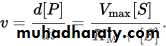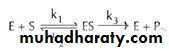محاضرات المرحلة الثانية كلية طب الاسنان للسنة الدراسية 2017- 2018
الدكتورة: نوال عبدالله مرتضى
Chemical Nature of Enzymes
All enzymes are protein in nature except ribozymes (RNA in nature) that cause reactions to happen in the body without raising the temperature. They are considered organic catalysts. Since they are proteins, their are made of chains of amino acids which in turn are controlled by sequences of DNA called genes located on chromosomes. Each enzyme is specific for one reaction. Note: Ribozymes (ribonucleic acid enzymes): are RNA molecules that are capable of catalyzing specific biochemical reactions, similar to the action of protein enzymes.Enzymes are biocatalysts• Catalysts are substances which accelerate the rate of chemical reactions, but do not change the equilibrium.• Lack of enzymes will lead to block in metabolic pathways causing inborn errors of metabolism.• The substance upon which an enzyme acts, is called the substrate.• The enzyme will convert the substrate into the product or products.
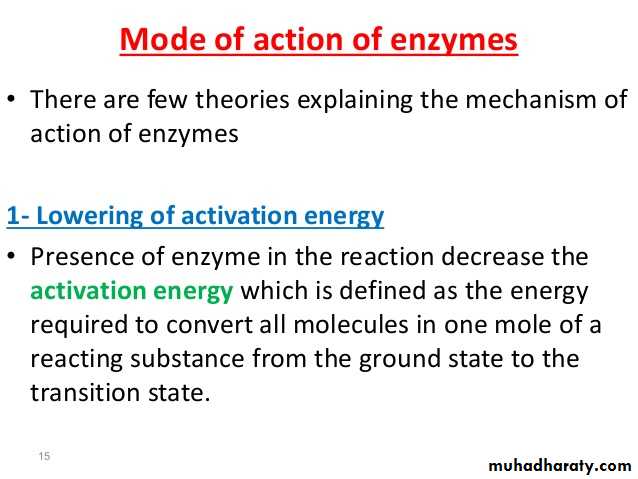
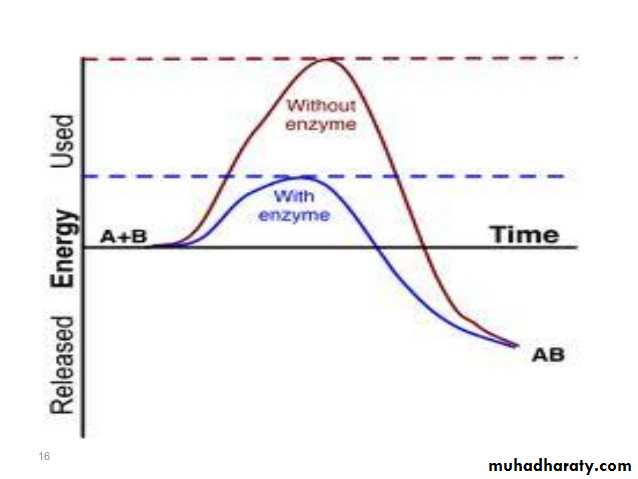
Activation energy may also be defined as the minimum energy required to start a chemical reaction. The activation energy of a reaction is usually denoted by Ea and given in units of kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the height of the potential barrier (sometimes called the energy barrier) separating two minima of potential energy (of the reactants and products of a reaction). For a chemical reaction to proceed at a reasonable rate, there should exist an appreciable number of molecules with translational energy equal to or greater than the activation energy.
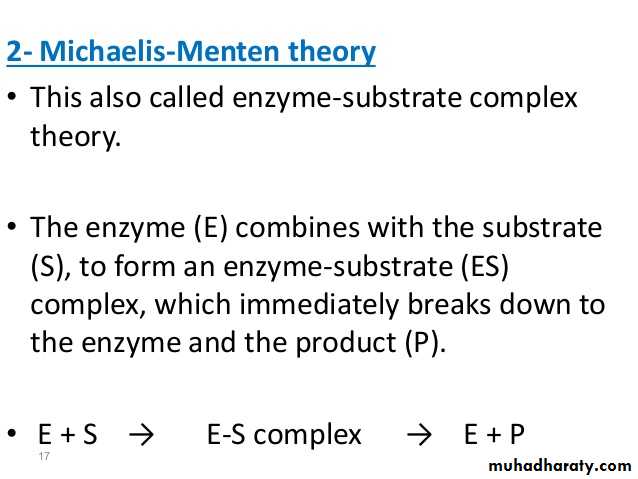
2- Michaelis – Menten theory:
Michaelis – Menten kinetics is one of the best-known models of enzyme kinetics.The model takes the form of an equation describing the rate of enzymatic reactions, by relating reaction rate v to [S], the concentration of a substrate S. Its formula is given by:
Enzyme kinetics and Km value:
The enzyme (E) and substrate (S) combine with each other to form an unstable enzyme-substrate complex (ES) for the formation of product (P).Here k1, k2 and k3 represent the velocity constants for the respective reactions, as indicated by arrows. Km, the Michaelis - Menten constant (or Brig’s and Haldane’s constant), is given by the formula:
Km or the Michaelis - Menten constant:
is defined as the substrate concentration (expressed in moles / lit) to produce half - maximum velocity in an enzyme catalyzed reaction. It indicates that half of the enzyme molecules (i.e. 50%) are bound with the substrate molecules when the substrate concentration equals the Km value. Km value is a constant and a characteristic feature of a given enzyme. It is a representative for measuring the strength of ES complex. A low Km value indicates a strong affinity between enzyme and substrate, whereas a high Km value reflects a weak affinity between them.Michaelis – Menten kinetics:
This equation is called the Michaelis – Menten equation. Here, Vmax represents the maximum rate achieved by the system, at saturating substrate concentration. The Michaelis constant KM is the substrate concentration at which the reaction rate is half of Vmax. Biochemical reactions involving a single substrate are often assumed to follow Michaelis – Menten kinetics, without regard to the model's underlying assumptions.
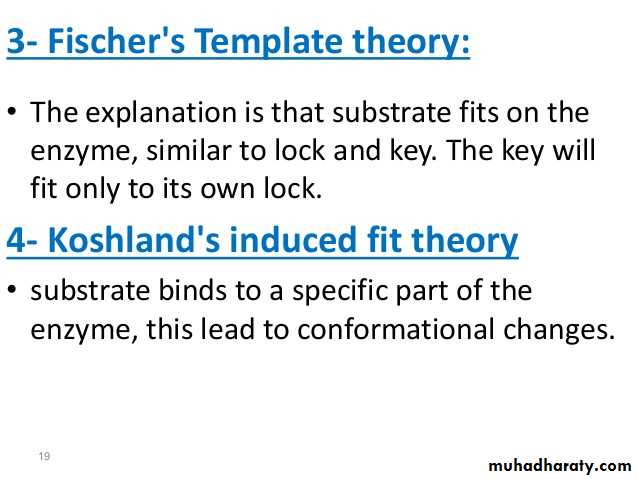
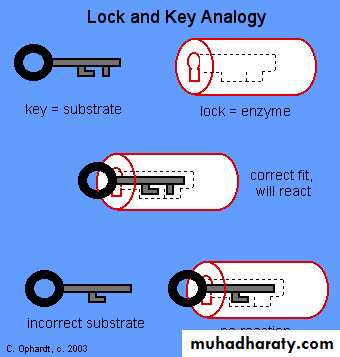
Lock and key" model 3-
To explain the observed specificity of enzymes, in 1894 Emil Fischer proposed that both the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. This is often referred to as "the lock and key" model. This early model explains enzyme specificity, but fails to explain the stabilization of the transition state that enzymes achieve.4- Induced fit model
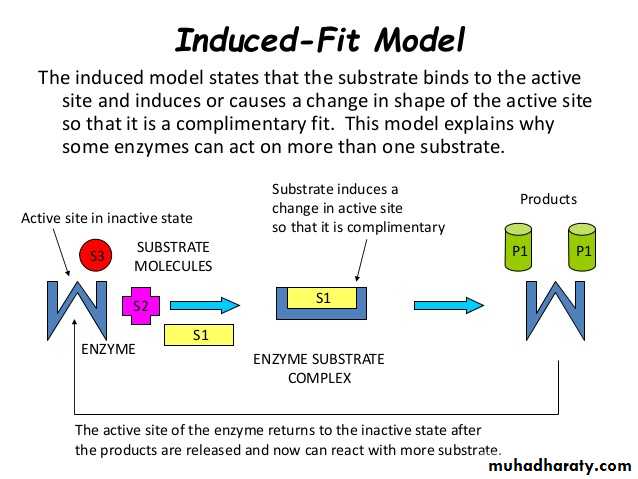
The Induced fit model describes the formation of the E-S as a result of the interaction between the substrate and a flexible active site. The substrate produces changes in the conformation on the enzyme, aligning properly the groups in the enzyme. It allows better binding and catalytic effects.Enzyme Classifications
Traditionally, enzymes were simply assigned names by the investigator who discovered the enzyme. As knowledge expanded, systems of enzyme classification became more comprehensive and complex. Currently enzymes are grouped into six functional classes by the International Union of Biochemists (I.U.B).
Biochemical Properties
Classification
Number
Act on many chemical groupings to add or remove hydrogen atoms.
Oxidoreductases
1
Transfer functional groups between donor and acceptor molecules. Kinases are specialized transferases that regulate metabolism by transferring phosphate from ATP to other molecules.
Transferases
2
Add water across a bond, hydrolyzing it.
Hydrolases
3
Add water, ammonia or carbon dioxide across double bonds, or remove these elements to produce double bonds.
Lyases
4
Carry out many kinds of isomerization: L to D isomerizations, mutase reactions (shifts of chemical groups) and others.
Isomerases
5
Catalyze reactions in which two chemical groups are joined (or ligated) with the use of energy from ATP.
Ligases
6
Specificity:
Specificity of Enzymes: One of the properties of enzymes that makes them so important as diagnostic and research tools is the specificity they exhibit relative to the reactions they catalyze. A few enzymes exhibit absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group. In general, there are four distinct types of specificity:
1- Absolute specificity - the enzyme will catalyze only one reaction. For example, lactase is an enzyme specific for the degradation of lactose into two sugar monosaccharides, glucose and galactose. Another example is Glucokinase, which is an enzyme involved in the phosphorylation of glucose to glucose-6-phosphate.
2- Group specificity - the enzyme will act only on molecules that have specific functional groups, such as amino, phosphate and methyl groups. One example is Pepsin, an enzyme that is crucial in digestion of foods ingested in our diet, that hydrolyzes peptide bonds in between hydrophobic amino acids, with recognition for aromatic side chains such as phenylalanine, tryptophan, and tyrosine.
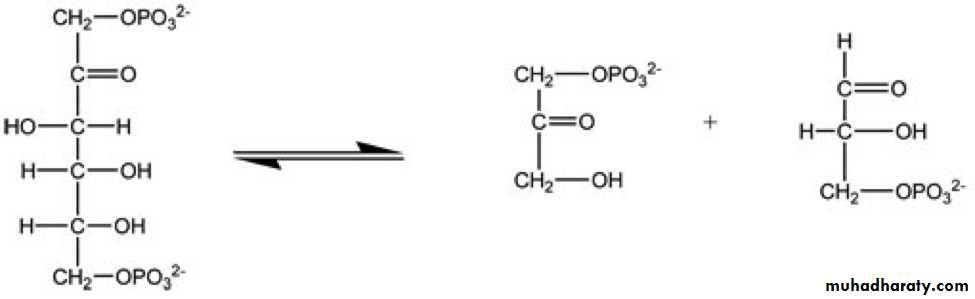
3- Linkage specificity - the enzyme will act on a particular type of chemical bond regardless of the rest of the molecular structure. Bond specificity, unlike group specificity, recognizes particular chemical bond types. Figure 1 is a reaction that illustrates an enzyme cleaving a specific bond of the reactant in order to create two products.
Figure 1
4- Stereochemical specificity - the enzyme will act on a particular steric or optical isomer. For example, beta-glycosidase will only react with beta-glycosidic bonds which are present in cellulose, but not present in starch and glycogen, which contain alpha-glycosidic linkages.Factors affecting Enzyme Activity
The activity of an Enzyme is affected by its environmental conditions. Changing these alter the rate of reaction caused by the enzyme. In nature, organisms adjust the conditions of their enzymes to produce an Optimum rate of reaction.
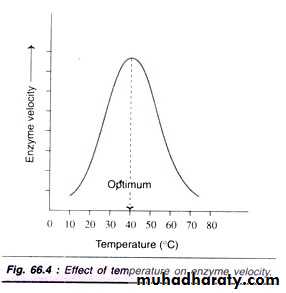
1- Temperature:
Increasing temperature increases the Kinetic Energy that molecules possess. Since enzymes catalyse reactions by randomly colliding with Substrate molecules, increasing temperature increases the rate of reaction, forming more product. As temperature increases, more bonds, especially the weaker Hydrogen and Ionic bonds, will break as a result of this strain. Breaking bonds within the enzyme will cause the Active Site to change shape. As temperature increases, more enzymes' molecules' Active Sites' shapes will be less Complementary to the shape of their Substrate, and more enzymes will be Denatured. This will decrease the rate of reaction. In summary, as temperature increases, initially the rate of reaction will increase, because of increased Kinetic Energy. However, the effect of bond breaking will become greater and greater, and the rate of reaction will begin to decrease. The temperature at which the maximum rate of reaction occurs is called the enzyme's Optimum Temperature. This is different for different enzymes. Most enzymes in the human body have an Optimum Temperature of around 37.0 °C.
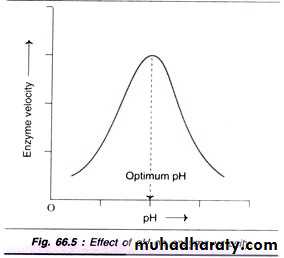
2- pH - Acidity and Basicity
pH measures the Acidity and Basicity of a solution. It is a measure of the Hydrogen Ion (H+) concentration, and therefore a good indicator of the Hydroxide Ion (OH-) concentration. It ranges from pH1 to pH14. Lower pH values mean higher H+ concentrations and lower OH- concentrations.Acid solutions have pH values below 7, and Basic solutions (alkalis are bases) have pH values above 7. Deionised water is pH7, which is termed 'neutral.
H+ and OH- Ions are charged and therefore interfere with Hydrogen and Ionic bonds that hold together an enzyme, since they will be attracted or repelled by the charges created by the bonds. This interference causes a change in shape of the enzyme, and importantly, its Active Site. Different enzymes have different Optimum pH values. This is the pH value at which the bonds within them are influenced by H+ and OH- Ions in such a way that the shape of their Active Site is the most Complementary to the shape of their Substrate. At the Optimum pH, the rate of reaction is at an optimum. Any change in pH above or below the Optimum will quickly cause a decrease in the rate of reaction, since more of the enzyme molecules will have Active Sites whose shapes are not (or at least are less) Complementary to the shape of their Substrate.
Concentration: 3-
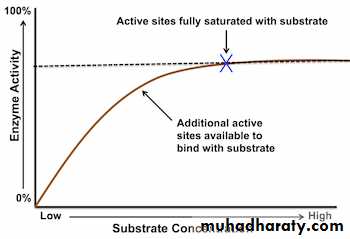
Changing the Enzyme and Substrate concentrations affect the rate of reaction of an enzyme - catalysed reaction. Controlling these factors in a cell is one way that an organism regulates its enzyme activity and so its Metabolism. Changing the concentration of a substance only affects the rate of reaction if it is the limiting factor: that is, it the factor that is stopping a reaction from preceding at a higher rate.A) Substrate Concentration
Increasing Substrate Concentration increases the rate of reaction. This is because more substrate molecules will be colliding with enzyme molecules, so more product will be formed.However, after a certain concentration, any increase will have no effect on the rate of reaction, since Substrate Concentration will no longer be the limiting factor. The enzymes will effectively become saturated, and will be working at their maximum possible rate.
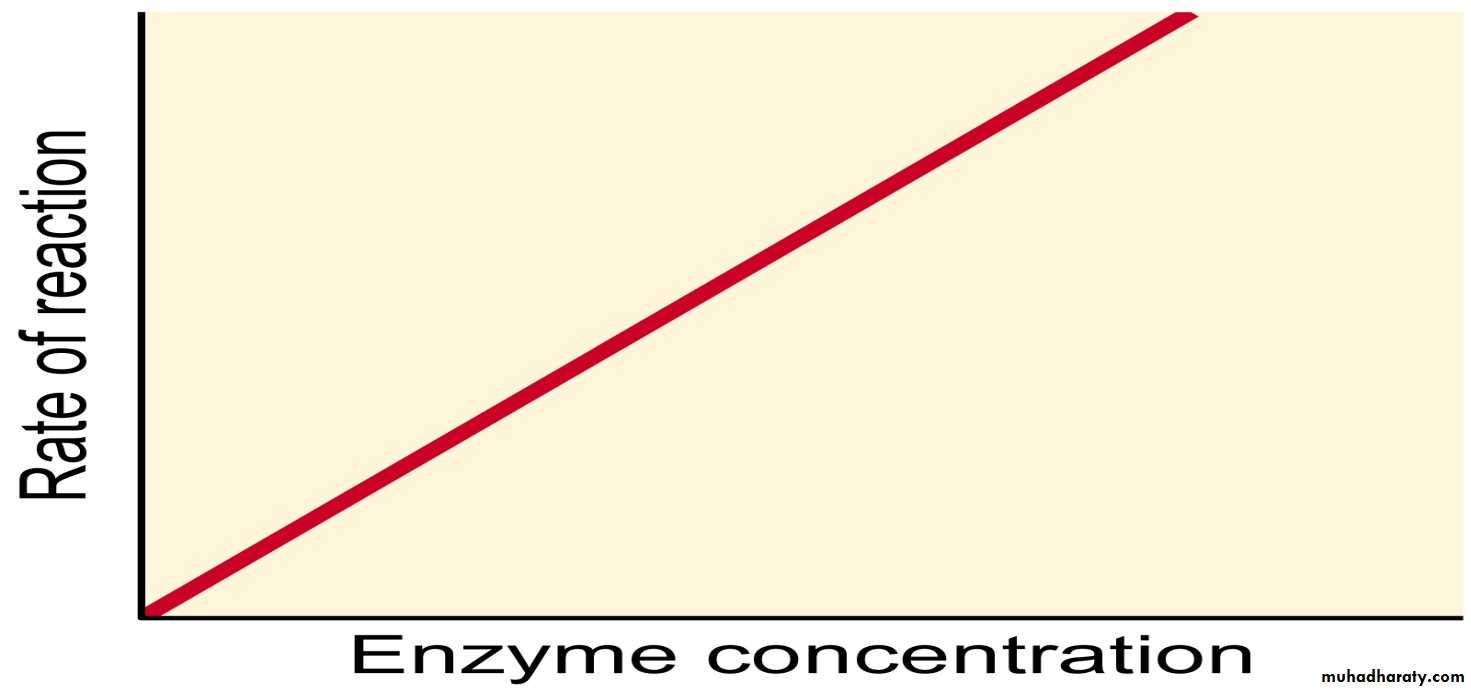
B) Enzyme Concentration
Increasing Enzyme Concentration will increase the rate of reaction, as more enzymes will be colliding with substrate molecules. However, this too will only have an effect up to a certain concentration, where the Enzyme Concentration is no longer the limiting factor.Isoenzyme: Enzymes that catalyze the same reaction may differ from one another in many ways ranging from small variations in secondary structure to broad differences in amino acid sequence and molecular weight.
Enzymes in the Diagnosis of Pathology:The measurement of the serum levels of numerous enzymes has been shown to be of diagnostic significance. This is because the presence of these enzymes in the serum indicates that tissue or cellular damage has occurred resulting in the release of intracellular components into the blood. Commonly assayed enzymes in the blood are the:
1- Aminotransferases (equally correct is the term transaminase): alanine transaminase, ALT (sometimes still referred to as serum glutamate-pyruvate transaminase, SGPT) and aspartate transaminase, AST (also referred to as serum glutamate-oxaloacetate transaminase, SGOT). Humans express a cytosolic AST and a mitochondrial AST, both of which function as homodimeric enzymes, and are encoded by distinct genes (cytosolic: GOT1; mitochondrial: GOT2). In addition, the enzymes cardiac troponin I (cTnI), lactate dehydrogenase (LDH) and creatine kinase (CK, also called creatine phosphokinase, CPK) are commonly measured in the diagnosis of cardiac infarct.
2- Gamma-glutamyltranferase (GGT, γ-glutamyltransferase; also called gamma-glutamyl transpeptidase) is involved in glutathione (GSH) metabolism and also in amino acid transport across membranes. Assessment of GGT levels in the blood is diagnostic for diseases of the liver and the biliary system as well as disease of the pancreas. Carbonic anhydrases are enzymes that catalyze the formation of carbonic acid (H2CO3) from CO2 and H2O, These enzymes are useful both as pharmacologic targets and as diagnostic tools in certain disease states. Other enzymes are assayed under a variety of different clinical situations but they will not be covered here. Liver Function Enzymes: The typical liver enzymes measured are aspartate transaminase (AST) and alanine transaminase (ALT). The diagnostically useful AST isoform is a cytoplasmic enzyme encoded by the GOT1 (glutamate oxaloacetate transaminase 1) gene while the ALT enzyme is encoded by the GPT (glutamate pyruvate transaminase) gene. A second GOT gene (GOT2) encodes a mitochondrial version of AST which participates in the malate-aspartate shuttle. ALT is particularly diagnostic of liver involvement as this enzyme is found predominantly in hepatocytes. When assaying for both ALT and AST the ratio of the level of these two enzymes can also be diagnostic. Normally in liver disease or damage that is not of viral origin the ratio of ALT/AST is less than 1. However, with viral hepatitis the ALT/AST ratio will be greater than 1. Measurement of AST is useful not only for liver involvement but also for heart disease or damage. Although measurement of AST is not, in and of itself, diagnostic for myocardial infarction, taken together with LDH and CK measurements, the level of AST is useful for timing of the infarct.
3- Cardiac Troponins: Troponins are complexes composed of three regulatory proteins, troponin C (TnC), troponin I (TnI), and troponin T (TnT) attached to tropomyosin and are found in the grooves between thin actin filaments in striated muscle tissue. The troponins are found in skeletal and cardiac muscle but not smooth muscle. The TnI subfamily is composed of three isoforms identified as TnI- skeletal-fast-twitch, TnI-skeletal-slow-twitch, and TnI- cardiac. The cardiac-specific TnI isoform is encoded by the TNNI3 (troponin I type 3, cardiac) gene. Cardiac troponins found in the serum, specifically troponin I and T, are excellent markers for myocardial infarction as well as for any other type of heart muscle damage. The measurement of plasma troponin I levels are highly diagnostic of necrosis of cardiac muscle. 4- Lactate Dehydrogenases: The measurement of lactate dehydrogenase (LDH) is especially diagnostic for myocardial infarction because this enzyme exists in five closely related, but slightly different forms (isozymes). These five isoforms are generated by combinations of two different subunits encoded by two different genes. The subunits are identified as the M form for muscle-specific (encoded by the LDHA gene) and the H form for heart-specific (encoded by the LDHB gene). Humans also express two additional LDH genes: LDHC (expression restricted to testis) and LDHD (mitochondrial enzyme specific for D-lactate). The five types of LDH used in diagnosis and their normal distribution and levels in non-disease/injury are listed below. LDH 1 (H4) – Found in heart and red-blood cells and is 17% – 27% of the normal serum total.
LDH 2 (H3M1) – Found in heart and red-blood cells and is 27% – 37% of the normal serum total.
LDH 3 (H2M2) – Found in a variety of organs and is 18% – 25% of the normal serum total.
LDH 4 (H1M3) – Found in a variety of organs and is 3% – 8% of the normal serum total.
LDH 5 (M4) – Found in liver and skeletal muscle and is 0% – 5% of the normal serum total.
5- Creatine Kinases: Creatine kinase (CK, or creatine phosphokinase, CPK) is found primarily in heart and skeletal muscle as well as the brain. Therefore, measurement of serum CPK levels is a good diagnostic for injury to these tissues. The muscle CPK isoform is expressed from the creatine kinase, muscle (CKM) gene while the brain isoform is expressed from the CKB gene. Dependent upon the tissue of expression, three major CPK isoforms are found in human tissues:
CPK3 (CPK-MM) is a homodimer of two CKM encoded proteins and is the predominant isoform in muscle and is 100% of the normal serum total.
CPK2 (CPK-MB) is a heterodimer of the CKM and CKB encoded proteins and this form accounts for about 35% of the CPK activity in cardiac muscle, but less than 5% in skeletal muscle and is 0% of the normal serum total.
CPK1 (CPK-BB) is a homodimer of two CKB encoded proteins and is the characteristic isoform in brain and is in significant amounts in smooth muscle and several other tissues and is 0% of the normal serum total.
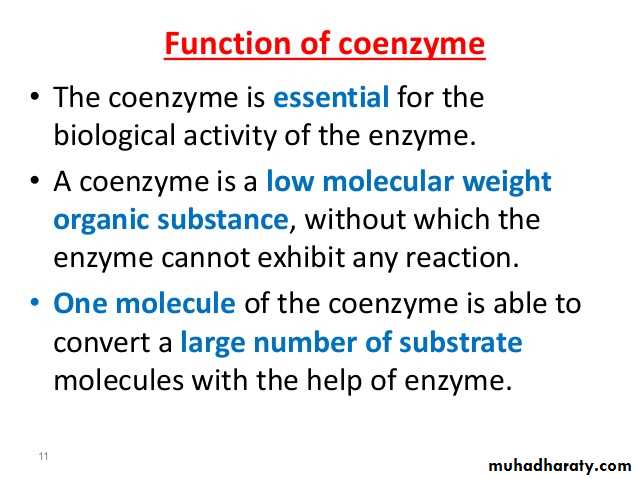
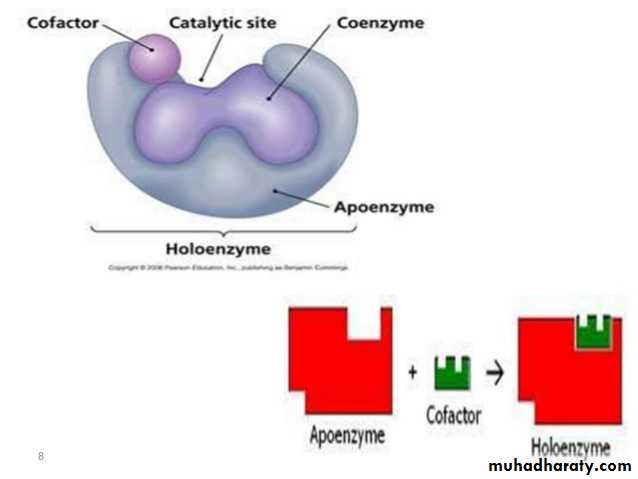
What is a Coenzyme
Enzymes make things happen faster. Enzymes don't always work alone, though. They have helpers called cofactors. Cofactors can be inorganic ions (like zinc) or non-protein, organic (carbon-containing) molecules. The latter are called coenzymes. Coenzymes bind to the enzyme and assist in enzyme activity.
Coenzyme Function and importance
There is a specific location on an enzyme which binds to substrates and helps turn them into products. This location, called the active site, is where coenzymes bind. There are several ways coenzymes assist in enzyme function, including changing their shape to activate, or turn on, enzymes, or aiding in chemical reactions by acting as carriers of energy or molecular groups. In order to occur, chemical reactions might require or release energy. Remember the First Law of Thermodynamics: energy can neither be created nor destroyed. So, for an enzyme to function, sometimes energy is needed. The cell likes to be efficient in its use of energy; therefore, it tries to capture and reuse energy. One of the ways it does this is through coenzymes. For example. The molecule ATP (adenosine triphosphate) can function as a coenzyme. When a phosphate group is removed, turning ATP into ADP (adenosine diphosphate), energy is released. Since many chemical reactions require energy, cells can use ATP to give energy to a reaction to assist in changing the substrate to product. The substrate can be temporarily phosphorylated, or have an added phosphate group. The phosphate group can then be removed and the product is formed partly through the addition and removal of a phosphate. Coenzymes often have long complicated names and are frequently shortened to acronyms or abbreviations. Coenzymes with shortened names include: NAD+/NADH, NADP+/NADPH, and FAD/FADH2. These function similarly to ATP, except instead of a molecular group, they remove or add electrons and hydrogen atoms. Hence, they have two different forms: NAD+ and NADH are the same molecule, except NADH has an added hydrogen. Also, the removal or addition of electrons can change their shape, allowing them to bind or dissociate (be removed) from an enzyme they are helping.
Enzyme activity:
Enzyme assays usually depend on the measurement the catalytic activity of the enzyme, rather than the concentration of the enzyme protein itself.
•One international unitis the amount of enzyme that will convert one micromole of substrate per minute per litreof sample and is abbreviated as U/L.
•Katal (catalytic activity)is defined as the number of mole of substrate transformed per second per litre of sample.