
Renal control of acid-base balance
The kidneys control acid-base balance by excreting either acidic or basic urine. Large
numbers of HCO
3
–
are filtered continuously into the tubules, and if they are excreted into the
urine, this removes base from the blood. Large numbers of H
+
are also secreted into the
tubular lumen by the tubular epithelial cells, thus removing acid from the blood. If more H
+
is secreted than HCO
3
–
is filtered, there will be a net loss of acid from the extracellular fluid
& vice versa.
The kidneys regulate extracellular fluid H
+
concentration through three fundamental
mechanisms which are accomplished through the same basic mechanism: (1) secretion of H
+
,
(2) reabsorption of filtered HCO
3
–
, and (3) production of new HCO
3
–
.
In
ECF H
+
conc. (alkalosis), the kidneys fail to reabsorb all the filtered bicarbonate, so
bicarbonate excretion which normally buffers hydrogen in the ECF, this loss of bicarbonate
is the same as adding an H
+
to ECF
H
+
conc. back toward normal.
In acidosis, the kidneys do not excrete bicarbonate into the urine but reabsorb all the filtered
bicarbonate and produce new bicarbonate, which is added back to ECF
ECF H
+
conc.
back toward normal.
● For each bicarbonate reabsorbed, an H
+
must be secreted, but different tubular segments
accomplish this task differently.
● About 80 to 90 % of the bicarbonate reabsorption (and H
+
secretion) occurs in the proximal
tubule, (the descending and ascending thin limbs of the loop of Henle, no bicarbonate
reabsorption and H
+
secretion). In the thick ascending loop of Henle, another 10 % of the
filtered bicarbonate is reabsorbed, and the remainder of the reabsorption takes place in the
distal tubule and collecting duct.
● The renal tubule secrete H
+
into the tubular fluid by sodium-hydrogen counter-transport &
by the hydrogen-ATPase mechanism at the intercalated cells (3.1 & 3.2).
The secretory process begins when CO
2
either diffuses into the tubular cells or is formed by
metabolism in the tubular epithelial cells. CO
2
, under the influence of the enzyme carbonic
anhydrase, combines with H
2
O to form H
2
CO
3
, which dissociates into HCO
3
–
and H
+
. The H
+
is secreted from the tubular cell into the lumen. The HCO
3
–
moves downhill across the
basolateral membrane into the renal interstitial fluid and the peritubular capillary blood. The
net result is that for every H
+
secreted into the tubular lumen, an HCO
3
–
enters the blood (Fig.
3.1).
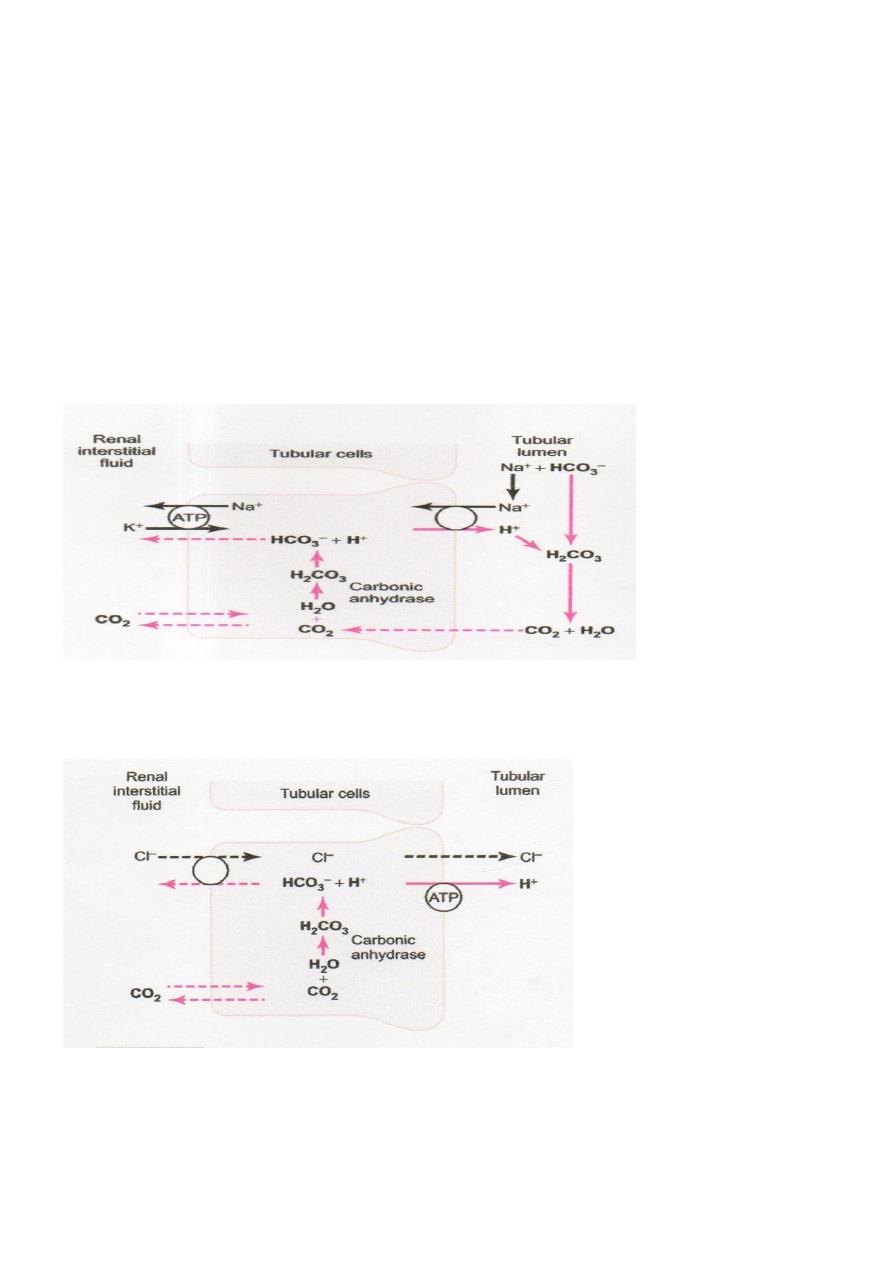
● Filtered bicarbonate ions by the glomerulus do not reabsorbed directly through the luminal
membranes of the renal tubular cells; Instead, HCO
3
–
is first combines with H
+
(secreted by
the tubular cells) to form H
2
CO
3
, which eventually becomes CO
2
and H
2
O (Fig. 3.1). The
CO
2
can move easily across the tubular membrane & diffuses into the tubular cell, where it
recombines with H
2
O, under the influence of carbonic anhydrase, to generate a new H
2
CO
3
molecule. This H
2
CO
3
in turn dissociates to form HCO
3
–
and H
+
; the HCO
3
–
then diffuses
through the basolateral membrane into the interstitial fluid and is taken up into the
peritubular capillary blood. The transport of HCO
3
–
across the basolateral membrane is
facilitated by two mechanisms: (1) Na
+
-HCO
3
–
co-transport and (2)Cl
–
-HCO
3
–
exchange.
Figure 3.1 Cellular mechanisms for active secretion of hydrogen ions into the renal
tubule.
Figure 3.2 Primary active secretion of hydrogen ions through the luminal membrane
●Under normal conditions, the rate of tubular H
+
secretion is about 4400 mEq/day, and the
rate of filtration by HCO
3
–
is about 4320 mEq/day (equal quantities), so HCO
3
–
and H
+

normally “titrate” each other in the tubules. But there is slight excess of H
+
(about 80
mEq/day) in the tubules to be excreted in the urine. Most of this H
+
is not excreted as free H
+
but rather in combination with other urinary buffers, especially phosphate and ammonia.
In metabolic alkalosis, the excess HCO
3
–
excreted into the urine. In acidosis, complete
reabsorption of the bicarbonate; the excess H
+
passes into the urine.
In the proximal tubules, the tubular fluid pH can be reduced to only about 6.7, although large
amounts of H
+
are secreted by this nephron segment. Although the secretion of H
+
in the late
distal tubule and collecting tubules accounts for only about 5 % of the total H
+
secreted, this
mechanism is important in forming a maximally acidic urine about 4.5 (high H
+
concentration) , which is the lower limit of pH that can be achieved in normal kidneys.
Combination of excess hydrogen ions with phosphate and ammonia buffers in the
tubule: in acidosis when H
+
is secreted in excess of the bicarbonate filtered into the tubular
fluid, only a small part of the excess H
+
can be excreted in the ionic form (H
+
) in the urine, to
keep the minimal urine pH is about 4.5. The large amounts of excess H
+
is excreted in urine
by combining the H
+
with buffers in the tubular fluid. The most important buffers are
phosphate buffer and ammonia buffer. There are other weak buffer systems, such as urate
and citrate that are much less important. And this results in the generation of new HCO
3
–
that
can also enter the blood (in addition to that the kidneys reabsorb all the filtered HCO
3
–
) to
reload the HCO
3
–
lost from the extracellular fluid in acidosis.
Phosphate is much effective as a buffer in the tubular fluid than extracellular fluid buffer
because:
1-The phosphate buffer (HPO
4
=
and H
2
PO
4
–
) become concentrated in the tubular fluid due to
their relatively poor reabsorption and the reabsorption of water from the tubular fluid.
2- The pK of this system is about 6.8 , so it normally functions near its most effective range
of pH.
The mechanism: any excess H
+
can combine with HPO
4
=
and other tubular buffers. After
the H
+
combines with HPO
4
=
to form H
2
PO
4
–
, it can be excreted as a sodium salt (NaH
2
PO
4
),
carrying with it the excess hydrogen (Fig. 3.3). Under normal conditions, much of the filtered
phosphate is reabsorbed and limited amount available for buffering H
+
. Therefore, much of
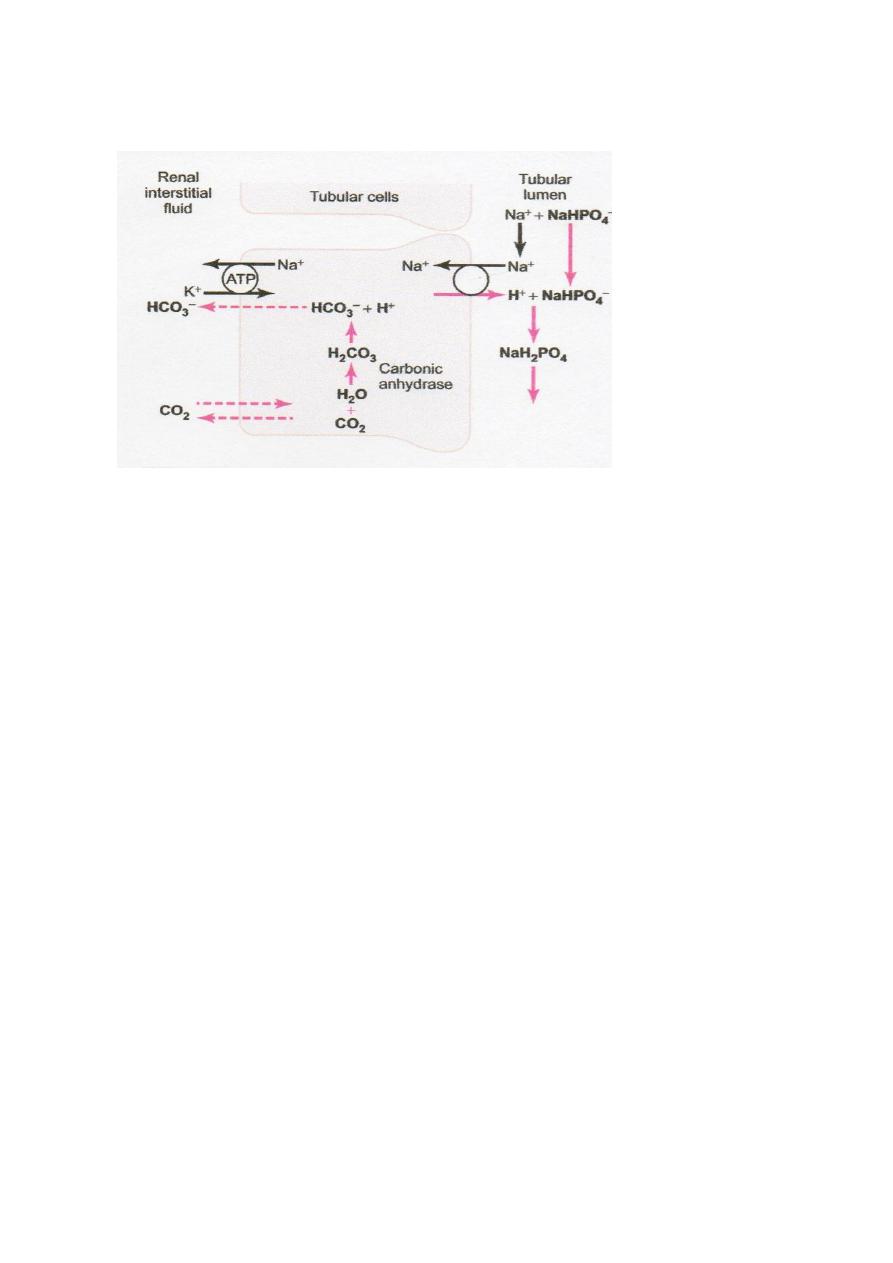
the buffering of excess H
+
in the tubular fluid in acidosis occurs through the ammonia buffer
system.
Figure 3.3 Buffering of secreted hydrogen ions by filtered phosphate (NaHPO
4
–).
Ammonia Buffer System: is composed of ammonia (NH
3
) and the ammonium ion (NH
4
+
).
Ammonium ion is synthesized from glutamine, which comes mainly from the metabolism of
amino acids in the liver. The mechanism: 1- generation two new HCO
3
–
, the filtered
glutamine by kidneys is transported into the epithelial cells of the proximal tubules, thick
ascending limb of the loop of Henle, and distal tubules (Fig. 3.4).
Once inside the cell, each molecule of glutamine is metabolized in a series of reactions
to ultimately form two NH
4
+
and two HCO
3
–
. The NH
4
+
is secreted into the tubular lumen by
a counter-transport mechanism in exchange for sodium. The HCO
3
–
is transported across the
basolateral membrane, along with the reabsorbed Na
+
, into the interstitial fluid and is taken
up by the peritubular capillaries.
2- generation one new HCO
3
–
by execreting H
+
, In the collecting tubules, H
+
is secreted by
the tubular membrane into the lumen, where it combines with NH
3
to form NH
4
+
, which is
then excreted in urine. The collecting duct is permeable to NH
3
but is much less permeable to
NH
4
+
; therefore the NH
4
+
is trapped in the tubular lumen and eliminated in the urine. For
each NH
4
+
excreted, a new HCO
3
–
is generated and added to the blood (Fig.3.5).
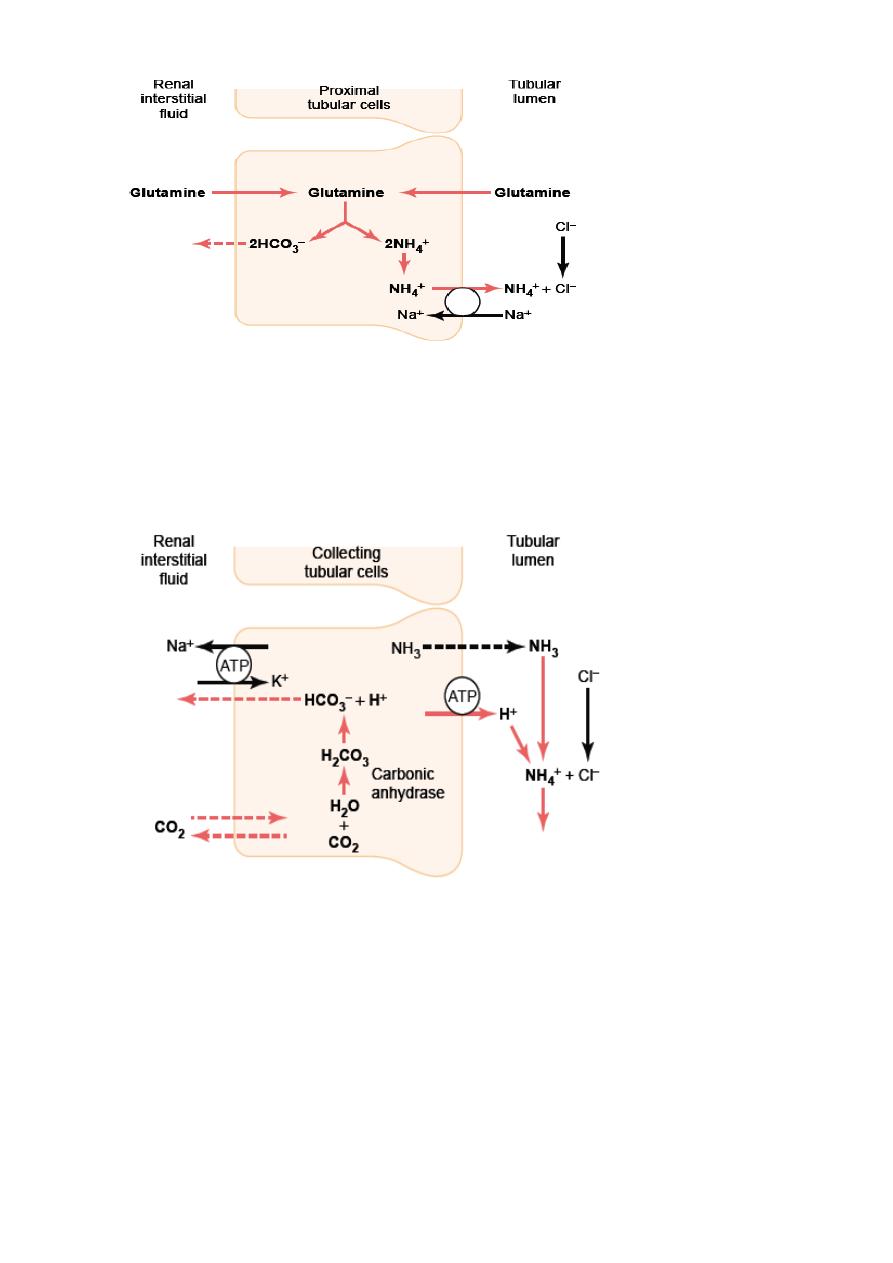
Figure 3.4 Production and secretion of ammonium ion (NH
4
+
) by proximal tubular
cells.
Figure 3.5 Buffering of hydrogen ion secretion by ammonia (NH
3
) in the collecting
tubules.
● An increase in ECF H
+
conc. stimulates renal glutamine metabolism and
the formation of
NH
4
+
and new HCO
3
–
to be used in H
+
buffering; and vice versa.

