Diabetes mellitus in children
Diabetes mellitus (DM)is a common, chronic, metabolic disease characterized by hyperglycemia as a cardinal biochemical feature
The major forms of diabetes are differentiated by insulin deficiency vs insulin resistance:
type 1 diabetes mellitus (T1DM) results from deficiency of insulin secretion because of pancreatic β-cell damage;
type 2 diabetes mellitus (T2DM) is a consequence of insulin resistance
TYPE 1 DIABETES MELLITUS
Formerly called insulin-dependent diabetes mellitus (IDDM) or juvenile diabetes, T1DM is characterized by:low or absent levels of endogenously produced insulin
by dependence on exogenous insulin to prevent development of ketoacidosis, an acute life threatening complication of T1DM.
The natural history includes 4 distinct stages:
preclinical β-cell autoimmunity with progressive defect of insulin secretiononset of clinical diabetes,
transient remission “honeymoon period,”
established diabetes during which there may occur acute and/or chronic complications and decreased life expectancy.
Peaks of presentation occur in 2 age groups:
at 5-7 yr of age
at the time of puberty.
The first peak may correspond to the time of increased exposure to infectious agents coincident with the beginning of school
The second peak may correspond to the pubertal growth spurt induced by
gonadal steroidsThe increased pubertal growth hormone secretion (which antagonizes insulin).
Autoantibodies to β-cell antigens such as
islet cell cytoplasm (ICA),insulin autoantibody (IAA),
antibodies to glutamic acid decarboxylase
are detected in serum from affected subjects.
These can be detected months to years prior to clinical onset of T1DM.
T1DM is associated with other autoimmune diseases such as thyroiditis, celiac disease, and Addison disease.
GENETICS
There is a clear familial clustering of T1DM, with prevalence in siblings approaching 6%.Risk of T1D increased when a parent has diabetes and this risk differs between the 2 parents;
the risk is 3-4% if the mother has diabetes but 5-6% when the father has diabetes
GENETICS
In monozygotic twins, the concordance rate ranges from 30-65%, whereas dizygotic twins have a concordance rate of 6-10%
85% of newly diagnosed type 1 diabetic patients do not have a family member with T1DM.
the known associations include the HLA DR3/4-DQ2/8 genotype.
ENVIRONMENTAL FACTORS
• Viral Infections:• It is possible that various viruses do play a role in the pathogenesis of T1DM, but no single virus, and no single pathogenic mechanism, stands out in the environmental etiology of T1DM.
ENVIRONMENTAL FACTORS
Congenital Rubella Syndrome:• Prenatal infection with rubella is associated with β-cell autoimmunity in up to 70%, with development of T1DM in up to 40% of infected children.
Mumps Virus, entero virus
ENVIRONMENTAL FACTORS
• DietBreastfeeding may lower the risk of T1DM, either directly or by delaying exposure to cow’s milk protein.
Early introduction of cow’s milk protein and early exposure to gluten are implicated in the development of autoimmunity.
VIT D, C, ZINC
ENVIRONMENTAL FACTORS
• Role of AutoantibodiesEven though T1DM does not occur as a direct consequence of autoantibody formation,
the risk of developing clinical disease increases dramatically with an increase in the number of antibodies.
ENVIRONMENTAL FACTORS
• Role of Autoantibodies
only 30% of children with 1 antibody will progress to diabetes, but this risk increases to 70% when 2 antibodies are present and 90% when 3 are present
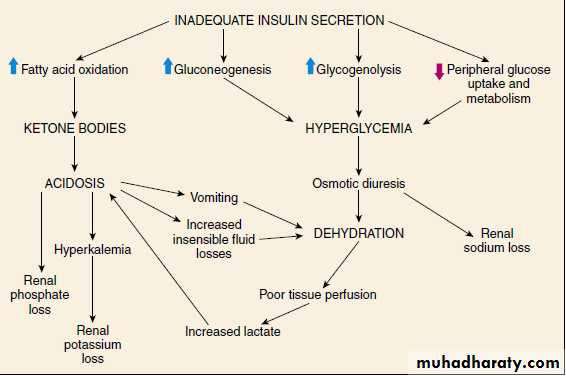
PATHOPHYSIOLOGY
In normal metabolism, there are regular swings between the postprandial, high-insulin anabolic state and the fasted, low-insulin catabolic state that affect liver, muscle, and adipose tissue.PATHOPHYSIOLOGY
T1DM is a progressive low insulin catabolic state in which feeding does not reverse, but rather exaggerates, these catabolic processes.With moderate insulinopenia, glucose utilization by muscle and fat decreases and postprandial hyperglycemia appears
PATHOPHYSIOLOGY
At even lower insulin levels, the liver produces excessive glucose via glycogenolysis and gluconeogenesis, and fasting hyperglycemia begins.Hyperglycemia produces an osmotic diuresis (glycosuria) when the renal threshold is exceeded (180 mg/dL)
The resulting loss of calories and electrolytes with the worsening dehydration, produces a physiologic stress with hypersecretion of stress hormones (epinephrine, cortisol, GH, and glucagon).
PATHOPHYSIOLOGY
These hormones contribute to the metabolic decompensation
by further impairing insulin secretion (epinephrine),by antagonizing its action (epinephrine, cortisol, GH),
By promoting glycogenolysis, gluconeogenesis, lipolysis, and ketogenesis (glucagon, epinephrine, GH, and cortisol)
decreasing glucose utilization and glucose clearance (epinephrine, GH, cortisol).
PATHOPHYSIOLOGY
The hormonal interplay of insulin deficiency and glucagon excess shunts the free fatty acids into ketone body formation;
the rate of formation of these ketone bodies, principally β-hydroxybutyrate and acetoacetate, exceeds the capacity for peripheral utilization and renal excretion.
PATHOPHYSIOLOGY
Patients with progressive β-cell destruction will eventually present with clinical T1DM.
It was thought that 90% of the total β-cell mass is destroyed by the time clinical disease develops, but later studies have revealed that this is not always the case.
It now appears that β-cell destruction is more rapid and more complete in younger children
PATHOPHYSIOLOGY
Accumulation of these keto acids results in metabolic acidosis (diabetic ketoacidosis [DKA]) and compensatory rapid deep breathing in an attempt to excrete excess CO2 (Kussmaul respiration)
PATHOPHYSIOLOGY
CLINICAL MANIFESTATIONS
Initially, when only insulin reserve is limited, occasional postprandial hyperglycemia occurs.When the serum glucose increases above the renal threshold, intermittent polyuria or nocturia begins.
With further β-cell loss, chronic hyperglycemia causes a more persistent diuresis, often with nocturnal enuresis, and polydipsia becomes more apparent.
Female patients may develop monilial vaginitis from the chronic glycosuria.
Calories are lost in the urine (glycosuria), triggering a compensatory hyperphagia.If this hyperphagia does not keep pace with the glycosuria, loss of body fat ensues, with clinical weight loss and diminished subcutaneous fat stores
CLINICAL MANIFESTATIONS
Diagnosis
Diabetic Ketoacidosis (DKA)
DKA is the end result of the metabolic abnormalities resulting from a severe deficiency of insulin or insulin effectiveness. The latter occurs during stress as counter-regulatory hormones block insulin actionDKA occurs in 20-40% of children with new-onset diabetes and in children with known diabetes who omit insulin doses or who do not successfully manage an intercurrent illness.
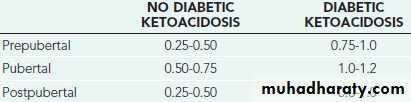
DKA may be arbitrarily classified as mild, moderate, or severe
Diabetic Ketoacidosis (DKA)
The range of symptoms depends on the depth of ketoacidosis. There is a large amount ofketonuria,
an increased ion gap,
a decreased serum bicarbonate (or total CO2) and pH,
an elevated effective serum osmolality, indicating hypertonic dehydration.
Diabetic Ketoacidosis (DKA)
Severity of DKA can be classified as following:
Diabetic Ketoacidosis (DKA)
TREATMENTTreatment can be divided according to type of presentation , whether present with classical signs and symptoms of T1D (polyuria,polydipsia.weight loss and others) which requires starting insulin only, or present with DKA which need to start a special protocol for treatment.
Insulin starting dose can be divided also according to this classification:
The optimal insulin dose can only be determined empirically, with frequent self-monitored blood glucose levels and insulin adjustment by the diabetes team.
TREATMENT
The insulin can be given in different regimens, which include:Multiple Daily Injection (MDI) Regimens,
Insulin Pump Therapy
NPH-Based Treatment Regimens (split-mixed regimen).
Bolus-basal treatment with multiple injections is better adapted to the physiologic profiles of insulin and glucose and can therefore provide better glycemic control than the conventional 2-3 dose regimen.
TREATMENT
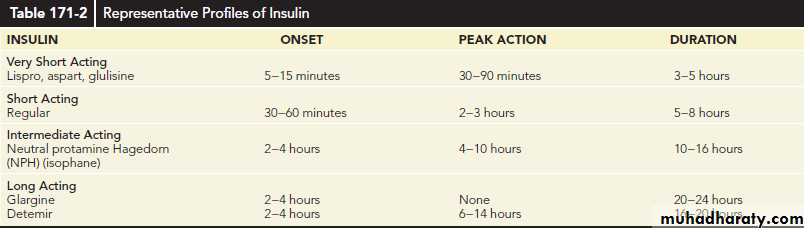
Insulin can be classified according to the source of production into 3 types:
a. insulin extracted from animal pancreases, specifically beef, beef–pork, and pork insulin (discontinued).b. human insulin produced by recombinant DNA technology.
c. insulin analogs, also produced by recombinant DNA technology with the introduction of molecule modifications that change the pharmacokinetic profile.
TREATMENT
Currently available insulin types are classified based on their duration of action as rapid, short, intermediate, and long acting.
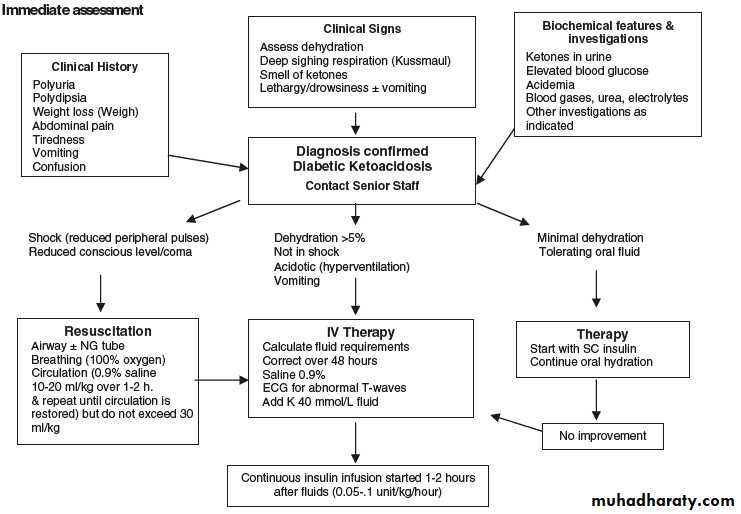
DKA treatment
Treatment of DKA
No need to give NaHco3 to every case of DKA; Increase serum osmolarity,free liberation of CO2 to brain,increase peripheral lactic acidosis ,induce more hypoklemia.Cerebral oedema commenest cause of morbidity and mortality in DKA
Cerberal oedema
Clinical presentation:disturbed iconsicousiness increase bl ressure,hypoventillation and bradycardia
Treatment 3% normal saline infusion or
Mannitol infusion
Dawn Phenomenon and Somogyi Phenomenon
Elevated morning fasting glucose mainly caused by these conditions.The dawn phenomenon is thought to be mainly caused by overnight growth hormone secretion and increased insulin clearance and therefore morning hyperglycemia and require increase insulin dose.
Dawn Phenomenon and Somogyi Phenomenon
Somogyi phenomenon:a theoretical rebound from late-night or early-morning hypoglycemia, thought to be from an exaggerated counter regulatory response and require decrease insulin dose.Mid night measurement of blood sugar can distinguish between these Two phenomenon.
Honeymoon Period
In some patients with new onset of DM1, the beta cell mass has not been completely destroyed. The remaining functional beta cells seem to recover function with insulin treatment.Honeymoon Period
When this occurs, exogenous insulin requirements decrease.This is a period of stable blood glucose control, often with nearly normal glucose concentrations.
This phase of the disease, known as the honeymoon period, usually starts in the first weeks of therapy, often continues for 3 to 6 months, and can last 2 years.
Complications
Patients with DM1 for more than 3 to 5 years should receive
an annual ophthalmologic examination for retinopathy.
Urine should be collected annually for assessment of microalbuminuria, which suggests early renal dysfunction and indicates a high risk of progression to nephropathy. Treatment with ACE I may halt the progression of microalbuminuria.
Complications
In children with DM1,annual cholesterol measurements
and periodic assessment of blood pressure are recommended.
Early detection of hypertension and high cholestrol with appropriate intervention can help limit future risk of coronary disease.
Other long term complicationss
Perripheral and autonomic neuropathyPoor growth and development
Mauriac syndrome consists of short stature hepatomegaly
Due to fatty infilteration and fibrosis with skin changes like wax ,lflexion deformities of the fingers
Lipid hypertrophy and atrophy at sites of improper insulin
injections
Acute complications
Diabetic Ketoacidosis occur in poorly controlled ,insufficient insulin dose to dietAnd to stress like infections
Hypoglycemia, due to excess insulin to diet, exercise ,vomiting or diarrhea it is dangerous in young child when the brain is vulnerable to hypoglycemia –brain injury
Prevention of acute complications
Recurent DKA, Education about insulin issues,diet,
Self-monitoring and how to increase and decrease insulin dose with dietory and infection
During sick days, vaccines for flue illness and Pneumococcal vaccine
Recurent hypoglycemia ,education how to decrease insulin dose during exercise and importance of measurement of 3-4am and splitting of conventional therapy
Acute complication
Teach the parents about manifestations of hypoglycemia like sweating, pallor , abdominal pain due to autonomic manifestations and headache abnormal movements, convulsion and coma.Teach how to treat hypoglycemia by giving glucose as syrup if they can drink it or glucose strip put in the mouth ,subcutaneous or IM Glucagon ,then give food to prevent further hypoglycemia
Follow up of diabetic children
Assess well being schooling growth and developmentAssess metabolic control by measurement of HBA1c
Non enzymatic attachment of glucose to Hemoglobin checked 3-4monthly ,its level depend s on the age ,a higher level is accepted at younger age to 8% but older child to 7% and adolescent 6%.
Screen for autoimmune disease like celiac and thyroid disease ,at onset and every 2 years and annually if abnormal