Respiratory regulation of acid-base balance
The second line of defence against acid-base disturbances is control of extracellular fluid
CO
2
concentration by the lungs. CO
2
is formed continually in the body cells by metabolism,
and then it diffuses to the interstitial fluids and blood flow which transports it to the lungs
alveoli and then expired by pulmonary ventilation.
If the rate of metabolic formation of CO
2
increases, the Pco
2
of the ECF is increased & vice
versa. If the metabolic formation of CO
2
remains constant, the only other factor that affects
Pco
2
in ECF is the rate of alveolar ventilation.
alveolar ventilation,
Pco
2
& vice versa.
When CO
2
concentration increases, the H
2
CO
3
concentration and H
+
concentration also
increase, thereby lowering ECF pH.
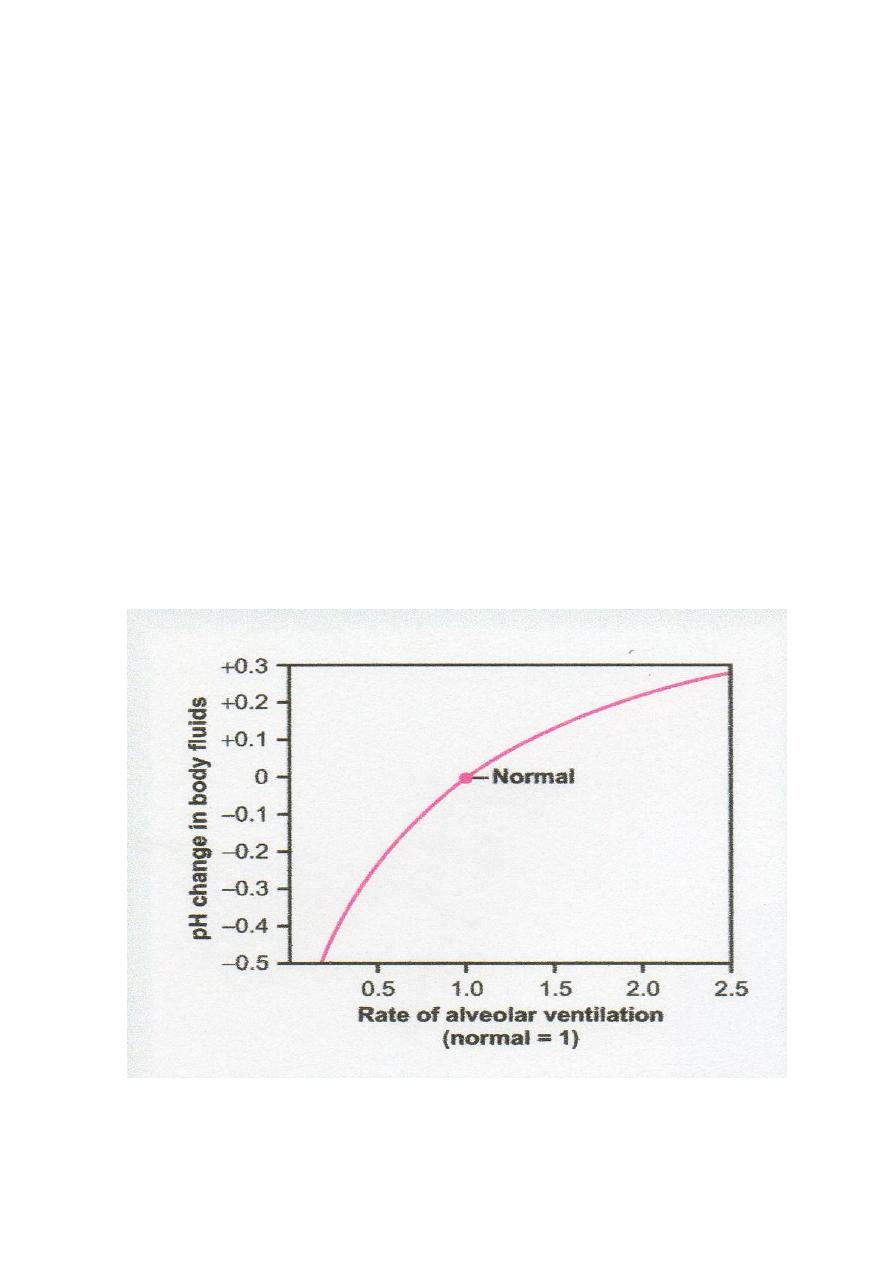
Alveolar ventilation affects pH (Fig.2.1). An increase in alveolar ventilation to twice normal
raises the pH of the ECF about 0.23 (7.63). Conversely, a decrease in alveolar ventilation to
one fourth normal reduces the pH by 0.45 (6.95) (note: the alveolar ventilation rate can
change markedly, from as low as 0 to as high as 15 times normal) .
Figure 2.1 Change in extracellular fluid pH caused by increased or decreased rate of
alveolar ventilation, expressed as times normal.
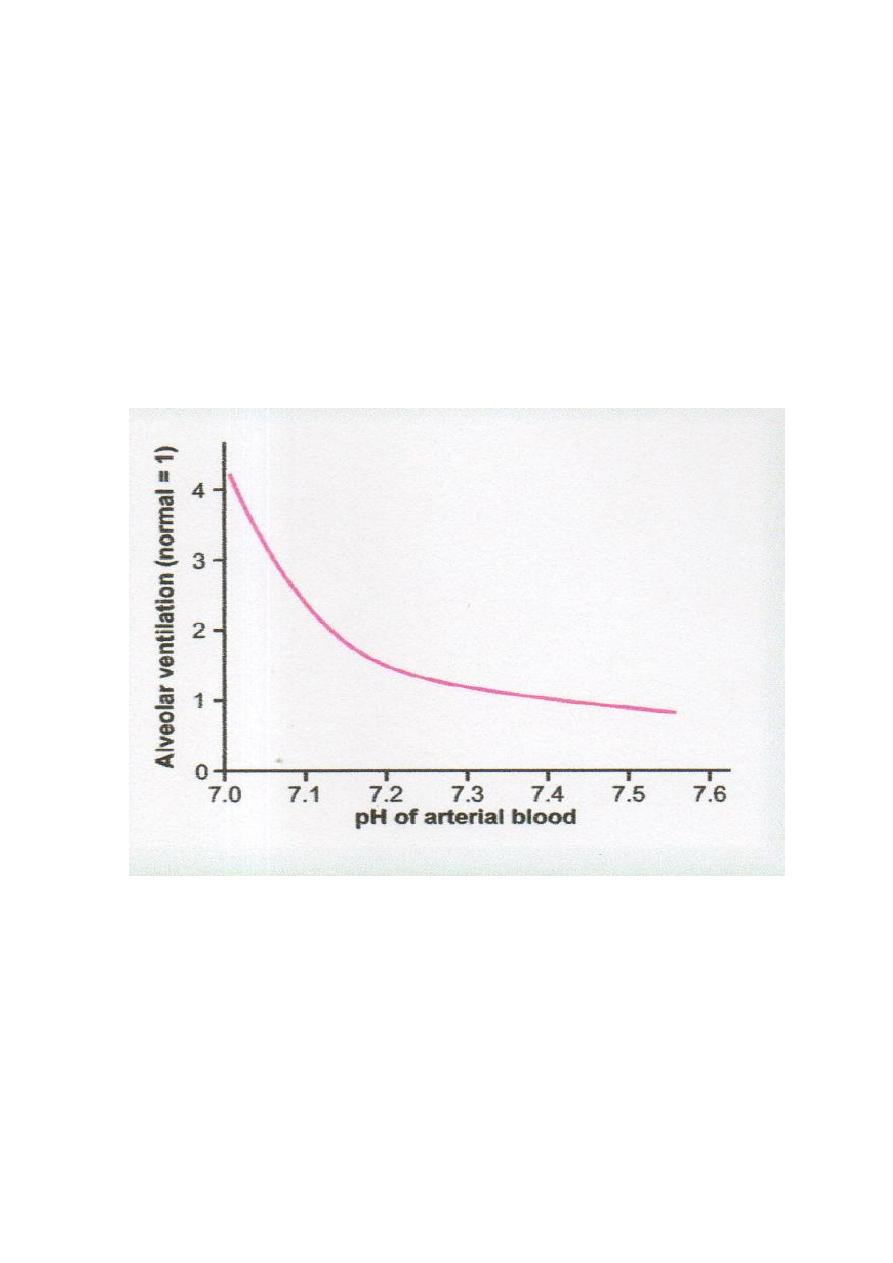
● also the H
+
concentration affects the rate of alveolar ventilation. As the pH
from the
normal value of 7.4 to the strongly acidic value of 7.0 (
H
+
concentration), the alveolar
ventilation rate
four to five times normal. Conversely, when plasma pH
above 7.4, this
causes a
in the ventilation rate (Fig.2.2). From the graph, reduced pH affect ventilation rate
much greater than
levels of pH
The reason for this is that as the alveolar ventilation rate
, due to an increase in pH (
H
+
concentration), the amount of oxygen added to the blood
and the partial pressure of oxygen
(PO
2
) in the blood also
, which stimulates the ventilation rate.
Figure 2.2 Effect of blood pH on the rate of alveolar ventilation
●Respiratory control cannot return the H
+
concentration all the way back to normal when a
disturbance outside the respiratory system has altered pH. The lung control H
+
concentration
with effectiveness between 50 and 75 % occurs within 3 to 12 minutes.
●the buffering power of the respiratory system is 1 to 2 times as great as that of all other
chemical buffers in the ECF combined. It acts rapidly and keeps the H
+
concentration from
changing too much until the slowly responding kidneys can eliminate the imbalance.
