1
Lectures in Community Medicine
Immunizations
Dr.Sijal Fadhil Farhood Al Joborae
F.I.C.M.S - M.Sc. - M.B.Ch.B

PART 1
• Vaccination is the
administration of
antigenic material
to stimulate an
individual's
immune system to
develop adaptive
immunity to a
pathogen.
what
is
a
vaccine
?
Introduction
• Vaccination is the most
effective method of
preventing infectious
diseases; widespread
immunity due to
vaccination is largely
responsible for the
worldwide eradication
of smallpox and the
restriction of diseases
such as polio, measles,
and tetanus from much
of the world.
what
is
a
vaccine
?
Introduction
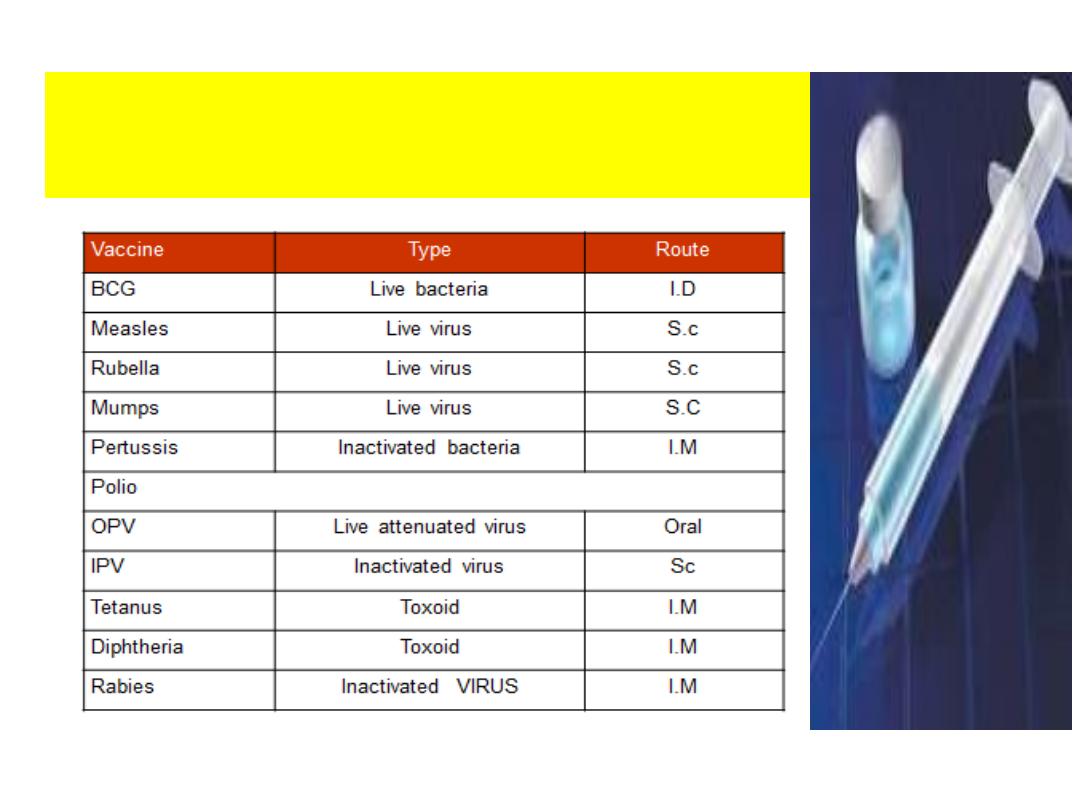
Types of Vaccines & Route
of Administration

Strategies:
1- Routine infant, child & CBAW immunization
2- Missed opportunities
3- Follow up of defaulters
4- NIDs campaign (National Immunization Days)
5- Mopping up campaign
6- Good cool chain
7- Active surveillance of E.P.I targeted diseases
8- Social mobilization

• The active agent of a vaccine
may be intact but inactivated
(non-infective) or attenuated
(with reduced infectivity)
forms of the causative
pathogens, or purified
components of the pathogen
that have been found to be
highly immunogenic (e.g.,
outer coat proteins of a
virus). Toxoids are produced
for immunization against
toxin-based diseases, such as
the modification of
tetanospasmin toxin of
tetanus to remove its toxic
effect but retain its
immunogenic effect
Constituents

1. Active Immunization antigens: examples
live virus, killed virus, live bacteria toxoid,
killed bacteria , polysaccharide .
2. Suspending fluid :
a. sterile water
b. saline
c. complex tissue culture fluid
Major constituents of vaccines:

3. Preservatives
Stabilizers
Antibiotics (e.g. Neomycin)
Trace amounts of chemicals (e.g. mercurials
thiomersal
)
These are necessary to prevent bacterial over
growth or to stabilize antigens .
4. Adjuvants: An aluminium salt is frequently used to increase
immunogenicity and to prolong the stimulatory effect.

Types of vaccines

Live Attenuated
Vaccines


•
Bacteria:
BCG
ORAL
TYPHOID
Viruses:
MMR, OPV,Varicella
zoster, Intranasal flu
vaccine.
•Produced by growing of
bacteria or viruses in
culture media then
inactivated with heat
and/or chemicals like
formalin
Inactivated vaccines

1- Whole cell vaccines
A -Viral-
polio, hepatitis A, rabies,
B-Bacterial-
pertussis, typhoid, cholera
2-Fractional Vaccines
Subunit—
HBV,Acellular Pertussis
Toxoid —Diphtheria,Tetanus

Polysaccharide vaccines
• A unique type of inactivated subunit vaccine
composed of long chains of sugar molecules that
make up the surface capsule of certain bacteria
• 1-Pneumoccacal vaccine(ppsv23)
• 2-Meningococcal vaccine
• 3-Salmonella typhi vaccine (VI)
Conjugated Vaccines
Polysaccharide is chemically bound with protein
molecule
•Heamophilus influenza
•Pneumoccacal vaccine(pcv13)
Recombinant vaccines
•Vaccine antigen produced by
genetic engineering
technology
through insertion
of a segment of the respective
viral gene into the gene of
yeast cell
.
Reassortment vaccines
• Tissue culture cells are infected with two ROTA
virus strains- a nonhuman and human parent
strains
This process is called
genetic reassortment

The term vaccine derives from Edward Jenner's 1796
use of cow pox (Latin variola vaccinia, adapted from
vacca, cow), to inoculate humans, providing them
protection against smallpox.

Vaccines in the 1900s
1923 diphtheria
1926 pertussis
1927 tuberculosis, tetanus
1935 yellow fever
1936 influenza
1955 polio Salk
1957 DTPw
1958 polio Sabin
*
i
m
p
a
c
t
o
f
a
n
t
i
b
i
o
t
i
c
s
o
n
v
a
c
c
i
n
e
d
v
p
t
.
*
c
e
l
l
c
u
l
t
u
r
e
t
e
c
h
n
o
l
o
g
y
1920s
1930s
1950s

Jonas Salk in 1955 holds bottles of a
culture used to grow polio vaccines.

Salk or Sabin vaccine?
WHO declares OPV the vaccine of choice for w/w
polio eradication; some EU countries/US use IPV
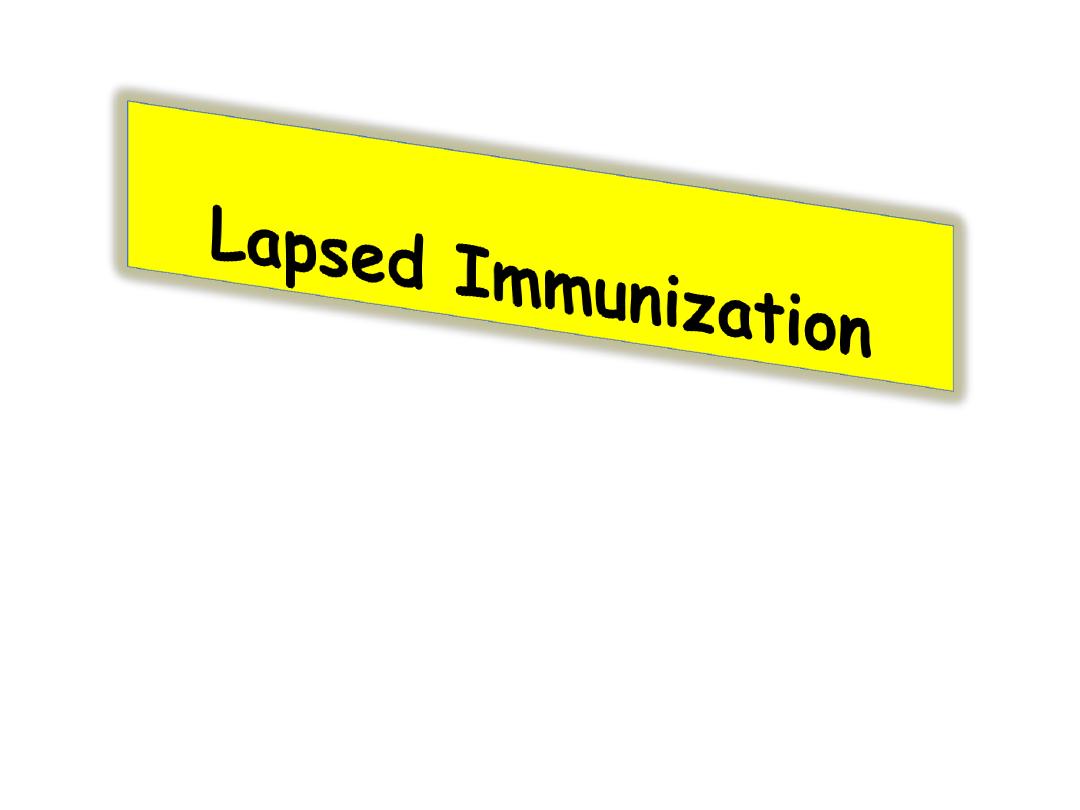
Immunization that does not
require re-institution of the entire
series .
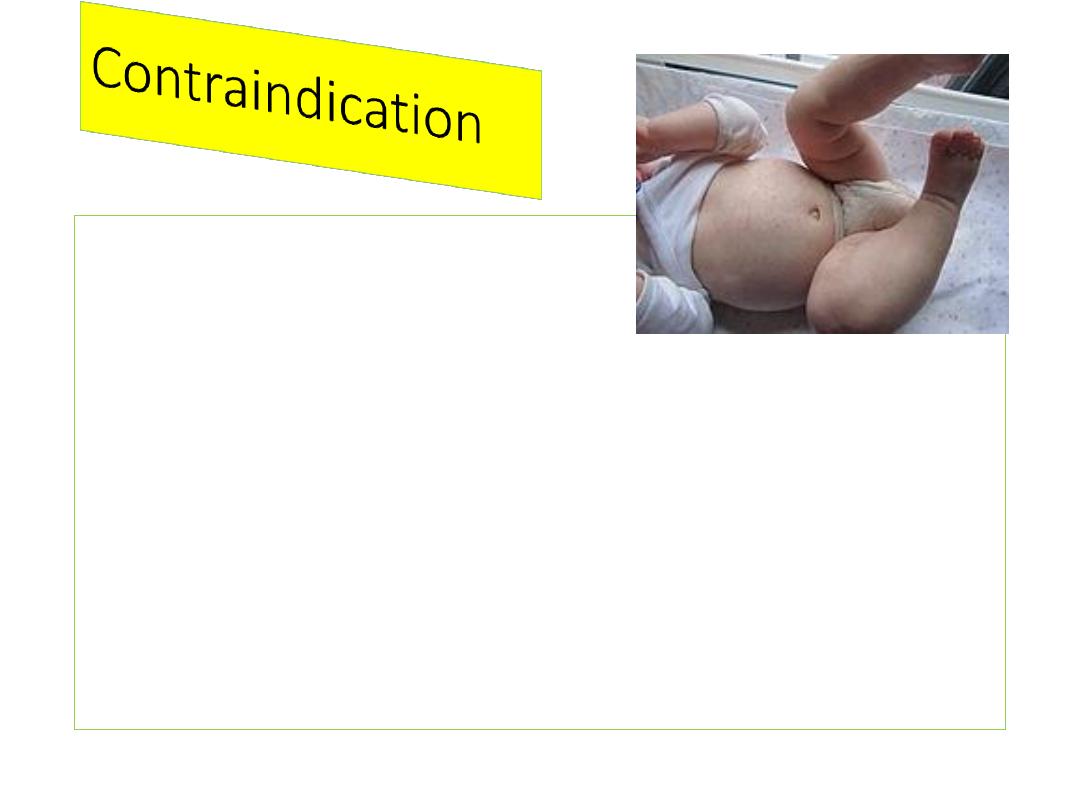
A condition in a recipient
that greatly increases the
chance of serious adverse
reaction

A condition in a recipient that
might increase the chance or
severity of an adverse reaction
OR
might compromise the ability of
the vaccine to produce immunity

Unknown or uncertain Immunization
status
No evidence indicate that
administration of MMR , Hep B ,polio to
already immune recipient is harmful .


• All vaccines
can
be administered at a visit as all other
vaccines
• Increasing the interval between doses of multidose
vaccines
does not
diminish the effectiveness of the
vaccine
• Decreasing the interval between doses of vaccines
may interfere
with antibody production and
protection
• Vaccine doses should not be given at intervals less
than the minimum intervals or earlier than the
minimum age
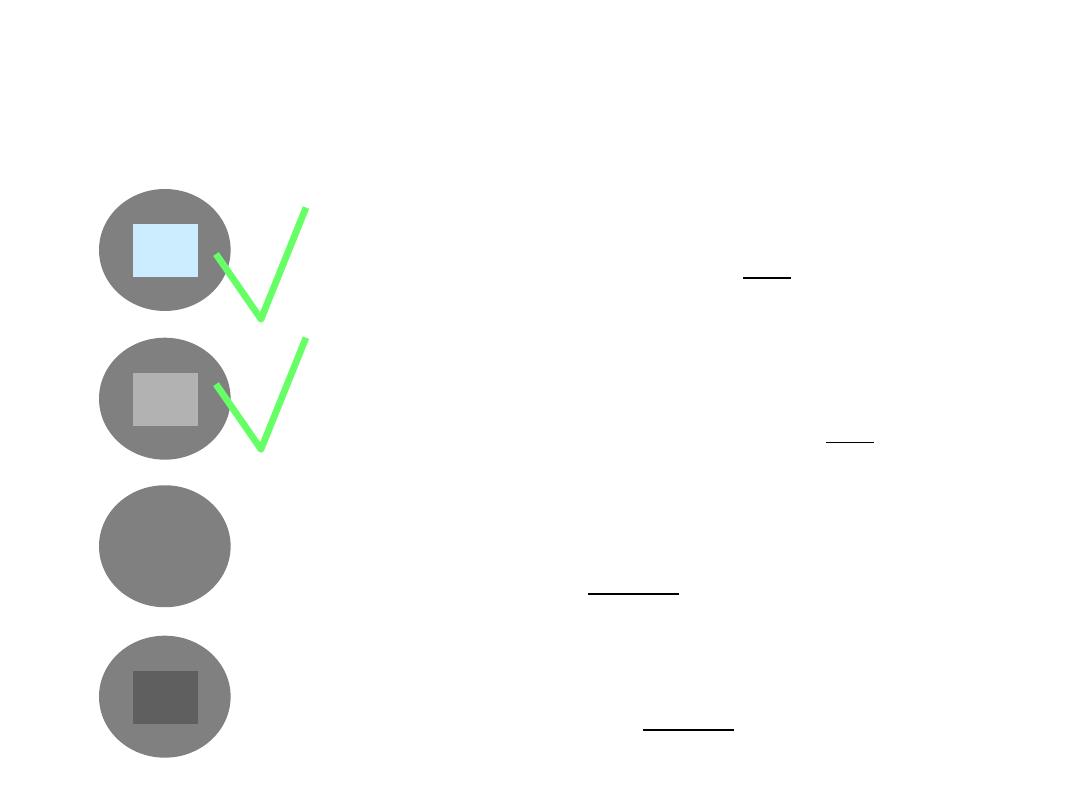
Vaccine vial monitors (VVMs)
inner square still lighter than outer ring;
if the expiry date is not passed, use the vaccine
inner square lighter than outer ring; if the
expiry date is not passed, use the vaccine
inner square matches the colour of outer ring;
discard point - do not use the vaccine
inner square darker than outer ring; beyond
the discard point - do not use the vaccine
x
x

11
Misconceptions concerning
vaccine contraindications


Most vaccines can be safely and effectively
administered simultaneously. No
contraindications are known .
Immune responses to one vaccine generally
do not interfere with those to other vaccines
;
exceptions include interference among the
three oral poliovirus serotypes in trivalent OPV
and concurrent administration of cholera and
yellow fever vaccines.
Mild and severe reactions to vaccines
Mumps Pain, tenderness, fever
parotitis
Aseptic meningitis
Rubella Pain, tenderness, fever
rash, lymphadenopathy
headache
Arthalgia
Arthritis
arthropathy
MMR
Fever, rash, parotitis
lymphadenopathy
OPV
VAPP, Gullian-Barre
ascptic meningitis,
transverse myelitis
Adverse events following vaccination
vaccine
mild
severe
BCG
Axillary, cervical, Lymph adenitis
Osteitis TB meningitis
Diphtheria
Redness, pain, fever
Urticaria, pruritis anaphylaxis
Tetanus
Pain, erythema sterile-abscess, fever
Urticaria, brachial neuritis Gullian-Barre
anaphylaxis
Pertussis
Pain, tenderness, Nodule erythema,
oedema fever, irritation, loss appetite,
vomiting
* persistant inconsolable cry
* unusual screams
* convulsion
* fever 40.5 ْ c
* hypotonic- hyporesponsive episodes
* encephalopathy
Hepatitis B
Fever, pain, swelling erythema, headache
Anaphylaxis Gullian-Barre Hair-loss
Diabetes type 1
Measles
Pain, tenderness, fever , rash
Urinary, encephalopathy, encephalitis, SSPE,
seizures Gullian-Barre thrombocytopenia,
Autism inflammatory bowel disease
Reactions to Vaccines

Part 2

• First identified as cause of diarrhea in 1973.
• Most common cause of severe diarrhea in
infants and children.
• Nearly universal infection by age 5 years.
• Responsible for up to 500.000 diarrheal
deaths each year worldwide.

Rota virus immunity:
Antibody against VP4 probably important for protection
First infection usually does not lead to permanent
immunity
Reinfection can occur at any age
Subsequent infections generally less severe

Rotavirus Clinical Features
• Incubation period 1-3 days
• Variable clinical presentation asymptomatic to
severe diarrhea
• First infection after age 3 months generally most
severe
• Illness not specific for rotavirus
• Confirmation requires laboratory testing

Rotavirus complications
• Severe diarrhea
• Dehydration
• Electrolyte imbalance
• Metabolic acidosis
• Immunodeficient children may have more severe or
persistent disease

Rotavirus Epidemiology
• Reservoir Human
• Transmission Fecal-oral, fomites
• Temporal pattern Fall and winter
(temperate areas)
• Communicability 2 days before to 10
days after onset

Characteristics
• In February 2006 , the food and drug
administration approved a new rotavirus vaccine
(Rota), Rota Teq , produced by Merck vaccine
division . Rota Teq is a live , oral vaccine that
contains five reassortant rotaviruses developed
from human and bovine parent rotavirus strains.

Rotavirus vaccine recommendations
• Routine immunization of all infants without
contraindications
• Administered at 2, 4 and 6 months of age
• Minimum age of first dose is 6 weeks
• First dose should be administered between 6 and
12 weeks of age (until age 13 weeks)
• Do not initiate series after 12 weeks of age

Rotavirus vaccine recommendations

Rotavirus vaccine recommendations
• Administer simultaneously with other indicated
vaccines
• Breastfeeding infants should be vaccinated on usual
schedule
• Vaccinated infants who have recovered from
documented rotavirus infection
• Do not repeat dose if infant spits out or
regurgitates vaccine –administer remaining doses
on schedule

• Vomiting 15%
• Diarrhea 24%
• Nasopharyngitis 7%
• Fever 43%
• No serious adverse
reactions reported
Rotavirus vaccine
adverse reactions

Severe allergic reaction to a
vaccine component or following a
prior dose of vaccine

Pneumonia
Otitis
Sinusitis
Diseases caused by Haemophilus influenzae
Major
syndromes:
Pneumonia
Meningitis
Septicaemia
Septicaemia
Meningitis
Arthritis
Peritonitis
Osteomyelitis

Global Burden of Hib diseases
1. morbidity:
A leading cause of infectious illnesses
• 3 million children with serious illness/ year
• Second most common cause of bacterial
pneumonia among < 5 years children
Hib accounts for ~20% of severe pneumonia (X ray proven) in
most studies

Global Burden of Hib diseases (cont’d)
2. Mortality
A leading cause of death
• 400,000 deaths/year
• 1 in 25 child deaths
Hib accounts for >1000 preventable deaths every days

3. Disability
15-35% of children who survive Hib meningitis
suffer lifelong consequences, including
paralysis
hearing loss
mental retardation
learning disabilities
Global Burden of Hib diseases (cont’d)
Are there serious adverse effects with Hib Vaccine?
•No
• But
• Moderate fever
• Moderate pain at injection site
• Swelling
• Discomfort
• Pain, Redness
Most side effects
are minor and are
the result of the
injection. They are
not due to the
vaccines.

Priorities in vaccination program
in iraq/2015-2016
• The main priorities are to
• 1-improve coverage by all vaccines to at least 90% by 2015
• 2-stop the ongoing measles outbreaks and other vaccine-
preventable diseases among internally displaced people
• 3- introduce pneumococcal conjugate vaccine and inactivated
polio vaccine by November 2015
• 4- and replace trivalent oral polio vaccine with bivalent oral
polio vaccine in April 2016
• 5- A comprehensive communications strategy for secure and
insecure areas needs to be developed for vaccinating all
children.

To eliminate the rare risks of vaccine-
associated paralytic polio (VAPP) and
circulating vaccine-derived poliovirus
(cVDPV).
The withdrawal of OPVs must occur in a
globally synchronized manner, starting in
April 2016 with a switch from trivalent OPV
(tOPV) to bivalent OPV (bOPV), removing
the type 2 component (OPV2) from
immunization programmes.
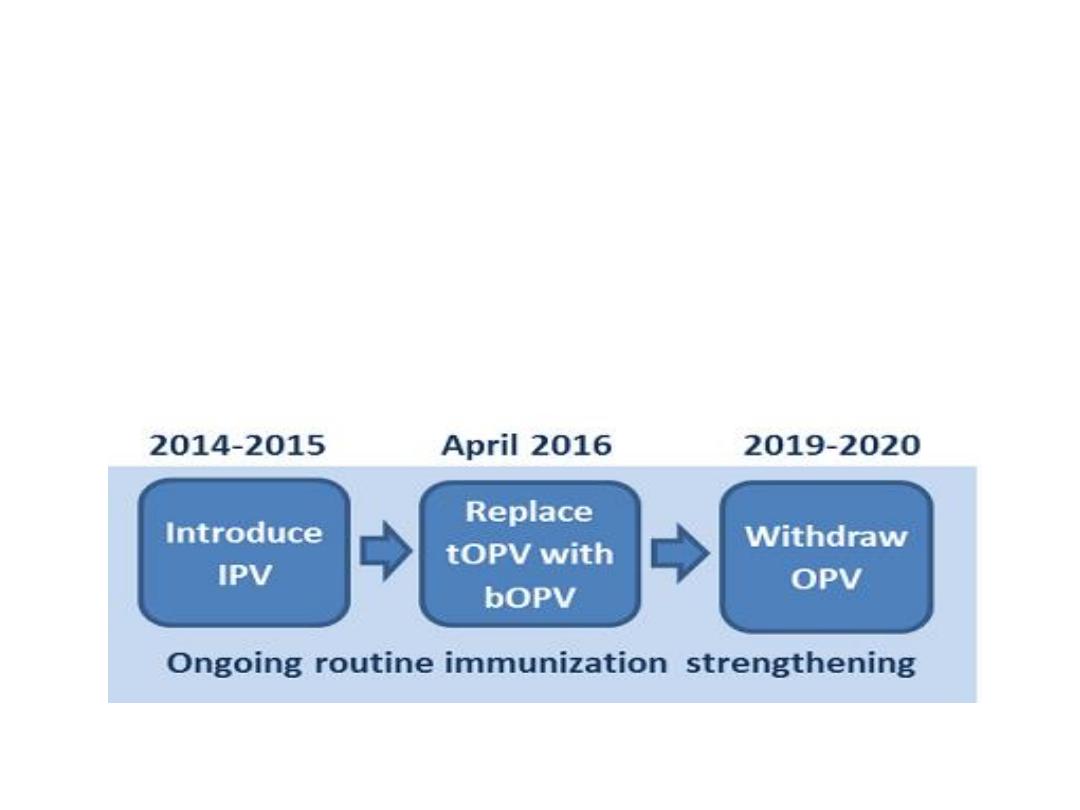
Preparation for the removal of OPVs also
includes the introduction of at least one dose
of inactivated polio vaccine (IPV) into routine
immunization programmes in all countries by
the end of 2015.

Why pneumococcal conjugate
vaccine?
• After introduction of the pneumococcal conjugate
vaccine in 2000, several studies described a decrease in
invasive pneumococcal disease in the United States.
One year after its introduction, a group of investigators
found a 69% drop in the rate of invasive disease in
those age less than 2 years of age. By 2004, all-cause
pneumonia admission rates had declined by 39% and
rates of hospitalizations for pneumococcal meningitis
decreased by 66% in children younger than 2.
• Interestingly, rates of invasive pneumococcal disease
among adults has also declined since the introduction
of the vaccine.

Vaccination schedule in Iraq
*At birth:BCG,OPV-0,HBV-1
*2 months completed:
HEXA-1,ROTA-1,Pneumococcal conjugated vaccine-1,
OPV-1
4months completed:
HEXA-2,ROTA-2,Pneumococcal conjugated vaccine-2,
OPV-2
6months completed:
HEXA-3,ROTA-3,Pneumococcal conjugated vaccine-3,
OPV-3

• 9months completed: Measles+Vit A 100000 IU
• 15months completed:MMR-1
• 18months completed:OPV,PENTA,Vit A 200000 IU
• 4-6 years:OPV,TETRA, MMR-2,Vit A 200000IU
