PREPARING SOLUTIONS AND REAGENTS
concentrationsIs weight over volume (w\v) relationship, it mean certain weight dissolved in certain volume of solvent.
The amount of a drug in a given volume of blood plasma, measured as the number of micrograms per milliliter.
One way of comparing drug potency is by the concentration at which 50% of the maximum effect is achieved. This is referred to as the 50% effective concentration or EC50. When two drugs are tested in the same individual, the drug with a lower EC50 would be considered more potent
For liquid drugs ,both oral and injectable
concentration is important factor in dosing.It refers to the amount of active drug(dose in mg) in a given volume vehicle usually a liquid but could be paste. e.g Suspension is 100mg\ml
e,.g ampoule contain 50mg\2ml
concentrations
1%--------------1gm in 100ml1:100,000--------------1gm in 100,000ml
1:80,000----------------1gm in 80,000ml
2%=2gm of lidocaine in 100ml of solvent
Dose calculation
Dose: is the measured portion of medicine to be taken at one time.
Factors influencing dosage
1- age: children and elderly2-sex:pregnancy and fetus
3- condition of the patient: resistance ,kidney and liver disease
4-enviromental factors:
5-method of administration:
6- genetic factor:
7-body weight:
8- severity of disease:
Dose calculation in children
1-Youngs rule:a Child dose= age of child(year)x average adult dose
age of child(year)+12
CHILD FROM 1-12 years
Dose calculation in children
2- Clarks rule:Child dose= weight of child(pound)x adult dose
150 pound
Dose calculation in children
3-For infant less than one year old use
FRIEDS RULE
child dose= age in months x adult dose
150 pound
Chemical Solutions (aqueous = water is the solvent)
• DEFINITIONS:• SOLUTES -- substances that are dissolved
• SOLVENTS -- substance in which solutes are dissolved (usually water)
• AMOUNT -- how much
Goals
Make solutionsDilute solutions
Convert between different concentrations of solutions
Facts of Life
• 1g = 1000mg = 1,000,000µg• 1L = 1000mL = 1,000,000 μL
Facts of life (cont’d)
Concentration means: amount of solute in a volume of solution
Expressed in many ways:
1. percent2. mg/ mL
3. molar
4. “X” solution
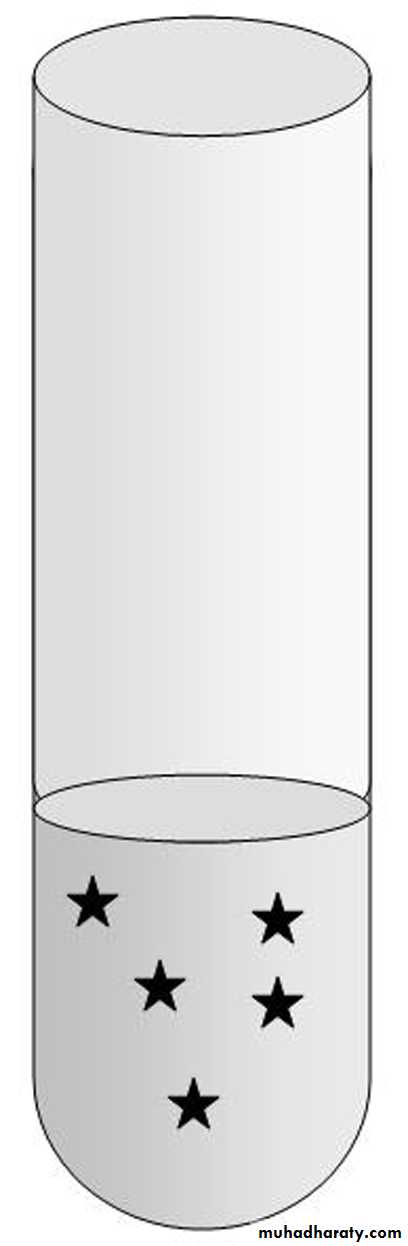
Each star represents 1 mg of NaCl.
What is the total amount of NaCl in the tube? _____What is the concentration of NaCl in the tube (in mg/mL)? _____
8 mL
5 mg = ?8 mL 1 mL
? = 0.63 mg, so the concentration is
0.63 mg/mLPercent Solutions
Per means “for every one”
Cent means 100
Example: a 5% sugar solution has
5 grams of sugar for 100ml of solution, orMake 250 mL of a 3% starch solution
3 g / 100 ml = 3 g / 100 mL because density of water is 1 g / mLSet up a ratio:
3 g / 100 mL = ?g / 250 mLUse 7.5 g of starch and bring to a volume of (BTV) 250 mL with distilled water
5 mg/mL has 5 milligrams of solute inone milliliter of solution
mg / mL Solutions