
Lecture 10
Effect of low arterial PO2 to stimulate alveolar ventilation when arterial carbon dioxide
and hydrogen ion concentrations remain normal
When there is a low arterial PO2 while PCO2 and the hydrogen ion concentration are kept
constant at their normal levels, only the ventilatory drive due to the effect of low oxygen on
the chemoreceptors is active which is quite strongly and lead to increase alveolar ventilation.
There is almost no effect on ventilation as long as the arterial PO2 remains greater than 100
mm Hg. But at pressures lower than 100 mm Hg, the ventilation start to increase until
approximately doubles when the arterial PO2 falls to 60 mm Hg and can increase as much as
fivefold at very low PO2s.
Effect of carbon dioxide and hydrogen ion concentration on chemoreceptor activity
The direct effect of increasing in either carbon dioxide concentration or hydrogen ion
concentration on respiratory centre is so much more powerful than their indirect effects
through chemoreceptors. The stimulation by way of the peripheral chemoreceptors occurs as
much as five times as rapidly as central stimulation, so that the peripheral chemoreceptors
might be especially important in increasing the rapidity of response to carbon dioxide at the
onset of exercise.
Acclimatization: Slowly mountain climbers over a period of days rather than hours, they
breathe much more deeply and therefore can tolerate far lower atmospheric oxygen
concentrations than when they ascend rapidly called acclimatization. The reason for that,
within 2 to 3days, the respiratory center in the brain stem loses about four fifths of its
sensitivity to changes in PCO2 and hydrogen ions. Therefore, the excess ventilatory blow-off
of carbon dioxide that normally would inhibit an increase in respiration fails to occur and
low oxygen can drive the respiratory system to a much higher level of alveolar ventilation
(400 to 500 %) than under acute conditions (70%). This helps greatly in supplying additional
oxygen to the mountain climber.
Regulation of respiration during exercise
In strenuous exercise, in the healthy athlete, alveolar ventilation increases in step with the
increased level of oxygen metabolism and to keep the arterial PO2, PCO2, and pH remain
almost exactly normal (so they are not the cause of increase ventilation). (1) It is believed
that the brain, on transmitting motor impulses to the exercising muscles, to transmit at the
same time collateral impulses into the brain stem to excite the respiratory center.
Occasionally, however, the nervous respiratory control signals are either too strong or too
weak. Then (2) chemical factors play a significant role in bringing about the final adjustment
of respiration required to keep the oxygen, carbon dioxide, and hydrogen ion concentrations
of the body fluids as nearly normal as possible.
At the onset of exercise, the alveolar ventilation increases immediately (signal from brain)
without an initial increase in arterial PCO2. This increase in ventilation is usually great
enough so that at first it actually decreases arterial PCO2 below normal, However, after
about 30 to 40 seconds, the amount of carbon dioxide released into the blood from the active

muscles approximately matches the increased rate of ventilation, and the arterial PCO2
returns essentially to normal toward the end of the 1-minute period of exercise even as the
exercise continues.
(3)
Many experiments suggest that there is a possibility that the neurogenic factor for control
of ventilation during exercise is a learned response.
Other factors that affect respiration
1- Voluntary control of respiration: we all know that for short periods of time,
respiration can be controlled voluntarily and that one can hyperventilate or
hypoventilate to such an extent that serious derangements in PCO2, pH, and PO2 can
occur in the blood.
2- Effect of irritant receptors in the airways: The epithelium of the trachea, bronchi,
and bronchioles is supplied with sensory nerve endings called pulmonary irritant
receptors that are stimulated by many incidents. These cause coughing and sneezing.
They may also cause bronchial constriction in such diseases as asthma and
emphysema.
3- Function of lung “J Receptors.” A few sensory nerve endings have been described in
the alveolar walls in juxtaposition to the pulmonary capillaries—hence the name “J
receptors.”They are stimulated especially when the pulmonary capillaries become
engorged with blood or when pulmonary edema occurs in such conditions as
congestive heart failure. Although the functional role of the J receptors is not clear,
their excitation may give the person a feeling of dyspnea.
4- Effect of brain edema. The activity of the respiratory center may be depressed or
even inactivated by acute brain edema resulting from brain concussion. As
the
damaged brain tissues swell, compressing the cerebral arteries against the cranial vault
and thus partially blocking cerebral blood supply.
5- Anesthesia: Overdosage with anesthetics (morphine) or narcotics cause respiratory
depression and respiratory arrest.
6- Hormonal effect:women hyperventilate during luteal phase of menstrual cycle and
pregnancy.
Periodic breathing: in this case the person breathes deeply for a short interval and then
breathes slightly or not at all for an additional interval, with the cycle repeating itself over
and over as in Cheyne-Stokes breathing, is characterized by slowly increasing and declining
respiration occurring about every 40 to 60 seconds.
Basic mechanism of Cheyne-Stokes breathing: When a person over breathes thus blowing
off too much carbon dioxide from the pulmonary blood while at the same time increasing
blood oxygen, it takes several seconds before the changed pulmonary blood can be
transported to the brain and inhibits the excess ventilation. By this time, the person has
already over ventilated for an extra few seconds. Therefore, when the over ventilated blood
finally reaches the brain respiratory center, the center becomes depressed an excessive
amount. Then the opposite cycle begins. That is, carbon dioxide increases and oxygen

decreases in the alveoli. Again, it takes a few seconds before the brain can respond to these
new changes. When the brain does respond, the person breathes hard once again, and the
cycle repeats. The basic cause of Cheyne-Stokes breathing occurs in everyone. However,
under normal conditions, this mechanism is highly “damped.”That is, the fluids of the blood
and the respiratory center control areas have large amounts of dissolved and chemically
bound carbon dioxide and oxygen , Therefore, normally, the lungs cannot build up enough
extra carbon dioxide or depress the oxygen sufficiently in a few seconds to cause the next
cycle of the periodic breathing. But in two different conditions, the damping factors can be
overridden, and Cheyne-Stokes breathing does occur:
1- Severe heart failure, where slow blood circulation to the brain.
2- Brain damage (increased negative feedback gain), increase the fold of ventilation when
slight increase PCO2 to many times than normal.
Sleep Apnea: The term apnea means absence of spontaneous breathing. Occasional apneas
occur during normal sleep, but in persons with sleep apnea, the frequency (300 to 500 times
each night) and duration are greatly increased (with episodes of apnea lasting for 10 seconds
or longer). Sleep apneas can be caused by: obstruction of the upper airways, especially the
pharynx, or by impaired central nervous system respiratory drive.
Clinical respiratory test and investigation
1) Blood gases & pH test: This important test determines the blood PO2, PCO2, and pH
which help in determining appropriate therapy for acute respiratory distress or acute
abnormalities of acid-base balance. All these three items measuring devices are built
into the same apparatus, and all these measurements can be made within a minute or so
using a single, droplet-size sample of blood.
2) Maximum expiratory flow: When a person first inhale as much air as possible and
then expires with maximum expiratory effort until he or she can expire at no greater
rate and it is more than 400 L/min. The effect of increased pressure applied to the
outsides of the alveoli and air passageways during forceful expiration caused by
compressing the chest cage. The arrows in (Fig. 23) indicate that the same pressure
compresses the outsides of both the alveoli and the bronchioles. Therefore, not only
does this pressure force air from the alveoli toward the bronchioles, but it also tends to
collapse the bronchioles at the same time, which will oppose movement of air to the
exterior. Once the bronchioles have almost completely collapsed, further expiratory
force can still greatly increase the alveolar pressure, but it also increases the degree of
bronchiolar collapse and airway resistance by an equal amount, thus preventing further
increase in flow and reach maximum expiratory flow.
●As the lung volume becomes smaller; the maximum expiratory flow rate also becomes less.

Because in the enlarged lung the bronchi and bronchioles are held open partially by way of
elastic pull on their outsides by lung structural elements; however, as the lung becomes
smaller, these structures are relaxed, so that the bronchi and bronchioles are collapsed more
easily by external chest pressure.
Maximum expiratory flow decreases in:
(1) Constricted lung diseases: as include fibrotic diseases of the lung itself, such as
tuberculosis and silicosis, and diseases that constrict the chest cage, such as kyphosis,
scoliosis, and fibrotic pleurisy (because the lung cannot expand to a normal maximum
volume, even with the greatest possible expiratory effort).
(2) Airway obstruction disease: as in asthma & some stages of emphysema (it is usually
much more difficult to expire than to inspire because the closing tendency of the airways is
greatly increased by the extra positive pressure required in the chest to cause expiration)
(Fig. 24).
3) Spirometery: this is useful clinical pulmonary test & simple, it can measure (fig. 25):
1. The forced expiratory vital capacity (FVC): Its maneuver, the person first inspires
maximally to the total lung capacity, and then exhales into the spirometer with
maximum expiratory effort as rapidly and as completely as possible (about 4600 ml).
2. The forced expiratory volume in first second (FEV1):
It is the volume of air expired
over the first second of forced vital capacity manoeuvre.
3. FEV1/FVC ratio: It is the ratio of forced expiratory volume in first second to force
vital capacity & is 80%. It decreases with airway obstruction.
In restrictive disease this
ratio show a normal because FEV1 as well as FVC are usually equivalent reduced.
4. The maximum expiratory flow over the middle 50% of the vital capacity (FEF
25–75%): it is a sensitive index of small airway function, it is important in monitoring
of asthma patients.
4) The transfer factor for carbon monoxide (TLCO): It reflects the efficiency of the
lung in transferring oxygen from the alveoli across the alveolar-capillary membrane
and into haemoglobin within the red blood cells. It measures the uptake of inhaled
carbon monoxide instead of oxygen across the lung alveolar capillary membrane,
which is limited by the rate of diffusion from alveolar space into the erythrocyte.
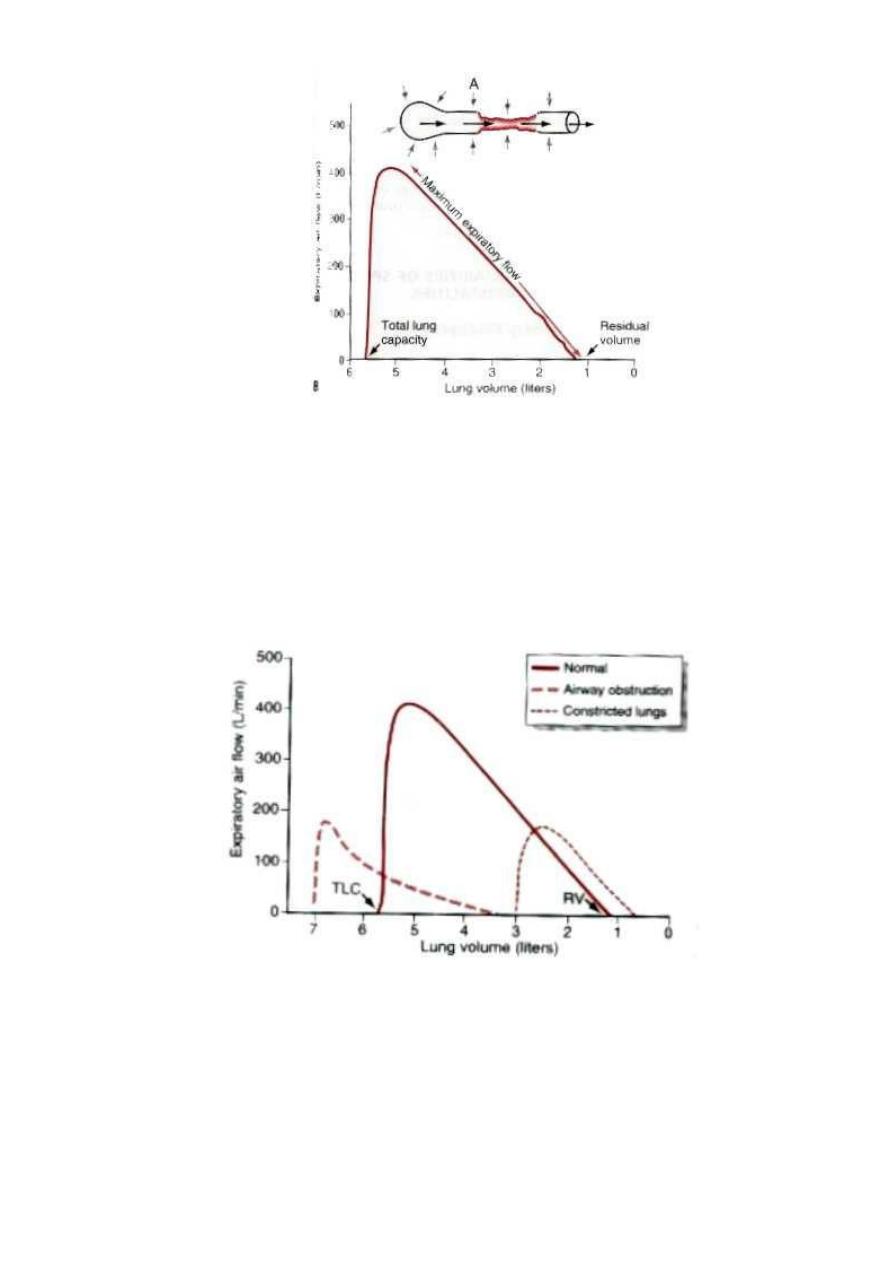
Figure 23 A, Collapse of the respiratory passageway during maximum expiratory effort, an effect that
limits expiratory flow rate. B, Effect of lung volume on the maximum expiratory air flow, showing
decreasing maximum expiratory air flow as the lung volume becomes smaller.
Figure 24 Effect of two respiratory abnormalities—constricted lungs and airway obstruction—-on the
maximum expiratory flow-volume curve. TLC, total lung capacity; RV, residual volume.
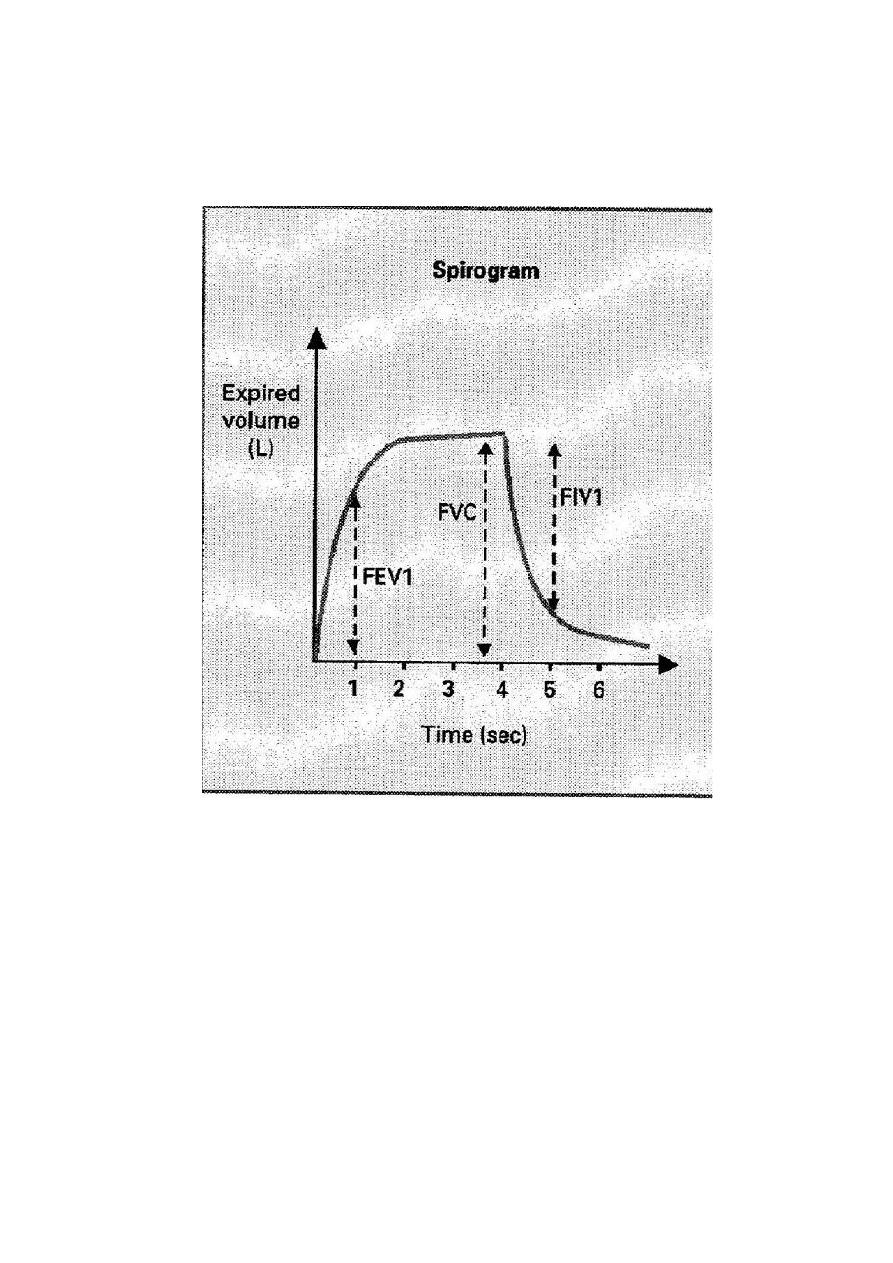
Figure 25 Recordings during the forced vital capacity maneuver
