
CARBOHYDRATES
Describe, Discuss, and compare the classification,
structure and function of carbohydrates and its
derivatives.
Dr. Noorhan Chelebi
1
ﻛﻳﻣﻳﺎء ﻧﻅﺭﻱ / ﺍﻭﻝ ﺍﺳﻧﺎﻥ ﻛﺭﻛﻭﻙ
ﺩ.ﻧﻭﺭﻫﺎﻥ
12/11/2018
88

CARBOHYDRATES
1 Role & Significance of
Carbohydrates
2 Monosaccharides
3 Oligosaccharides
4 Polysaccharides
5 Glyconoconjugates
2

ROLE OF CARBOHYDRATES
As a major energy source for living organisms
(
glucose is a principal energy source in animal and plants)
As means of transporting energy
( sucrose in plant tissues)
As a structural material
( cellulose in plants, chitin in insects, building blocks of
nucleotides).
As a precursor for other biomolecules
(
purine, pyrimide)
3

SIGNIFICANCE OF CARBOHYDRATES
Carbohydrates are the most important biomolecules in
nature, having a direct link between solar energy
and the chemical bond energy in living organisms.
Source of rapid energy production.
Structural building blocks of cells.
Components of several metabolic pathways.
Recognition of cellular phenomena, such as cell
recognition and binding (e.g., by other cells,
hormones, and viruses)
4

Carbohydrate: compounds contains H, C & O with the
compound:
(CH
2
O)
n
→(Hydrate of carbon)
Carbohydrates:
→Consist of sugar (saccharum)
Sugars are compounds that contains alcohol & carbonyl
functional group
Carbonyl func.group : >C=O
Adehyde
→ aldose
Ketone
→ ketose
CARBOHYDRATES
5
Examples:
6
cellulose, chitin,
starch, glycogen,
glucoaminoglycan
s
disaccharides
Glycoproteins
(bacterial cell
walls
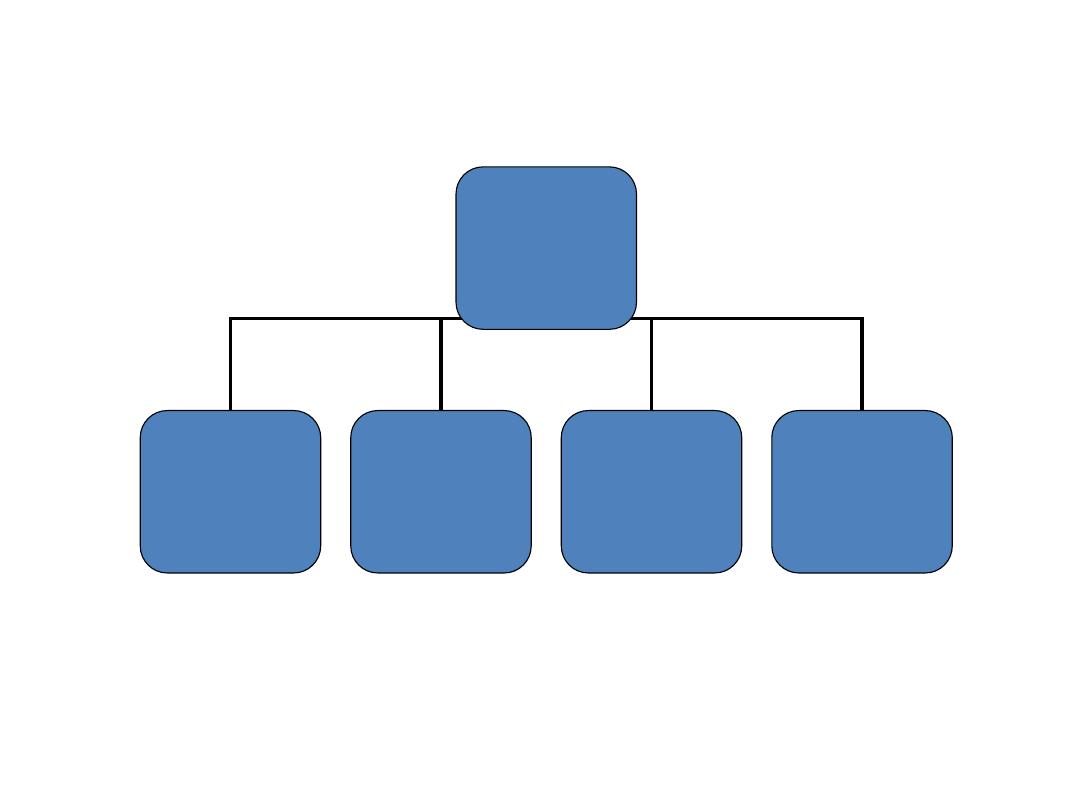
Classification
Carbohydrate
Mono
saccharide
Oligo
saccharide
Poly
saccharide
Glyconoconjugate
s
Glucose, fructose
Ribose
(aldopentose)
Deoxy ribose
glycoproteins
and
proteoglycans
7

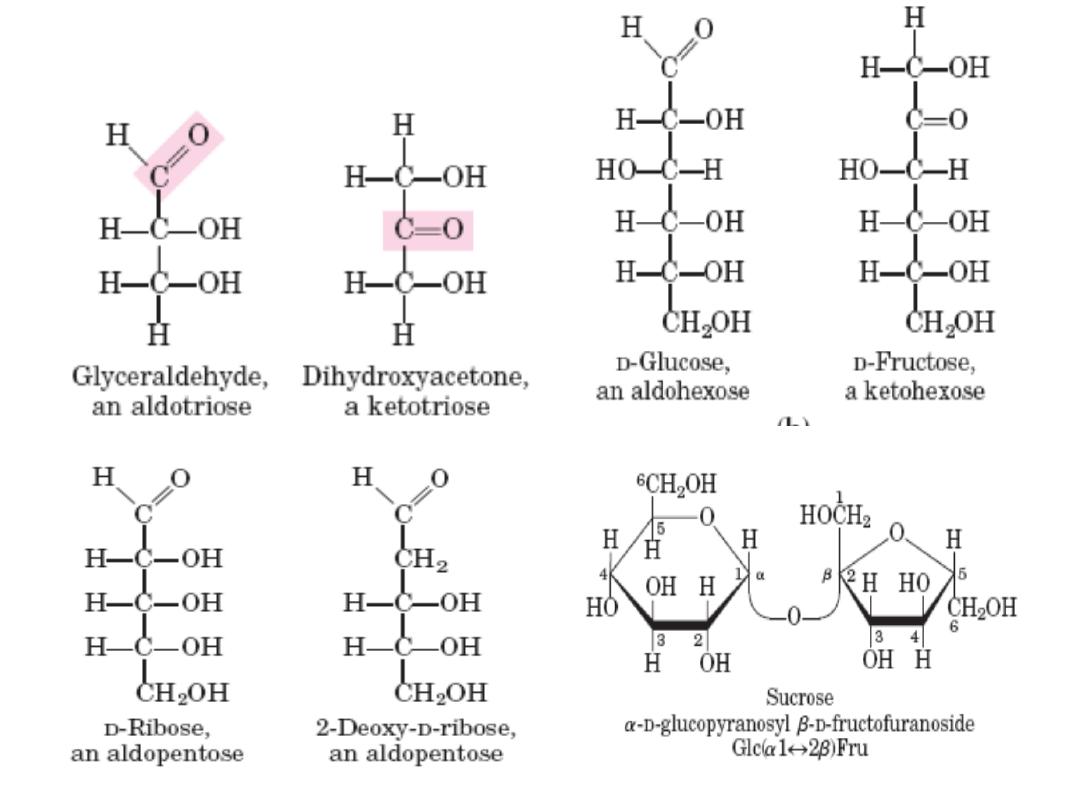
Monosach Properties & classification
• Colorless, crystalline solids.
• Soluble in water but insoluble in nonpolar solvents.
• One of the carbon atoms is double‐bonded to an
oxygen atom to form a carbonyl group; each of the
other carbon atoms has a hydroxyl group.
– Carbohydrates with an aldehyde (‐CHO) functional group
are called aldoses
– e.g. glyceraldehyde (CH
2
OH‐CHOH‐CHO)
Those with a keto group (‐C=O) are ketoses e.g.
Dihydroxyacetone (CH2OH‐C=O‐CH2OH)
– Classified according to the number of carbon atoms they
contain
8
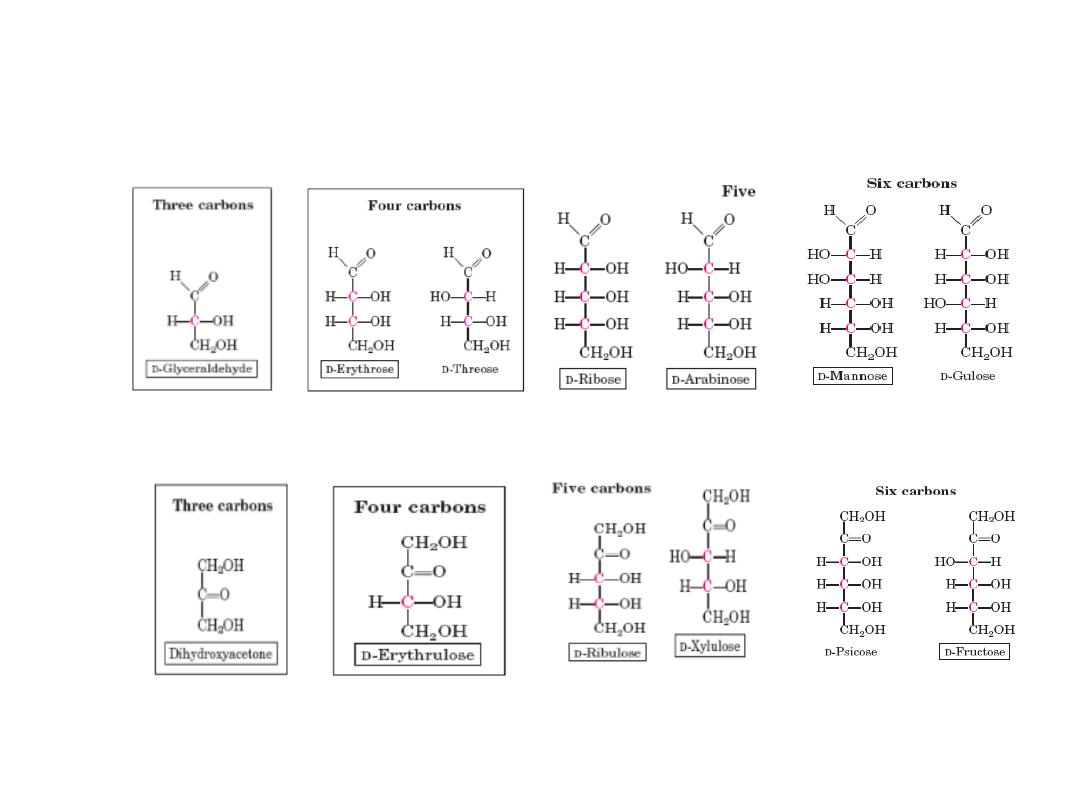
Monosacharides : Exp. aldoses & ketoses
Aldotetros
e
Aldotrios
e
Aldopentose
s
Ketotrios
e
Ketotetros
e
Ketopentos
e
Ketohexos
e
Aldohexos
e
9
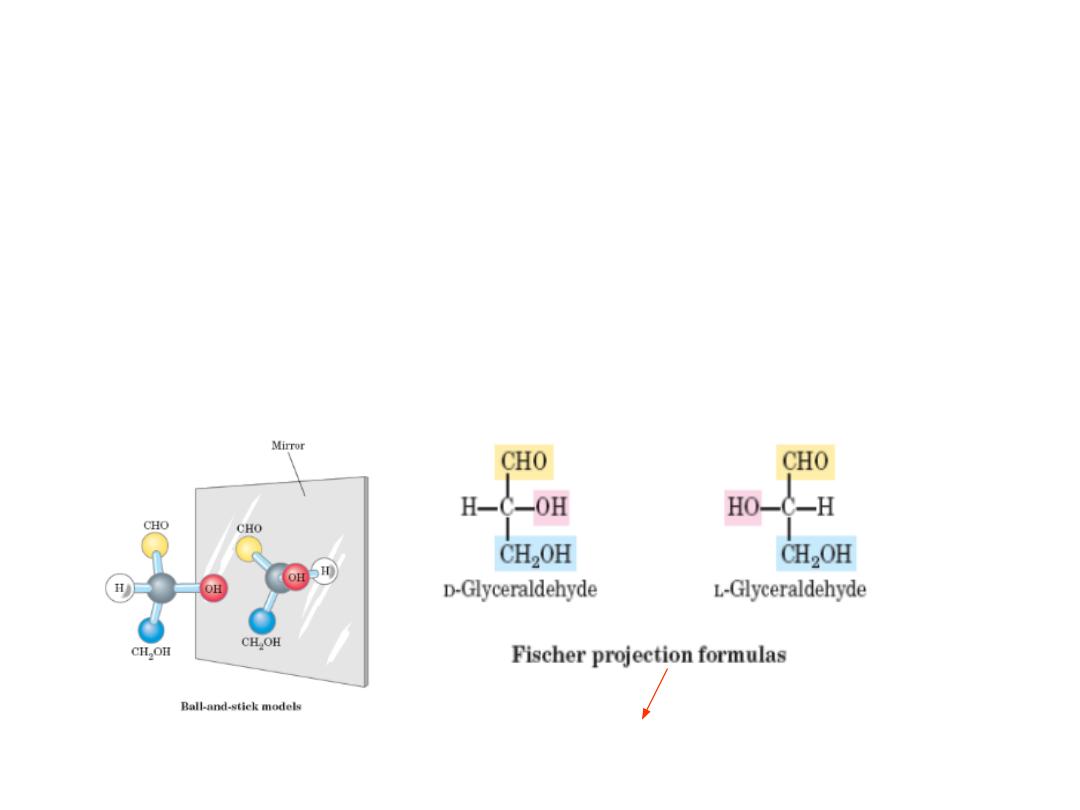
MONOSACCHARIDES STEREOISOMERS
• Isomers: same chemical formulas, different structures
• Conformation : the spatial arrangement of substituent groups
• Chiral centers: asymmetric carbons, i.e carbon atom with four different
substituents
• Enantiomers : mirror images Stereoisomers
• The simplest aldose, glyceraldehyde, contains one chiral center (the
middle carbon atom) and has two different optical isomers, or
enantiomers
the projection in which the carbohydrate backbone is
drawn vertically with the carbonyl shown on the top.
10
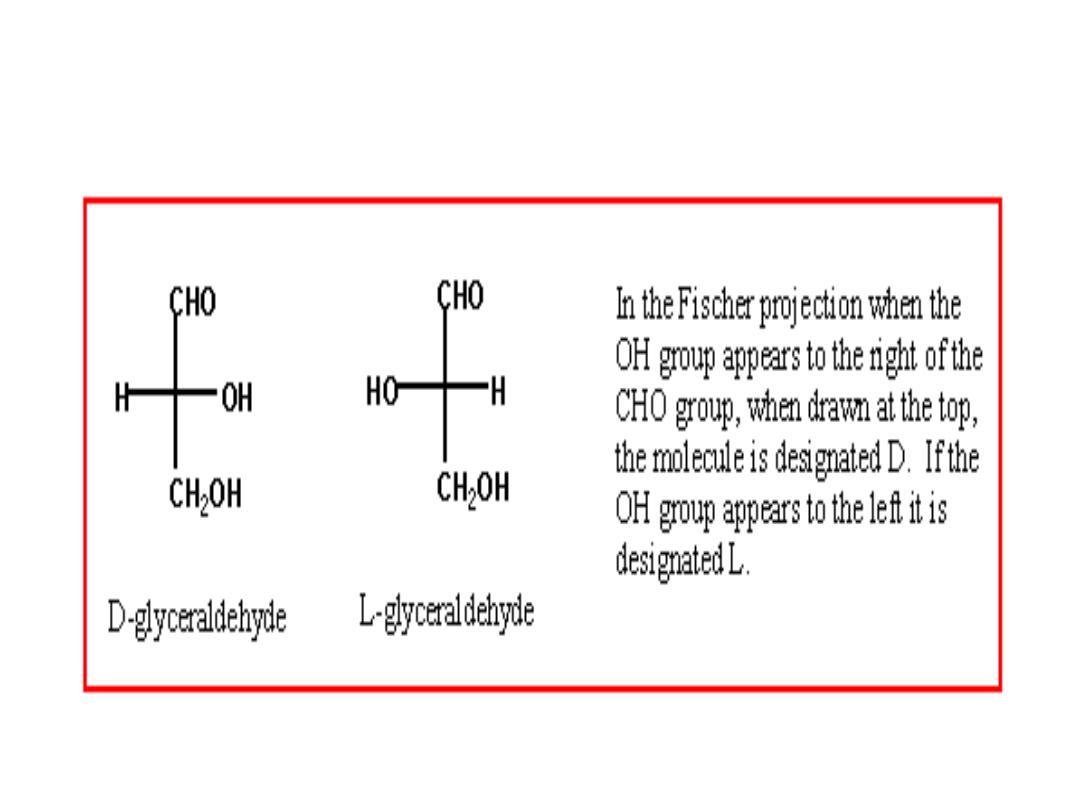
D‐ and L‐ enantiomers
11
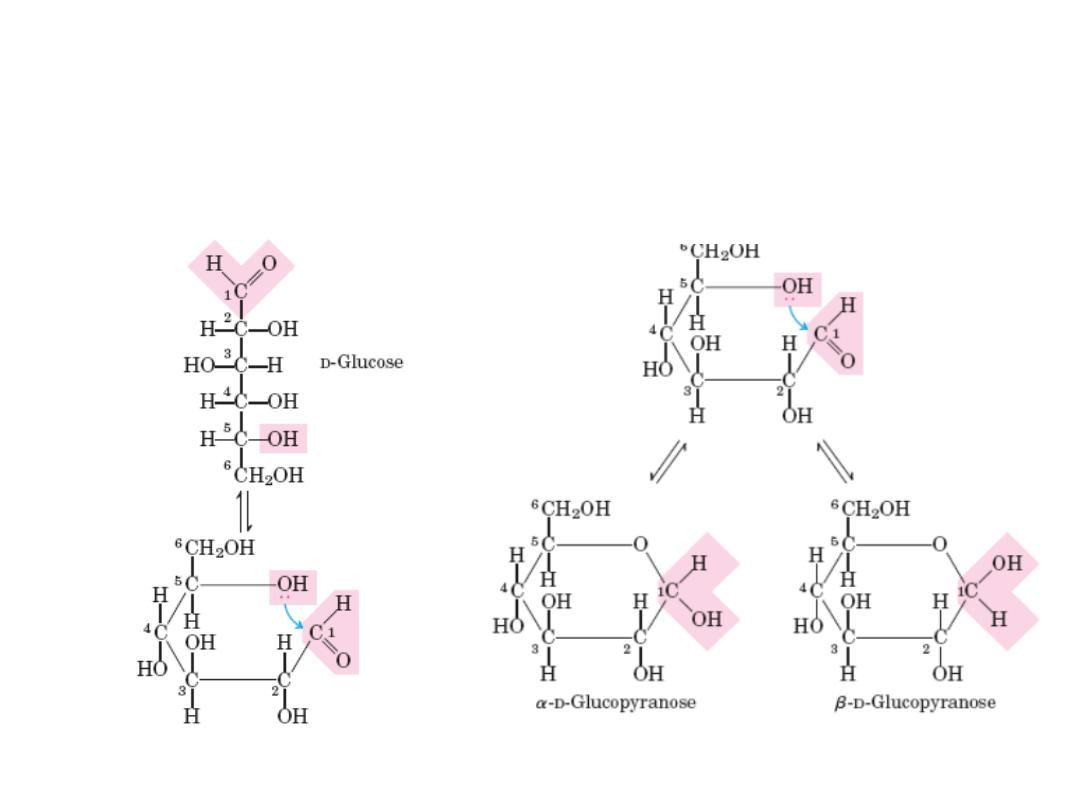
Cyclic structure of monosacharides
In aqueous solution, monosaccharides with 5 or more C atoms in the backbone occur
predominantly as cyclic (ring) structures in which the carbonyl group has formed a
covalent bond with the oxygen of a hydroxyl group along the chain.
The new chiral center in cyclic (c1) is called anomeric
carbon
12
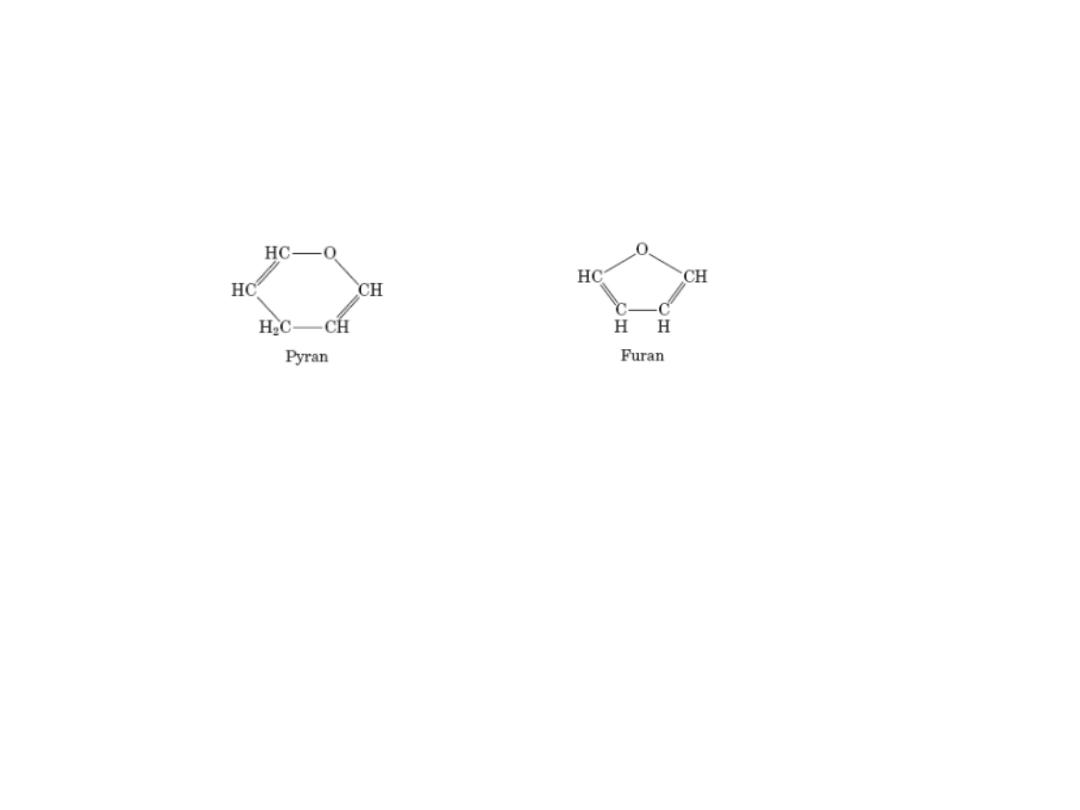
Pyranoses & Furanoses
Pyranoses: sixmembered ring compounds ( resemble pyrann)
Furanoses : fivemembered rings, (resemble furan)
The structure systematic names glucose & fructose become
13
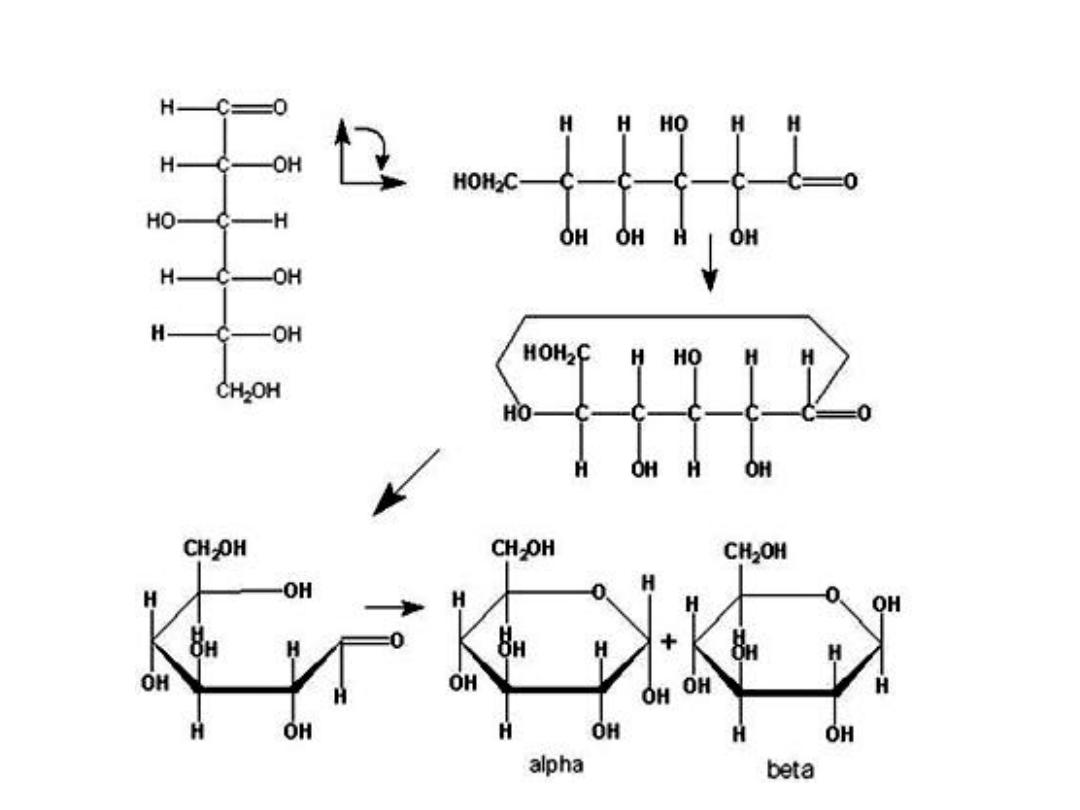
FISHER AND HAWORTH FORMS OF SUGAR
• .
14
SUMMARY OF SUGAR STRUCTURES
• ISOMERS‐ compounds that have the same chemical formula e.g. fructose,
glucose, mannose, and galactose are isomers of each other having formula
C
6
H
12
O
6
.
• EPIMERS‐ refer to sugars whose configuration differ around one specific
carbon atom e.g. glucose and galactose are C‐4 epimers and glucose and
mannose are C‐2 epimers.
• ENANTIOMERS‐ a special type of isomerism found in pairs of structures that
are mirror images of each other. The mirror images are termed as
enantiomers and the two members are designated as D‐ and L‐ sugar. The
vast majority of sugars in humans are D‐sugars.
• CYCLIZATION OF SUGARS‐ most monosaccharides with 5 or more carbon
atoms are predominately found in a ring form, where the aldehyde or
ketone group has reacted with an alcoholic group on the same sugar group
to form a hemiacetal or hemiketal ring.
Pyranose ring‐ if the ring has 5 carbons and 1 oxygen.
Furanose ring‐ if the ring is 5‐membered (4 carbons and 1 oxygen
15

IMPORTANT REACTIONS IN MONOSACCHARIDES
Monosaccharides undergo the following reactions :
1. Mutarotation
2. Oxidation
3. Reduction
4. Isomerization
5. Esterification
6. Glycoside
formation
16

IMPORTANT REACTIONS IN MONOSACCHARIDES
Details
1. Mutarotation –
alfa and beta forms of sugars are readily interconverted
when dissolved in water
2 Oxidation and reduction
in presence of oxidising agents, metal ions (Cu2+) and
enzymes, monosacchs undergo several oxidation reactions e.
g. Oxidation of aldehyde group (R‐CHO) yields aldonic acid
3 REDUCTION
reduction of the aldehyde and ketone groups of
monosacchs yield sugar alcohols (alditols) Sugar alcohols e.g.
sorbitol, are used commercially in processing foods and
pharmaceuticals. a molecule to form a cyclic ester lactone.
17
.
4 ISOMERIZATION
Monosaccharides undergo several types of isomerization e.g.
D‐glucose in alkaline solution for several hours containn D‐
mannose and D‐fructose. The conversion of glucose to
mannose is termed epimerization.
5 ESTERIFICATION
Free OH groups of carbohydrates react with acids to form
esters. This reaction will change the physical and chemical
propteries of sugar.
6 GLYCOSIDE FORMATION‐
Hemiacetals and hemiketals reaction with alcohols to form
the corressponding aceta or ketal. On the contrary when a
cyclic hemiacetal or hemiketal form of monosaccharide
reacts with alcohol, the new linkage is called glycosidic
linkage and the compound is called glycoside.
18

19
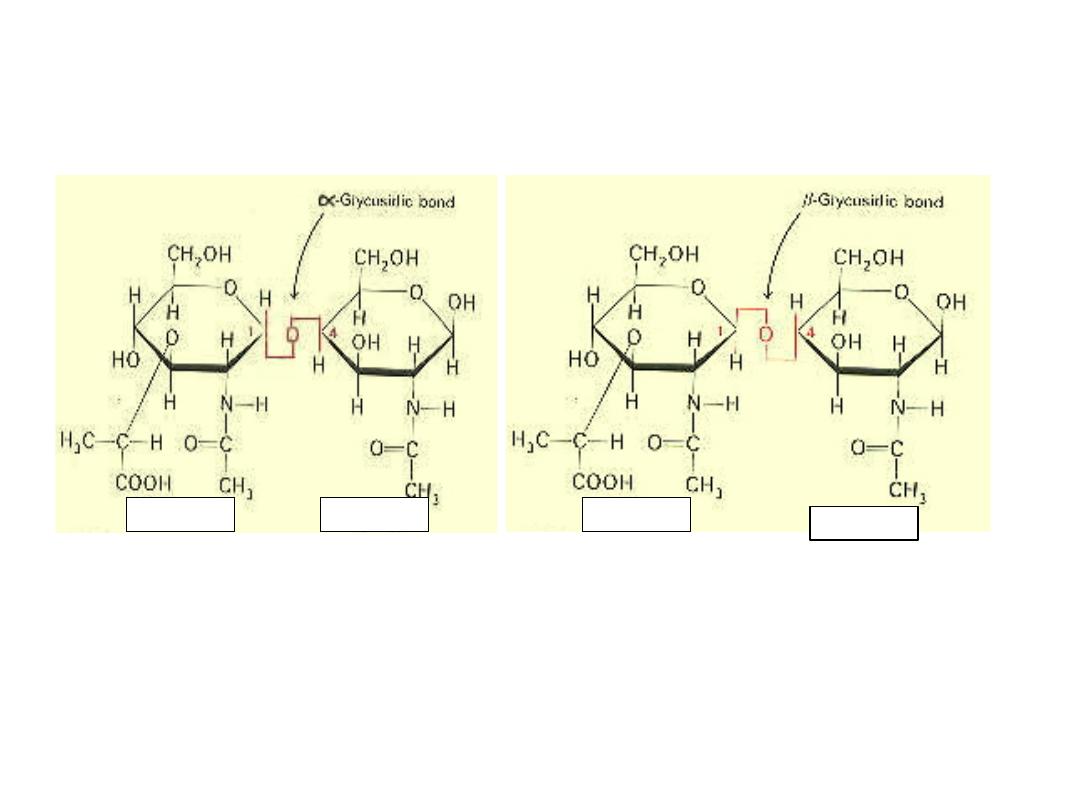
Alfa & beta GLYCOCIDIC BOND
20

REDUCING SUGARS
• All monosacchs are reducing sugars.
• They can be oxidised by weak oxidising agent
such as Benedict’s reagent
• Benedict's reagent is a solution of copper
sulfate, sodium hydroxide, and tartaric acid.
Aqueous glucose is mixed with Benedict's
reagent and heated. The reaction reduces the
blue copper (II) ion to form a brick red
precipitate of copper (I) oxide. Because of this,
glucose is classified as a reducing sugar.
21

IMPORTANT MONOSACCHARIDES
• GLUCOSE
• FRUCTOSE
• GALACTOSE
• DGlucose:
➢ Dglucose (dextrose) is the primary fuel in living cells
especially in brain cells that have few or no
mitochondria.
➢ Cells such as eyeballs have limited oxygen supply
and use large amount of glucose to generate energy
➢ Dietary sources include plant starch, and the
disaccharides lactose, maltose, and sucrose
22

DIABETES (Diabetes mellitus)
• Characterized by high blood glucose levels that leaks over
into the urine
• These high glucose levels impairs the insulin‐stimulated
glucose entry into cells and starve the cells of insulin.
• This leads to ketosis or high levels of ketone bodies (acids)
that hinders the buffering capacity of the blood in the kidney,
which controls blood pH (by excreting excess H+ ions into the
urine).
• The H+ excretion is accompanied by the excretion ammonia,
sodium,potassium, and phosphate ions causing severe
dehydration
• This leads to excessive thirst symptom of diabetes and life‐
threatening decrease in blood volume.
23

Important monosaccharides.
• FRUCTOSE
– D‐fructose (levulose) is often referred as fruit sugar and is
found in some vegetables and honey
– This molecule is an important member of ketose member
of sugars
– It is twice as sweet as sucrose (per gram basis) and is used
as sweeting agent in processed food products
– It is present in large amounts in male reproductive tract
and is synthesised in the seminal vesicles.
24
Important monosaccharides.
• GALACTOSE
– is necessary to synthesize a variety of biomolecules
(lactose‐in mammalary glands, glycolipids, certain
phospholipids, proteoglycans, and glycoproteins)
– Galactose and glucose are epimers at carbon 4 and
interconversion is catalysed by enzyme epimerase.
– Medical problems – galactosemia (genetic disorder)
where enzyme to metabolize galactose is missing;
accumulation of galactose in the body can cause liver
damage, cataracts, and severe mental retardation
25

Mono sugar derivatives
• AMINO SUGARS –
– Sugars in which a hydroxyl group (common on
carbon 2) is replaced by an amino group e.g. D‐
glucosamine and D‐galactosamine
– common constituents of complex carbohydrate
molecule found attached to cellular proteins and
lipids
– Amino acids are often acetylated e.g. N‐acetyl‐
glucosamine.
26

Mono sugar derivatives
• DEOXYSUGARS
– monosaccharides in which an ‐ H has replaced an – OH
group
– Important sugars: L‐fucose (formed from D‐mannose by
reduction reactions) and 2‐deoxy‐D‐ribose
– L‐fucose – found among carbohydrate components of
glycoproteins, such as those of the ABO blood group
determinates on the surface of red blood cells
– 2‐deoxyribose is the pentose sugar component of DNA.
27

GLYCOSIDES
• Monosaccharides can be linked by glycosidic bonds (joining
of 2 hydroxyl groups of sugars by splitting out water
molecule) to create larger structures.
• Disaccharides contain 2 monosaccharides e.g. lactose
(galactose+glucose); maltose (glucose+glucose);
sucrose (glucose+fructose)
• Oligosaccharides – 3 to 12 monosaccharides units e.g.
glycoproteins
• Polysaccharides – more than 12 monosaccharides units e.g.
glycogen (homopolysaccharide) having hundreds of sugar
units; glycosaminoglycans (heteropolysaccharides) containing
a number of different monosaccharides species
.
28

Section 3
DISACCHARIDES
AND
OLIGOSACCHARIDES
29

DISACCHARIDES AND OLIGOSACCHARIDES
• Cnfigurations: alfa or beta ( 1,4, glycosidic
bonds or linkages; other linkages 1,1; 1,2; 1,3;
1,6)
• Digestion aided by enzymes. Defficiency of
any one enzyme causes unpleasant
symptoms (fermentation) in colon produces
gas [bloating of cramps].
• Most common defficiency, an ancestoral
disorder, lactose intolerance caused by
reduced synthesis of lactase
30
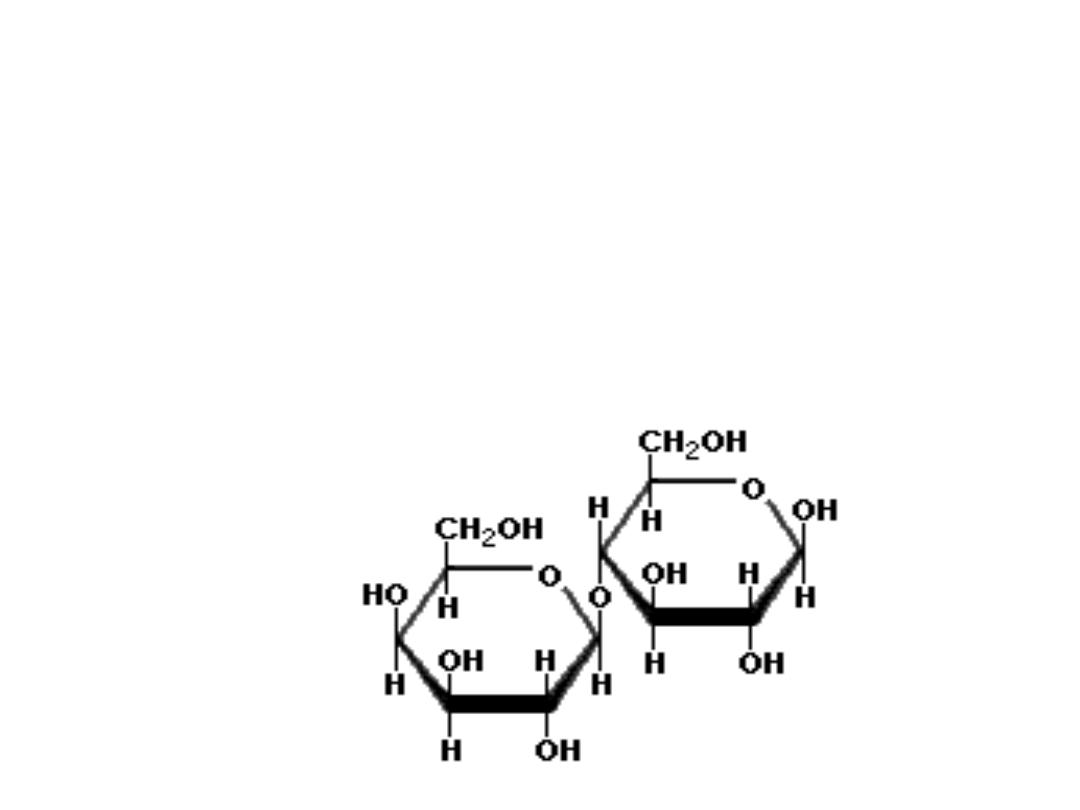
Important sugars of Disaccharides
• LACTOSE
(milk sugar) disaccharide found in milk; composed of one
molecule of galactose and glucose linked through beta(1,4)
glycosidic linkage; because of the hemiacetal group of the
glucose component, lactose is a reducing sugar
31

Lactose intolerance
• Lactose (milk sugar) in infants is hydrolyzed by
intestinal enzyme lactase to its component
monosacch for absorption into the bloodstream
(galactose epimerized to glucose).
• Most adult mammals have low levels of beta‐
galactosidase. Hence, much of the lactose they
ingest moves to the colon, where bacterial
fermentation generates large quantities of CO2, H2
and irritating organic acids.
• These products cause painful digestive upset known
as lactose intolerance and is common in the African
and Asian decent.
32

❑ MALTOSE ( malt sugar)
An intermediate product of starch hydrolysis; it is a disaccharide with an
alfa(1,4) glycosidic linkage between two Dglucose molecules; in solution the
free anomeric carbon undergoes mutarotation resulting in an equilibrium
mixture of alfa and beta – maltoses; it does not occur freely in nature
33
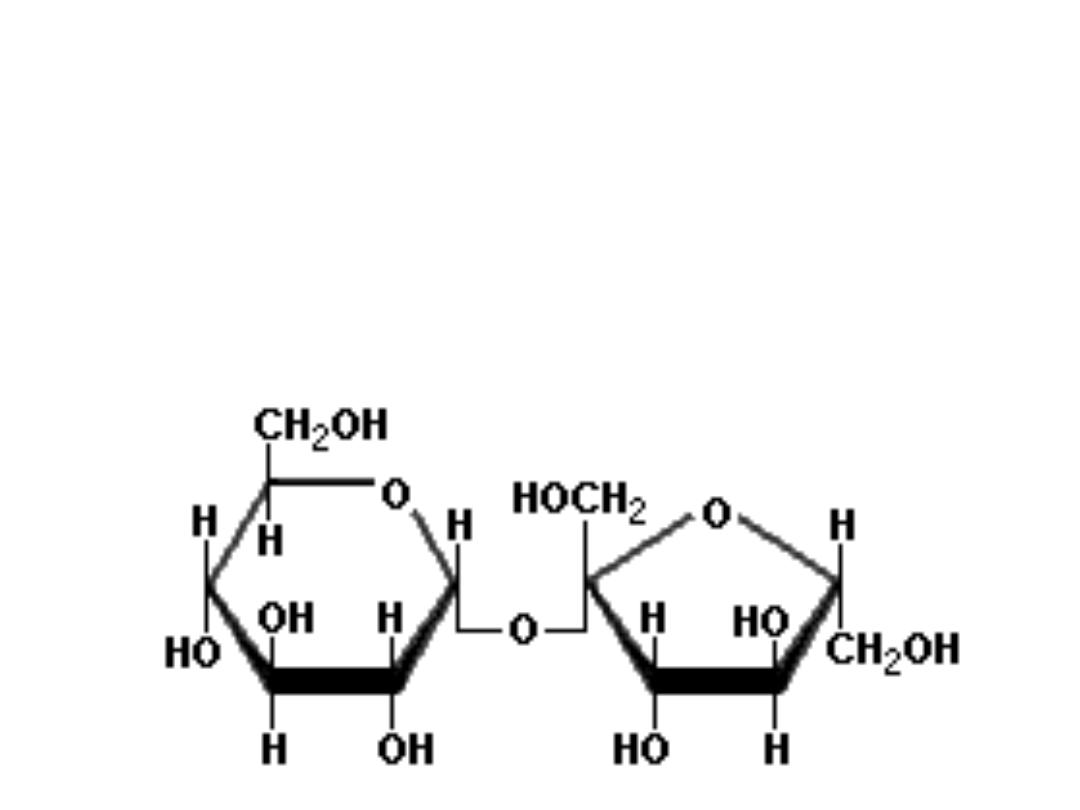
• SUCROSE
common table sugar: cane sugar or beet sugar produced in
the leaves and stems of plants; it is a disaccharide containing
both alfa‐glucose and beta‐fructose residues linked by alfa,
beta(1,2)glycosidic bond.
34

• CELLOBIOSE
degradation product of cellulose containing
two molecules of glucose linked by a beta
(1,4) glycosidic bond; it does not occur freely
in nature
35
OLIGOSACCHARIDE SUGARS
• Oligosaccharides are small polymers often
found attached to polypeptides in
glycoproteins and some glycolipids.
• They are attached to membrane and
secretory proteins found in endoplasmic
reticulum and Golgi complex of various cells
• Two classes: N‐linked and O‐linked
36

Section 4
POLYSACCHARIDES
4.1. Intro to Polysaccharides
4.2. Classification of
Polisacharides
4.2.1. Homosacharides
4.2.2.
Heteropolysacharides
37

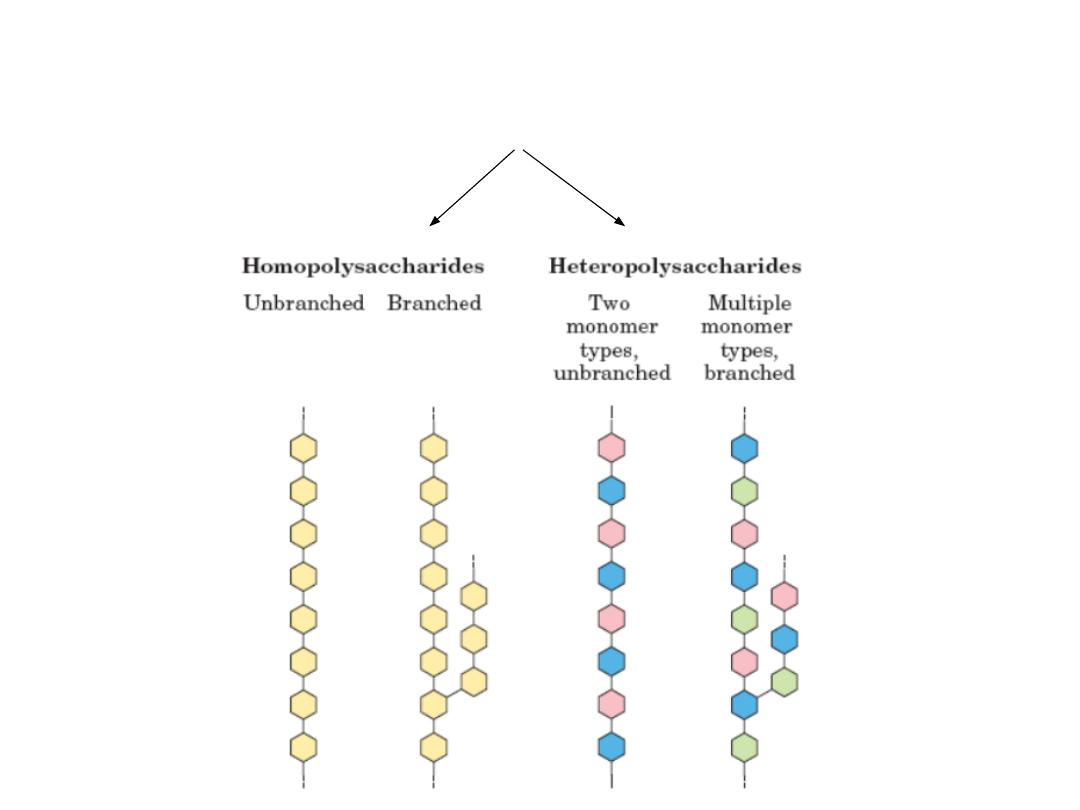
4.1. Intro to Polysaccharides
• Composed of large number of monosaccharide units
connected by glycosidic linkages
• Classified on the basis of their main monosaccharide
components and the sequences and linkages between them,
as well as the anomeric configuration of linkages, the ring
size (furanose or pyranose), the absolute configuration (D‐ or
L‐) and any other substituents present.
(http://www.lsbu.ac.uk/water/
hypol.html)
• Polysaccharides are more hydrophobic if they have a greater
number of internal hydrogen bonds, and as their
hydrophobicity increases there is less direct interaction with
water
• Divided into homopolysaccharides (e.g.Starch, glycogen,
cellulose, and chitin) & heteropolysaccharides
(glycoaminoglycans or GAGs, murein).
38
4.2. Classification of Polisacharides
39

4.2.1.HOMOSACCHARIDES
• Found in abundance in nature
• Important examples: starch, glycogen, cellulose, and
chitin
• Starch, glycogen, and cellulose all yield D‐glucose
when they are hydrolyzed
• Cellulose ‐ primary component of plant cells
• Chitin – principal structural component of
exoskeletons of arthropods and cell walls of many
fungi; yield glucose derivative N‐acetyl glucosamine
when it is hydrolyzed.
40

STARCH (Homosaccharide)
• A naturally abundant nutrient carbohydrate, (C
6
H
10
O
5
)n, found chiefly in
the seeds, fruits, tubers, roots, and stem pith of plants, notably in corn,
potatoes, wheat, and rice, and varying widely in appearance according to
source but commonly prepared as a white amorphous tasteless powder.
• Any of various substances, such as natural starch, used to stiffen cloth, as
in laundering.
• Two polysaccharides occur together in starch: amylose and amylopectin
• Amylose – unbranched chains of D‐glucose residues linked with
alfa(1,4,)glycosidic bonds
• Amylopectin – a branched polymer containing both alfa(1,4,) and
alfa(1,6) glcosidic linkages; the alfa(1,6) branch points may occur every
20‐25 glucose residues to prevent helix formation
• Starch digestion begins in the mouth; alfa‐amylase in the saliva initiates
hydrolysis of the gycosidic linkages
41

GLYCOGEN (Homosaccharide)
• Glycogen is the storage form of glucose in animals and humans which is
analogous to the starch in plants.
• Glycogen is synthesized and stored mainly in the liver and the muscles.
• Structurally, glycogen is very similar to amylopectin with alpha acetal
linkages, however, it has even more branching and more glucose units
are present than in amylopectin.
• Various samples of glycogen have been measured at 1,700‐600,000 units
of glucose.
• The structure of glycogen consists of long polymer chains of glucose units
connected by an alpha acetal linkage.
• The branches are formed by linking C # 1 to a C # 6 through an acetal
linkages.
• In glycogen, the branches occur at intervals of 8‐10 glucose units, while in
amylopectin the branches are separated by 12‐20 glucose units.
42
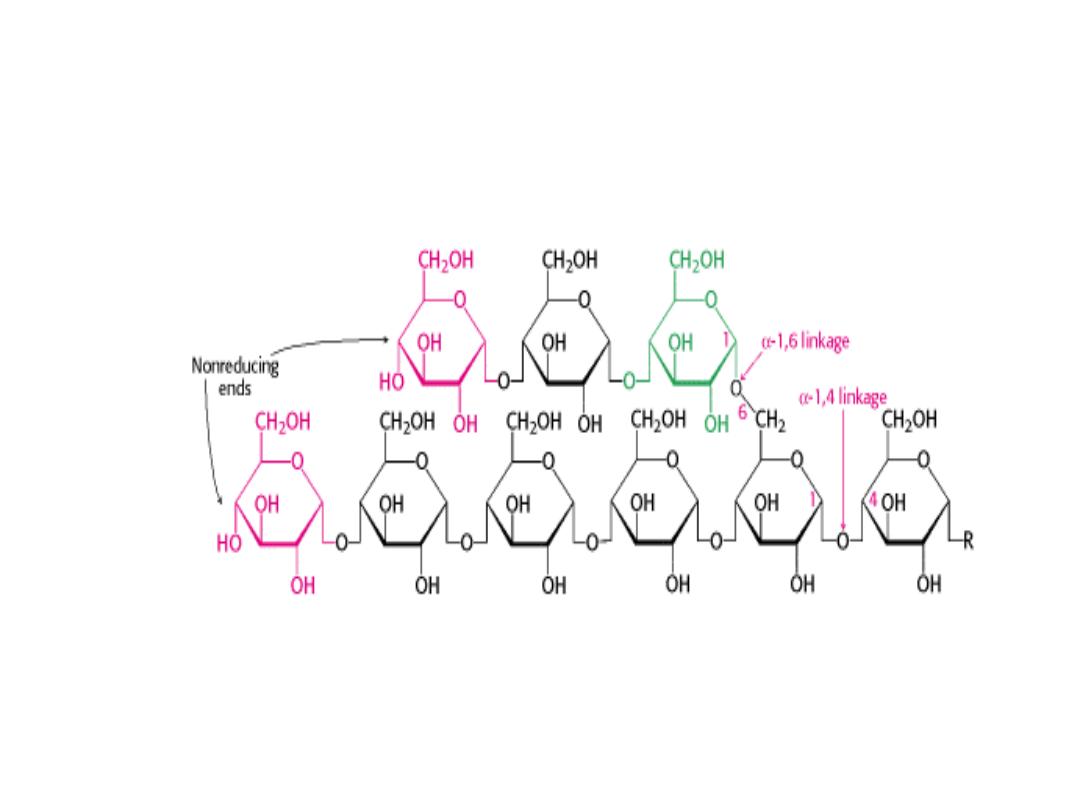
STRUCTURE OF GLYCOGEN
43

CELLULOSE (Homosaccharide)
• Cellulose is found in plants as microfibrils (2‐20 nm diameter and 100 ‐ 40
000 nm long). These form the structurally strong framework in the cell
walls.
• Cellulose is mostly prepared from wood pulp
• Cellulose is a linear polymer of β-(1 4)‐D‐glucopyranose units in
4
C
1
conformation. The fully equatorial conformation of β-linked
glucopyranose residues stabilizes the chair structure, minimizing its
flexibility
• Cellulose has many uses as an anticake agent, emulsifier, stabilizer,
dispersing agent, thickener, and gelling agent but these are generally
subsidiary to its most important use of holding on to water.
• Water cannot penetrate crystalline cellulose but dry amorphous cellulose
absorbs water becoming soft and flexible.
• Purified cellulose is used as the base material for a number of water‐
soluble derivatives e.g. Methyl cellulose, carbomethycellulose
44
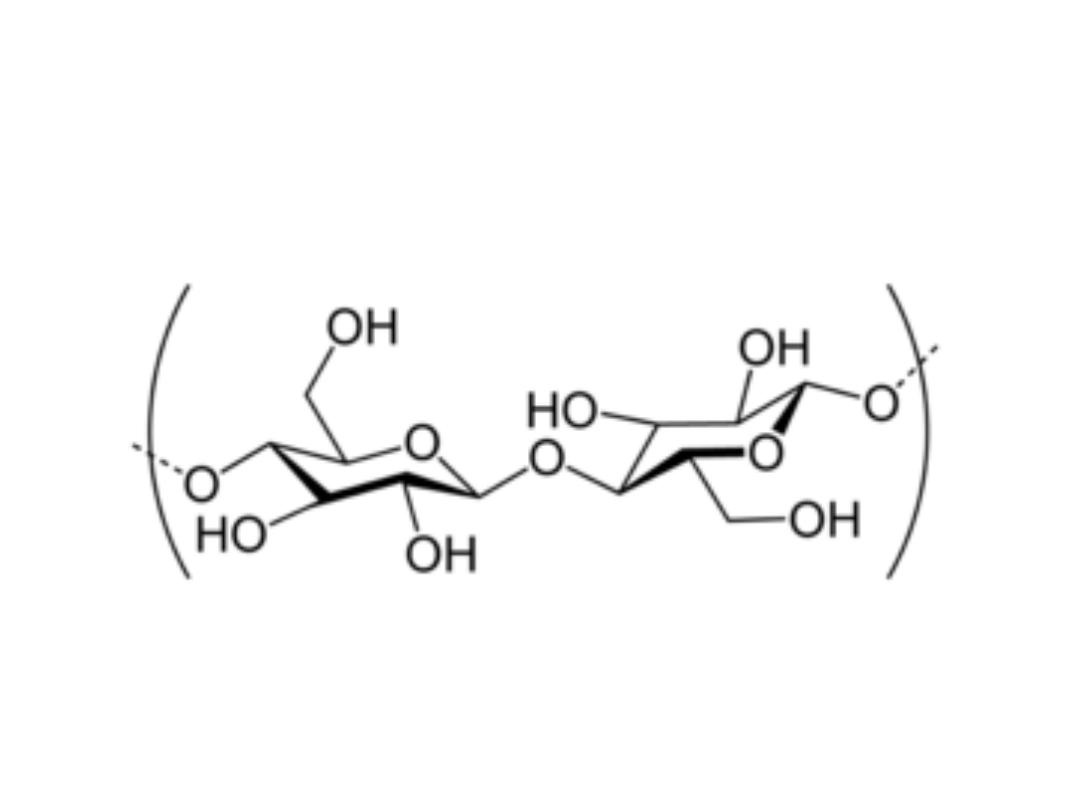
Cellulose as polymer of β-D‐glucose
45
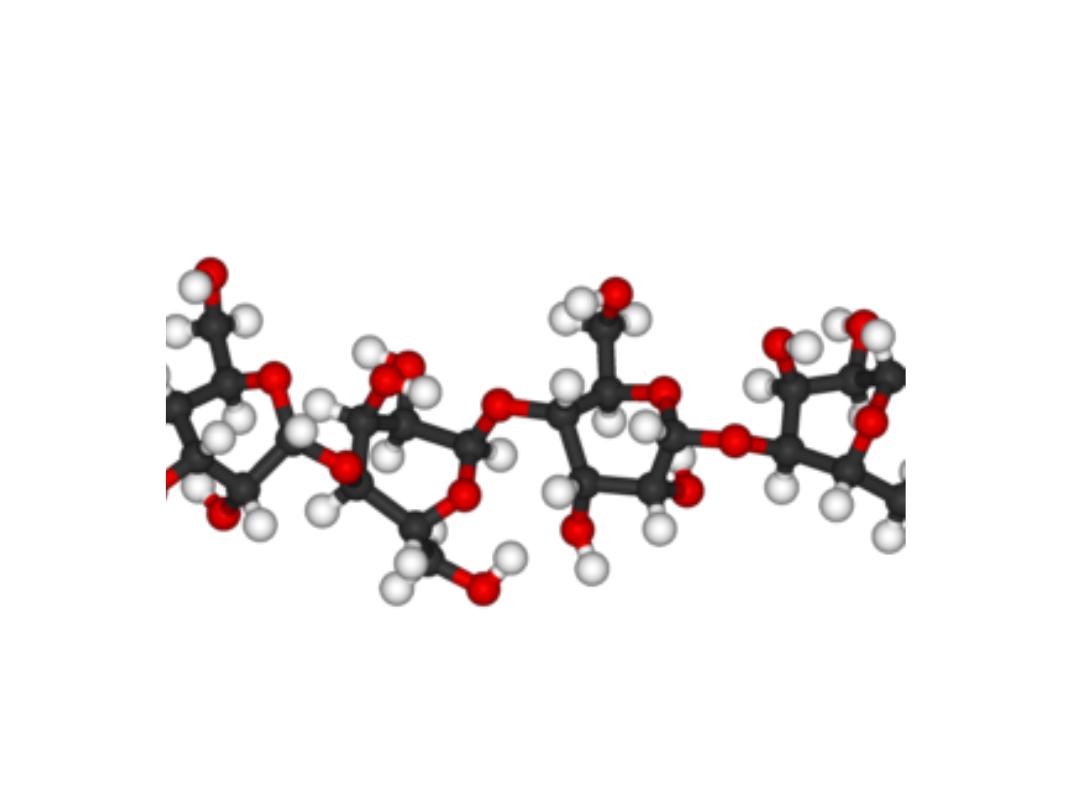
Cellulose in 3D
46
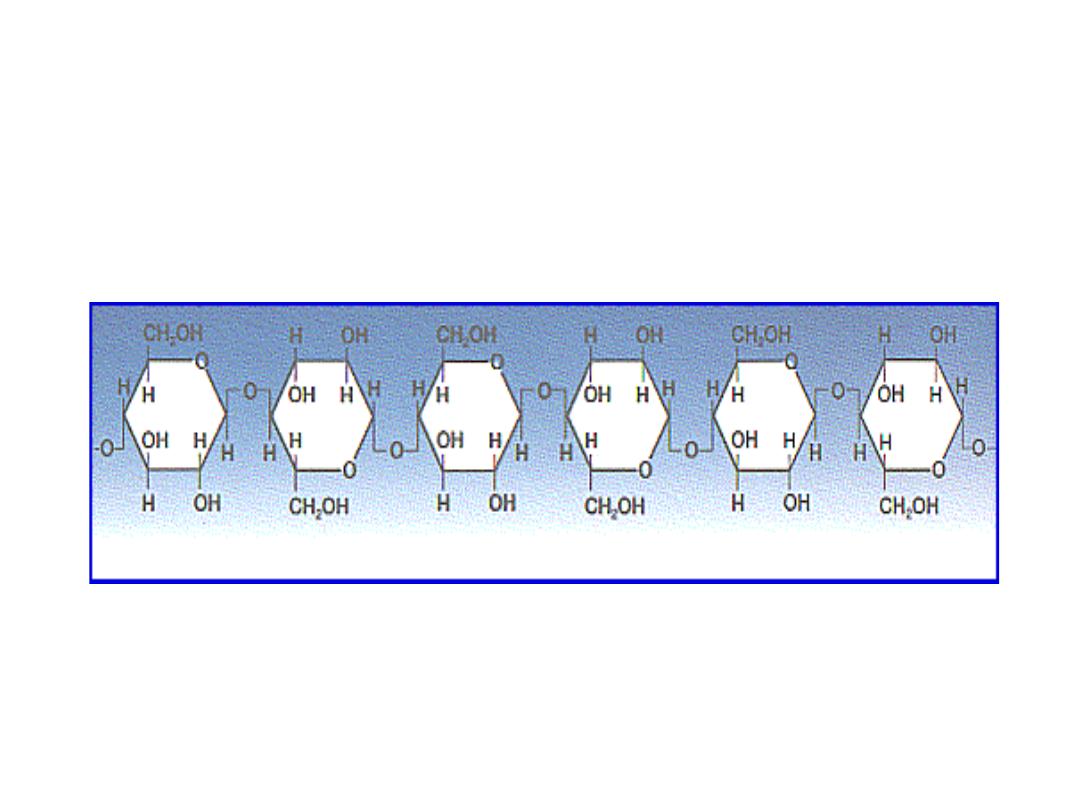
CELLULOSE
47
CHITIN (Homosaccharide)
• Chitin is a polymer that can be found in anything from the
shells of beetlesto webs of spiders. It is present all around us,
in plant and animal creatures.
• It is sometimes considered to be a spinoff of cellulose,
because the two are very molecularly similar.
• Cellulose contains a hydroxy group, and chitin contains
acetamide.
• Chitin is unusual because it is a "natural polymer," or a
combination of elements that exists naturally on earth.
• Usually, polymers are man‐made. Crabs, beetles, worms and
mushrooms contain large amount of chitin.
• Chitin is a very firm material, and it help protect an insect
against harm and pressure
48
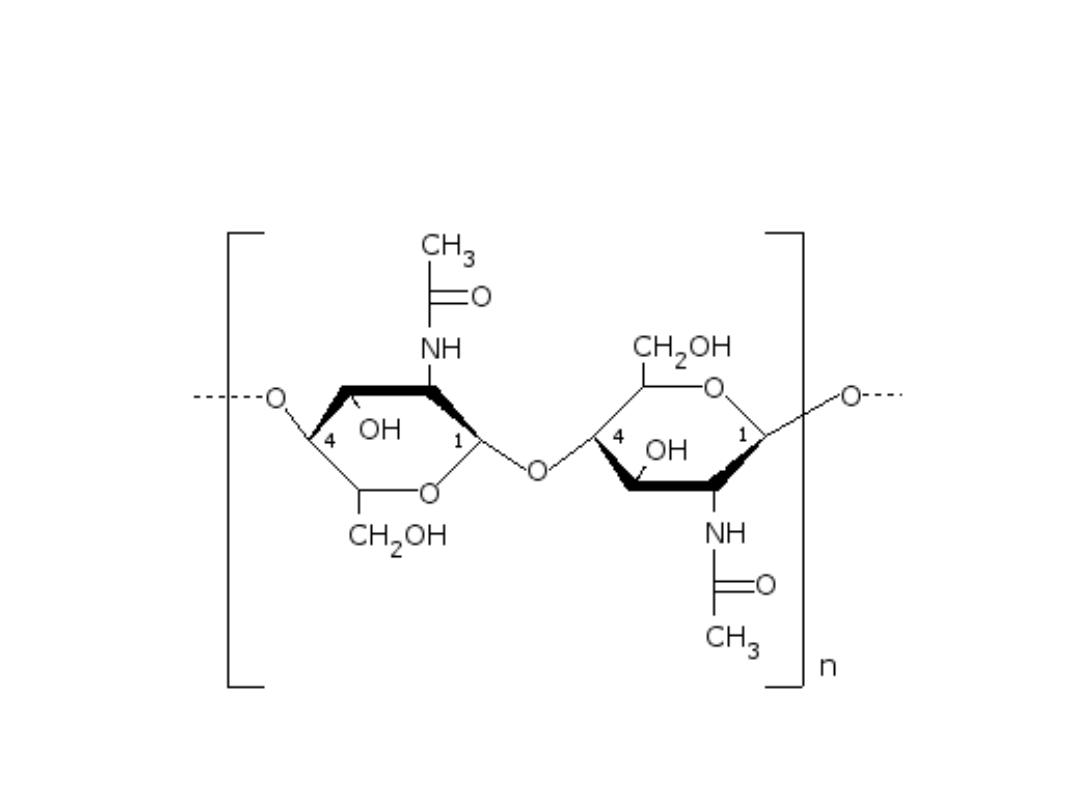
Structure of the chitin molecule, showing two of the N‐
acetylglucosamine units that repeat to form long chains in beta‐1,4
linkage.
49

4.2.2.HETEROPOLYSACCHARIDES
• Are high‐molecular‐weight carbohydrate
polymers more than one kind of
monosaccharide
• Important examples include
glycosaminoglycans (GAGs) – the principle
components of proteoglycans and murein, a
major component of bacterial cell walls.
50

SECTION 5
GLYCOCONJUGATES
5.1. Intro to glycoconjugates
5.2. glycoproteins
5.3. glycosylation
5.4. proteoglycans
51

5.1. Intro to glycoconjugates
• They are compounds that result from covalent
linkages of carbohydrate molecules to both proteins
and lipids.
• They have a profound effects on the functions of
individual cells as weell as cell‐cell interactions of
multicellular organisms.
• Two classes of carbohydrate‐protein conjugate:
glycoproteins and proteoglycans.
• The glycolipids (oligosaccharide‐containing lipid
molecules) are found predominately on the outer
surface of plasma membrane.
52

5.2.glycoproteins
• Glycoprotein carbohydrate chains are highly diverse. They
are formed by glycosylation and classified into two groups:
1. N‐linked oligosaccharides
2. O‐linked oligosaccharides
• The N‐linked oligosaccharides have a minimum of 5 sugar
residues
• N‐linked attached to polypeptides by an N‐glycosidic bond
with a chain amide group of amino acid and asparagine
• O‐linked oligosaccharides are generally short (1‐4 sugar
residues)
• O‐linked are attached to polypeptides by the side chain
hydroxyl group of amino acids serine or threonine in
polypeptide chains or hydroxyl groups of membrane lipids
53
5.3.glycosylation
• Glycosylation is the process or result of addition of
saccharides to proteins and lipids
• The process plays an important role in the synthesis of
membrane and secreted proteins
• Majority of proteins synthesized in the rough ER undergo
glycosylation
• It is an enzyme ‐directed site‐specific process.
• Two types of glycosylation exist: N‐linked glycosylation to the
amide nitrogen of asparagine side chains and O‐linked
glycosylation to the hydroxy oxygen of serine and threonine
side chains.
• Glycosylation may play a role in cell‐cell adhesion (a
mechanism employed by cells of the immune system), as
well.
54
GLYCOPROTEIN FUNCTIONS
• Types of glycoproteins: asparagine‐linked carbohydrate;
mucin‐type cabohydrate
• Examples: glycophorin (membrane protein, source – human
RBC, % carbohydrate ‐ 60); potato lectin (lectin, carbohydrate
binding proteins, source – potato, % carbohydrate – 50)
• Functions: Many glycoproteins have structural functions:
constituent of the cell wall; form connective tissues such as
collagen; found in gastrointestinal mucus secretions; used as
protective agents and lubricants ;found abundantly in the
blood plasma.
• The human blood groups A, B, AB, and O depend on the
oligosaccharide part of the glycoprotein on the surface of
erythrocyte cells. The terminal monosaccharide of the
glycoprotein at the nonreducing end determines blood group.
55

BLOOD GROUPS
TYPE
TERMINAL SUGAR
A
N‐acetylgalactosamine
B
α‐D‐galactose
AB
both the above
O
neither of the above
O
is the “universal donor”
AB
is the “universal
acceptor”
56

Oligosaccharides are antigeneic
determinants
• Carbohydrates on cell surfaces are immunochemical
markers.
• ABO blood group antigens are oligosaccharide
components of glycoproteins and glycolipids on the
surfaces of individual cells, besides blood cells.
• Individuals with type A cells have A antigens on
their cell surfaces and carry anti‐B antibodies
• Those with type B cells which bear B antigens, carry
anti‐A antibodies
57

• Those with type AB cells, which have both A and B
antigens, carry neither anti‐A nor anti‐B antibodies
• Type O individuals whose cells bear neither antigen,
carry both anti‐A and anti‐B antibodies.
• Transfusion of type A blood group into a type B
individual, results in an anti‐A antibody‐ A antigen
reaction.
• This reaction clumps together (agglutinates) the
transfused erythrocytes, resulting in an often fatal
blockage of blood vessels
58

Functions of glycoproteins (elaborated)
http://www.cs.stedwards.edu/chem/Chemistry/CHEM43/CHEM43/Glycoproteins/Glycoproteins.HTML
Carbohydrates and proteins by themselves serve in a vast
number of biological functions,linking the two together
results in a macromolecule with an extremely large number
of functions.
• Structural: Glycoproteins are found throughout matrices.
They act as receptors on cell surfaces that bring other cells
and proteins (collagen) together giving strength and support
to a matrix.
• In nerve tissue glycoproteins are abundant in gray matter
and appear to be associated with synaptosomes, axons, and
microsomes.
• Protection: Human lacrimal glands produce a glycoprotein
which protects the corneal epithelium from desiccation and
foreign particles. Human sweat glands secrete glycoproteins
which protect the skin from the other excretory products
that could harm the skin
59

Functions of glycoproteins (further elaborated‐1)
• Prothrombin, thrombin, and fibrinogen are all glycoproteins
that play an intricate role in the blood clotting mechanism
• In certain bacteria the slime layer that surrounds the
outermost components of cell walls are made up of
glycoproteins of high molecular weight. In addition to
forming these s‐layers, glycoproteins also function as
bacterial flagella. These are made up of bundles of
glycoproteins protruding from the cell's surface. Their
rotation provides propulsion.
• In plants, glycoproteins have roles in cell wall formation,
tissue differentiation, & embryogenesis.
• Reproduction: Glycoproteins found on the surface of
spermatozoa appear to increase a sperm cell's attraction for
the egg by altering the electrophoretic mobility of the plasma
membrane.
60

Functions of glycoproteins (further elaborated‐2)
• Adhesion: Glycoproteins serve to adhere cells to
cells and cells to substratum.
• Hormones: There are many glycoproteins that
function as hormones such as human chorionic
gonadotropin (HCG) which is present in human
pregnancy urine. Another example is erythropoietin
which regulates erythrocyte production
• Enzymes: Glycoprotein enzymes are of three types.
These are oxidoreductases, transferases, and
hydrolases.
61

Functions of glycoproteins (further elaborated‐3)
• Carriers: Glycoproteins can bind to certain molecules and
serve as vehicles of transport. They can bind to vitamins,
hormones, cations, and other substances.
• Inhibitors: Many glycoproteins in blood plasma have shown
antiproteolytic activity. For example, the glycoprotein a1‐
antichymotrypsin inhibits chymotrypsin.
• Immunological: The interaction of blood group substances
with antibodies is determined by the glycoproteins on
erythrocytes. Many immunoglobulins are actually
glycoproteins.
B and T cells contain surface glycoproteins that attract
bacteria to these sites and bind them. In much the same
manner, glycoproteins can direct phagocytosis. Because the
HIV virus recognizes the receptor protein CD4, it binds to
helper T cells which contain it.
62

CELL MEMBRANE
• The cell membrane is a fluid mosaic of lipids, proteins, and
carbohydrates.
• Membrane carbohydrates are usually branched
oligosaccharides with fewer than 15 sugar units.
• Some of these oligosaccharides are covalently bonded to
lipids, forming molecules called glycolipids.
• Most are covalently bonded to proteins, which are thereby
glycoproteins.
• Plants produce pectin, major component of cell wall.
• The oligosaccharides on the external side of the plasma
membrane vary from species to species
• The diversity of the molecules and their location on the cell's
surface enable oligosaccharides to function as markers that
distinguish one cell from another.
63
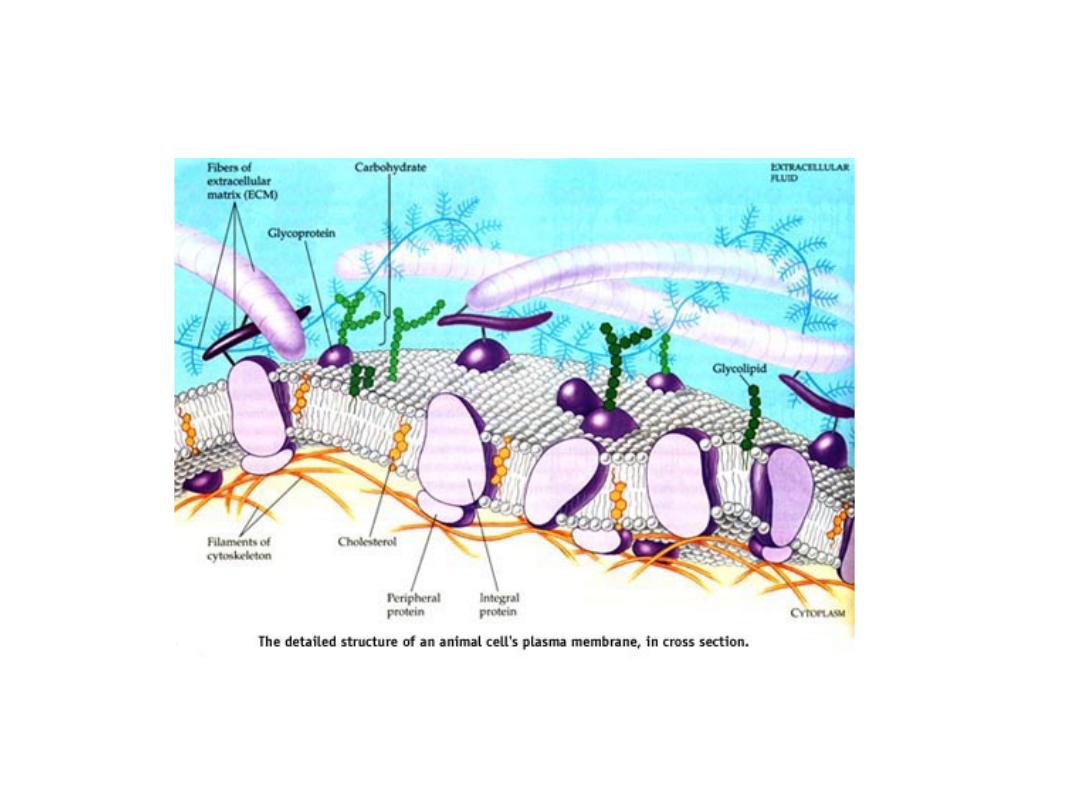
THE MOSAIC OF CELL’S MEMBRANE
64

CELL MEMBRANE MATRIX
• The biological membrane is a collage of many
different proteins embedded in the fluid matrix of
the lipid bilayer.
• The lipid bilayer is the main fabric of the membrane,
and its structure creates a semi‐permeable
membrane.
• The hydrophobic core impedes the transport of
hydrophilic structures, such as ions and polar
molecules but enable hydrophobic molecules, which
can dissolve in the membrane, cross it with ease.
65

Protein layer of cell membrane
• Proteins determine most of the membrane's
specific functions.
• The plasma membrane and the membranes
of the various organelles each have unique
collections of proteins.
• For example, to date more than 50 kinds of
proteins have been found in the plasma
membrane of red blood cells.
66

5.4. PROTEOGLYCANS
• Proteoglycans represent a special class of glycoproteins that
are heavily glycosylated.
• They consist of a core protein with one or more covalently
attached glycosaminoglycan chain(s).
• These glycosaminoglycan (GAG) chains are long, linear
carbohydrate polymers that are negatively charged under
physiological conditions, due to the occurrence of sulphate
and uronic acid groups.
• Proteoglycans can be categorised depending upon the
nature of their glycosaminoglycan chains. These chains may
be:
1. chondroitin sulfate and dematan sulfate
2. heparin and heparin sulfate
3. keratan sulfate
67
• Proteoglycans can also be categorised by size.
• Example of large proteoglycan is aggrecan.
• Aggrecan, is the major proteoglycan in
cartilage, present in many adult tissues
including blood vessels and skin.
• The small leucine rich repeat proteoglycans
(SLRPs) include decorin, biglycan,
fibromodulin and lumican.
68
SYNTHESIS OF PROTEOGLYCANS
• The protein component of proteoglycans is
synthesized by
ribosomes
The protein component of
proteoglycans is synthesized by ribosomes and
translocated
The protein component of
proteoglycans is synthesized by ribosomes and
translocated into the lumen of the
rough
endoplasmic reticulum
.
• Glycosylation of the proteoglycan occurs in the
Golgi
apparatus
Glycosylation of the proteoglycan occurs
in the Golgi apparatus in multiple
enzymatic
steps.
• First a special link tetrasaccharide is attached to a
serine
side chain on the core protein to serve as a
primer for polysaccharide growth.
• Then sugars are added one at the time by glycosyl
transferase.
• The completed proteoglycan is then exported in
secretory
vesicles
to the extracellular matrix of the
69
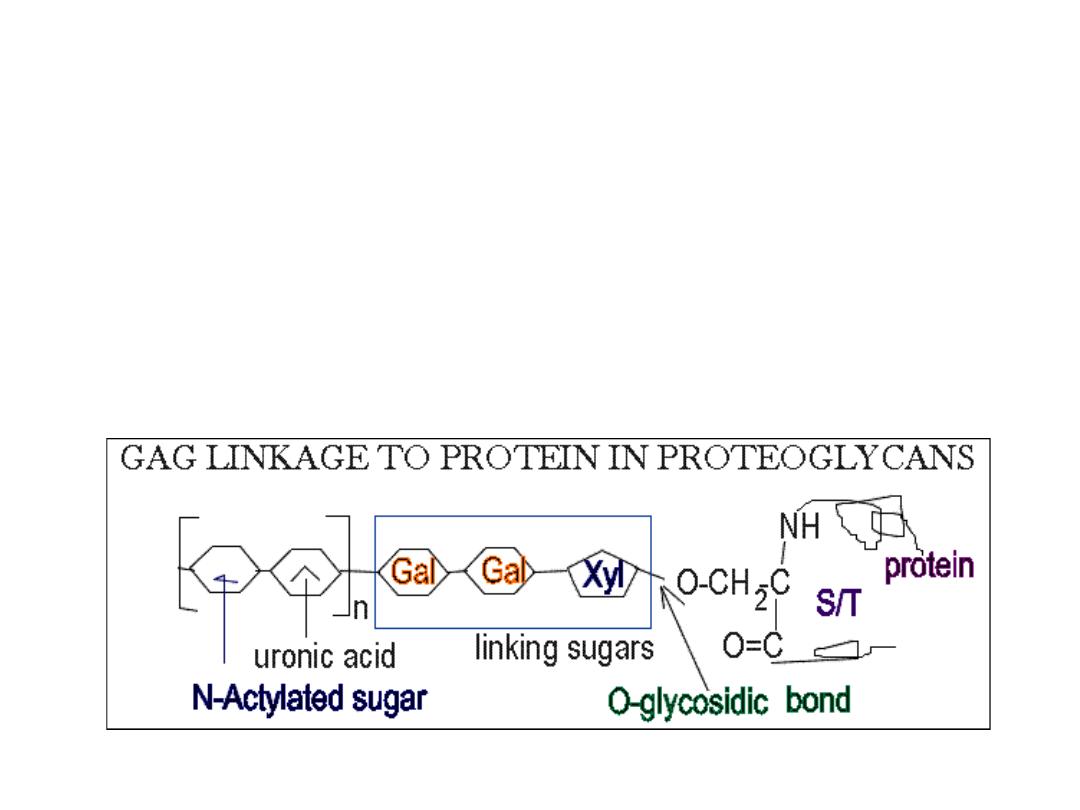
Structure of proteoglycans
• The GAGs extend perpendicular from the core protein in a bottlebrush‐
like structure.
• The linkage of GAGs such as (heparan sulfates and chondroitin sulfates) to
the protein core involves a specific trisaccharide linker
• Some forms of keratan sulfates are linked to the protein core through an
N‐asparaginyl bond.
• The protein cores of proteoglycans are rich in Ser and Thr residues which
allows multiple GAG attachment.
70
FUNCTION OF PROTEOGLYCANS
• Proteoglycans are a major component of the animal
extracellular matrix
Proteoglycans are a major component of
the animal extracellular matrix, the 'filler' substance existing
between
cells
in an organism.
• Individual functions of proteoglycans can be attributed to
either the protein core or the attached GAG chain.
• In extracellular matrix, they form large complexes, with other
proteoglycans, to
hyaluronan
In extracellular matrix, they
form large complexes, with other proteoglycans, to
hyaluronan and to fibrous matrix proteins (such as
collagen
).
• They are also involved in binding
cations
They are also
involved in binding cations (such as
sodium
They are also
involved in binding cations (such as sodium,
potassium
They
are also involved in binding cations (such as sodium,
potassium and
calcium
They are also involved in binding
cations (such as sodium, potassium and calcium) and
water
,
and also regulating the movement of molecules through the
matrix.
• They can affect the activity and stability of proteins and
71

ROLE OF PROTEOGLYCANS
http://www.cryst.bbk.ac.uk/pps97/assignments/projects/emilia/Proteoglycans.HTM
• GAG dependent functions can be divided into two classes:
the biophysical and the biochemical.
• The biophysical functions depend on the unique properties
of GAGs : the ability to fill the space, bind and organize water
molecules and repel negatively charged molecules. Because
of high viscosity and low compressibility they are ideal for a
lubricating fluid in the joints. On the other hand their rigidity
provides structural integrity to the cells and allows the cell
migration due to providing the passageways between cells.
For example the large quantities of chondroitin sulfate and
keratan sulfate found on aggrecan play an important role in
the hydration of cartilage. They give the cartilage its gel‐like
properties and resistance to deformation.
72

Role of proteoglycans contd.
• The other, more biochemical functions of GAGs are
mediated by specific binding of GAGs to other
macromolecules, mostly proteins. Proteoglycans
participate in cell and tissue development and
physiology.
• Hurler’s syndrome: (refer Text) disease related with
proteoglycan metabolism where excessive
accumulation of dermatin sulfate due to deficiency
of a specific enzyme may cause mental retardation,
skeletal deformity ansd death in early childhood.
73

SUMMARY
• Monosaccharides, the simplest carbohydrates, are classified
as aldoses or ketoses.
• The cyclic hemiacetal and hemiketal forms of monosacchs
have either alfa or beta configuration at their anomeric
carbon.
• Monosacch derivatives include aldonic acids, uronic acids,
deoxy sugars, amino sugars, alfa & beta glycosides.
• Disaccharides simplest polysaccharides occuring as hydrolysis
products of larger molecules e.g. Lactose,sucrose
• Oligosaccharides play important roles in determining protein
structure and in cell‐surface recognition phenomena.
Oligosacchs with 3 or more sugar residues are mostly found
in plants.
74
Summary contd. ‐1
• POLOYSACCHARIDES consist of monosacchs linked
by glycosidic bonds.
• Cellulose and chitin are structural polysacchs with
beta(1‐4) linkages that adopt rigid and extended
structures.
• The storage polysacchs starch and glycogen consist
of alfa‐glycosidically linked glucose residues
• Glycosaminoglycans are unbranched polysacchs
containing uronic acids and amino sugars that are
often sulfated
75
Summary contd.‐2
• GLYCOCONJUGATES:
Proteoglycans & glycoproteins
play important roles in information transfer in living
organisms.
• Proteoglycans are enormous molecules consisting
of hyaluronate with attached core proteins that
bear numerous glycosaminoglycans and
oligosaccharides. Found in the extracellular matrix
of tissues
• Bacterial cell walls are made up of peptidoglycan, a
network of polysaccharide and poypeptide chains.
76

Summary contd.‐3
• Glycoproteins or Glycosylated proteins may contain
N‐linked oligosacchs attached to Asn (asparagine) or
O‐linked oligosacchs attached to Ser or Thr (serine
or threonine) or both
• Different molecules of glycoproteins may contain
different sequences and locations of
oligosaccharides.
• Occur in cells in both soluble and membrane bound
forms, and in extracellular fluids
77

END NOTES
• The destiny of a nation depends on the
manner in which it feeds itself.
• We eat to live, NOT, live to eat.
• Lower your carbohydrate consumption, but
balance it with the right amount of protein
and fat.
78
