1
Course: Medical Microbiology
Lecturer: Dr. Weam Saad
Subject: Anatomy and Genetic of bacteria
Anatomy of bacteria
All bacteria, both pathogenic and saprophytic, are unicellular organisms
that reproduce by binary fission. Most bacteria are capable of independent
metabolic existence and growth, but species of Chlamydia and Rickettsia are
obligate intracellular organisms. Bacterial cells are extremely small and are
most conveniently measured in microns (10-6 m). They range in size from
large cells such as Bacillus anthracis (1.0 to 1.3 µm X 3 to 10 µm) to very
small cells such as Pasteurella tularensis (0.2 X 0.2 to 0.7 µm), Mycoplasmas
(atypical pneumonia group) are even smaller, measuring 0.1 to 0.2 µm in
diameter.
Bacteria have characteristic shapes. The common microscopic
morphologies are cocci (round cells, such as Staphylococcus aureus or
Streptococcus sp.; rods, such as Bacillus and Clostridium species; long,
filamentous branched cells, such as Actinomyces species; and comma-shaped
and spiral cells, such as Vibrio cholerae and Treponema pallidum.
The arrangement of cells is also typical of various species or groups of
bacteria (Fig. below). Some rods or cocci characteristically grow in chains;
some, such as Staphylococcus aureus, form grapelike clusters of spherical
cells; some round cocci form cubic packets. Bacterial cells of other species
grow separately. The microscopic appearance is therefore valuable in
classification and diagnosis.
The Nucleoid
The prokaryotic nucleoid is equivalent for the eukaryotic nucleus, it is
structurally simpler than the true eukaryotic nucleus, the electron micrograph

2
shows the bacterial nucleoid with no surrounding nuclear membrane and
contains the DNA fibrils.
The DNA is a single, continuous, "giant" circular molecule with a
molecular weight of approximately 3 X 109. The unfolded nuclear DNA
would be about 1 mm long (compared with an average length of 1 to 2 µm for
bacterial cells). The bacterial nucleoid, then, is a structure containing a single
chromosome. The number of copies of this chromosome in a cell depends on
the stage of the cell cycle (chromosome replication, cell enlargement,
chromosome segregation, etc). Bacterial chromatin does not contain basic
histone proteins, but low-molecular-weight polyamines and magnesium ions
may fulfill a function similar to that of eukaryotic histones.

3
Surface Appendages
Two types of surface appendage can be recognized on certain bacterial
species: Flagella, which are organs of locomotion, and Pili ( in Latin =hairs),
which are also known as fimbriae ( in Latin = fringes). Flagella occur on both
Gram-positive and Gram-negative bacteria, and their presence can be useful in
identification. For example, they are found on many species of bacilli but
rarely on cocci. In contrast, pili occur almost exclusively on Gram-negative
bacteria and are found on only a few Gram-positive organisms (e.g.,
Corynebacterium renale).
Some bacteria have both flagella and pili. The electron micrograph in Fig.
below shows the characteristic wavy appearance of flagella and two types of
pili on the surface of Escherichia coli.
1. Flagella
Structurally, bacterial flagella are long (3 to 12 µm), filamentous surface
appendages about 12 to 30 nm in diameter. The protein subunits of a flagellum
form a cylindrical structure with a core.
A flagellum consists of three parts:
(1) the long filament, which lies external to the cell surface.
(2) the hook structure at the end of the filament.
(3) the basal body, to which the hook is anchored and which imparts
motion to the flagellum.

4
The basal body traverses the outer wall and membrane structures. It
consists of a rod and one or two pairs of discs; the counterclockwise rotation
of bacterial flagellum is due to the basal body, which causes the helically
twisted filament to whirl. The ability of bacteria to swim by means of the
propeller-like action of the flagella provides them with the mechanical means
to perform chemotaxis (movement in response to attractant and repellent
substances in the environment).
Chemically, flagella are constructed of a class of proteins called flagellins.
Flagellins are immunogenic and constitute a group of protein antigens called
the H antigens, which are characteristic of a given species, strain, or variant of
an organism. The species specificity of the flagellins reflects differences in the
primary structures of the proteins. Antigenic changes of the flagella known as
the phase variation of H1 and H2 occurs in Salmonella typhimurium.
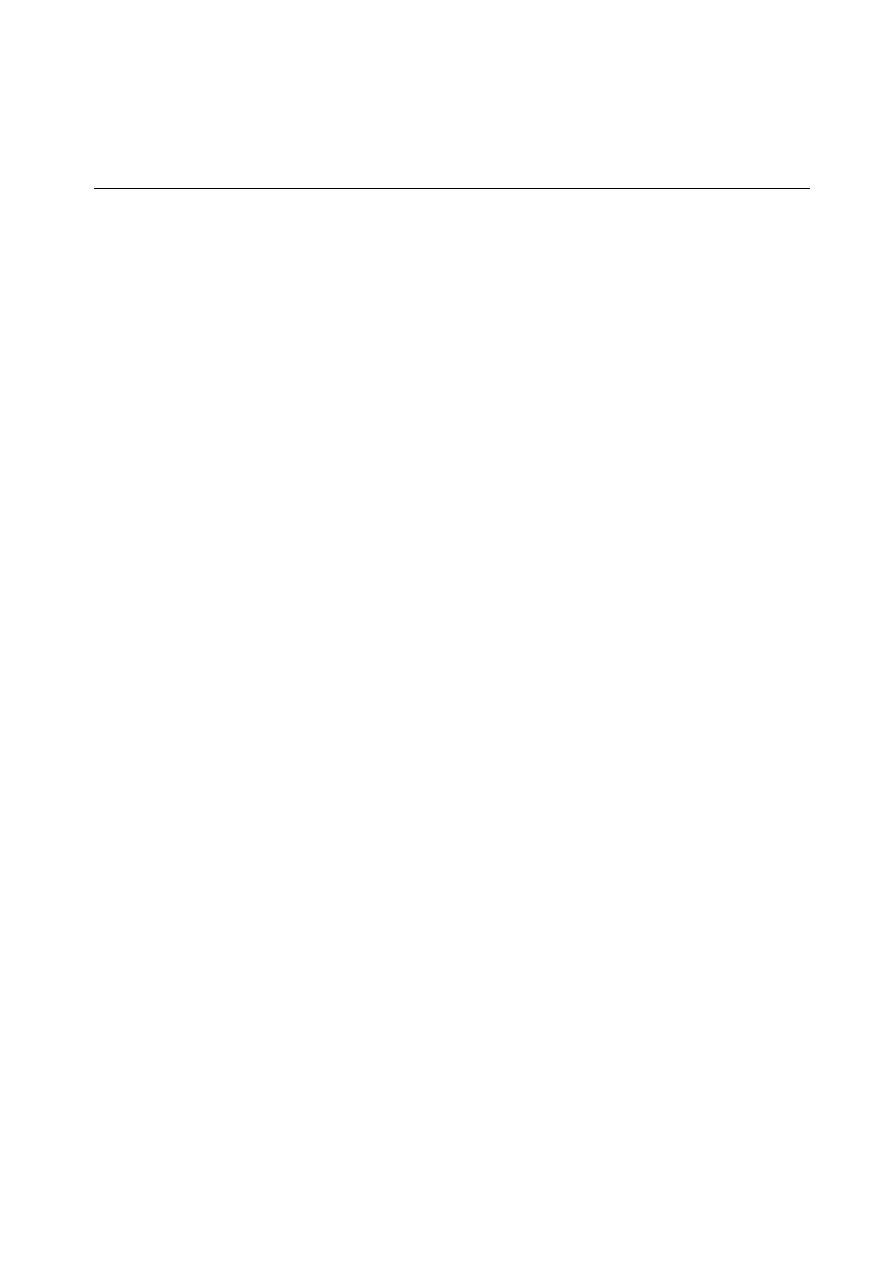
The number and distribution of flagella on the bacterial surface are
characteristic for a given species and hence are useful in identifying and
classifying bacteria. Figure below illustrates typical arrangements of flagella
on or around the bacterial surface. For example, V. cholerae has a single
flagellum at one pole of the cell (i.e., it is monotrichous), whereas Proteus
vulgaris and E. coli have many flagella distributed over the entire cell surface
(i.e., they are peritrichous). The flagella of a peritrichous bacterium must
aggregate as a posterior bundle to propel the cell in a forward direction.
Flagella can be sheared from the cell surface without affecting the viability
of the cell. The cell then becomes temporarily nonmotile. In time it
synthesizes new flagella and regains motility. The protein synthesis inhibitor
chloramphenicol, however, blocks regeneration of flagella.
5
2. Pili
The terms pili and fimbriae are usually used to describe the thin, hairlike
appendages on the surface of many Gram-negative bacteria and proteins of pili
are referred to as pilins. Pili are more rigid in appearance than flagella. In
some organisms, such as Shigella species and E coli, pili are distributed over
the cell surface, with as many as 200 per cell.
As is easily recognized in strains of E coli, pili can come in two types:
short, abundant common pili, and a small number (one to six) of very long pili
known as sex pili. Sex pili can be distinguished by their ability to bind male-
specific bacteriophages (the sex pilus acts as a specific receptor for these
bacteriophages). The sex pili attach male to female bacteria during
conjugation.
Pili in many enteric bacteria confer adhesive properties on the bacterial
cells, enabling them to adhere to various epithelial surfaces, to red blood cells
(causing hemagglutination), and to surfaces of yeast and fungal cells. These
adhesive properties of piliated cells play an important role in bacterial
colonization of epithelial surfaces and are therefore referred to as colonization
factors.

6
Surface Layers
The principal surface layers are capsules and loose slime, cell wall and
plasma (cytoplasmic) membranes.
1. Capsules and Loose Slime
Some bacteria form capsules, they are thick layer of viscous gel. Capsules
may be up to 10 µm thick. Some organisms lack a well-defined capsule but
have loose, amorphous slime layers external to the cell wall or cell envelope.
The hemolytic Streptococcus mutans, the primary organism found in dental
plaque is able to synthesis a large extracellular mucoid glucans from sucrose.
Not all bacterial species produce capsules; the capsules of encapsulated
pathogens are often important determinants of virulence. Encapsulated species
are found among both Gram-positive and Gram-negative bacteria. In both
groups, most capsules are composed of high molecular-weight viscous
polysaccharides that are retained as a thick gel outside the cell wall or
envelope.
The capsule of Bacillus anthracis (the causal agent of anthrax) is
unusual in that it is composed of a g-glutamyl polypeptide. Mutational loss of
enzymes involved in the biosynthesis of the capsular polysaccharides can
result in the smooth-to-rough variation seen in the pneumococci.
The capsule is not essential for viability. Viability is not affected when
capsular polysaccharides are removed enzymatically from the cell surface, the
exact function is resistance to phagocytosis and provide the bacterial cell with
protection against host defenses against invasion.
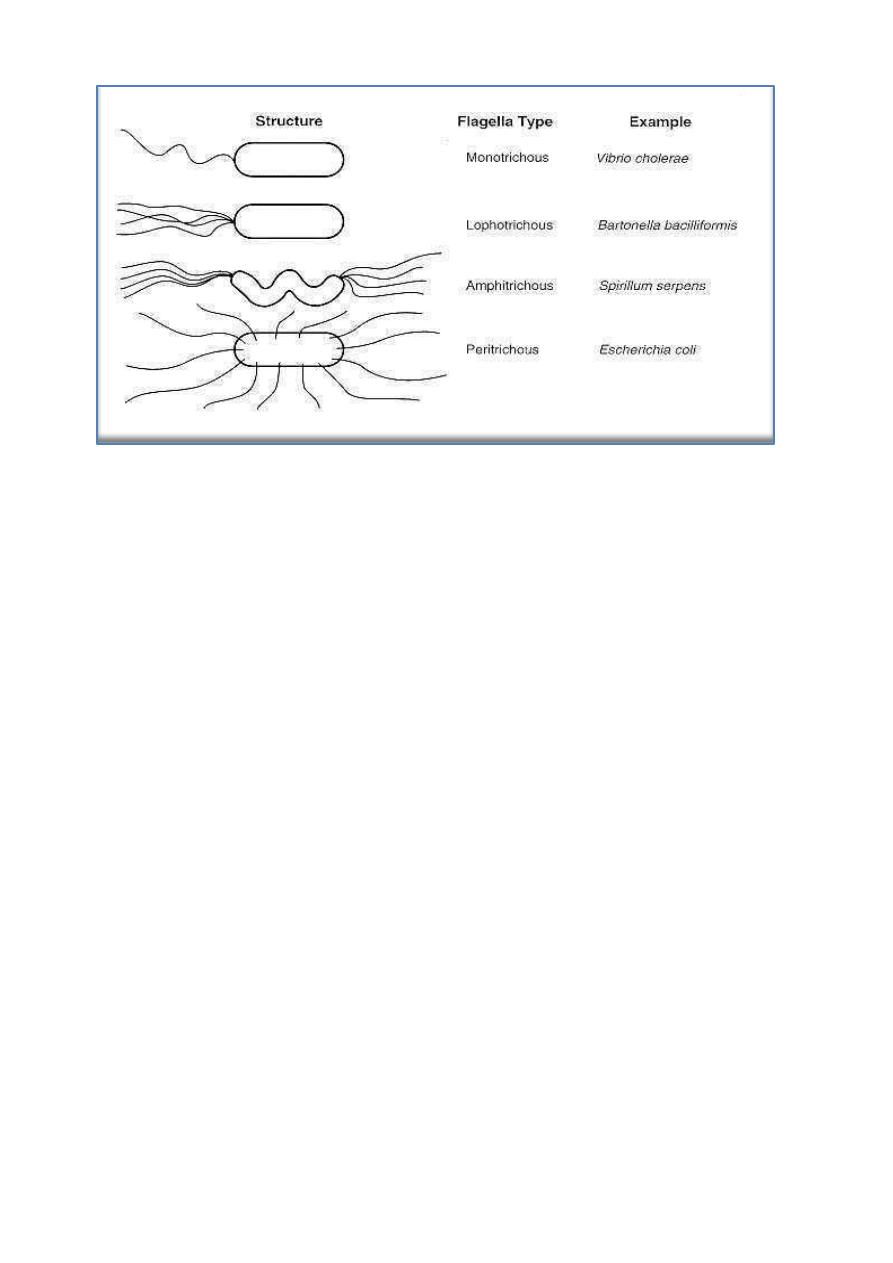
2. Cell Wall
The Gram stain differentiates bacteria into Gram-positive and Gram-
negative groups. Gram-positive and Gram-negative organisms differ in the
structures outside the plasma membrane, see figure below:
7
Most Gram-positive bacteria have a relatively thick (about 20 to 80 nm),
continuous cell wall (often called the sacculus), which is composed largely of
peptidoglycan (also known as mucopeptide or murein). In thick cell walls,
other cell wall polymers (such as the teichoic acids, polysaccharides, and
peptidoglycolipids) are covalently attached to the peptidoglycan. In contrast,
the peptidoglycan layer in Gram-negative bacteria is thin (about 5 to 10 nm
thick); in E coli, the peptidoglycan is probably only a monolayer thick.
Outside the peptidoglycan layer in the Gram-negative envelope is an outer
8
membrane structure. In most Gram-negative bacteria, this membrane structure
is anchored noncovalently to lipoprotein molecules (Braun's lipoprotein),
which, in turn, are covalently linked to the peptidoglycan. The
lipopolysaccharides of the Gram-negative cell envelope form part of the outer
leaflet of the outer membrane structure.
The basic differences in surface structures of Gram-positive and Gram-
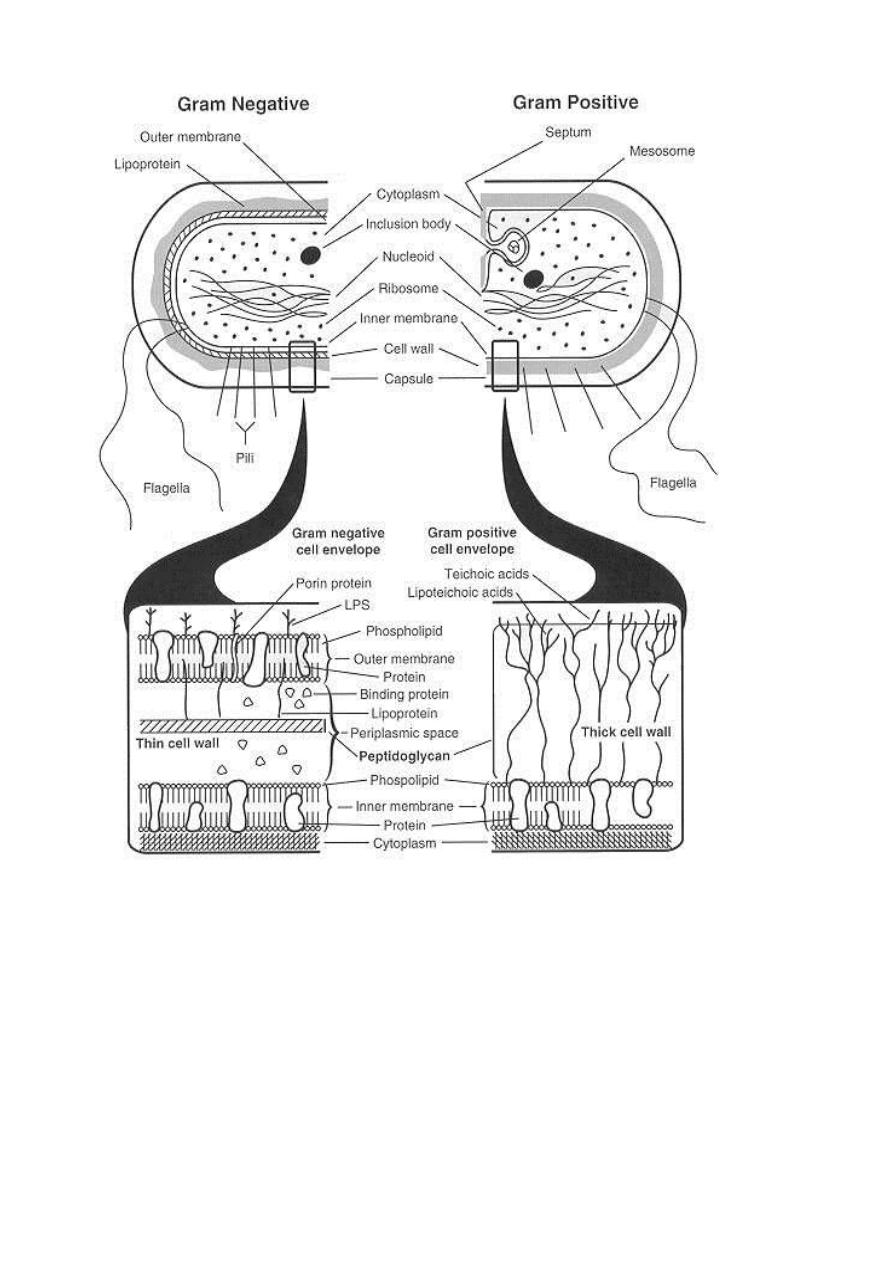
negative bacteria explain the results of Gram staining. Both Gram-positive and
Gram-negative bacteria take up the same amounts of crystal violet (CV) and
iodine (I). The CV-I complex, is trapped inside the Gram-positive cell by the
dehydration and reduced porosity of the thick cell wall as a result of the
differential washing step with 95% ethanol or other solvent mixture. Then the
thin peptidoglycan layer at the membrane adhesion sites; do not stop the
solvent extraction of the CV-I complex from the Gram-negative cell.
The mechanism of the Gram staining based on the structural differences
between the two groups of bacteria. The sequence of steps in the Gram stain
differentiation is illustrated diagrammatically in Figure below:

9
1. Peptidoglycan
Unique features of almost all prokaryotic cells (except for mycoplasmas)
are cell wall peptidoglycan, this layer serves in mechanical protection and
there are specific enzymes involved in its biosynthesis. These enzymes are
target sites for inhibition of peptidoglycan synthesis by specific antibiotics.
The primary chemical structures of peptidoglycans of both Gram-positive and
Gram-negative bacteria consist of a polymer glycan backbone of repeating
groups of disaccharides of N-acetylmuramyl-N-acetylglucosamine and are
linked through the carboxyl group by amide linkage of muramic acid residues
of the glycan chains.
There are two groups of bacteria that lack the protective cell wall
peptidoglycan structure, the Mycoplasma species, one of which causes
atypical pneumonia and some genitourinary tract infections and the L-forms,
which originate from Gram-positive or Gram-negative bacteria and are so
designated because of their discovery and description at the Lister Institute,
London. The mycoplasmas and L-forms are all Gram-negative and insensitive
to penicillin and are bounded by a surface membrane structure.
2. Teichoic Acids
Wall teichoic acids are found only in certain Gram-positive bacteria
(such as Staphylococci, Streptococci, Lactobacilli, and Bacillus spp); they
have not been found in gram- negative organisms. Teichoic acids are polyol
phosphate polymers, with either ribitol or glycerol linked by phosphodiester
bonds. Substituent groups on the polyol chains can include D-alanine (ester
linked), N-acetylglucosamine, N-acetylgalactosamine, and glucose; the
substituent is characteristic for the teichoic acid from a particular bacterial
species and can act as a specific antigenic determinant. Teichoic acids are
covalently linked to the peptidoglycan. These highly negatively charged
polymers of the bacterial wall can serve as a cation-sequestering mechanism.
3. Lipopolysaccharides
A characteristic feature of Gram-negative bacteria is possession of various
types of complex macromolecular lipopolysaccharide (LPS), only one Gram-
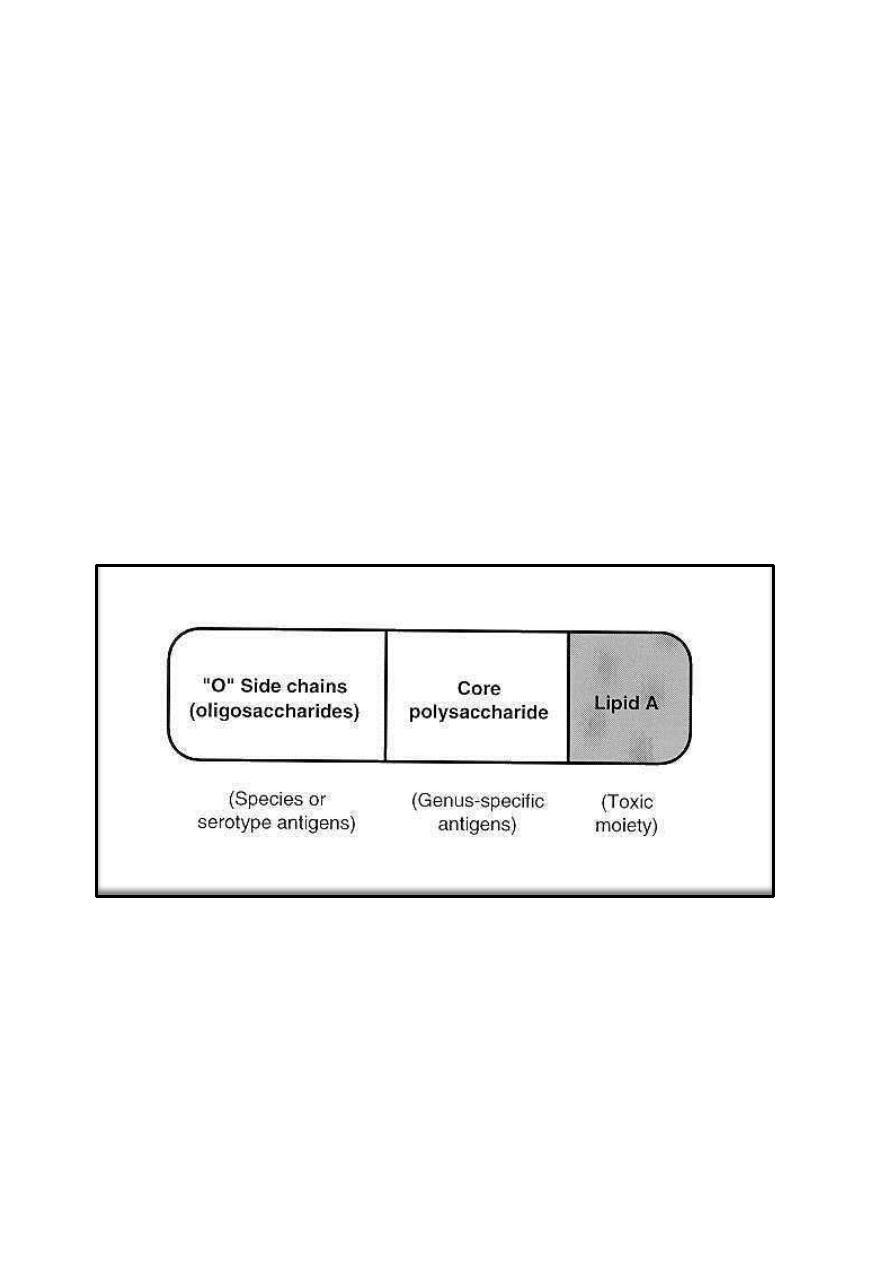
11
positive organism, Listeria monocytogenes, has been found to contain LPS.
The LPS are also called endotoxins, they are cell-bound, heat-stable toxins and
differ from heat-labile, protein exotoxins secreted into culture media.
Endotoxins possess an array of powerful biologic activities and play an
important role in the pathogenesis of many Gram-negative bacterial infections.
In addition to causing endotoxic shock, LPS is pyrogenic, can activate
macrophages and complement, is mitogenic for B lymphocytes, induces
interferon production, causes tissue necrosis and tumor regression, and has
adjuvant properties. The endotoxic properties of LPS reside largely in the lipid
A components. Usually, the LPS molecules have three regions: The lipid A
attached to the core composed of polysaccharide chains which are linked to the
O-antigens responsible for serologic specificity of the Gram-negative bacteria.
Intracellular Components
1. Plasma (Cytoplasmic) Membranes
Bacterial plasma membranes, the functional equivalents of eukaryotic
plasma membranes, are referred to variously as cytoplasmic, protoplast, or (in
Gram-negative organisms) inner membranes, they are composed primarily of

11
proteins and lipids (phospholipids). Protein-to-lipid ratios of bacterial plasma
membranes are approximately 3: 1, close to those for mitochondrial
membranes,cytoplasmic membranes from Gram-positive bacteria possess a
class of macromolecules not present in the Gram-negative membranes. Many
Gram-positive bacterial membranes contain membrane-bound lipoteichoic
acid.
Plasma membranes are the site of active transport, respiratory chain
components, energy-transducing systems, the ATPase of the proton pump, and
membrane stages in the biosynthesis of phospholipids, peptidoglycan, LPS,
and capsular polysaccharides. In essence, the bacterial cytoplasmic membrane
is a multifunction structure similar to mitochondrial transport and biosynthetic
functions of eukaryotic cells. The plasma membrane is also the anchoring site
for DNA.
2. Mesosomes
Thin sections of Gram-positive bacteria reveal the presence of vesicular or
tubular-vesicular membrane structures called mesosomes, which are
apparently formed by an invagination of the plasma membrane. These
structures equivalent to bacterial mitochondria; and may be related to events in
the cell division cycle.
3. Other Intracellular Components
In addition to the nucleoid and cytoplasm (cytosol), the intracellular
compartment of the bacterial cell is densely packed with ribosomes of the 70S
type. These ribonucleoprotein particles are not arranged on a membranous
rough endoplasmic reticulum as they are in eukaryotic cells
Endospores are highly heat-resistant, dehydrated resting cells formed
intracellularly in members of the genera Bacillus and Clostridium.
Sporulation, the process of forming endospores, is an unusual property of
certain bacteria. The series of biochemical and morphologic changes that
occur during sporulation represent true differentiation within the cycle of the
bacterial cell. The process, which usually begins in the stationary phase of the
vegetative cell cycle, is initiated by depletion of nutrients (usually readily

12
utilizable sources of carbon or nitrogen, or both). The cell then undergoes a
highly complex, well-defined sequence of morphologic and biochemical
events that ultimately lead to the formation of mature endospores. stages have
been recognized by morphologic and biochemical studies of sporulating
Bacillus species: stage 0, vegetative cells with two chromosomes at the end of
exponential growth; stage I, formation of axial chromatin filament and
excretion of exo-enzymes, including proteases; stage II, forespore septum
formation and segregation of nuclear material into two compartments; stage
III, spore protoplast formation and elevation of tricarboxylic acid and
glyoxylate cycle enzyme levels; stage IV, cortex formation of spore; stage V,
spore coat protein formation; stage VI, spore maturation, modification of
cortical peptidoglycan, uptake of dipicolinic acid (a unique endospore product)
and calcium, and development of resistance to heat and organic solvents; and
stage VII, final maturation and liberation of endospores from mother cells (in
some species).
Some strains produce autolysins that digest the walls and liberate free
endospores. The spore protoplast, or core, contains a complete nucleus,
ribosomes, and energy generating components that are enclosed within a
modified cytoplasmic membrane. The peptidoglycan spore wall surrounds the
spore membrane; on germination, this wall becomes the vegetative cell wall.
Surrounding the spore wall is a thick cortex that contains an unusual type of
peptidoglycan, which is rapidly released on germination. A spore coat of
keratinlike protein encases the spore contained within a membrane (the
exosporium). During maturation, the spore protoplast dehydrates and the spore
becomes refractile and resistant to heat, radiation, pressure, desiccation, and
chemicals; these properties correlate with the cortical peptidoglycan and the
presence of large amounts of calcium dipicolinate.
Genetic Information In Bacteria
The genetic material of bacteria and plasmids is DNA. Bacterial viruses
(bacteriophages or phages) have DNA or RNA as genetic material. The two
essential functions of genetic material are replication and expression. Genetic
material must replicate accurately so that progeny inherit all of the specific

13
genetic determinants (the genotype) of the parental organism. Expression of
specific genetic material under a particular set of growth conditions determines
the observable characteristics (phenotype) of the organism. Bacteria have few
structural or developmental features that can be observed easily, but they have
a vast array of biochemical capabilities and patterns of susceptibility to
antimicrobial agents or bacteriophages. These latter characteristics are often
selected as the inherited traits to be analyzed in studies of bacterial genetics.
Nucleic Acid Structure
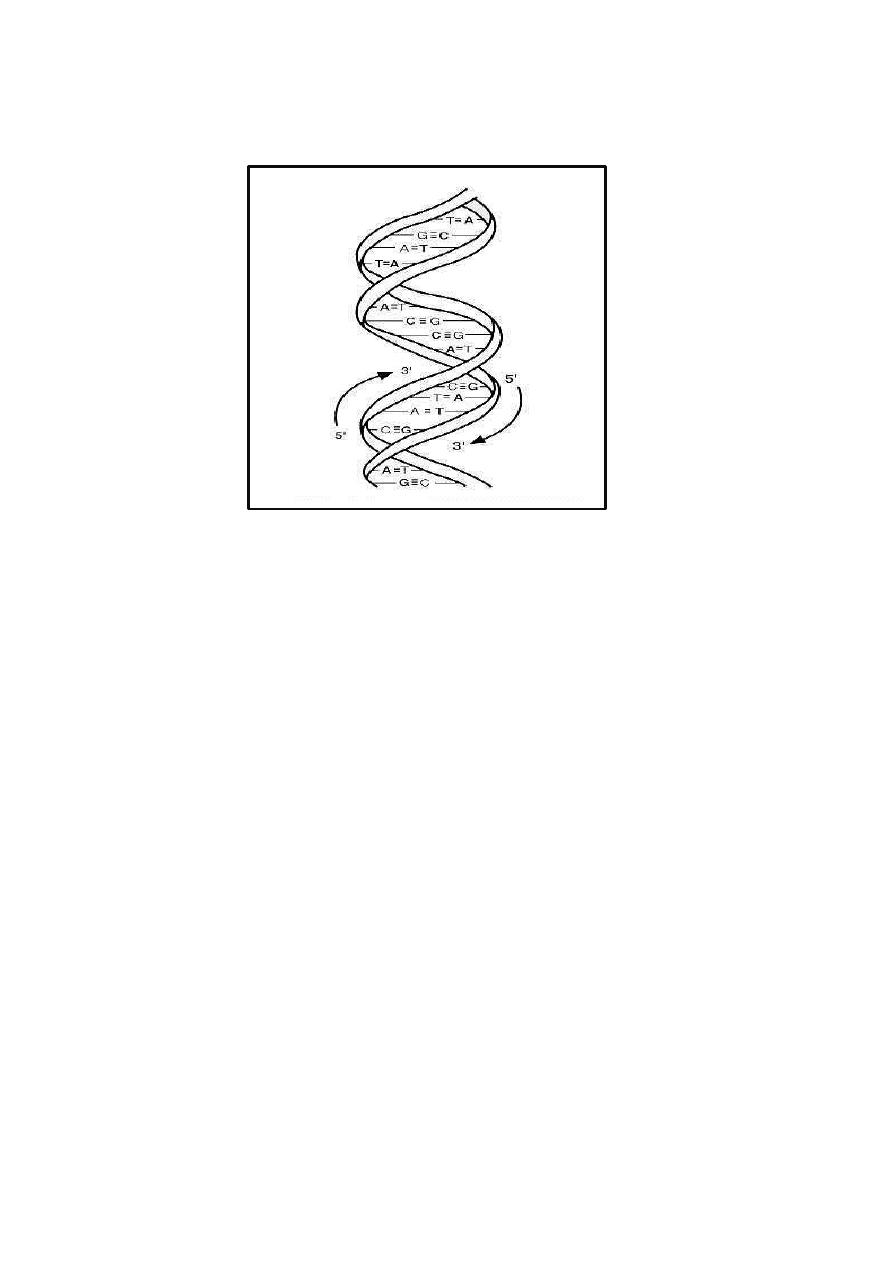
Nucleic acids are large polymers consisting of repeating nucleotide units (Fig.
below). Each nucleotide contains one phosphate group, one pentose or
deoxypentose sugar, and one purine or pyrimidine base. In DNA the sugar is
D-2-deoxyribose; in RNA the sugar is D-ribose. In DNA the purine bases are
adenine (A) and guanine (G), and the pyrimidine bases are thymine (T) and
cytosine (C). In RNA, uracil (U) replaces thymine. Chemically modified
purine and pyrimidine bases are found in some bacteria and bacteriophages.
The repeating structure of polynucleotides involves alternating sugar and
phosphate residues, with phosphodiester bonds linking the 3'-hydroxyl group
of one nucleotide sugar to the 5'-hydroxyl group of the adjacent nucleotide
sugar. These asymmetric phosphodiester linkages define the polarity of the
polynucleotide chain.
Double-stranded DNA is helical, and the two strands in the helix are
antiparallel. The double helix is stabilized by hydrogen bonds between purine
and pyrimidine bases on the opposite strands. At each position, A on one
strand pairs by two hydrogen bonds with T on the opposite strand, or G pairs
by three hydrogen bonds with C. The two strands of double-helical DNA are,
therefore, complementary. Because of complementarity, double-stranded DNA
contains equimolar amounts of purines (A + G) and pyrimidines (T + C), with
A equal to T and G equal to C, but the mole fraction of G + C in DNA varies
widely among different bacteria. Information in nucleic acids is encoded by
the ordered sequence of nucleotides along the polynucleotide chain, and in
double-stranded DNA the sequence of each strand determines what the
sequence of the complementary strand must be. The extent of sequence
14
homology between DNAs from different microorganisms is important for
determining how closely they are related.
DNA Replication
During replication of the bacterial genome, each strand in double-helical
DNA serves as a template for synthesis of a new complementary strand. Each
daughter double-stranded DNA molecule thus contains one old polynucleotide
strand and one newly synthesized strand. This type of DNA replication is
called semiconservative. Replication of chromosomal DNA in bacteria starts at
a specific chromosomal site called the origin and proceeds bi-directionally
until the process is completed. When bacteria divide by binary fission after
completing DNA replication, the replicated chromosomes are partitioned into
each of the daughter cells carrying all the genetic material to be inherited by
each daughter cell at the time of cell division.
Gene Expression
Genetic information encoded in DNA is expressed by synthesis of
specific RNAs and proteins, and information flows from DNA to RNA to
protein. The DNA-directed synthesis of RNA is called transcription. Because
the strands of double-helical DNA are antiparallel and complementary, only

15
one of the two DNA strands can serve as template for synthesis of a specific
mRNA molecule. Messenger RNAs (mRNAs) transmit information from
DNA, and each mRNA in bacteria functions as the template for synthesis of
one or more specific proteins.
The process by which the nucleotide sequence of an mRNA molecule
determines the primary amino acid sequence of a protein is called translation.
Ribosomes, complexes of ribosomal RNAs (rRNAs) and several ribosomal
proteins, translate each mRNA into the corresponding polypeptide sequence
with the aid of transfer RNAs (tRNAs), amino-acyl tRNA synthesases,
initiation factors and elongation factors. All of these components of the
apparatus for protein synthesis function in the production of many different
proteins.
A gene is a DNA sequence that encodes a protein, rRNA, or tRNA
molecule (gene product). The genetic code determines the nucleotides in
mRNA which is specific to amino acids in a polypeptide by the help of
trinucleotides (codons), while any of the three codons (UAG, UAA or UGA)
results in termination of translation. They are also called nonsense codons
because they do not specify any amino acids.
Genome Organization
DNA molecules that replicate as genetic units in bacteria are called
replicons. In some Escherichia coli strains, the chromosome is the only
replicon present in the cell. Other bacterial strains have additional replicons,
such as plasmids and bacteriophages.
Chromosomal DNA
Bacterial genomes differ in size from about 0.4 x 109 to 8.6 x 109
daltons (Da), some of the smallest being obligate parasites (Mycoplasma) and
the largest belonging to bacteria capable of complex differentiation such as
Myxococcus. The amount of DNA in the genome determines the maximum
amount of information that it can encode. Most bacteria have a haploid
genome, a single chromosome consisting of a circular, double stranded DNA
molecule. However linear chromosomes have been found in Gram-positive

16
Borrelia and Streptomyces spp., and one linear and one circular chromosome
is present in the Gram-negative bacterium Agrobacterium tumefaciens.
The E coli genome is only about 0.1% as large as the human genome,
accounting for about 2 to 3 % of the dry weight of the cell, but it is sufficient
to code for several thousand polypeptides of average size (40 kDa or 360
amino acids), the DNA is supercoiled and tightly packaged in the bacterial
nucleoid. The time required for replication of the entire chromosome is about
40 minutes, which is approximately twice the shortest division time for this
bacterium. DNA replication must be initiated as cells divide, so in rapidly
growing bacteria a new round of chromosomal replication begins before an
earlier round is completed. Thus, the chromosome in rapidly growing bacteria
is replicating at more than one point. The replication of chromosomal DNA in
bacteria is complex and involves many different proteins.
Plasmids
Plasmids are replicons, extrachromosomal genetic elements in bacteria.
They are usually much smaller than the bacterial chromosome. Plasmids
usually encode properties that are not essential for bacterial viability, and
replicate independently of the chromosome.
Most plasmids are supercoiled, circular, double-stranded DNA
molecules, but linear plasmids have also been demonstrated in Borrelia and
Streptomyces. Conjugative plasmids, large plasmids, that also promote transfer
of the bacterial chromosome from the donor bacterium to other recipient
bacteria are called fertility plasmids, and are discussed below. While small
plasmids are usually non-conjugative.
Many plasmids control medically important properties of pathogenic
bacteria, including resistance to one or several antibiotics, production of
toxins, and synthesis of cell surface structures required for adherence or
colonization. Plasmids that determine resistance to antibiotics are often called
R plasmids (or R factors). Representative toxins encoded by plasmids include
heat-labile and heat-stable enterotoxins of E coli, exfoliative toxin of
Staphylococcus aureus, and tetanus toxin of Clostridium tetani. Some

17
plasmids are cryptic and have no recognizable effects on the bacterial cells that
harbor them.
Bacteriophages
Bacteriophages (bacterial viruses, phages) are infectious agents that
replicate as obligate intracellular parasites in bacteria. Extracellular phage
particles are metabolically inert and consist principally of proteins plus nucleic
acid (DNA or RNA, but not both). The proteins of the phage particle form a
protective shell (capsid) surrounding the tightly packaged nucleic acid
genome.
Phage genomes are different in size, consist of double-stranded DNA,
single-stranded DNA, or RNA. Phage genomes, like plasmids, encode
functions required for replication in bacteria, but unlike plasmids they also
encode capsid proteins and nonstructural proteins required for phage.
A single cycle of phage growth is initiated by adsorption of phage to
specific receptors on the surface of susceptible host bacteria. The capsids
remain at the cell surface, and the DNA or RNA genomes enter the target cells
(penetration). The infecting phage RNA or DNA is replicated to produce many
new copies of the phage genome, and phage-specific proteins are produced.
Phages are classified into two major groups: virulent and temperate. For
example of bacteriophages, the prophage of bacteriophage l in E coli is
integrated into the bacterial chromosome at a specific site and replicates as
part of the bacterial chromosome, whereas the prophage of bacteriophage P1
in E coli replicates as an extrachromosomal plasmid.
The production of diphtheria toxin by Corynebacterium diphtheriae,
erythrogenic toxin by Streptococcus pyogenes (group A beta-hemolytic
streptococci), botulinum toxin by Clostridium botulinum, and Shiga-like
toxins by E coli; in each of these examples the gene which encodes the
bacterial toxin is present in a temperate phage genome.

18
Phage typing is the testing of strains of a particular bacterial species for
susceptibility to specific bacteriophages. The patterns of susceptibility to the
set of typing phages provide information about the possible relatedness of
individual clinical isolates. Such information is particularly useful for
epidemiological investigations.
Mutation and Selection
Mutations are heritable changes in the genome. Spontaneous mutations
in individual bacteria are rare. Some mutations cause changes in phenotypic
characteristics. In microbial genetics specific references organisms are
designated as wild-type strains and that have mutations in their genomes are
called mutants. Thus, mutants are characterized by the inherited differences
between them and their ancestral wild-type strains. Variant forms of a specific
genetic determinant are called alleles.
Detection of Mutant Phenotypes
Selective and differential media are helpful for isolating bacterial
mutants. Some selective media permit particular mutants to grow, but do not
allow the wild-type strains to grow. Rare mutants can be isolated by using
such selective media. Differential media permit wild-type and mutant bacteria
to grow and form colonies that differ in appearance
Spontaneous and Induced Mutations
The mutation rate in bacteria is determined by the accuracy of DNA
replication, the occurrence of damage to DNA, and the effectiveness of
mechanisms for repair of damaged DNA.
For a particular bacterial strain under defined growth conditions, the
mutation rate for any specific gene is constant and is expressed as the
probability of mutation per cell division. In a population of bacteria grown
from a small inoculum, the proportion of mutants usually increases
progressively as the size of the bacterial population increases.

19
Mutations in bacteria can occur spontaneously and independently of the
experimental methods used to detect them.
Exchange of Genetic Information
Genetic interactions between microbes enable their genomes to evolve
much more rapidly than by mutation alone. Representative phenomena of
medical importance that involve exchanges of genetic information or genomic
rearrangements include the rapid emergence and dissemination of antibiotic
resistance plasmids, flagellar phase variation in Salmonella, and antigenic
variation of surface antigens in Neisseria and Borrelia.
Sexual processes in bacteria involve transfer of genetic information from
a donor to a recipient and result either in substitution of donor alleles for
recipient alleles or addition of donor genetic elements to the recipient genome.
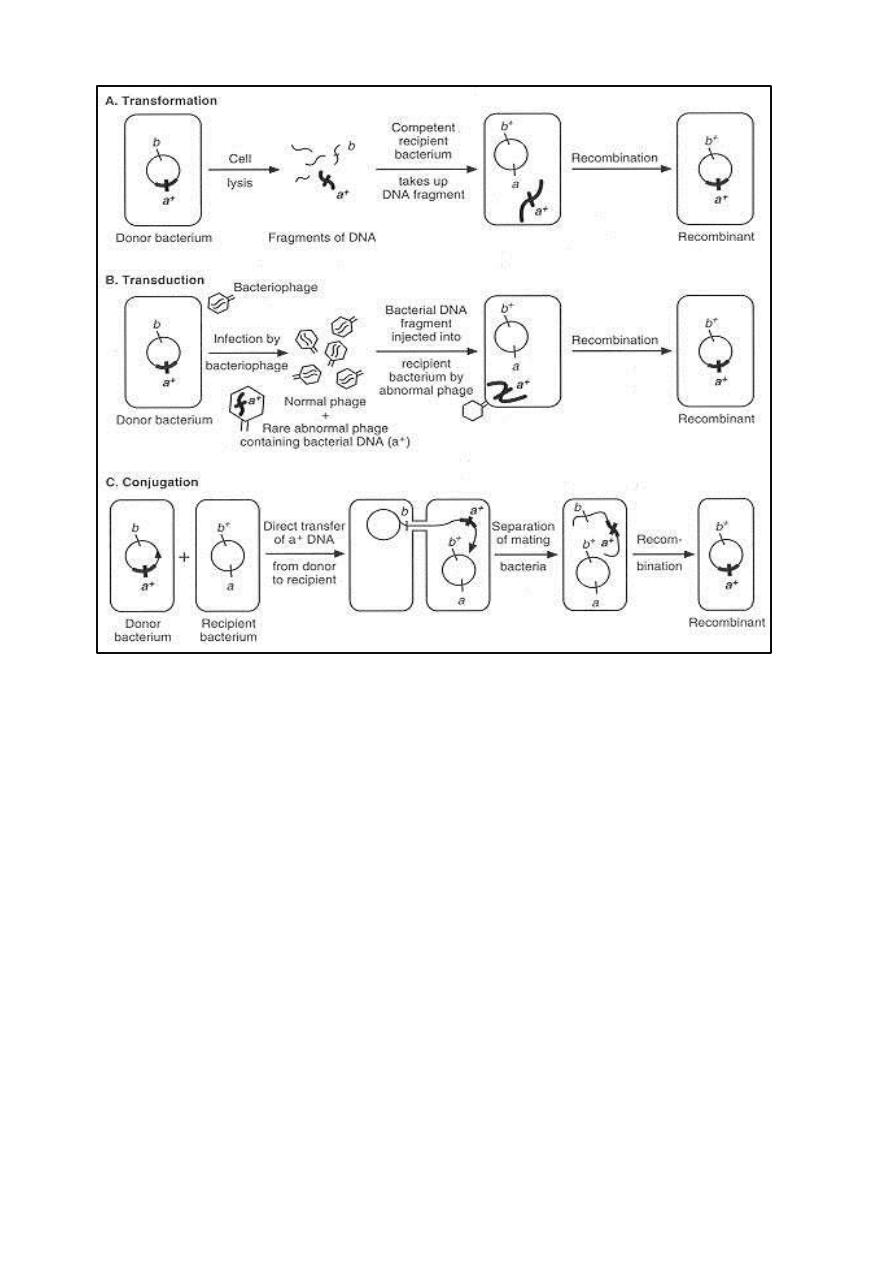
Transformation, transduction, and conjugation are sexual processes that use
different mechanisms to introduce donor DNA into recipient Recombination is
most likely to occur when the donor and recipient bacteria are from the same
or closely related species.
For a recombinant to be detected, its phenotype must be different from
both parental phenotypes. Growth or cell division may be required before the
recombinant phenotype is expressed. Genetic and physical mapping are also
used to analyze extrachromosomal replicons such as bacteriophages and
plasmids.
21
1. Transformation
In transformation, pieces of DNA released from donor bacteria are taken up
directly from the extracellular environment by recipient bacteria.
Recombination occurs between single molecules of transforming DNA and the
chromosomes of recipient bacteria. To be active in transformation, DNA
molecules must be at least 500 nucleotides in length, and transforming activity
is destroyed rapidly by treating DNA with deoxyribonuclease. Transformation
was discovered in Streptococcus pneumoniae and occurs in other bacterial
genera including Haemophilus, Neisseria, Bacillus, and Staphylococcus.
2. Transduction
In transduction, bacteriophages function as vectors to introduce DNA from
donor bacteria into recipient bacteria by infection. Each individual transducing

21
phage carries a different set of closely linked genes, representing a small
segment of the bacterial genome. Transduction mediated by populations of
such phages is called generalized transduction, because each part of the
bacterial genome has approximately the same probability of being transferred
from donor to recipient bacteria.
When a generalized transducing phage infects a recipient cell, expression of
the transferred donor genes occurs, complete transduction is characterized by
production of stable recombinants that inherit donor genes and retain the
ability to express them. In abortive transduction the donor DNA fragment does
not replicate, and among the progeny of the original transductant only one
bacterium contains the donor DNA fragment.
3. Conjugation
In conjugation, direct contact between the donor and recipient bacteria
leads to establishment of a cytoplasmic bridge between them and transfer of
part or all of the donor genome to the recipient. Donor ability is determined by
specific conjugative plasmids called fertility plasmids or sex plasmids.
The F plasmid (also called F factor) of E coli is the prototype for fertility
plasmids in Gram-negative bacteria. Strains of E coli with an
extrachromosomal F plasmid are called F+ and function as donors, whereas
strains that lack the F plasmid are F- and behave as recipients.
The conjugative functions of the F plasmid are specified by a cluster of at
least 25 transfer (tra) genes which determine expression of F pili, synthesis
and transfer of DNA during mating, interference with the ability of F+ bacteria
to serve as recipients, and other functions. Each F+ bacterium has 1 to 3 F pili
that bind to a specific outer membrane protein on recipient bacteria to initiate
mating.
An intercellular cytoplasmic bridge is formed, and one strand of the F
plasmid DNA is transferred from donor to recipient, beginning at a unique
origin and progressing in the 5' to 3' direction. The transferred strand is
converted to circular double-stranded F plasmid DNA in the recipient

22
bacterium, and a new strand is synthesized in the donor to replace the
transferred strand. Both of the ex-conjugant bacteria are F+, and the F plasmid
can therefore spread by infection among genetically compatible populations of
bacteria. In addition to the role of the F pili in conjugation, they also function
as receptors for donor-specific (male-specific) phages.
Because the F plasmid and the bacterial chromosome are both circular
DNA molecules, reciprocal recombination between them produces a larger
DNA circle consisting of F plasmid DNA inserted linearly into the
Conjugation also occurs in Gram-positive bacteria. Gram-positive donor
bacteria produce adhesins that cause them to aggregate with recipient cells, but
sex pili are not involved. In some Streptococcus species, recipient bacteria
produce extracellular sex pheromones that cause the donor phenotype to be
expressed by bacteria that harbor an appropriate conjugative plasmid, and the
conjugative plasmid prevents the donor cells from producing the
corresponding pheromone.
Recombination
Recombination involves breakage and joining of parental DNA
molecules to form hybrid, recombinant molecules. Several distinct kinds of
recombination have been identified that depend on different features of the
participating genomes and require the activities of different gene products.
Specific enzymes that act on DNA (for example, exonucleases, endonucleases,
polymerases, ligases) participate in recombination.
Transposons
Transposons are segments of DNA that can move from one site in a
DNA molecule to other target sites in the same or a different DNA molecule.
The process is called transposition and occurs by a mechanism that is
independent of generalized recombination. Transposons are important genetic
elements because they cause mutations, mediate genomic rearrangements,
function as portable regions of genetic homology, and acquire new genes and

23
contribute to their dissemination within bacterial populations. Each transposon
encodes the functions necessary for its transposition, including a transposase
enzyme that interacts with specific sequences at the ends of the transposon.
Recombination DNA and Gene Cloning
Many methods are available to make hybrid DNA molecules in vitro
(recombinant DNA) and to characterize them. Such methods include isolating
specific genes in hybrid replicons, determining their nucleotide sequences, and
creating mutations at designated locations (site-directed mutagenesis). Gene
cloning is the process of incorporating foreign genes into hybrid DNA
replicons. Cloned genes can be expressed in appropriate host cells, and the
phenotypes that they determine can be analyzed.
The first step in gene cloning is to make fragments of the donor DNA by
mechanical or enzymatic methods. Certain restriction endonucleases,
designated as class II, are particularly useful for preparing defined fragments
of DNA molecules. By choosing appropriate restriction enzymes, specific
DNA molecules, including bacterial chromosomes, plasmids, and phage
genomes, can be digested into sets of restriction fragments that have
appropriate sizes for specific applications.
The second step in gene cloning is to create hybrid replicons consisting
of donor DNA fragments and a cloning vector. Cloning vectors are small
plasmid or phage replicons that have one or more restriction sites into which
foreign DNA can be inserted. Hybrid replicons are produced by using DNA
ligase to join the restricted vector DNA with donor DNA fragments that have
compatible ends, or, alternatively, synthetic oligonucleotides are used as
linkers to create compatibility between donor and vector DNA molecules with
different ends. Plasmid and phage vectors are used mainly to clone small
inserts.
The final steps in gene cloning are to introduce hybrid replicons into
appropriate recipient cells and test them for expression of donor genes of
interest. Prokaryotic cells (including bacteria) or eukaryotic cells (including
yeast, animal or plant cells) can be used as recipients, but they differ in post-
translational modifications of protein structure that they can accomplish.

24
Many methods are available to identify bacteria that contain
recombinant DNA molecules. Most cloning vectors have genes for traits that
can be positively selected, such as resistance to antibiotics.
Applications of DNA cloning are expanding rapidly in all fields of
biology and medicine. In medical genetics such applications range from the
prenatal diagnosis of inherited human diseases to the characterization of
oncogenes and their roles in carcinogenesis. Pharmaceutical applications
include large-scale production from cloned human genes of biologic products
with therapeutic value, such as polypeptide hormones, interleukins, and
enzymes. Applications in public health and laboratory medicine include
development of vaccines to prevent specific infections and probes to diagnose
specific infections by nucleic acid hybridization or polymerase chain reaction
(PCR). The latter process uses oligonucleotide primers and DNA polymerase
to amplify specific target DNA sequences during multiple cycles of synthesis
in vitro, making it possible to detect rare target DNA sequences in clinical
specimens with great sensitivity.
