1
Course: Immunology
Lecturer: Dr. Weam Saad
Lecture: Immunoglobulins, structure and function and Antigens- Antibody Reactions
Immunoglobulins; Structure and Function
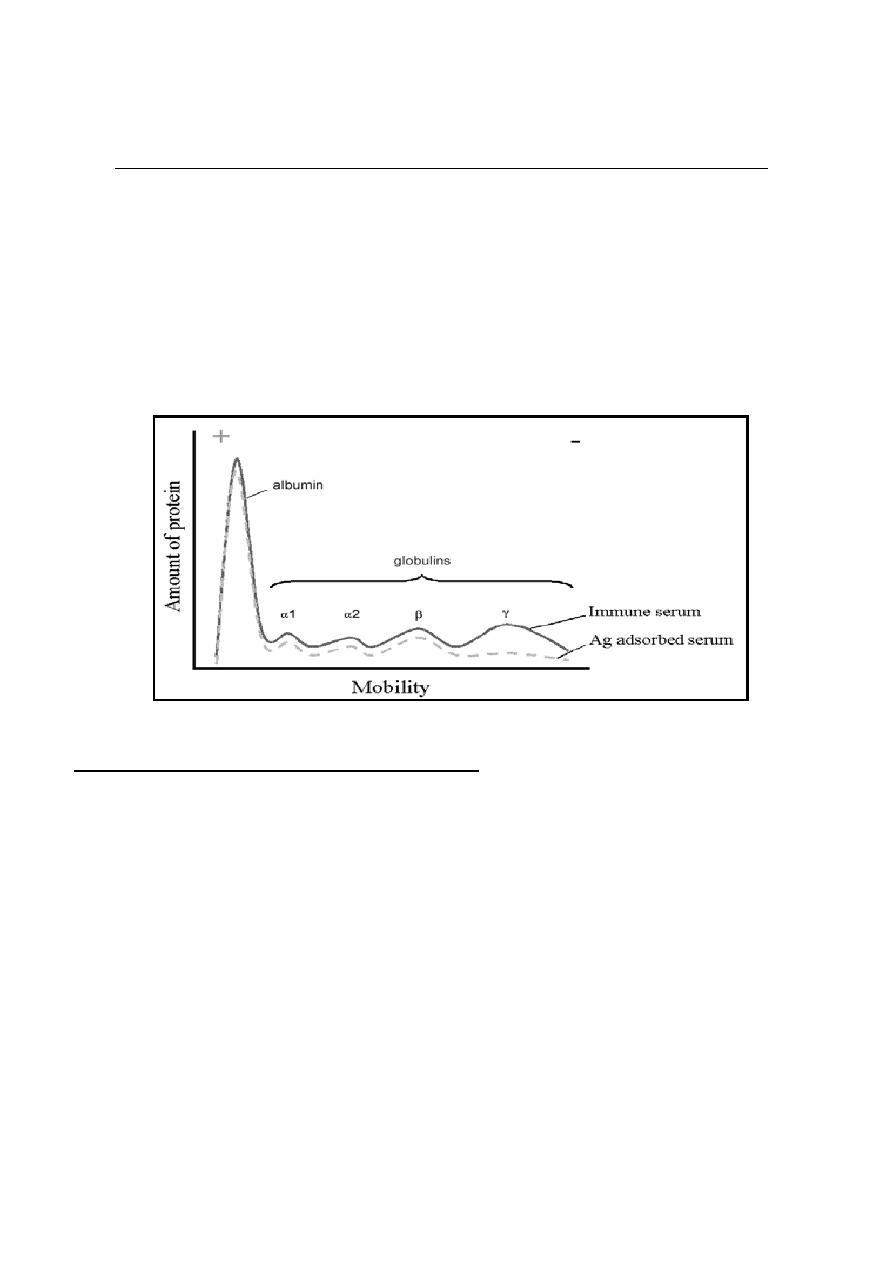
Immunoglobulins (Ig) or (Antibodies Ab) are Glycoprotein molecules, which
produced by plasma cells in response to an immunogen and which function as
antibodies and responsible for immunity. The immunoglobulins derive their name
because when serum containing antibodies placed in an electrical field then the
antibodies migrated with the globular proteins.
General functions of Immunoglobulins:
The general function is Ag binding - Immunoglobulins bind specifically to one or
a few closely related antigens. Each immunoglobulin binds to a specific antigenic
determinant. Antigen binding by antibodies is the primary function of antibodies
and can result in protection of the host. Other effecter functions are:
1. Fixation of complement - lysis of cells, release of biologically active
molecules.
2. Binding to various cell types - phagocytic cells, lymphocytes, platelets, mast
cells, and basophils have receptors that bind immunoglobulins and the binding
can activate the cells to perform some function. Some immunoglobulins also
bind to receptors on
placental trophoblasts. The binding results in transfer of
the immunoglobulin across the placenta and the transferred maternal antibodies
provide immunity to the fetus and newborn.
2
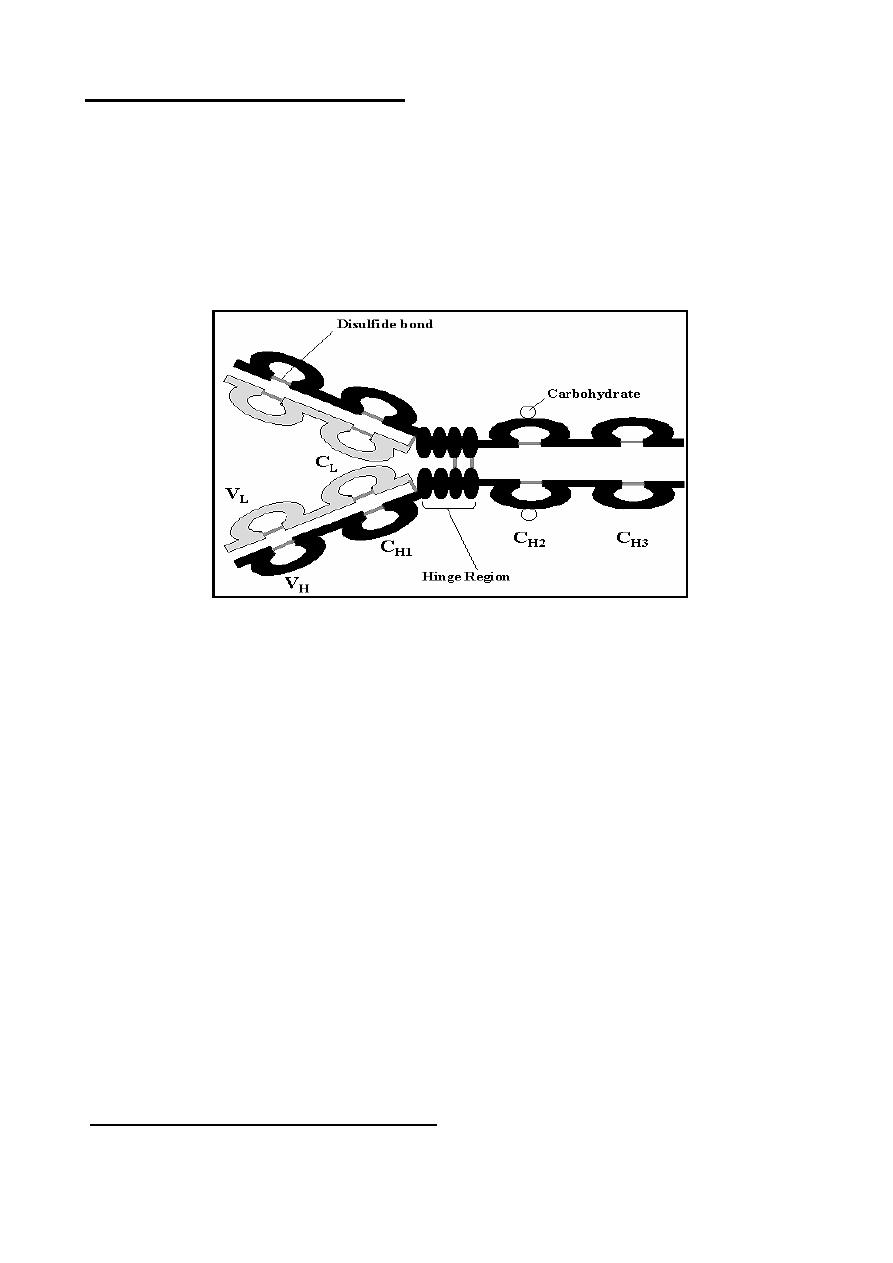
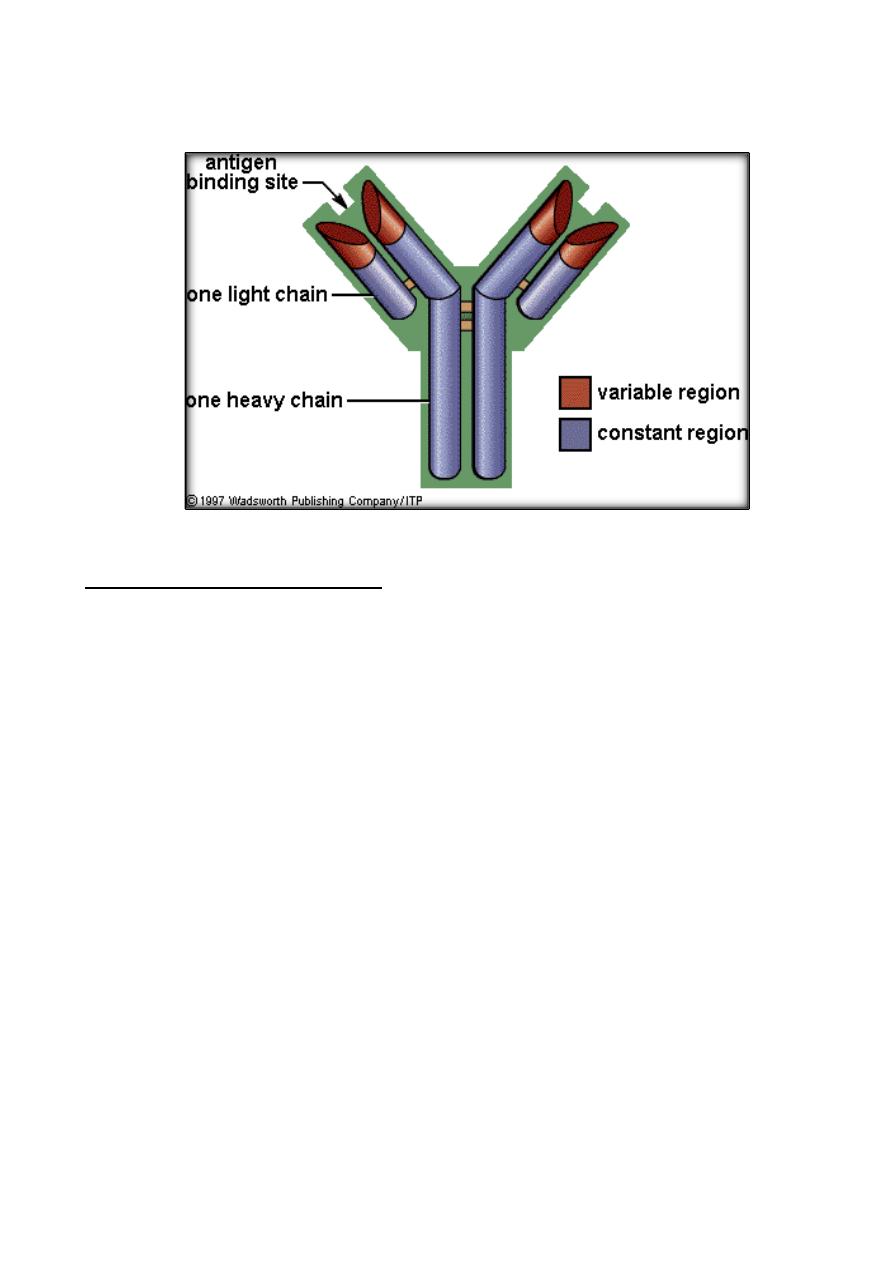
Structure of Immunoglobulins:
The basic structure of the immunoglobulins is illustrated in the Figure bellow.
Although different immunoglobulins can differ structurally, they are built from the
same basic unit.
A
. Heavy and Light Chains
- All immunoglobulins have a four chain structure
as their basic unit. They are composed of two identical light chains (23Kd)
and two identical heavy chains (50-70Kd)
B. Disulfide bonds
:
1. Inter-chain
2. Intra-chain
C. Variable (V) and Constant (C) Regions
:
They could be divided into two regions based on variability in the amino acid
sequences.
1. Light Chain - VL (110 aa) and CL (110 aa).
2. Heavy Chain - VH (110 aa) and CH (330-440 aa).
D. Hinge Region
:
The region at which the arms of the antibody molecule forms a
Y
is called the
hinge region because there is some flexibility in the molecule at this point.
E. Domains
F. Oligosaccharides
Structure of the variable regions:
These regions composed from:
A. Hypervariable (HVR) regions
3
B. Framework regions
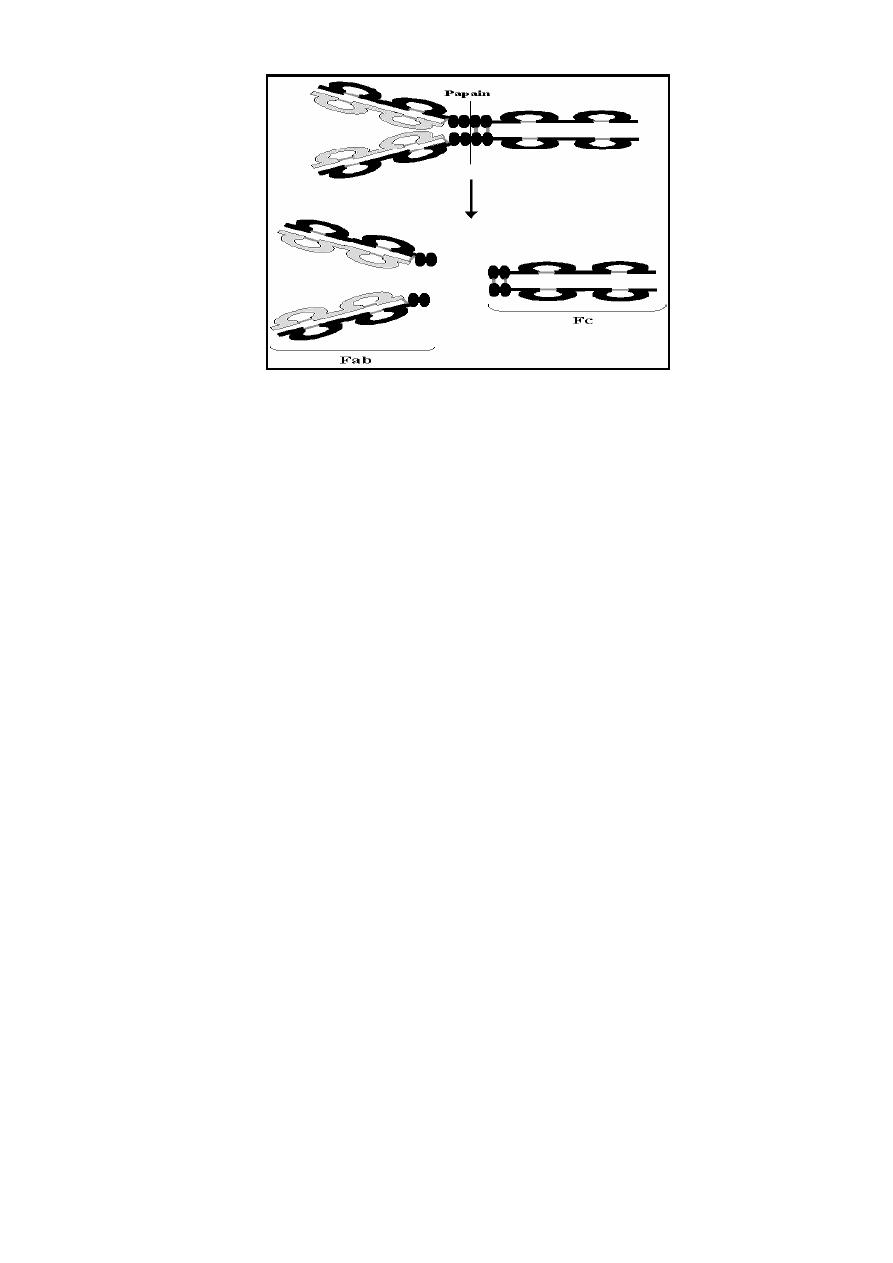
Immunoglobulin Fragments:
Immunoglobulin fragments produced by proteolytic digestion are useful in
understanding structure/function relationships in immunoglobulins.
1)
Fab region: can results after digestion with papain and breaks the
immunoglobulin molecule in the hinge region before the H-H inter-chain
disulfide bond. These regions are Antigen binding sites of the antibodies.
2)
Fc region: can results after digestion with papain which produces a
fragment that contains the remainder of the two heavy chains this
fragment was called Fc because it was easily crystallized. This region
has receptors on immune cells surfaces help in attaching of
Immunoglobulins on immune cells surface.
4
General Immunoglobulins classes:
The immunoglobulins can be divided into 5 different classes based on differences
in the amino acid sequences in the constant region of the heavy chains. All
immunoglobulins within a given class will have very similar heavy chain constant
regions. These differences can be detected by sequence studies or more commonly
by serological techniques (i.e. by the use of antibodies directed to these
differences).
1. IgG - Gamma (γ) heavy chains
2. IgM - Mu (μ) heavy chains
3. IgA - Alpha (α) heavy chains
4. IgD - Delta (δ) heavy chains
5. IgE - Epsilon (ε) heavy chains
In addition, the classes of immunoglobulins can be divided into subclasses based
on small differences in the amino acid sequences in the constant region of the heavy
chains. These subclasses can be differentiated serologically too.
Isotypes: They are antigenic determinants that characterize classes and subclasses
of heavy chains and types and subtypes of light chains.
Allotypes: They are antigenic determinants specified by allelic forms of the Ig
genes.

5
Properties of Immunoglobulins (Abs) classes:
1. IgG immunoglobulin:
Structure: Monomer, have4 subclasses (IgG1, IgG2, IgG3 and IgG4), the
subclasses differ in the number of disulfide bonds and length of the hinge region.
Properties:
1) IgG is the major Ig in serum - 75% of serum Ig is IgG.
2) IgG is the major Ig in extra vascular spaces.
3) Placental transfer - IgG is the only class of Ig that crosses the placenta.
Transfer is mediated by receptor on placental cells for the Fc region of IgG.
Not all subclasses cross equally; IgG2 does not cross well.
4) Able to fix complement - Not all subclasses fix equally well; IgG4 does
not fix complement
5) Binding to cells - Macrophages, monocytes, PMN's and some lymphocytes
have Fc receptors for the Fc region of IgG. Not all subclasses bind equally
well; IgG2 and IgG4 do not bind to Fc receptors. A consequence of binding
to the Fc receptors on PMN's, monocytes and macrophages is that the cell
can now internalize the antigen better. The antibody has prepared the
antigen for eating by the phagocytic cells. The term opsonin is used to
describe substances that enhance phagocytosis. IgG is a good opsonin.
Binding of IgG to Fc receptors on other types of cells results in the
activation of other functions.
2. IgM immunoglobulin:
Structure - IgM normally exists as a pentamer but it can also exist as a
monomer. IgM has an extra domain (another protein molecule) covalently bound
via the S-S bond called the J chain. This chain functions in polymerization of the
molecule into a pentamer.
Properties
a) IgM is the third most common serum Ig.
b) IgM is the first Ig to be made by the fetus and the first Ig to be made by B
cells when it is stimulated by antigen.
c) Due to its pentameric structure, IgM is a good complement fixing Ig. Thus,
IgM antibodies are very efficient in leading to the lysis of microorganisms.

6
d) IgM is also a good agglutinating Ig. Thus, IgM antibodies are very good in
clumping microorganisms for elimination from the body. IgM binds to
some cells via Fc receptors. Cell surface IgM functions as a receptor for
antigen on B cells.
3. IgA immunoglobulin:
Structure - Serum IgA is a monomer but IgA found in secretions is a
dimer. When IgA is found in secretions is also has another protein
associated with it called the secretory piece or T piece (sIgA), this secretory
piece is made in epithelial cells and is added to the IgA as it passes into the
secretions. The secretory piece helps IgA to be transported across mucosa
and also protects it from degradation in the secretions.
Properties:
a) IgA is the second most common serum Ig.
b)
IgA is the major class of Ig in secretions - tears, saliva, colostrum,
mucus. Since it is found in secretions secretory IgA is important in
local (mucosal) immunity.
c) IgA does not fix complement.
4) IgD immunoglobulin:
Structure: IgD exists only as a monomer.
Properties:
a) IgD is found in low levels in serum; its role in serum uncertain.
b) IgD is primarily found on B cell surfaces where it functions as a
receptor for antigen.
c) IgD does not bind complement.
5) IgE immunoglobulin:
Structure: IgE exists as a monomer.
Properties:
a)
IgE is the least common serum Ig since it binds very tightly to Fc receptors
on basophils and mast cells even before interacting with antigen.
b) Involved in allergic reactions - As a consequence of its binding to basophils
and mast cells, IgE is involved in allergic reactions. Binding of the allergen
(allergy antigen) to the IgE on the cells results in the release of many

7
pharmacological mediators and cytokines that result in allergic symptoms
e.g.histamin.
c) IgE also plays a role in parasitic helminth diseases. Since serum IgE levels
rise in parasitic diseases, measuring IgE levels is helpful in diagnosing
parasitic infections. Eosinophils have Fc receptors for IgE and binding of
eosinophils to IgE-coated helminths results in killing of the parasite.
d) IgE does not fix complement.
Antigens- Antibody Reactions
It is a specific reaction between an antibody (produced by B-cells
and secreted by plasma cells) and any antigen then forming Ag-Ab
Complex. The antigenic determinant or epitopes are recognized by
the Ag binding site of the antibody at the variable region, then Antigens
are bound to antibodies through weak and non-covalent interactions such
as electrostatic interactions, hydrogen bonds, Van der Waals forces,
and hydrophobic interactions.
Ag+Ab → Ag-Ab Complex
Types of Antigens- Antibody Reactions
1. Precipitation (when the Ag is soluble).
2. Agglutination (when the Ag is insoluble or particulate).
3. Complement Fixation (during Complement classical pathway
activation).
4. Neutralization (when the Ag is Toxin)
5. Opsonization (when the Ag is still on the cell surface and the
Ab with the help of complement increase the susceptibility of
foreign cell to be engulfed by Macrophages).
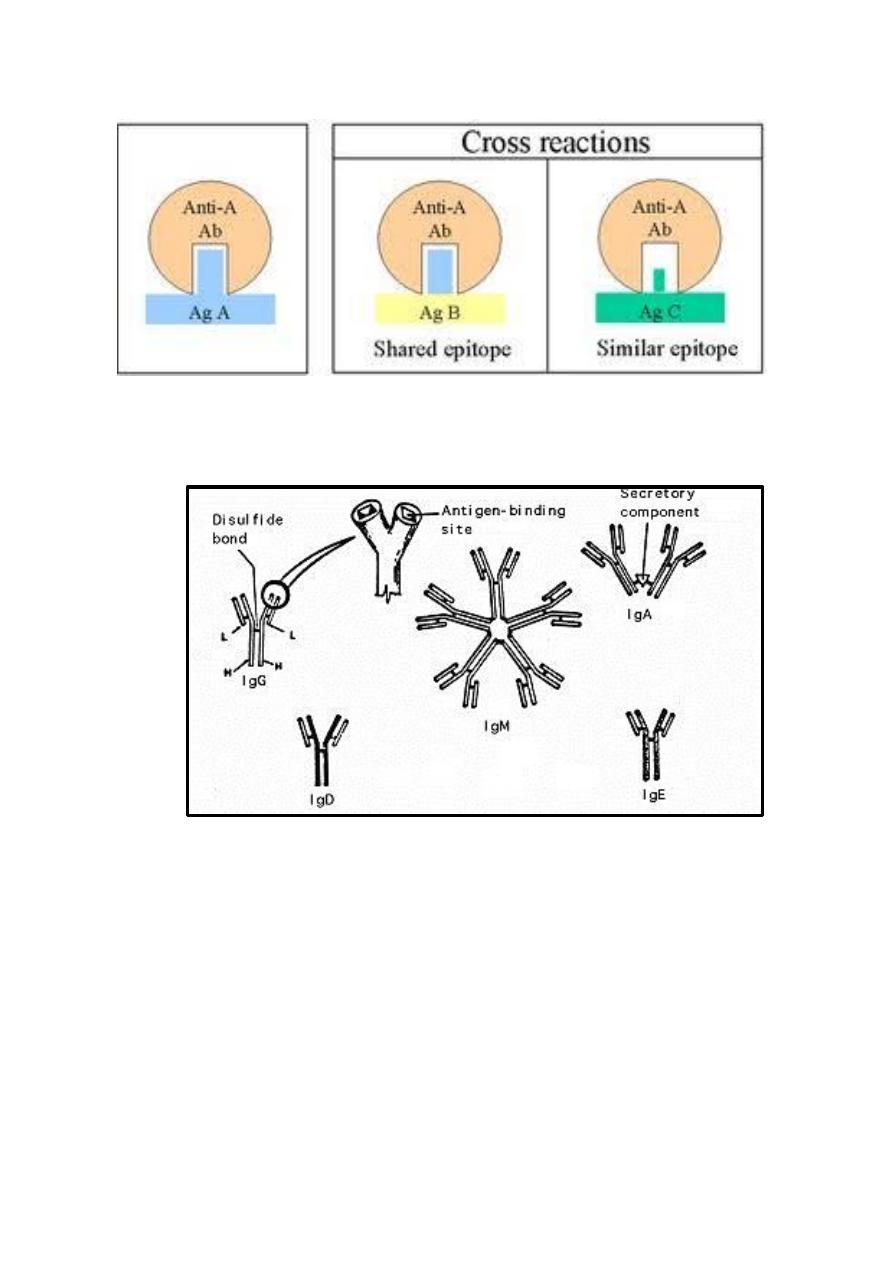
Cross reactivity - Cross reactivity refers to the ability of an antibody
combining site to react with more than one antigenic determinant. Cross
reactions occurs because the cross reacting antigen shares an epitope in
common with the immunizing antigen or because it has an epitope which
is similar in structure to one epitop on the immunizing antigen and that
is called (multispecificity).
8
