
Dr. Ahmed Saleem
FICMS
TUCOM / 3rd Year / 2015
FLUID AND ELECTROLYTE MANAGEMENT OF THE SURGICAL PATIENT
Fluid and electrolyte management is paramount to the care of the surgical patient. Changes in both fluid
volume and electrolyte composition occur preoperatively, intraoperatively, and postoperatively, as well as
in response to trauma and sepsis.
Body Fluids
Total Body Water
Water constitutes approximately 50 to 60% of total body weight. The relationship between total body
weight and total body water (TBW) is relatively constant for an individual and is primarily a reflection of
body fat. In an average young adult male 60% of total body weight is TBW, whereas in an average young
adult female it is 50%.
The highest percentage of TBW is found in newborns, with approximately 80% of
their total body weight comprised of water. This decreases to approximately 65% by 1 year of age and
thereafter remains fairly constant.
Fluid Compartments
TBW is divided into three functional fluid compartments: plasma, extravascular interstitial fluid, and
intracellular fluid. The extracellular fluids (ECF), plasma and interstitial fluid together comprise about one
third of the TBW and the intracellular compartment the remaining two thirds. The extracellular water
comprises 20% of the total body weight and is divided between plasma (5% of body weight) and interstitial
fluid (15% of body weight). Intracellular water makes up approximately 40% of an individual's total body
weight, with the largest proportion in the skeletal muscle mass.
Composition of Fluid Compartments
The ECF compartment is balanced between sodium, the principal cation, and chloride and bicarbonate, the
principal anions. The intracellular fluid compartment is comprised primarily of the cations potassium and
magnesium, and the anions phosphate and proteins. Although the movement of ions and proteins between
the various fluid compartments is restricted, water is freely diffusible. Water is distributed evenly
throughout all fluid compartments of the body; so that a given volume of water increases the volume of any
one compartment relatively little. Sodium, however, is confined to the ECF compartment, and because of
its osmotic and electrical properties, it remains associated with water. Therefore, sodium-containing fluids
are distributed throughout the ECF and add to the volume of both the intravascular and interstitial spaces.
Although the administration of sodium-containing fluids expands the intravascular volume, it also expands
the interstitial space by approximately three times as much as the plasma.
Page 1 of 11

Osmolality
The osmolality of the intracellular and extracellular fluids is maintained between 290 and 310 mOsm in
each compartment. Because cell membranes are permeable to water, any change in osmotic pressure in
one compartment is accompanied by a redistribution of water until the effective osmotic pressure between
compartments is equal. For example, if the ECF concentration of sodium increases, there will be a net
movement of water from the intracellular to the extracellular compartment. Conversely, if the ECF
concentration of sodium decreases, water will move into the cells. Although the intracellular fluid shares in
losses that involve a change in concentration or composition of the ECF, an isotonic change in volume in
either one of the compartments is not accompanied by the net movement of water as long as the ionic
concentration remains the same. For practical clinical purposes, most significant gains and losses of body
fluid are directly from the extracellular compartment.
The principal determinants of osmolality are the concentrations of sodium, glucose, and urea (blood urea
nitrogen, or BUN):
Calculated serum osmolality = 2 sodium + (glucose/18) + (BUN/2.8)
Body Fluid Changes
Normal Exchange of Fluid and Electrolytes
The healthy person consumes an average of 2000 mL of water per day, approximately 75% from oral intake
and the rest extracted from solid foods. Daily water losses include 800 to 1200 mL in urine, 250 mL in stool,
and 600 mL in insensible losses. Insensible losses of water occur through both the skin (75%) and lungs
(25%), and can be increased by such factors as fever, hypermetabolism, and hyperventilation. Sensible
water losses such as sweating or pathologic loss of GI fluids vary widely, but these include the loss of
electrolytes as well as water. To clear the products of metabolism, the kidneys must excrete a minimum of
500 to 800 mL of urine per day, regardless of the amount of oral intake.
Classification of Body Fluid Changes
Disorders in fluid balance may be classified into three general categories: disturbances in (a) volume, (b)
concentration, and (c) composition. Although each of these may occur simultaneously, each is a separate
entity with unique mechanisms demanding individual correction. Isotonic gain or loss of salt solution results
in extracellular volume changes, with little impact on intracellular fluid volume. If free water is added or lost
from the ECF, water will pass between the ECF and intracellular fluid until solute concentration or
osmolarity is equalized between the compartments. Unlike with sodium, the concentration of most other
ions in the ECF can be altered without significant change in the total number of osmotically active particles,
producing only a compositional change. For instance, doubling the serum potassium concentration will
profoundly alter myocardial function without significantly altering volume or concentration of the fluid
spaces.
Page 2 of 11

A - Disturbances in Fluid Balance (Volume changes)
Volume Deficit
Extracellular volume deficit is the most common fluid disorder in surgical patients and can be either acute
or chronic. Acute volume deficit is associated with cardiovascular and central nervous system signs,
whereas chronic deficits display tissue signs, such as a decrease in skin turgor and sunken eyes, in addition
to cardiovascular and central nervous system signs (Table 1). Laboratory examination may reveal an
elevated blood urea nitrogen level if the deficit is severe enough to reduce glomerular filtration and
hemoconcentration. Urine osmolality usually will be higher than serum osmolality, and urine sodium will be
low, typically <20 mEq/L. Serum sodium concentration does not necessarily reflect volume status and
therefore may be high, normal, or low when a volume deficit is present. The most common cause of volume
deficit in surgical patients is a loss of GI fluids (Table 2) from nasogastric suction, vomiting, diarrhea, or
enterocutaneous fistula. In addition, sequestration secondary to soft tissue injuries, burns, and intra-
abdominal processes such as peritonitis, obstruction, or prolonged surgery can also lead to massive volume
deficits.
Volume Excess
Extracellular volume excess may be iatrogenic or secondary to renal dysfunction, congestive heart failure,
or cirrhosis. Both plasma and interstitial volumes usually are increased. Symptoms are primarily pulmonary
and cardiovascular (Table 1). In fit patients, edema and hyperdynamic circulation are common and well
tolerated. However, the elderly and patients with cardiac disease may quickly develop congestive heart
failure and pulmonary edema in response to only a moderate volume excess.
Table (1) Signs and Symptoms of Volume Disturbances
System
Volume Deficit
Volume Excess
Generalized
Weight loss
Decreased skin turgor
Weight gain
Peripheral edema
Cardiac
Tachycardia
Orthostasis/hypotension
Collapsed neck veins
Increased cardiac output
Increased central venous pressure
Distended neck veins
Murmur
Renal
Oliguria
Azotemia
—
GI
Ileus
Bowel edema
Pulmonary
—
Pulmonary edema
Page 3 of 11
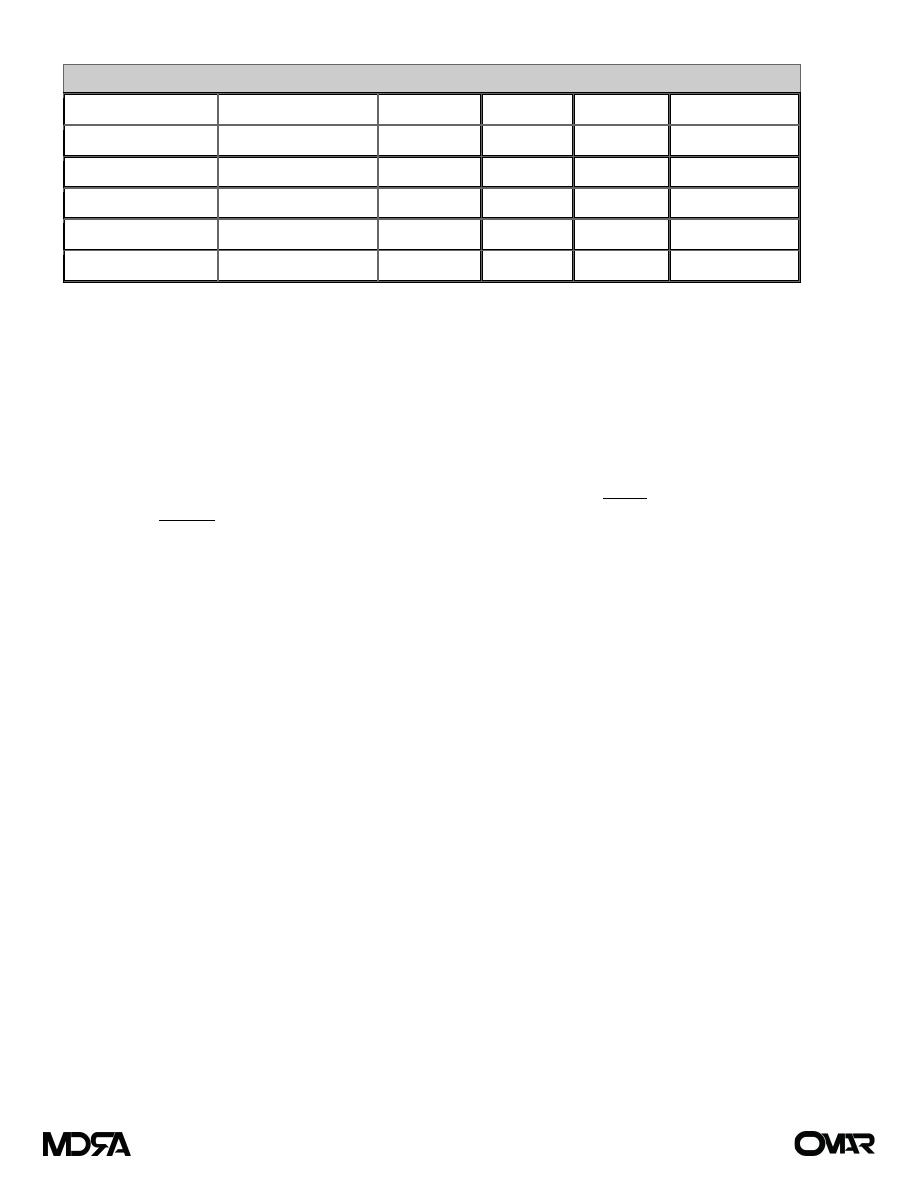
Table (2) Composition of GI Secretions ) (لألطالع
Type of Secretion
Volume (mL/24 h)
Na (mEq/L) K (mEq/L) Cl (mEq/L) HCO
3
–
(mEq/L)
Stomach
1000–2000
60–90
10–30
100–130
0
Small intestine
2000–3000
120–140
5–10
90–120
30–40
Colon
—
60
30
40
0
Pancreas
600–800
135–145
5–10
70–90
95–115
Bile
300–800
135–145
5–10
90–110
30–40
B - Concentration Changes
Changes in serum sodium concentration (135–145 mmol/L) are inversely proportional to TBW. Therefore,
abnormalities in TBW are reflected by abnormalities in serum sodium levels.
Hyponatremia
A low serum sodium level occurs when there is an excess of extracellular water relative to sodium.
Extracellular volume (not water) can be high, normal, or low. In most cases of hyponatremia, sodium
concentration is decreased as a consequence of either sodium depletion or dilution.
Dilutional hyponatremia frequently results from excess extracellular water and therefore is associated with
a high extracellular volume status. Excessive oral water intake or iatrogenic IV excess free water
administration can cause hyponatremia. Postoperative patients are particularly prone to increased
secretion of antidiuretic hormone (ADH), which increases reabsorption of free water from the kidneys with
subsequent volume expansion and hyponatremia. This is usually self-limiting in that both hyponatremia and
volume expansion decrease ADH secretion. Physical signs of volume overload usually are absent, and
laboratory evaluation reveals hemodilution.
Depletional hyponatremia is associated with either a decreased intake or increased loss of sodium-
containing fluids. A concomitant ECF volume deficit is common. Causes include decreased sodium intake,
such as consumption of a low-sodium diet or use of enteral feeds, which are typically low in sodium; GI
losses from vomiting, prolonged nasogastric suctioning, or diarrhea; and renal losses due to diuretic use or
primary renal disease.
Hyponatremia also can be seen with an excess of solute relative to free water, such as with untreated
hyperglycemia or mannitol administration. Glucose exerts an osmotic force in the extracellular
compartment, causing a shift of water from the intracellular to the extracellular space. Hyponatremia
therefore can be seen when the effective osmotic pressure of the extracellular compartment is normal or
even high. When hyponatremia in the presence of hyperglycemia is being evaluated, the corrected sodium
concentration should be calculated as follows:
For every 100 mg/dL increment in plasma glucose above normal, the plasma sodium should decrease by 1.6 mEq/L
Page 4 of 11
Lastly, extreme elevations in plasma lipids and proteins can cause pseudohyponatremia, because there is
no true decrease in extracellular sodium relative to water.
Signs and symptoms of hyponatremia (Table 3) are dependent on the degree of hyponatremia and the
rapidity with which it occurred. Clinical manifestations primarily have a central nervous system origin and
are related to cellular water intoxication and associated increases in intracranial pressure. Oliguric renal
failure also can be a rapid complication in the setting of severe hyponatremia.
Most cases of hyponatremia can be treated by free water restriction and, if severe, the administration of
sodium. In patients with normal renal function, symptomatic hyponatremia does not occur until the serum
sodium level is ≤120 mEq/L. If neurologic symptoms are present, 3% normal saline should be used to
increase the sodium by no more than 1 mEq/L per hour until the serum sodium level reaches 130 mEq/L or
neurologic symptoms are improved. Correction of asymptomatic hyponatremia should increase the sodium
level by no more than 0.5 mEq/L per hour to a maximum increase of 12 mEq/L per day, and even more
slowly in chronic hyponatremia. The rapid correction of hyponatremia can lead to pontine myelinolysis and
may result in permanent brain damage and death.
Table (3) Clinical Manifestations of Abnormalities in Serum Sodium Level
Body System
Hyponatremia
Central nervous
system
Headache, confusion, hyperactive or hypoactive deep tendon reflexes, seizures, coma,
increased intracranial pressure
Musculoskeletal
Weakness, fatigue, muscle cramps/twitching
GI
Anorexia, nausea, vomiting, watery diarrhea
Cardiovascular
Hypertension and bradycardia if significant increases in intracranial pressure
Tissue
Lacrimation, salivation
Renal
Oliguria
Body System
Hypernatremia
Central nervous
system
Restlessness, lethargy, ataxia, irritability, tonic spasms, delirium, seizures, coma
Musculoskeletal
Weakness
Cardiovascular
Tachycardia, hypotension, syncope
Tissue
Dry sticky mucous membranes, red swollen tongue, decreased saliva and tears
Renal
Oliguria
Metabolic
Fever
Page 5 of 11

Hypernatremia
Hypernatremia results from either a loss of free water or a gain of sodium in excess of water. Like
hyponatremia, it can be associated with an increased, normal, or decreased extracellular volume.
Hypervolemic hypernatremia usually is caused either by iatrogenic administration of sodium-containing
fluids, including sodium bicarbonate, or mineralocorticoid excess as seen in hyperaldosteronism, Cushing's
syndrome, and congenital adrenal hyperplasia. Urine sodium concentration is typically >20 mEq/L and urine
osmolarity is >300 mOsm/L.
Normovolemic hypernatremia can result from renal causes, including diabetes insipidus, diuretic use, and
renal disease, or from non-renal water loss from the GI tract or skin, although the same conditions can
result in hypovolemic hypernatremia. With non-renal water loss, the urine sodium concentration is <15
mEq/L and the urine osmolarity is >400 mOsm/L.
Symptomatic hypernatremia usually occurs only in patients with impaired thirst or restricted access to fluid,
because thirst will result in increased water intake. Symptoms are rare until the serum sodium
concentration exceeds 160 mEq/L but, once present, are associated with significant morbidity and
mortality. Because symptoms are related to hyperosmolarity, central nervous system effects predominate
(Table 3). Central nervous system symptoms can range from restlessness and irritability to seizures, coma,
and death. The classic signs of hypovolemic hypernatremia, (tachycardia, orthostasis, and hypotension) may
be present, as well as the unique findings of dry, sticky mucous membranes.
Treatment of hypernatremia usually consists of treatment of the associated water deficit. In hypovolemic
patients, volume should be restored with normal saline before the concentration abnormality is addressed.
Once adequate volume has been achieved, the water deficit is replaced using a hypotonic fluid such as 5%
dextrose, 5% dextrose in 1/4 normal saline, or enterally administered water. The formula used to estimate
the amount of water required to correct hypernatremia is as follows:
Water deficit (L) =
(Serum Na - 140) / 140
* TBW
Estimate TBW as 50% of lean body mass in men and 40% in women
C - Composition Changes:
Potassium Abnormalities
Extracellular potassium is maintained within a narrow range (3.5 – 5 mEq/L), principally by renal excretion
of potassium. Although only 2% of the total body potassium is located within the extracellular
compartment, this small amount is critical to cardiac and neuromuscular function; thus, even minor
changes can have major effects on cardiac activity. The intracellular and extracellular distribution of
potassium is influenced by a number of factors, including surgical stress, injury, acidosis, and tissue
catabolism.
Page 6 of 11
Hyperkalemia
Hyperkalemia is defined as a serum potassium concentration above the normal range of 3.5 to 5.0 mEq/L. It
is caused by excessive potassium intake, increased release of potassium from cells, or impaired potassium
excretion by the kidneys (Table 4).
Symptoms of hyperkalemia are primarily GI, neuromuscular, and cardiovascular (Table 5). Early
cardiovascular signs may be apparent from electrocardiogram (ECG) changes and eventually lead to
hemodynamic symptoms of arrhythmia and cardiac arrest. ECG changes that may be seen with
hyperkalemia include high peaked T waves (early), widened QRS complex, flattened P wave, prolonged PR
interval (first-degree block), sine wave formation, and ventricular fibrillation.
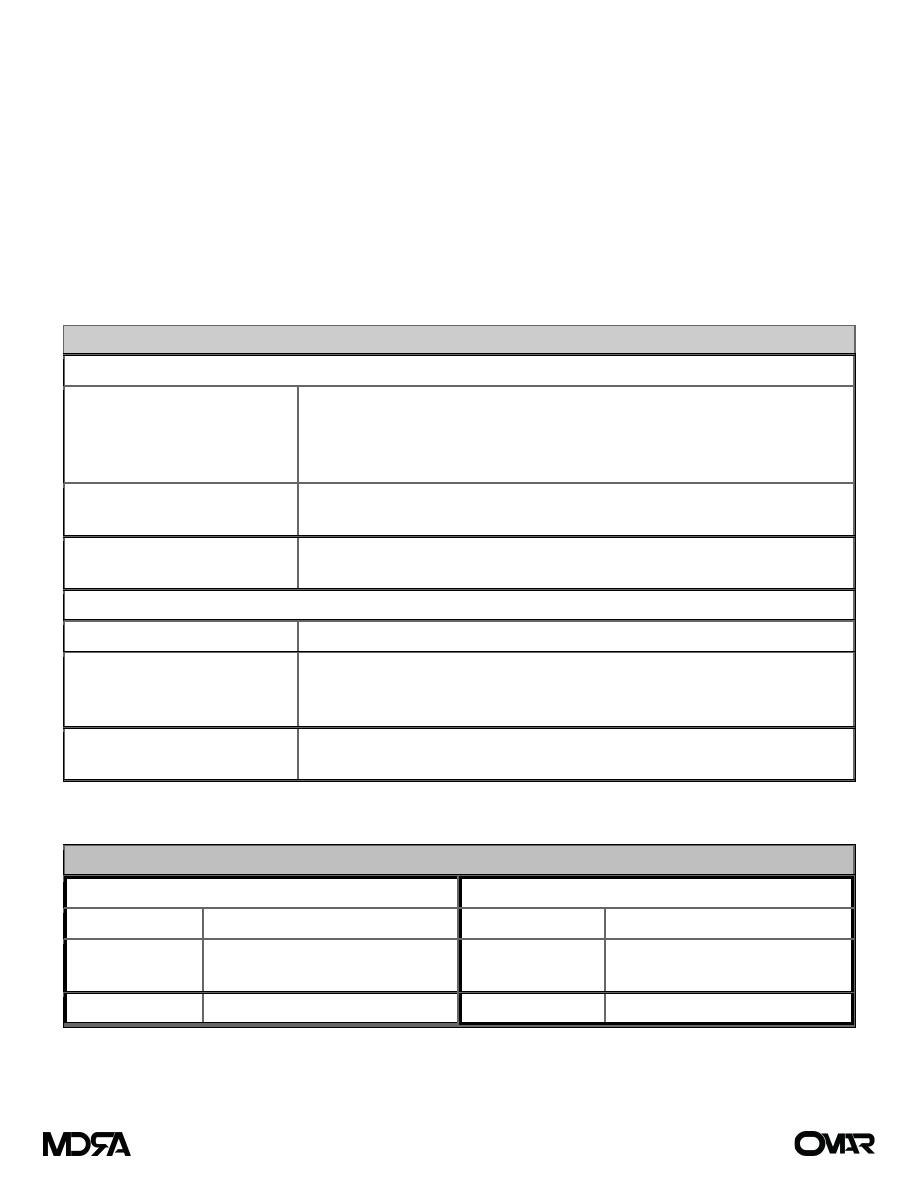
Table (4) Etiology of Potassium Abnormalities
Hyperkalemia
Increased intake
Potassium supplementation
Blood transfusions
Endogenous load/destruction: hemolysis, rhabdomyolysis, crush injury,
gastrointestinal hemorrhage
Increased release
Acidosis
Rapid rise of extracellular osmolality (hyperglycemia or mannitol)
Impaired excretion
Potassium-sparing diuretics
Renal insufficiency/failure
Hypokalemia
Inadequate intake
Dietary, potassium-free intravenous fluids, potassium-deficient TPN
Excessive potassium excretion Hyperaldosteronism
Medications
Renal loss of potassium
GI losses
Direct loss of potassium from GI fluid: diarrhea and gastric fluid, either as
vomiting or high nasogastric output)
Table (5) Clinical Manifestations of Abnormalities in Potassium Levels
Increased Serum Levels
Decreased Serum Levels
GI
Nausea/vomiting, colic, diarrhea
GI
Ileus, constipation
Neuromuscular Weakness, paralysis,
respiratory failure
Neuromuscular Decreased reflexes, fatigue,
weakness, paralysis
Cardiovascular Arrhythmia, arrest
Cardiovascular Arrest
Page 7 of 11

The goals of therapy include 1-reducing the total body potassium, 2-shifting potassium from the
extracellular to the intracellular space, and 3-protecting the cells from the effects of increased potassium.
For all patients exogenous sources of potassium should be removed, including potassium supplementation
in IV fluids and enteral and parenteral solutions. Potassium can be removed from the body using a cation-
exchange resin such as Kayexalate that binds potassium in exchange for sodium. It can be administered
orally, in alert patients, or rectally. Immediate measures also should include attempts to shift potassium
intracellularly with glucose and bicarbonate infusion. Nebulized albuterol (10 to 20 mg) may also be used.
Use of glucose alone will cause a rise in insulin secretion, but in the acutely ill this response may be blunted,
and therefore both glucose and insulin may be necessary. When ECG changes are present, calcium chloride
or calcium gluconate (5 to 10 mL of 10% solution) should be administered immediately to counteract the
myocardial effects of hyperkalemia. All of the aforementioned measures are temporary, lasting from 1 to
approximately 4 hours. Dialysis should be considered in severe hyperkalemia when conservative measures
fail.
Hypokalemia
Hypokalemia is much more common than hyperkalemia in the surgical patient (see Table 4). The symptoms
of hypokalemia, like those of hyperkalemia, are primarily related to failure of normal contractility of GI
smooth muscle, skeletal muscle, and cardiac muscle (see Table 5). ECG changes suggestive of hypokalemia
include U waves, T-wave flattening, ST-segment changes, and arrhythmias (with digitalis therapy).
Treatment for hypokalemia consists of potassium repletion, the rate of which is determined by the
symptoms. Oral repletion is adequate for mild, asymptomatic hypokalemia. If IV repletion is required,
usually no more than 10 mEq/h is advisable in an unmonitored setting. This amount can be increased to 40
mEq/h when accompanied by continuous ECG monitoring, and even more in the case of imminent cardiac
arrest from a malignant arrhythmia associated hypokalemia. Caution should be exercised when oliguria or
impaired renal function is coexistent.
Calcium Abnormalities
The vast majority of the body's calcium is contained within the bone matrix, with <1% found in the ECF.
Serum calcium is distributed among three forms: protein pound (40%), complexed to phosphate and other
anions (10%), and ionized (50%). It is the ionized fraction that is responsible for neuromuscular stability and
can be measured directly. When total serum calcium levels are measured, the albumin concentration must
be taken into consideration:
Adjust total serum calcium down by 0.8 mg/dL for every 1 g/dL decrease in albumin.
Unlike changes in albumin, changes in pH will affect the ionized calcium concentration. Acidosis decreases
protein binding, thereby increasing the ionized fraction of calcium.
Daily calcium intake is 1 to 3 g/d. Most of this is excreted via the bowel, with urinary excretion relatively
low. Total body calcium balance is under complex hormonal control, but disturbances in metabolism are
relatively long term and less important in the acute surgical setting. However, attention to the critical role
of ionized calcium in neuromuscular function often is required.
Page 8 of 11

Hypercalcemia
Hypercalcemia is defined as a serum calcium level above the normal range of 8.5 to 10.5 mEq/L (2.12–2.65
mmol/L). Primary hyperparathyroidism in the outpatient setting and malignancy in hospitalized patients,
from either bony metastasis or secretion of parathyroid hormone–related protein, account for most cases
of symptomatic hypercalcemia. The initial clinical manifestations of hypercalcemia are nonspecific:
weakness, fatigue, anorexia, nausea, and vomiting. As serum calcium increases, severe headaches, diffuse
musculoskeletal pain, polyuria, and polydipsia develop. The combination of decreased oral intake, vomiting,
and polyuria leads to hypovolemia and dehydration, which may become pronounced. Cardiac symptoms
can be manifest as hypertension, cardiac arrhythmias, and a worsening of digitalis toxicity. When serum
calcium increases to 15 mg/dL, and above, confusion and depression progress to somnolence, stupor, and
coma. This degree of hypercalcemia results in death unless it is corrected promptly.
Treatment is required when hypercalcemia is symptomatic, which usually occurs when the serum level
exceeds 12 mg/dL. The critical level for serum calcium is 15 mg/dL, when symptoms noted earlier may
rapidly progress to death. The initial treatment is aimed at repleting the associated volume deficit and then
inducing a brisk diuresis with normal saline.
Hypocalcemia
Hypocalcemia is defined as a serum calcium level below 8.5 mEq/L or a decrease in the ionized calcium level
below 4.2 mg/dL. The causes of hypocalcemia include pancreatitis, massive soft tissue infections such as
necrotizing fasciitis, renal failure, pancreatic and small bowel fistulas, Surgically induced
hypoparathyroidism (transient or permanent), toxic shock syndrome and abnormalities in magnesium
levels. Calcium precipitation with organic anions is also a cause of hypocalcemia and may occur during
hyperphosphatemia from tumor lysis syndrome or rhabdomyolysis. Massive blood transfusion with citrate
binding is another mechanism.
Hypocalcemia rarely results solely from decreased intake, because bone
reabsorption can maintain normal levels for prolonged periods.
Asymptomatic hypocalcemia may occur when hypoproteinemia results in a normal ionized calcium level.
Conversely, symptoms can develop with a normal serum calcium level during alkalosis, which decreases
ionized calcium. In general, neuromuscular and cardiac symptoms do not occur until the ionized fraction
falls below 2.5 mg/dL. Clinical findings may include paresthesias of the face and extremities, muscle cramps,
carpopedal spasm, stridor, tetany, and seizures. Patients will demonstrate hyperreflexia and may exhibit
positive Chvostek's sign (spasm resulting from tapping over the facial nerve) and Trousseau's sign (spasm
resulting from pressure applied to the nerves and vessels of the upper extremity with a blood pressure
cuff). Hypocalcemia may lead to decreased cardiac contractility and heart failure.
Acute symptomatic hypocalcemia should be treated with IV 10% calcium gluconate to achieve a serum
concentration of 7 to 9 mg/dL. Associated deficits in magnesium, potassium, and pH must also be
corrected. Hypocalcemia will be refractory to treatment if coexisting hypomagnesemia is not corrected first.
Routine calcium supplementation is no longer recommended in association with massive blood
transfusions.
Page 9 of 11

Acid–Base Balance
Acid–base balance is in effect the body’s management of large amounts of endogenously produced
hydrogen ion. Normally, the free hydrogen ion concentration of ECFs is maintained at a pH of 7.40 ± 0.05.
Maintenance at this level is accomplished by the combined action of three mechanisms:
1. Buffering systems that are present in all body fluids and that immediately offset changes in hydrogen
concentration. Important buffers include intracellular proteins and phosphates and the bicarbonate–
carbonic acid buffer system in ECF is one of the most important components.
2. Pulmonary ventilation changes that can promptly adjust the excretion of CO2
3. Renal tubular function, which, over time, can contribute by modulating the urinary excretion or
conservation of acid or base
Disorders of Acid–Base Balance
The pH disorders of blood can be grouped into two broad categories, respiratory and metabolic.
Base excess, base deficit, and anion gap
These are derived numbers, calculated by blood gas analyzers, quantifying changes in metabolic or fixed
acids, but because they depend on several assumptions, they do not always reflect the true acid–base
balance.
• Base excess is defined as the mmol/L of acid that would be required to titrate the blood pH back to 7.4 if
the pCO2 were normal.
• Base deficit (negative base excess) is defined as the mmol/L of base to titrate the blood pH back to 7.4 if
the pCO2 were normal.
A base deficit is negative and a base excess is positive by convention. Normal values are –2mmol/L to
+2mmol/L. A base deficit greater than this (e.g. –6mmol/L) indicates a metabolic acidosis.
• The anion gap is the difference between measured cations and measured anions = (K
+
+ Na
+
)–(Cl
-
+ HCO3).
This is made up of metabolic acids: ketones, lactate, and phosphates. The anion gap is normally 8 - 16
mmol/L; an increase in anion gap indicates a metabolic acidosis.
Diagnosing acid–base abnormalities
An arterial blood pH that is <7.35 signifies acidemia, while pH > 7.45 signifies alkalemia.
The normal limits of PaCO2 are 37 to 45 mm Hg (4.7–6.0kPa). Look at the PaCO2
is there a
change in keeping with the pH derangement? If so, the derangement is a respiratory one.
Look at the base deficit (or anion gap). This will tell you if there is a metabolic derangement
Mixed acid–base disorders include all possible combinations. For example, a patient may develop metabolic
acidosis and respiratory acidosis simultaneously. Another patient may have a combination of respiratory
alkalosis and metabolic acidosis. In clinical practice, the presence of an isolated acid–base disorder is
unusual. With normal kidney and lung function, compensation occurs. The Flenley nomogram (see Fig. 1) is
a useful diagnostic aid where mixed metabolic and respiratory derangements are present.
Page 10 of 11
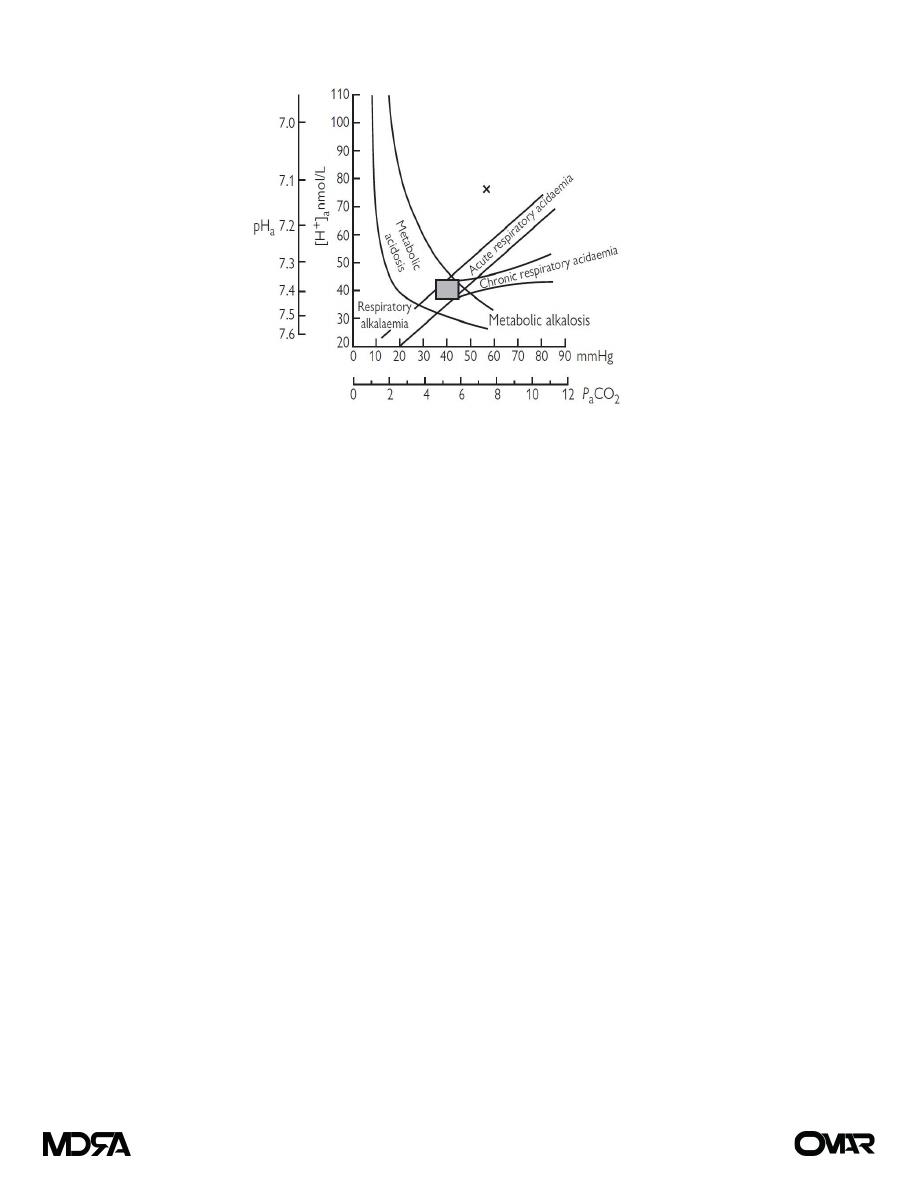
Figure (1) The Flenley nomogram
Metabolic acidosis
Metabolic acidosis due to increased metabolic acids (
anion gap)
• Lactic acid (global and/or regional hypoperfusion, hypoxia, sepsis, hepatic failure as the liver normally
metabolizes lactate).
• Uric acid (renal failure).
• Ketones (diabetic ketoacidosis, alcoholic and starvation ketoacidosis).
• Drugs/toxins (salicylates, sodium nitroprusside overdose).
Due to loss of bicarbonate or hyperchloremia (normal anion gap)
• Renal tubular acidosis (loss of bicarbonate).
• Diarrhea, high output ileostomy (loss of bicarbonate).
• Pancreatic fistulae (loss of bicarbonate).
• Hyperchloremic acidosis (excessive saline administration).
Metabolic alkalosis
• Loss of H+ from gut (vomiting, NG tube suction).
• Renal loss of H+ (diuretics),
reabsorption of HCO3
-
(hypochloremia).
• Administration of base (NaHCO3, citrate in blood transfusions).
Respiratory acidosis
• Any cause of respiratory failure or hypoventilation.
• Increased production of CO2, e.g. sepsis, malignant hyperpyrexia.
• Rebreathing CO2 (circuit misconnections in ventilators, soda lime exhaustion).
Respiratory alkalosis
• Hyperventilation: deliberate, inadvertent in ventilated patients or in non-ventilated patients caused by
stroke, anxiety, pulmonary embolism (PE), pneumonia, asthma, pulmonary edema.
Page 11 of 11
