CORONARY HEART DISEASE
Coronary heart disease (CHD) is the most common form of heart disease and the single most important cause of
premature death in Europe. By 2020 it is estimated that it will be the major cause of death in all regions of the world.
The death rates from CHD in the UK are amongst the highest in Western Europe but are falling, particularly in younger age
groups; in the last 10 years CHD mortality has fallen by 42% among UK men and women aged 16-64. However, in Eastern
Europe and much of Asia, the rates of CHD are rapidly rising.
Disease of the coronary arteries is almost always due to atheroma and its complications, particularly thrombosis .
Occasionally, the coronary arteries are involved in other disorders such as aortitis, polyarteritis and other connective tissue
disorders.
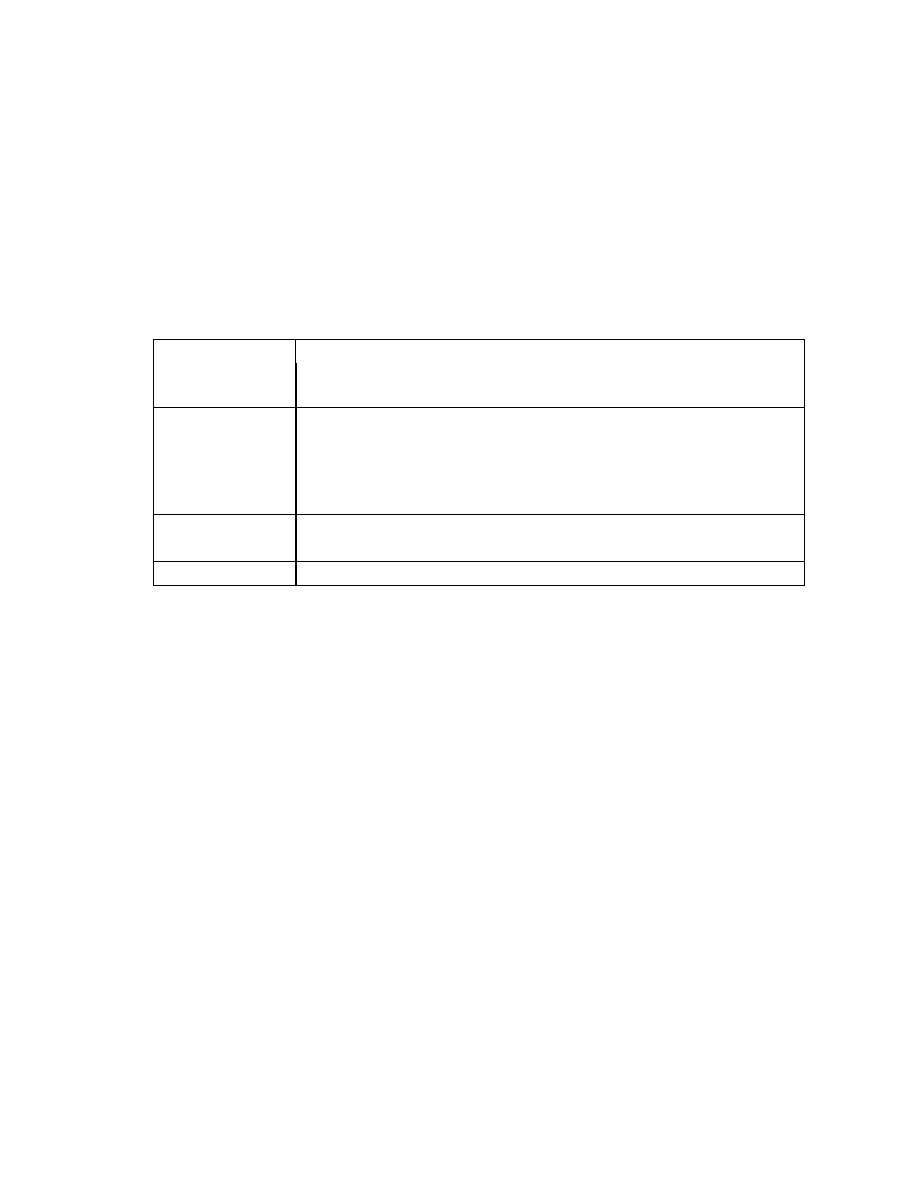
Coronary heart disease: clinical manifestations and pathology
Clinical problem Pathology
Stable angina
Ischaemia due to fixed atheromatous stenosis of one or more
coronary arteries
Unstable angina Ischaemia caused by dynamic obstruction of a coronary artery due to
plaque rupture or erosion with superimposed thrombosis
Myocardial
infarction
Myocardial necrosis caused by acute occlusion of a coronary artery
due to plaque rupture or erosion with superimposed thrombosis
Heart failure
Myocardial dysfunction due to infarction or ischaemia
Arrhythmia
Altered conduction due to ischaemia or infarction
Sudden death
Ventricular arrhythmia, asystole or massive MI
Cardiovascular risk is predicted from special premade charts which depends on the patient's age, sex, smoking habit, BP
and cholesterol ratio. The ratio of total to high-density lipoprotein (HDL) cholesterol can be determined in a non-fasting
blood sample. Where HDL cholesterol concentration is unknown, it should be assumed to be 1 mmol/L; the lipid scale
should be used as total serum cholesterol. Current guidelines suggest initiation of primary prevention in individuals with a
10-year cardiovascular risk ≥ 20%. Patients with diabetes mellitus should be assumed to have a 10-year cardiovascular risk
of ≥ 20% and receive secondary prevention therapy.
Use of statins in prevention of atherosclerotic disease
1- Primary prevention : In patients without evidence of coronary disease but with high serum cholesterol concentrations,
cholesterol-lowering with statins does not lower mortality but does prevent coronary events (angina and MI).
2- Secondary prevention :In patients with established coronary disease (MI or angina), statin therapy can safely reduce the
5-year incidence of all-cause death as well as major coronary events, coronary revascularisation and stroke.
ACE inhibitors and secondary prevention of atherosclerotic disease :- ACE inhibitor therapy reduces the risk of death,
MI and stroke in patients with atherosclerotic vascular disease without apparent left ventricular systolic dysfunction or heart
failure.
Aspirin and secondary prevention in atherosclerotic vascular disease:-In patients with established coronary heart
disease, peripheral vascular disease or thrombotic stroke, aspirin is effective in reducing morbidity and mortality (non-fatal
MI, stroke and cardiovascular death). In patients at high risk of future vascular events, the overall risk reduction is 22%.
Stable angina
Angina pectoris is the symptom complex caused by transient myocardial ischaemia and constitutes a clinical syndrome rather
than a disease. It may occur whenever there is an imbalance between myocardial oxygen supply and demand . Coronary

atheroma is by far the most common cause of angina, although the symptom may be a manifestation of other forms of heart
disease, particularly aortic valve disease and hypertrophic cardiomyopathy.
Factors influencing myocardial oxygen supply and demand
Oxygen demand: cardiac work
Heart rate
BP
Myocardial contractility
Left ventricular hypertrophy
Valve disease, e.g. aortic stenosis
Oxygen supply: coronary blood flow
Duration of diastole
Coronary perfusion pressure (aortic diastolic minus coronary sinus or right atrial diastolic pressure)
Coronary vasomotor tone
Oxygenation
o
Haemoglobin
o
Oxygen saturation
Activities precipitating angina
Common
Physical exertion
Cold exposure
Heavy meals
Intense emotion
Uncommon :- Lying flat (decubitus angina) and
Vivid dreams (nocturnal angina)
The history is by far the most important factor in making the diagnosis . Stable angina is characterised by central
chest pain, discomfort or breathlessness that is precipitated by exertion or other forms of stress , and is promptly relieved by
rest . Some patients find that the discomfort comes when they start walking, and that later it does not return despite greater
effort ('warm-up angina')
Physical examination is frequently unremarkable but should include a careful search for evidence of valve disease
(particularly aortic), important risk factors (e.g. hypertension, diabetes mellitus), left ventricular dysfunction (cardiomegaly,
gallop rhythm), other manifestations of arterial disease (carotid bruits, peripheral vascular disease) and unrelated conditions
that may exacerbate angina (anaemia, thyrotoxicosis).
Investigations
Resting ECG
The ECG may show evidence of previous MI but is often normal, even in patients with severe coronary artery
disease. Occasionally, there is T-wave flattening or inversion in some leads, providing non-specific evidence of
myocardial ischaemia or damage. The most convincing ECG evidence of myocardial ischaemia is the
demonstration of reversible ST segment depression or elevation, with or without T-wave inversion, at the time
the patient is experiencing symptoms (whether spontaneous or induced by exercise testing).
Exercise ECG
An exercise tolerance test (ETT) is usually performed using a standard treadmill or bicycle ergometer protocol
while monitoring the patient's ECG, BP and general condition. Planar or down-sloping ST segment depression of
≥ 1 mm is indicative of ischaemia . Up-sloping ST depression is less specific and often occurs in normal
individuals.
Exercise testing is also a useful means of assessing the severity of coronary disease and identifying high-risk
individuals . For example, the amount of exercise that can be tolerated and the extent and degree of any ST
segment change provide a useful guide to the likely extent of coronary disease. Exercise testing may produce

false positive results in the presence of digoxin therapy, left ventricular hypertrophy, bundle branch block or
WPW syndrome. The predictive accuracy of exercise testing is lower in women than men. The test should be
classed as inconclusive (rather than negative) if the patient cannot achieve an adequate level of exercise because
of locomotor or other non-cardiac problems.
Risk stratification in stable angina
High risk Low risk
Post-infarct angina Predictable exertional angina
Poor effort tolerance Good effort tolerance
Ischaemia at low workload Ischaemia only at high workload
Left main or three-vessel disease Single-vessel or two-vessel disease
Poor LV function Good LV function
Other forms of stress testing
1-
Myocardial perfusion scanning. This may be helpful in the evaluation of patients with an equivocal or
uninterpretable exercise test and those who are unable to exercise. It includes obtaining scintiscans of the
myocardium at rest and during stress (either exercise testing or
pharmacological stress, such as a controlled infusion of dobutamine) after the administration of an intravenous
radioactive isotope, such as 99technetium tetrofosmin. Thallium and tetrofosmin are taken up by viable perfused
myocardium. A perfusion defect present during stress but
not at rest provides evidence of reversible myocardial ischaemia, whereas a persistent perfusion defect seen
during both phases of the study is usually indicative of previous MI.
2- Stress echocardiography. This is an alternative to myocardial perfusion scanning and can achieve similar
predictive accuracy. It uses transthoracic echocardiography to identify ischaemic segments of myocardium and
areas of infarction . The former characteristically exhibit reversible defects in contractility during exercise or
pharmacological stress, and the latter do not contract at rest or during stress.
Coronary arteriography
This provides detailed anatomical information about the extent and nature of coronary artery disease, and is
usually performed with a view to coronary artery bypass graft (CABG) surgery or percutaneous coronary
intervention (PCI). In some patients, diagnostic coronary angiography may be indicated when non-invasive tests
have failed to establish the cause of atypical chest pain. The procedure is performed under local anaesthesia and
requires specialised radiological equipment, cardiac monitoring and an experienced operating team.
Management
: general measures
The management of angina pectoris involves:
1-a careful assessment of the likely extent and severity of arterial disease
2- the identification and control of risk factors such as smoking, hypertension and hyperlipidaemia
3- the use of measures to control symptoms
4- the identification of high-risk patients for treatment to improve life expectancy.
Management should start with a careful explanation of the problem and a discussion of the potential lifestyle and
medical interventions that may relieve symptoms and improve prognosis . Anxiety and misconceptions often
contribute to disability; for example, some patients avoid all forms of exertion because they believe that each
attack of angina is a 'mini heart attack' that results in permanent damage. Effective management of these
psychological factors can make a huge difference to the patient's quality of life.
Advice to patients with stable angina
1- Do not smoke
2- Aim for ideal body weight
3- Take regular exercise (exercise up to, but not beyond, the point of chest discomfort is beneficial and may
promote collateral vessels)
4- Avoid severe unaccustomed exertion, and vigorous exercise after a heavy meal or in very cold weather
5- Take sublingual nitrate before undertaking exertion that may induce angina

Antiplatelet therapy
Low-dose (75 mg) aspirin reduces the risk of adverse events such as MI and should be prescribed for all patients
with coronary artery disease indefinitely. Clopidogrel (75 mg daily) is an equally effective antiplatelet agent that
can be prescribed if aspirin causes troublesome dyspepsia or other side-effects.
Anti-anginal drug treatment
Five groups of drug are used to help relieve or prevent the symptoms of angina: nitrates, β-blockers, calcium
antagonists, potassium channel activators and an I
f
channel antagonist.
Nitrates
These drugs act directly on vascular smooth muscle to produce venous and arteriolar dilatation. Their beneficial
effects are due to a reduction in myocardial oxygen demand (lower preload and afterload) and an increase in
myocardial oxygen supply (coronary vasodilatation). Sublingual glyceryl
trinitrate (GTN) administered from a metered-dose aerosol (400 μg per spray) or as a tablet (300 or 500 μg) will
relieve an attack of angina in 2-3 minutes. Side-effects include headache, symptomatic hypotension and, rarely,
syncope.
Patients should be encouraged to use the drug prophylactically before taking exercise that is liable to provoke
symptoms. Sublingual GTN has a short duration of action ; however, a variety of alternative nitrate preparations
can provide a more prolonged therapeutic effect. GTN can be given transcutaneously as a patch (5-10 mg daily),
or as a slow-release buccal tablet (1-5 mg 6-hourly). GTN undergoes extensive first-pass metabolism in the liver
and is ineffective when swallowed. Other nitrates such as isosorbide dinitrate (10-20 mg 8-hourly) and isosorbide
mononitrate (20-60 mg once or twice a day) can be given by mouth. Headache is common but tends to diminish
if the patient perseveres with the treatment Continuous nitrate therapy can cause pharmacological tolerance. This
can be avoided by a 6-8-hour nitrate-free period, best achieved at night when the patient is inactive. If nocturnal
angina is a predominant symptom, long-acting nitrates can be given at the end of the day.
Beta-blockers
These lower myocardial oxygen demand by reducing heart rate, BP and myocardial contractility, but they may
provoke bronchospasm in patients with asthma. In theory, non-selective β-blockers may aggravate coronary
vasospasm by blocking the coronary artery β2-adrenoceptors and so a once-daily
cardioselective preparation is used (e.g. slow-release metoprolol 50-200 mg daily, bisoprolol 5-15 mg daily).
Beta-blockers should not be withdrawn abruptly because this may have a rebound effect and precipitate
dangerous arrhythmias, worsening angina or MI: the β-blocker withdrawal syndrome.
Calcium channel antagonists
These drugs inhibit the slow inward current caused by the entry of extracellular calcium through the cell
membrane of excitable cells, particularly cardiac and arteriolar smooth muscle, and lower myocardial oxygen
demand by reducing BP and myocardial contractility. Dihydropyridine calcium antagonists, such as nifedipine
and nicardipine, often cause a reflex tachycardia. This may be counterproductive and it is best to use them in
combination with a β-blocker. In contrast, verapamil and diltiazem are particularly suitable for patients who are
not receiving a β-blocker (e.g. those with airways obstruction) because they slow SA node firing, inhibit
conduction through the AV node and tend to cause a bradycardia. Calcium channel antagonists reduce
myocardial contractility and can aggravate or precipitate heart failure. Other unwanted effects include peripheral
oedema, flushing, headache and dizziness.
Potassium channel activators
These have arterial and venous dilating properties but do not exhibit the tolerance seen with nitrates. Nicorandil
(10-30 mg 12-hourly orally) is the only drug in this class currently available for clinical use.
I
f
channel antagonist
Ivabradine is the first of this class of drug. It induces bradycardia by modulating ion channels in the sinus node.
In contrast to β-blockers and rate-limiting calcium antagonists, it does not have other cardiovascular effects. It
appears to be safe to use in patients with heart failure.
Although each of these anti-anginal drugs is superior to placebo in relieving the symptoms of angina, there is
little evidence that one group is more effective than another. It is conventional to start therapy with low-dose
aspirin, a statin, sublingual GTN and a β-blocker, and then add a calcium channel antagonist or a long-acting
nitrate later if needed. The goal is the control of angina with minimum side-effects and the simplest possible drug
regimen. There is little evidence that prescribing multiple anti-anginal drugs is of benefit, and revascularisation
should be considered if an appropriate combination of two or more drugs fails to achieve an acceptable
symptomatic response.

Invasive treatment
Percutaneous coronary intervention (PCI)
This is performed by passing a fine guidewire across a coronary stenosis under radiographic control and using it
to position a balloon which is then inflated to dilate the stenosis. A coronary stent is a piece of coated metallic
'scaffolding' that can be deployed on a balloon and used to maximise and maintain dilatation of a stenosed vessel.
The routine use of stents in appropriate vessels reduces both acute
complications and the incidence of clinically important restenosis . PCI provides an effective symptomatic
treatment but definitive evidence that it improves survival in patients with chronic stable angina is lacking. It is
mainly used in single or two-vessel disease. Stenoses in bypass grafts can be dilated, as well as those in the
native coronary arteries. The technique is often used to provide palliative therapy for patients with recurrent
angina after CABG. Coronary surgery is usually the preferred option in patients with three-vessel or left main
stem disease, although recent trials have demonstrated that PCI is also feasible in such patients. The main acute
complications of PCI are occlusion of the target vessel or a side branch by thrombus or a loose flap of intima
(coronary artery dissection), and consequent myocardial damage. This occurs in about 2-5% of procedures and
can often be corrected by deploying a stent; however, emergency CABG is sometimes required. Minor
myocardial damage, as indicated by elevation of sensitive intracellular markers (troponins), occurs in up to 10%
of cases. The main long-term complication of PCI is restenosis , which occurs in up to one-third of cases. This is
due to a combination of elastic recoil and smooth muscle proliferation (neo-intimal hyperplasia) and tends to
occur within 3 months. Stenting substantially reduces the risk of restenosis, probably because it allows the
operator to achieve more complete dilatation in the first place. Drug-eluting stents can reduce this risk even
further by allowing an antiproliferative drug such as sirolimus or paclitaxel to elute slowly from the coating and
prevent neo-intimal hyperplasia and in-stent restenosis. There is an increased risk of late stent thrombosis with
drug-eluting stents, although the absolute risk is small (< 0.5%). Recurrent angina (affecting up to 15-20% of
patients receiving an intracoronary stent at 6 months) may require further PCI or bypass grafting.
Coronary artery bypass grafting (CABG)
The internal mammary arteries, radial arteries or reversed segments of the patient's own saphenous vein can be
used to bypass coronary artery stenoses . This usually involves major surgery under cardiopulmonary bypass, but
in some cases, grafts can be applied to the beating heart: 'off-pump' surgery. The operative mortality is
approximately 1.5% but risks are higher in elderly patients, those with poor left ventricular function and those
with significant comorbidity, such as renal failure.
Approximately 90% of patients are free of angina 1 year after CABG surgery, but fewer than 60% of patients are
asymptomatic after 5 or more years. Early post-operative angina is usually due to graft failure arising from
technical problems during the operation, or poor 'run-off' due to disease in the
distal native coronary vessels. Late recurrence of angina may be due to progressive disease in the native coronary
arteries or graft degeneration. Less than 50% of vein grafts are patent 10 years after surgery. However, arterial
grafts have a much better long-term patency rate, with more than 80% of internal mammary artery grafts patent
at 10 years. This has led many surgeons to consider total arterial revascularisation during CABG surgery. Aspirin
(75-150 mg daily) and clopidogrel (75 mg daily) have both been shown to improve graft patency, and one or
other should be prescribed indefinitely if well tolerated. Intensive lipid-lowering therapy slows the progression of
disease in the native coronary arteries and bypass grafts, and reduces clinical cardiovascular events. There is
substantial excess cardiovascular morbidity and mortality in patients who continue to smoke after bypass
grafting. Persistent smokers are twice as likely to die in the 10 years following surgery than those who give up at
surgery. CABG improves survival in symptomatic patients with left main stem stenosis or three-vessel coronary
disease (i.e. involving LAD, CX and right coronary arteries) or two-vessel disease involving the proximal LAD
coronary artery. Improvement in survival is most marked in those with impaired left ventricular function or
positive stress testing prior to surgery and those who have undergone left internal mammary artery grafting.
Neurological complications are common, with a 1-5% risk of perioperative stroke. Between 30% and 80% of
patients develop short-term cognitive impairment that is often mild and typically resolves within 6 months. There
are also reports of long-term cognitive decline that may be evident in more than 30% of patients at 5 years.
Comparison of PCI and CABG
PCI CABG

Death < 0.5% < 1.5%
Myocardial infarction* 2% 10%
Hospital stay 12-36 hrs 5-8 days
Return to work 2-5 days 6-12 wks
Recurrent angina 15-20% at 6 mths 10% at 1 yr
Repeat revascularisation 10-20% at 2 yrs 2% at 2 yrs
Neurological complications Rare Common
Other complications Emergency CABG, Diffuse myocardial damage;
vascular damage infection
Prognosis
Symptoms are a poor guide to prognosis; nevertheless, the 5-year mortality of patients with severe angina
(NYHA class III or IV) is nearly double that of patients with mild symptoms. Exercise testing and other forms of
stress testing are much more powerful predictors of mortality.
In general, the prognosis of coronary artery disease is related to the number of diseased vessels and the
degree of left ventricular dysfunction. A patient with single-vessel disease and good left ventricular function has
an excellent outlook (5-year survival > 90%), whereas a patient with severe left ventricular dysfunction and
extensive three-vessel disease has a poor prognosis (5-year survival < 30%) without revascularisation.
Spontaneous symptomatic improvement due to the development of collateral vessels is common.
Angina with normal coronary arteries
Approximately 10% of patients who report stable angina on effort will have angiographically normal coronary
arteries. Many of these patients are women and the mechanism of their symptoms is often difficult to establish. It
is important to review the original diagnosis and explore other potential causes.
Coronary artery spasm
Vasospasm in coronary arteries may coexist with atheroma, especially in unstable angina ; in < 1% of cases,
vasospasm may occur without angiographically detectable atheroma. This form of angina is sometimes known as
variant angina, and may be accompanied by spontaneous and transient ST elevation on the ECG (Prinzmetal's
angina). Calcium channel antagonists, nitrates and other coronary vasodilators are the most useful therapeutic
agents but may be ineffective.
Syndrome X
The constellation of typical angina on effort, objective evidence of myocardial ischaemia on stress testing, and
angiographically normal coronary arteries is sometimes known as syndrome X. This disorder is poorly
understood but carries a good prognosis and may respond to treatment with anti-anginal therapy.
Acute coronary syndrome
Acute coronary syndrome is a term that encompasses both unstable angina and MI. Unstable angina is
characterised by new-onset or rapidly worsening angina (crescendo angina), angina on minimal exertion or
angina at rest in the absence of myocardial damage. In contrast, MI occurs when symptoms occur at rest and
there is evidence of myocardial necrosis, as demonstrated by an elevation in cardiac troponin or creatine kinase-
MB isoenzyme.
Universal definition of myocardial infarction*
The term 'myocardial infarction' should be used when there is evidence of myocardial necrosis in a clinical
setting consistent with myocardial ischaemia, in which case any one of the following meets the diagnosis for
MI:-
Detection of rise and/or fall of cardiac biomarkers (preferably troponin), with at least one value above the
99th percentile of the upper reference limit, together with at least one of the following:
o
Symptoms of ischaemia
o
ECG changes indicative of new ischaemia (new ST-T changes or new left bundle branch block)
o
Development of pathological Q waves
o
Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality

Sudden unexpected cardiac death, involving cardiac arrest, often with symptoms suggestive of
myocardial ischaemia, and accompanied by presumably new ST elevation or new left bundle branch block,
and/or evidence of fresh thrombus by coronary angiography and/or at autopsy, but death occurring before blood
samples could be obtained or before the appearance of cardiac biomarkers in the blood
Pathological findings of an acute MI
An acute coronary syndrome may present as a new phenomenon or against a background of chronic stable
angina. The culprit lesion is usually a complex ulcerated or fissured atheromatous plaque with adherent platelet-
rich thrombus and local coronary artery spasm. This is a dynamic process whereby the degree of obstruction may
either increase, leading to complete vessel occlusion, or regress due to the effects of platelet disaggregation and
endogenous fibrinolysis. In acute MI, occlusive thrombus is almost always present at the site of rupture or
erosion of an atheromatous plaque. The thrombus may undergo spontaneous lysis over the course of the next few
days, although by this time irreversible myocardial damage has occurred. Without treatment, the infarct-related
artery remains permanently occluded in 20-30% of patients. The process of infarction progresses over several
hours and most patients present when it is still possible to salvage myocardium and improve outcome.
Clinical features
Pain is the cardinal symptom of an acute coronary syndrome but breathlessness, vomiting, and collapse are
common features. The pain occurs in the same sites as angina but is usually more severe and lasts longer; it is
often described as a tightness, heaviness or constriction in the chest. In acute MI, the pain can be excruciating,
and the patient's expression and pallor may vividly convey the seriousness of the situation.
Most patients are breathless and in some this is the only symptom. Indeed, MI may pass unrecognised.
Painless or 'silent' MI is particularly common in older patients or those with diabetes mellitus. If syncope occurs,
it is usually due to an arrhythmia or profound hypotension. Vomiting and sinus bradycardia are often due to
vagal stimulation and are particularly common in patients with inferior MI. Nausea and vomiting may also be
caused or aggravated by opiates given for pain relief. Sometimes infarction occurs in the absence of physical
signs.
Sudden death, from VF or asystole, may occur immediately and often within the first hour. If the patient survives
this most critical stage, the liability to dangerous arrhythmias remains, but diminishes as each hour goes by.
Symptoms
Prolonged cardiac pain: chest, throat, arms, epigastrium or back
Anxiety and fear of impending death
Nausea and vomiting
Breathlessness
Collapse/syncope
Physical signs
Signs of sympathetic activation: pallor, sweating, tachycardia
Signs of vagal activation: vomiting, bradycardia
Signs of impaired myocardial function
o
Hypotension, oliguria, cold peripheries
o
Narrow pulse pressure
o
Raised JVP
o
Third heart sound
o
Quiet first heart sound
o
Diffuse apical impulse
o
Lung crepitations
Signs of tissue damage: fever
Signs of complications: e.g. mitral regurgitation, pericarditis

Diagnosis and risk stratification
The differential diagnosis is wide and includes most causes of central chest pain or collapse. The
assessment of acute chest pain depends on an analysis of the character of the pain and its associated features,
evaluation of the ECG, and serial measurements of biochemical markers of cardiac damage, such as troponin I
and T. A 12-lead ECG is mandatory and defines the initial triage, management and treatment. Patients with ST
segment elevation or new bundle branch block require emergency reperfusion therapy. In patients with acute
coronary syndrome without ST segment elevation, the ECG may show transient or persistent ST/T wave changes
including ST depression and T-wave inversion.
Approximately 12% of patients will die within 1 month and a fifth within 6 months of the index event.
The risk markers that are indicative of an adverse prognosis include recurrent ischaemia, extensive ECG changes
at rest or during pain, the release of biochemical markers (creatine kinase or troponin), arrhythmias, recurrent
ischaemia and haemodynamic complications (e.g. hypotension, mitral regurgitation) during episodes of
ischaemia.
Investigations
Electrocardiography
The ECG is central to confirming the diagnosis but may be difficult to interpret if there is bundle branch block or
previous MI. The initial ECG may be normal or non-diagnostic in one-third of cases. Repeated ECGs are
important, especially where the diagnosis is uncertain or the patient has recurrent or persistent symptoms.
The earliest ECG change is usually ST-segment deviation. With proximal occlusion of a major coronary artery,
ST-segment elevation (or new bundle branch block) is seen initially with later diminution in the size of the R
wave, and in transmural (full thickness) infarction, development of a Q wave. Subsequently, the T wave becomes
inverted because of a change in ventricular repolarisation; this change persists after the ST segment has returned
to normal. These sequential features are sufficiently reliable for the approximate age of the infarct to be
deduced.
In non-ST segment elevation acute coronary syndrome, there is partial occlusion of a major vessel or complete
occlusion of a minor vessel, causing unstable angina or partial-thickness (subendocardial) MI. This is usually
associated with ST-segment depression and T-wave changes. In the presence of infarction, this may be
accompanied by some loss of R waves in the absence of Q waves .
The ECG changes are best seen in the leads that 'face' the ischaemic or infarcted area. When there has been
anteroseptal infarction, abnormalities are found in one or more leads from V
1
to V
4
, while anterolateral infarction
produces changes from V
4
to V
6
, in aVL and in lead I. Inferior infarction is best shown in leads II, III and aVF,
while at the same time leads I, aVL and the anterior chest leads may show 'reciprocal' changes of ST depression .
Infarction of the posterior wall of the LV does not cause ST elevation or Q waves in the standard leads, but can
be diagnosed by the presence of reciprocal changes (ST depression and a tall R wave in leads V
1
-V
4
). Some
infarctions (especially inferior) also involve the RV. This may be identified by recording from additional leads
placed over the right precordium.
Plasma cardiac markers
In unstable angina, there is no detectable rise in cardiac markers or enzymes, and the initial diagnosis is made
from the clinical history and ECG only. In contrast, MI causes a rise in the plasma concentration of enzymes and
proteins that are normally concentrated within cardiac cells. These biochemical markers are creatine kinase (CK),
a more sensitive and cardiospecific isoform of this enzyme (CK-MB), and the cardiospecific proteins, troponins
T and I. Admission and serial (usually daily) estimations are helpful because it is the change in plasma
concentrations of these markers that confirms the diagnosis of MI .
CK starts to rise at 4-6 hours, peaks at about 12 hours and falls to normal within 48-72 hours. CK is also present
in skeletal muscle, and a modest rise in CK (but not CK-MB) may sometimes be due to an intramuscular
injection, vigorous physical exercise or, particularly in older people, a fall. Defibrillation causes significant
release of CK but not CK-MB or troponins. The most sensitive markers of myocardial cell damage are the
cardiac troponins T and I, which are released within 4-6 hours and remain elevated for up to 2 weeks.
Other blood tests
A leucocytosis is usual, reaching a peak on the first day. The erythrocyte sedimentation rate (ESR) and C-
reactive protein (CRP) are also elevated.

Chest X-ray
This may demonstrate pulmonary oedema that is not evident on clinical examination . The heart size is often
normal but there may be cardiomegaly due to pre-existing myocardial damage.
Echocardiography
This is useful for assessing left and right ventricular function and for detecting important complications such as
mural thrombus, cardiac rupture, ventricular septal defect, mitral regurgitation and pericardial effusion.
Immediate management: the first 12 hours
Patients should be admitted urgently to hospital because there is a significant risk of death or recurrent
myocardial ischaemia during the early unstable phase, and appropriate medical therapy can reduce the incidence
of these by at least 60%.
Patients are usually managed in a dedicated cardiac unit where the necessary expertise, monitoring and
resuscitation facilities can be concentrated. If there are no complications, the patient can be mobilised from the
second day and discharged from hospital after 3-5 days.
Analgesia
Adequate analgesia is essential not only to relieve distress, but also to lower adrenergic drive and thereby reduce
vascular resistance, BP, infarct size and susceptibility to ventricular arrhythmias. Intravenous opiates (initially
morphine sulphate 5-10 mg or diamorphine 2.5-5 mg) and antiemetics (initially metoclopramide 10 mg) should
be administered and titrated by giving repeated small aliquots until the patient is comfortable. Intramuscular
injections should be avoided because the clinical effect may be delayed by poor skeletal muscle perfusion, and a
painful haematoma may form following thrombolytic or anti-thrombotic therapy.
Antithrombotic therapy
Antiplatelet therapy
In patients with acute coronary syndrome, oral administration of 75-300 mg aspirin daily improves survival, with
a 25% relative risk reduction in mortality. The first tablet (300 mg) should be given orally within the first 12
hours and therapy should be continued indefinitely if there are no side-effects. In combination with aspirin, the
early (within 12 hours) use of clopidogrel 600 mg, followed by 150 mg daily for 1 week and 75 mg daily
thereafter, confers a further reduction in ischaemic events . In patients with an acute coronary syndrome with or
without ST-segment elevation, ticagrelor (180 mg followed by 90 mg 12-hourly) is more effective than
clopidogrel in reducing vascular death, MI or stroke, and all-cause death without affecting overall major bleeding
risk.
Glycoprotein IIb/IIIa receptor antagonists, such as tirofiban and abciximab, block the final common pathway of
platelet aggregation and are potent inhibitors of platelet-rich thrombus formation. They are of particular benefit
in patients with acute coronary syndromes who undergo PCI, those with recurrent ischaemia and those at
particularly high risk, such as patients with diabetes mellitus or an elevated troponin concentration.
Anticoagulants
Anticoagulation reduces the risk of thromboembolic complications, and prevents reinfarction in the absence of
reperfusion therapy or after successful thrombolysis . Anticoagulation can be achieved using unfractionated
heparin, fractioned (low molecular weight) heparin or a pentasaccharide. Comparative clinical trials suggest that
the pentasaccharides (subcutaneous fondaparinux 2.5 mg daily) have the best safety and efficacy profile, with
low molecular weight heparin (subcutaneous enoxaparin 1 mg/kg 12-hourly) being a reasonable alternative.
Anticoagulation should be continued for 8 days or until discharge from hospital or coronary revascularisation.
A period of treatment with warfarin should be considered if there is persistent atrial fibrillation or evidence of
extensive anterior infarction, or if echocardiography shows mobile mural thrombus, because these patients are at
increased risk of systemic thromboembolism.
Anti-anginal therapy
Sublingual glyceryl trinitrate (300-500 μg) is a valuable first-aid measure in unstable angina or threatened
infarction, and intravenous nitrates (GTN 0.6-1.2 mg/hour or isosorbide dinitrate 1-2 mg/hour) are useful for the
treatment of left ventricular failure and the relief of recurrent or persistent ischaemic pain.
Intravenous β-blockers (e.g. atenolol 5-10 mg or metoprolol 5-15 mg given over 5 mins) relieve pain,
reduce arrhythmias and improve short-term mortality in patients who present within 12 hours of the onset of
symptoms. However, they should be avoided if there is heart failure (pulmonary oedema), hypotension (systolic
BP < 105 mmHg) or bradycardia (heart rate < 65/min).
A dihydropyridine calcium channel antagonist (e.g. nifedipine or amlodipine) can be added to the β-blocker
if there is persistent chest discomfort but may cause an unwanted tachycardia if used alone. Because of their rate-

limiting action, verapamil and diltiazem are the calcium channel antagonists of choice if a β-blocker is
contraindicated.
Reperfusion therapy
Non-ST segment elevation acute coronary syndrome
Immediate emergency reperfusion therapy has no demonstrable benefit in patients with non-ST segment
elevation MI and thrombolytic therapy may be harmful. Selected medium- to high-risk patients do benefit from
in-hospital coronary angiography and coronary revascularisation but this does not need to take place in the first
12 hours.
ST segment elevation acute coronary syndrome
Immediate reperfusion therapy restores coronary artery patency, preserves left ventricular function and improves
survival. Successful therapy is associated with pain relief, resolution of acute ST elevation and sometimes
transient arrhythmias (e.g. idioventricular rhythm).
Primary percutaneous coronary intervention (PCI)
This is the treatment of choice for ST segment elevation MI . Outcomes are best when it is used in combination
with glycoprotein IIb/IIIa receptor antagonists and intracoronary stent implantation. In comparison to
thrombolytic therapy, it is associated with a greater reduction in the risk of death, recurrent MI or stroke . The
universal use of primary PCI has been limited by availability of the necessary resources to provide this highly
specialised emergency service. Thus, intravenous thrombolytic therapy remains the first-line reperfusion
treatment in many hospitals, especially those in rural or remote areas. When primary PCI cannot be achieved
within 2 hours of diagnosis, thrombolytic therapy should be administered.
Thrombolysis
The appropriate use of thrombolytic therapy can reduce hospital mortality by 25-50% and this survival advantage
is maintained for at least 10 years . The benefit is greatest in those patients who receive treatment within the first
few hours: 'minutes mean muscle'.
Alteplase (human tissue plasminogen activator or tPA) is a genetically engineered drug that is given over 90
minutes (bolus dose of 15 mg, followed by 0.75 mg/kg body weight, but not exceeding 50 mg, over 30 mins and
then 0.5 mg/kg body weight, but not exceeding 35 mg, over 60 mins). Its use is associated with better survival
rates than other thrombolytic agents, such as streptokinase, but carries a slightly higher risk of intracerebral
bleeding
Analogues of tPA, such as tenecteplase and reteplase, have a longer plasma half-life than alteplase and can be
given as an intravenous bolus. Tenecteplase (TNK) is as effective as alteplase at reducing death and MI whilst
conferring similar intracerebral bleeding risks. However, other major bleeding and transfusion risks are lower
and the practical advantages of bolus administration may provide opportunities for prompt treatment in the
emergency department or in the pre-hospital setting. Reteplase (rPA) is administered as a double bolus and also
produces a similar outcome to that achieved with alteplase, although some of the bleeding risks appear slightly
higher.
Thrombolytic therapy reduces short-term mortality in patients with MI if it is given within 12 hours of the onset
of symptoms and the ECG shows bundle branch block or characteristic ST segment elevation > 1 mm in the limb
leads or 2 mm in the chest leads . Thrombolysis appears to be of little net benefit and may be harmful in those
who present more than 12 hours after the onset of symptoms and in those with a normal ECG or ST depression.
The benefit is greatest for patients treated within the first 2 hours.
The major hazard of thrombolytic therapy is bleeding. Cerebral haemorrhage causes 4 extra strokes per 1000
patients treated and the incidence of other major bleeds is between 0.5% and 1%.
Relative contraindications to thrombolytic therapy
Ischaemic stroke > 3 months previously
Dementia
Noncompressible vascular puncture
Uncontrolled hypertension (SBP>180mmHg and/or DBP>110 mmHg)
Recent surgery (within 3 weeks)
Recent trauma (including traumatic resuscitation> 10 min)
Internal bleeding within the preceeding 2-4 weeks or active peptic ulcer
Pregnancy
Current warfarin therapy

For streptokinase or anistreptase-a prior exposure( more than 5 dayspreviously) or
allergic reaction to theses drugs
Absolute contraindication for thrombolytic therapy
Haemorrhagic stroke at any time
Ischaemic stroke in preceeding 3 months with the exception of acute ischaemic stroke
within 3 hours
CNS vascular malformation or neoplasm
Significant closed- head or facial injury in preceeding 3 weeks
Active bleeding or bleeding diathesis
Aortic dissection
Complications of acute coronary syndrome
1-Arrhythmias
Common arrhythmias in acute coronary syndrome
Ventricular fibrillation
Ventricular tachycardia
Accelerated idioventricular rhythm
Ventricular ectopics
Atrial fibrillation
Atrial tachycardia
Sinus bradycardia (particularly after inferior MI)
Atrioventricular block
Ventricular fibrillation
This occurs in about 5-10% of patients who reach hospital and is thought to be the major cause of death in those
who die before receiving medical attention. Prompt defibrillation will usually restore sinus rhythm and is life-
saving. The prognosis of patients with early ventricular fibrillation (within the first 48 hours) who are
successfully and promptly resuscitated is identical to that of patients who do not suffer ventricular fibrillation.
Atrial fibrillation
This is common but frequently transient, and usually does not require emergency treatment. However, if the
arrhythmia causes a rapid ventricular rate with hypotension or circulatory collapse, prompt cardioversion by
immediate synchronised DC shock is essential. In other situations, digoxin or a β-blocker is usually the treatment
of choice. Anticoagulation is required if atrial fibrillation persists.
Bradycardia
This does not usually require treatment, but if there is hypotension or haemodynamic deterioration, atropine (0.6-
1.2 mg i.v.) may be given. AV block complicating inferior infarction is usually temporary and often resolves
following reperfusion therapy. If there is clinical deterioration due to second-degree or complete AV block, a
temporary pacemaker should be considered. AV block complicating anterior infarction is more serious because
asystole may suddenly supervene; a prophylactic temporary pacemaker should be inserted .
2-Ischaemia
Patients who develop recurrent angina at rest or on minimal exertion following an acute coronary syndrome are
at high risk and should be considered for prompt coronary angiography with a view to revascularisation. Patients
with dynamic ECG changes and ongoing pain should be treated with intravenous glycoprotein IIb/IIIa receptor
antagonists. Patients with resistant pain or marked haemodynamic changes should be considered for intra-aortic
balloon counterpulsation and emergency coronary revascularisation.
Post-infarct angina occurs in up to 50% of patients treated with thrombolysis. Most patients have a residual
stenosis in the infarct-related vessel despite successful thrombolysis, and this may cause angina if there is still
viable myocardium downstream. For this reason, all patients who have received successful thrombolysis should
be considered for early (within the first 6-24 hours) coronary angiography with a view to coronary
revascularisation.
3-Acute circulatory failure

Acute circulatory failure usually reflects extensive myocardial damage and indicates a bad prognosis. All the
other complications of MI are more likely to occur when acute heart failure is present.
4-Pericarditis
This only occurs following infarction and is particularly common on the second and third days. The patient may
recognise that a different pain has developed, even though it is at the same site, and that it is positional and tends
to be worse or sometimes only present on inspiration. A pericardial rub may be audible. Opiate-based analgesia
should be used. Non-steroidal and steroidal anti-inflammatory drugs may increase the risk of aneurysm formation
and myocardial rupture in the early recovery period, and so should be avoided.
The post-MI syndrome (Dressler's syndrome) is characterised by persistent fever, pericarditis and pleurisy, and
is probably due to autoimmunity. The symptoms tend to occur a few weeks or even months after the infarct and
often subside after a few days; prolonged or severe symptoms may require treatment with high-dose aspirin,
NSAIDs or even corticosteroids.
5-Mechanical complications
Rupture of the papillary muscle can cause acute pulmonary oedema and shock due to the sudden onset of
severe mitral regurgitation, which presents with a pansystolic murmur and third heart sound. In the
presence of severe valvular regurgitation, the murmur may be quiet or absent. The diagnosis is confirmed
by echocardiography and emergency mitral valve replacement may be necessary. Lesser degrees of mitral
regurgitation due to papillary muscle dysfunction are common and may be transient.
Rupture of the interventricular septum causes left-to-right shunting through a ventricular septal defect.
This usually presents with sudden haemodynamic deterioration accompanied by a new loud pansystolic
murmur radiating to the right sternal border, but may be difficult to distinguish from acute mitral
regurgitation. However, patients with an acquired ventricular septal defect tend to develop right heart
failure rather than pulmonary oedema. Doppler echocardiography and right heart catheterisation will
confirm the diagnosis. Without prompt surgery, the condition is usually fatal.
Rupture of the ventricle may lead to cardiac tamponade and is usually fatal , although it may rarely be
possible to support a patient with an incomplete rupture until emergency surgery is performed.
6-Embolism
Thrombus often forms on the endocardial surface of freshly infarcted myocardium. This can lead to systemic
embolism and occasionally causes a stroke or ischaemic limb.
7-Impaired ventricular function, remodelling and ventricular aneurysm
Acute transmural MI is often followed by thinning and stretching of the infarcted segment (infarct expansion).
This leads to an increase in wall stress with progressive dilatation and hypertrophy of the remaining ventricle
(ventricular remodelling). As the ventricle dilates, it becomes less efficient and heart failure may supervene.
Infarct expansion occurs over a few days and weeks but ventricular remodelling can take years. ACE inhibitor
therapy reduces late ventricular remodelling and can prevent the onset of heart failure.
A left ventricular aneurysm develops in approximately 10% of patients with MI and is particularly common
when there is persistent occlusion of the infarct-related vessel. Heart failure, ventricular arrhythmias, mural
thrombus and systemic embolism are all recognised complications of aneurysm formation. Other clinical features
include a paradoxical impulse on the chest wall, persistent ST elevation on the ECG, and sometimes an unusual
bulge from the cardiac silhouette on the chest X-ray. Echocardiography is usually diagnostic. Surgical removal of
a left ventricular aneurysm carries a high morbidity and mortality but is sometimes necessary.
Later in-hospital management
Risk stratification and further investigation
Lifestyle modification
Cessation of smoking
Regular exercise
Diet (weight control, lipid-lowering)
Secondary prevention drug therapy
Antiplatelet therapy (aspirin and/or clopidogrel)

β-blocker
ACE inhibitor/ARB
Statin
Additional therapy for control of diabetes and hypertension
Aldosterone receptor antagonist
Rehabilitation Devices
Implantable cardiac defibrillator (high-risk patients)
Risk stratification and further investigation
Simple clinical tools can be used to identify medium- to high-risk patients. The GRACE score is a simple
method of calculating early mortality that can help guide which patients should be selected for intensive therapy,
and specifically early inpatient coronary angiography.
The prognosis of patients who have survived an acute coronary syndrome is related to the extent of residual
myocardial ischaemia, the degree of myocardial damage and the presence of ventricular arrhythmias.
Left ventricular function
The degree of left ventricular dysfunction can be assessed from physical findings (tachycardia, third heart sound,
crackles at the lung bases, elevated venous pressure and so on), ECG changes and chest X-ray (size of the heart
and presence of pulmonary oedema). However, assessment with echocardiography should be undertaken in the
early recovery phase.
Ischaemia :
Patients with early ischaemia following an acute coronary syndrome should undergo coronary angiography with
a view to revascularisation. Low-risk patients without spontaneous ischaemia should undergo an exercise
tolerance test approximately 4 weeks after the acute coronary syndrome.
Arrhythmias
The presence of ventricular arrhythmias during the convalescent phase of acute coronary syndrome may be a
marker of poor ventricular function and may herald sudden death. Although empirical anti-arrhythmic treatment
is of no value and even hazardous, selected patients may benefit from electrophysiological testing and specific
anti-arrhythmic therapy (including implantable cardiac defibrillators.
Mobilisation and rehabilitation
When there are no complications, the patient can mobilise on the second day, return home in 3-5 days
and gradually increase activity with the aim of returning to work in 4-6 weeks. The majority of patients
may resume driving after 4-6 weeks,
Emotional problems, such as denial, anxiety and depression, are common and must be addressed. Many
patients mistakenly believe that 'stress' was the cause of their heart attack and may restrict their activity
inappropriately. The patient's spouse or partner will also require emotional support, information and
counselling. Formal rehabilitation programmes based on graded exercise protocols with individual and
group counselling are often very successful, and in some cases have been shown to improve the long-term
outcome.
Secondary prevention drug therapy
Aspirin and clopidogrel
Low-dose aspirin therapy reduces the risk of further infarction and other vascular events by approximately
25% and should be continued indefinitely if there are no unwanted effects. Clopidogrel should be given in
combination with aspirin for at least 3 months. If patients are intolerant of long-term aspirin, clopidogrel is
a suitable alternative.
Beta-blockers
Continuous treatment with an oral β-blocker reduces long-term mortality by approximately 25% among
the survivors of acute MI . Unfortunately, a minority of patients do not tolerate β-blockers because of
bradycardia, AV block, hypotension or asthma.
ACE inhibitors
Long-term treatment with an ACE inhibitor (e.g. enalapril 10 mg 12-hourly or ramipril 2.5-5 mg 12-

hourly) can counteract ventricular remodelling, prevent the onset of heart failure, improve survival, reduce
recurrent MI and avoid rehospitalisation. In patients intolerant of ACE inhibitor therapy, angiotensin
receptor blockers (e.g. valsartan 40-160 mg 12-hourly or candesartan 4-16 mg daily) are suitable
alternatives and are better tolerated.
Patients with acute MI and left ventricular dysfunction (ejection fraction < 35%) and either
pulmonary oedema or diabetes mellitus further benefit from additional aldosterone receptor
antagonism (e.g. eplerenone 25-50 mg daily).
Coronary revascularisation
Most low-risk patients stabilise with aspirin, clopidogrel, anticoagulation and anti-anginal therapy, and
can be rapidly mobilised. Coronary angiography should be considered with a view to revascularisation in
all patients at moderate or high risk, including those who fail to settle on medical therapy, those with
extensive ECG changes, those with an elevated plasma troponin and those with severe pre-existing stable
angina.
Device therapy
Implantable cardiac defibrillators are of benefit in preventing sudden cardiac death in patients who have
severe left ventricular impairment (ejection fraction ≤ 30%) after MI .
Prognosis
In almost one-quarter of all cases of MI, death occurs within a few minutes without medical care. Half the
deaths occur within 24 hours of the onset of symptoms and about 40% of all affected patients die within
the first month. The prognosis of those who survive to reach hospital is much better, with a 28-day
survival of more than 85%. Patients with unstable angina have a mortality approximately half those with
MI.
Early death is usually due to an arrhythmia and is independent of the extent of MI. However, late
outcomes are determined by the extent of myocardial damage and unfavourable features include poor left
ventricular function, AV block and persistent ventricular arrhythmias. The prognosis is worse for anterior
than for inferior infarcts. Bundle branch block and high cardiac marker levels both indicate extensive
myocardial damage. Old age, depression and social isolation are also associated with a higher mortality.
DISORDERS OF HEART RATE, RHYTHM AND CONDUCTION
The heart beat is normally initiated by an electrical discharge from the sinoatrial (sinus) node. The atria and
ventricles then depolarise sequentially as electrical depolarisation passes through specialised conducting tissues.
The sinus node acts as a pacemaker and its intrinsic rate is regulated by the autonomic nervous system; vagal
activity slows the heart rate, and sympathetic activity accelerates it via cardiac sympathetic nerves and
circulating catecholamines.
If the sinus rate becomes unduly slow, a lower centre may assume the role of pacemaker. This is known as an
escape rhythm and may arise in the AV node or His bundle (junctional rhythm) or the ventricles (idioventricular
rhythm).
A cardiac arrhythmia is a disturbance of the electrical rhythm of the heart. Arrhythmias are often a manifestation
of structural heart disease but may also occur because of abnormal conduction or depolarisation in an otherwise
healthy heart. A heart rate > 100/min is called a tachycardia and a heart rate < 60/min is called a bradycardia.
There are three main mechanisms of tachycardia:
Increased automaticity. The tachycardia is produced by repeated spontaneous depolarisation of an ectopic

focus, often in response to catecholamines.
Re-entry. The tachycardia is initiated by an ectopic beat and sustained by a re-entry circuit. Most
tachyarrhythmias are due to re-entry.
Triggered activity. This can cause ventricular arrhythmias in patients with coronary heart disease. It is a
form of secondary depolarisation arising from an incompletely repolarised cell membrane.
Bradycardia may be due to:
Reduced automaticity, e.g. sinus bradycardia.
Blocked or abnormally slow conduction, e.g. AV block.
An arrhythmia may be 'supraventricular' (sinus, atrial or junctional) or ventricular. Supraventricular
rhythms usually produce narrow QRS complexes because the ventricles are depolarised normally through the AV
node and bundle of His. In contrast, ventricular rhythms produce broad, bizarre QRS complexes because the
ventricles are activated in an abnormal sequence. However, occasionally a supraventricular rhythm can produce
broad or wide QRS complexes due to coexisting bundle branch block or the presence of accessory conducting
tissue .
Bradycardias tend to cause symptoms that reflect low cardiac output: fatigue, lightheadedness and syncope.
Tachycardias cause rapid palpitation, dizziness, chest discomfort or breathlessness. Extreme tachycardias can
cause syncope because the heart is unable to contract or relax properly at extreme rates. Extreme bradycardias or
tachycardias can precipitate sudden death or cardiac arrest.
Sinus rhythms
Sinus arrhythmia
Phasic alteration of the heart rate during respiration (the sinus rate increases during inspiration and slows during
expiration) is a consequence of normal parasympathetic nervous system activity and can be pronounced in
children. Absence of this normal variation in heart rate with breathing or with changes in posture may be a
feature of autonomic neuropathy .
Sinus bradycardia
A sinus rate < 60/min may occur in healthy people at rest and is a common finding in athletes. Asymptomatic
sinus bradycardia requires no treatment. Symptomatic acute sinus bradycardia usually responds to intravenous
atropine 0.6-1.2 mg. Patients with recurrent or persistent symptomatic sinus bradycardia should be considered for
pacemaker implantation.
Sinus tachycardia
This is defined as a sinus rate > 100/min, and is usually due to an increase in sympathetic activity associated with
exercise, emotion, pregnancy or pathology. Young adults can produce a rapid sinus rate, up to 200/min, during
intense exercise.
Atrial tachyarrhythmias
Atrial ectopic beats (extrasystoles, premature beats)
These usually cause no symptoms but can give the sensation of a missed beat or an abnormally strong beat. The
ECG shows a premature but otherwise normal QRS complex; if visible, the preceding P wave has a different
morphology because the atria activate from an abnormal site. In most cases these are of no consequence,
although very frequent atrial ectopic beats may herald the onset of atrial fibrillation. Treatment is rarely
necessary but β-blockers can be used if symptoms are intrusive.
Atrial tachycardia
Atrial tachycardia may be a manifestation of increased atrial automaticity, sinoatrial disease or digoxin toxicity.
It produces a narrow complex tachycardia with abnormal P-wave morphology, sometimes associated with AV
block if the atrial rate is rapid. It may respond to β-blockers which reduce automaticity, or class I or III anti-
arrhythmic drugs. The ventricular response in rapid atrial tachycardias may be controlled by AV node-blocking
drugs. Catheter ablation can be used to target the ectopic site and should be offered as an alternative to anti-
arrhythmic drugs in patients with recurrent atrial tachycardia.
Atrial flutter
Atrial flutter is characterised by a large (macro) re-entry circuit, usually within the RA encircling the tricuspid

annulus. The atrial rate is approximately 300/min, and is usually associated with 2:1, 3:1 or 4:1 AV block (with
corresponding heart rates of 150, 100 or 75/min). Rarely, in young patients, every beat is conducted, producing a
heart rate of 300/min and potentially haemodynamic compromise. The ECG shows saw-toothed flutter waves .
When there is regular 2:1 AV block, it may be difficult to identify flutter waves which are buried in the QRS
complexes and T waves. Atrial flutter should always be suspected when there is a narrow complex tachycardia of
150/min. Carotid sinus pressure or intravenous adenosine may help to establish the diagnosis by temporarily
increasing the degree of AV block and revealing the flutter waves .
Management
Digoxin, β-blockers or verapamil can be used to control the ventricular rate . However, in many cases it may be
preferable to try to restore sinus rhythm by direct current (DC) cardioversion or by using intravenous
amiodarone. Beta-blockers or amiodarone can also be used to prevent recurrent episodes of atrial flutter.
Although flecainide can also be used for acute treatment or prophylaxis, it should be avoided because there is a
risk of slowing the flutter circuit and facilitating 1:1 AV nodal conduction. This can cause a paradoxical
tachycardia and haemodynamic compromise. If used, it should always be prescribed along with an AV node-
blocking drug, such as a β-blocker. Catheter ablation offers a 90% chance of complete cure and is the treatment
of choice for patients with persistent, troublesome symptoms.
Atrial fibrillation
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, with an overall prevalence of
0.5% in the adult population of the UK. The prevalence rises with age, affecting 2-5% and 8% of those aged over
70 and 80 years respectively. Atrial fibrillation is a complex arrhythmia characterised by both abnormal
automatic firing and the presence of multiple interacting re-entry circuits looping around the atria. Episodes of
atrial fibrillation are usually initiated by rapid bursts of ectopic beats arising from conducting tissue in the
pulmonary veins or from diseased atrial tissue. AF becomes sustained because of initiation of re-entrant
conduction within the atria or sometimes because of continuous ectopic firing . Re-entry is more likely to occur
in atria that are enlarged, or in which conduction is slow (as is the case in many forms of heart disease). During
episodes of AF, the atria beat rapidly but in an uncoordinated and ineffective manner. The ventricles are
activated irregularly at a rate determined by conduction through the AV node. This produces the characteristic
'irregularly irregular' pulse. The ECG shows normal but irregular QRS complexes; there are no P waves but the
baseline may show irregular fibrillation waves.
AF can be classified as paroxysmal (intermittent, self-terminating episodes), persistent (prolonged
episodes that can be terminated by electrical or chemical cardioversion) or permanent. In patients with AF seen
for the first time, it can be difficult to identify which of these is present. Unfortunately for many patients,
paroxysmal AF will become permanent as the underlying disease process that predisposes to AF progresses.
Electrophysiological changes occur in the atria within a few hours of the onset of AF that tend to maintain
fibrillation: electrical remodelling. When AF persists for a period of months, structural remodelling occurs
with atrial fibrosis and dilatation that further predispose to AF. Thus early treatment of AF will prevent this and
reinitiation of the arrhythmia.
AF may be the first manifestation of many forms of heart disease , particularly those that are associated
with enlargement or dilatation of the atria. Alcohol excess, hyperthyroidism and chronic lung disease are also
common causes of AF, although multiple aetiological factors often coexist such as the combination of alcohol,
hypertension and coronary disease. About 50% of all patients with paroxysmal AF and 20% of patients with
persistent or permanent AF have structurally normal hearts; this is known as 'lone atrial fibrillation'.
AF can cause palpitation, breathlessness and fatigue. In patients with poor ventricular function or valve disease it
may precipitate or aggravate cardiac failure because of loss of atrial function and heart rate control. A fall in BP
may cause lightheadedness, and chest pain may occur with underlying coronary disease. However, AF is often
completely asymptomatic, in which case it is usually discovered as a result of a routine examination or ECG.
AF is associated with significant morbidity and a twofold increase in mortality that are largely attributable to the
effects of the underlying heart disease and the risk of cerebral embolism. Careful assessment, risk stratification
and therapy can improve the prognosis.
Common causes of atrial fibrillation
Coronary artery disease (including acute MI)
Valvular heart disease, especially rheumatic mitral valve disease
Hypertension
Sinoatrial disease

Hyperthyroidism
Alcohol
Cardiomyopathy
Congenital heart disease
Chest infection
Pulmonary embolism
Pericardial disease
Idiopathic (lone AF)
Management
Assessment of patients with newly diagnosed AF includes a full history, physical examination, 12-lead
ECG, echocardiogram and thyroid function tests. Additional investigations such as exercise testing may be
needed to determine the nature and extent of any underlying heart disease. Biochemical evidence of
hyperthyroidism is found in a small minority of patients with otherwise unexplained AF.
When AF complicates an acute illness (e.g. chest infection, pulmonary embolism), effective treatment of the
primary disorder will often restore sinus rhythm. Otherwise, the main objectives are to restore sinus rhythm as
soon as possible, prevent recurrent episodes of AF, optimise the heart rate during periods of AF, minimise the
risk of thromboembolism and treat any underlying disease.
Paroxysmal atrial fibrillation
Occasional attacks that are well tolerated do not necessarily require treatment. Beta-blockers are normally
used as first-line therapy if symptoms are troublesome, and are particularly useful for treating patients with AF
associated with ischaemic heart disease, hypertension and cardiac failure. Beta-blockers reduce the ectopic
firing that normally initiates AF. Class Ic drugs , such as propafenone or flecainide, are also effective at
preventing episodes but should not be given to patients with coronary disease or left ventricular dysfunction.
Flecainide is usually prescribed along with a rate limiting β-blocker because it occasionally precipitates atrial
flutter. Amiodarone is the most effective agent for preventing AF but its side-effects restrict its use to patients in
whom other measures fail. Digoxin and verapamil are not effective drugs for preventing paroxysms of AF,
although they serve to limit the heart rate when AF occurs by blocking the AV node. In patients with AF in
whom β-blockers or class Ic drugs are ineffective or cause side-effects, catheter ablation can be considered.
Ablation is used to isolate electrically the pulmonary veins from the LA, preventing ectopic triggering of AF.
Sometimes ablation is used to create lines of conduction block within the atria to prevent re-entry. Ablation
prevents AF in approximately 70% of patients with prior drug-resistant episodes, although drugs may
subsequently be needed to maintain sinus rhythm. Ablation for AF is an evolving treatment which is associated
with a small risk of embolic stroke or cardiac tamponade. Specialised 'AF suppression' pacemakers have been
developed which pace the atria to prevent paroxysms but this has not proved to be as effective as was initially
hoped.
Persistent and permanent atrial fibrillation
There are two options for treating persistent AF:
rhythm control: attempting to restore and maintain sinus rhythm
rate control: accepting that AF will be permanent and using treatments to control the ventricular rate and
to prevent embolic complications.
Rhythm control
An attempt to restore sinus rhythm is particularly appropriate if the arrhythmia has precipitated
troublesome symptoms and there is a modifiable or treatable underlying cause. Electrical cardioversion is
initially successful in three-quarters of patients but relapse is frequent (25-50% at 1 month and 70-90% at 1
year). Attempts to restore and maintain sinus rhythm are most successful if AF has been present for < 3 months,
the patient is young and there is no important structural heart disease.
Immediate DC cardioversion after the administration of intravenous heparin is appropriate if AF has been
present for < 48 hours. An attempt to restore sinus rhythm by infusing intravenous flecainide (2 mg/kg over 30
minutes, maximum dose 150 mg) is a safe alternative to electrical cardioversion if there is no underlying
structural heart disease. In other situations, DC cardioversion should be deferred until the patient has been

established on warfarin, with an international normalised ratio (INR) > 2.0 for a minimum of 4 weeks, and any
underlying problems, such as hypertension or alcohol excess, have been eliminated. Anticoagulation should be
maintained for at least 3 months following successful cardioversion; if relapse occurs, a second (or third)
cardioversion may be appropriate. Concomitant therapy with amiodarone or β-blockers may reduce the risk of
recurrence. Catheter ablation is sometimes used to help restore and maintain sinus rhythm in resistant cases, but
it is a less effective treatment for persistent AF than for paroxysmal AF.
Rate control
If sinus rhythm cannot be restored, treatment should be directed at maintaining an appropriate heart rate.
Digoxin, β-blockers or rate-limiting calcium antagonists such as verapamil or diltiazem will reduce the
ventricular rate by increasing the degree of AV block. This alone may produce a striking improvement in overall
cardiac function, particularly in patients with mitral stenosis. Beta-blockers and rate-limiting calcium antagonists
are often more effective than digoxin at controlling the heart rate during exercise and may have additional
benefits in patients with hypertension or structural heart disease. Combination therapy (e.g. digoxin + atenolol) is
often advisable.
In exceptional cases, poorly controlled and symptomatic AF can be treated by deliberately inducing complete
AV nodal block with catheter ablation; a permanent pacemaker must be implanted beforehand. This is known as
the 'pace and ablate' strategy.
Prevention of thromboembolism
Loss of atrial contraction and left atrial dilatation cause stasis of blood in the LA and may lead to thrombus
formation in the left atrial appendage. This predisposes patients to stroke and other forms of systemic embolism.
The annual risk of these events in patients with persistent AF is approximately 5% but it is influenced by many
factors and may range from less than 1% to 12% .
Several large randomised trials have shown that treatment with adjusted-dose warfarin (target INR 2.0-3.0)
reduces the risk of stroke by about two-thirds, at the cost of an annual risk of bleeding of approximately 1-1.5%,
whereas treatment with aspirin reduces the risk of stroke by only one-fifth . Warfarin is thus indicated for
patients with AF who have specific risk factors for stroke. For patients with intermittent AF, the risk of stroke is
proportionate to the frequency and duration of AF episodes. Those with frequent, prolonged (> 24 hours)
episodes of AF should be considered for warfarin anticoagulation.
How to assess risk of thromboembolism in atrial fibrillation: the CHA2DS2-VASc
score
Congestive heart failure (1 point)
Hypertension (1 point)
Age > 75 (2 point)
Diabetes mellitus (1 point)
Stroke or transient ischaemic attack (2 points)
Vascular disease (previous MI , peripheral arterial disease or aortic plaque)
(1point)
Age (65-74) ( 1point )
Sex category ( female) ( 1 point )
Score: 0 = aspirin therapy only, 1 = warfarin or aspirin, ≥ 2 = warfarin
An assessment of the risk of embolism helps to define the possible benefits of antithrombotic
therapy, which must be balanced against its potential hazards. Echocardiography is valuable in risk
stratification. Warfarin is indicated in patients at high or very high risk of stroke, unless anticoagulation
poses unacceptable risks. Comorbid conditions that may be complicated by bleeding, such as peptic
ulcer, uncontrolled hypertension, alcohol misuse, frequent falls, poor drug compliance and potential
drug interactions, are all relative contraindications to warfarin. Patients at moderate risk of stroke may
be treated with warfarin or aspirin after discussing the balance of risk and benefit with the individual.
Young patients (under 65 years) with no evidence of structural heart disease have a very low risk of
stroke; they do not require warfarin but may benefit from aspirin.

