Dr.Mushriq A. Hussein
M.B.CH.B,F.I.B.M.SPoisoning
ETIOLOGY
The most common agents ingested by young children include family members' medications.
The most common poisonings that lead to hospitalization are due to acetaminophen, lead, and antidepressant medications.
Fatal childhood poisonings are commonly caused by carbon monoxide, hydrocarbons, medications (iron, cardiovascular drugs, cyclic anti-depressants), drugs of abuse, and caustic ingestions.
EPIDEMIOLOGY
more than 50% of poisoning annually occur in children <6 yr old. Almost all of these exposures are unintentional and reflect the propensity for young children to put virtually anything in their mouths.
Poisoning exposures in children 6-12 yr old are much less common, involving only ~ 6% of reported pediatric exposures
More than 90% of toxic exposures in children occur in the home, produce no (82%) or minor (17%) toxicity; 0.01% are fatal.
Approximately 50% of cases involve nondrug substances, such as cosmetics, personal care items, cleaning solutions, plants, and foreign bodies.
COMMON NONTOXIC AND MINIMALLY TOXIC* PRODUCTS
Antacids, non-salicylate-containing
Antibiotics, topical
Antifungals, topical
Body conditioners
Contraceptives (oral) without iron
Corticosteroids, topical
Cosmetics …..etc..
Approach to the patient
The initial approach to the patient with a witnessed or suspected poisoning should be no different than that in any other sick child, starting with stabilization and rapid assessment of the airway, breathing, circulation, and mental status . A serum dextrose concentration should be obtained early in the evaluation of any patient with altered mental status.
History:
historical features such as age of the child (toddler or adolescent), acute onset of symptoms without prodrome, sudden alteration of mental status, multiple system organ dysfunction, or highs levels of household stress
Description of the Exposure
Whether wittnessed in the site,referred from other hospital
Time,amount of substance ingested,it is better to be overestimated.
Symptoms
1.ODOR:
Bitter almonds
Acetone
Garlic
2.OCULAR SIGNS:
Miosis
Mydriasis
Nystagmus
Lacrimation
Retinal hyperemia
3.CUTANEOUS SIGNS
Diaphoresis
Alopecia
Erythema
Cyanosis (unresponsive to oxygen)
4.ORAL SIGNS
Salivation
Oral Burns
Gum lines
5.GASTROINTESTINAL SIGNS:
Diarrhea
Hematemesis
6.CARDIAC SIGNS:
Bradycardia
Hypertension
Hypotension
tachycardia
7.RESPIRATORY SIGNS
Depressed respirations
Tachypnea
8. CNS Signs.
Past Medical History
Social History
Physical Examination
COMPLICATIONS :
A poisoned child can exhibit any one of six basic clinical patterns: coma, toxicity, metabolic acidosis, heart rhythm aberrations, gastrointestinal symptoms, and seizures.
Laboratory Evaluation
For select intoxications (salicylates, some anticonvulsants, acetaminophen, iron, digoxin, methanol, lithium, theophylline, ethylene glycol, carbon monoxide), quantitative blood concentrations are integral to confirming the diagnosis and formulating a treatment plan.. Based on the clinical presentation, additional labs tests that may be helpful include electrolytes and renal function (an elevated anion gap suggests a number of ingestions), serum osmolarity (toxic alcohols), complete blood count, liver function tests, urinalysis (crystals), co-oximetry, and a serum creatine kinase level.
Additional Diagnostic Testing
An electrocardiogram (ECG) is a quick and noninvasive bedside test that can yield important clues to diagnosis and prognosis.A widened QRS interval suggests blockade of fast sodium channels, as may be seen after ingestion of tricyclic antidepressants, diphenhydramine, cocaine, propoxyphene, and carbamazepine, among others
Chest x-ray may reveal signs of pneumonitis (e.g., hydrocarbon ingestion), pulmonary edema (e.g., salicylate toxicity), or a foreign body. Abdominal x-ray can suggest the presence of a bezoar, demonstrate radiopaque tabletsRADIOPAQUE SUBSTANCE ON KUB (MNEMONIC = CHIPPED), or reveal drug packets in a body packer
Hospital admission
Children who have features of poisoning should generally be admitted to hospital.
Children who have taken poisons with delayed actions should also be admitted, even if they appear well.Delayed-action poisons include aspirin, iron, paracetamol, tricyclic antidepressants, and co-phenotrope (diphenoxylate with atropine, Lomotil);
the effects of modified-release preparations are also delayed
Principles of Management
The four principles of management of the poisoned patient are decontamination, enhanced elimination, antidotes, and supportive care.
Decontamination
The goal of decontamination is to prevent absorption of the toxic substance.
decontamination should not be routinely employed for every poisoned patien.
Dermal and ocular decontamination begin with removal of any contaminated clothing and particulate matter, followed by flushing of the affected area with tepid water or normal saline.
Dermal decontamination, especially after exposure to adherent or lipophilic (e.g., organophosphates) agents, should include thorough cleansing with soap and water. Water should not be used for decontamination after exposure to highly reactive agents.such as elemental sodium, phosphorus, calcium oxide, and titanium tetrachloride. After an inhalational exposure, decontamination involves moving the patient to fresh air and administering supplemental oxygen if indicated.
Gastrointestinal (GI) decontamination is a controversial , In general, GI decontamination strategies are most likely to be effective in the first hour after an acute ingestion, GI absorption may be delayed after ingestion of agents that slow GI motility (anticholinergic medications, opioids), massive pill ingestions, sustained-release preparations, and ingestions of agents that can form pharmacologic bezoars (e.g., enteric-coated salicylates) .
Described methods of GI decontamination include induced emesis with ipecac, gastric lavage, cathartics, activated charcoal, and whole-bowel irrigation (WBI).
Syrup of ipecac contains 2 emetic alkaloids that work in both the central nervous system (CNS) and locally in the GI tract to produce vomiting.
Criteria for use:
the ipecac can be administered within 30-90 min of the ingestion.
There is a substantial risk of serious toxicity to the patient.
There are no contraindications to the use of ipecac .
There is no alternative therapy available to decrease GI absorption.
The use of ipecac will not adversely affect more definitive therapy that may be provided at the hospital.
Gastric Lavage
in most clinical scenarios, the use of gastric lavage is no longer recommended?why
lavage may be considered in the extremely rare instance of a child who presents very soon (30-60 min) after an ingestion of a highly toxic agent for which antidotal therapy or supportive care is unlikely to be of substantial benefit.
Single-Dose Activated Charcoal :
activated charcoal is thought to potentially be the most useful.
toxins are adsorbed onto its surface, thus preventing absorption from the GI tract. Charcoal is most likely to be effective when given within 1 hr of ingestion.
The dose of activated charcoal is 1 g/kg in children
Some toxins, including heavy metals, iron, lithium, hydrocarbons, cyanide, and low-molecular-weight alcohols, are not significantly bound to charcoal. Charcoal administration should also be avoided after ingestion of a caustic substance.
one must ensure that the patient's airway is intact or protected and that he or she has a benign abdominal exam.Constipation is another common side effect of activated charcoal, and in extreme cases, bowel perforation has been reported.
Cathartics (sorbitol, magnesium sulfate, magnesium citrate) have been used in conjunction with activated charcoal to prevent constipation and accelerate evacuation of the charcoal-toxin complex.
WBI involves instilling large volumes (35 mL/kg/hr in children or 1-2 L/hr in adolescents) of a polyethylene glycol electrolyte solution (e.g., GoLYTELY) to “cleanse” the entire GI tract.Complications of WBI include vomiting, abdominal pain, and abdominal distention
Enhanced Elimination
Multiple-Dose Activated Charcoal
MDAC is typically given as 0.5 g/kg every 4-6 hr (for ≤24 hr) and continued until there is significant clinical improvement. the airway and abdominal exam should be assessed before each dose. MDACs enhance elimination via 2 proposed mechanisms: interruption of enterohepatic recirculation and “GI dialysis,”which uses the intestinal mucosa as the dialysis membrane and pulls toxins from the bloodstream back into the intraluminal space, where they are adsorbed to the charcoal.
Urinary Alkalinization :Urinary alkalinization is accomplished with a continuous infusion of sodium bicarbonate–containing intravenous fluids, with a goal urine pH of 7.5-8. Alkalinization of the urine is most useful in managing salicylate and methotrexate toxicity. Alkalinization may also be beneficial in managing phenobarbital toxicity
complications of urinary alkalinization include electrolyte derangements, such as hypokalemia and hypocalcemia, Serum pH should be closely monitored because a serum pH of >7.55 is potentially dangerous to cellular functions. volume overload could cccur after treatment. This method of enhanced elimination is contraindicated in patients who are unable to tolerate the large volumes of fluid needed to achieve alkalinization, including patients with heart failure, kidney failure, pulmonary edema, or cerebral edema.
Hemodialysis:
Toxins that are amenable to dialysis have the following properties: low volume of distribution (<1 L/kg), low molecular weight, low degree of protein binding, and high degree of water solubility. toxins for which dialysis may be useful include methanol and ethylene glycol, as well as large symptomatic ingestions of salicylates, theophylline, bromide, or lithium
Intralipid Emulsion Therapy
A potentially life-saving intervention of infusing Intralipid emulsions
is a means of sequestering fat-soluble drugs and decreasing their
impact at target organs. Initial experience regarding this intervention
has been developed by anesthesiologists as a reversal agent for asystole
resulting from inadvertent intravenous injection of bupivacaine.
.Using the same 20% Intralipid used for total parenteral nutrition,
a bolus dose of 1.5 mL/kg is given over 3 min, followed by an infusion
of 0.25 mL/kg/min until recovery or a total of 10 mL/kg has been
infused. Lipophilic drugs are potentially bound by Intralipid
emulsions, including calcium channel blockers (verapamil and diltiazem)
and tricyclic antidepressants.
Antidotes:
Glucagon and/or insulin and glucose
Calcium channel antagonists
Diphenhydramine and/or benztropine
Dystonic reactions
Calcium salts
Fluoride, calcium channel blockers
Protamine
Heparin
Folinic acid
Methotrexate, trimethoprim, pyrimethamine
Sodium bicarbonate
Sodium channel blockade (tricyclic antidepressants, type 1 antiarrhythmics)
Acetaminophen
N-Acetylcysteine
Anticholinergics
Physostigmine
Supportive Care
The goal is to support the vital functions of the patient until the patient can eliminate the toxin from the system. If the level of consciousness is depressed, and a toxic substance is suspected, glucose (1 g/kg IV), 100% oxygen, and naloxone should be administered.
PROGNOSIS : Mortality from poisoning is rare .The most common exposures resulting in death include carbon monoxide, hydrocarbons, and opioids (all of which interfere with oxygen delivery to tissues). Medications that affect cardiac function (calcium channel antagonists, β-adrenergic sustained-release medications, tricyclic antidepressants and adrenergic ingestions) also are in the top 10 causes of death from poisoning.
PREVENTION
Properly educating parents to use childproof medication containers, to store toxic substances in locked cabinets, and to label toxic chemicals properly is necessary for preventing ingestionsPoisoning
lec:2
Selected Compounds Commonly Involved in Pediatric Poisonings
Acetaminophen
it can be unintentionally ingested by young children, taken in an intentional overdose by adolescents and adults, or inappropriately dosed in all ages.Pathophysiology
Acetaminophen(APAP) toxicity results from the formation of a highly reactive intermediate metabolite, N-acetyl-p-benzoquinone imine (NAPQI) which is then immediately conjugated with glutathione to form a nontoxic mercapturic acid conjugate.
The single acute toxic dose of acetaminophen is generally considered to be >200 mg/kg in children and >7.5-10 g in adolescents and adults.
Clinical and Laboratory Manifestations:
The fowllowing are the 4 stages of the clinical course of acetaminophen toxicity
Stage I…..
0.5-24 hr onset after ingestion
Anorexia, nausea, vomiting, malaise, pallor, diaphoresis
Labs typically normal, except for acetaminophen level
Stage II ……24-48 hr
Resolution of earlier symptoms; right upper quadrant abdominal pain and ; elevated bilirubin, prothrombin time, and hepatic enzymes; oliguria
Stage III……..3-5 days
Peak liver function abnormalities; fulminant hepatic failure; multisystem organ failure and potential death.
stage IV……4 days-2 wk
Resolution of liver function abnormalities
Clinical recovery precedes histologic recovery
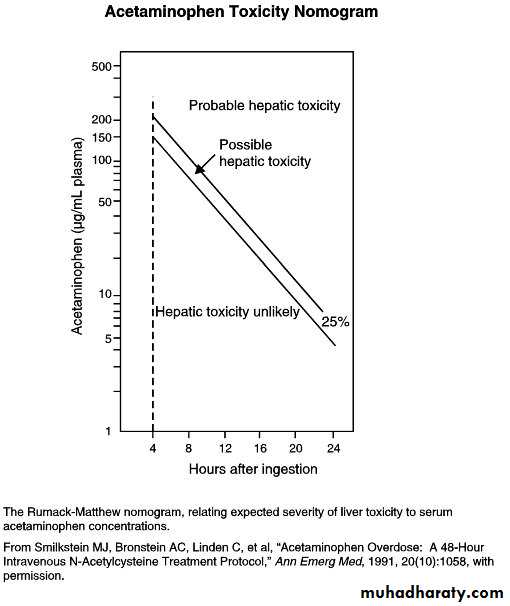
Acetaminophen levels obtained <4 hr after ingestion are difficult to interpret and cannot be used to estimate the potential for toxicity
Assessment of the patient who presents with an unknown time of ingestion or a history of chronic supratherapeutic ingestion is more complicated.
Treatment
Initial treatment should focus on the ABCs and consideration of decontamination with activated charcoal in patients who present within 1-2 hr of ingestion.The antidote for acetaminophen poisoning is NAC, which works primarily via replenishing hepatic glutathione stores.
NAC is continued for at least 21-24 hr and until the patient is clinically well, with improving transaminases, normalizing synthetic function, and acetaminophen level <10 microg/mL. Patients who develop hepatic failure in spite of NAC therapy may be candidates for liver transplantation
Transaminases, synthetic function, and renal function should be followed daily while the patient is being treated with NAC.
Side effects:anaphylaxis,mild increase in INR.
1. Prophylactic: By definition, these patients have a normal aspartate aminotransferase (AST). If the APAP level is known and the ingestion is within 24 hr of the level being drawn, then treatment.Any patient with a serum APAP level in the possible or probable hepatotoxicity range per the nomogram should be treated with N-acetylcysteine (NAC). If the AST is normal and the APAP becomes nondetectable, then treatment may be discontinued. If the AST becomes elevated, then the patient moves into the next category of treatment (injury). If APAP is still present, treatment should be continued until the level is nondetectable
2. Hepatic Injury: These patients are exhibiting evidence of
hepatocellular necrosis, manifested first as elevated livertransaminases (AST rises first, then the alanine aminotransferase),followed by a rise in the INR. Any patient in this category requires therapy with NAC (IV or oral).
3. Acute Liver Failure: patients should be referred for consideration of liver transplant.
4. Repeated Supratherapeutic Ingestion: In the asymptomatic patient, if the AST is normal and the APAP is <10 μg/mL, then no therapy is indicated. A normal AST and an elevated APAP warrants NAC dosing for at least long enough for the drug to metabolize while the AST remains normal.An elevated AST puts the patient in the “hepatic injury” category described above.
N-acetylcysteine (NAC(
Prevents binding of NAPQI to hepatocytesReduces NAPQI
Conjugates NAPQI
Increases sulfation metabolism
Smells like rotten eggs
Oral formulation may need to be given via NGT
Dilute with juice
Use metoclopramide or ondansetron if not tolerated due to vomiting
Salicylates
The incidence of salicylate poisoning in young children has declined dramatically .
Pathophysiology
Salicylates lead to toxicity by direct stimulation of the respiratory center, uncoupling of oxidative phosphorylation, inhibition of the tricarboxylic acid cycle, and stimulation of glycolysis and gluconeogenesis. The acute toxic dose of salicylates is generally considered to be >150 mg/kg, Therapeutic salicylate levels are 10-20 mg/dL, and levels >30 mg/dL warrant treatment.
Clinical and Laboratory Manifestations Salicylate ingestions are classified as acute or chronic.
Early signs of acute salicylism include nausea, vomiting, diaphoresis, and tinnitus. Chronic salicylism can have a more insidious presentation, and patients can show marked toxicity at significantly lower salicylate levels than in acute toxicity.
BGA,LFT,ELECTROLYTE,B.G,SALICYLATE LEVEL,ACETAMINOPHEN LEVEL, Serum and urine pH ,CXR.
Treatment
For the patient who presents soon after an acute ingestion, initial treatment should include gastric decontamination with activated charcoal. Initial therapy focuses on aggressive volume resuscitation and prompt initiation of sodium bicarbonate therapy in the symptomatic patient, even before obtaining serum salicylate levels. The primary mode of therapy for salicylate toxicity is urinary alkalinization .Alkalinization is achieved by administration of a sodium bicarbonate infusion at approximately 1.5 times maintenance fluid rates. The goals of therapy include a urine pH of 7.5-8, a serum pH of 7.45-7.55. In cases of severe toxicity, dialysis may be required
Digoxin
Digoxin is a cardiac glycoside extracted from the leaves of Digitalis lanata .
Therapeutically, digoxin is used in the management of heart failure and some supraventricular tachydysrhythmias.
Acute overdose can occur in the setting of dosing errors (especially in younger children), unintentional or intentional medication ingestion. Chronic toxicity can result from alteration of the digoxin dose, alteration in digoxin clearance due to renal impairment, or drug interactions.
Pathophysiology
Digoxin blocks the Na+, K+-ATPase pump, leading to intracellular loss of K+ and gain of Na+ and Ca2 → rise in ca → improve intropy → subsequent increase in atrial,nodal and ventricular ectopy.
Digoxin also affects nodal conduction, leading to slowed conduction through the AV node.
Digoxin has a very narrow therapeutic index. Therapeutic plasma digoxin concentrations are 0.5-2.0 ng/mL; a level of >2 ng/mL is considered toxic .
Medications known to increase serum digoxin concentrations include the macrolides, spironolactone
Clinical and Laboratory Manifestations :
Nausea and vomiting are common initial symptoms of acute digoxin toxicity, manifesting within 6 hr of overdose. Cardiovascular manifestations include bradycardia, heart block, and a wide variety of dysrhythmias. CNS manifestations consist of lethargy, confusion. Chronic toxicity is more insidious and manifests with GI symptoms, altered mental status, and visual disturbances. Investigations:
should include an ECG, serum digoxin level, serum potassium, and kidney function tests. The serum digoxin level should be assessed at least 6 hr after ingestion.
Treatment
good general supportive care and gastric decontamination with activated charcoal if the ingestion was recent. An antidote for digoxin, digoxin-specific Fab antibody fragments (Digibind or Digifab) .Fab fragments bind free digoxin in both the intravascular and the interstitial spaces to form a pharmacologically inactive complex that is subsequently renally eliminated.
Indications for Fab …….
If Digibind or Digifab are not readily available, phenytoin or lidocaine may be beneficial in managing ventricular irritability. Atropine is potentially useful in managing symptomatic bradycardia.
Consultation with a cardiologist.
Cholinesterase-Inhibiting Insecticides
The most commonly used insecticides are organophosphates and carbamates; both are inhibitors of cholinesterase enzymes (acetylcholinesterase,pseudocholinesteras, and erythrocyte acetylcholinesterase).
Pathophysiology
Organophosphates and carbamates produce toxicity by binding to and inhibiting acetylcholinesterase, preventing the degradation of acetylcholine and resulting in its accumulation at nerve synapses.
Clinical and Laboratory Manifestations
Clinical manifestations of toxicity relate to the accumulation of acetylcholine at peripheral nicotinic and muscarinic synapses and in the CNS. symptoms of cholinergic excess at muscarinic receptors is DUMBBELS, which stands for diarrhea/defecation, urination, miosis, bronchorrhea/bronchospasm, bradycardia, emesis, lacrimation, and salivation. Nicotinic signs and symptoms include muscle weakness, fasciculations, tremors, hypoventilation (diaphragm paralysis), hypertension, tachycardia, and dysrhythmias. Severe manifestations include coma, seizures, shock, arrhythmias and resp. failure.
Diagnosis : is based primarily on history and physical exam findings. Red blood cell cholinesterase and pseudocholinesterase concentrations.
Treatment
Basic decontamination should be performed, including washing all exposed skin with soap and water and immediately removing all exposed clothing.
Administering activated charcoal after ingestion of insecticides is controversial,
Basic supportive care
Two antidotes are useful in treating cholinesterase inhibitor poisoning: atropine and pralidoxime.
**Atropine 0.05-0.1 mg/kg repeated q5-10min
as needed via IV/ET(endotracheal tube) S/E: Tachycardia, dry mouth, blurred vision, urinary retention
**Pralidoxime 25-50 mg/kg over 5-10 min (max:200 mg/min); can be repeated after 1-2 hr, then q10-12hr as needed through IV/IM S/E :Nausea, dizziness, headache,tachycardia, muscle rigidity,bronchospasm (with rapid administration).
Even with treatment, some patients develop a delayed polyneuropathy and a range of chronic neuropsychiatric symptoms.
Hydrocarbons
Hydrocarbons include a wide array of chemical substances found in thousands of commercial products .
Pathophysiology
The most important manifestation of hydrocarbon toxicity is aspiration pneumonitis via inactivation of the type II pneumocytes and resulting surfactant deficiency .
Compounds with low viscosity, such as mineral spirits, naphtha, kerosene, gasoline, and lamp oil, spread rapidly across surfaces and cover large areas of the lungs when aspirated
Only small quantities (<1 mL) of low-viscosity hydrocarbons need be aspirated to produce significant injury.
Pneumonitis does not result from dermal absorption of hydrocarbons or from ingestion in the absence of aspiration. Gasoline and kerosene are poorly absorbed, but they often cause considerable irritation of the GI mucosa as they pass through the intestines.
A number of volatile hydrocarbons, including toluene, propellants, refrigerants, and volatile nitrites, are commonly abused by inhalation. ), can sensitize the myocardium to the effects of endogenous catecholamines. Another hydrocarbons is Methylene chloride, found in some paint removers, is metabolized to carbon monoxide. Benzene is known to cause cancer, most commonly acute myelogenous leukemia, after long-term exposure.
Clinical and Laboratory Manifestations
Transient, mild CNS depression
Aspiration is characterized by coughing, which usually is the first clinical finding.
Chest radiographs may initially be normal, but they often show abnormalities within 6 hr of exposure in patients who have aspirated. . Pneumatoceles can appear on the chest radiograph 2-3 wk after.
Respiratory symptoms. can remain mild or progress rapidly to (ARDS) .
Fever and leukocytosis.
Treatment
Emesis and lavage are contraindicated given the risk of aspiration.
Activated charcoal is not useful because it does not bind the common hydrocarbons and can also induce vomiting.
respiratory treatment is supportive, Neither corticosteroids nor prophylactic antibiotics have shown any clear benefit .
Patients with dysrhythmias should be treated with β-blockers (usually esmolol).
Poisoning lec:3
Iron
Historically, iron was a common cause of childhood poisoning deaths. The severity of an exposure is related to the amount of elemental iron ingested. Ferrous sulfate contains 20% elemental iron, ferrous gluconate 12%, and ferrous fumarate 33%.
Pathophysiology:
Iron is directly corrosive to the GI mucosa, leading to hematemesis, melena, ulceration, infarction, and potential perforation.
Early iron-induced hypotension is due to massive volume losses, increased permeability of capillary membranes, and venodilation mediated by free iron.
Iron accumulates in tissues, including the Kupffer cells of the liver and myocardial cells, leading to hepatotoxicity, coagulopathy, and cardiac dysfunction
Pediatric patients who ingest >40 mg/kg of elemental iron should be referred to medical care for evaluation, though moderate to severe toxicity is typically seen with ingestions of >60 mg/kg.
Clinical and Laboratory Manifestations:
Iron toxicity is classically described in 4, often overlapping, stages
The initial stage, 30 min to 6 hr after ingestion, consists of profuse vomiting and diarrhea (often bloody), abdominal pain, and significant volume losses leading to potential hypovolemic shock.
The second stage, 6 to 24 hr after ingestion, is the quiescent phase, as GI symptoms typically resolve. careful clinical exam can reveal subtle signs of hypoperfusion, including tachycardia, pallor, and fatigue
During the third stage, occurring 12 to 24 hr after ingestion, patients develop multisystem organ failure, shock, hepatic and cardiac dysfunction, acute lung injury or ARDS, and profound metabolic acidosis. Death occurs most commonly during this stage.
the fourth stage (4 to 6 wk after ingestion) is marked by formation of strictures and signs of GI obstruction.
Symptomatic patients and patients with a large exposure by history should have serum iron levels drawn 4-6 hr after ingestion.
Other lab. Invstigations….
blood gas, complete blood count, serum glucose level, liver function tests, and coagulation parameters
An abdominal x-ray might reveal the presence of iron tablets.
Careful attention should be paid to ongoing monitoring of the patient's hemodynamic status.
Treatment
Close clinical monitoring, combined with aggressive supportive and symptomatic care, is essential to the management of iron poisoning.
Activated charcoal does not adsorb iron, and WBI remains the decontamination strategy of choice.
Deferoxamine, a specific chelator of iron, is the antidote for moderate to severe iron intoxication.
Indications for deferoxamine treatment include a serum iron concentration of >500 mg/dL or moderate to severe symptoms of toxicity, regardless of serum iron concentration.
Deferoxamine is preferably given via continuous IV infusion at a rate of 15 mg/kg/hr. Hypotension is a common side effect of deferoxamine infusion and is managed by slowing the rate of the infusion and administering fluids and/or vasopressors as needed
Prolonged deferoxamine infusion (>24 hr) has been associated with pulmonary toxicity (acute respiratory distress syndrome) and Yersinia sepsis.
The deferoxamine-iron complex can color the urine reddish (“vin ros?”), though this is an unreliable indicator of iron excretion, therapy is typically continued until clinical symptoms resolve.
Lead Poisoning
Lead is a metal that exists in four isotopic forms. Clinically, it is purely a toxicant; no organism has an essential function that is lead-dependent.. The blood lead level (BLL) is the gold standard for determining health effects. Previously BLL of 10 microg/dL or greater regarded as a level of concern for public health purposes. The Centers for Disease Control and Prevention (CDC),recognizing that a BLL of 10 μg/dL qualifies neither as a threshold of toxicity nor as a protective parameter of children with lead exposure,changed its standard. It no longer refers to a level of concern or toxicity but has designated 5 μg/dL as the “reference value based on the 97.5th percentile of the population BLL in children aged 1-5 years to identify children living or staying for long periods in environments that expose them to lead hazards.”
Although stated as a reference value, it is likely that clinicians and departments of health will consider this a threshold for action. It is important to recognize that lead toxicity occurs at levels below 5 μg/dL; no safe level has been identified. In part, this reflects the accuracy limitations of available clinical laboratory methodologies.
Sources of Exposure
Several hundred products contain lead, including batteries, cable sheathing, cosmetics, mineral supplements, plastics, toys,lead-based paint. As paint deteriorates, it chalks, flakes, and turns to dust .The dust can coat all surfaces, including children's hands. All of these forms of lead can be ingested.
SOURCES OF LEAD
Paint chips
Dust
Soil
Parent's or older child's occupational exposure (auto repair, smelting, construction, remodeling, plumbing, gun/bullet exposure, painting)
Glazed ceramics
Home remedies, including antiperspirants
Stored battery casings (or living near a battery smelter)
Lead-based gasoline
Indoor firing ranges
Lead-based cosmetics (kohl)
Lead plumbing (water)
Imported foods in lead-containing cans
Imported toys
Home renovations
Antique toys or furniture
Metabolism
The nonnutritive hand-to-mouth activity of young children is the most common pathway by which lead enters the body. In nearly all cases, lead is ingested.
After absorption, lead is disseminated throughout the body. Most retained lead accumulates in bone, where it may reside for years. It circulates bound to erythrocytes; about 97% in blood is bound on or in the red blood cells. The plasma fraction is too small to be measured by conventional techniques; it is presumably the plasma portion that may enter cells and induce toxicity. Thus, clinical laboratories report the blood lead level, not the serum or plasma lead level.
Lead has multiple effects in cells………
It binds to enzymes, particularly those with available sulfhydryl groups, changing the contour and diminishing function.
The heme pathway, present in all cells, has three enzymes susceptible to lead inhibitory effects.
The last enzyme in this pathway, ferrochelatase, enables protoporphyrin to chelate iron, thus forming heme.
Protoporphyrin is readily measurable in red blood cells. Levels of protoporphyrin higher than 35 microg/dL are abnormal and are consistent with lead poisoning, iron deficiency, or recent inflammatory disease.
Measurement of the erythrocyte protoporphyrin (EP) level is, therefore, a useful tool for monitoring biochemical lead toxicity
A second mechanism of lead toxicity works via its competition with calcium. Many calcium-binding proteins have a higher affinity for lead than for calcium. Lead bound to these proteins may alter function, resulting in abnormal intracellular and intercellular signaling.
a third mechanism prevents the development of the normal tertiary brain structure. A longitudinal study of childhood lead poisoning that followed a cohort from birth and into their 20s performed MRI and functional MRI confirmed the association of early childhood exposure and subsequent decreased gray and white matter volume and neuronal function. The investigators concluded that early lead exposure in life causes persistent reorganization of brain architecture and diminished function.
Failure to construct the appropriate tertiary brain structure during the first few years of life may result in a permanent abnormality.
Clinical Effects
The BLL is the best-studied measure of the lead burden in children
Hearing and height are inversely related to BLLs in children; in neither case, however, does the lead effect reach a level that would bring an individual child to medical attention.
As BLLs increased in the study population, more sound (at all frequencies) was needed to reach the hearing threshold. Children with higher BLLs are slightly shorter than those with lower levels; for every 10 microg increase in the BLL, the children are 1 cm shorter. Chronic lead exposure also may delay puberty.
there is agreement that BLLs, expressed as either a level obtained at around 2 yr of age or a measure that integrates multiple BLLs drawn from a subject over time, are inversely related to cognitive test scores. Because the BLLs from early childhood are predictors of the cognitive test results performed years later, this finding implies that the effects of lead can be permanent.
The effect of in utero lead exposure is less clear maternal blood lead levels between 0 and 10microg/dL even as early as the first trimester were associated with about a 6-point drop in cognitive test score results when the children were tested up to age 10 yr.
Behavior also is adversely affected by lead exposure. Hyperactivity is noted in young school-aged children with histories of lead poisoning or with concurrent elevations in BLL. Older children with higher bone lead content are more likely to be aggressive and to have behaviors that are predictive of later juvenile delinquency.
These studies support the concept that early exposure to lead can result in long-term deficits in cognition and behavior; they also hold out the possibility that reductions in lead burden may be associated with improvement in cognitive test scores.
Clinical Symptoms
Gastrointestinal Tract and Central Nervous System
GI symptoms of lead poisoning include anorexia, abdominal pain, vomiting, and constipation, often occurring and recurring over a period of weeks.
CNS symptoms are related to worsening cerebral edema and increased intracranial pressure. Headaches, change in mentation, lethargy, papilledema, seizures, and coma leading to death are rarely seen at levels lower than 100 microg/dL but have been reported in children with a BLL as low as 70 microg/dL.. There is no clear cutoff BLL value for the appearance of hyperactivity, but it is more likely to be observed in children who have levels higher than 20 microg/dL.
At high levels (>100 microg/dL), renal tubular dysfunction is observed. Lead may induce a reversible Fanconi syndrome.
at high BLLs, red blood cell survival is shortened, possibly contributing to a hemolytic anemia, although most cases of anemia in lead-poisoned children are due to other factors, such as iron deficiency and hemoglobinopathies.
Older patients may develop a peripheral neuropathy.
Diagnosis
ScreeningIt is estimated that 99% of lead-poisoned children are identified by screening procedures rather than through clinical recognition of lead-related symptoms
Because of substantial regional and local variations in the prevalence of elevated lead levels, the recommendation to screen all children is controversial.
In general, it is advisable to screen high-risk children, including those with the following characteristics:
1.Having a sibling or playmate with an elevated lead level
2.Living with an adult whose job or hobby involves lead
3.Living near an industry that is likely to release lead (e.g., smelting plant, battery-recycling plant
4. the child is a recent immigrant from a country that still permits use of leaded gasoline
5.or the child has pica or developmental delay
Venous sampling is preferred to capillary sampling because the chances of false-positive and false-negative results are less with the former.
Interpretation of Blood Lead Levels
The threshold for lead effects and the level of concern for risk management purposes are not the same.
A screening value at or above 2microg/dL is consistent with exposure and requires a second round of testing for a diagnosis and to determine the appropriate intervention. The timing for the “repeat” evaluation depends on the initial value . If the diagnostic (second) test confirms that the BLL is elevated, then further testing is required by the recommended schedule . A confirmed venous BLL of 45 microg/dL or higher requires prompt chelation therapy.
NOT RECOMMENDED AT ANY BLOOD LEAD CONCENTRATION
Searching for gingival lead lines
Evaluation of renal function (except during chelation with CaNa2EDTA [ethylenediaminetetraacetic acid])
Testing of hair, teeth, or fingernails for lead
Radiographic imaging of long bones
X-ray fluorescence of long bones
BLL determinations remain the gold standard for evaluating children.
.
Radiographs of long bones may show dense bands at the metaphyses, which may be difficult to distinguish from growth arrest lines but, if caused by lead, are indicative of months to years of exposure.
For children with acute symptoms,, a kidneys-ureters-bladder (KUB) radiograph may reveal radiopaque flecks in the intestinal tract, a finding that is consistent with recent ingestion of lead-containing plaster or paint chips.
An elevated EP value that cannot be attributed to iron deficiency or recent inflammatory illness is both an indicator of lead effect and a useful means of assessing the success of the treatment.
Treatment
Once lead is in bone, it is released only slowly and is difficult to remove even with chelating agents. Because the cognitive/behavioral effects of lead may be irreversible, the main effort in treating lead poisoning is to prevent it from occurring and to prevent further ingestion by already-poisoned children.
The main components in the effort to eliminate lead poisoning are universally applicable to all children (and adults), as follows..
(1) identification and elimination of environmental sources of lead exposure,
(2) behavioral modification to reduce nonnutritive hand-to-mouth activity, and
(3) dietary counseling to ensure sufficient intake of the essential elements calcium and iron.
(4)For the small minority of children with more severe lead poisoning, drug treatment is available that enhances lead excretion.
Parental efforts at reducing the hand-to-mouth activity of the affected child are necessary to reduce the risk of lead ingestion, handwashing is best limited to the period immediately before nutritive hand-to-mouth activity occurs.
Because there is competition between lead and essential minerals, it is reasonable to promote a healthy diet that is sufficient in calcium and iron.
In general, for children 1 yr of age and up a calcium intake of about 1 g per day is sufficient .Iron requirements also vary with age, ranging from 6 mg/day for infants to 12 mg/day for adolescents.Drug treatment to remove lead is lifesaving for children with lead encephalopathy. In nonencephalopathic children, it prevents symptom progression.
<25 μg/dL
Chelation is not indicated.
25-45 μg/dL
Chelation is not routinely indicated because no evidence exists that chelation prevents or reverses neurotoxicity. Some patients may benefit from (oral) chelation (e.g., succimer), especially if elevated levels persist despite aggressive environmental intervention and abatement
45-70 μg/dL
Chelation is indicated with either succimer or CaNa2EDTA (if no clinical symptoms suggestive of encephalopathy are present [e.g., headache, persistent vomiting]). If symptoms of encephalopathy are seen, chelation With dimercaprol and CaNa2EDTA are indicated. Before chelation, an abdominal radiograph scan should be taken to evaluate for the possible removable of enteral lead.
>70 μg/dL
Inpatient chelation therapy with dimercaprol and CaNa2EDTA is indicated.
Four d rugs are available :
2,3-dimercaptosuccinic acid (DMSA [succimer])CaNa2EDTA (versenate)
penicillamine
British antilewisite (BAL [dimercaprol]),
DMSA and penicillamine can be given orally, whereas CaNa2EDTA and BAL can be administered only parenterally .
The choice of agent is guided by the severity of the lead poisoning, the effectiveness of the drug, and the ease of administration.
NAME
SYNONYMDOSE
TOXICITY
Succimer
Chemet, 2,3-dimercaptosuccinic acid (DMSA)
350 mg/m2 body surface area/dose (not 10 mg/kg) q8h, PO for 5 days, then q12h for 14 days
Gastrointestinal distress, rashes; elevated LFTs, depressed white blood cell count
Edetate*
CaNa2EDTA (calcium disodium edetate), versenate
1,000-1,500 mg/m2 body surface area/d; IV infusion—continuous or intermittent; IM divided q6h or q12h for 5 days
Proteinuria, pyuria, rising blood urea nitrogen/creatinine—all rare Hypercalcemia if too rapid an infusion Tissue inflammation if infusion infiltrates
British antilewisite (BAL)
Dimercaprol, British antilewisite
300-500 mg/m2 body surface area/ day; IM only divided q4h for 3-5 days. Only for BLL ≥ 70 ?g/dL
GI distress, altered mentation; elevated LFTs, hemolysis if glucose-6-phosphate dehydrogenase deficiency; no concomitant iron treatment
D-Pen
Penicillamine
10 mg/kg/d for 2 wk increasing to 25-40 mg/kg/d; oral, divided q12h.For 12-20 wk
Rashes, fever; blood dyscrasias, elevated LFTs, proteinuriaAllergic cross-reactivity with penicillin
All of the drugs are effective in reducing BLLs when given in sufficient doses and for the prescribed time. These drugs also may increase lead absorption from the gut and should be administered to children in lead-free environments. None of these agents removes all lead from the body. Within days to weeks after completion of a course of therapy the BLL rises, even in the absence of new lead ingestion. The source of this rebound in the BLL is believed to be bone.
With successful intervention, BLLs decline, with the greatest fall in BLL occurring in the first 2 mo after therapy is initiated. Subsequently the rate of change in BLL declines slowly so that by 6-12 mo after identification, the BLL of the average child with moderate lead poisoning (BLL >20 microg/dL) will be 50% lower.
Early screening remains the best way of avoiding and therefore obviating the need for the treatment of lead poisoning.
Good luck
Caustic ingestions may cause dysphagia, epigastric pain, oral mucosal burns, and low-grade fever. Patients with esophageal lesions may have no oral burns or may have significant signs and symptoms. Treatment depends on the agent ingested and the presence or absence of esophageal injury.Alkali agents may be solid, granular, or liquid (e.g., Liquid Drano, 9.5% sodium hydroxide, or Liquid Plumer, 8% potassium hydroxide). These liquid agents are tasteless and produce full-thickness liquefaction necrosis of the esophagus or oropharynx. When the esophageal lesions heal, strictures form. Ingestion of these agents also creates a long-term risk of esophageal carcinoma. Many clinicians do not recommend the routine use of diluents as a first aid measure because of the lack of proven efficacy and the potential risk of induced vomiting. Subsequent treatment includes antibiotics if there are signs of infection and dilation of late-forming (2 to 3 weeks later) strictures.
Acid agents, such as Lysol Toilet Bowl Cleaner (8.5% hydrochloric acid) or Vanish Toilet Bowl Cleaner (65% sodium acid sulfate), can injure the lungs (with hydrochloric acid fumes), oral mucosa, esophagus, and stomach. Because acids taste sour, children usually stop drinking the solution, limiting the injury. Acids produce a coagulation necrosis, which limits the chemical from penetrating into deeper layers of the mucosa and damages tissue less severely than alkali.
Initial treatment of caustic exposures includes thorough
removal of the product from the skin or eye by flushing with
water. Emesis and lavage are contraindicated. Activated charcoal
should not be used because it does not bind these agents and can
predispose the patient to vomiting and subsequent aspiration. Stridor or other signs of respiratory distress should alert the provider to the need for a thorough evaluation of the airway for potential intubation or surgical airway management. Endoscopy can be performed within12-24 hr of ingestion for prognostic and diagnostic purposes in symptomatic patients or those in whom injury is suspected on the basis of history and knowncharacteristics of the ingested product. Endoscopy is contraindicated in any patient with signs of peritonitis. The use of corticosteroids or prophylactic antibiotics is not beneficial
Ingested button batteries also may produce a caustic mucosal injury from sodium hydroxide, potassium hydroxide, or mercuric oxide. If the batteries pass to the stomach, no further therapy is needed because they are likely to be passed in the stool within 1 week. Batteries that remain in the esophagus may cause esophageal burns and erosion and should be removed with an endoscope.