
1
Course: Microbial Physiology
Lecturer: Dr. Weam Saad
Lecture: Microbial Metabolism
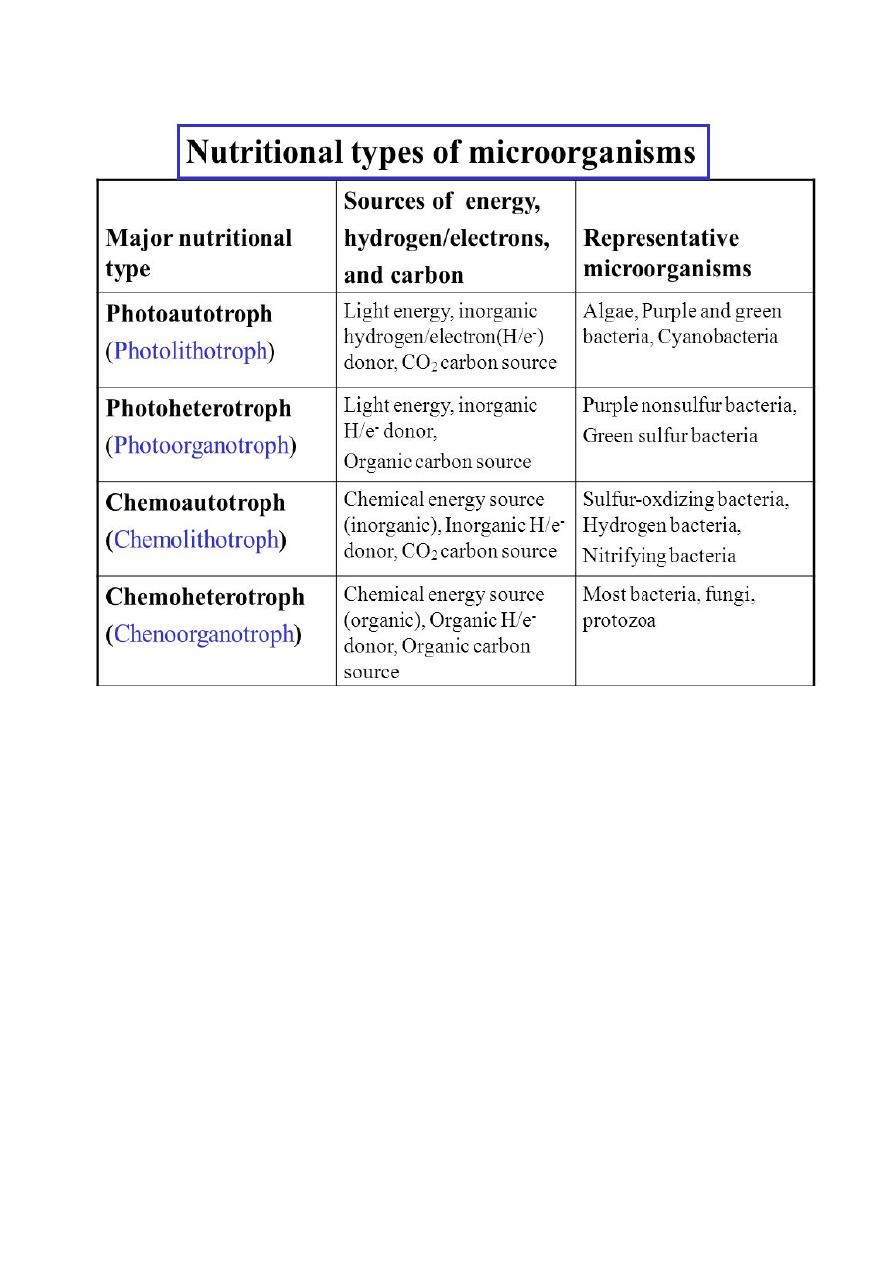
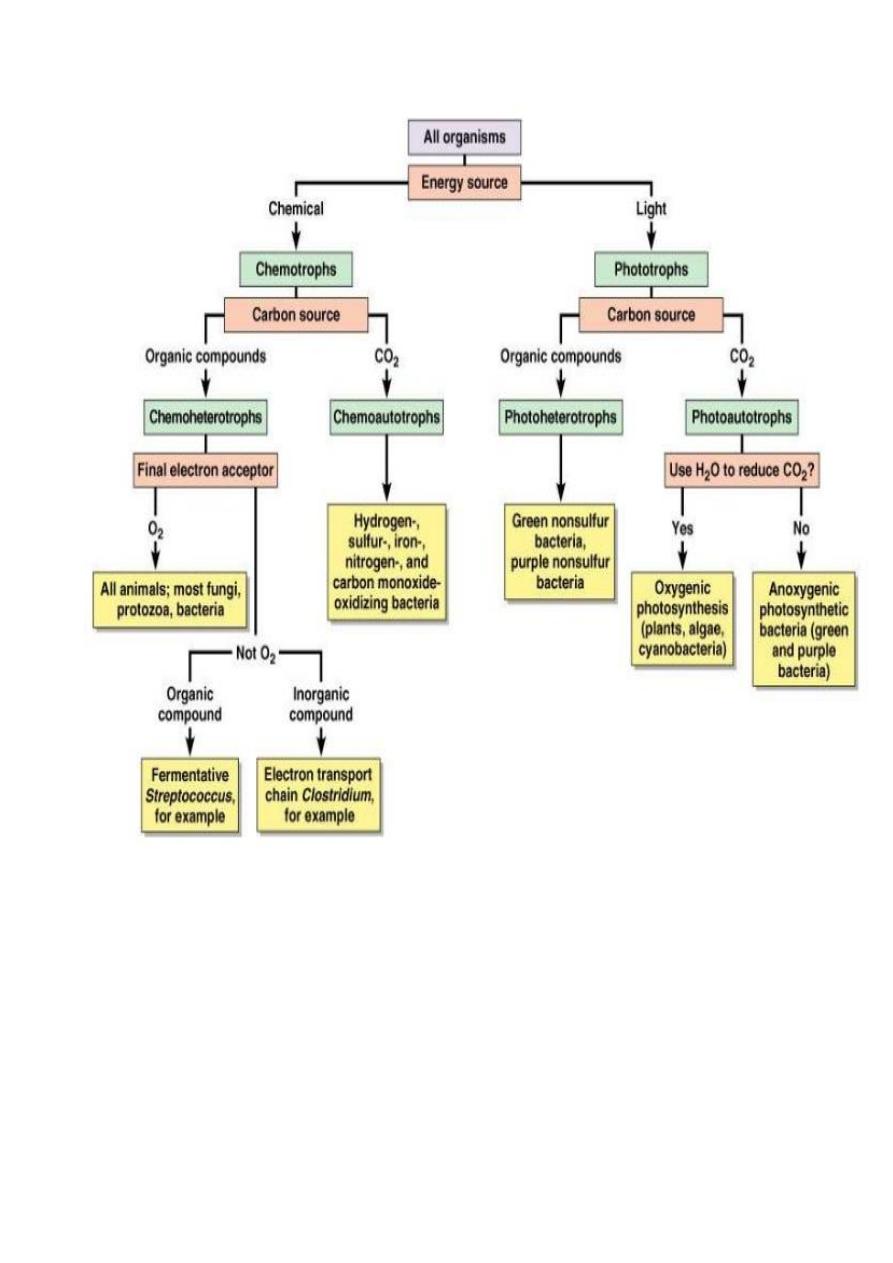
The Diversity of Metabolism in Prokaryotes
The diversity of prokaryotes is due to their great difference in energy production
and metabolism. The eukaryotes and prokaryotes produce energy (ATP) through
the following ways:
1. Alcohol fermentation (e.g. yeast).
2. Lactic acid fermentation (e.g. muscle cells, neutrophils).
3. Aerobic respiration (e.g. molds, protozoa, animals).
4. Oxygenic photosynthesis (e.g. algae, plants).
5. Fermentations through the Embden-Meyerhof pathway (prokaryotes
only)
6. Other fermentation pathways such as the phosphoketolase (heterolactic)
and Entner-Doudoroff pathways (prokaryotes only).
7. Anaerobic respiration: respiration that uses substances other than O
2
as
a final electron acceptor (prokaryotes only).
8. Lithotrophy: use of inorganic substances as sources of energy
(prokaryotes only).
9. Photoheterotrophy: use of organic compounds as a carbon source during
bacterial photosynthesis (prokaryotes only).
10. Anoxygenic photosynthesis: photophosphorylation in the absence of O
2
(prokaryotes only).
11. Methanogenesis: an ancient type of metabolism used by archabcateria
that use H
2
as an energy source and produces methane (prokaryotes only).
12. Light-driven non-photosynthetic photophosphorylation: especial
metabolism used by archabacteria that converts light energy into chemical
energy (prokaryotes only).
Even within a prokaryotic species, there may be great versatility in metabolism.
Consider Escherichia coli. The bacterium can produce energy for growth by

2
fermentation or respiration. It can respire aerobically using O
2
as a final electron
acceptor, or it can respire under anaerobic conditions, using NO
3
or fumarate as
a final electron acceptor. E. coli can use glucose or lactose as a sole carbon source
for growth, with the metabolic ability to transform the sugar into all the necessary
amino acids, vitamins and nucleotides that make up cells. Rhodospirillum
rubrum, has all the heterotrophic abilities like E. coli, plus the ability to grow by
photoautotrophic, photoheterotrophic or lithotrophic ways and need one growth
factor biotin that must be added to the growth media.
Energy-Generating Metabolism
The term metabolism refers to all the biochemical reactions required for energy
generation and the use of energy to synthesize cell material from small molecules
in the environment, metabolism includes catabolism, and anabolism. Catabolic
reactions produce energy as ATP, which can be used in anabolic reactions to
build cell material from nutrients in the environment.
ATP
ATP - adenosine triphosphate, contain high energy bonds release an energy
about 8 kcal/mole during the hydrolysis of ATP to ADP releases 8 kcal. It is a
coenzyme in most energy producing reactions in cells.
NAD
Another coenzyme commonly involved in energy-producing metabolism
(oxidation/reduction reactions), usually functions as the electron carrier, it is
derived from the vitamin niacin, is the pyridine nucleotide, NAD (Nicotinamide
Adenine Dinucleotide). The oxidized form of NAD is NAD; the reduced forms
(NADH, NADH
2
or NADH + H
+)
.
NAD
+
+ 2H----->NADH + H
+
Coenzyme A
Coenzyme A is another coenzyme involved in energy-generating metabolism of
prokaryotes. Coenzyme A is involved in some fermentative bacteria and in all

3
respiratory organisms. The oxidations of pyruvate and alpha ketoglutamate, TCA
cycle involve Coenzyme A.
ATP Synthesis in Prokaryotes
The goal of a catabolic pathway is to make ATP: to transform the chemical
energy or electromagnetic (light) energy into the chemical energy by forming the
high-energy bonds of ATP. Cells can produce ATP in two ways:
1. Substrate level phosphorylation (SLP) is the very simple way, oldest
way to make ATP. In a substrate level phosphorylation, ATP is made
during the conversion of an organic molecule from a form to another and
release energy in the form of ATP and occurs during fermentations and
respiration (the TCA cycle).
2. Electron Transport Phosphorylation (ETP) occurs during respiration,
photosynthesis, lithotrophy and possibly other types of bacterial
metabolism and need electron transport system (ETS) within a membrane.
The electrons are transferred through the ETS to some final electron
acceptor in the membrane (like O
2
in aerobic respiration).
Heterotrophic Types of Metabolism
Heterotrophy (e.g. chemoheterotrophy) is the use of an organic compound as a
source of carbon and energy. It is the complete metabolism, cells oxidize organic
molecules in order to produce energy (catabolism) and then use the energy to
synthesize cellular material from these organic molecules (anabolism).
Many Bacteria and few Archaea are heterotrophs (Archaea that live in
associations with animals). Heterotrophic bacteria are the decomposers and help
in biodegradation in the environment. Heterotrophic metabolism have two
metabolic processes: fermentations and respirations.
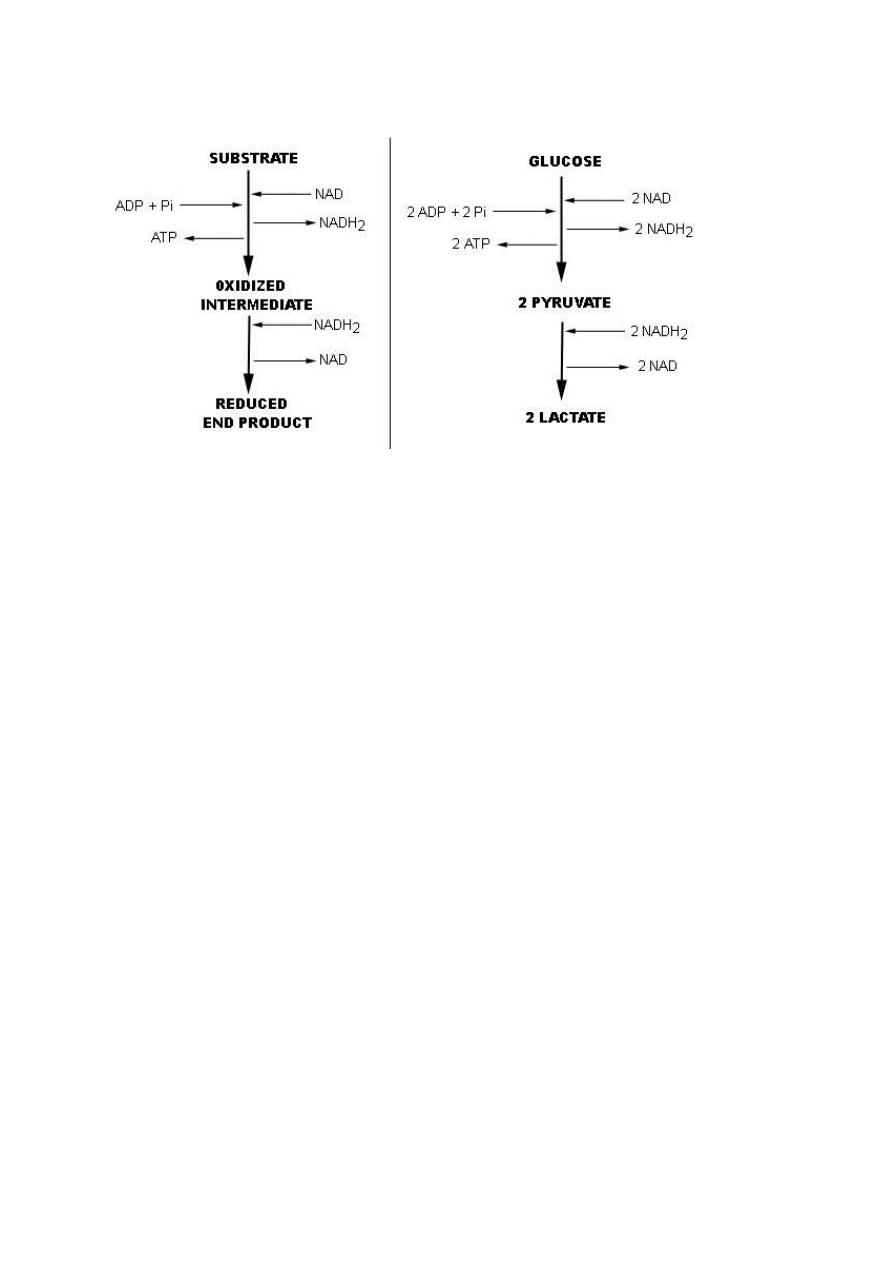
A. Fermentation
Fermentation is an ancient mode of metabolism, energy is formed from the partial
oxidation of an organic compound using organic intermediates as electron donors
and electron acceptors. No outside electron acceptors are involved; no membrane
or electron transport system is required; all ATP is produced by substrate level
phosphorylation. E.g. fermentation by Lactobacillus, the substrate (glucose) is
oxidized to pyruvate, and pyruvate becomes reduced to lactic acid.
4
In prokaryotes there are three pathways of glycolysis (the dissimilation of
sugars) used by bacteria:
1) The classic Embden-Meyerhof pathway, which is also used by most
eukaryotes, including yeast (Saccharomyces).
2) The phosphoketolase or heterolactic pathway, the hexose-pentose
shunt.
3) The Entner-Doudoroff pathway.
1) The Embden-Meyerhof Pathway
This pathway of glycolysis is most used by Saccharomyces to produce ethanol
and CO
2
as eukaryote microorganism, and is used by the (homo)lactic acid
bacteria to produce lactic acid, and it is used by many other bacteria to produce
different fatty acids, alcohols and gases. Some end products of Embden-
Meyerhof fermentations are important in foods and some are useful fuels and
industrial solvents. Diagnostic microbiologists use bacterial fermentation profiles
(e.g. testing an organism's ability to ferment sugars and the end products) for the
diagnosis.
Lactic acid bacteria reduce the pyruvate to lactic acid; yeast reduce the pyruvate
to alcohol (ethanol) and CO2. The oxidation of glucose to lactate yields a total
of 56 kcal per mole of glucose. Since the cells get 2 ATP (16 kcal) as energy for
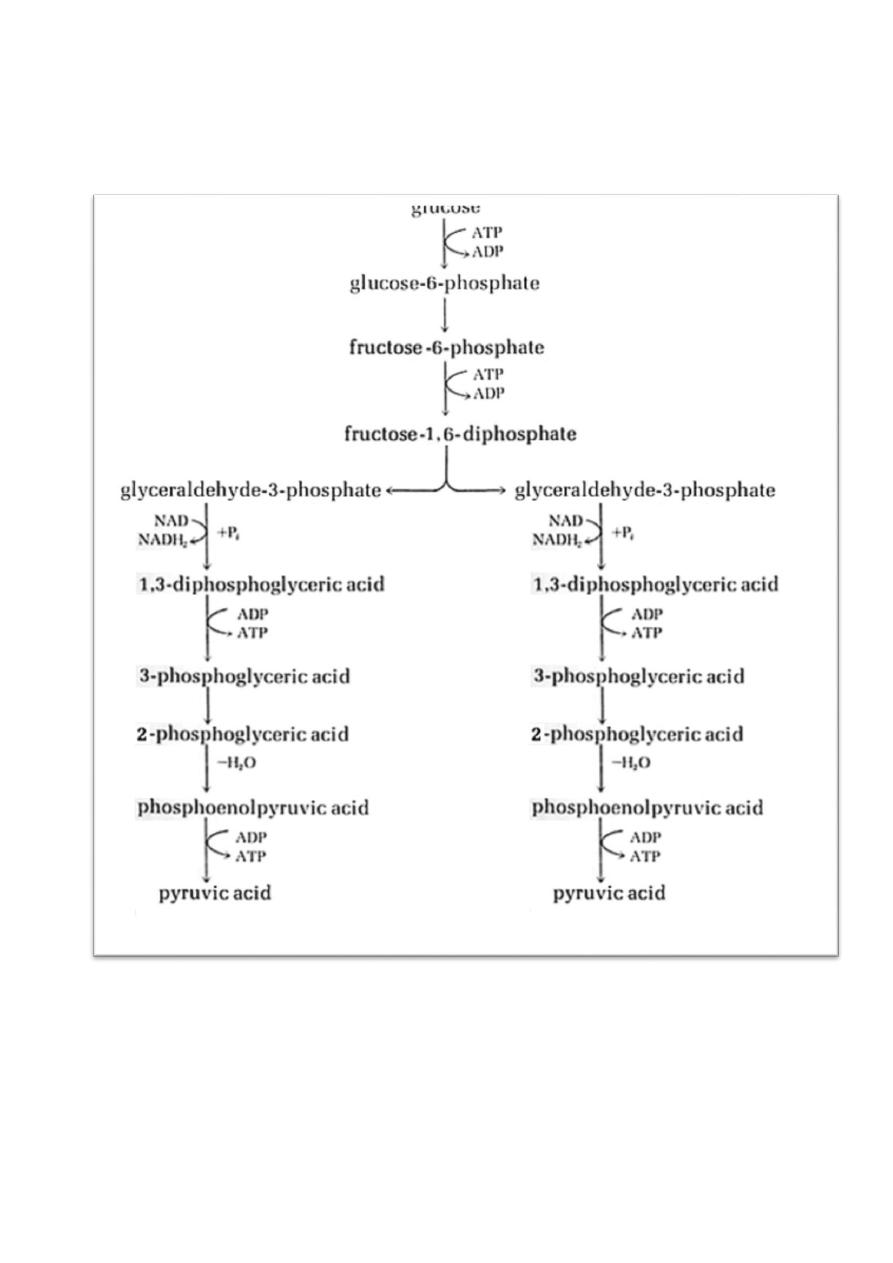
5
use, the efficiency of the lactate fermentation is about 29% (16/56). Ethanol
fermentations have a similar efficiency
.
Embden-Meyerhof fermentations in bacteria lead to many end products as the
following groups:
1. Homolactic Fermentation. Lactic acid is the end product. E.g.
Lactobacillus and most Streptococci). The bacteria are used to ferment milk and
milk products in the manufacture of yogurt and cheese.

6
2.
Mixed
Acid
Fermentations.
Mainly
the
pathway
of
the Enterobacteriaceae. End products are a mixture of lactic acid, acetic
acid, formic acid, succinate and ethanol, with the possibility of gas formation
(CO
2
and H
2
). The microbiologists have specific tests to detect low acid and
acetoin in order to distinguish non fecal enteric bacteria such
as Klebsiella and Enterobacter from fecal enterics that form mixed acid after
fermentation such as E. coli, Salmonella and Shigella.
3. Butyric acid fermentations. E.g. Clostridium sp. which form acetic acid,
CO
2
and H
2
from the fermentation of sugars.
4. Propionic acid fermentation. This is an unusual fermentation and used only
by the propionic acid bacteria which include
Corynebacteria, Propionibacterium and Bifidobacterium..
2) The Heterolactic (Phosphoketolase) Pathway
The overall reaction is Glucose ---------->1 lactic acid + 1 ethanol +1 CO
2
with
a net production of 1 ATP. The efficiency is about half that of the E-M
pathway.
Glucose -------> Lactic acid + ethanol + CO
2
+ 1 ATP (net)
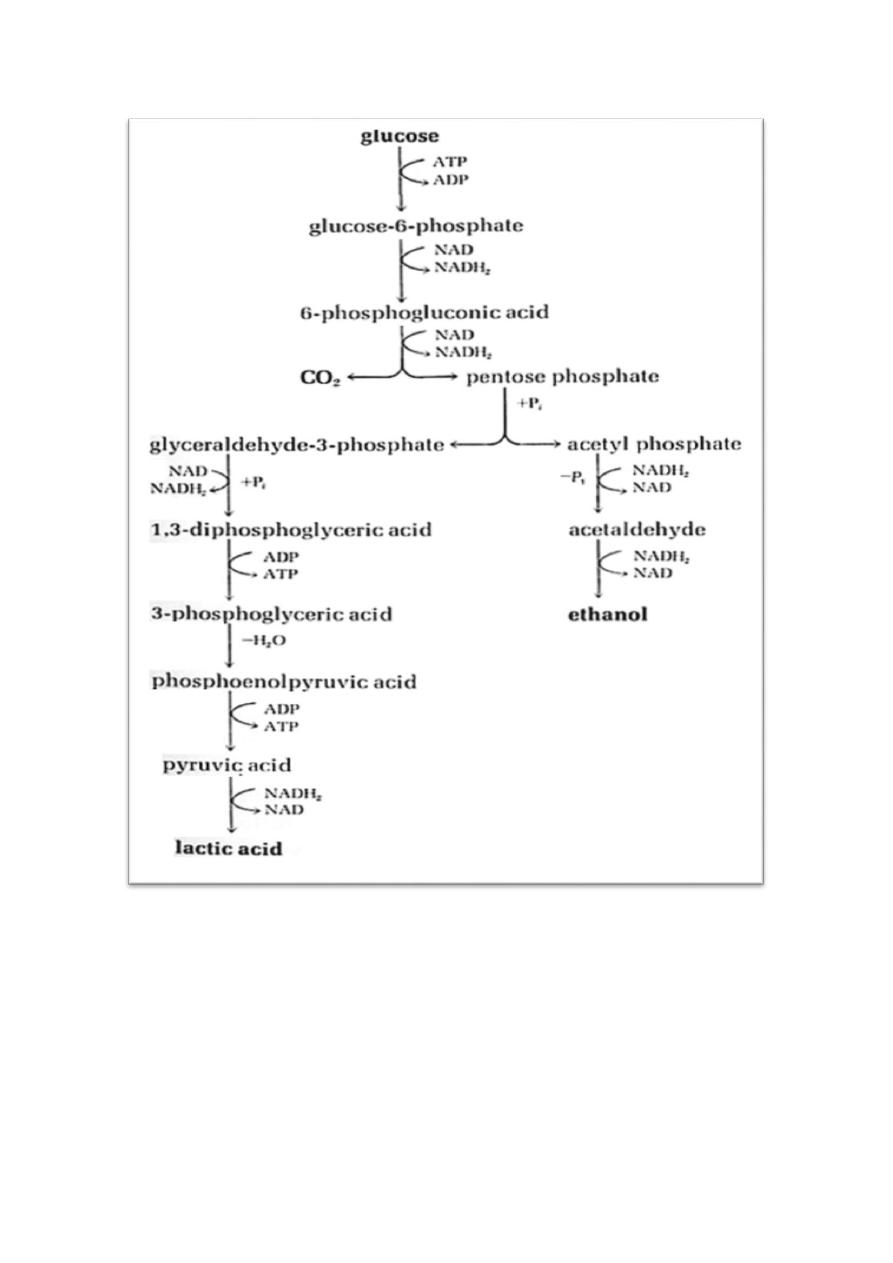
7
).
3) The Entner-Doudoroff Pathway
Only used by Zymomonas bacteria and called yeast-like bacterium, this E-D
pathway yields 2 pyruvic acid from glucose (same as the E-M pathway).
Glucose -------> 2 ethanol + 2 CO
2
+ 1 ATP (net).
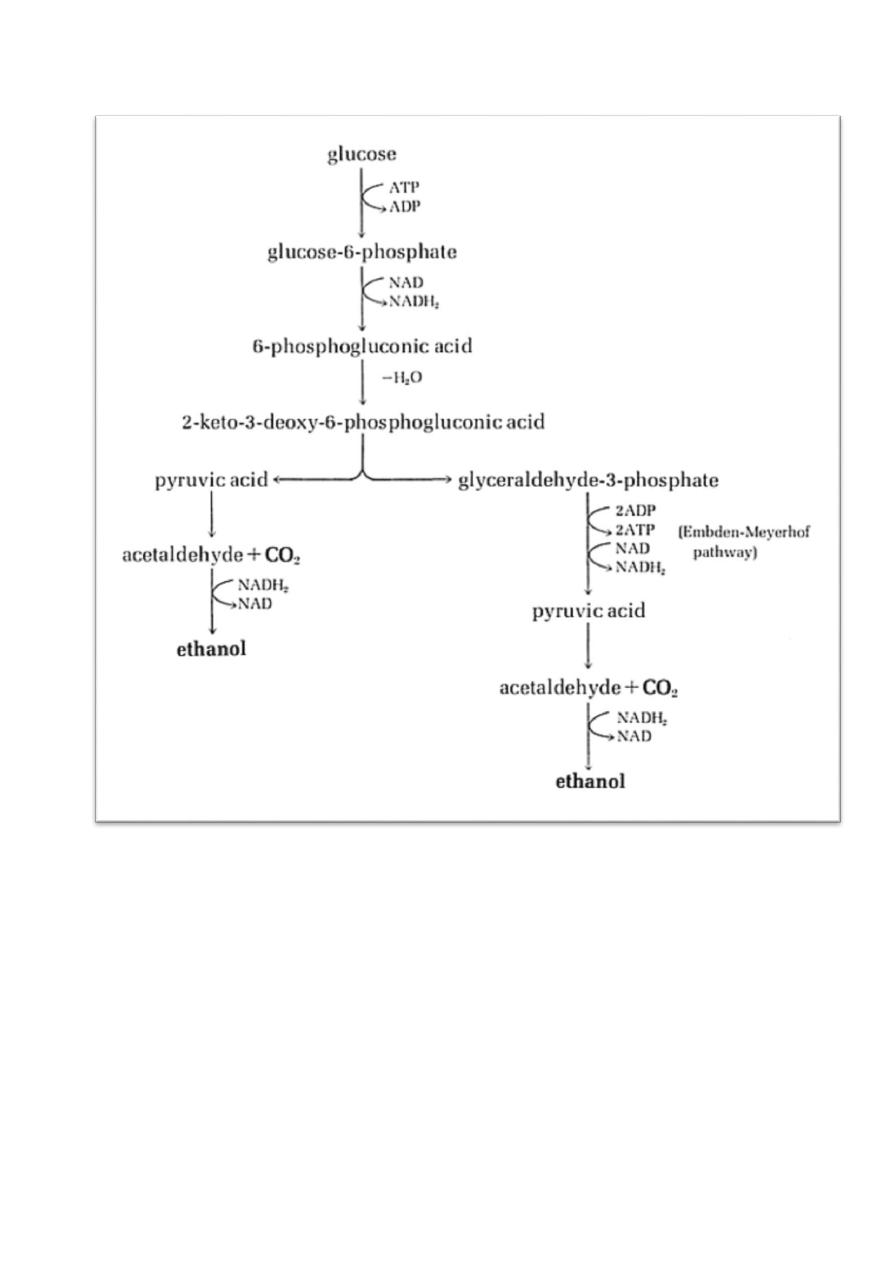
8

9
Oxidative pathways of glycolysis employed by various bacteria.
Bacterium
Embden-
Meyerhof
pathway
Phosphoketolase
(heterolactic) pathway
Entner-
Doudoroff
pathway
Acetobacter aceti
-
+
-
Azotobacter
vinelandii
-
-
+
Bacillus subtilis
major
Minor
-
Escherichia coli
+
-
-
Lactobacillus
acidophilus
+
-
-
Leuconostoc
mesenteroides
-
+
-
Pseudomonas
aeruginosa
-
-
+
Vibrio cholera
minor
-
Major
Zymomonas
mobilis
-
-
+
End product yields in microbial fermentations.
Pathway
Key enzyme
Ethanol Lactic Acid CO
2
ATP
Embden-Meyerhof
Saccharomyces
fructose 1,6-diP aldolase 2
0
2
2
Embden-Meyerhof
Lactobacillus
fructose 1,6-diP aldolase 0
2
0
2
Heterolactic
Streptococcus
phosphoketolase
1
1
1
1
Entner-Doudoroff
Zymomonas
KDPG aldolase
2
0
2
1

11
B. Respiration
Respirations is the complete oxidation of the substrate by an outside electron
acceptor. In addition to the pathway of glycolysis (respiration occurs after
glycolysis) four metabolic parts are needed for respiration:
1. The tricarboxylic acid (TCA) cycle (also known as the citric acid cycle or
the Kreb's cycle): when an organic compound is utilized as a substrate, the TCA
cycle is used for the complete oxidation of the substrate. The end product that
always results from the complete oxidation of an organic compound is CO
2
.
2. A membrane and an associated electron transport system (ETS). The
ETS is a sequence of electron carriers in the plasma membrane that
transports electrons taken from the substrate through the chain of carriers to a
final electron acceptor and release energy in the process of ATP synthesis by
the mechanisms of electron transport phosphorylation. The operation of the
ETS establishes a proton motive force (pmf).
3. An outside electron acceptor. For aerobic respiration the electron acceptor
is O
2
. But in the anaerobic respiration of bacteria, the final electron acceptors
may be SO
4
or S or NO
3
or NO
2
or organic compound, such as fumarate.
4. A transmembranous ATPase enzyme (ATP synthetase). This enzyme
utilizes the proton motive force on the membrane to synthesize ATP in the
process of electron transport phosphorylation. The produced energy usually
used in flagella movment.
ADP + Pi + 2 H
+
<----------> ATP.

11
Model of Aerobic respiration.
The overall reaction for the aerobic respiration of glucose is
Glucose + 6 O
2
----------> 6 CO
2
+ 6 H
2
0 + (38 ATP) 688 kcal (total)
Which can be written as:
Glucose ----------> 6 CO
2
+ 10 NADH
2
+ 2 FADH
2
+ 4 ATP
(2NADH
2
from glycolysis, 8NADH
2
from two turns of TCA, 2 FADH
2
from two
turns of TCA; 2ATP (net) from glycolysis, 2 ATP (GTP) from two turns of TCA)
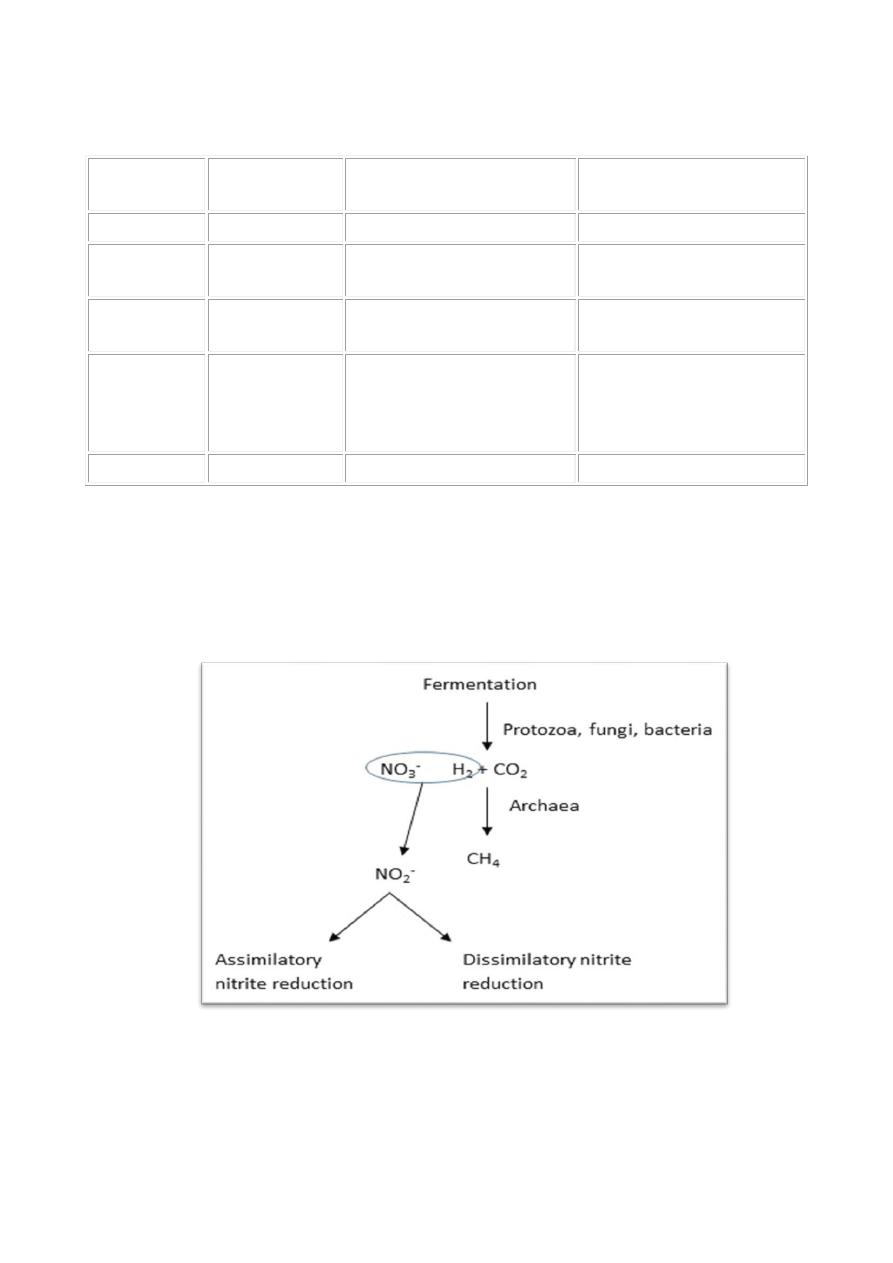
Anaerobic respiration:
The use of some compound (not O
2
) as a final electron acceptor in the electron
transport chain, it is used by prokaryote. Electron acceptors are used by
prokaryotes for two processes respiration and methanogenesis.
12
Electron acceptors for respiration and methanogenesis in procaryotes
electron
acceptor
reduced end
product
name of process
Organism
O
2
H
2
O
aerobic respiration
Escherichia, Streptomyces
NO
3
NO
2
, N
2
O or
N
2
anaerobic respiration:
denitrification
Bacillus, Pseudomonas
SO
4
S or H
2
S
anaerobic respiration:
sulfate reduction
Desulfovibrio
fumarate
Succinate
anaerobic respiration:
using an organic e-
acceptor
Escherichia
CO
2
CH
4
Methanogenesis
Methanococcus
Methanogenesis is the source of methane (CH
4
natural gas) on the planet.
Methane is produced in anaerobic conditions, and oxygen is needed to oxidize
the CH
4
molecule. Methanogenesis is not anaerobic respiration, but it is a type of
energy production metabolism needs an outside electron acceptor CO
2
.
Denitrification is an important process in agriculture because it removes
NO
3
from the soil. NO
3
is a source of nitrogen fertilizer in agriculture. The nitrate
is the respiratory electron acceptor (not oxygen O
2
). E. coli can use NO
3
(and
13
fumarate) as a respiratory electron acceptor and so this bacteria is able to respire
in the anaerobic intestinal habitat.
Note: CO
2
is the only source of carbon for the methanogens and the nitrification
bacteria.
Sulfate reduction: It is an obligatory process that occurs only under anaerobic
conditions. Anaerobic respiration bacteria and methanogens play an important
role in the cycles of carbon, nitrogen and sulfur. The lithotrophic prokaryotes
metabolize the reduced forms of nitrogen and sulfur to a more oxidized state in
order to produce energy.
Lithotrophic Types of Metabolism
Lithotrophy is the use of an inorganic compound as a source of energy. Most
lithotrophic bacteria do aerobic respiration and produce energy, they remove
electrons from a substrate and put them through an electron transport system that
will produce ATP by electron transport phosphorylation. Lithotrophs occur to get
electrons from an inorganic compound (not organic compound like heterotrophs),
e.g. most of the Archaea are lithotrophs.

14
Physiological groups of lithotrophs
physiological
group
energy
source
oxidized end
product
organism
hydrogen bacteria H
2
H
2
O
Alcaligenes, Pseudomonas
methanogens
H
2
H
2
O
Methanobacterium
carboxydobacteria CO
CO
2
Azotobacter
nitrifying bacteria NH
3
NO
2
Nitrosomonas
nitrifying bacteria NO
2
NO
3
Nitrobacter
sulfur oxidizers
H
2
S or S
SO
4
Thiobacillus, Sulfolobus
iron bacteria
Fe
++
Fe
+++
Thiobacillus
, Gallionella
Physiological groups of lithotrophs
1. The hydrogen bacteria oxidize H
2
(hydrogen gas) as an energy source.
The hydrogen bacteria are facultative lithotrophs e.g. Pseudomonas sp.
Which have hydrogenase enzyme that will oxidize H
2
using the respiratory
ETS.
2. The methanogens are Archaea. They are able to oxidize H
2
as the only
source of energy and transferring the electrons from H
2
to CO
2
.
Metabolism of the methanogens is special. Methanogens use H
2
and CO
2
to
produce cell material and methane. They have special coenzymes and
electron transport processes never seen in the Bacteria, and their
mechanism of autotrophic CO
2
fixation is very rare.
3. The carboxydobacteria are able to oxidize CO (carbon monoxide) to CO
2
,
using an enzyme CODH (carbon monoxide dehydrogenase). The
carboxydobacteria are not obligate CO users, the enzyme CODH used by
the carboxydobacteria to oxidize CO to CO
2
, is used by the methanogens
for the reverse reaction - the reduction of CO
2
to CO - during CO
2
fixation
by the CODH pathway.
4. The
nitrifying
bacteria
are
represented
by
two
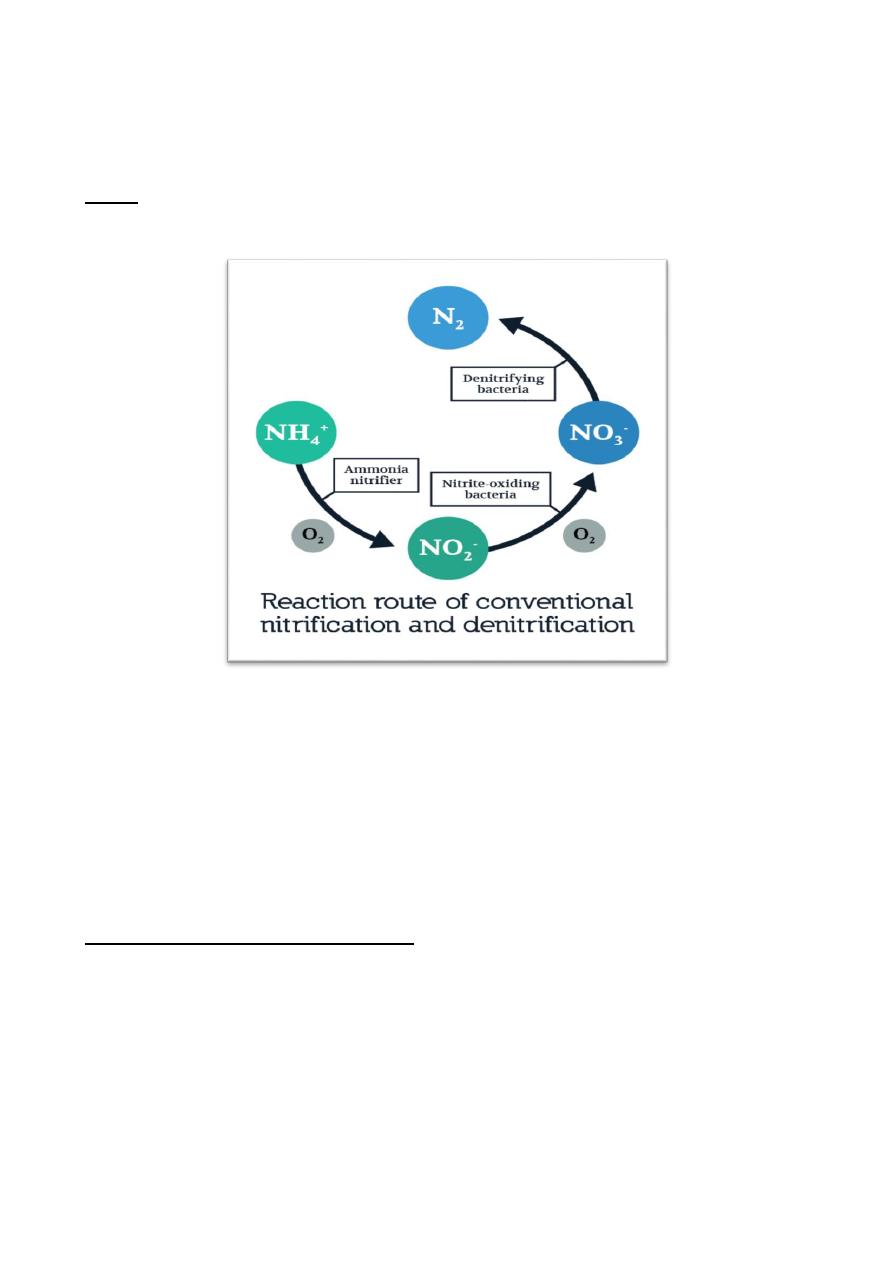
bacteria Nitrosomonas and Nitrobacter, these bacteria together do

15
oxidation of NH
3
to NO
3
, called nitrification. No single organism can
carry out the whole oxidative process. Nitrosomonas oxidizes ammonia to
NO
2
and Nitrobacter oxidizes NO
2
to NO
3
. Most of the nitrifying bacteria
are obligate lithoautotrophs. Nitrifying bacteria grow in environments
rich in ammonia or places of protein decomposition and play role in
Nitrogen cycle.
5. Lithotrophic sulfur oxidization microorganisms, they can produce
energy from an inorganic compound and fix CO
2
as autotrophs, include
both Bacteria (e.g. Thiobacillus) and Archaea (e.g. Sulfolobus), they
oxidize H
2
S (sulfide) or S (elemental sulfur) as a source of energy, the
purple and green sulfur bacteria oxidize H
2
S or S as an electron donor for
photosynthesis, and use the electrons for CO
2
fixation (the dark reaction of
photosynthesis). Lithoautotrophic sulfur oxidizers are found in
environments rich in H
2
S, such as volcanic hot springs and deep-sea
thermal vents.
6. Iron bacteria oxidize Fe
++
(ferrous iron) to Fe
+++
(ferric iron), two bacteria
oxidize Fe
++
as a source of energy and electrons and are capable of
lithoautotrophic growth: the bacterium Gallionella, which forms rust-
colored colonies attached to objects in nature, and Thiobacillus
ferrooxidans,
Phototrophic Metabolism
Phototrophy is the use of light as a source of energy for growth, turn light energy
into chemical energy in the form of ATP. Phototrophy Prokaryotes include
cyanobacteria, the purple and green bacteria and the halobacteria of archaea. The
cyanobacteria like plant can do photosynthesis, called oxygenic photosynthesis;
the purple and green bacteria can do bacterial photosynthesis or anoxygenic
photosynthesis; the extreme halophilic archaea use a type of non-
photosynthetic photophosphorylation to use light energy in production of
ATP.
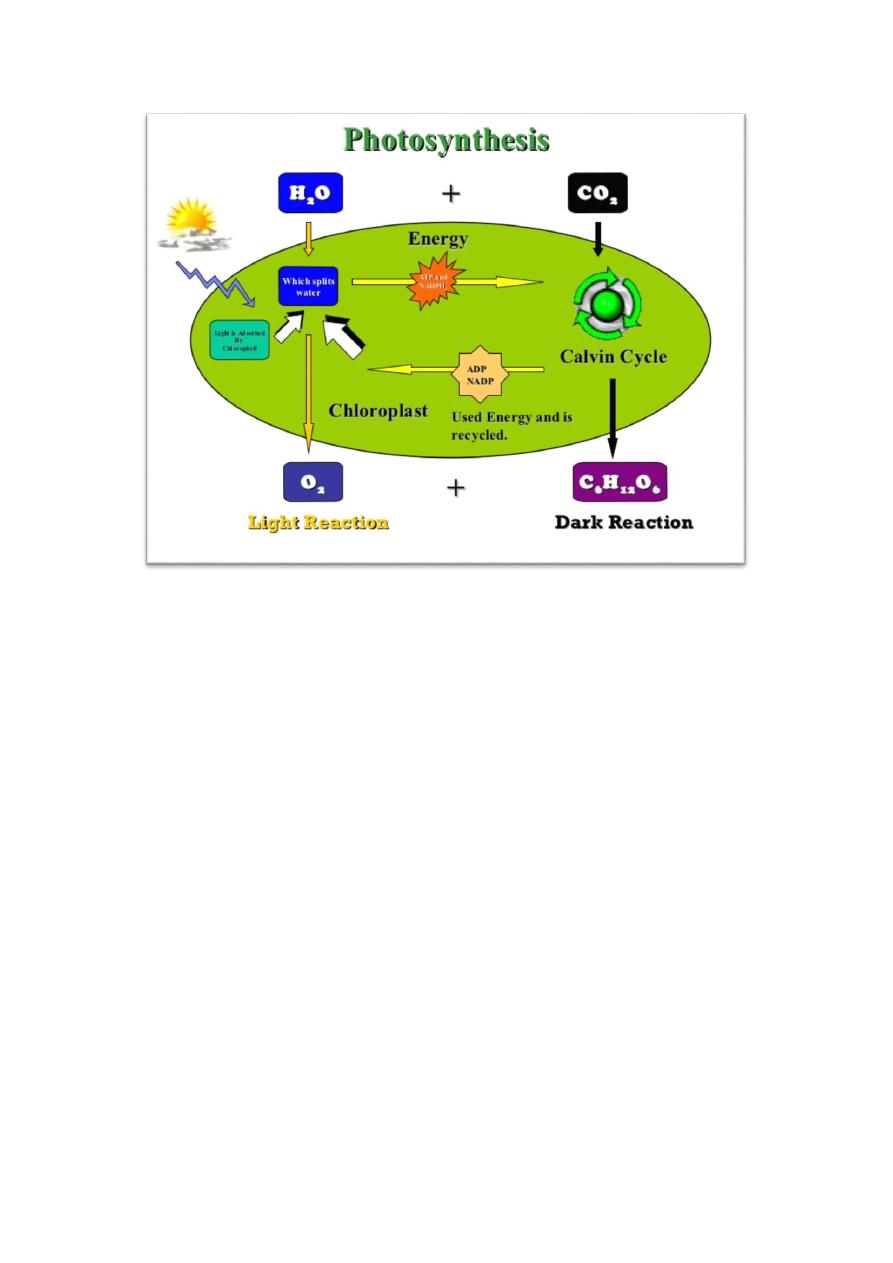
Photosynthesis is the turning of light energy into chemical energy that used in
the formation of cellular material from CO
2
. Photosynthesis is a type of
metabolism. The catabolic reactions of photosynthesis is the light reaction, the

16
anabolic reactions involves the fixation of CO
2
and its use as a carbon source for
growth, called the dark reaction. In plant photosynthesis, the photosynthetic
electron donor is H
2
O, the photosystem II and the production of O
2
. In
photosynthetic prokaryotes there are two types of photosynthesis and two types
of CO
2
fixation.
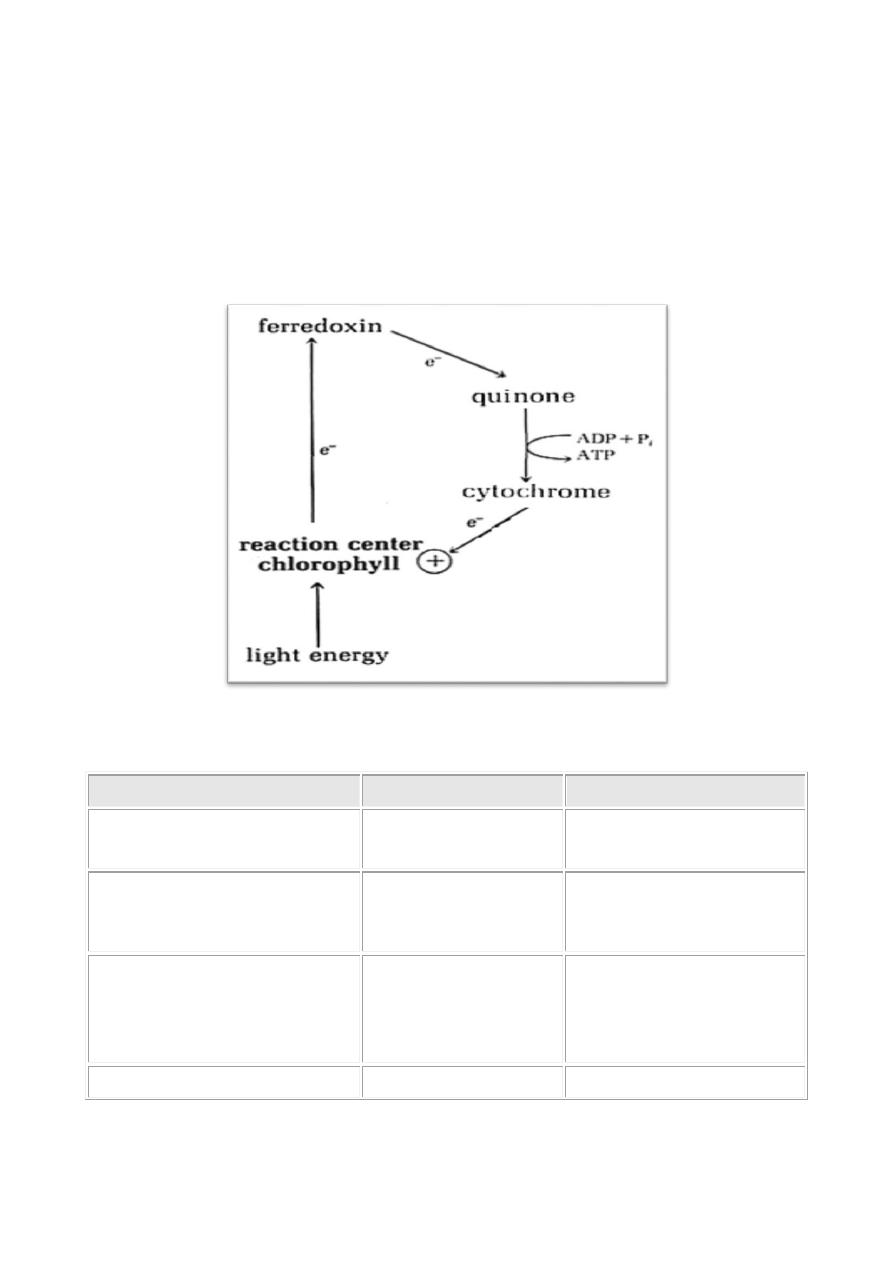
The Light Reactions depend on the chlorophyll, the primary light-harvesting
pigment in the membrane of photosynthetic organisms. Absorption of a light by
a chlorophyll molecule causes the movement of an electron at the reaction center.
The electron is an energy source and moves through the membrane
photosynthetic electron transport system and reach the cytochrome and back to
chlorophyll. When the electron move, a proton motive force is activated on the
membrane, and ATP is synthesized by an ATPase enzyme. This process of
converting
light
energy
into
chemical
energy
is
called
cyclic
photophosphorylation (transfers electrons from H
2
O and produces O
2
).
The important parts of the photochemical system are light harvesting pigments,
a membrane electron transport system, and an ATPase enzyme. The
photosynthetic electron transport system is similar to a respiratory ETS, but with
low ferredoxin.
Cyanobacteria have chlorophyll a, the same as plants and algae. The
chlorophylls of the purple and green bacteria, called bacteriochlorophylls and
chemically different than chlorophyll a in their side chains and that cause
different light absorption spectra. Chlorophyll a absorbs light in two regions of
the spectrum, one around 450nm and the other between 650 -750nm; bacterial
chlorophylls absorb from 800-1000nm.
Carotenoids they are secondary light-harvesting pigments, absorbing light in
the blue-green region between 400-550 nm. Carotenoids transfer energy to
chlorophyll and stop oxidative effects of oxygen radical produced during
reactions between chlorophyll and O
2
. Some nonphotosynthetic bacterial
pathogens, i.e., Staphylococcus aureus, produce carotenoids that protect the cells
from lethal oxidations by oxygen radicals inside phagocytic cells.
17
Phycobiliproteins are the major light harvesting pigments of the cyanobacteria
and some groups of algae. They may be red or blue, absorbing light in the middle
of the spectrum between 550 and 650nm, transfer light energy to the chlorophyll
at the reaction center.
Differences between plant and bacterial photosynthesis
plant photosynthesis bacterial photosynthesis
Organisms
plants, algae,
cyanobacteria
purple and green bacteria
type of chlorophyll
chlorophyll a
absorbs 650-750nm
bacteriochlorophyll
absorbs 800-1000nm
Photosystem I
(cyclic
photophosphorylation)
Present
Present
Photosystem I
Present
Absent

18
(noncyclic
photophosphorylation)
Produces O
2
Yes
No
Photosynthetic electron
donor
H
2
O
H
2
S, other sulfur
compounds or
certain organic compounds
The extreme halophiles or archaea that live in Dead Sea and the Great Salt Lake
at very high salt concentration (25% of NaCl) they have purple membrane as
light-harvesting pigment in the plasma membrane. The pigment is
called bacteriorhodopsin which reacts with light and forms ATP. The high
concentration of NaCl in their environment decrease the availability of O
2
for
respiration so they are able to produce ATP by converting light energy into ATP
using bacteriorhodopsin.
Autotrophic CO
2
fixation
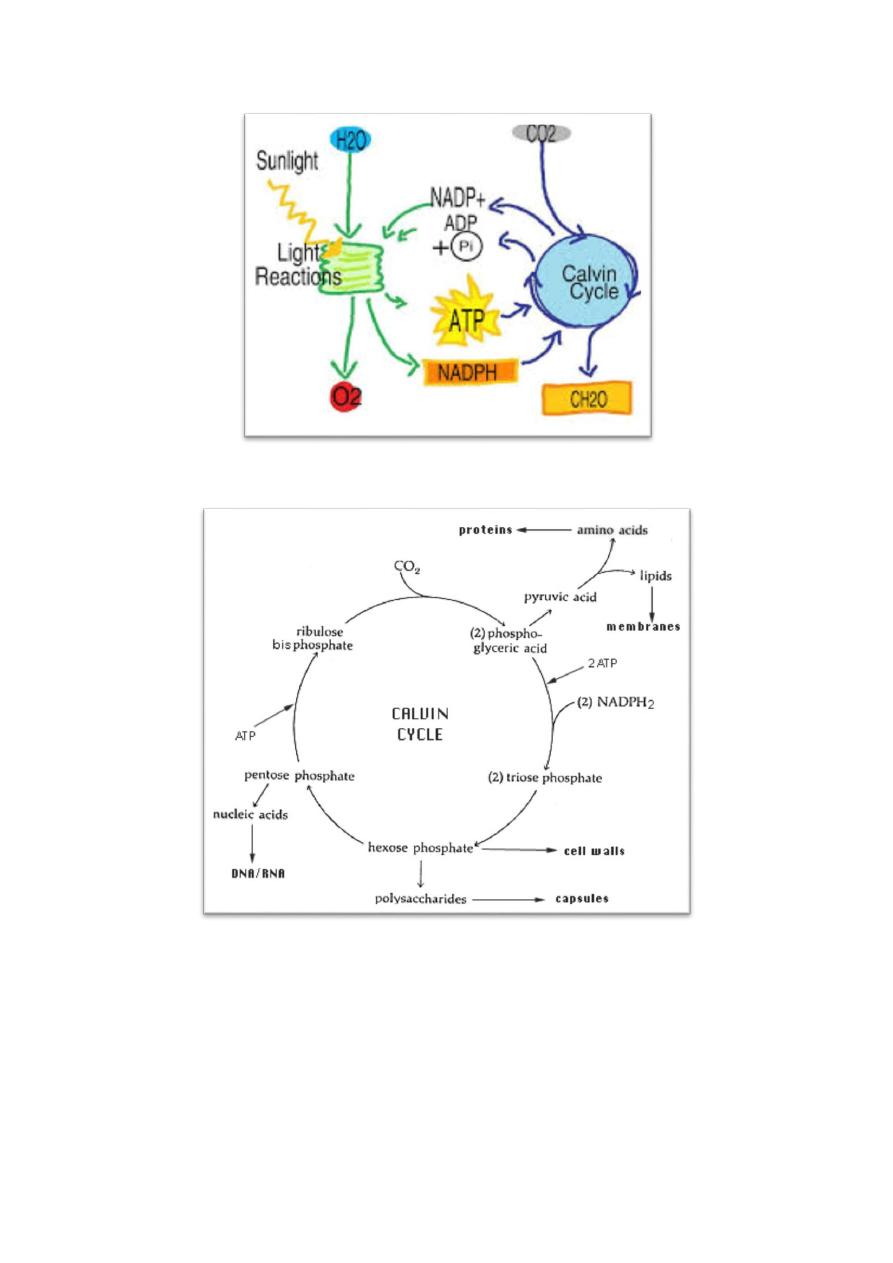
The use of RUBP carboxylase and the Calvin cycle is the most common
mechanism for CO
2
fixation among autotrophs, RUBP carboxylase is the first
enzyme and the nitrogenase which fixes N
2
is the second. This is the only
mechanism of autotrophic CO
2
fixation among eukaryotes, and it is used also by
cyanobacteria and purple bacteria. The green bacteria and the methanogens do
not use RUBP carboxylase.
RUBP carboxylase (ribulose bisphosphate carboxylase) uses ribulose
bisphosphate (RUBP) and CO
2
as substrates and start the Calvin cycle. An
important function of the Calvin cycle is to provide the organic molecules for the
biosynthesis of cell material (anabolic pathway). Intermediates must be
constantly withdrawn from the Calvin cycle in order to make cell material. The
Calvin cycle is fixation of CO
2
to the level of glucose (C
6
H
12
O
6
) and requires 18
ATP and 12 NADPH
2
.
19
21
Biosynthesis
These pathways of metabolism include glycolysis, Embden-Meyerhof pathways
and the TCA cycle, they produce the macromolecules for the biosynthesis of cell
material and produce energy ATP and called amphibolic pathway.
Biosynthesis in prokaryotic cells give the followings:
1. Polysaccharide capsules or inclusions are polymers of glucose.
2. Cell wall peptidoglycan from glucose phosphate.
3. Amino acids for the proteins have many sources, like pyruvic acid, alpha
ketoglutaric acid and oxalacetic acid.
4. Nucleotides (DNA and RNA) are synthesized from ribose
phosphate. ATP and NAD are part of purine (nucleotide) metabolism.
5. Triose-phosphates for glycerol synthesis, and acetyl CoA is for
lipids synthesis for cell membranes.
6. Vitamins and coenzymes are synthesized in many pathways.
21
22
