
Preventive Dentistry
5
th
stage
Lec.3 Dr. Jihan Abdulhussein
Fluoride and dental caries
Since 1930 fluoride (F), was demonstrated for its anti caries effect.
Fluoride in small doses has a remarkable influence on dental system. It
causes a strong inhibition of dental caries, while in large concentration it
may cause disturbances of the enamel formation (dental fluorosis).
What is fluoride?
Fluoride was earlier known as fluorine, which is derived from a Latin
term (fluore) that is to flow. It is the 17th element in order of frequency of
occurrence of elements representing 0.06% - 0.09 % of the earth crust. In
rocks and soil, fluorine may occur in combined form in different minerals
as:
- Fluorspar (CaF2).
- Fluoroapatite (Ca10(PO4)6F2).
- Cryolite (Na3AlF6).
Fluoride is highly reactive it forms salts of almost all metals, it is rarely
occurs as F ions in nature.
Fluoride is widely distributed in the atmosphere originating from:
- Dusts of fluoride containing soils.
- Gaseous industrial wastes.
- Gases emitted in areas of volcanic activity.

It enters the hydrosphere by leaching from soils and minerals in to ground
water or entry with surface water. The concentration in ground water is
10 – 67 ppm, and sea water 0.8 – 1.0 ppm.
Fluoride enters vegetations by:
- Uptake from soils.
- Water.
- Absorption from air.
- Deposition from the atmosphere.
- Rain.
Concentration of fluoride in vegetation is range from two part per million
(ppm) to 20 ppm dry weight. This depends on species of the plant, age of
leafs, soils, fertilizers and pollution. Fluoride returns to soils through
plant wastes or it may enter the food chain and be returned as animals
waste. Fluoride may enter the environment indirectly by industrial air
pollutions as coal burning, production of aluminum steel, phosphate
fertilizers, manufacture of glass etc.
In biological tissues fluoride is present in traces, thus called a trace
element.
Sources of fluoride intake in Man:
1- Water: The greater part of F intake originates from water ingested
each day. Fluoride is naturally present in rivers, oceans and ground water.
The concentration may range from 0.1 – 10 ppm.
2- Food: It may present in various concentration in soft drinks, different
infants and adult food. Fluoride concentration in various foods reflects its
concentration in water where the product has been prepared.
For infants the daily fluoride intake is determined by feeding pattern, as
breast milk or formula milk. In human breast milk F concentration is 6 -
12 mg/ml this is in fluoridated and non fluoridated area. In cow's milk the
concentration is less than 0.019 ppm. In formula milk and cereals the

concentration depends on the product and on F concentration in water
these are prepared.
For adults food F concentrations vary, but usually less than 0.5 ppm. A
higher concentration may be found in tea (0.5 – 4ppm) and in fish and
shell fish.
3- Drugs and dental products: Some drugs contain a high concentration
of F as diuretics and anesthetics. Dental products as dentifrices and mouth
rinses
4- Pollutions: F is present in high concentration in the vicinity of metal
industries, about 25 – 1000 times the normal.
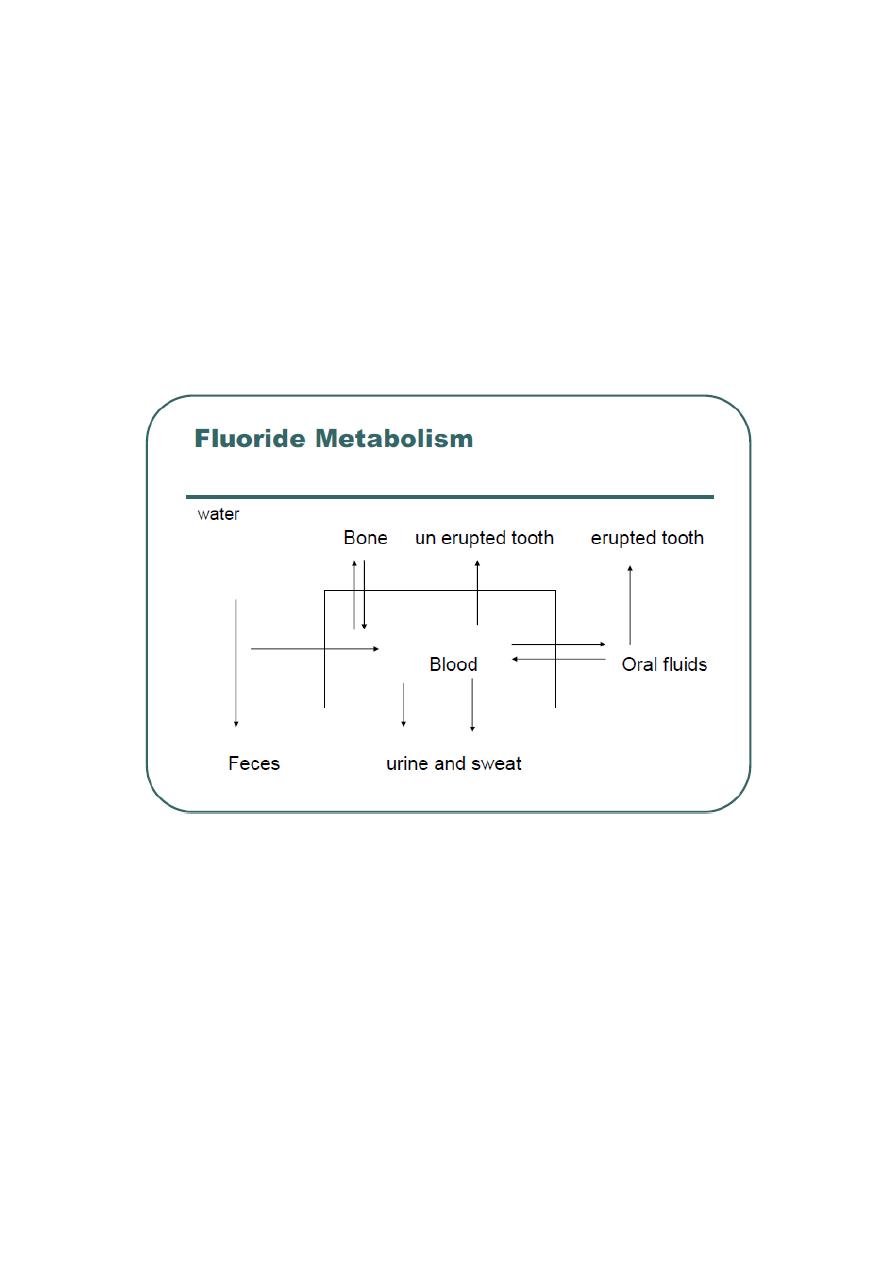
Fluoride Metabolism:
1- Absorption:
After intake of F it will be absorbed in the gastro intestinal tract, it is also
absorbed via lungs. There are factors affecting the rate of F absorption,
these are:
- Solubility and degree of ionizations of the components. This will
determine the amounts of F ions released. Only F in ionic form is of
importance to health. NaF is more soluble than CaF2 thus the rate and
degree of absorption of NaF is more than CaF2.
- Dose and F concentration. Following absorption there will be an
elevation in plasma F level. The height of plasma peak is proportional to
F dose ingested and rate and degree of absorption in addition to body
weight (with increase body weight there will be a lower plasma peak).
- Presence of food in the stomach. Presence of certain dietary items as Ca
may lead to formation of insoluble salts with F. Food acts as a physical
barrier that retards absorption of F from GIT.
- Gastric acidity. There is an inverse relation between gastric acidity and
absorption of F from GIT.
Note: The bioavailability of F of the most of dental products is 100%.
Ingestion of F is
- Empty stomach ………………100%
- In presence of glass of milk…… 70%
- Ca- rich breakfast ………… 60%
Milk may retard absorption of F from the stomach in the first hour, later
absorption will continue at higher levels for longer period of time.
2- Retention and distribution in the body:
The maximum plasma concentration of F is reached in 30 minutes. The
plasma peak will be reduced as F distributed in the body. Fluoride is a
calcified tissue seeker, more than 99% of F in the body is found in
calcified tissues. F is rapidly distributed to bone, teeth, heart, kidney and
liver. While, fluoride is slowly distributed to skeletal muscles and adipose
tissues.
The uptake of F is affected by age factor, younger the age the greater will
be the uptake of F. Not all F in bone is firmly held as some of F is
subsequently lost again by the osteoclastic resorption of bone.
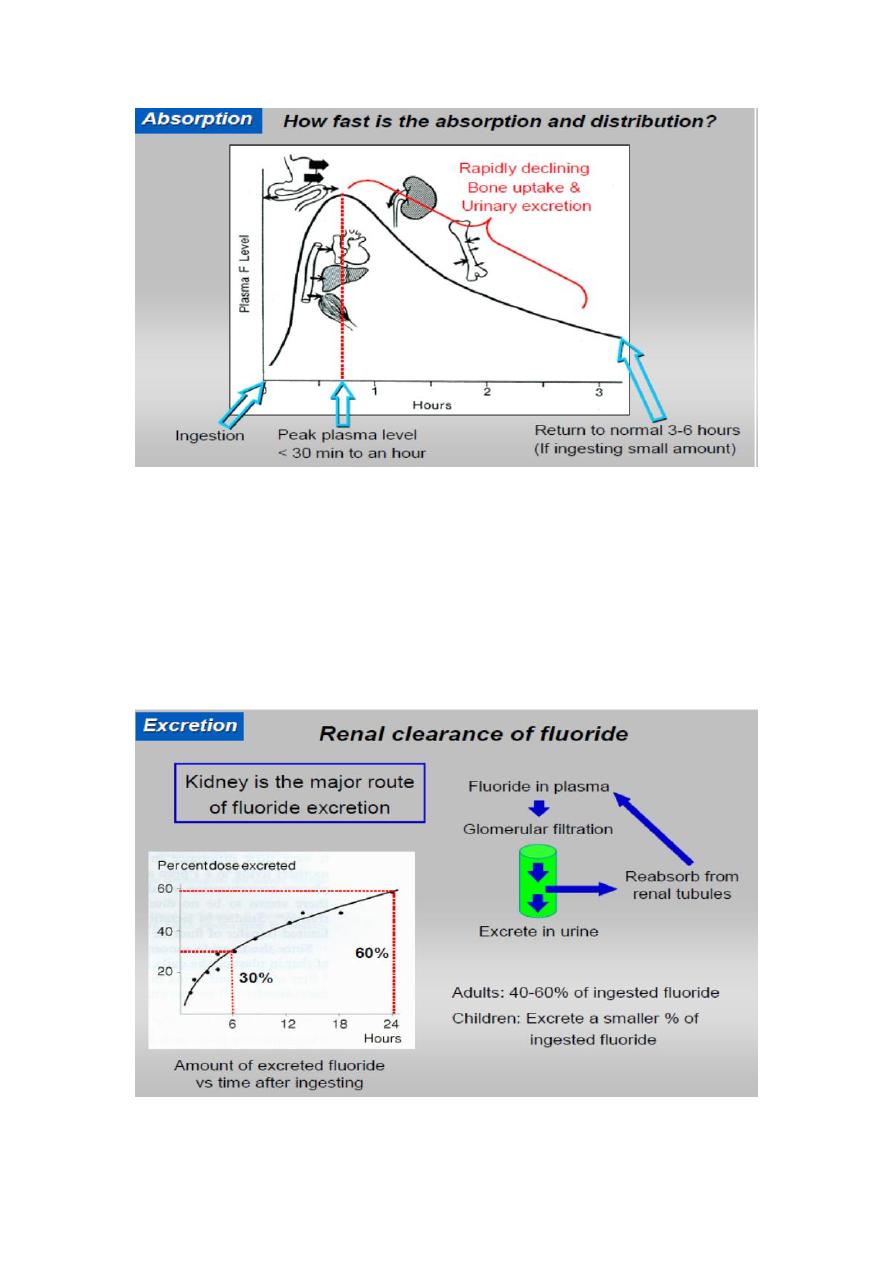
3- Excretion:
The major route of excretion is by kidneys. After entering the renal
tubules some of F ions will be re absorbed and return to the circulatory
system, while the remainder of ions will be excreted by the urine. The
renal clearance of F is 50 ml/minute. About 10% of F is removed by feces
this amount is never absorbed, also a less quantity is excreted by sweat,
saliva and gingival exudates, tears.
