Al-Mustansiriyah University
College of Medicine - Department of MedicineDivision of Radiology
IMAGING OF ENDOCRINE SYSTEM
DR. ABDULLATEEF AL-BAYATITeaching Board Member
CABMS-RAD
10
IMAGING OF THE PITUITARY GLAND
The pituitary gland consists of two functionally separate components:
• Anterior lobe: secretes hormones including adrenocorticotropic hormone (ACTH), thyroid stimulating hormone (TSH), luteinizing hormone (LH), follicle stimulating hormone (FSH) and growth hormone.
• Posterior lobe: stores hormones secreted by hypothalamus (vasopressin and oxytocin).
• The pituitary stalk connects the pituitary gland to the hypothalamus and contains axons that transport releasing hormones.
Tumors of the pituitary region include:
• Pituitary adenomas, classified on hormonal status and on size criteria• Macroadenoma: >10 mm
• Microadenoma: <10 mm
• Pituitary carcinoma (rare)
• Meningioma
• Craniopharyngioma
• Metastasis
• Optic chiasm glioma (commonly associated with neurofibromatosis, type 1).
Pituitary region tumors may present clinically in multiple ways depending on the size, location and hormonal status of the tumor:
• Compression of structures
• Pituitary: hypopituitarism
• Optic chiasm: bitemporal hemianopia
• Raised intracranial pressure due to a large tumor or obstructive hydrocephalus (Headache, nausea, vomiting).
• Endocrine syndromes.
Endocrine syndromes associated with pathology of the pituitary gland and hypothalamus include:
• Pituitary dwarfism
• Hypoplasia of adenohypophysis
• Ectopic neurohypophysis
• Absent pituitary stalk
• Central diabetes insipidus
• Dysfunction of neurohypophysis or hypothalamus due to (tumor, Langerhans cell histiocytosis, infection or trauma).
• Precocious puberty
• Hamartoma or other neoplasm of hypothalamus
• Hypersecretion syndromes
• Pituitary adenomas.
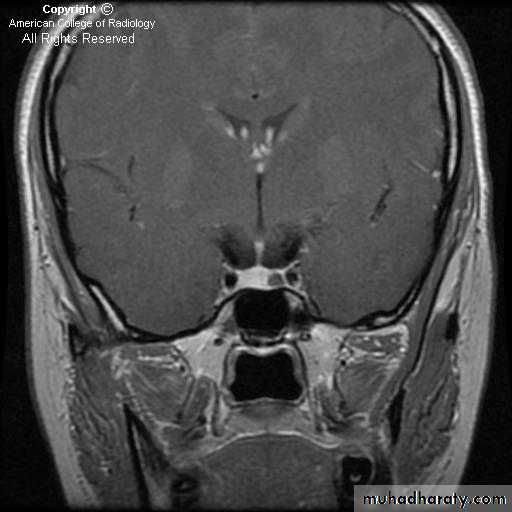
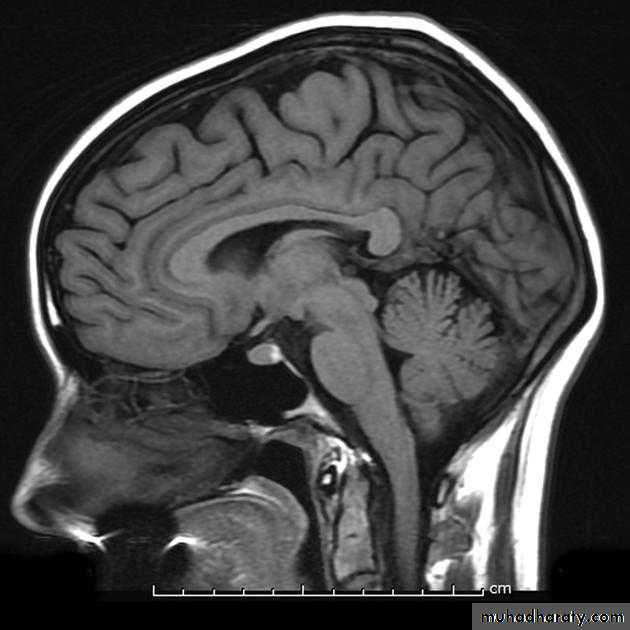
MRI is the investigation of choice for imaging of the pituitary gland and adjacent structures.
• Sagittal T1-weighted images demonstrate the lobar anatomy of the pituitary:
• Adenohypophysis of similar signal intensity to brain tissue
• Neurohypophysis seen as a posterior ‘bright spot’.
• Coronal T1-weighted scans with dynamic contrast enhancement are particularly useful for the diagnosis of microadenomas. Where MRI is unavailable or contraindicated CT may be used, however it is much less sensitive.
THYROID IMAGING
Common clinical indications for imaging of the thyroid gland include:
• Hyperthyroidism (Thyrotoxicosis).
• Diffuse thyroid enlargement.
• Focal thyroid mass or nodule.
The most commonly used imaging techniques for the investigation of thyroid diseases are:
• Ultrasound.• US-guided fine needle aspiration (FNA).
• Scintigraphy with 99mTc or radioiodine.
• CT or MRI may be used to outline the anatomy of large goiters prior to surgical removal, particularly where there is retrosternal extension into the upper mediastinum.
Hyperthyroidism (thyrotoxicosis)
Thyrotoxicosis refers to a spectrum of clinical and biochemical findings that occur due to excess thyroid hormone:• Heat intolerance, inability to sleep, weight loss and palpitations
• Elevated serum triiodothyronine (T3) and/or thyroxine (T4)
• Rarely, elevated TSH levels may indicate a TSH secreting pituitary adenoma
• More commonly, TSH levels are low indicating a thyroid problem.
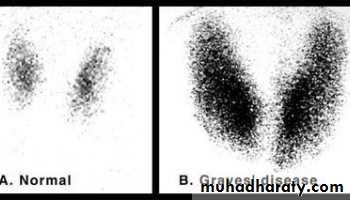
Imaging investigation of Grave’s disease includes:
• Scintigraphy with 99mTc or radioiodine
• It is the investigation of choice.• 99mTc is more widely available than radioiodine and has a lower radiation dose.
• Scintigraphy with 99mTc is useful for differentiating Grave’s disease from other causes of thyrotoxicosis, including subacute thyroiditis, multinodular goiter and toxic adenoma.
• Scintigraphy with 99mTc shows intensely increased uptake.
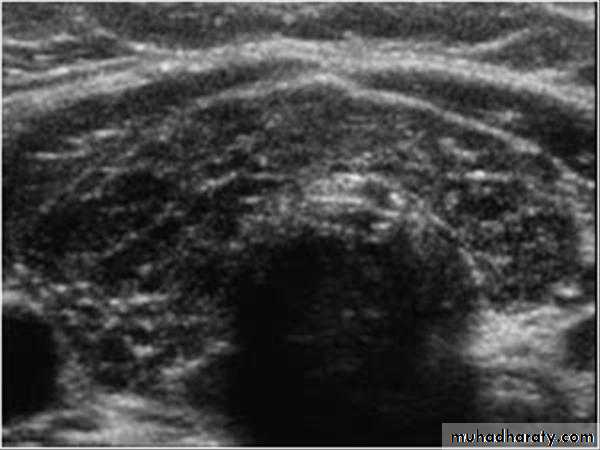
• Ultrasound: enlarged, hypoechoic and hypervascular thyroid.
Diffuse thyroid enlargement
Causes of diffuse thyroid enlargement include:
• Grave’s disease
• Hashimoto thyroiditis
• Subacute thyroiditis
• Multinodular goiter.
Diagnosis is often achieved by clinical history and examination, plus laboratory tests for thyroid function and antibodies. These may be complemented by thyroid scintigraphy with 99mTc, and US with color Doppler.
Thyroid nodules and masses
A thyroid nodule is defined as a discrete mass lesion in the thyroid gland, distinguishable from thyroid parenchyma on US.
Thyroid nodules include:
• Benign colloid nodules.
• Follicular adenoma is a true benign neoplasm of the thyroid gland.
• Thyroid carcinoma is usually of epithelial origin and is classified into (papillary, follicular, medullary and anaplastic). Papillary carcinoma is the most common type (75–80 per cent); it has an excellent prognosis with a 30-year survival rate of 95 per cent.
• Thyroid nodules are extremely common, found in up to 40% of adults on US examination.
• Thyroid nodules may be assessed clinically and with imaging, the aim being to decide which nodules may be malignant.
Clinical factors associated with an increased likelihood of a nodule being malignant include:
• Family history of thyroid cancer
• History of irradiation to the neck
• Patient age <20 or >60
• Rapid growth of the nodule
• Fixation to adjacent structures
• Vocal cord paralysis
• Adjacent suspicious lymphadenopathy.
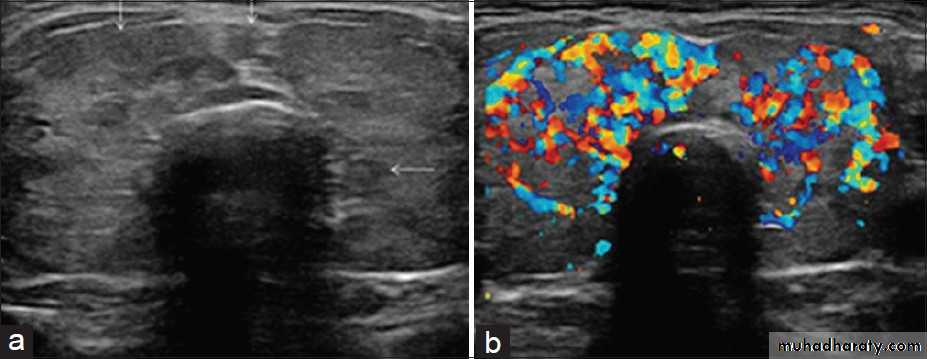
• US is the imaging investigation of choice for characterization of thyroid nodules.
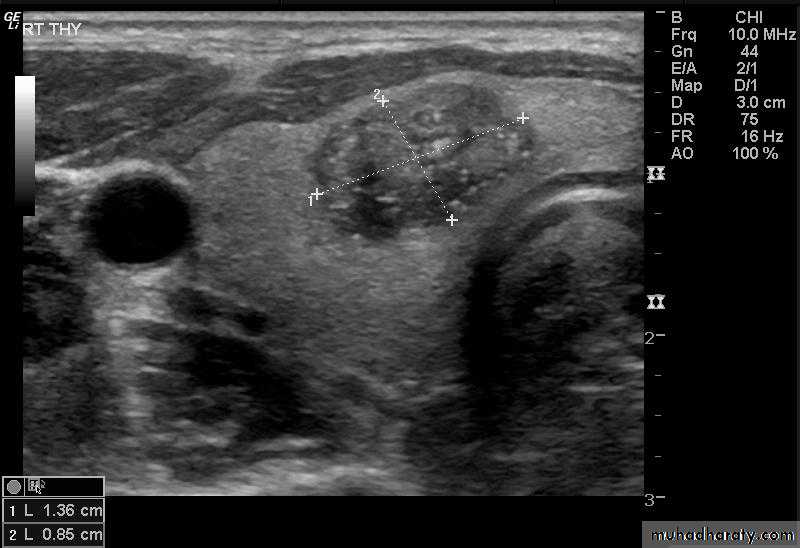
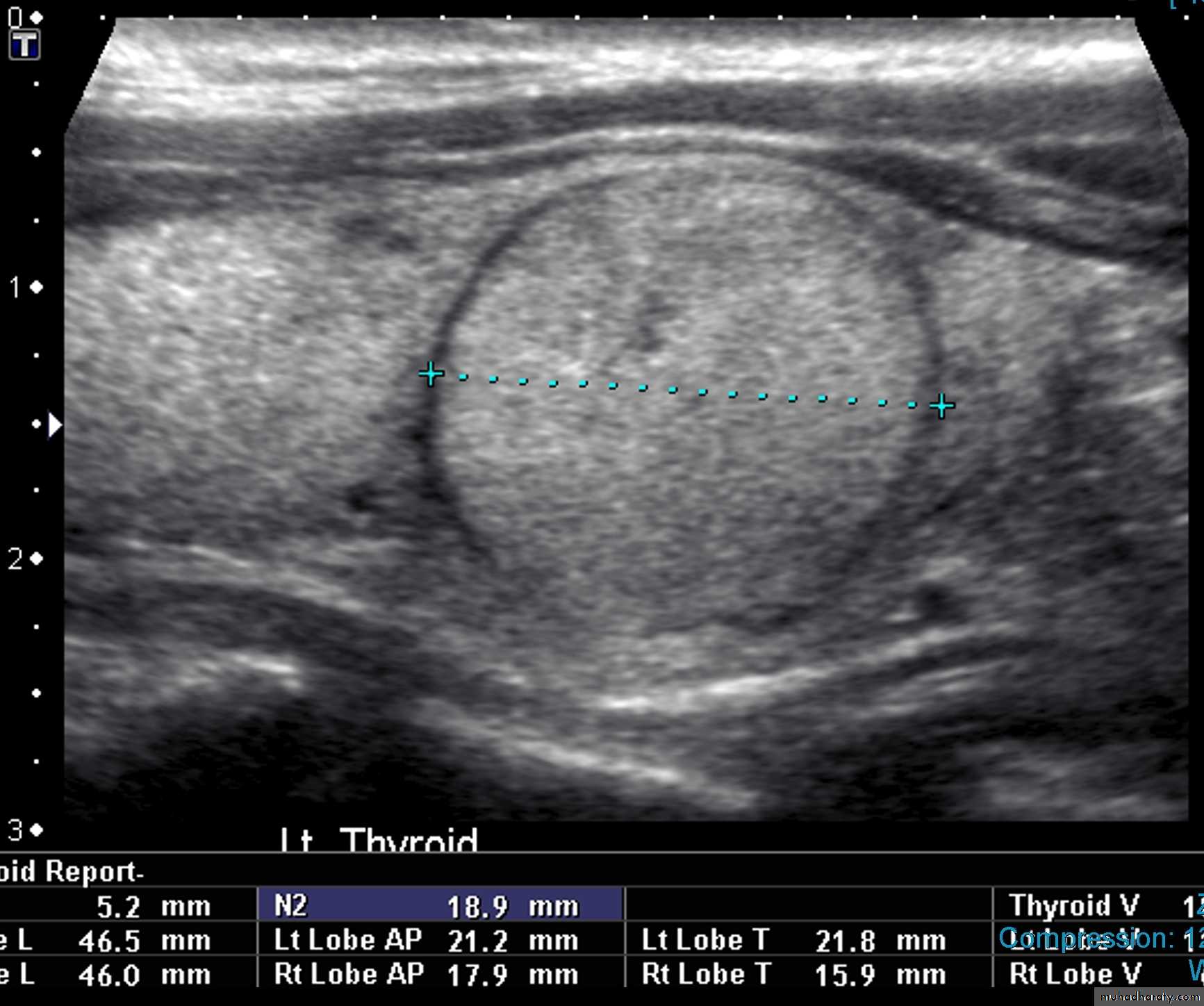
• Features of thyroid nodules assessed with US include:• Size: the incidence of cancer in nodules smaller than 1 cm is extremely low
• Composition: cystic, solid or mixed
• Margins: well-defined margin or ‘halo’; irregular margins
• Calcification: coarse or fine
• Vascularity.
• US-guided FNA is usually definitive.
• FNA is indicated for solitary nodules with the following features:• >1.0 cm with fine calcifications
• >1.5 cm if predominantly solid or with coarse calcifications
• >2.0 cm if mixed solid and cystic components.
• In multiple nodules, each nodule is judged on its merits using the criteria above.
• Nodules not meeting the above criteria may still be referred for FNA if there is suspicion of malignancy on clinical grounds.PRIMARY HYPERPARATHYROIDISM
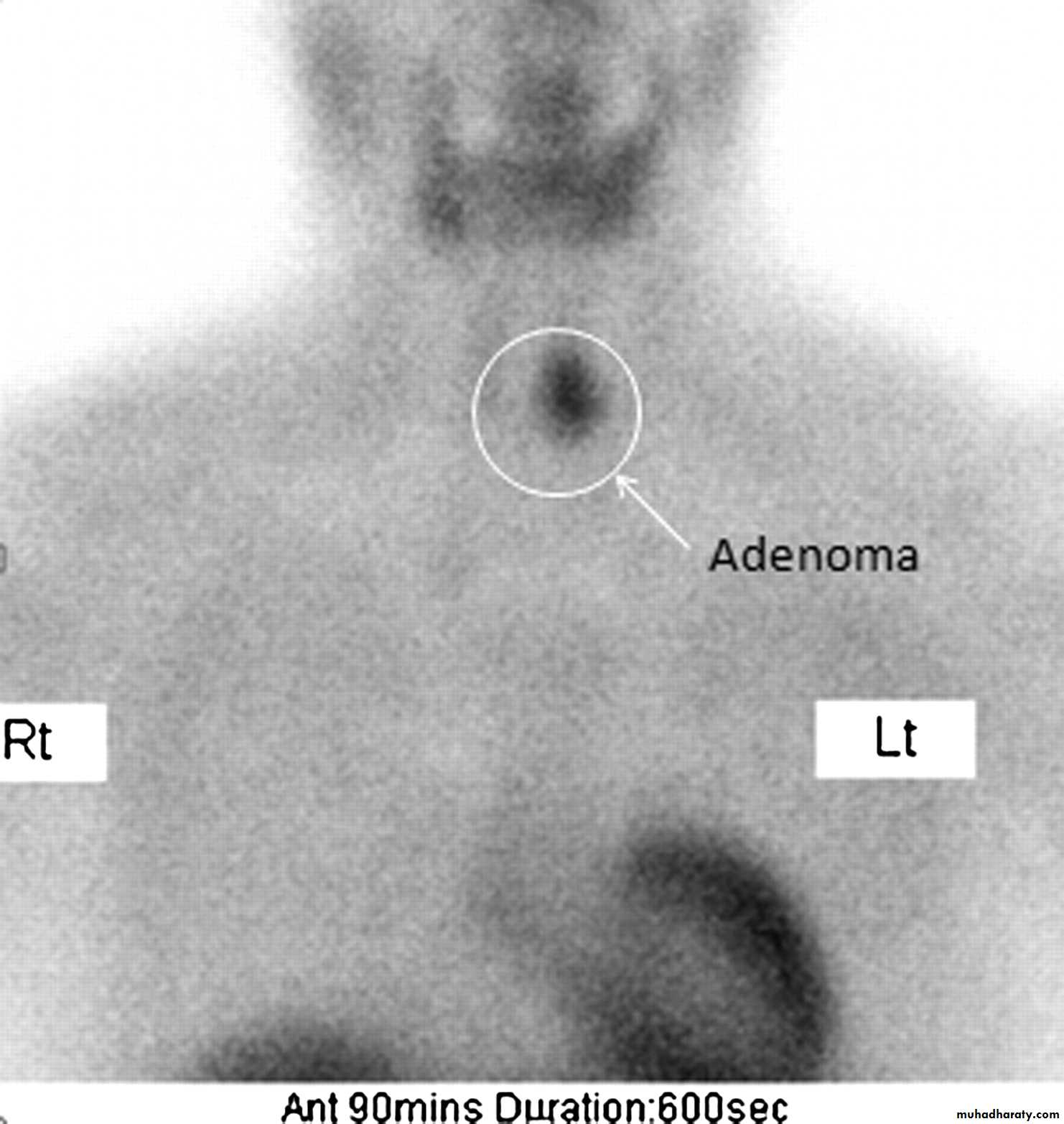
• Primary hyperparathyroidism is the most common indication for imaging of the parathyroid glands.
• Causes of primary hyperparathyroidism:
• Solitary parathyroid adenoma: 80 %• Multiple parathyroid adenomas: 7 %
• Parathyroid hyperplasia: 10 %
• Parathyroid carcinoma: 3 %
• Imaging is indicated in hyperparathyroidism to localize the causative lesion prior to surgery.
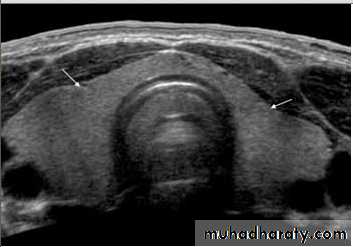
• US is the investigation of first choice.
• US with high-resolution equipment has a high sensitivity (80–90 %) for the detection of parathyroid adenoma.• US appearance of parathyroid adenoma is a well-defined hypoechoic mass usually of around 1.0–1.5 cm in diameter.
• Most parathyroid adenomas lie behind or immediately below the thyroid gland.
• The principal cause of a false-negative US is ectopic adenoma, which may be present in up to 10 % of cases; ectopic sites include mediastinum and carotid sheath.
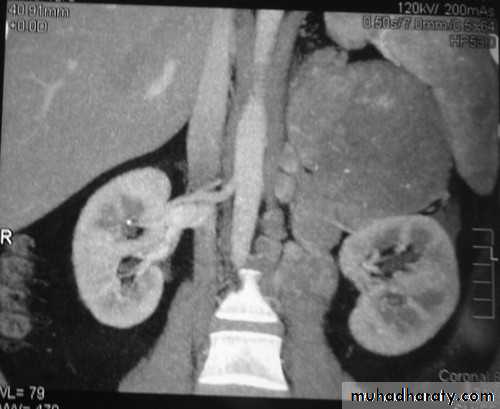
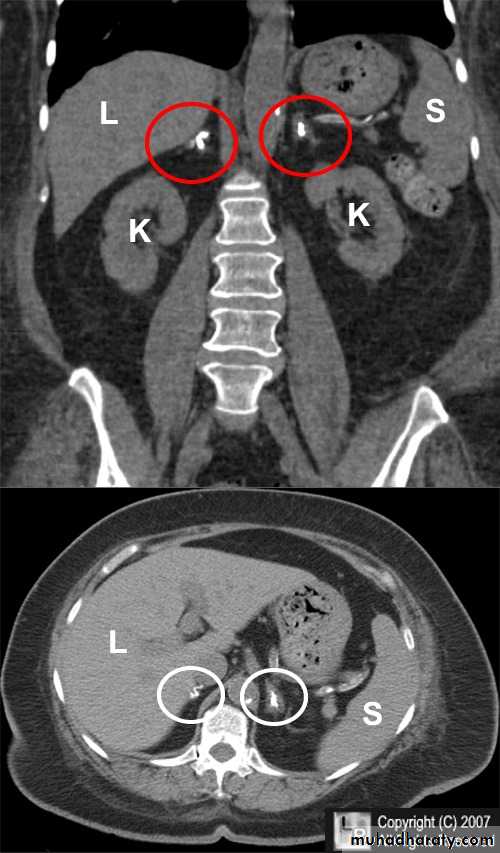
ADRENAL IMAGING
• The adrenal gland is essentially made up of two separate organs, the adrenal cortex and the adrenal medulla:
• Adrenal cortex: endocrine gland composed of fat-rich cells Secretes cortisol, aldosterone and androgenic steroids
• Adrenal medulla: develops from the neural crest Secretes adrenaline and noradrenaline.
•
• Indications for imaging of the adrenal glands include:
• Endocrine syndromes
• Cushing syndrome
• Hyperaldosteronism
• Suspected phaeochromocytoma
• Incidentally discovered adrenal mass.
• CT is the investigation of choice for imaging of the adrenal glands.
• MRI and scintigraphy may occasionally be useful for specific indications.
• More specialized techniques, such as adrenal vein sampling and percutaneous biopsy, rarely may be required.
ADRENAL ENDOCRINE SYNDROMES
Cushing syndrome
Causes of Cushing syndrome:
• Excessive ACTH production (70 %) (Cushing disease): usually due to pituitary adenoma
• Ectopic ACTH production by tumors (10 %): bronchial carcinoid, thymoma, islet cell tumor
• Adrenal disease (20 %)
• Bilateral adrenal hyperplasia (70 %)
• Unilateral adrenocortical adenoma (20 %)
• Adrenal carcinoma (10 %).
• Biochemical assessment of the patient with Cushing syndrome includes
• Measurement of serum ACTH levels.
• Elevated ACTH indicates pituitary or ectopic production
• Low or undetectable levels of ACTH indicate adrenal disease. Patients
• High dose dexamethasone suppression test.
• Cortisol suppression indicates a pituitary source of ACTH.
• Lack of cortisol suppression suggests an ectopic source.
• Imaging investigation of Cushing syndrome guided by biochemical testing is therefore as follows:
• Suspected pituitary source of ACTH: MRI of the pituitary gland
• Suspected ectopic source of ACTH: CT of chest and abdomen
• Suspected adrenal disease: CT of the adrenal glands.
Primary hyperaldosteronism (Conn syndrome)
• Primary hyperaldosteronism is thought to be responsible for up to 10 per cent of cases of hypertension. The diagnosis of primary hyperaldosteronism is established biochemically with measurement of plasma aldosterone concentration to plasma renin activity ratio (PAC/ PRA).
• Causes of primary hyperaldosteronism (Conn syndrome):
• Solitary unilateral adrenal adenoma (70 %)• Multiple adrenal adenomas (20 %)
• Bilateral adrenal hyperplasia (10 %)
• Adrenal carcinoma (rare).
• CT of the adrenal glands is the initial investigation of choice.
• When bilateral adrenal disease is seen on CT, or when CT is normal, bilateral selective adrenal vein sampling for aldosterone levels may be helpful to localize a small abnormality and therefore guide management.
Primary adrenal insufficiency (Addison disease)
• Causes of primary adrenal insufficiency (Addison disease):
• Usual cause: idiopathic (probably autoimmune) adrenal atrophy
• TB
• Sarcoidosis
• Bilateral adrenal haemorrhage
• Birth trauma, hypoxia, and sepsis in neonates
• Anticoagulation therapy or sepsis in adults.
• CT of the adrenal glands is the investigation of choice.
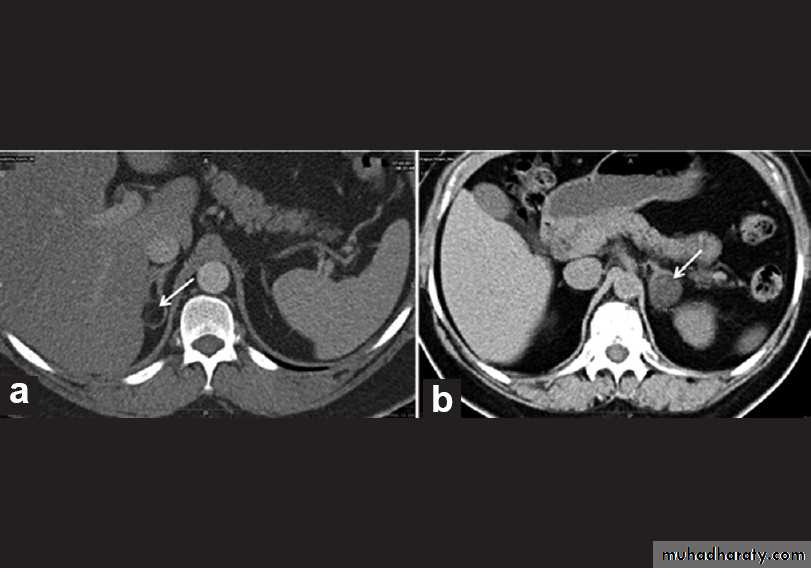
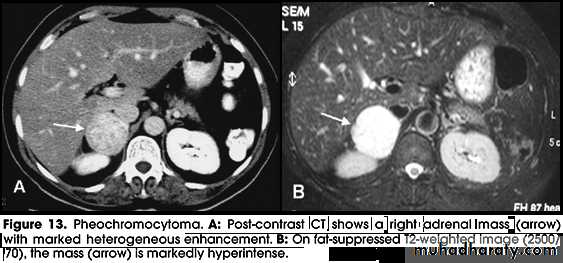
Phaeochromocytoma
• Phaeochromocytoma is a tumour arising from chromaffin cells of the adrenal medulla.
• Ninety per cent occur in the adrenal gland and 10 per cent in ectopic extra-adrenal locations.
• Phaeochromocytoma usually presents with symptomatology related to excess catecholamine production:
• Paroxysmal or sustained hypertension
• Headaches, sweating, flushing
• Nausea and vomiting
• Abdominal pain.
• Urine analysis reveals elevated levels of vanillylmandelic acid (VMA).
• Phaeochromocytoma may also be discovered due to a hypertensive crisis brought on by surgery or some other stress.
• Ten per cent arise as part of a syndrome, e.g. multiple endocrine neoplasia, familial phaeochromocytoma, tuberous sclerosis, and Von Hippel–Lindau disease, neurofibromatosis.
• Ten per cent are malignant.
• Phaeochromocytomas are usually large tumors, measuring up to 12 cm with an average around 5 cm.
•
• Imaging of Phaeochromocytomas includes:
• CT of the abdomen, with particular attention to the adrenal glands is the initial imaging investigation of choice in the diagnosis of phaeochromocytoma.
• When the adrenal glands are normal on CT and no obvious mass is seen elsewhere, whole body scintigraphy with iodine labelled metaiodobenzylguanidine (131I-/123I-MIBG) may be useful.
• MRI of the abdomen may be used where MIBG is not available.
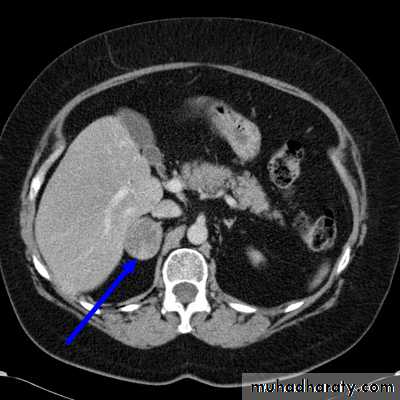
DIFFERENTIATION BETWEEN BENIGN AND MALIGNANT ADRENAL MASS
• It is important to differentiate benign from malignant masses, specifically adrenal adenoma from adrenal metastasis or carcinoma.
•
• CT features of a benign adrenal adenoma:
• Small size (<3 cm)
• Smooth contour
• Low density on unenhanced scans due to the high fat content of normal adrenal cells.
•
• CT features of adrenal carcinoma/metastasis include:
• Relatively large size (>5 cm)
• Higher density on unenhanced scans with low density centrally due to necrosis
• Other evidence of malignancy, such as liver metastases, lymphadenopathy, venous invasion.
• Equivocal lesions may be further assessed with multiphase contrast-enhanced CT. Attenuation value of the adrenal mass is measured pre-contrast injection and then at 60 seconds and 15 minutes after injection.
• Adrenal adenomas show rapid enhancement and subsequent washout with intravenous contrast material (Adrenal adenoma will show low attenuation pre-contrast, high attenuation at 60 seconds, and significantly reduced attenuation at 15 minutes).
• MRI, including chemical shift imaging may also be useful in differentiating adrenal adenoma from malignancy.
• Occasionally, when imaging is unable to confidently classify an adrenal mass as either benign or malignant, percutaneous biopsy under CT guidance may be required for definitive diagnosis.