De- Novo Synthesis of Purine Nucleotides
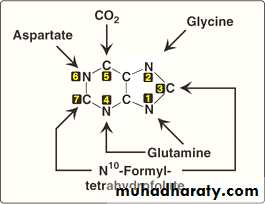
The atoms of the purine rings (i.e. the six- sided and the five- sided chains) are contributed by a number of compounds, including:
1. amino acids (aspartic acid, glycine, and glutamine),
2. CO2,
3. N10–formyltetrahydrofolate
through a series of hepatic reactions that will add the donated atoms to the already preformed ribose 5-phosphate (by the pentose phosphate pathway or HMP shunt).
Keep in your mind that during synthesis of purine nucleotides, we have to assemble the pentose- phosphate moiety (backbone) first and then the atoms of the nitrogenous base will be added consequently over it.
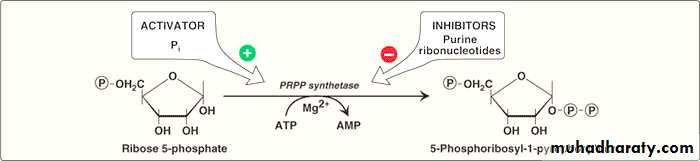
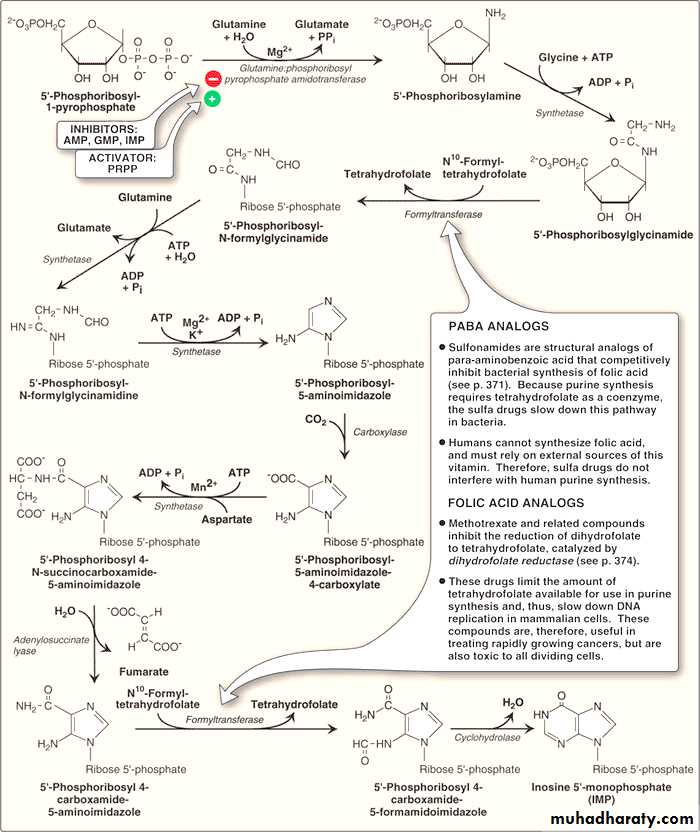
A. Synthesis of 5-phosphoribosyl-1-pyrophosphate (PRPP)
PRPP is an “activated pentose” that participates in the synthesis and salvage of purines and pyrimidines.Its synthesis requires the presence of ATP as a source of phosphate and ribose -5- phosphate.
This reaction is catalyzed by an X-linked enzyme called PRPP synthetase or (ribose phosphate pyrophosphokinase).
This reaction is activated by inorganic phosphate and inhibited by purine nucleotides (end-product inhibition).
NB:When deoxyribonucleotides are required for DNA synthesis, the ribose sugar moiety is reduced first.
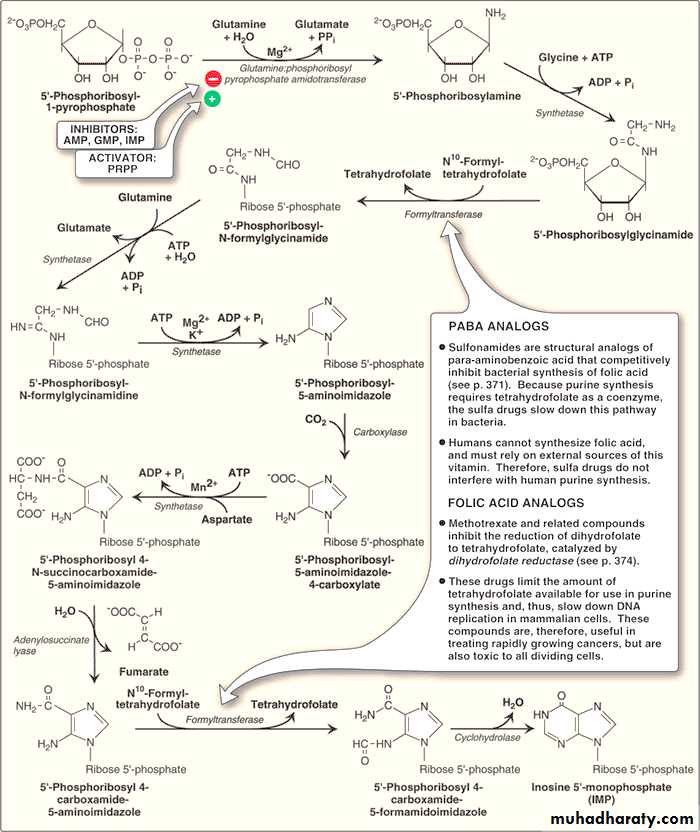
B. Synthesis of 5′-phosphoribosylamine
This compound is synthesized from PRPP and glutamine. The amide group of glutamine replaces the pyrophosphate group of PRPP at carbon atom no.1. The reaction is catalyzed by the enzyme glutamine:phosphoribosyl pyrophosphate amidotransferase.This enzyme is inhibited by the end products of this pathway AMP, GMP, and inosine monophosphate (IMP).
This is the committed step in purine nucleotide biosynthesis and its rate is controlled by the intracellular concentration of PRPP.
C. Synthesis of inosine monophosphate, the “parent” purine nucleotide
Once 5′-phosphoribosylamine is formed, it goes through 9 steps leading to synthesis of IMP (whose nitrogenous base is hypoxanthine).This pathway requires 4 ATP molecules as an energy source. Two steps in the pathway require N10-formyltetrahydrofolate.Synthetic Inhibitors of Purine Nucleotides
Some synthetic inhibitors of purine synthesis are designed to inhibit the growth of rapidly dividing microorganisms without interfering with human cell functions (e.g sulfonamides like sulfamethoxazole). Sulfonamides and other related compounds are structural analogues of PABA (para-aminobenzoic acid)
The analogues of PABA (para-aminobenzoic acid) can competitively inhibit bacterial synthesis of folic acid, and hence they reduce the synthesis of “tetrahydrofolate” which is an essential co-enzyme for purine synthesis leading to slow down this synthetic pathway in bacteria.
As human beings can not synthesize folic acid (but they get it from diet), therefore; sulfa drugs do not interfere with human purine synthesis.
Other purine synthesis inhibitors include structural analogs of folic acid which are used pharmacologically for selective inhibition of bacterial activity because of its selective inhibition of bacterial dihydrofolate reductase e.g. an antibiotic Trimethoprim which again does not interfere with human cell functions.
Other structural analogs of folic acid (for example, methotrexate), are used pharmacologically to control the spread of cancer by interfering with the synthesis of nucleotides and, therefore, of DNA and RNA again through inhibiting dihydrofolate reductase and thus reducing the amount of tetrahydrofolate.
Inhibitors of human purine synthesis are extremely toxic to normal tissues, especially to rapidly developing structures such as in:
fetus,
cell types that normally replicate rapidly, including:
bone marrow,
skin,
gastrointestinal (GI) tract,
immune system,
hair follicles.
As a result, individuals taking such anticancer drugs can experience adverse effects, including anemia, scaly skin, GI tract disturbance, immunodeficiencies, and baldness.
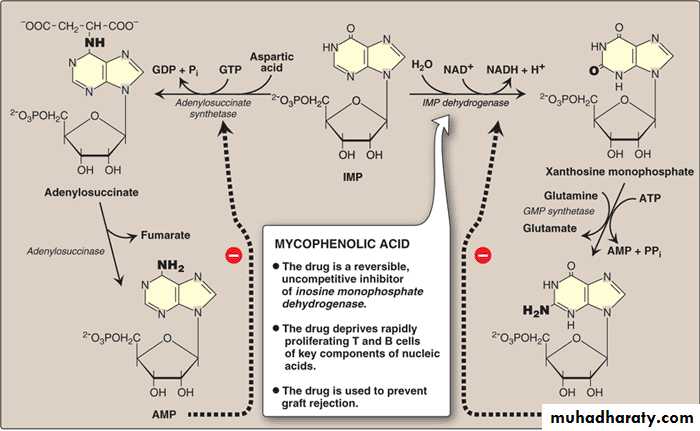
D. Conversion of IMP to AMP and GMP
The conversion of IMP to either AMP or GMP uses a two-step, energy-requiring pathway (i.e it utilizes ATP and GTP as energy sources).The increase in the amount of any of the two bioproducts causes a feedback inhibition to the first step reaction in this pathway. This shifts the IMP to the synthesis of the species of purine present in lesser amounts.
When both AMP and GMP are present in adequate amounts, the De Novo pathway of purine is turned off at the Glutamin:PRPP amidotransferase step.
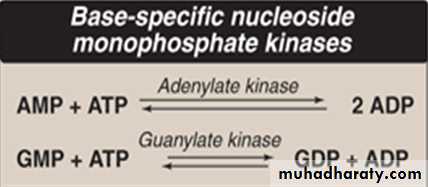
Conversion of nucleoside monophosphates to nucleoside diphosphates and triphosphates
Nucleoside diphosphates (NDP) are synthesized from the corresponding nucleoside monophosphates (NMP) by base-specific nucleoside monophosphate kinases. Usually ATP is the source of transferred phosphate.Adenylate kinase is particularly active in liver and muscle, where the turnover of energy from ATP is high. Its function is to maintain an equilibrium among AMP, ADP, and ATP.
Nucleoside diphosphates and triphosphates are interconverted by “nucleoside diphosphate kinase”—an enzyme that, unlike the monophosphate kinases, has broad specificity (i.e. not base- specific).
خاطره تحفيزيه :-
سورة يوسف تقول لك أن تستمر بالمحاولة..لا أن تتوهم أنك ستكون مثل يوسف لمجرد أنك دخلت السجن ....
د.احمد خيري العمري...
By: A. Al-jalili.