
FIFTH YEAR
FEVER AND HEPATOMEGALY
(AIDS AND KALA AZAR)
9-10-2013
Dr. Nadhim M. Mosa
HIV DISEASE IN CHILDEN
The WHO estimates that approximately 2.3 million children
are living with the human immunodeficiency virus (HIV) as of
2006.
In 2006 alone, 530,000 children were newly infected, an
improvement from the 640,000 newly infected in 2004.
This is most prevalent in sub-Saharan Africa, where 18 million
children are predicted to be orphaned by AIDS by the end of
2010.
Worldwide, the United Nations Children's Fund (UNICEF)
predicts the number of children orphaned and made vulnerable
by HIV/AIDS is expected to reach 25 million by the end of the
decade.
HIV infection in children progresses more rapidly than in adults,
and some untreated children die within the 1st 2 yr of life
(higher viral burden and faster depletion of infected CD4
lymphocytes).
ETIOLOGY & PATHOGENESIS
HIV-1 and HIV-2 are members of the Retroviridae family
and belong to the Lentivirus genus, which includes cytopathic
viruses causing diverse diseases in several animal species.
The HIV-1 genome contains 2 copies of single-stranded RNA.
Although 2 strains of HIV have currently been identified, most
patients who have AIDS are positive for HIV type 1 (HIV-1) or
are positive for both HIV-1 and HIV type 2 (HIV-2).
HIV-1 is a retrovirus that exhibits a variety of structural
and nonstructural proteins that determine the interaction of the
virus with the host's immune system and cellular components.
The HIV virus attaches to the host cell by the association of a
surface glycoprotein to the CD4 molecule; therefore, it primarily
infects CD4+ lymphocytes and macrophages. Once the virus
core enters the cell cytoplasm of the host, viral reverse
transcriptase copies viral RNA to the DNA of the host.

The viral DNA is then transported into the nucleus and
incorporated into the DNA of that cell. If activated, viral
expression can result in new viral RNA and proteins. New viral
core proteins, enzymes, and viral RNA molecules can induce
budding, with additional cell infection.
The reduction in cell-mediated immunity and secondary B-
cell dysfunction result in the immunocompromised state and in
the proliferation of opportunistic infections and malignancies.
An elevated level of activation-induced cell death resulting from
apoptosis of T cells occurs in patients who are HIV positive.
EPIDEMIOLOGY
An estimated 2.3 million children worldwide younger
than15 years are living with HIV/AIDS. As of 2007, 90% of the
newly infected children are infants who acquire HIV from their
infected mothers.
A vanishing minority of children were infected through
receipt of contaminated blood products and/or clotting factors,
primarily before 1985, when screening of the blood supply was
instituted.
Alarmingly, 90% of babies who acquire the disease from
infected mothers are found in sub-Saharan Africa. The
prevalence of HIV infection among undernourished children has
been estimated to be as high as 25%.
In 2004, more than half a million children younger than 15
years died from HIV/AIDS. In 2006, this number decreased to
380,000. In 2002, HIV/AIDS was the seventh leading cause of
mortality in children in developing countries.
Transmission
Transmission of HIV-1 occurs via sexual contact,
parenteral exposure to blood, or vertical transmission from
mother to child. The primary route of infection in the pediatric
population is vertical transmission, accounting for almost all
new cases. Rates of transmission of HIV from mother to child
varied among countries. Vertical transmission of HIV can occur
before (intrauterine), during (intrapartum), or after delivery
(through breast-feeding). Intrauterine transmission has been
suggested by identification of HIV by culture or polymerase
chain reaction (PCR) in fetal tissue as early as 10 wk.
It is generally accepted that 30-40% of infected newborns
are infected in utero, because this percentage of infants has
laboratory evidence of infection (positive viral culture or PCR)
within the 1st wk of life.
The highest percentage of HIV-infected children acquire
the virus intrapartum (exposure to infected blood and
cervicovaginal secretions in the birth canal, where HIV is found
in high titers during late gestation and delivery), evidenced by
the fact that 60-70% of infected infants do not demonstrate
detectable virus before 1 wk of age. breast-feeding is an
important transmission route in developing countries.
WHO Clinical Staging of HIV/AIDS for Children with
Confirmed HIV Infection
CLINICAL STAGE 1
- Asymptomatic
- Persistent generalized lymphadenopathy
CLINICAL STAGE 2
- Unexplained persistent hepatosplenomegaly
- Papular pruritic eruptions
- Fungal nail infection
- Angular cheilitis
- Lineal gingival Erythema
- Extensive wart virus infection
- Extensive molluscum contagiosum
- Recurrent oral ulceration
- Unexplained persistent parotid enlargement
- Herpes zoster
- Recurrent or chronic upper respiratory tract infections (otitis
media, otorrhoea, sinusitis, tonsillitis).

CLINICAL STAGE 3
- Unexplained moderate malnutrition or wasting not adequately
responding to standard therapy
- Unexplained persistent diarrhea (14 days or more)
- Unexplained persistent fever (above 37.5°C intermittent or
constant, for longer than one month)
- Persistent oral candidiasis (after first 6–8 weeks of life)
- Oral hairy leukoplakia
- Acute necrotizing ulcerative gingivitis or periodontitis
- Lymph node tuberculosis
- Pulmonary tuberculosis
- Severe recurrent bacterial pneumonia
- Symptomatic lymphoid interstitial pneumonitis
- Chronic HIV-associated lung disease including brochiectasis
- Unexplained anemia (<8 g/dl), neutropaenia (<0.5 × 109 per
litre) or chronic thrombocytopenia (<50 × 109 per litre).
CLINICAL STAGE 4
- Unexplained severe wasting, stunting or severe malnutrition
not responding to standard therapy
- Pneumocystis pneumonia
- Recurrent severe bacterial infections (such as empyema,
bone or joint infection or meningitis but excluding
pneumonia)
- Chronic herpes simplex infection (orolabial or cutaneous of
more than one month’s duration or visceral at any site)
- Esophageal candidiasis (or of trachea, bronchi or lungs)
- Extrapulmonary / disseminated tuberculosis
- Kaposi’s sarcoma
- Cytomegalovirus infection: retinitis or cytomegalovirus
infection affecting another organ, with onset at age older
than one month
- Extrapulmonary cryptococcosis (including meningitis)
- Central nervous system toxoplasmosis (after one month of
life)
- HIV encephalopathy

- Disseminated
endemic
mycosis
(extrapulmonary
histoplasmosis, coccidiomycosis)
- Disseminated non-tuberculous mycobacterial infection
- Chronic cryptosporidiosis (with diarrhea)
- Chronic isosporiasis
- HIV associated tumors including Cerebral or B-cell non-
Hodgkin lymphoma
- Progressive multifocal leukoencephalopathy
- Symptomatic
HIV-associated
nephropathy
or
cardiomyopathy.
Laboratory diagnosis
HIV DNA PCR Preferred test to diagnose HIV-1 infection in
infants and children younger than 18 mo of age; highly sensitive
and specific by 2 wk of age and available; performed on
peripheral blood mononuclear cells.
HIV p24 Ag Less sensitive, false-positive results during 1 mo of
Iife,variable results; not recommended
HIV culture Expensive, not easily available, requires up to 4
wk to do test; not recommended
HIV RNA PCR Not recommended for routine testing of infants
and children younger than 18 mo of age because a negative
result cannot be used to exclude HIV infection definitively
Antibody to HIV In children ≥18 months of age, antibody testing
should be done in the same manner as in adults. Detected by
EIA, immunoblot, or immunoflurescent techniques..
%CD4 and lymphocyte count.
WHO Case Definition for HIV Infection in Children
Diagnosis of HIV infection is based on laboratory criteria
• Children younger than 18 months:
• positive virological test for HIV or its components
(HIV-
RNA or HIV-DNA or ultrasensitive HIV p24 antigen)
confirmed by a second virological test obtained from a
separate determination taken more than four weeks after

birth. Positive antibody testing is not recommended for
definitive or confirmatory diagnosis of HIV infection in
children until 18 months of age.
• Children 18 months or older:
• positive HIV antibody testing (rapid or laboratory-
based
enzyme immunoassay). This is usually confirmed by a
second HIV antibody test (rapid or laboratory-based
enzyme immunoassay) relying on different antigens or of
different operating characteristics
• OR
• positive virological test for HIV or its components
(HIV-
RNA or HIV-DNA or ultrasensitive HIV p24 antigen)
confirmed by a second virological test obtained from a
separate determination.
WHO Case Definition of AIDS in Children
AIDS is defined clinically or immunologically in children
with confirmed HIV infection
Confirmed HIV infection AND clinical
diagnosis (presumptive
or definitive) of any stage 4 condition
OR
Confirmed HIV Infection AND first ever
documented:
• %CD4+ <25 among infants younger than 12 months of
age
• %CD4+ <20 among children aged 12-35 months
• %CD4+ <15 among children aged 36-59 months
• CD4 cell count of less than 200/ mm3 or % CD4+ <15
among children 5 years or older
AIDS case reporting for surveillance is no longer required if
HIV infection or advanced HIV infection is reported.

TREATMENT
specific, supportive
specific: The currently available therapy does not eradicate the
virus and cure the patient; it only suppresses the virus for
extended periods of time and changes the course of the disease
to a chronic process. Decisions about anti-retroviral therapy for
pediatric HIV-infected patients are based on the magnitude of
viral replication (viral load), CD4 lymphocyte count or
percentage, and clinical condition.
Anti-retroviral drugs are categorized by their mechanism
of action, such as the ability to inhibit the HIV reverse
transcriptase or protease enzymes.
Within the reverse transcriptase inhibitors, further
subdivision can be made:
1. nucleoside
(or
nucleotide)
reverse
transcriptase
inhibitors (zidovudine, lamivudine ) and
2. non-nucleoside
reverse
transcriptase
inhibitors
(nevirapine, efavirenz).
3. The protease inhibitors (lopinavir, nelfinavir).
Supportive Care: A multidisciplinary team approach is
desirable for successful management. Close attention should be
paid to nutrition, oral hygiene, development, and pain
management.
All HIV-exposed and infected children should receive
standard pediatric immunizations. In general, live oral polio
vaccine and live bacterial vaccines (BCG) should not be given.
Neither varicella nor MMR vaccines are recommended to
severely immunocompromised children.
Prophylactic regimens are integral for the care of HIV-infected
children. All infants between 6 wk and 1 yr of age who are
proven to be HIV infected should receive prophylaxis (e.g.
TMP/SMZ, IVIG).
All HIV-exposed children should have skin testing (5TU PPD)
for T.B. at 1 yr of age and be retested every 2 yr.
PROGNOSIS
In general, the best prognostic indicators are the sustained
suppression of plasma viral load and CD4' lymphocytes. A high
viral load (>100,000 copies/mL) over time is associated with
greater risk for disease progression and death. CD4 lymphocyte
percentage is another prognostic indicator, and the mortality rate
is higher in patients with a CD4 lymphocyte percentage of
<I5%.
Children with opportunistic infections, encephalopathy, or
wasting syndrome have the worst prognosis. Persistent fever
and/or oral thrush, serious bacterial infections (meningitis,
pneumonia, sepsis), hepatitis, persistent anemia and/or
thrombocytopenia also suggest a poor outcome.
In
contrast,
lymphadenopathy,
splenomegaly,
hepatomegaly, lymphoid interstitial pneumonitis, and parotitis
are indicators of a better prognosis.
PREVENTION.
Interruption of perinatal transmission from mother to child
has been achieved by administering ZDV chemoprophylaxis to
the pregnant woman (started as early as 4 wk of gestation) and
continued during delivery and in the newborn for the 1st 6 wk of
life. Educational efforts about avoidance of risk factors are
essential for older school-aged children and adolescents.
Visceral Leishmaniasis
(KALA AZAR)
Leishmaniasis is a zoonotic infection caused by protozoa
that belong to the genus Leishmania. transmitted by sandflies
(Phlebotomus species). In the human host, Leishmania are
intracellular parasites that infect the mononuclear phagocytes.
Epidemiology
The Leishmania species that infect humans are mainly
leishmania donovani, which causes visceral leishmaniasis (kala
azar).

Geographical distribution of leishmaniasis is restricted to
tropical and temperate regions (natural habitat of the sandfly).
Leishmaniases are considered endemic in 88 countries (16
developed countries, 72 developing countries) on 5 continents:
Africa, Asia, Europe, North America, and South America. A
total of 350 million people are at risk. The incidence of
leishmaniasis is increasing, mainly because of man-made
environmental changes that increase human exposure to the
sandfly vector. Poverty and malnutrition play a major role in the
increased susceptibility to the disease. The immune deficiency
has lead to increased susceptibility to infections, including
leishmaniasis (AIDS). Another risk factor is the movement of
susceptible populations into endemic areas, including large-
scale migration of populations for economic reasons. The
predominant mode of transmission is a sandfly bite which act as
vectors. Uncommon modes of transmission include congenital
transmission, blood transfusion, and, rarely, inoculation of
cultures.
Pathophysiology
Leishmaniasis infections are considered zoonotic diseases
because the infection is maintained in dogs, wild rodents, and
other animals in endemic areas. Leishmania are obligatory
intracellular parasites and are transmitted by the bite of the
sandfly belonging to the genera Phlebotomus .
Life cycle:
The parasite has 2 forms: the amastigote form and the
promastigote form. The amastigote form occurs in humans,
whereas the promastigote form occurs in the vector (sandfly).
Only the female sandfly transmits the protozoan, infecting itself
with the Leishmania parasites contained in the blood it sucks
from its human or mammalian host. The parasite continues its
development inside the sandfly into the promastigote form.
Following the bite, some of the flagellates (promastigote )
enter the cells of the reticuloendothelial system, where they
change into the amastigote form. The amastigote forms also

multiply. The multiplication continues until the host cell is
packed with the parasites and ruptures, liberating the
amastigotes into the circulation. The free amastigotes then
invade fresh cells, thus repeating the cycle and, in the process,
infecting the entire reticuloendothelial system. Some of the free
amastigotes are drawn by the sandfly during its blood meal, thus
completing the cycle.
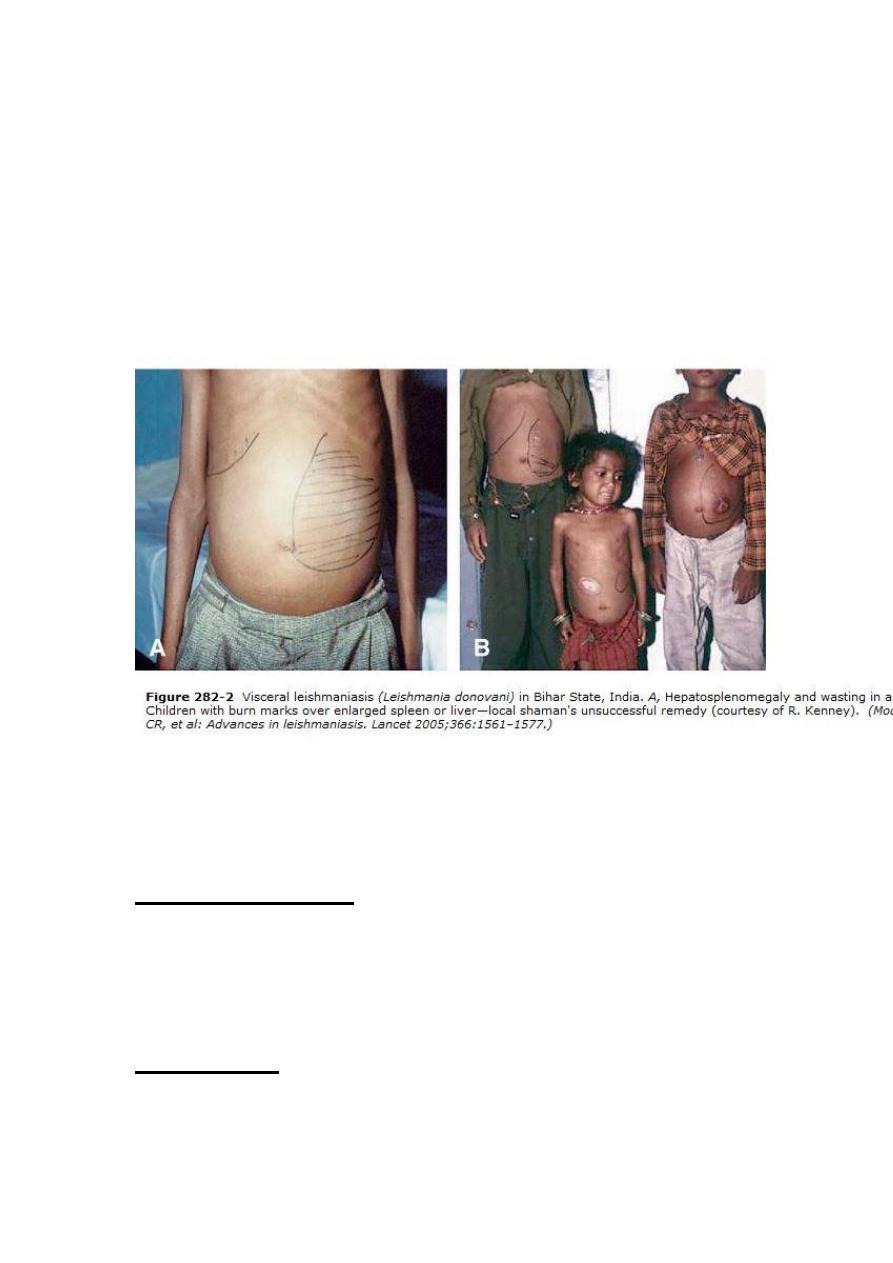
CLINICAL FEATURES
The spectrum of illness ranges from asymptomatic
infection to severe life-threatening infection. The disease is also
known as kala azar, Dumdum fever, Assam fever, and infantile
splenomegaly. It is the most severe form of leishmaniasis and is
usually fatal within 2 years if left untreated. The incubation
period is usually 3-6 months but can be months or years. Young
malnourished children are most susceptible to developing
progressive infection.
The disease has an insidious onset with pyrexia(continuous
or remittent ) which becomes intermittent at a later stage. It is
characteristically described as a double rise in 24 hours. Waves
of pyrexia may be followed by a period without fever. Children
presenting later in the course of the disease may present with
edema, hemorrhage, or growth failure.
The skin is dry, thin, and scaly, and hair is lost. As the disease
progresses, the skin on the hands, feet, abdomen and face may
become darkened, which is why the disease is also termed kala
azar or black fever. Petechiae and ecchymosis may be seen in
the extremities. Fulminant forms of visceral leishmaniasis is
manifested by features that include pancytopenia and hepatic
failure.
Splenic enlargement is a striking feature that is often
considerable.
Hepatomegaly
and
Lymphadenopathy
are
associated findings. Jaundice with mildly elevated enzyme
levels is rarely seen and is considered a bad prognostic sign.
Anemia is almost always present and is usually severe.
Leucopenia is also observed and may contribute to secondary
infections. Thrombocytopenia contributes to the hemorrhagic
tendency observed in some cases.
Hypergammaglobinemia, circulating immune complexes,
and rheumatoid factors are present in sera of most patients with
visceral leishmaniasis.
If untreated, death occurs within 2 years and is often
caused by bacterial pneumonia, septicemia, dysentery,
tuberculosis, cancrum oris, and uncontrolled hemorrhage or its
sequelae.
Differential diagnosis
Visceral leishmaniasis - Includes other conditions
associated with massive splenomegaly, including malaria,
tropical splenomegaly syndrome, typhoid, miliary tuberculosis,
portal hypertension, leukemia and lymphomas, hemolytic
anemia .
Lab Studies
• Direct evidence of infection: The parasite can be detected
through direct evidence from peripheral blood, bone
marrow, or splenic aspirates, as explained below.
• Indirect evidence of infection
*Detection of hypergammaglobinemia by aldehyde and the
antimony tests.
*Immunological tests The direct agglutination test, which
detects the specific immunoglobulin M (IgM) antibody at an
early stage, has been found to be useful in the detection of both
clinical and subclinical infections.
* Nonspecific tests: The direct agglutination test,
immunofluorescent antibody test, complement fixation, and
counterimmunoelectrophoresis are the various tests used in
diagnosis of kala azar. Polymerase chain reaction (PCR) has
also been used in the diagnosis of leishmaniasis. An
immunochromatographic test for detection of anti–rK-39
antibodies has been reported with high sensitivity and specificity
in diagnosis of visceral leishmaniasis. Leishmanin skin test
(Montenegro test) is useful only for epidemiological purposes,
indicating prior exposure to infection.
• Supportive tests: Hematological parameters include : a
normochromic
normocytic
anemia,
leucopenia,
neutropenia, thrombocytopenia, elevated gamma globulin
levels, and a reversal of the albumin/globulin.
TREATMENT
Sodium stibogluconate, a pentavalent antimonial compound
is the drug of choice in the treatment of visceral leishmaniasis.
The dose 20 mg/kg/d IV/IM for 28 days. meglumine
antimonate is also used. The earliest sign of improvement is an
improvement in symptoms. Regression of splenomegaly takes a
few months.
Supportive treatment includes rest, blood transfusions, and
treatment of secondary infections.
A high-protein and high-calorie diet is required during the
course of treatment.

Liposomal amphotericin B, Pentamidine, paromomycin,
Ketoconazole, Itraconazole, Allopurinol, Interferon gamma,
and miltefosine( the first oral antileishmanial agent licensed for
use) are other alternatives used in treatment especially in
resistant cases.
Prevention
Personal protection using repellants and nets is an important
aspect. In endemic areas, spraying with DDT and other
insecticides is effective in sandfly control.
Prognosis
If untreated, death occurs within 2 years and is often caused
by bacterial pneumonia, septicemia, dysentery, tuberculosis, and
uncontrolled hemorrhage or its sequelae.
