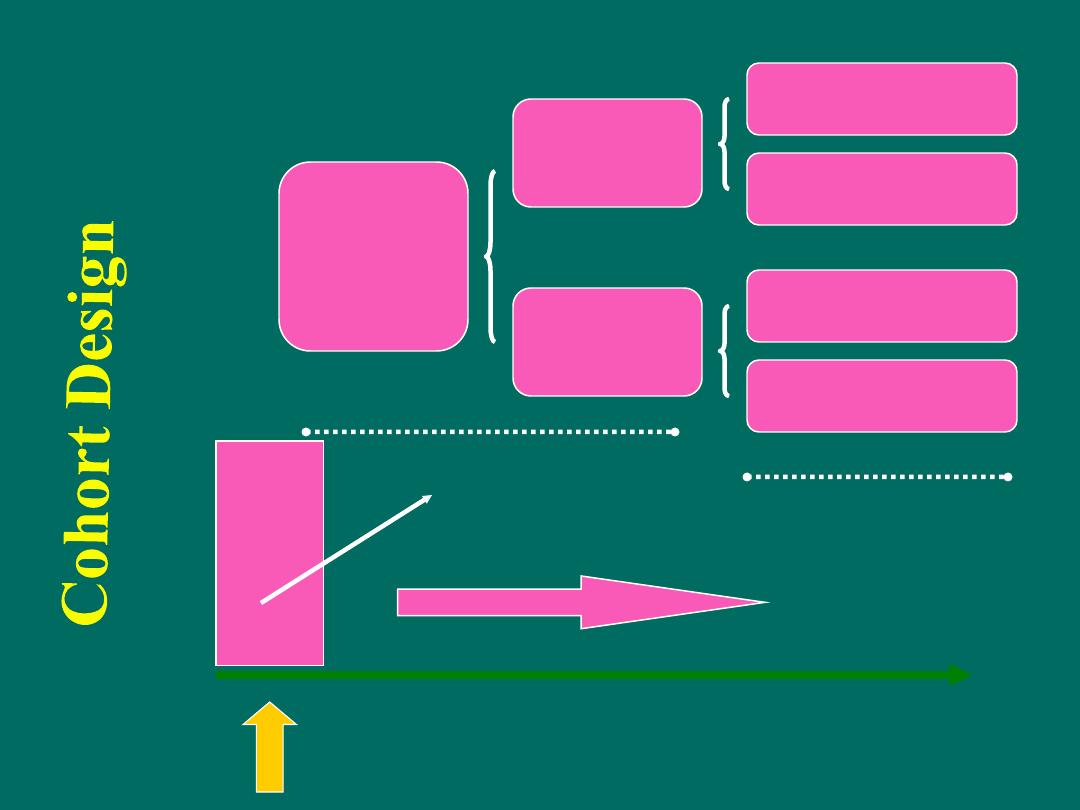
time
Study begins here
Study
population
free of
disease
Factor
present
Factor
absent
disease
no disease
disease
no disease
present
future
Timeframe of Studies
• Prospective Study
- looks forward,
looks to the future, examines future
events, follows a condition, concern or
disease into the future
time
Study begins here
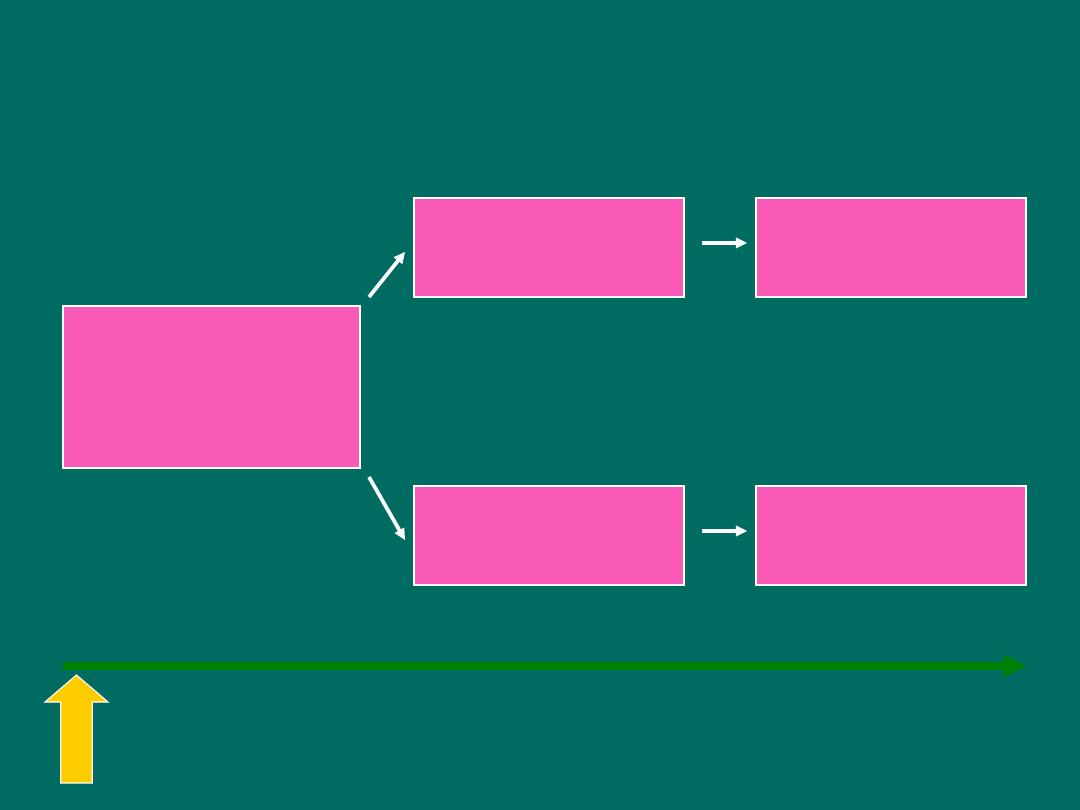
Prospective Cohort study
Measure exposure
and confounder
variables
Exposed
Non-exposed
Outcome
Outcome
Baseline
time
Study begins here
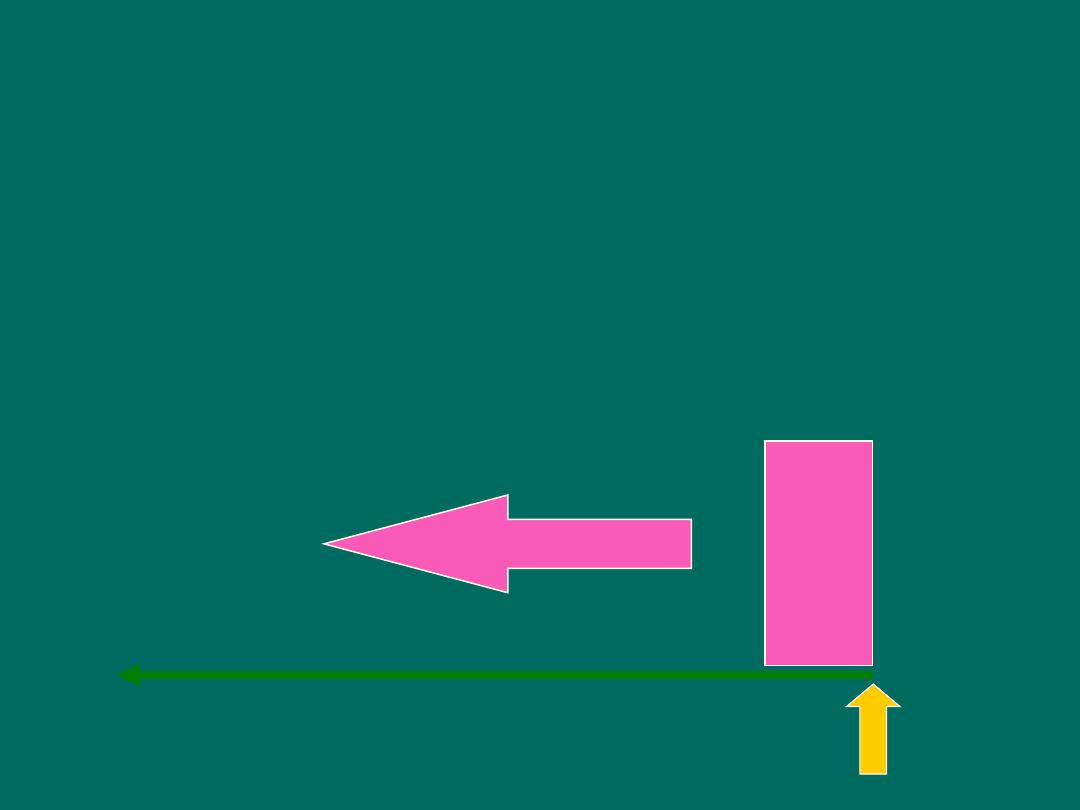
Timeframe of Studies
• Retrospective Study
-
“to look back”,
looks back in time to study events that
have already occurred
time
Study begins here
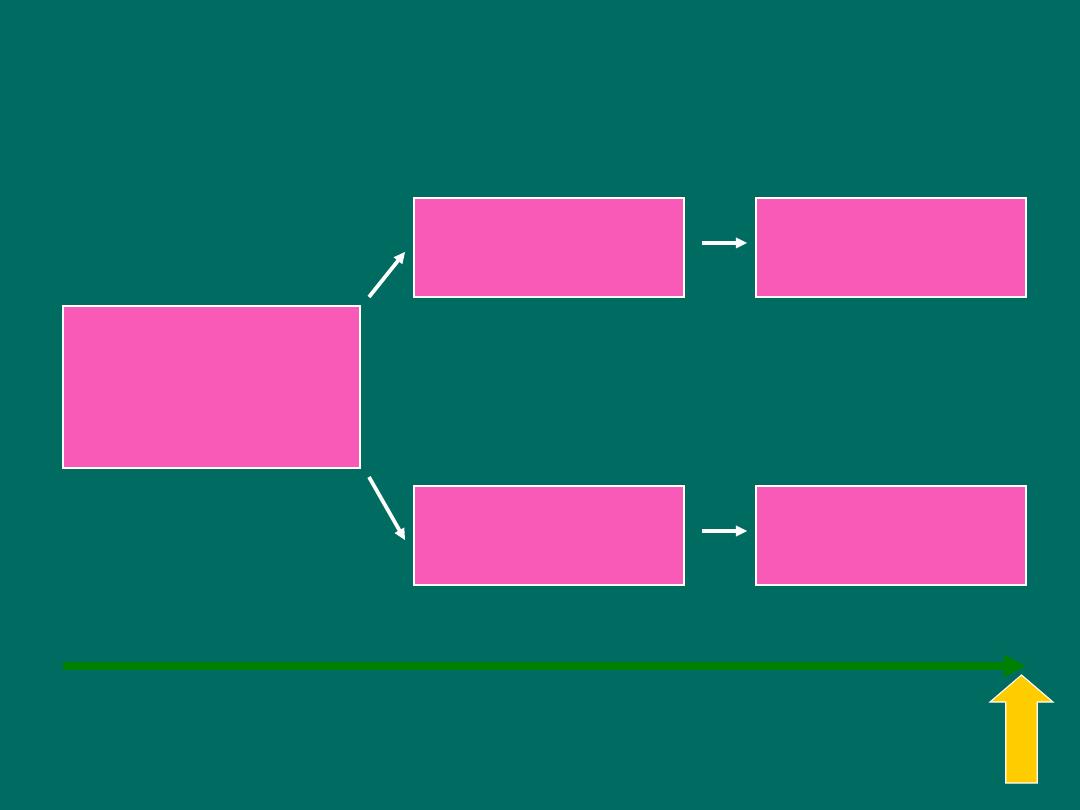
Retrospective Cohort study
Measure exposure
and confounder
variables
Exposed
Non-exposed
Outcome
Outcome
Baseline
time
Study begins here

Cohort Study
• Strengths
– Exposure status determined before disease
detection
– Subjects selected before disease detection
– Can study several outcomes for each exposure
• Limitations
– Expensive and time-consuming
– Inefficient for rare diseases or diseases with
long latency
– Loss to follow-up
