Dental Chemistry
ProfDr. Amer A. Taqa
Cements
Classification of materials in dentistry
The materials may be classification as follows:
a-acid base reaction cement
b- polymerizing materials
1-acrylic polymer
2-cyanoacrylates
3-dimethacrylate polymers
4-polymer-ceramic composites
C-other materials
1-calicium hydroxide
2-Gutta percha
3-varnishes
Acid base reaction cements
Chief constituents
Dental cements are formulated as powders and liquid.
The powders are amphoteric or basic (proton acceptors) and the
liquid are acid or proton donors.
On mixing the two together a viscous paste is formed, which
subsequently hardens to a solid mass. The cements can be classified
by the nature of the cement powder.
a/ Zinc oxide: this can react with a range of liquids as detailed later.
b/ Ion –leachable glasses, particularly fluorine containing
aluminosilicate.
General reactions:
A cement reaction is the interaction between an acid and base in
general
MO+H
2
A→MA+H
2
O
MOXSiO
2
+H
2
A →MA+XSiO
2
+H
2
O
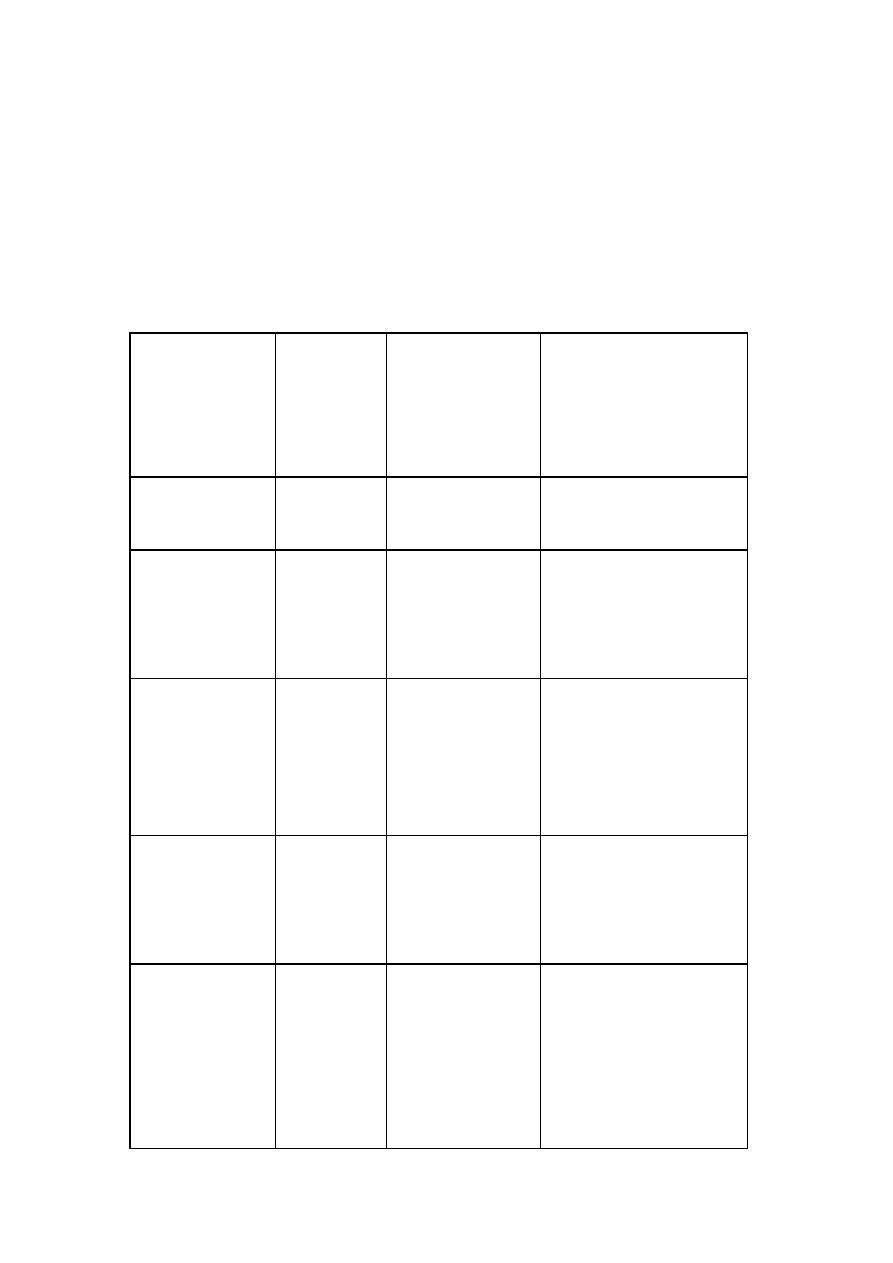
Table: chief constituents of acid –base reaction cements
Cement
powder
(proton
acceptor)
Cement
liquid
(proton
donor)
Type of cement Related materials
Zinc oxide
Eugenol
Zinc oxide-
eugenol(ZOE)
EBA cement
Zinc oxide
Aqueous
solution of
phosphoric
acid
Zinc phosphate Copper and silver
cement
Zinc oxide
Aqueous
solution of
poly
acrylic
acid
Zinc
polycarboxylate
or polyacrylate
Poly acrylic acid may
be in solid form as a
powder components
Fluorine
containing
aluminosilicate
glass
Aqueous
solution of
phosphoric
acid
Silicate cement Silicaphosphatcement
Fluorine
containing
aluminosilicate
Aqueous
solution of
poly
acrylic
acid or
polymer
Glass-ionomer

General structure
Set cements are heterogeneous, only part of the powder reacts with
the liquids and the final set material is composed of a core un
reacted powder surrounded by a matrix of reaction products, i.e. the
gel-salt.
Requirements of cements
1-They should be non-toxic, and non irritant to the pulp and other
tissues.
2-Insoluble in saliva and liquid taken into the mouth.
3-Mechanical properties: these must meet the requirements for their
particular applications, for example , for a cavity lining , a cement
should develop sufficient strength rapidly to enable a filling materials
to be packed on it.
4-Protection of the pulp from effect of other restoration materials
Zinc phosphate cement
The compositions of powder and liquid in a typical cement are given
in table:
Powder ZnO 90%
Other metallic oxide 10%
Liquid aqueous solution of phosphoric acid 50-60%
Al
3
(PO
4
)
2
up to 10% as
buffers
Zn
3
(PO
4
)
2
Setting reaction
On mixing the powder and liquid together a vigorous reaction
occurs, resulting in the formation of a relatively insoluble zinc
phosphate
3ZnO+2H
3
PO
4
+H
2
O →Zn
3
(PO
4
)
2
+4H
2
O
Only the surface layers of the zinc oxide particles react leaving

unconsumed cores bound together by the phosphate matrix (the
reaction is exothermic)
Copper cements
These are similar to phosphate cement except that the powder
contains a copper compound in addition to zinc oxide. If copper (I)
oxide (copprous oxide) is used, the cement is red, while
copper(II)oxide(cupric oxide) gives black materials.
The black copper cements have the following properties
1-Their effect on the pulp may be even greater than that of an
unmodified phosphate cement.
2-They are considered to be bactericidal, so have been used to
prolong the life of deciduous teeth. If all the carious tooth substance
cannot be removed.
They are sometimes used in the cementation of splints and fixed
orthodontic appliances.
Silver cements
Same phosphate type cements contain silver salt in an endeavor to
render there bactericidal.
— Cements based on zinc oxide
— Zinc oxide-eugenol and related materials
— Composition
— a-powder
— Zinc oxide
— Magnesium oxide may be present in small quantities, it react with
eugenol in a similar manner to zinc oxide.
— Zinc acetate( or other salts) in quantities up to about 1%, are used
accelerators for the setting reaction.
— b-liquid
— Eugenol, the major constituent of oil of cloves.
— Olive oil, up to 15%.
— Some times acetic acid , to act as an accelerator.
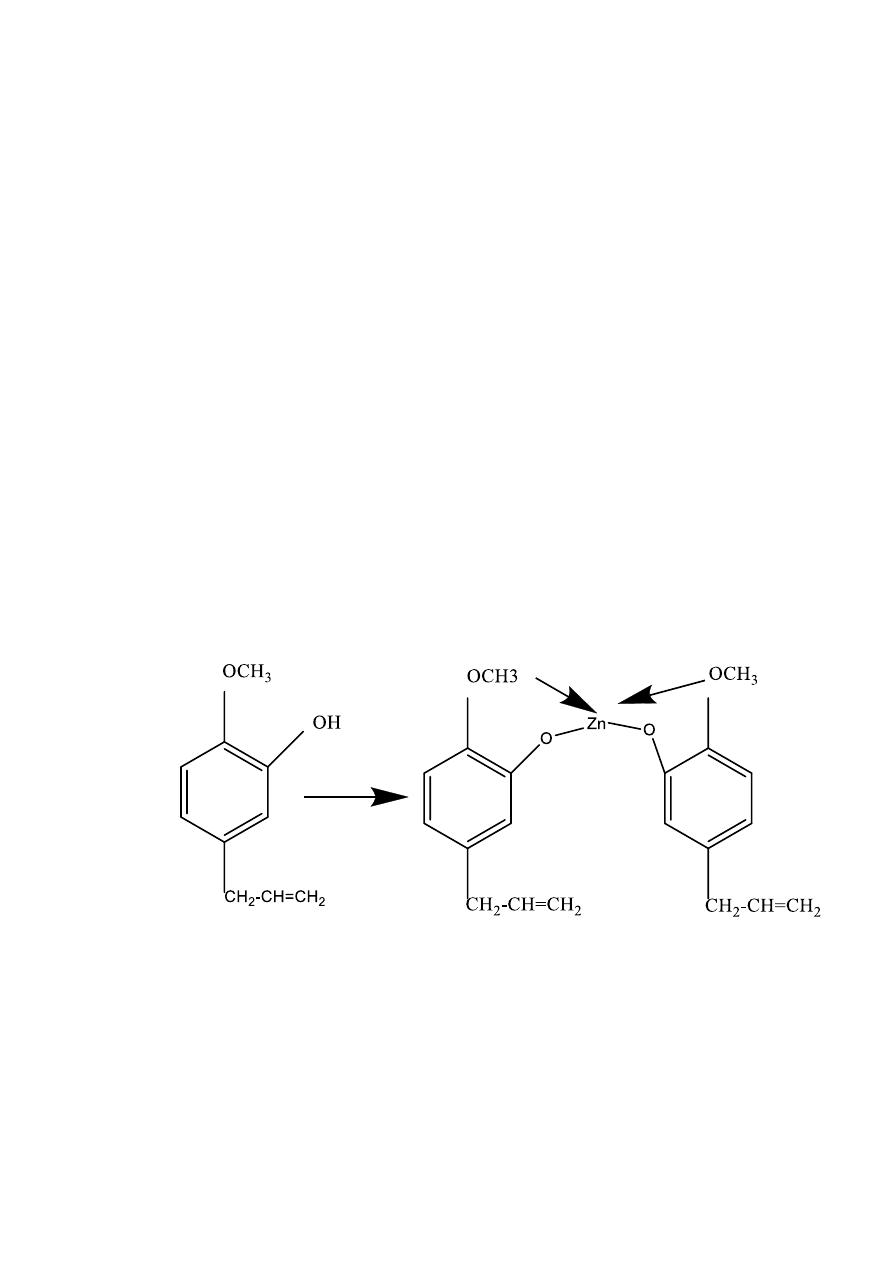
Setting reaction
One or both of two things may occur on mixing the powder and liquid:
— Chemical reaction, to form a compound called zinc eugenolate.
The reaction between zinc oxide and eugenol begins gives the structural
formula of eugenol. The basis of the reaction is that the phenolic –OH of
the eugenol act as a weak acid and undergoes an acid –base reaction with
zinc oxide to form a salt, zinc eugenolate, as follows:
— 2C
10
H
12
O
2
+ ZnO Zn(C
10
H
11
O
2
)
2
+H
2
O
— Two molecules of eugenol reacts with zinc oxide to form the salt.
The structural formula of zinc eugenolate.
— The ionic salt bonds are formed between zinc and the phenolic
oxygen of each molecule of eugenol. Two further co-ordinate
bonds are formed by donation of pairs of electrons from the
methoxy oxygens to zinc. These bonds are indicated by the arrows
in figure
Complex of zinc eugenolate
— Absorption of the eugenol by the zinc oxide may also occur other
factors to be noted are:

—
— 1-the setting reaction between pure zinc oxide and pure eugenol,
will not occur in the absence of water. Thus , a mixture of zinc
oxide and eugenol, without add(accelerators), can be kept in a
desiccators for several days without undergoing much change.
— 2- the set materials contains both some unreacted oxide and
eugenol.
—
— Setting time
— This depends on
— The powder ( its method to preparation, the particles size of the
powder
— The accelerating additives
— The powder/liquid ratio a thicker mixing gives a faster setting
materials.
— Exposure to moister on mixing or the addition of water will
accelerator the reaction.
— An increase in temperature also causes faster setting.
— Properties of cement
— All the cement(include ZOE) must be have the following
requirements
— Effect on the pulp is small, thus the material has been
recommended for use in deep cavities near the pulp.
— Chemical properties: the solubility of the set cement in water is
high- by the far the highest off all dental cements-mainly due to the
elution of eugenol.
— Mechanical properties: (ZOE cement)these are the weakest of all
the cement(except calicium hydroxide).
— Protection of the pulp.

— Optical properties.
— Adhesion : ZOE cement do not adhere to enamel and dentine. This
is one reason why they are not frequently used for the final
cementation of dental restoration
— ZOE cement are bacteriostatic and abundant.
— Thermal insulation: a cement is used under a large metallic
restoration (e.g. amalgam) to protect the pulp from temperature
changes.
— Chemical protection : a cement should be able to prevent
penetration into the pulp of harmful chemicals from the restorative
material.
— Electrical insulation under a metallic restoration to minimize
galvanic effects.
— Optical properties: for cementation of translucent restoration (for
example, a porcelain crown) the optical properties of the cement
should parallel these of tooth substance.
— A cement should ideally be adhesion to enamel and dentine, and to
gold alloys, porcelain and acrylic, but not to dental instruments.
— A cement should be bacteriostatic if inserted in a cavity with
residual caries.
— Cement should be have an abundant effect on the pulp.
— Rheological properties are important, a luting cement should have
sufficiently low viscosity to give a low film thickness and should
have a adequate working time at mouth temperature to prevent
placement of the restoration
— Dental amalgam
An amalgam consist of a mixture of two or more metals, one of
which is mercury combined with a powdered silver-tin alloy.
Mercury is a liquid at R.T. and is able to form a workable mass
when mixed with the alloy. This behavior renders the material
suitable for use in dentistry.
The reaction between mercury and alloy which follows mixing is
termed an “amalgamation’ reaction . dental amalgam is the most
widely used of all the available filling materials.
Chemical composition
Mercury used in dental amalgam is purified by distillation, this ensure the
elimination of impurities which would adversely affect the setting
characterization and physical properties of the set amalgam.
The composition of the alloy powder particles varies from one product to
another. Many alloys conveniently described as “conventional alloys”
have a composition in which the concentration of the component metals
are given in table:
Composition of conventional amalgam alloy
Metals
Wt%
Silver
65
Tin
29
Zinc
6 max
copper
2max
Mercury
3 max
— The quantities of silver and tin specified ensure a preponderance of
the silver/tin inter metallic compound Ag
3
Sn, this compound
known as the δ (gamma) phase of the silver-tin system, is formed
over only a small composition range and is particularly
advantageous since it readily undergoes an amalgamation reaction
with mercury.

— The role of zinc is a “scavenger” during the production of the alloy.
The alloys is formed by melting all the constituents metals
together. At the elevated temperatures required for this purpose
there is a tendency for oxidation to occur.
— Oxidation of tin, copper or silver would seriously affect the
properties of the alloy and amalgam. Zinc reacts rapidly and
preferentially with the available oxygen, forming a slag of zinc
oxide which is easily removed. many alloys and oxidation during
melting is preventing by carrying out majority in an inert
atmosphere.
— The majority of alloys powders contain no mercury . those product
containing up to 3%. Mercury are called pre-amalgamated alloys.
They are said to react more rapidly when mixed with mercury.
— The reactions which take place when alloys powder and mercury
are mixed is complex. Mercury diffuses into the alloys particles,
very small particles may become totally dissolved in mercury. The
alloys structure of the surface layer is broken down and the
constituent metals undergo amalgamation with mercury. The
reaction products crystallize to give new phase in the set amalgam.
A large quantity of the initial alloys remains unreacted at the
completion of setting. The structure of the set materials is such
that the unreacted cores of alloy particles remain embedded in a
matrix of reaction products.
— In simplified terms, the reaction for conventional amalgam alloys
may be given by the following unbalanced equation
—
— Ag
3
Sn +Hg Ag
2
Hg
2
+Sn
x
Hg +Ag
3
Sn
— Or δ Hg δ
1
+ δ
2
+ δ
—
— The primary reaction products are a silver-mercury phase (the δ
1
phase) and a tin –mercury phase(the δ
2
phase) . the δ
2
phase has a
rather imprecise structure and the value of X in the formula Sn
x
Hg

may vary from 7 to 8. The equation emphasizes the fact that
considerable quantities of unreacted alloy (δ phase) remain
unconsumed.
— For copper –enriched alloys the reaction may be presented by
— Ag
3
Sn + Cu +Hg Ag
2
Hg
3
+ Cu
6
Sn
5
+Ag
3
Sn
Or
δ + Cu + Hg δ
1
+ Cu
6
Sn
5
+ δ
— the essential difference between this and the reaction for
conventional alloys is the replacement of the tin-mercury, δ
2
phase
in the reaction product with a copper-tin phase.
— In the case of the dispersion-modified, copper-enriched material. It
is believed that the particles of conventional lathe-cut alloy
initially reacts to form δ
1
and δ
2
phases. The δ
2
phase than reacts
with copper from the silver-copper eutectic spheres to form the
copper- tin phase. Thus in these materials, the δ
2
phase exists as an
intermediate reaction product for a short time during setting.
— The reaction rate is quite slow and some times takes several days
or even weeks to reach completion. This is reflected in the rate of
development of mechanical properties.
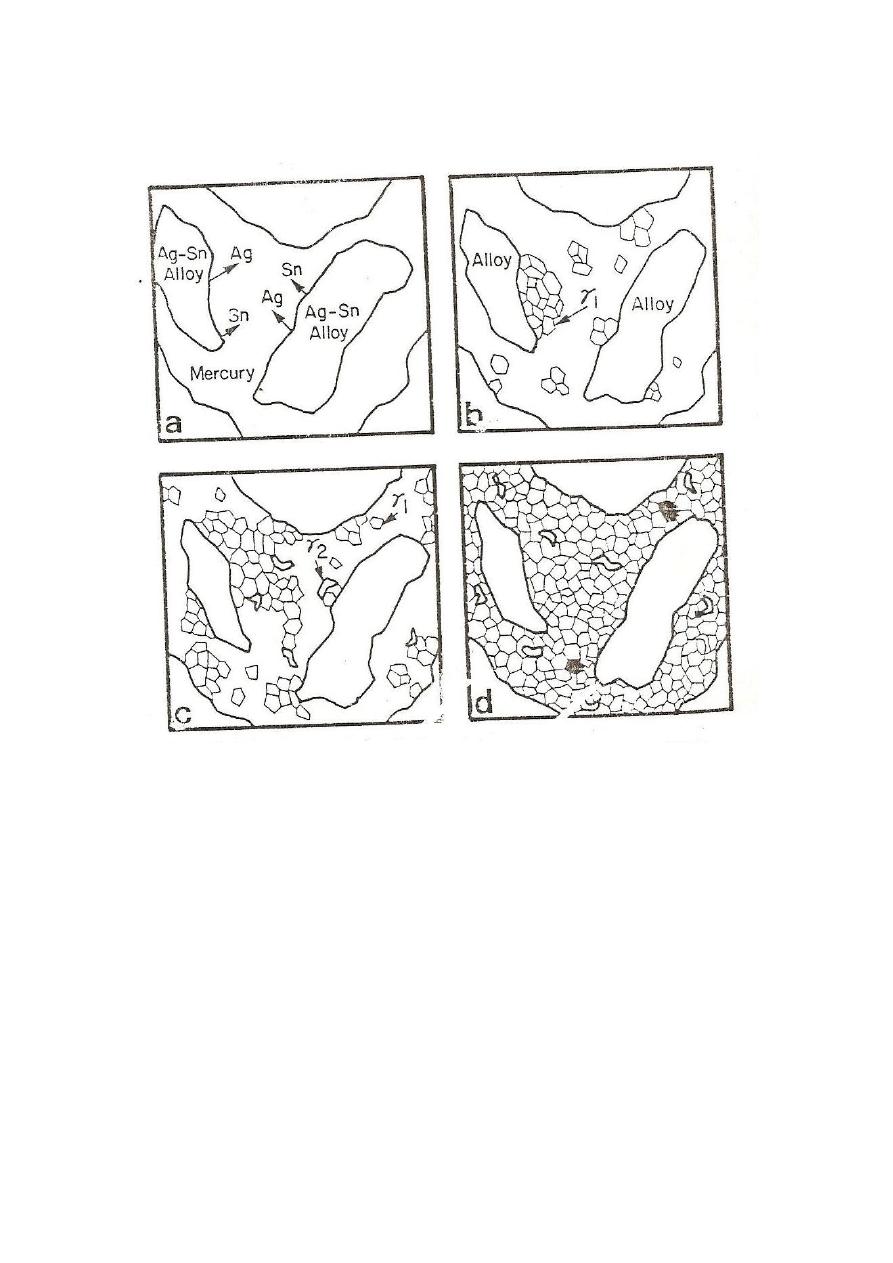
— Amalgamation and resulting structure(Low copper)
—
— Amalgamation occurs when mercury comes into contact with the
surface of Ag-Sn alloy particles. When a powder is triturated, the
silver and tin in the outer proton of the particles dissolve into
mercury. At the same time mercury diffuse into the alloy particles.
The mercury has a limited solubility for silver (0.035wt%) and tin
(0.6wt%).