
1
Preventive dentistry
Topical fluoride therapy
Lec. Dr.Jihan Abdulhussein
The term of Topical fluoride therapy refers to the use of systems
containing relatively large concentrations of fluoride that are applied
locally or topically, to the erupted tooth surface to prevent the formation
of dental caries.
Topical fluoride advocated for home use contains comparatively less
amount of fluoride & are used daily. Professionally applied F agents
contain very high amount of F & applied less frequently, majority being
biannually.
Advantages
1. Does not cause fluorosis.
2. Cariostatic for people of all ages.
3. Available only to people who desire it.
4. Easy to use.
Disadvantages
1. Person must remember to use.
2. Per capita cost is high compared to water fluoridation.
3. More concentrated professional use products can cause short-term side
effects like nausea immediately after use.
The efficacy of topical fluoride depends on:
a. The concentration of fluoride used.
b. The frequency with which it is applied and the duration of application.
c. The specific fluoride compound used
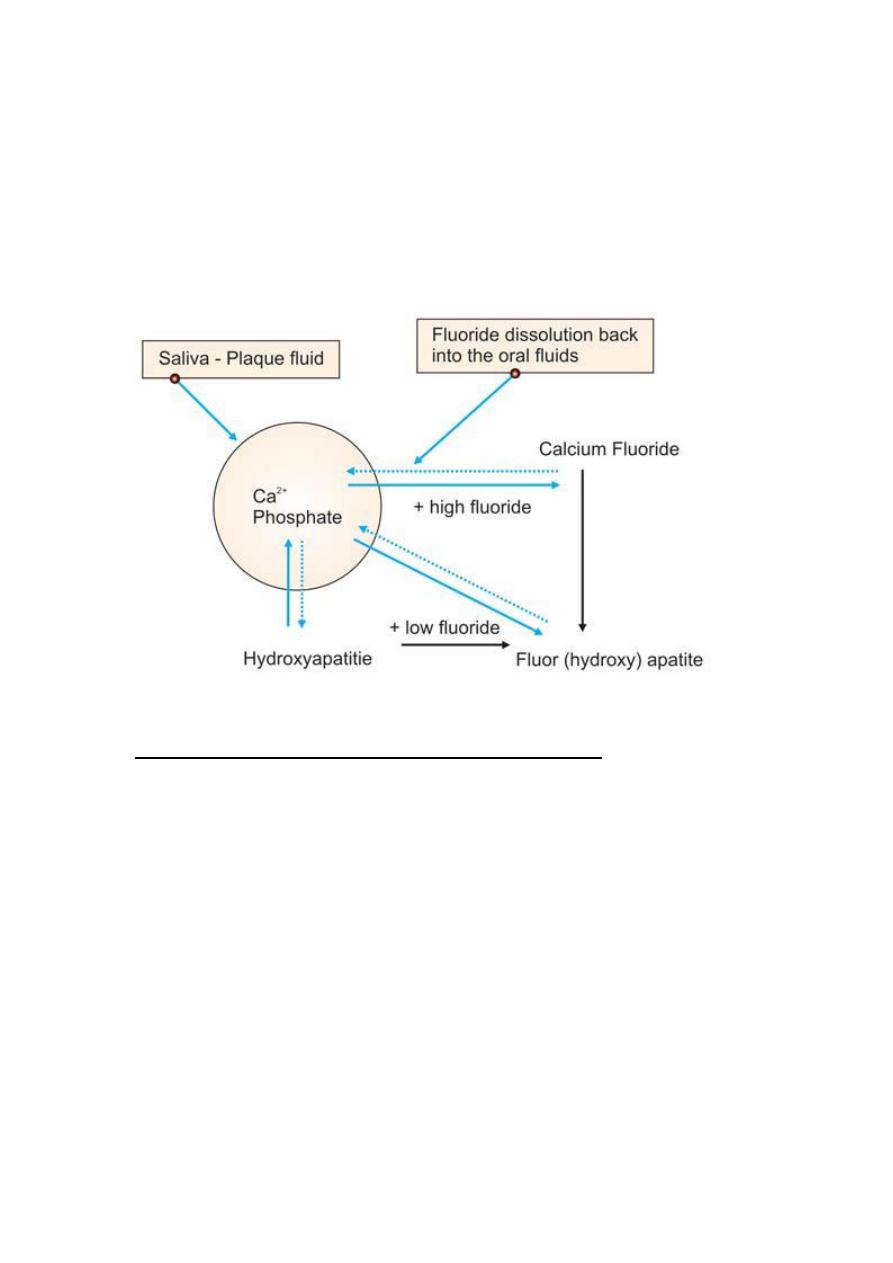
MECHANISM OF ACTION
When concentrated topical fluoride agents react with enamel there is
formation of calcium fluoride. The presence of elevated concentration of
fluoride in enamel surface makes tooth surface more resistant to
development of dental caries. Fluoride ions when substituted into the
2
hydroxyapatite crystals fit more perfectly than do hydroxyl ions. Also the
greater bonding potential of fluoride makes the apatite crystals more
compact and more stable, thereby more resistant to the acid dissolution.
Ca10 [PO4]6[OH] 2 + 20F– ↔ 10 CaF2 + 6[HPO4–] 3 + 2[OH]–
Hydroxyapatite Calcium fluoride
Mechanism of action of fluoride on enamel
FLUORIDE APPLICATION TECHNIQUES
Polishing is not necessary before fluoride application.Since all
fluoride products are diluted when mixed with saliva, a dry field
ensures more effective fluoride uptake. Techniques followed for
application of fluoride in the dental office are:
Paint on technique, by which fluoride material applied to teeth
by cotton applicator of brush. For patients who cannot tolerate
tray application, this technique is indicated. While more time
consuming, the gag reflex is greatly reduced. It is the most
appropriate method when fluoride solutions are used, but may
also be used with gels and foams.
Tray technique: a small amount of fluoride is added to a tray
then inserted in the patient mouth. Trays come in different

3
shapes and types as foam lined or paper, custom vinyl etc. this
technique allows simultaneous application to both maxillary and
mandibular teeth, and is the most appropriate method for gels
and foams.
For both techniques: - Teeth are cleaned first (scaling and
polishing) to remove dental plaque, calculus, stain and debris if
present. These may interfere with the uptake of fluoride ions and
reduce its effectiveness. - Teeth are isolated using cotton roll
and saliva ejector. Patient should be seated upright & the head
of the patient tilted forward to avoid accidental swallowing of
the materials. - The fluoridated agent applied following dryness
of teeth for 1 – 4 minutes. The amount of agent used must not
exceed 4 ml to prevent acute toxicity. - Use un waxed dental
floss to push the material between teeth. - Following treatment
ask the patient to expectorate several times. - Instruct the patient
not eat or drink for at least 30 minutes.
Acidulated phosphate fluoride should not be used on patients
with porcelain and composite restorations.
Classification
Fluorides Applied by Dentist/Professionally Applied
A. Aqueous solutions
• Sodium fluoride - 2 %
• Stannous fluoride - 8%
B. Fluoride Gels
• Acidulated phosphate fluoride - 1.23 %
C. Fluoride varnishes
• Duraphat
• Fluorprotector
D. Fluoride prophylactic paste
4
E. Restorative materials containing fluoride
F. Fluoride containing devices (slow release)
Self-Applied fluoride
• Fluoride dentifrices
• Fluoride mouth rinses.
Professionally applied fluorides
Dental personnel have been applying fluoride agents on teeth since 1940.
It was seen that when fluoride was applied to teeth, it gets deposited in
the outer enamel, making it more resistant to dissolution by acids.
Although it is now known that frequency and availability of low
concentration of fluoride is more important in caries prevention, but
studies have shown to support the beneficial effect of infrequent
professional application of agents for prevention of dental caries. Topical
fluoride applications are indicated for patients with active smooth surface
caries and those patients in high caries risk groups . This includes special
patient groups, such as those undergoing orthodontic treatment and in
high-risk groups.
Indications for use of professionally applied topical fluorides
• Patients who are at high risk for caries on smooth tooth surfaces
• Patients who are at high risk for caries on root surfaces
• To reduce tooth sensitivity
• White spots
• Active decay
• Special patient groups, such as:
a) Orthodontic patients
b) Patients undergoing head and neck irradiation
c) Patients with decreased salivary flow
• Children whose permanent molars should, but cannot be sealed

5
• Additional protection if necessary for children in areas without
fluoridated drinking water
They may be in the form of sodium fluoride, stannous fluoride or APF.
Thixotropic gels are better than solution due to their high viscosity and
inherent property to flow under pressure. They contain methyl cellulose
that is responsible for their viscosity. Use of foam reduces the risk of
overdosage.
A. Aqueous Solutions
Sodium Fluoride
:
2% NaF is used
• Neutral pH
• 9,200 ppm of available fluoride
• 29% effective in caries reduction
[Knutson's Technique]
Method of preparation: It can be prepared by dissolving 0.2 gm of
powder in 10 ml [20 gm in 1 liter] of distilled water. The prepared
solution has a basic pH and is stable if stored in plastic bottle. If stored in
glass bottle, the fluoride ion of prepared solution can react with silica of
glass forming SiF2 [silicon fluoride], thus reducing the availability of free
active fluoride. Hence reducing its anti caries action.
Recommended ages: It is recommended that a series of 4-weekly
applications of 2 percent NaF be given at ages 3,7,11 and 13. coinciding
with the eruption of different groups of primary and permanent teeth.
Mechanism of Action of Sodium Fluoride
When sodium fluoride is applied on the tooth surface there is rapid influx
of fluoride leading to the formation of calcium fluoride. The calcium
fluoride forms a layer on the tooth surface blocking further entry of
fluoride ions. This sudden stop of the entry of fluoride is termed as “
Chocking off effect”. Fluoride then slowly leaches from the calcium
fluoride. Thus calcium fluoride acts a s a reservoir for fluoride release
and that is the reason why sodium fluoride is kept untouched on the tooth
for 4 minutes.

6
The chemical reaction involved is:
Ca10 (P04)6(0H)2 + 20 F- ↔ 10CaF2 + 6P04- + 20H-
CaF2 + 2Ca5 (P04)3 OH → 2Ca5 (PO4)3F + Ca (OH)2
Advantages
1. Relatively stable when stored in plastic containers.
2. Taste is acceptable.
3. Non-irritating to gingiva and does not cause discoloration of tooth
structures.
Disadvantage: Patient has to make four visits in relatively short period of
time.
Stannous Fluoride
• 8% SnF2 is used
• 2.4-2.8 pH
• 19,500 ppm of available fluoride and 32% effective in caries reduction
(Muhler's Technique)
Available in powder form either in bulk containers or pre-weighed
capsules. The recommended and approved concentration is 8 %.
Method of Preparation
The solution has to be freshly prepared as they are not stable.It can be
prepared by dissolving 0.8 gm of powder in 10 ml of distilled water. The
solution is acidic, with a pH of 2.8. the left over solution should be
discarded after application.
Recommended Schedule;A six monthly interval treatment schedule is
advised
Mechanism of Action
Stannous fluoride reacts with hydroxy apatite and in addition to fluoride,
the Tin of solution also reacts with enamel and form Stannous tri-
fluorophosphate. which is more resistant to carious attack.
Chemical reaction

7
at low concentration is:
Ca5(P04)30H + 2SnF2 2CaF2 + Sn2(OH)P04 + Ca3(PO4 )
At High concentration:
Ca5(P04)30H + 16SnF2 → CaF2 + 2Sn3F3P04
(Tin tri-fluorophosphate)
+ Sn2(OH)PO4
(Tin hydroxyl phosphate)
+ 4CaF2(SnF3)2
(Calcium trifluorostannate)
Tin hydroxy phosphate gets dissolved in oral fluids and is responsible for
the metallic taste. Tin trifluorophosphate which is the main end product is
responsible for making the tooth structure more stable and less
susceptible to decay.
Calcium fluoride [CaF2] so formed further reacts with hydroxyapatite
and some fluorhydroxyapatite also gets formed. SnF2 has produced
significantly greater caries reduction (59%) than sodium fluoride (30%).
Advantages
1. Rapid penetration of fluoride to the deeper layer of enamel.
2. Highly insoluble tin fluorophosphates complex form on the enamel
surface that acts as a protective layer for the enamel decay.
Disadvantages
1. Unstable in aqueous solution and undergoes rapid oxidation so should
be prepared fresh for each patient.
2. It is highly acidic in nature (pH 2.1-2.3)
3. It has metallic taste which is unacceptable to most of the children and
patient.
4. It may cause gingival irritation particularly to dehydrated and diseased
gingival tissues.
8
5. SnF2 produces discoloration of hypocalcified area of teeth.
6. It will produce staining on the margins of the restorations
Fluoride Gels
Fluoride gels and foams contain a high concentration of fluoride,
typically up to 12.3 mg fluoride
Acidulated Phosphate Fluoride
1.23% is used
• 12,300 ppm of available fluoride
• 3.0 pH
• 28% effective in caries reduction
[Brudevolds Solution]
This is available as either as a solution or gel. Both are stable.
Method of Preparation
Solution: It is prepared by dissolving 20 gms of sodium fluoride in 1 liter
of 0.1 M phosphoric acid. To this is added 50 percent hydrofluoric acid to
maintain a pH of 3.0 and fluoride ion concentration at 1.23 percent.
Gel: for preparation of gel [APF], a gelling agent methylcellulose or
hydroxyethyl cellulose is added to the solution and the pH is adjusted 4-5.
Another form of APF Thixotropic gels is available. Thixotropic denotes a
solution that sets in a gel like state but is not a true gel. Upon the
application if pressure, thixotropic gels behave like solutions.
Recommended frequency of APF application is twice a year topically
Mechanism of Action
APF when applied on teeth initially leads to dehydration and shrinkage in
the volume of hydroxyapatite crystals. There is further hydrolysis and
formation of di-calcium phosphate dehydrate (DCPD), which is highly
reactive The fluoride ions start penetrating into the deeper crystalline
structure of enamel and forms fluorapatite which is stronger to acid
dissolution.

9
Advantages
1. It is stable when stored in a plastic container.
2. No staining of teeth.
3. Gels can self applied.
4. Cheap
Disadvantages
1. Cannot be stored in glass container because it may remove minerals
from the glass [etch].
2. Repeated exposure of porcelain or composite restorations to APF can
lead to loss of material leading to surface roughening and cosmetic
changes hence not advisable to use acidic topical fluoride agent in
patients with these type of restorations.
3. It has an acidic taste.
4. Repeated application necessitates the use of suction, limiting its use in
field programs.
For patients with porcelain or resin restorations, neutral sodium fluoride
is recommended to prevent etching of restorations.
FluorideVarnishes
Fluoridated varnishes were introduced into the market in the 1960s, and
are intended for professional application only.
The main advantages of varnishes are
The prolonged contact time between fluoride and the tooth surfaces
(increases fluoride uptake by dental hard tissues, as well as
The formation of CaF2 reservoirs),and
The possibility of using very small amounts of the product (a thin
layer), which minimizes the risk of excessive fluoride ingestion.
These products are much more concentrated than gels, with typical
concentrations of 22,600 ppm fluoride (in NaF varnishes) 7,000 ppm
fluoride (in
Fluor protector [Silane fluoride] varnishes)
Duraphat are the most used and studied products. In order to achieve the
maximum benefits for caries prevention, varnishes must be applied (2– 4)
times/year, depending on caries risk considerations.
10
Despite having higher fluoride concentrations, varnishes can be regarded
as a safer option when compared to gels, due to the small amount used
during application. Fluoride concentrations in plasma and urine of
children were reported to be lower than toxic levels after the application
of a fluoride varnish.
Method of Varnish Application
1. Oral prophylaxis is done.
2. Teeth are dried and but not isolated with cotton rolls as varnish sticks
to cotton.
3. First lower arch is taken up for application and then upper arch as
saliva collects rapidly on the lower arch.
4. Dispense a small amount of varnish (0.3 ml to 0.5 ml, or 2 drops, for
the entire primary dentition) to the applicator dish or pad.
5. Application is done with single tufted brush starting with proximal
surfaces (Dental floss can be used to ensure that the varnish reaches
interproximal areas) (Fig. 30.21).
6. Since varnish sets rapidly when they come in contact with saliva, no
drying is necessary.
7. After application, patient is made to sit with mouth open for 4 minutes.
8. Patient is instructed not to rinse or drink anything for 1 hour, and not to
eat anything solid and avoid brushing till next morning. Patient is advised
to take liquids or semisolids only, as contact between varnish and tooth
surface is maintained for about 18 hours. It is for prolonged interaction
between fluoride and enamel.
Fluoride Prophylactic Paste
The major functions of prophylactic paste are:
1. To clean the tooth surface through the removal of all exogenous
deposits.
2. Polish the dental hard tissues, including restorations.
Prophylactic paste contains abrasive particles which abrade the deposits
and debris from tooth surface. Now a days APF-silicone dioxide paste
and SnF2 - Zirconium silicate paste are also available. Studies have
shown that their use alone cannot be considered as an effective cariostatic
method. A thorough polishing may remove a thin, but highly mineralized
11
outer layer of enamel. If prophylaxis is required for periodontal reason or
cosmetic reasons then fluoride prophylactic past recommended, as it may
help replenish the minerals that abraded during polishing. They may have
a modest carious effect.
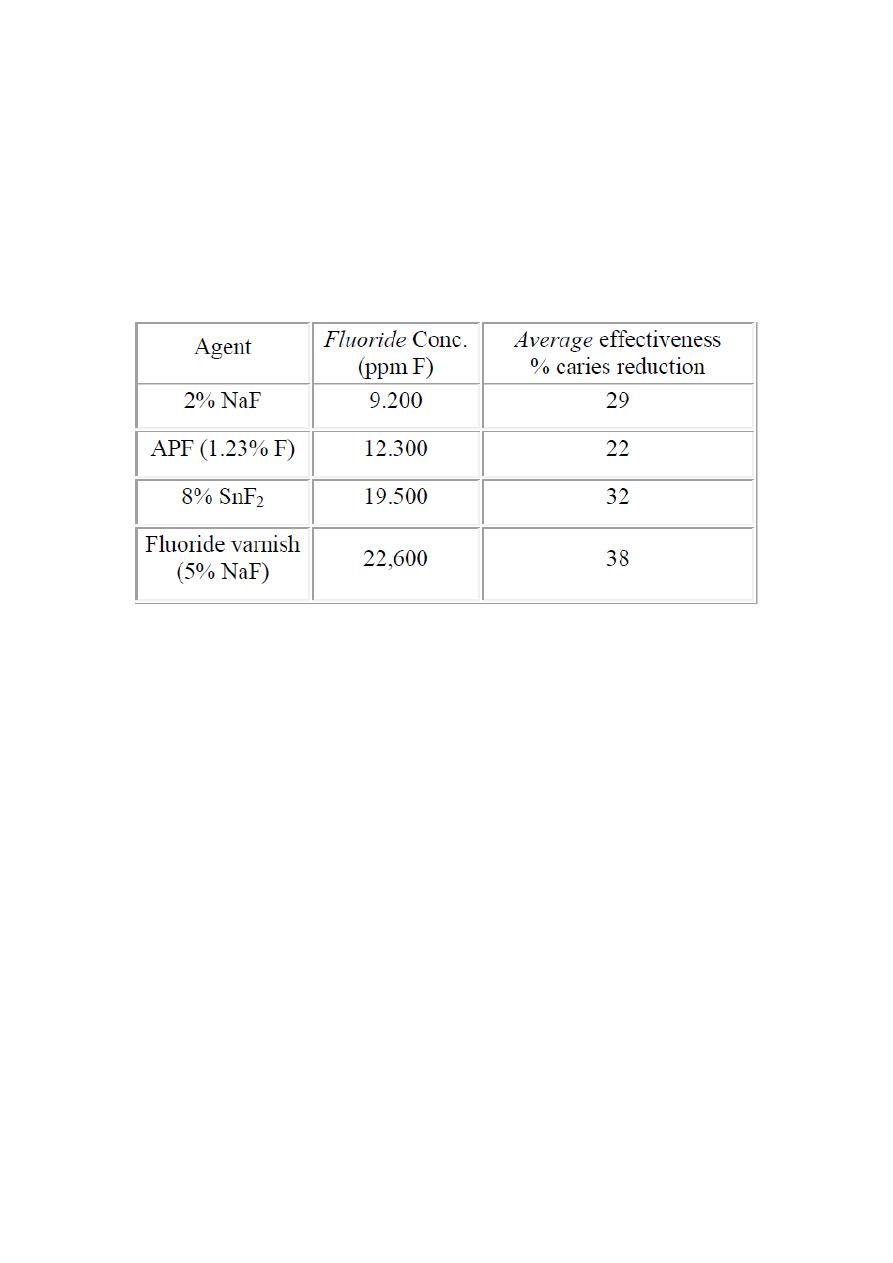
Comparative effectiveness of professionally applied topical fluoride
agents
