Anemia in Pregnancy
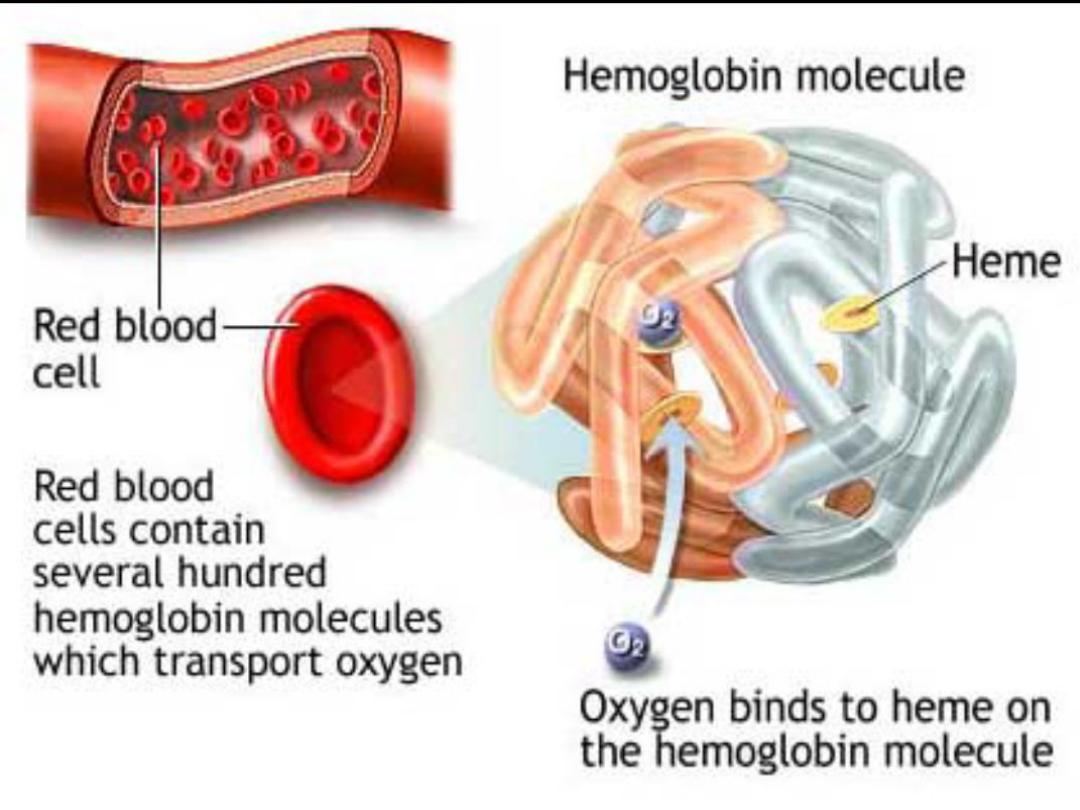
Definition :anaemia is a pathological condition in
which the oxygen-carrying capacity of red blood cells
is insufficient to meet the body needs .
WHO : haemoglobin concentration of < 11.0 g/dL in the
1
st
trimester & < 10.5 g/dL in 2
nd
& 3
rd
trimesters.
Incidence:
30-50% of women become anaemic during pregnancy,
Iron deficiency anaemia responsible for more than 90%
of cases.
Folate deficiency is 5%.
Screening during pregnancy
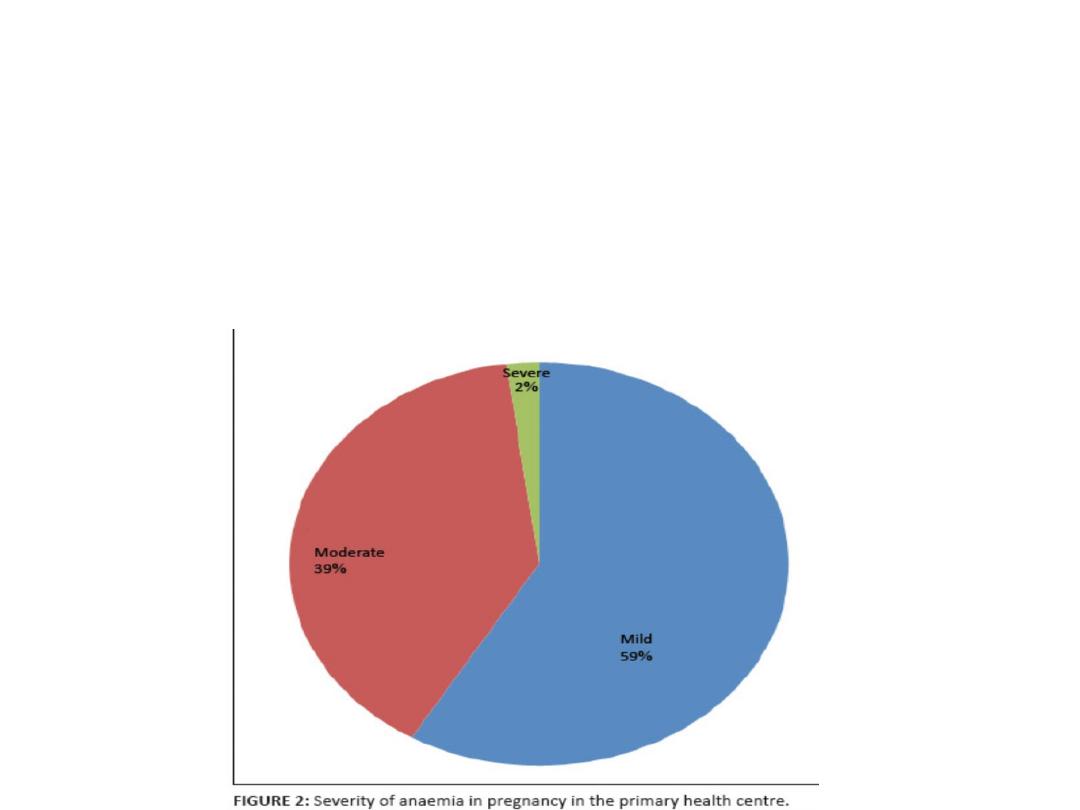
Severity of Anemia:
Mild : Hb 10-10.9 g/dL
Moderate : Hb 7-9.9 g/dL
Severe :4-6.9 g/dL
Very sever : < 4 g/dL
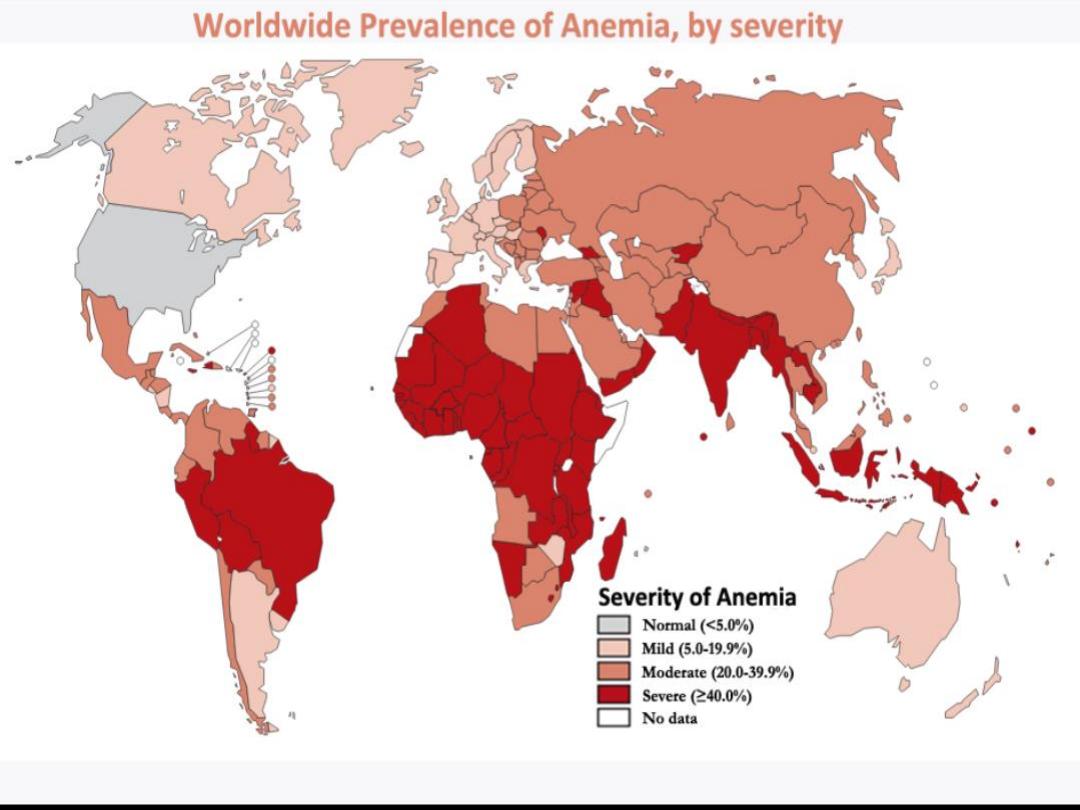
Clinical features :
Symptoms: tiredness, dizziness, fainting,
lethargy.
Signs: Pallor, koilonychia (IDA), delayed capillary
refilling.
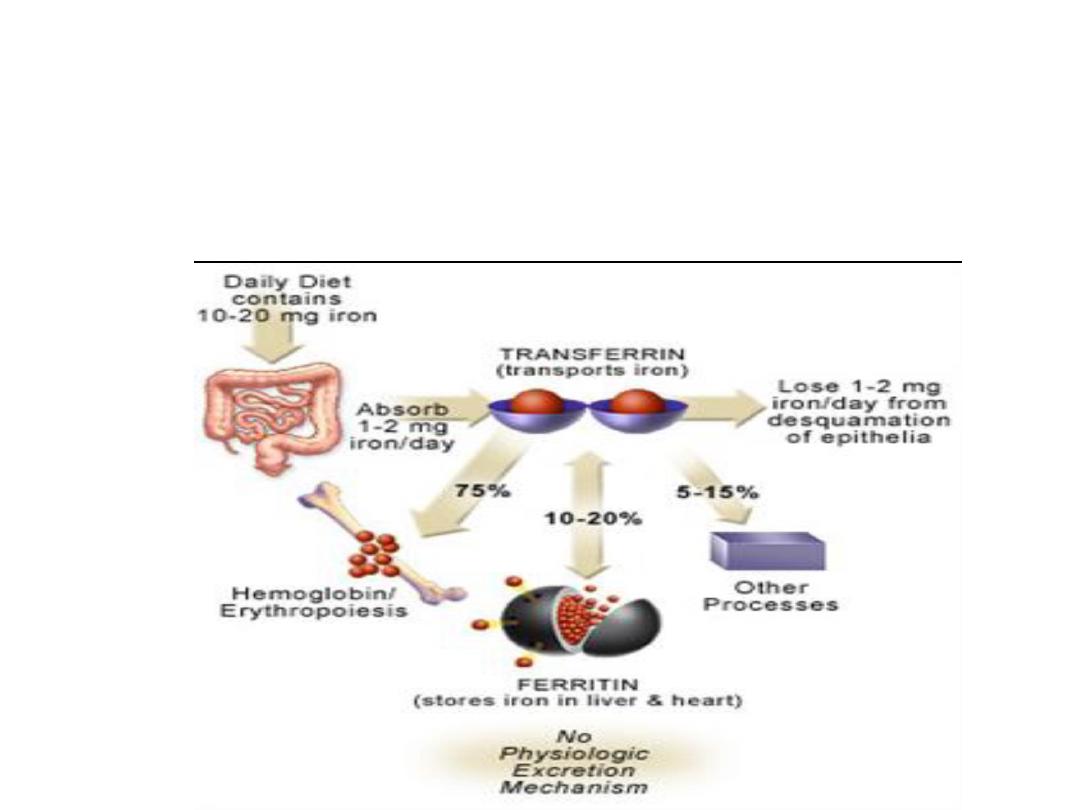
Iron deficiency anaemia (microcytic
anaemia):
• Iron demand in pregnancy increases from 2 mg to 4
mg daily. A healthy diet contains 10 mg.
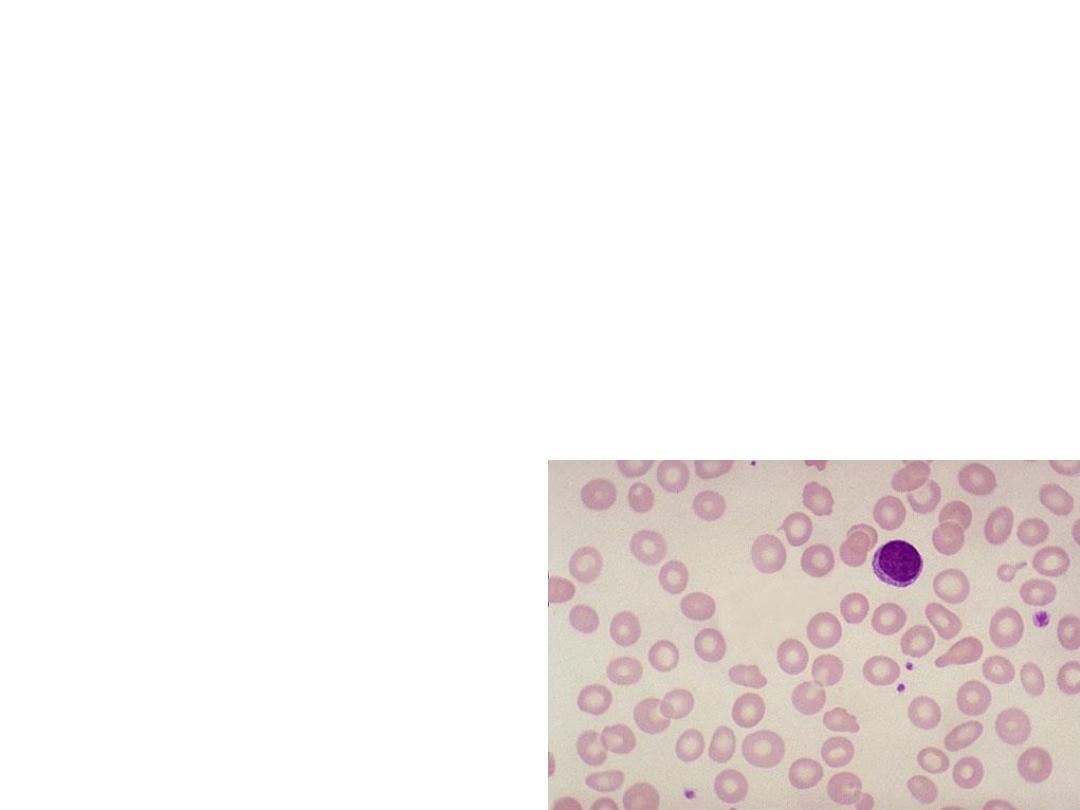
Investigations:
• Complete Blood count :the mean corpuscular
volume (MCV) is low <85 fL.
• Blood Film: hypochromic microcytic
erythrocytes
• Low levels of serum iron & ferritin with
increased TIBC
• Hb electrophoresis is
normal.

Complications:
• Impaired function of iron-dependent enzymes
is the basis for the explanation of the link
between iron deficiency anaemia & preterm
delivery, infection, medical intervention during
labour & postpartum haemorrhage.

• Prevention: good balanced diet &
identification & treatment of iron deficiency
anaemia prior to pregnancy are optimal. A
policy of routine iron supplementation during
pregnancy is also adopted.
• Prophylaxis: 30-60 mg elemantal iron.
• Treatment:
• Oral iron replacement is usually effective if there is
enough time ( maximum increase in Hb=0.8 g/dL per
week).
• the recommended dose of elemental iron is 120-200
mg daily. prophylactic doses until 3 months
postpartum to ensure that iron stores are replenished.
• Therapy failure: malabsorption, loss exceed intake,
poor compliance, wrong diagnosis.
• Parenteral iron therapy for those with
intolerable side effect & those with poor
compliance. Response is not faster than oral
iron. careful administration is required because
of the possibility of anaphylaxis.
• Blood transfusion is indicated if the woman has
severe anemia beyond 36 weeks of gestation
Folate deficiency
• folic acid is present in many food stuffs such as green
vegetables, fruits & liver.
• increased MCV (> 100 fL) (macrocytic anaemia).
• Diagnosed by decreased red cell folate concentration

• Folate requirement is increased in pregnancy
as all tissues require it for manufacture of
DNA.
• Folate deficiency can occur in those with
malabsorption syndrome or those with
increased demand due to multiple gestation,
haemolytic conditions & those taking
anticonvulsant medications
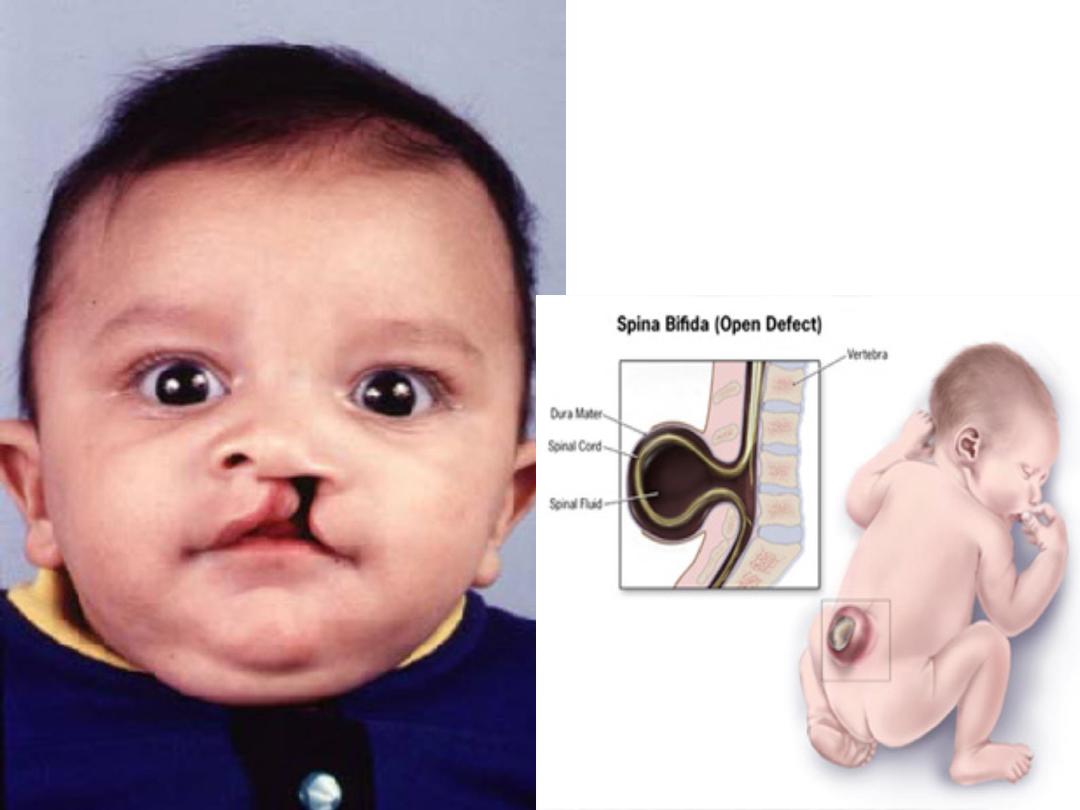

• Prophylaxis: All women considering pregnancy
should be encouraged to use folate supplementation
(400 µg) 3 month before pregnancy & the first three
moonths of pregnancy , as it has been shown to
reduce the incidence of neural tube defect.
• (5mg) is required for women receiving anticonvulsant
medication due to their antifolate activity or those
with a history of previous child with neural tube
defect & multiple pregnancy.
• Treatment: folic acid 5 mg daily continued for up to 4
weeks in the peurperium.


Vitamin B12 deficiency:
• Causes: pernicious anemia, malabsorption,
Chron’s disease, tapeworm infestation
• macrocytic anemia, such cases are more likely
present with infertility.
• diagnosed by increased MCV > 100 fL &
decreased serum vit B12 level.
• Treatement: weekly intramuscular injection of
1000 µg B12 until anemia resolved.
Haemoglobinopathies:
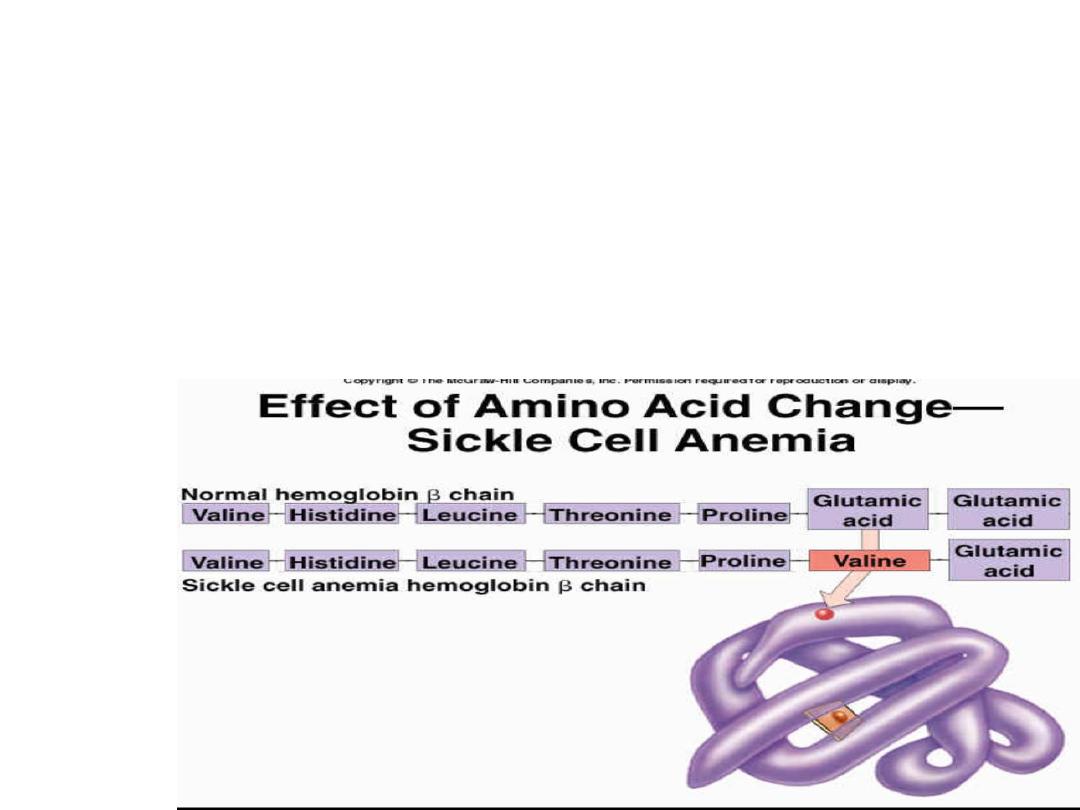
• Sickle-cell anaemia: an inherited disease with
autosomal recessive inheritance in which abnormal
haemoglobin (HbS) contains beta-chains with an
amino acid substitution of valine in place of
glutamine.

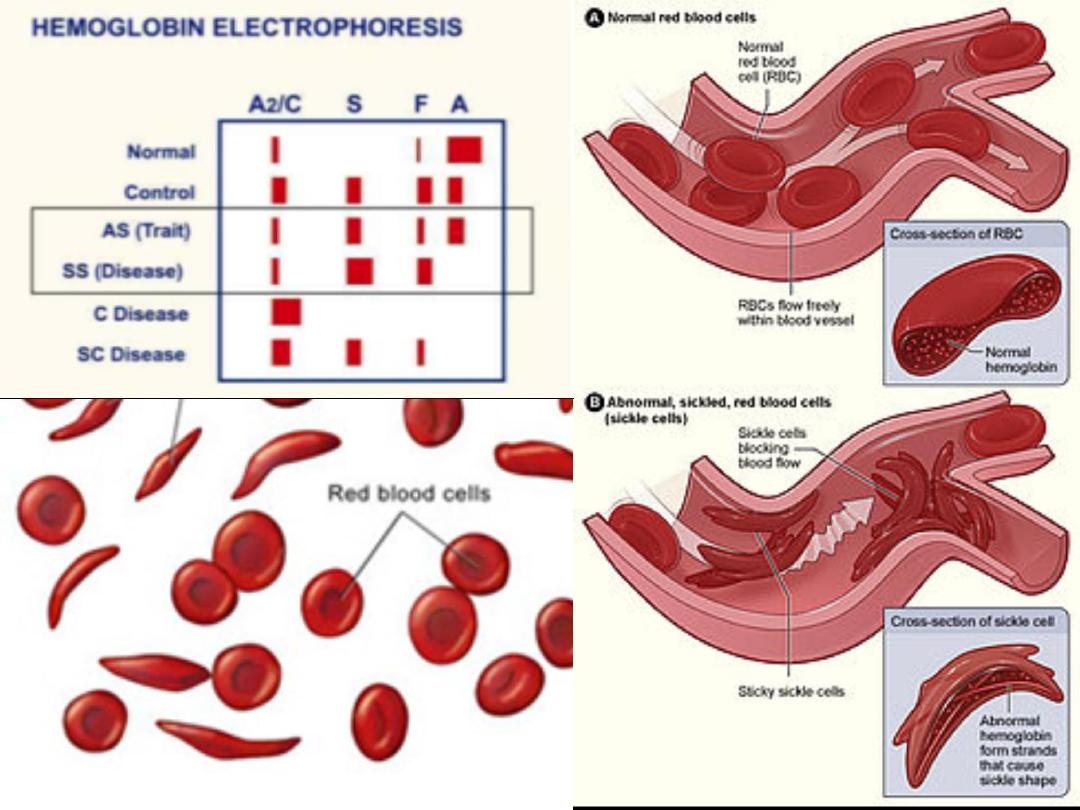
• In its deoxygenated state HbS becomes
insoluble giving the red blood cells sickle
shape, because of their rigid structure sickled
cells block small blood vessels leading to
sickling crises.
• It can be homozygous ( sickle cell disease) or
heterozygous ( sickle cell trait).

Sickle-cell disease
• HbSS is a severe condition & in pregnancy women
are at high risk of complications.
• Pregnancy is associated with increased incidence of
sickle-cell crises that may result in episodes of severe
pain, typically affecting the bones or lungs.
• crisis may be precipitated by hypoxia, stress,
infection & haemorrhage.
• Mothers are at increased risk of miscarriage, pre-
eclampsia, chest & urinary tract infection & preterm
labour. The fetal loss rate is higher than normal, as is
the incidence of growth restriction.

Sick-cell trait :
Carriers of the trait are usually fit & well, but are
at increased risk of urinary tract infection.
Sickle-cell Haemoglobin C Disease
:
may
cause mild degree of anemia but is associated
with very severe crises that are more common
in pregnancy

Antenatal management
• Women should be screened at booking to detect
haemoglobinopathies.
• No specific treatment exists to prevent sickle-cell
crises;
• hypoxia, infection & dehydration should be avoided
by aggressive treatment with adequate analgesia,
antibiotic, oxygen & rehydration.
• Hb concentration of at least 10 g/dL with 60%
normal HbA will minimize the risk of crises.
• Vaginal delivery should be the aim & epidural
analgesia advised, to reduce the stress of labour.

Thalassaemia:
• The defect is a reduced production of normal
haemoglobin
• The syndromes are divided into the alpha &
beta types depending on which globin chain is
affected.
• Normal haemoglobin consist mostly of HbA
(2α 2β), with a small percentage of HbA2 ( 2α
2δ) & HbF (2α 2γ)
Beta-thalassaemia
• results from defects in the normal production of beta
chains for which two genes are responsible
• If one of the genes for beta-chain is missing the patient
will have beta-thalassaemia minor, If the two genes are
missing the
patient will have
beta-thalassaemia major.
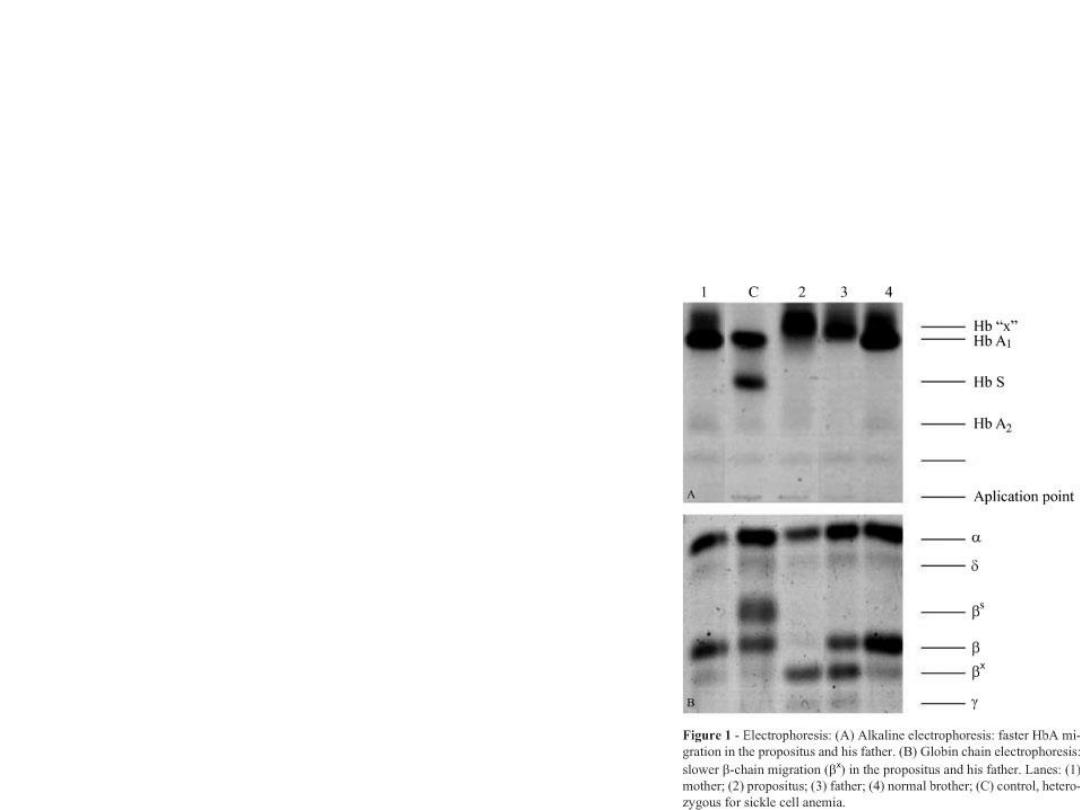
• These conditions can be
diagnosed using haemoglobin
electrophoresis.

• Beta-thalassaemia minor is not a problem
antenatally, although women tend to be mildly
anaemic with low MCV
• Oral iron & folate should be given & the partner
should be screened.
• If the partner has beta-thalassaemia trait, there is
1:4 chance that the fetus has beta-thalassaemia
major.
• The fetus produces HbF in utero while in postnatal
life, normal HbA cannot be produced & severe
anaemia develops requiring serial blood transfusion
• Eventually this leads to problems of iron overload &
death.

alpha-thalassaemia minor
• there is a deletion of one, two or three of the
four normal alpha genes required for
haemoglobin production.
• effected individual is chronically anaemic,
• it rarely produces obstetric complications
except in cases of severe blood loss

alpha-thalassaemia major
• there is no functional alpha chains,
• no normal haemoglobin is synthesized & the
condition is incompatible with life
• The fetus develops marked hydrops, &
pregnacies are complicated by
polyhydramnios & preterm delivery.
• These pregnancies may also be complicated
by severe pre-eclampsia related to the
enlarged & hydropic placenta.
