
Lecture 8 Dr. Janan Alrefaee
2-Thirst mechanism: It regulates fluid intake. The thirst centre located in hypothalamus
(Fig.7.4, last lecture).
Stimuli for Thirst
1-Increased extracellular fluid osmolarity causes intracellular dehydration in the thirst
centers, thereby stimulating the sensation of thirst.
2- Decreases in extracellular fluid volume and arterial pressure.
3-
angiotensin II.
4- Dryness of the mouth and mucous membranes of the esophagus.
5- Gastrointestinal and pharyngeal stimuli influence thirst (gastric distension
thirst).
The threshold for drinking
When the sodium concentration increases only about 2 mEq/L above normal, the thirst
mechanism is activated, causing a desire to drink water.
●An increase in sodium intake six times normal, slightly increase plasma sodium
concentration as long as the ADH and thirst mechanisms are both or one of them functioning
normally and there is fluid intakes.
●angiotensin II and aldosterone: normally have little increases in sodium concentration
(except under disease conditions) because that: 1-they increase both sodium and water
reabsorption by the renal tubules. 2- The ADH-thirst system regulates sodium concentration
when it increases due to them.
Salt-appetite mechanism for controlling extracellular fluid sodium concentration and
volume
Humans can survive and function normally on 10 to 20 mEq/day sodium intake.
The two primary stimuli that increase salt appetite are (1) decreased extracellular fluid
sodium concentration and (2) decreased blood volume or blood pressure, associated with
circulatory insufficiency.
The neuronal mechanism for salt appetite is analogous to that of the thirst mechanism.
Regulation of potassium (k+) excretion and potassium concentration (conc.) in
extracellular fluid (ECF)
Normally ECF k+ conc. is about 4.2 mEq/L (± 0.3 mEq/L).
Many cell functions are very
sensitive to changes in ECF k+ conc. (an increase in plasma k+ conc. can cause heart
problem). Over 98 % of the total body k+ is intracellular and only 2 % in the ECF.
Redistribution of k+ between the intra- and ECF compartments provides a first line of
defence against changes in ECF k+ conc.

Factors shift potassium into cells (decrease extracellular potassium concentration)
1-Insulin (In diabetes mellitus,
there is a rise in plasma potassium concentration after eating a
meal).
2-aldosterone (Increased potassium intake stimulates secretion of aldosterone).
3-
β-adrenergic stimulation.
4-
Metabolic alkalosis
Factors shift potassium out of cells (increases extracellular potassium concentration)
1- β-adrenergic blockade.
2-
Metabolic acidosis.
3- Cell lysis.
4-
During prolonged exercise (shift k+ out of skeletal muscle, rarely this may be severe
enough to cause cardiac arrhythmias and sudden death).
5-Increased ECF osmolarity (This because osmotic flow of water out of the cells
cellular
dehydration increases intracellular potassium concentration
diffusion of potassium out of
the cells as in diabetes mellitus
in plasma glucose) & vice versa.
Renal potassium excretion
The filtered k+ is reabsorbed as fallow: 65 % in the proximal tubule, 25 to 30% in the loop of
Henle, especially in the thick ascending part. The most of the day-to-day regulation of k+
excretion occurs at the late distal tubules and cortical collecting tubules
where k+ can be
either reabsorbed or secreted, depending on the needs of the body.
With a normal k+ intake of 100 mEq/day, the kidneys must excrete about 92 mEq/day (the
remaining 8 mEq are lost in the feces). About one third (31 mEq/day) of this amount of k+ is
secreted into the distal and collecting tubules, this amount modify depending on body need.
Potassium secretion by principal cells of late distal and cortical collecting tubules (Fig.
8.1): Secretion of k+ from blood into interstitium then by sodium-potassium ATPase pump
into tubular cell then passively into tubular lumen (downhill).The luminal membrane of the
principal cells has channels that specifically permeable to potassium ions.
Factors that regulate potassium secretion by the principal cells
1-
Increased ECF potassium concentration (rise above 4.1 mEq/L, slightly less than the
normal concentration) stimulates potassium secretion and excretion. It also stimulates
aldosterone secretion which further stimulates potassium secretion.
2-
Aldosterone: It especially increases the permeability of the luminal membrane for
potassium and encourages potassium secretion and reduces ECF k+ conc. back toward
normal occur when potassium intake is raised & increase ECF k+ conc. (Figure 8.2).

3-increased distal tubular flow rate stimulates potassium secretion and viceversa
The mechanism: With normal tubular flow rate, the potassium is secreted into the tubular
fluid
the luminal potassium concentration
potassium secretion. With
tubular flow
rate, the secreted potassium is continuously flushed down the tubule which minimizes the
luminal potassium concentration
net potassium secretion.
This is important to preserve normal potassium excretion during changes in sodium intake.
For e.g., with
sodium intake
aldosterone secretion
potassium secretion and
excretion. However, the high distal tubular flow rate that occurs with a high sodium intake
tends to increase potassium secretion.
Therefore, these two effects of high sodium intake,
counterbalance each other, so that there is little change in potassium excretion and viceversa.
4-Acute acidosis decreases potassium Secretion
Acute increases in hydrogen ion concentration of the ECF (acidosis) reduce potassium
secretion (by reducing the activity of the sodium-potassium ATPase pump) & vice versa.
With more prolonged acidosis, lasting over a period of several days, there is an increase in
urinary potassium excretion due to inhibit proximal tubular sodium chloride and water
reabsorption, which increases distal volume delivery, thereby stimulating the secretion of
potassium. This effect reverses the inhibitory effect of hydrogen ions on the sodium-
potassium ATPase pump.
Intercalated cells: through them potassium are reabsorbed in the late distal and collecting
tubules with severe potassium depletion & potassium secretion is stopped.
This by a
hydrogen-potassium ATPase transport mechanism located in the luminal membrane.
Then
the potassium diffuses through the basolateral membrane of the cell into the blood.
But under
normal conditions this transporter plays a small role in controlling the excretion of
potassium.
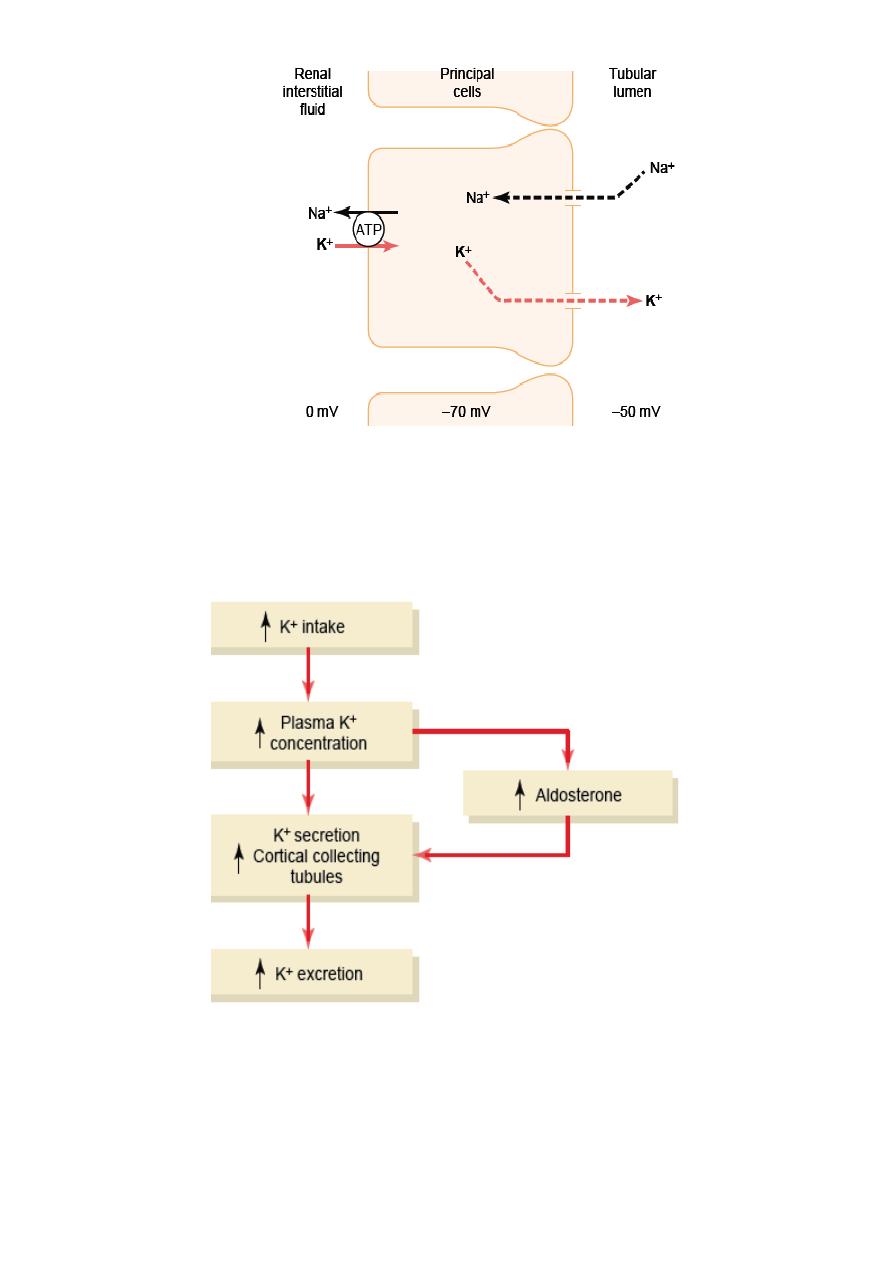
Figure 8.1 Mechanisms of potassium secretion and sodium reabsorption by the
principal cells of the late distal and collecting tubules.
Figure 8.2 Primary mechanisms by which high potassium intake raises potassium
excretion
