190
MODULE - 7
Chemistry
Notes
Chemistry of Organic
Compounds
28
ALCOHOLS, PHENOLS
AND ETHERS
S
o far you have learnt the chemistry of hydrocarbons which serve as basic skeleton for
the attachment of various functional groups to give a large number of their derivatives. In
the last lesson, we discussed one such class of compounds viz halogen derivatives of
hydrocarbons. Another very useful and important catagory of hydrocarbon derivatives is
that of compounds containing functional groups in which the carbon atom is linked to an
oxygen atom.
We have devoted two lessons for the study of these compounds. In this lesson, you will
study about compounds containing carbon-oxygen single bond (
C
O
) whereas the
next lesson deals with compounds containing carbon-oxygen double-bond (
—
O
C
—
).
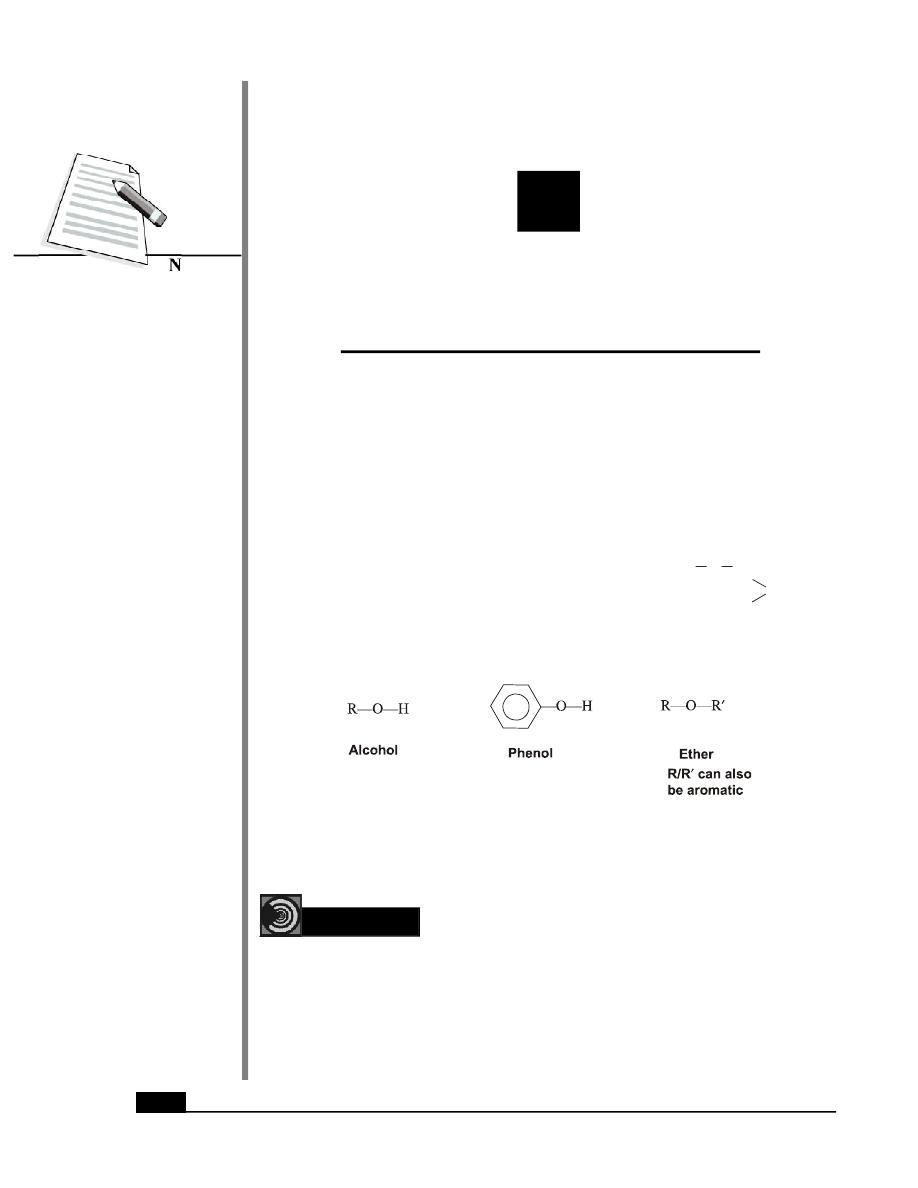
Among the compounds with carbon-oxygen single bond are the classes of alcohols, phenols
and ethers having the following general structures.
These are very important categories of compounds both in the industry and in the synthesis
of other organic compounds. You will study each of these classes of compounds in this
Lesson.
Objectives
After reading this lesson, you should be able to
Classify alcohols as primary, secondary or tertiary;
Name simple alcohols according to IUPAC system of nomenclature;
List general methods of preparation of alcohols;
Discuss the properties of alcohols in the light of their structure;
191
MODULE - 7
Alcohols, Phenols and Ethers
Notes
Chemistry of Organic
Compounds
Explain various reactions exhibited by alcohols to give other categories of organic
compounds;
Give the names of common phenolic compounds;
Describe the laboratory and industrial methods of preparation of phenols;
Explain the greater acidity of phenols as compared to alcohols;
Discuss the reactions of phenols;
Name ethers according to the IUPAC system of nomenclature;
Describe the general methods of preparation of ethers and
Explain the important reactions of ethers.
28.1 Alcohols
Alcohols are organic compounds that have one or more hydroxy (-OH) groups bonded to
the carbon atoms in aliphatic compounds. They occur widely in nature and have many
industrial and pharmaceutical applications. For example, methanol and ethanol are two
industrially important alcohols.
3
3
2
CH
OH
CH CH
OH
Methanol
Ethanol
(Methyl alcohol)
(Ethyl alcohol)
28.1.1 Classification and Nomenclature of Alcohols
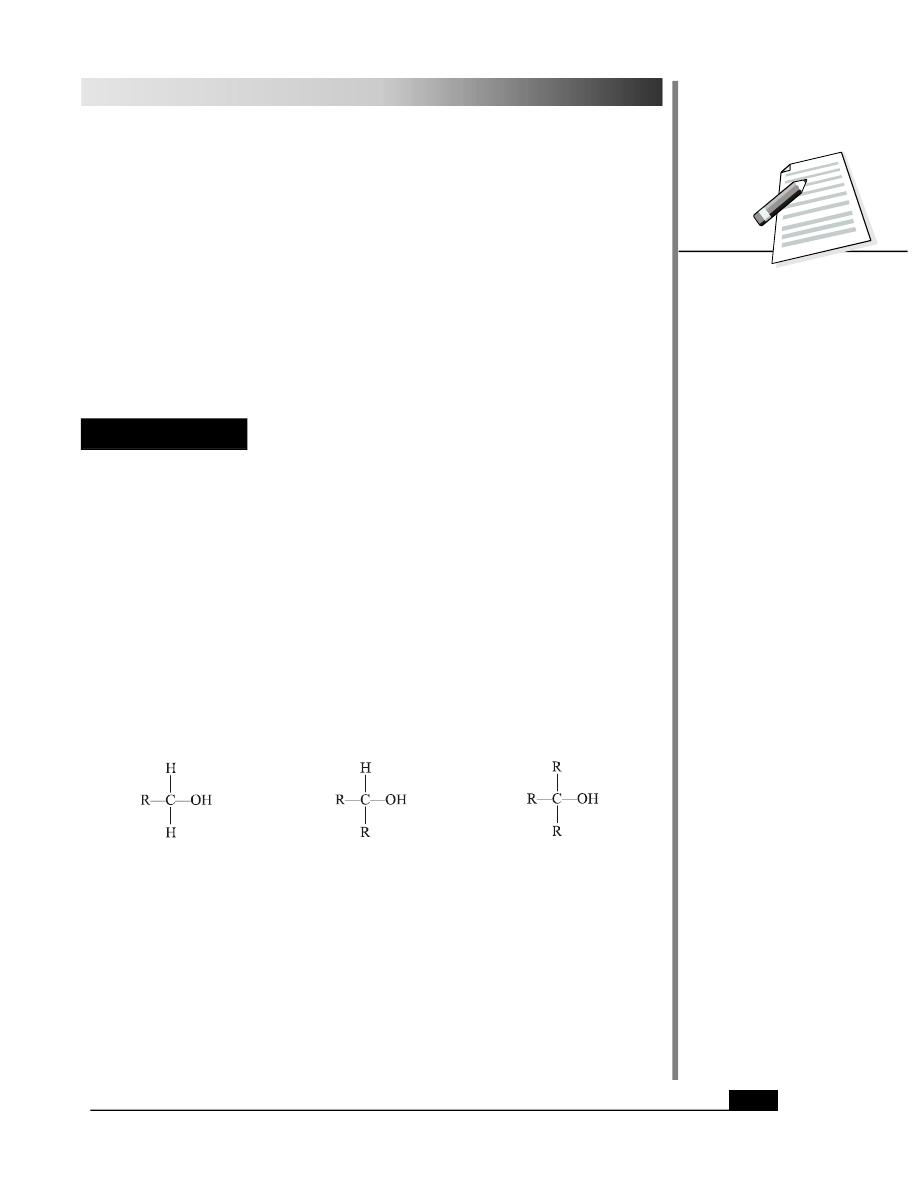
Alcohols are classified as primary (1º), secondary (2º) or tertiary (3º) depending upon
whether the number of alkyl groups bonded to the carbon atom bearing the hydroxy group
is one, two or three, respectively.
primary alcohol
secondary alcohol
tertiary alcohol
According to the IUPAC system of nomenclature, alcohols are called alkanols. They
are named as the derivatives of the corresponding alkane in which the -e of the alkane is
replaced by -ol .
The procedure for nomenclature involves the following steps:
Step 1: Select the longest carbon chain which contains the carbon atom bearing the –OH
group. Count the number of carbon atoms and identify the corresponding alkane. From
the name of this alkane, drop the final e and suffix -ol in its place. This gives the root
name or the parent name.
192
MODULE - 7
Chemistry
Notes
Chemistry of Organic
Compounds
Step 2: Number the carbon chain starting from the end nearest to the hydroxy group. The
number of the carbon atom bearing the hydroxy group is indicated before -ol in the name.
Step 3: Number the other substituents according to their position on the chain.
Step 4: Write the name of the alcohol by listing the substituents in the alphabetical order
alongwith their position.
You may remember from Lesson 25 that the hydroxyl group takes precedence over
double and triple bonds.
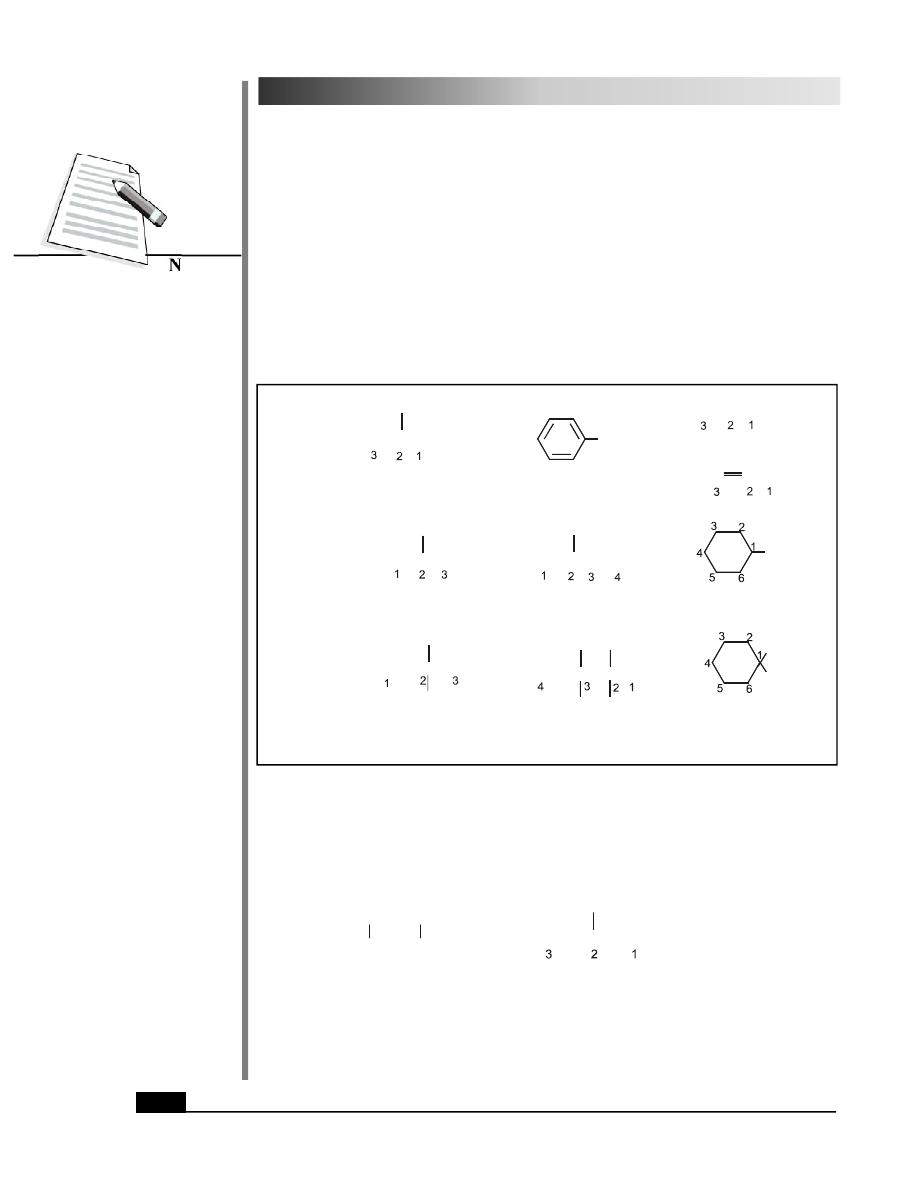
Table 28.1 illustrates some common alcohols and their IUPAC and common names. Go
through them in light of the steps given above for nomenclature.
Table 28.1 : Some common Alcohols and their Names
2
H C
2
CHCH OH
3
2
CH CHCH OH
1-Propanol
( -Propyl alcohol)
n
Prop-2-en-1-ol
3
2
CH CHCH OH
3
CH
2-Methylpropan
ol
-1-
(Isobutyl alcohol)*
2
CH OH
Phenylmethanol
(Benzyl alcohol)
3
3
CH CHCH
OH
Propan- ol
2-
(
propyl alcohol)
Iso
3
2
3
CH CHCH CH
OH
Butan- ol
2-
(
Butyl alcohol)
sec
OH
Cyclohexanol
(Cyclohexyl alcohol)
OH
3
CH
—C—
3
CH
3
CH
2-Methylpropan
ol
-2-
(
-Butyl alcohol)
tert
3
CH
—C
3
CH
OH
3
CH
—C—
3
CH
3
CH
2,3,3-Trimethylbutan
ol
-2-
Primary
Alcohol
Secondary
Alcohol
Tertiary
Alcohol
OH
3
CH
1-Methylcyclohex-1-ol
* The names given in the brackets are common names.
In the above examples, only one -OH group is present in the molecule. These alcohols are
called monohydric alcohols. Alcohols having two hydroxyl groups in a molecule are
known as dihydric alcohols or diols or glycols. Examples of some diols are shown
below :
CH
OH
—
2
CH
OH
2
—CH
3
CH
OH
—
2
CH OH
Ethane-1,2-diol
Propane-1,2-diol
(Ethylene glycol)
(Propylene glycol)
Note that the term glycol generally means 1,2-diol or a vicinal diol. In these diols, the two
hydroxyl groups are present on the adjacent carbon atoms.
Similarly, alcohols having three hydroxyl groups are called trihydric alcohols. 1,2,3-
propanetriol which is commanly known as glycerol, is a trihydric alcohol.
193
MODULE - 7
Alcohols, Phenols and Ethers
Notes
Chemistry of Organic
Compounds
OH
—
2
CH —OH
HO —
2
CH
CH
—
1,2,3-Propanetriol
(Glycerol)
28.1.2 General Methods of Preparation
Alcohols are synthesized by the following general methods. You might have come across
some of these methods in previous lessons. Let us now study these methods.
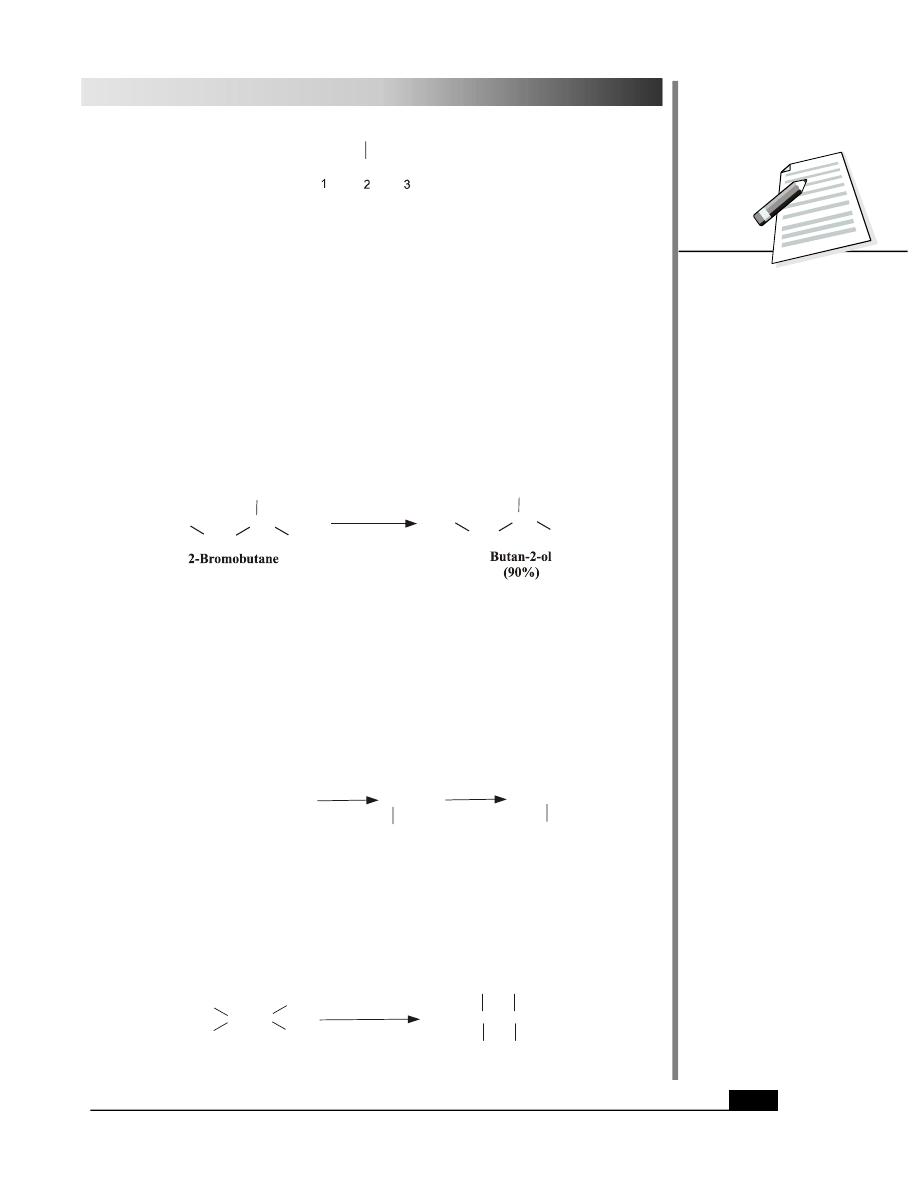
1. Hydrolysis of Haloalkanes
Haloalkanes can be converted to corresponding alcohols using aqueous sodium or potassium
hydroxide or water as nucleophiles.
3
2
3
2
CH CH Cl
NaOH(aq.)
CH CH OH
NaCl
Chloroethane
Ethanol
Br
OH
3
CH
2
CH
CH
3
CH
aq. NaOH
3
CH
2
CH
CH
3
CH
2. From hydration of Alkenes
Hydration means addition of water molecule. In case of alkenes, hydration is the addition
of H
+
and OH
–
across the double bond to give alcohols.
Alkenes can be hydrated by the following methods:
(i) Acid-catalysed Hydration
Alkenes can be hydrated to yield alcohols in the presence of acid catalysts.
OH
—
2
4
H SO
3
2
CH CH
3
H O
+
2
CH
2
H C
—
+
4
HSO
3
2
CH CH
Ethene
Ethyl hydrogen
Ethanol
sulphate
The reaction proceeds via alkyl hydrogen sulphate and this method is used for the industrial
preparation of ethanol.
In case of unsymmetric alkenes, the addition follows Markovniokov’s rule.
3
CH
—
C
C
—
H
H
3
CH
3
CH
C
—
—
C
H
—
H
H
3
CH
OH
2
H / H O
+
2-Methylpropene
2-Methyl-2-propanol
194
MODULE - 7
Chemistry
Notes
Chemistry of Organic
Compounds
(ii) Oxymercuration-demercuration
Alkenes react with mercury (II) acetate, i.e. mercuric acetate [
3 2
Hg(OCCH )
O
also
represented as Hg(OAc)
2
] in aqueous tetrahydrofuran (THF) solvent to give hydroxyalkyl
mercury compounds which are reduced to alcohols by sodium borohydride.
Step 1: Oxymercuration
3
CH COOH
— C
C —
C
—
— C
Hg
OAc
THF
+
2
H O +
2
Hg(OAc)
HO
+
—
—
Step 2: Demercuration
Hg
C
C —
C
—
— C
H
+
+
4
NaBH
HO
+
HO
Hg – OAc
—
—
—
3
CH COO
+
OH
This method gives very good yield of alcohols and here also, the addition takes place in
Markovnikov’s fashion.
OH
2
CH
—
—
2
CH
—
3
2 2
CH (CH ) CH
3
2 2
CH (CH ) CH
2
Hg(OAc)
HgOAc
4
NaBH
OH
3
2 2
3
CH (CH ) CHCH
Hg
+
OH
aq. THF
Pent-1-ene
Pentan-2-ol
(93%)
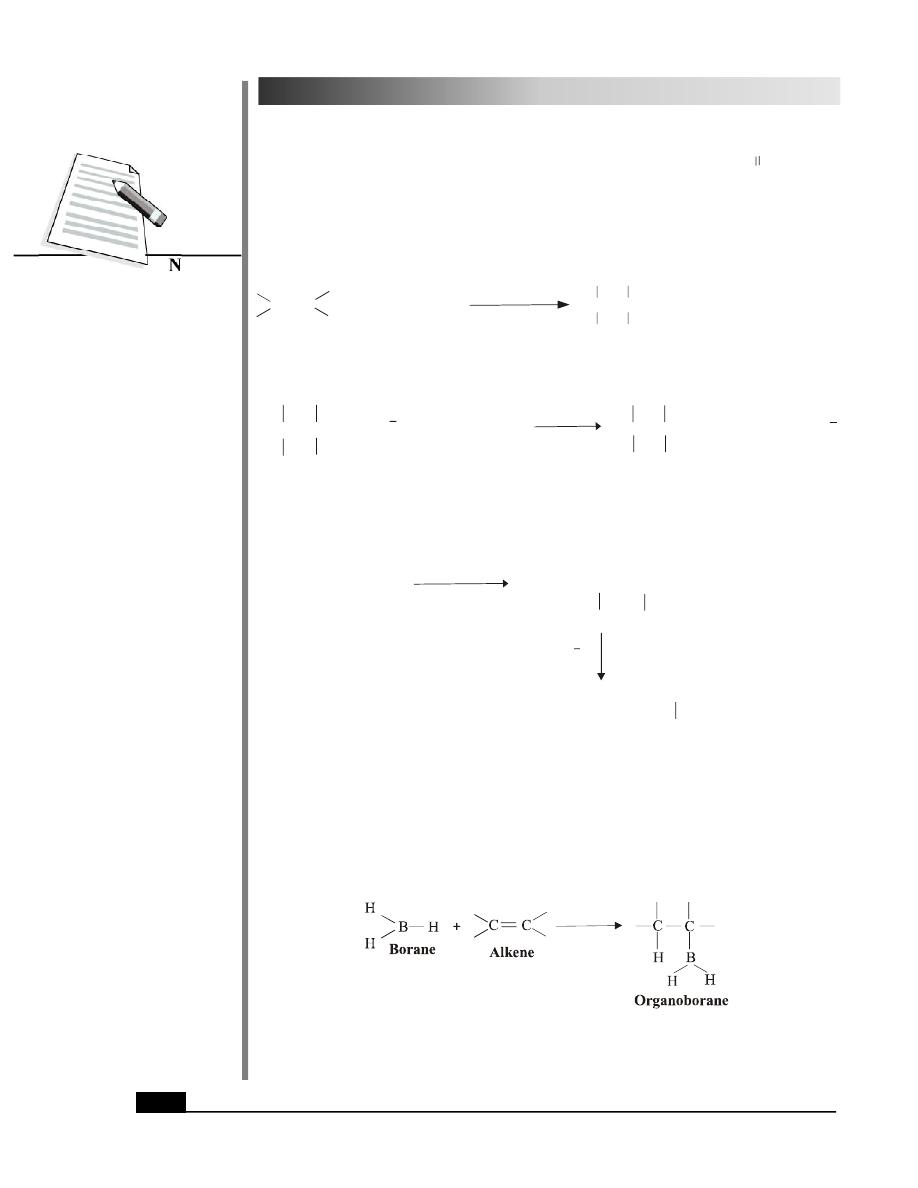
(iii) Hydroboration - Oxidation
When an alkene reacts with BH
3
(a boron hydride) in THF solution, an organoborane is
obtained.
Since BH
3
has three hydrogens, above addition can occur three times to give trialkylborane
(R
3
B). This is shown below for propene.
195
MODULE - 7
Alcohols, Phenols and Ethers
Notes
Chemistry of Organic
Compounds
—
H
3
CH CH
2
CH
2
BH
—
—
2
CH
3
CH CH
—
3
(CH
2
2 2
CH CH ) B
Dipropylborane
Propylborane
Tripropylborane
3
2
2 3
(CH CH CH ) B
3
BH
+
—
2
CH
3
CH CH
—
Propene
—
2
CH
3
CH CH
—
The trialkylborane so obtained is oxidised using alkaline hydrogen peroxide solution to give
three molecules of alcohols and boric acid.
(CH
3
CH
2
CH
2
)
3
B
2
2
H O / OH
3CH
3
CH
2
CH
2
OH + B(OH)
3
Tripropylborane
Propanol
Boric acid
Note that hydroboration-oxidation yields the anti-Markovnikov addition of water although
the reaction proceeds according to Markonikov’s rule.
3. Reduction of Carbonyl Compounds
Carbonyl compounds (which contain –C–
O
group) such as aldehydes, ketones, carboxylic
acids and esters can be reduced to alcohols.
Aldehydes give primary alcohols while ketones yield secondary alcohols on reduction.
C
— —
H
C
H
O
R
O
H
H
R
C
— —
H
C
R'
O
R
O
H
R
R'
Primary alcohol
Secondary alcohol
Reduction
Reduction
Aldehyde
Ketone
H /Pd
2
NaBH
4
Carboxylic acids and esters also give primary alcohols on reduction.
C
— —
H
C
OH
O
R
OH
H
R
Reduction
C
— —
H
C
OR '
O
R
O
H
H
R
Reduction
or
+
—
Carboxylic acid
Primary alcohol
Primary alcohol
Ester
R '
OH
LiAlH
4
196
MODULE - 7
Chemistry
Notes
Chemistry of Organic
Compounds
The reduction is carried out using hydride reagents such as lithium aluminium hydride
(LiAIH
4
) and sodium borohydride (NaBH
4
). LiAIH
4
is stronger and reacts explosively
with water while NaBH
4
is convenient to handle and reacts slowly.
Lithium aluminium hydride reduces all of the above classes of compounds while sodium
borohydride reduces only aldehydes and ketones and does not reduce carboxylic acids and
esters. Hence, it can be used to selectively reduce aldehydic/ketonic carbonyl group in
presence of carboxylic acid/ester function. Some examples below illustrate the use of
these reagents.
O
3
2
2
CH CH CH CH
4
2
5
1. NaBH , C H OH
3
2.H O
+
3
2
2
2
CH CH CH CH OH
Butanol
Butan-1-ol
4
1.LiAlH , ether
3
2.H O
+
O
H
OH
Cyclohex-2-enone
Cyclohex-2-enol
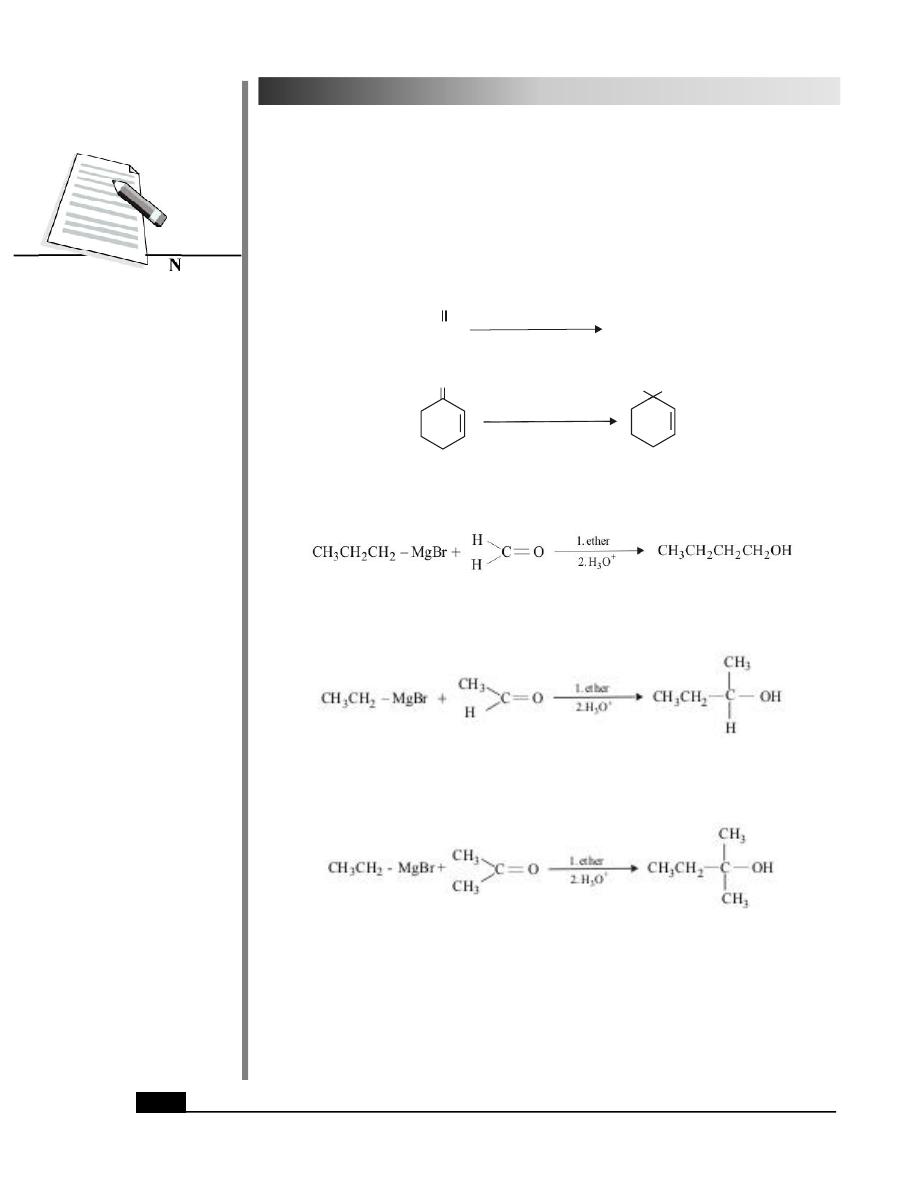
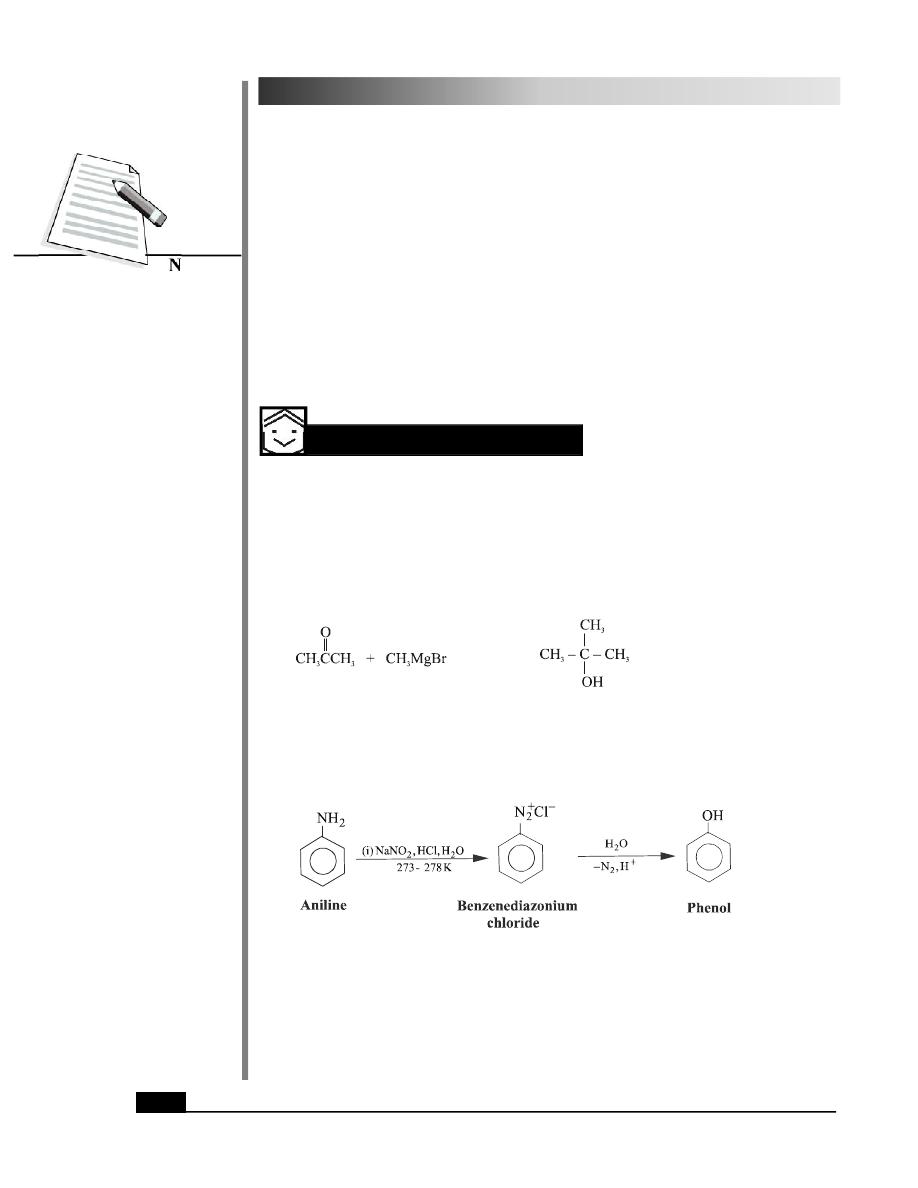
4. From Aldehydes and Ketones using Grignard Regents
Grignard reagents react with methanal (or formaldehyde) to give a primary alcohol.
Propyl magnesium bromide Methanal
Butan-1-ol
(Primary alcohol)
All other aldehydes yield secondary alcohols on reaction with Grignard reagents.
Ethyl magnesium
Ethanal
Butan-2-ol
bromide
(Acetaldehyde)
(Secondary alcohol)
With ketones, Grignard reagents give tertiary alcohols.
Ethyl magnesium
Propanone
2-Methylbutan-2-ol
bromide
5. Diazotization of Primary Aliphatic Amines
This reaction also yields alchols and will be discussed in Lesson 30.
6. Fermentation
Ethanol is prepared on a large scale using fermentation. It involves breaking down large
molecules into simpler ones using enzymes. Usually, yeast is added as a source of enzymes.
197
MODULE - 7
Alcohols, Phenols and Ethers
Notes
Chemistry of Organic
Compounds
The fermentation of sugar is shown below :
C H O + H O
12
22
11
2
Sugar
Investase
C H O + C H O
6
12
6
6
12
6
Glucose
Fructose
Zymase
2 C H OH + 2 CO
2
5
2
Ethanol
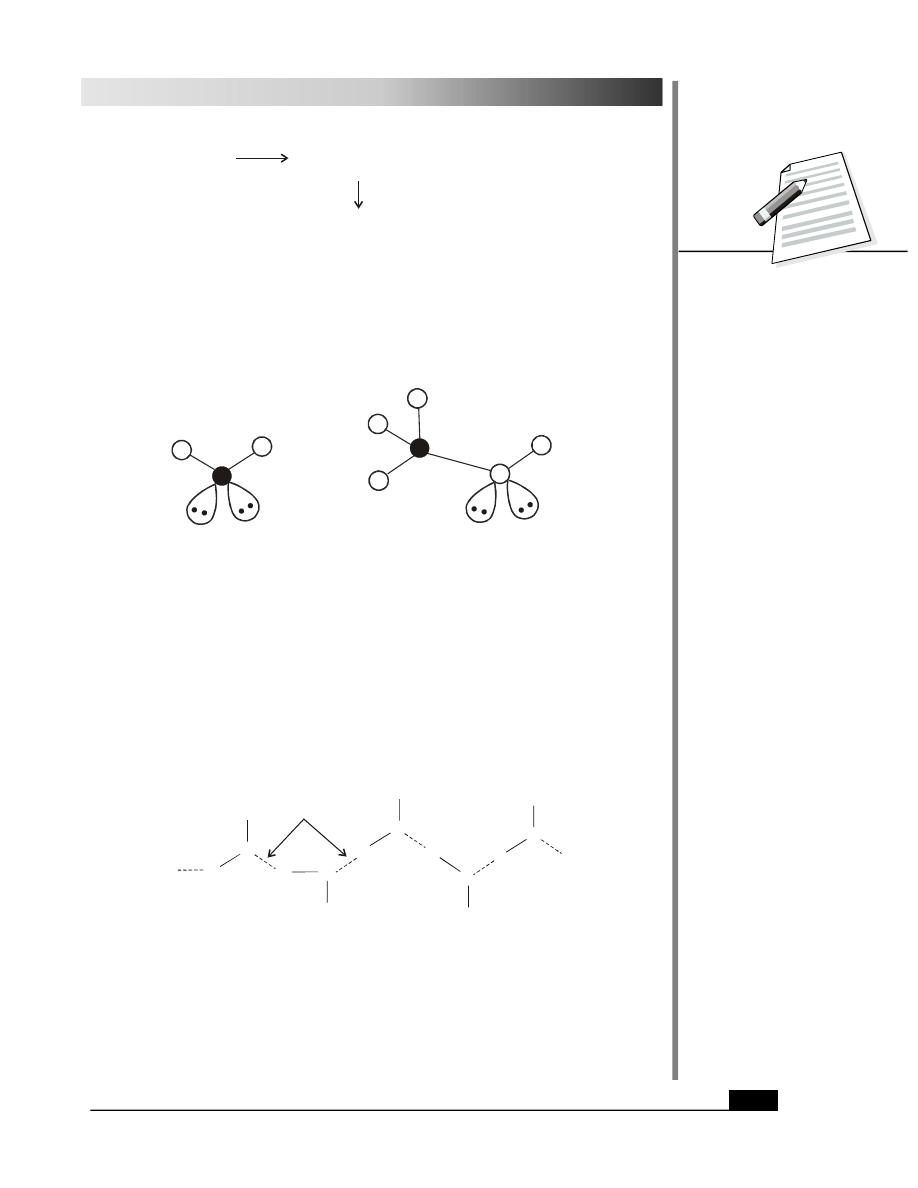
28.1.3 Structure and Physical Properties
The structure of alcohols is similar to that of water. The structures of water and methanol
molecules are shown in Fig. 28.1.
H
H
H
H
H
H
O
C
C
Fig. 28.1: Water and Methanol molecule
You know that the electronegativity of oxygen is more than that of hydrogen. Therefore,
in alcohols, the O–H bond is polar in nature. In other words, oxygen has a slight negative
charge on it whereas hydrogen has a slight positive charge. This bond polarity alone
cannot explain the higher boiling points of alcohols as compared to hydrocarbons or similar
haloalkanes, as listed in Table 28.2.
Normally, hydrogen bonding is responsible for higher boiling points of alcohols. Hydrogen
bonding amongst alcohol molecules is depicted in Fig. 28.2.
H
H
O
H
O
H
H
O
R
R
R
R
R
O
O
+
–
–
–
–
+
+
–
+
H
yd
ro
ge
n
bo
nd
in
g
Fig. 28.2: Hydrogen bonding in alcohol molecules
You can see that the negatively polarised oxygen atom of one alcohol molecule attracts
the positively polarised hydrogen atom of the other molecule. Thus, alcohol molecules are
associated or are held together. This force of attraction is to be overcome before a molecule
is set free from the liquid state and vaporises. Thus, more heat energy is required to break
the hydrogen bonds and hence, the boiling points of alcohols are higher than alkanes and
haloalkanes of comparable molecular mass.

198
MODULE - 7
Chemistry
Notes
Chemistry of Organic
Compounds
Table 28.2: Physical Properties of some Alcohols, Hydrocarbons and related Haloalkanes
Compound
IUPAC Name Melting Point
Boiling Point
Solubility
(K)
(K)
g/100 mL of water
CH OH
3
Methanol
175.2
322.8
CH4
Methane
90.5
181.13
–
CH Cl
3
Chloromethane
175.3
248.8
–
CH CH OH
3
2
Ethanol
158.3
351.5
CH CH
3
3
Ethane
189.7
184.4
–
CH CH Cl
3
2
Chloroethane
136.6
285.3
–
CH CH CH OH
3
2
2
Propan-1-ol
378.04
CH CH CH
3
2
3
Propane
85.3
230.9
–
CH
3
CH CH
3
OH
Propan-2-ol
184
355
CH CH CH CH OH
3
2
2
2
Butan-1-ol
183
391
8.3
CH
3
CH
2
CH CH
3
OH
Butan-2-ol
159
373
10.0
From the last column of Table 28.2, you must have noticed that alcohols have high solubilities
in water. The lower alcohols are completely miscible and their solubilities decrease as the
hydrocarbon portion of the molecule becomes larger. The higher solubility of alcohols can
be again attributed to the hydrogen bonding. In this case, hydrogen bonding takes place
between the alcohol and water molecules as is shown below in Fig. 28.3.
R – O H – O H – O H – O
H
H
R
H
Fig. 28.3: Hydrogen bonding in a solution of methanol and water
28.1.4 Reactions of Alcohols
Alcohols exhibit the following reactions:
1. Acidic and Basic behaviour
Alcohol behave both as acids and bases. They are weakly acidic. A strong base such as a
hydride ion (H
–
) in sodium hydride (NaH), can remove the proton from the alcohol molecule
and an alkoxide ion results.
R
H
+
B
R
+
B
H
O
. .
. .
O
. .
. .
Alcohol
Base
Alkoxide ion Protonated base
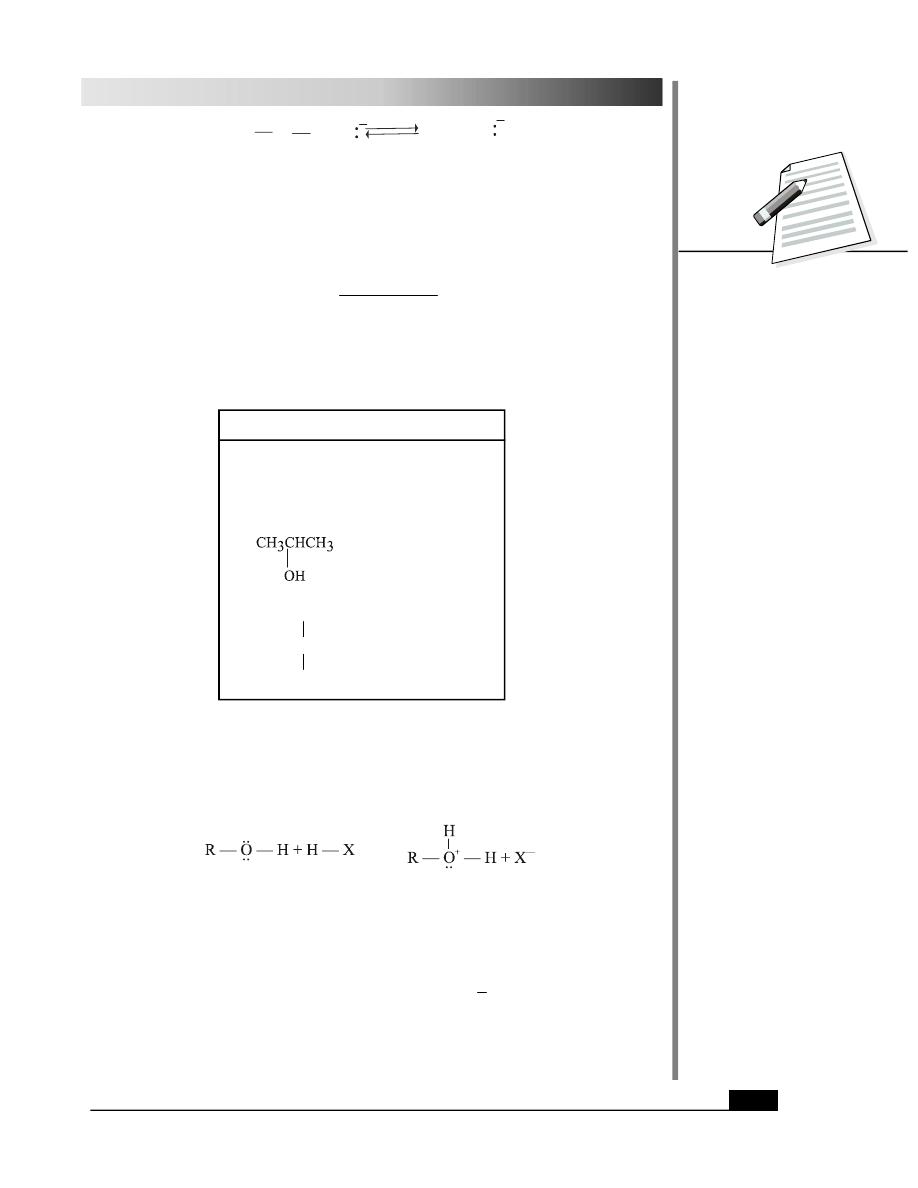
199
MODULE - 7
Alcohols, Phenols and Ethers
Notes
Chemistry of Organic
Compounds
H
+
B
+
BH
O
. .
. .
O
. .
. .
3
2
CH CH
3
2
CH CH
Ethanol
Base
Ethoxide ion Protonated base
When water is used as a base, the acid dissociation constant (K
a
) and pK
a
can be
written as follows:
2
3
R
O
H
H O
R
O
H O
a
K
3
[H O ]]RO ]
[ROH]
a
K
pK
a
= – log K
a
Some pK
a
values are listed in Table 28.3.
Table 28.3: pK
a
values of some compounds
Compound
pK
a
CH OH
3
15.5
H O
2
15.74
CH CH OH
3
2
15.9
16.5
CH
3
– C – OH
CH
3
CH
3
18.0
Remember that the lower the pK
a
value, higher is the acidity of the compound.
Alcohols can behave as weak bases also. They have lone pair of electrons on oxygen
atom and hence they can be protonated by strong acids to give oxonium ions as shown
below:
Alcohol
Acid
Oxonium ion
2. Formation of Alkoxides
Alcohols react with sodium or potassium metals to give the respective alkoxides.
3
2
3
2
2
1
CH CH OH
Na
CH CH O Na
H (g)
2
Ethanol
Sodium
Sodium
metal
ethoxide
200
MODULE - 7
Chemistry
Notes
Chemistry of Organic
Compounds
3 3
3 3
2
1
(CH ) C OH
K
(CH ) C O K
H (g)
2
tert-Butyl alcohol
Potassium
Potassium
metal
tert-butoxide
Alkoxides are used in the synthesis of organic compounds.
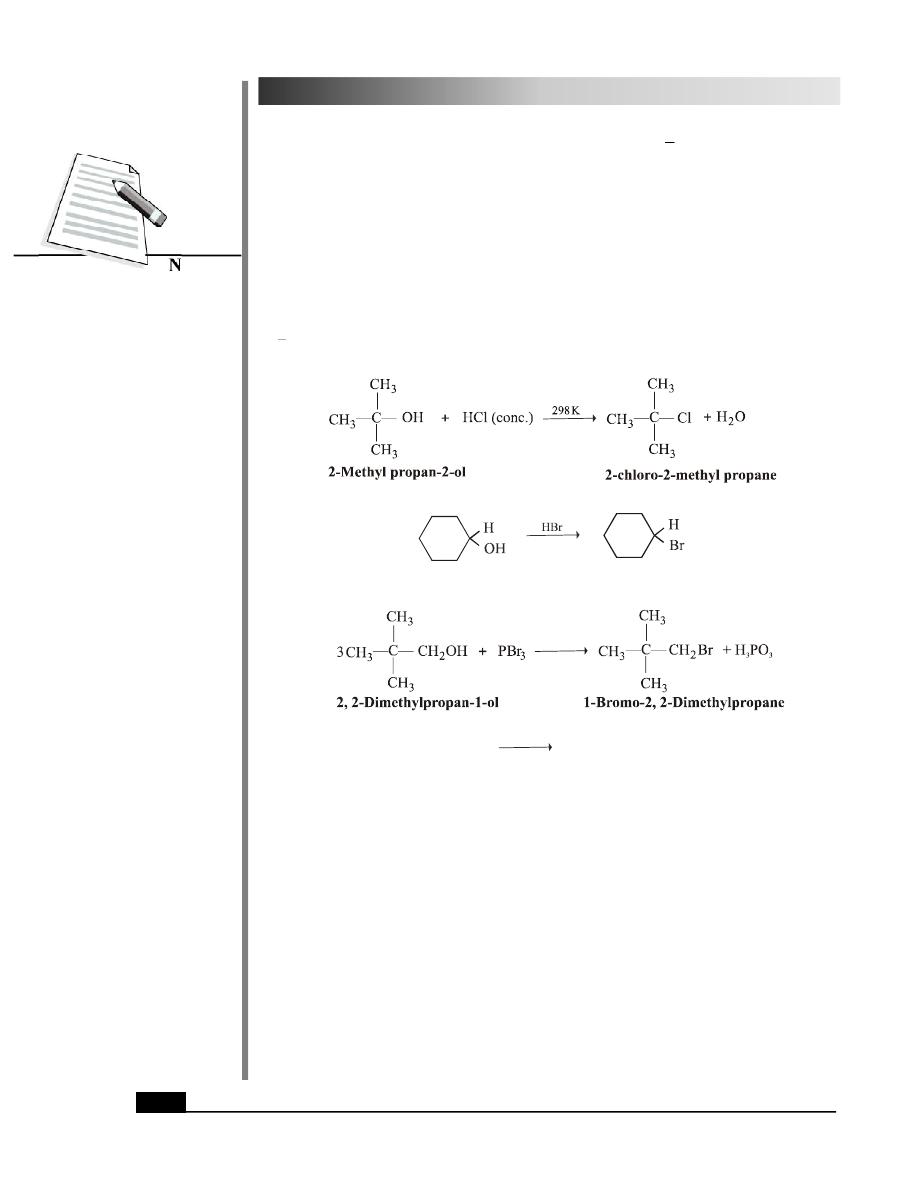
3. Conversion to Alkyl Halides
You have already studied in Lesson 27 that alcohols react with a variety of reagents to
form alkyl halides. These are hydrogen halides (such as HCl, HBr or HI), phosphorus
tribromide (PBr
3
) and thionyl chloride (SOCl
2
). The reaction involves the breaking of
OH
R
bond of alcohol molecule.
+ H
2
O
Cyclohexanol
Bromocyclohexane
3
2
2
2
CH CH CH OH SOCl
+
3
2
2
2 (g)
(g)
CH CH CH Cl SO
HCl
+
+
Propan-1-ol
1-Chloropropane
Tertiary alcohols are readily converted to alkyl halides by HCl or HBr while the best
method with primary and secondary alcohols is by using PBr
3
or SOCl
2
as the reagents.
Another advantage of using SOCl
2
is that both the by-products in this reaction, i.e. SO
2
and
HCl are gases and hence can be easily eliminated to yield pure alkyl halide.
Lucas Test
The formation of alkyl halides from alcohols is the basis of this test. In involves the
reaction of the alcohol with Lucas reagent (i.e. anhyd. ZnCl
2
+ conc. HCl). Since the
reactivity of alcohols is in the following order :
primary alcohols
<
secondary alcohols
<
tertiary alcohols
With primary alcohols turbidity does not appear. In case of secondary alcohols, turbidity
appears within 5 mintues whereas it appears immediately with tertiary alcohols. The turbidity
is due to the formation of alkyl chlorides from the corresponding alcohols.
201
MODULE - 7
Alcohols, Phenols and Ethers
Notes
Chemistry of Organic
Compounds
4. Formation of Alkenes
Alcohols can be dehydrated to alkenes. This reaction requires an acidic catalyst and is
favoured at higher tempratures. Usually sulphuric and phosphoric acid are used as acidic
catalysts. You have come across this reaction in Lesson 26 also. The ease of dehydration
follows the following order amongst alcohols.
tertiary alcohols
>
secondary alcohols
>
primary alcohols
5. Dehydration to form Ethers
Intermolecular dehydration of alcohols yields ethers. This reaction takes place at a lower
temperature than that for dehydration to give alkenes.
The formation of ethers by dehydration is a substitution type of reaction and gives only
symmetrical ethers. You will study a better method of synthesis of ethers later under the
section of ethers in this lesson.
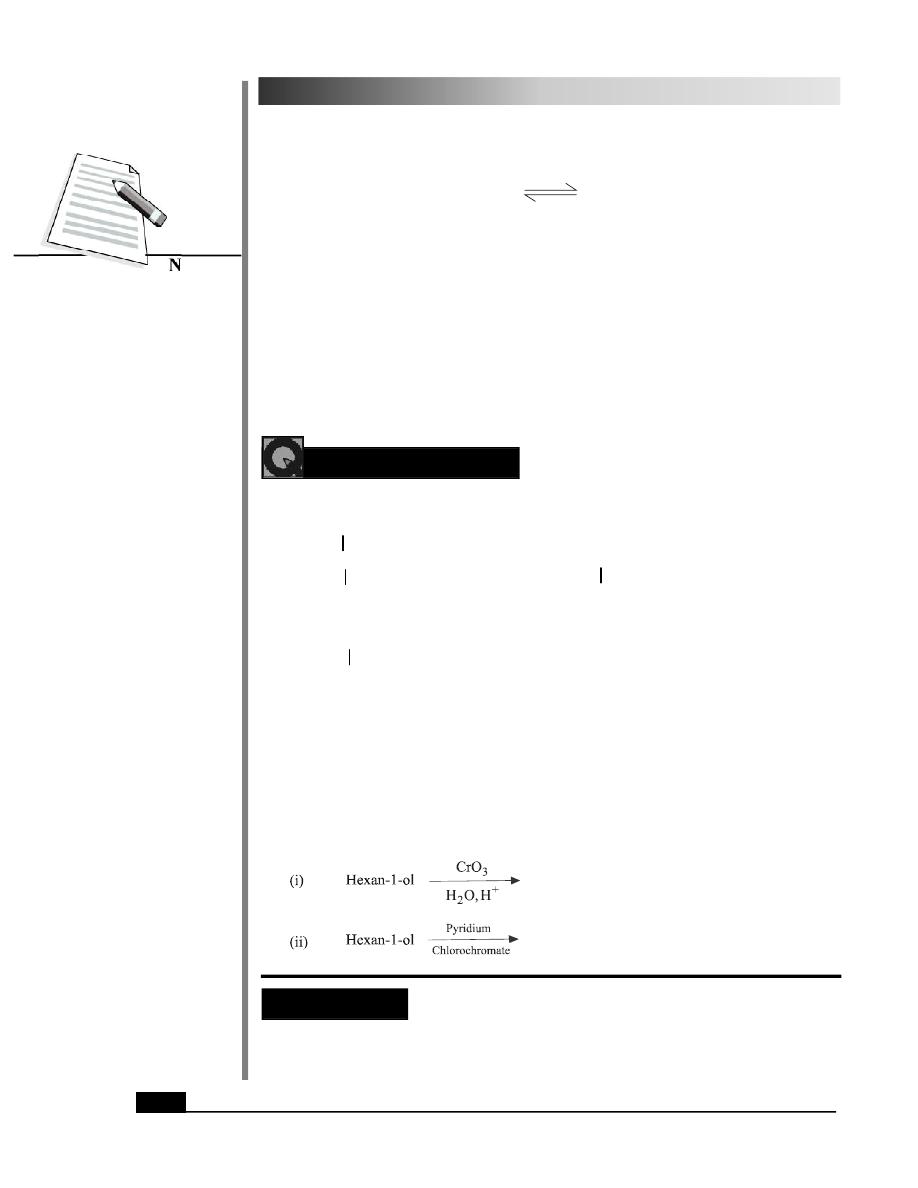
6. Oxidation
Alcohols can be oxidised to carbonyl compounds. Primary alcohols give aldehydes or
carboxylic acids on oxidation while secondary alcohols yield ketones. The tertiary alcohols
do not usually undergo oxidation. Normally KMnO
4
, CrO
3
and Na
2
Cr
2
O
7
or K
2
Cr
2
O
7
are used as oxidising agents.
2
2
7
2
4
2
K Cr O ,H SO
Further oxidation
3
2
2
3
2
3
2
H O
CH CH CH OH
CH CH CHO
CH CH COOH
Propan-1-ol
Propanal
Propanoic acid
The aldehydes obtained by oxidation of the primary alcohols get further oxidised to carboxylic
acids as shown above. You will study more about these classes of compounds in the next
lesson.
The oxidation can be controlled and aldehydes are obtained as the products by using
pyridium chlorochromate (PCC) which is a mild reagent.
PCC
3
2 8
2
3
2 8
CH (CH ) CH OH
CH (CH ) CHO
Decanol
Decanal
Secondary alcohols can be oxidised to ketones as shown below :
H
OH
Cyclohexanol
2
2
7
2
4
Na Cr O
H SO
O
Cyclohexanone
202
MODULE - 7
Chemistry
Notes
Chemistry of Organic
Compounds
7. Formation of Esters
Alcohols react with carboxylic acids to form esters. This reaction is discussed in the next lesson.
3
3
2
CH COOH
CH CH OH
+
3
2
3
2
CH COOCH CH
H O
+
H+
Ethanoic acid
Ethanol
Ethyl ethanoate
Water
This reaction is called esterification reaction and is reversible in nature.
U s e s
Alcohols find a large variety of uses as follows :
1. As solvents
2. As laboratory reagents
3. In medicines
4. As thinners in paints, varnishes, etc.
Intext Questions 28.1
1. Give the IUPAC names of the following alcohols :
(i)
OH
CH
3
CCH
2
CH
2
CH
3
CH
3
(ii) CH
3
CH == CCH
2
CH
3
CH
2
OH
(iii)
OH
CH
3
CHCH
2
CH
2
CH
2
OH
....................................................................................................................................
2. How will you prepare propan-1-ol from propanal?
....................................................................................................................................
3. Give the synthesis of 2-methylpropan-2-ol using Grignard reagent.
....................................................................................................................................
4. Give the product of the following reactions:
28.2 Phenols
The name phenol is specifically used for the following compound (hydroxybenzene) in
which one hydroxyl group is attached to the benzene ring.
203
MODULE - 7
Alcohols, Phenols and Ethers
Notes
Chemistry of Organic
Compounds
OH
Phenol
It is also used as a general name for the class of compounds derived from the above
compound. Phenol is a disinfactant. Phenols are widely distributed in nature. They are
also important in the synthesis of organic compounds such as aspirin and in the preparation
of dyes. Phenol is also used in the manufacture of bakelite which is a very useful polymer.
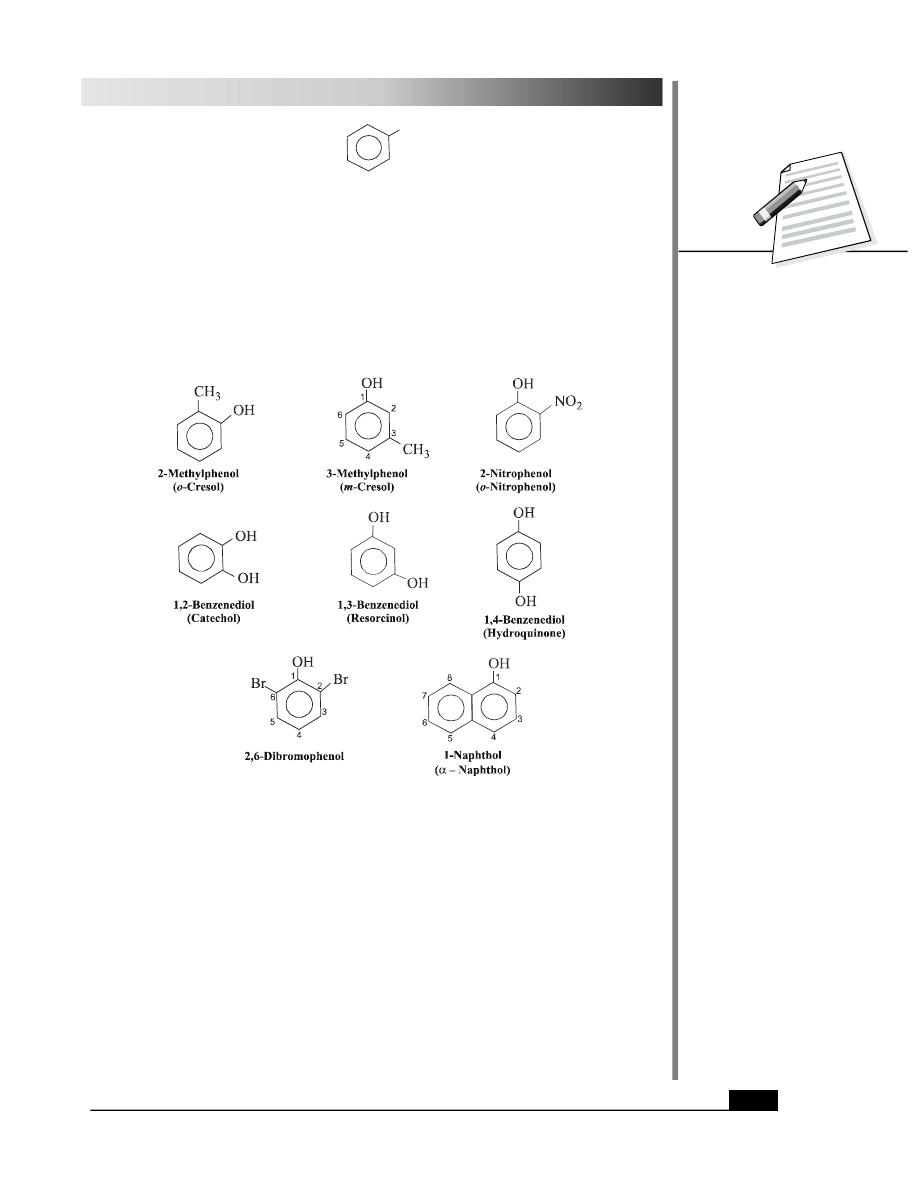
28.2.1 Nomenclature of Phenols
Some representative examples of phenolic compounds are given below:
Note that the term phenol is used as a parent name and the other substituents present in
the compound are given a specific number according to their position on the aromatic ring.
As done before the common names of the above compounds are given in the brackets
below their IUPAC names.
28.2.2 General Methods of Preparation
We can categorise the methods of preparation as methods of laboratory synthesis and
industrial synthesis of phenols.
A. Laboratory Synthesis of Phenols
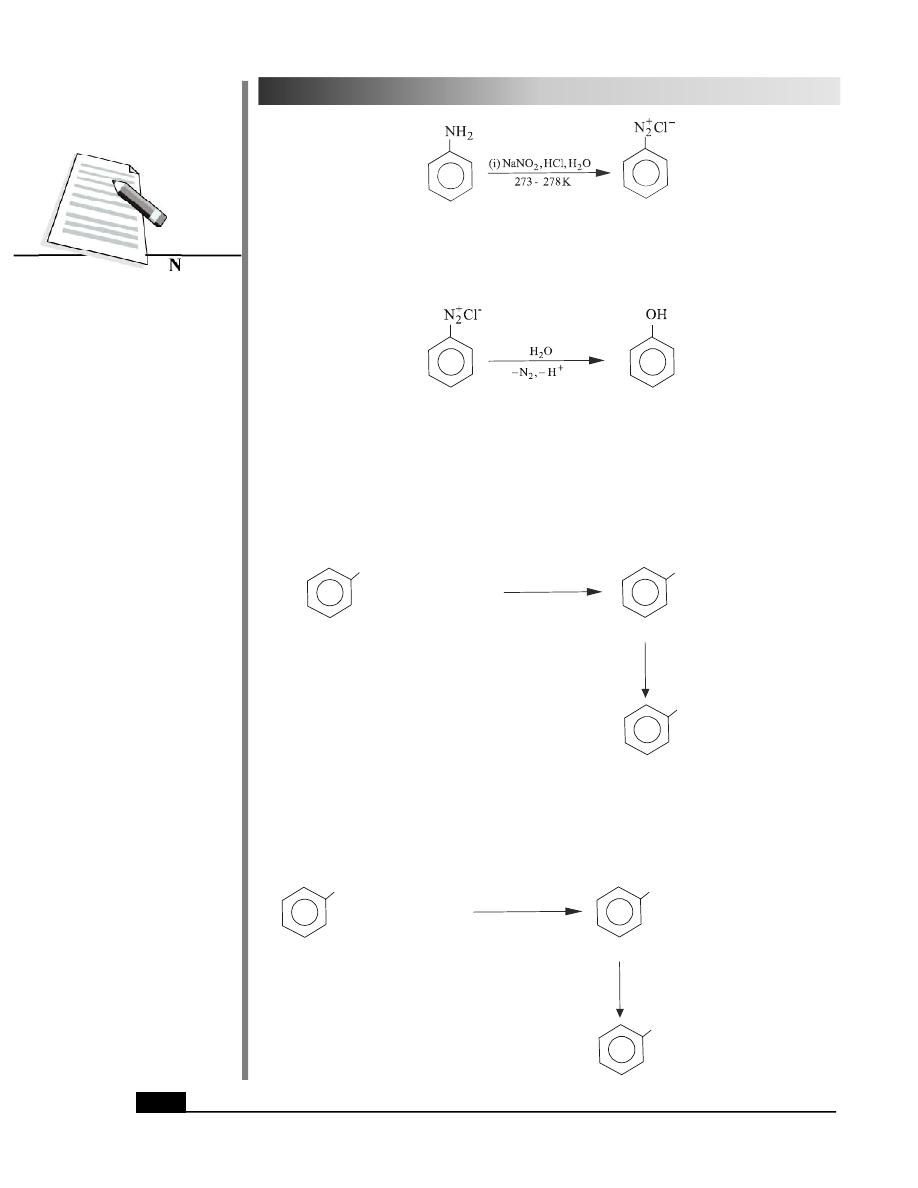
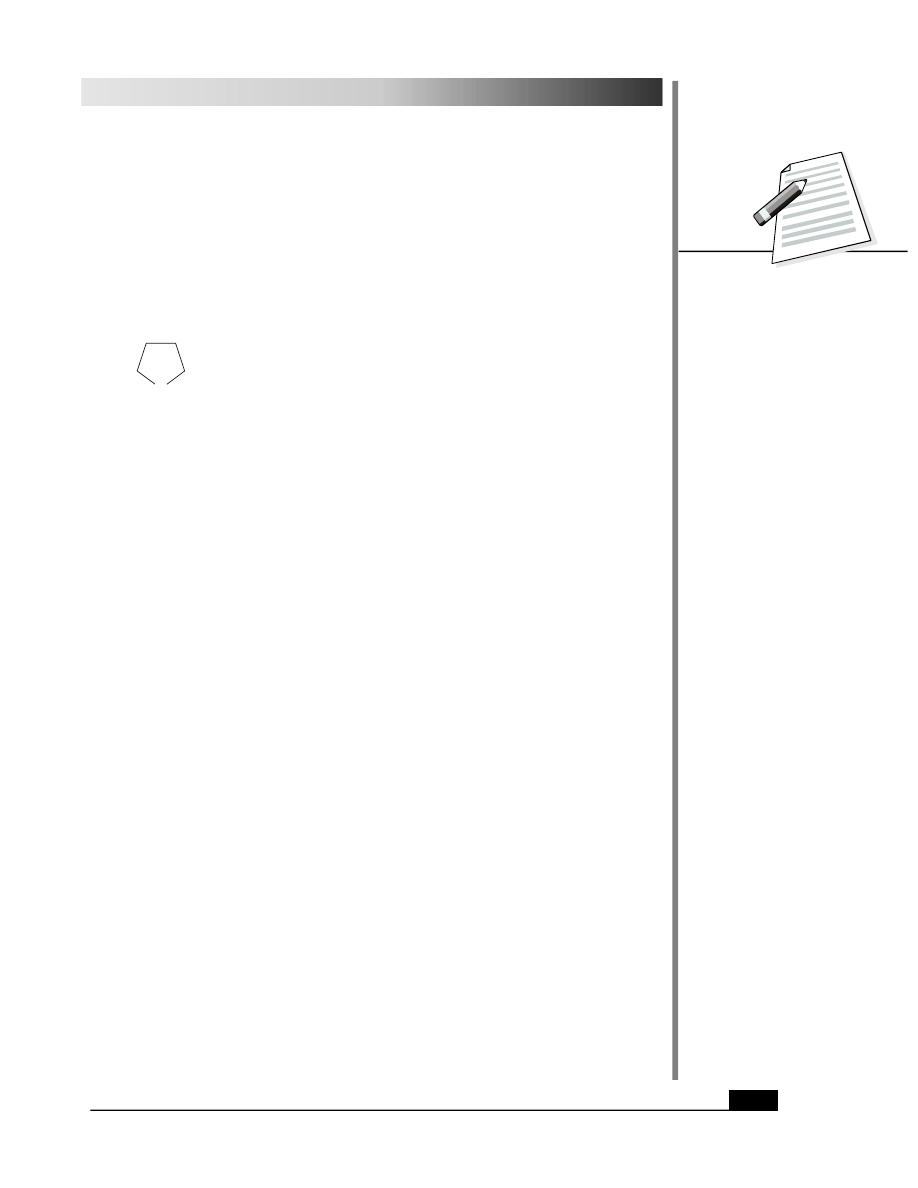
1. From Arenediazonium Salts
It is the most general method of preparation of phenols and requires mild conditions.
Arenediazonium salts or aromatic diazonium salts are obtained by the diazotization of
primary aromatic amines as given below :
204
MODULE - 7
Chemistry
Notes
Chemistry of Organic
Compounds
Benzenamine
Benzenediazonium chloride
(Aniline)
(an aromatic amine)
The arenediazonium salt on hydrolysis yields phenol.
Benzenediazonium chloride
Phenol
2. Alkali Fusion of Sodium Benzenesulphonate
This was the first commercial synthesis of phenol developed in Germany in 1890. It can
also be used as a laboratory method for synthesis of phenol.
Sodium benzenesulphonate is fused with sodium hydroxide to give sodium phenoxide which
on acidification yields phenol.
–
3
SO Na
+
623 K
2NaOH
+
2
3
Na SO
+
2
H O
+
OH
Sodium phenoxide
Sodium benzenesulphonate
3
H O
+
Phenol
O Na
–
+
B. Industrial Synthesis of Phenols
1. Dow Process
In this process, chlorobenzene is heated with aqueous sodium hydroxide under pressure.
Sodium phenoxide so produced on acidification gives phenol.
2
H O
Cl
623 K
2NaOH
+
NaCl
+
+
OH
Sodium phenoxide
Chlorobenzene
3
H O
+
Phenol
Pressure (300 atm)
O Na
–
+
205
MODULE - 7
Alcohols, Phenols and Ethers
Notes
Chemistry of Organic
Compounds
This method was in use for many years but now phenol is synthesised via cumene
hydroperoxide which is discussed below.
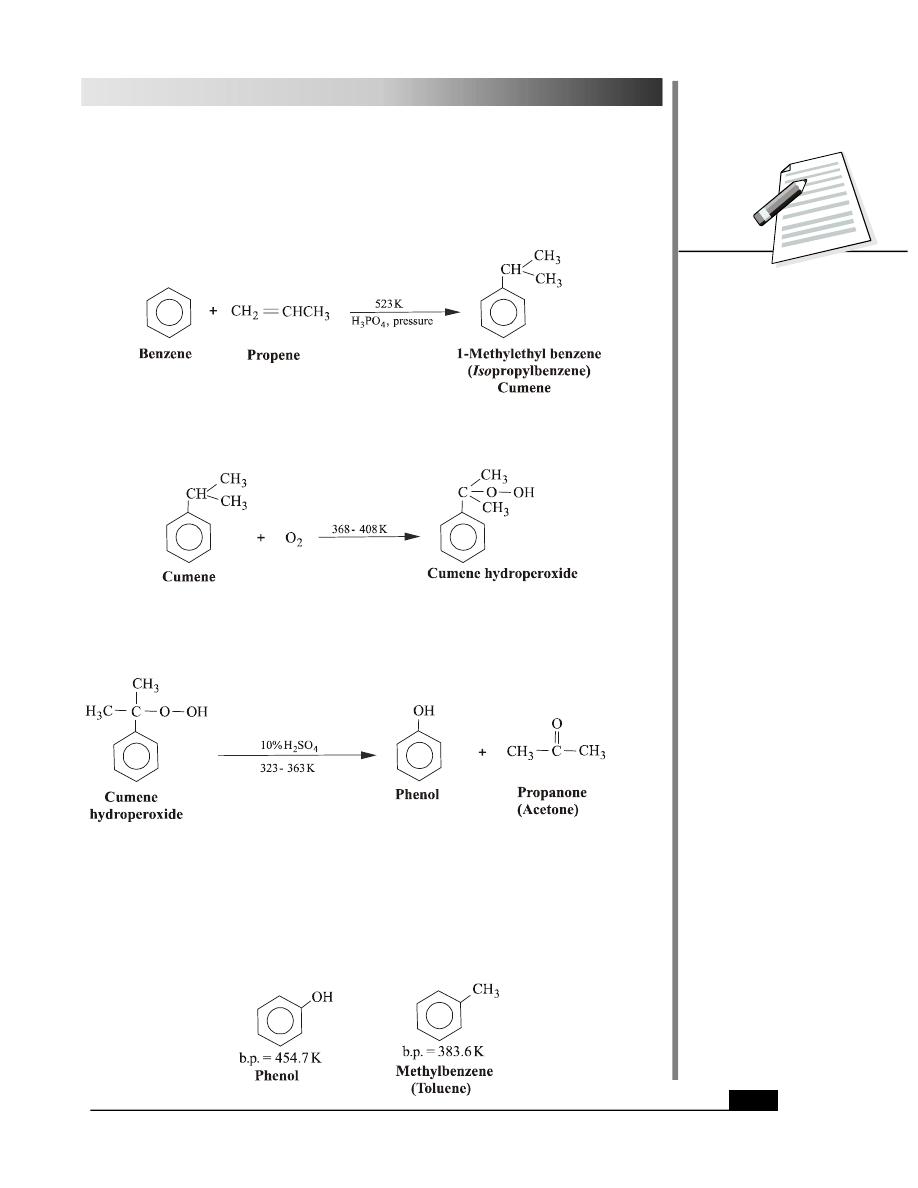
2. From Cumene Hydroperoxide
The reaction between benzene and propene in presence of phosphoric acid yields cumene.
Cumene is then oxidised to cumene hydroperoxide by air.
In the final step, cumene hydroperoxide is treated with 10% sulphuric acid to give phenol
and acetone on hydrolytic rearrangement.
Note that propanone is obtained as a valuable byproduct in this reaction.
28.2.3 Physical Properties
Similar to alcohols, phenols also have hydrogen atom linked to the electronegative oxygen
atom. Thus, phenols also exhibit hydrogen bonding and hence have higher boiling points as
compared to the hydrocarbons of similar molecular weight.
206
MODULE - 7
Chemistry
Notes
Chemistry of Organic
Compounds
Due to their ability to form hydrogen bonds, phenols show some water solubility. For
example, the solubility of phenol is 9.3 g per 100
mL
of water..
28.2.4 Reactions of Phenols
Let us now study the reactions exhibited by phenols.
1. Acidic and Basic Nature
Phenols are much more acidic than alcohols. pK
a
values of some phenols are listed in
Table 28.4.
Table 28.4: pK
a
values of phenols
Name
pK
a
Phenol
9.89
2- Methylphenol
10.20
2-Chlorophenopl
8.11
3-Chlorophenol
8.80
2-Nitrophenol
7.17
3-Nitrophenol
8.28
4-Nitrophenol
7.15
2,4,6-Trinitrophenol
0.38
(Picric acid)
Since phenols are acidic in nature, they are soluble in dilute sodium hydroxide.
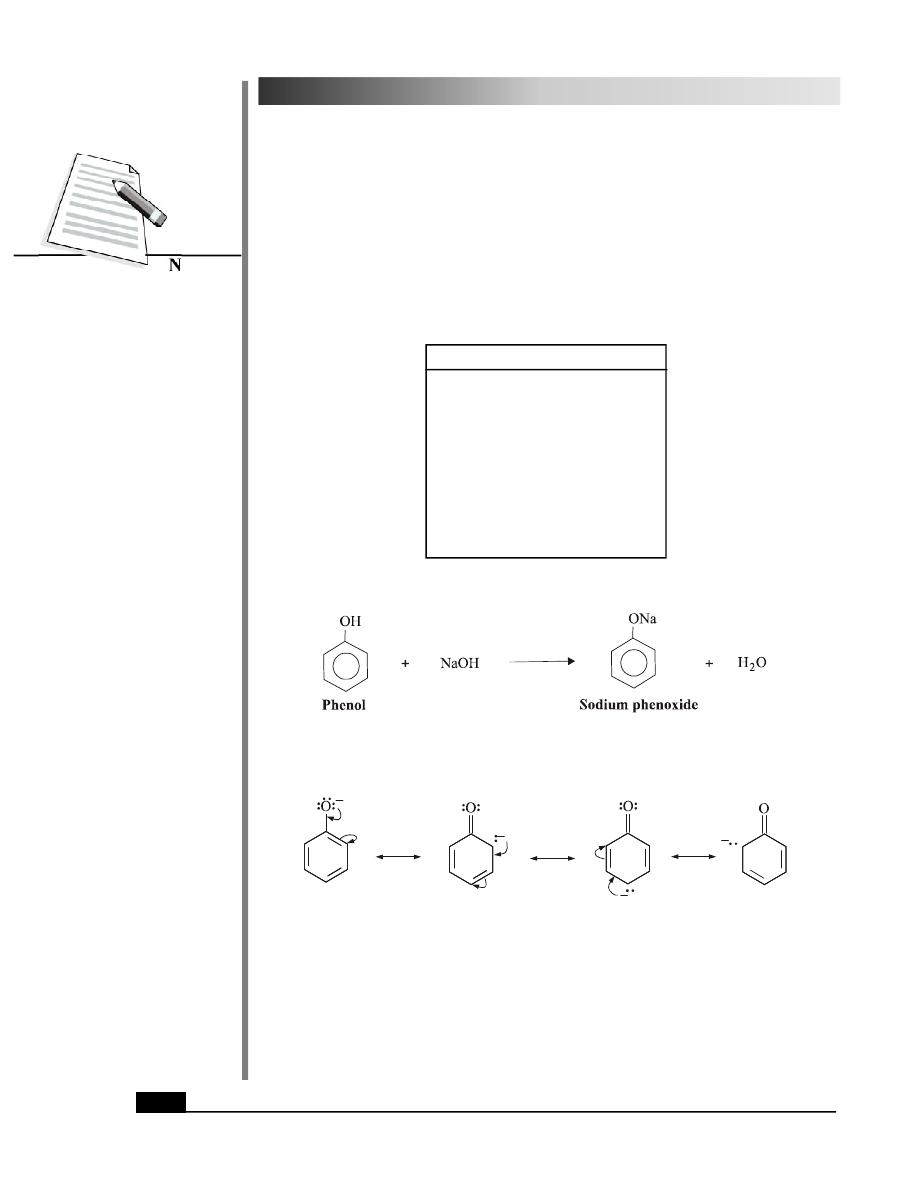
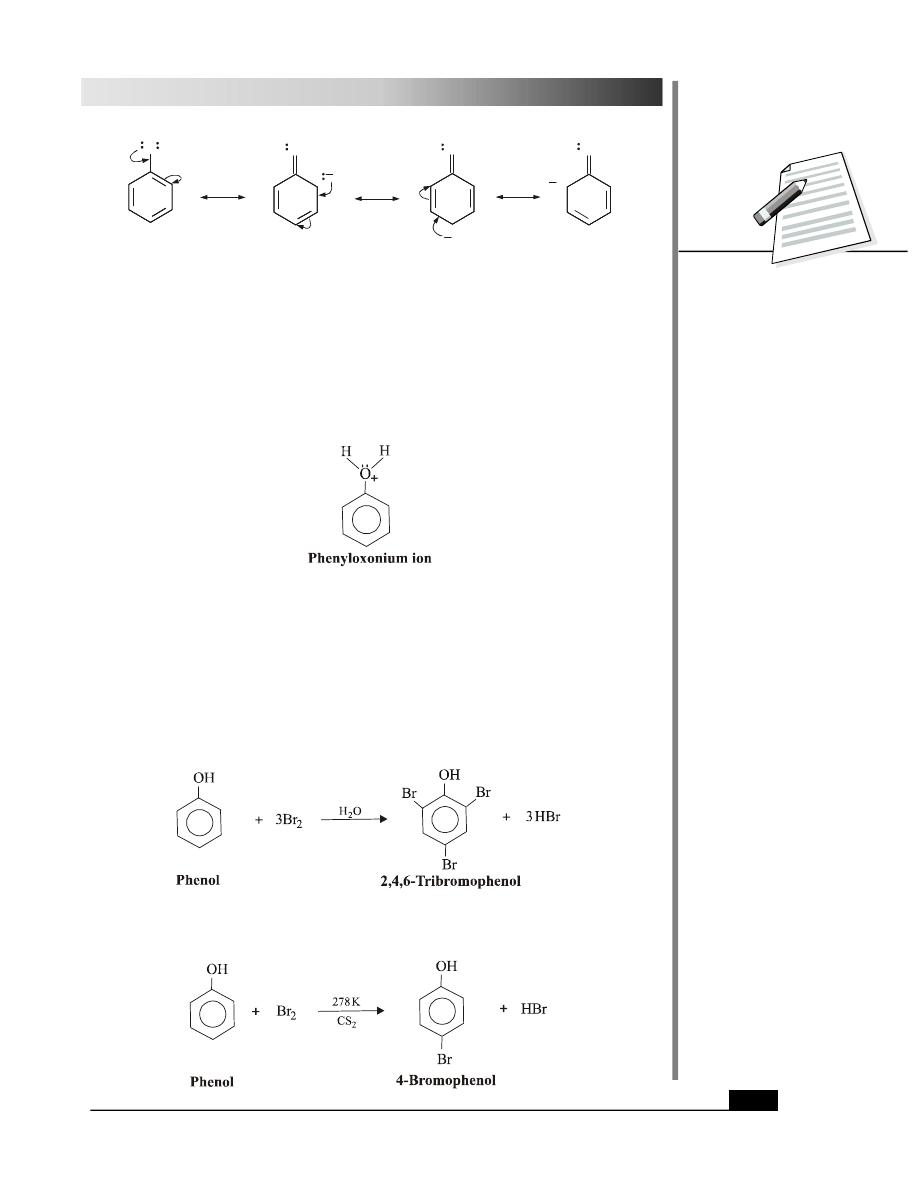
The greater acidity of phenols can be attributed to the resonance stablisation of the phenoxide
ion. The resonance structures of pheoxide ion are shown in Fig. 28.4.
Fig. 28.4: Resonance structures of phenoxide ion
The delocalisation of the negative charge over the benzene ring stabilises the phenoxide
ion. No such stabilisation is possible, in case of alkoxide ions.
Similar resonance is also shown in phenol itself, see Fig 28.5. But the resonance structures
of phenol are less stable as compared to those of phenoxide ion as they involve the separation
of charge.
207
MODULE - 7
Alcohols, Phenols and Ethers
Notes
Chemistry of Organic
Compounds
O H
OH
+
OH
+
OH
+
. .
. .
. .
. .
. .
. .
Fig. 28.5: Resonance structures of phenol
If you carefully go through the pK
a
values given in Table 28.4, you would see that the
electron donating substituents such as methyl group decrease the acidity of phenol and
hence alkylphenols have greater pK
a
values as compared to phenol itself. On the other
hand, electron withdrawing substituents increase the acidity and phenols having these
substituents (–Cl, –NO
2
, etc.) have lower pK
a
values than phenol. In fact, 2,4,6-trinitrophenol
is more acidic than many carboxylic acids.
Phenols behave as weak bases also. Similar to alcohols, they can also be protonated to
give phenyloxonium ion.
2. Electrophilic Substitution Reactions
The hydroxyl group is a powerful activating group and hence phenols readily undergo
electrophilic substitution reactions. In this reaction, an electrophile (electron loving species)
attacks the benzene ring and replaces one of its hydrogen atoms. Since the ortho and
para positions of the phenol are electron rich, the substitution takes place at these positions.
Two such reactions are halogenation and nitration reactions. Let us now study them in
details.
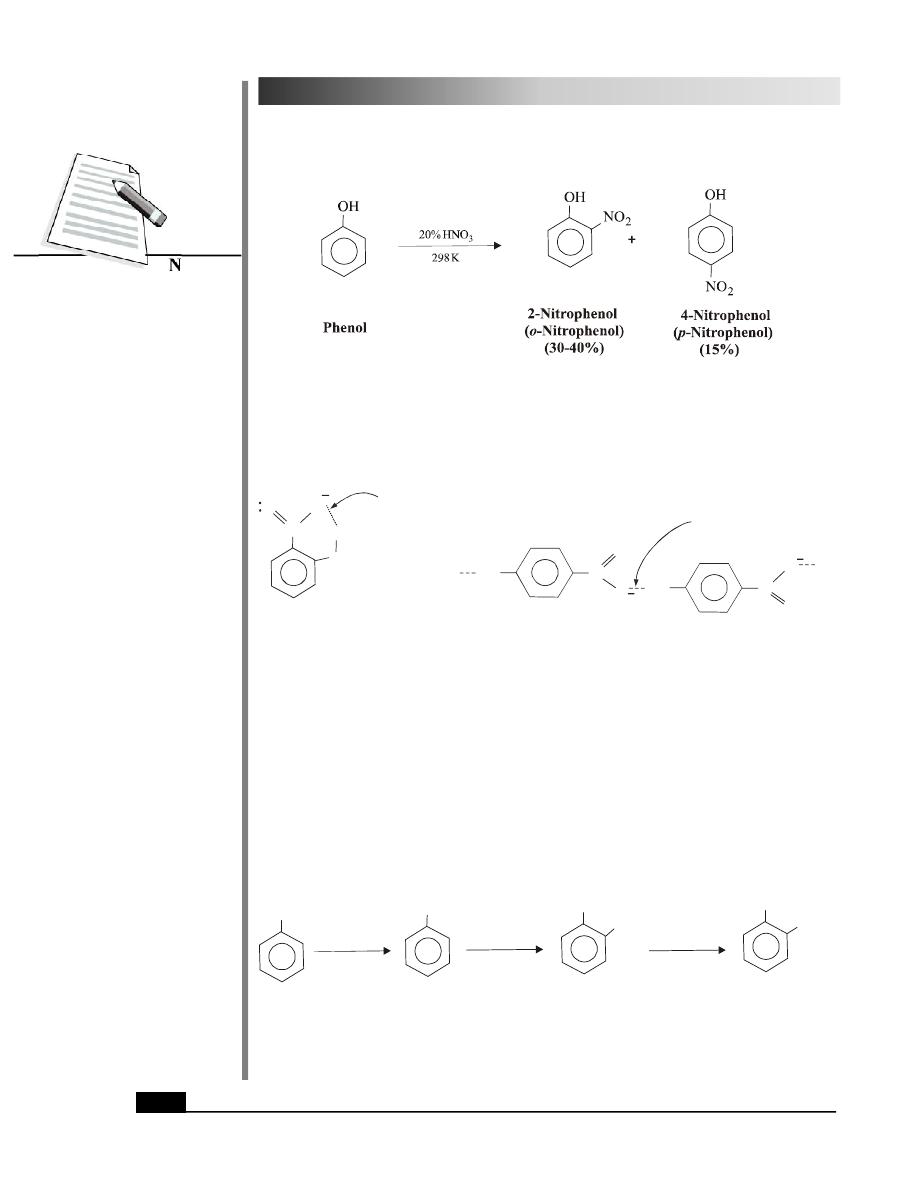
(i) Halogenation: Phenol reacts with bromine in aqueous solution to give 2,4,6-
tribromophenol in about 100% yield.
Bromination can be limited to monobromination to give mainly 4-bromophenol using low
temprature and less polar solvent such as carbon disulphide. The other product formed in
minor quantity is 2-bromophenol.
208
MODULE - 7
Chemistry
Notes
Chemistry of Organic
Compounds
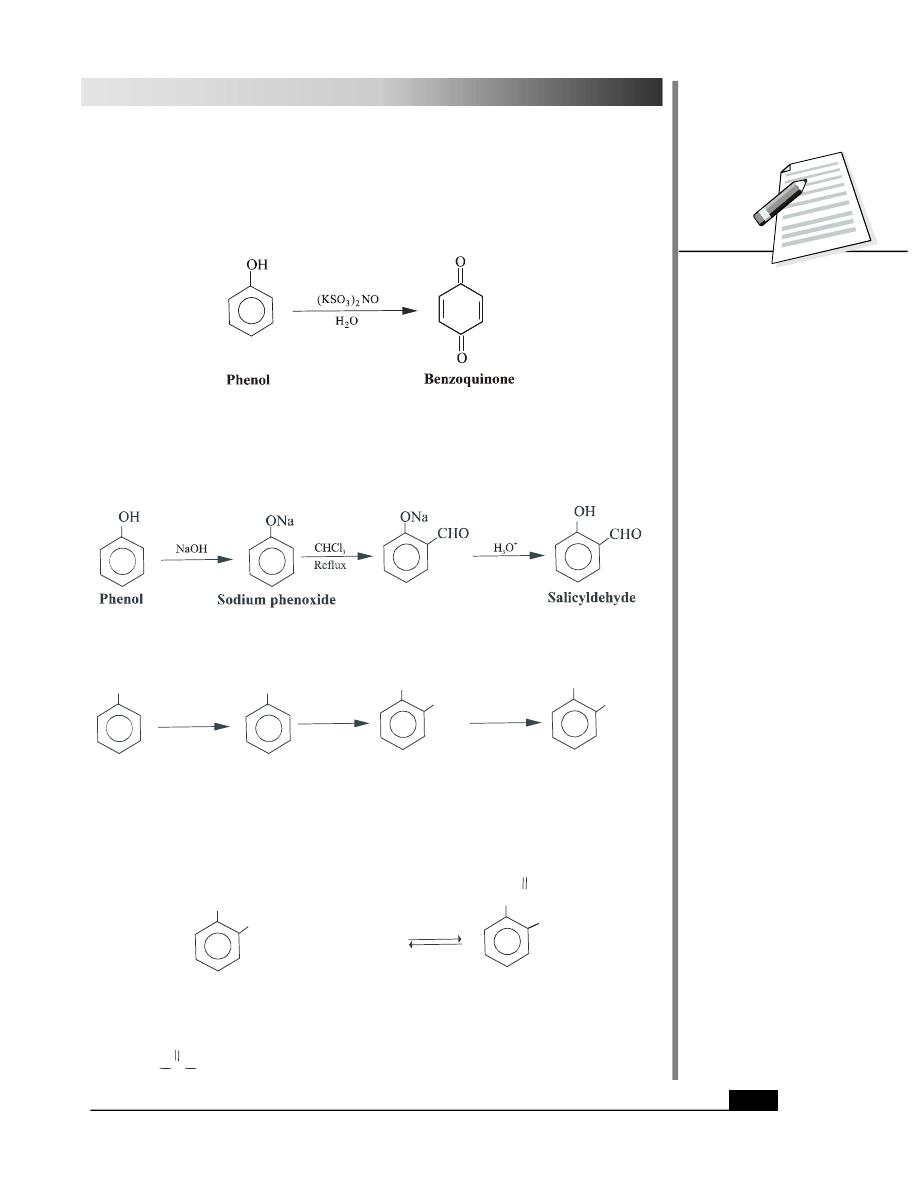
(ii) Nitration: Phenol gives a mixture of 2-nitro and 4-nitrophenols on nitration with dilute
nitric acid.
The mixture of nitrophenols so obtained is separated using steam distillation. Both these
products show hydrogen bonding. In case of 2-nitrophenol, the hydrogen bonding is
intramolecular (in the same molecule) whereas in case of 4-nitrophenol, it is
intermolecular (between different molecules). These are depicted in Fig. 28.5.
O
2-Nitrophenol
N
O
O
. .
. .
. .
..
H
intramolecular
hydrogen bonding
4-Nitrophenol
O
N
+
O
HO
O
N
+
O
HO
Intermolecular
hydrogen bonding
. .
. .
. .
. .
. .
. .
Fig. 28.5 : Intramolecular and intermolecular hydrogen bonding in nitrophenols
2-Nitrophenol is steam volatile and distills out on passing steam whereas 4-nitrophenol is
less volatile due to intermolecular hydrogen bonding.
Treatment of phenol with a mixture of conc. nitric acid and conc. sulphuric acid at 323K
yields 2,4,6-trinitrophenol also known as picric acid.
3. Kolbe Reaction
It involves sodium phenoxide which is allowed to absorb carbon dioxide and then heated
under a pressure of CO
2
to 398 K. Sodium salicylate so obtained on acidification yields
salicylic acid.
NaOH
OH
ONa
OH
OH
2
CO
398K
pressure
(4-7 atm)
Sodium
salicylate
Salicylic acid
COONa
COOH
3
H O
+
Phenol
Sodium
phenoxide
By reaction with acetic anhydride, salicylic acid yields aspirin, which is the common
pain reliever.
209
MODULE - 7
Alcohols, Phenols and Ethers
Notes
Chemistry of Organic
Compounds
4. Oxidation
Phenols undergo oxidation reactions to give products which are diffrent from those obtained
by alcohols. They can be oxidised using a variety of oxidising agents such as sodium
dichromate or silver oxide to give quinones. These days Fremy’s salt [(KSO
3
)
2
NO] is
preferred for oxidation.
5. Reimer Tiemann Reaction
Phenols react with chloroform in the presence of sodium hydroxide (or potassium hydroxide)
solution followed by acidification to give hydroxy aldehydes.
Use of carbon tetrachloride in place of chloroform gives salicylic acid.
OH
ONa
Salicylicacid
ONa
COOH
OH
Sodium phenoxide
Phenol
NaOH
CCl
4
H O
3
+
COOH
6. Esterification
Similar to alcohols, phenols react with carboxylic acids to give esters.
COOH
OH
3
CH COOH
H
+
+
O–C–CH
3
Acetyl salicylic acid
COOH
O
Ethanoic acid
2-Hydroxybenzoic acid
This reaction is an acetylation reaction as the H of –OH the phenol is replaced by the
acetyl (
C
3
CH
O
) group.
210
MODULE - 7
Chemistry
Notes
Chemistry of Organic
Compounds
7. Coupling Reaction
Phenols react with aromatic diazonium salts in slightly alkaline conditions to give azo
compounds. These azo compounds are brightly coloured and are used as azo dyes.
N
N
=
+
orange solid
-Phenylazophenol
p
( -Hydroxyazobenzene)
p
Phenol
Benzenediazonium
chloride
–
2
N Cl
+
OH
2
NaOH, H O
273K
OH
U s e s
1. Phenol is used as a disinfectant.
2. It is also used in the synthesis of polymers.
3. Phenols are used in the synthesis of many organic compounds.
4. Substituted phenols are used in dyeing and tanning industries.
Intext Questions 28.2
1.
How will you convert aniline to phenol?
...............................................................................................................................
2.
What is the starting material in Dow’s process?
...............................................................................................................................
3.
Arrange the following in the increasing order of their acidity:
Phenol, 2-Methylphenol, 2-Chlorophenol
...............................................................................................................................
4.
How will you prepare salicylic acid from phenol?
...............................................................................................................................
5.
What is an azo dye?
...............................................................................................................................
28.3 Ethers
Ethers are organic compounds in which an oxygen atom is bonded to two alkyl groups or
aryl groups. Thus, ethers can be represented as
R
O
R
where
R
and
R
may be alkyl
or aryl groups. When the two substituent groups (R and
R
) are identical, then the ether is
called a symmetrical ether, otherwise if these two groups are different, then the ether is
known as an unsymmetrical ether.
216
MODULE - 7
Chemistry
Notes
Chemistry of Organic
Compounds
(i) CH
3
CH
2
CH
2
Cl + NaOH (aq.)
..............
(ii) CH
3
CHO
4
3
1. LiAlH , ether
2. H O
..............
(iii) CH
3
OH + Na
..............
5.
How is ethanol prepared using fermentation?
6.
What is Lucas test? What is its use?
7.
Which reagent is used for oxidising primary alcohols to aldehydes?
8.
Why are phenols more acidic than alcohols? Explain.
9.
Why are ethers polar in nature?
Answers to Intext Questions
28.1
1.
(i) 2-Methylpentan-2-ol
(ii) 2-Ethylbut-2-en-1-ol
(iii) 1, 4-Pentanediol
2.
By reduction with NaBH
4
or LiAlH
4
3.
3
1. Ether
2. H O
4.
(i) Hexanoic Acid
(ii) Hexanal
28.2
1.
2.
Chlorobenzene
3.
2-Methylphenol < Phenol < 2-Chlorophenol
4.
By Kolbe reaction
5.
Azo dyes are azo compounds formed by the reaction of phenols with aromatic
diazonium salts. They are brightly coloured.
217
MODULE - 7
Alcohols, Phenols and Ethers
Notes
Chemistry of Organic
Compounds
28.3
1.
(i) 2-Methoxybutane
(ii) Methoxymethane
2.
(i)
3
2
2
3
CH CH CH O
CH Br
CH
3
CH
2
– O – CH
3
+ Br
–
(ii) Methoxypropane
3.
They may explode due to the presence of peroxides.
4.
Because they are unreactive in nature.
5.
It is a cyclic ether.
O
It is used as a solvent.
