
Stomach Pathology
Gastritis
• Acute
– Causes: infectious, chemical
– Histologically: Edema, hyperemia, mucosal erosions and
hemorrhage.
• Chronic
– Helicobacter pylori infection (type B, antral, chronic active
with lymphoid follicles)
– Autoimmune (type A, body, atrophy, pernicious anemia)
– Chemical (induced by drugs, bile reflux, alcohol(
Nonspecific symptoms, abdominal pain

The pathogenesis of acute ulceration
• NSAID-induced ulcers are caused by direct chemical
irritation as well as cyclooxygenase inhibition, which
prevents prostaglandin synthesis.
This eliminates the protective effects of prostaglandins,
which include enhanced bicarbonate secretion and
increased vascular perfusion.
• Lesions associated with intracranial injury are thought
to be caused by direct stimulation of vagal nuclei, which
causes gastric acid hypersecretion.

•Systemic acidosis, a frequent finding in critically
ill patients, also may contribute to mucosal
injury by lowering the intracellular pH of
mucosal cells.
•Hypoxia and reduced blood flow caused by
stress-induced splanchnic vasoconstriction also
contribute to acute ulcer pathogenesis.

Acute gastric ulcers
Macroscopic finding
It range in depth from shallow erosions caused by superficial
epithelial damage to deeper lesions that penetrate the
mucosa.
Acute ulcers are rounded and typically are less than 1 cm in
diameter.
The ulcer base frequently is stained brown to black by acid
digested extravasated red cells, in some cases associated
with transmural inflammation and local serositis. While these
lesions may occur singly, more often multiple ulcers are
present within the stomach and duodenum.

Acute stress ulcers are sharply demarcated,
with essentially normal adjacent mucosa,
although there may be suffusion of blood into
the mucosa and submucosa and some
inflammatory reaction.
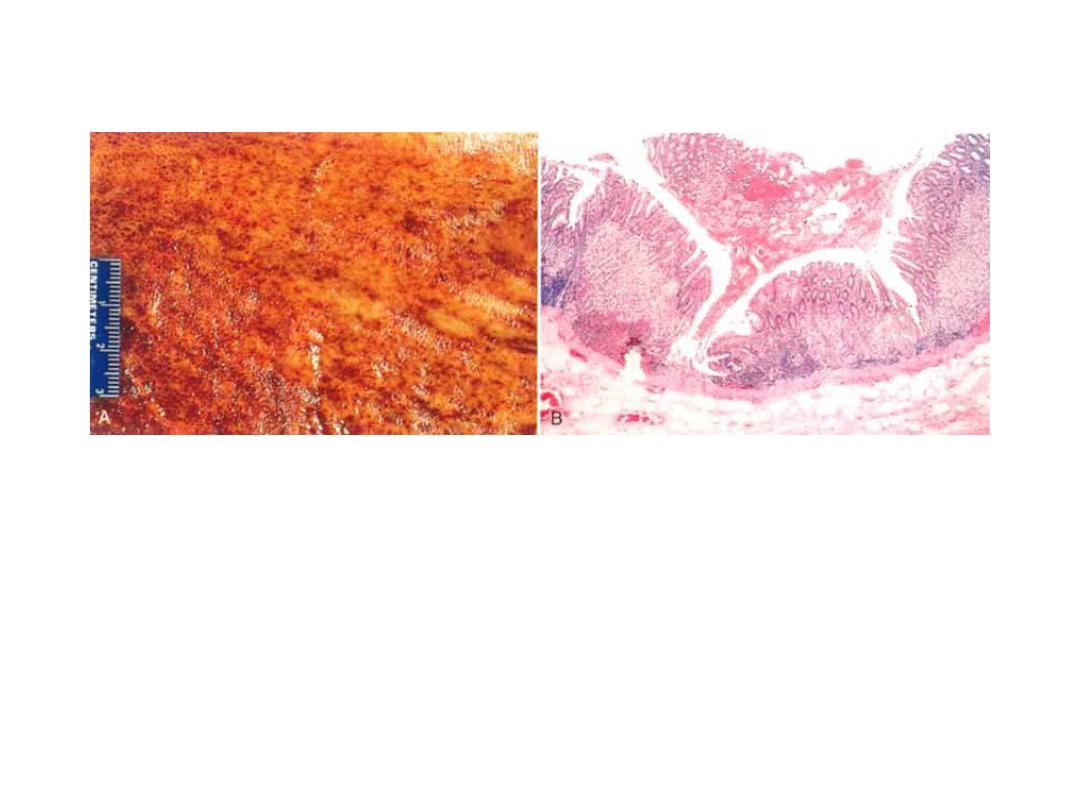
Acute gastritis
A,
Gross view showing punctate erosions in an otherwise
unremarkable mucosa; adherent blood is dark due to exposure to
gastric acid.
B,
Low-power microscopic view of focal mucosal disruption with
hemorrhage; the adjacent mucosa is normal.

Chronic gastritis
• Epithelial damage: increase of nuclei, distorsion of foveolae
and glands)
• Mucosal infiltrates of lymphocytes and plasma cells
• Active process: neutrophil granulocytes (acute damage of
epithelial cell)
• Atrophy: chief and parietal cell loss and replacement by
intestinal type epithelial cells
• Intestinal metaplasia: acid mucin containing goblet cells,
microvilli, Panneth cells
• Regenerative changes: foveolar hyperplasia, increase of
myofibroblasts and capillaries
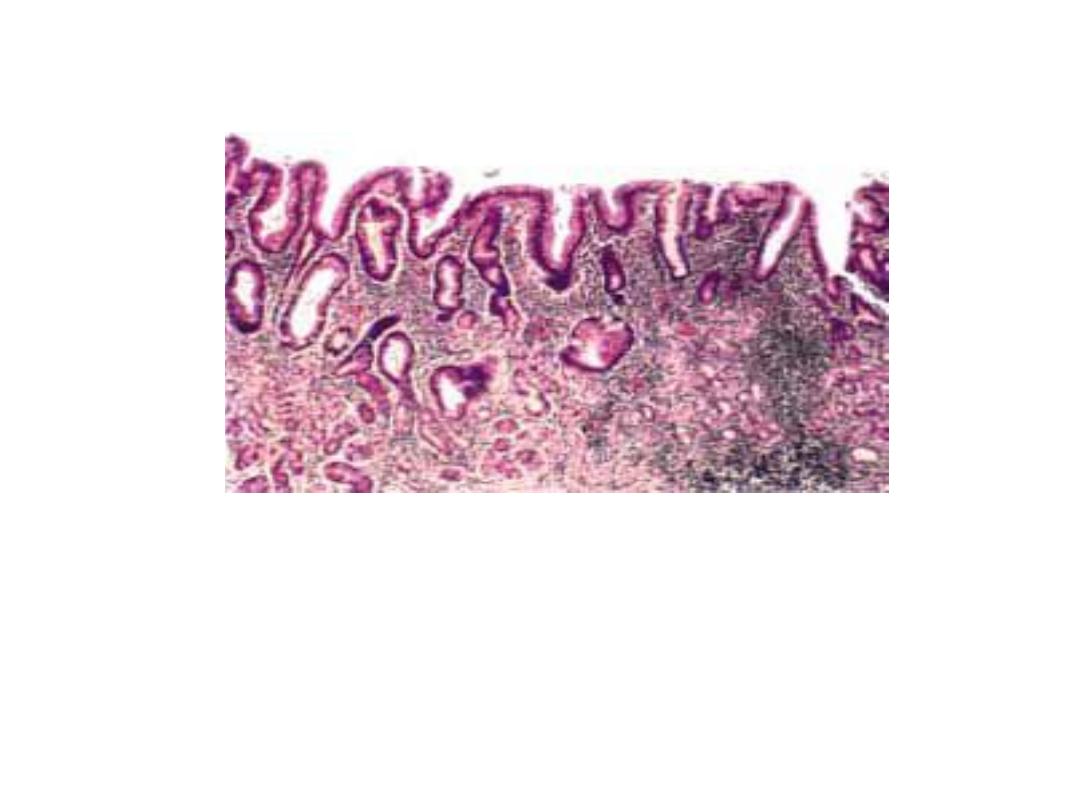
Chronic gastritis
partial replacement of the gastric mucosal epithelium by intestinal
metaplasia (upper left) and inflammation of the lamina propria
(right) containing lymphocytes and plasma cells.

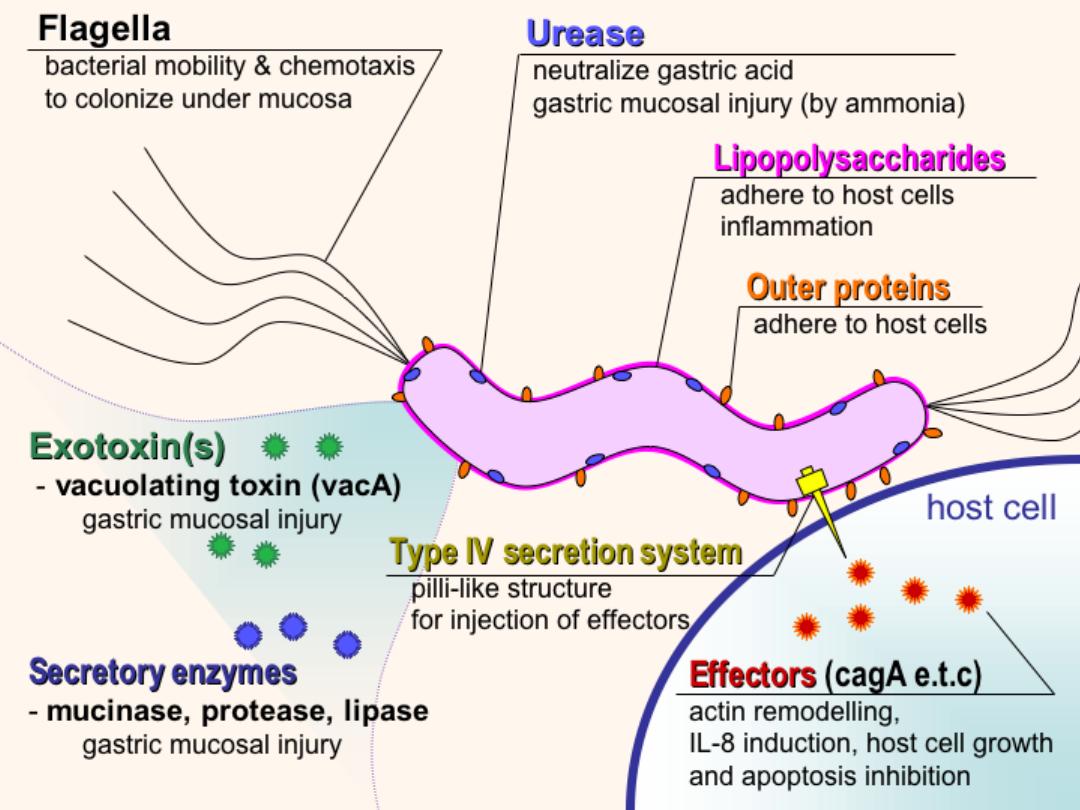
Helicobacter associated chronic gastritis
• Gross: red mucosa, coarser texture than normal,
may have thickened rugal folds or thin/flat
mucosa; with long term disease, mucosa may be
thin/flat; usually affects antrum (particularly in
children), and cardia .

Micro:
Bacteria is curved, spirochete-like, in
superficial mucus layer and along microvilli of
epithelial cells; are (not invasive); are usually
not seen in areas of intestinal metaplasia;
associated
with
chronic
inflammatory
infiltrate with germinal centers (follicular
gastritis) and plasma cells in lamina propria;
active inflammation if neutrophils in
glandular or surface epithelial layer

Helicobacter pylori.
Steiner silver stain demonstrates the numerous darkly stained
Helicobacter
organisms along the luminal surface of the gastric
epithelial cells.
Note that there is no tissue invasion by bacteria.
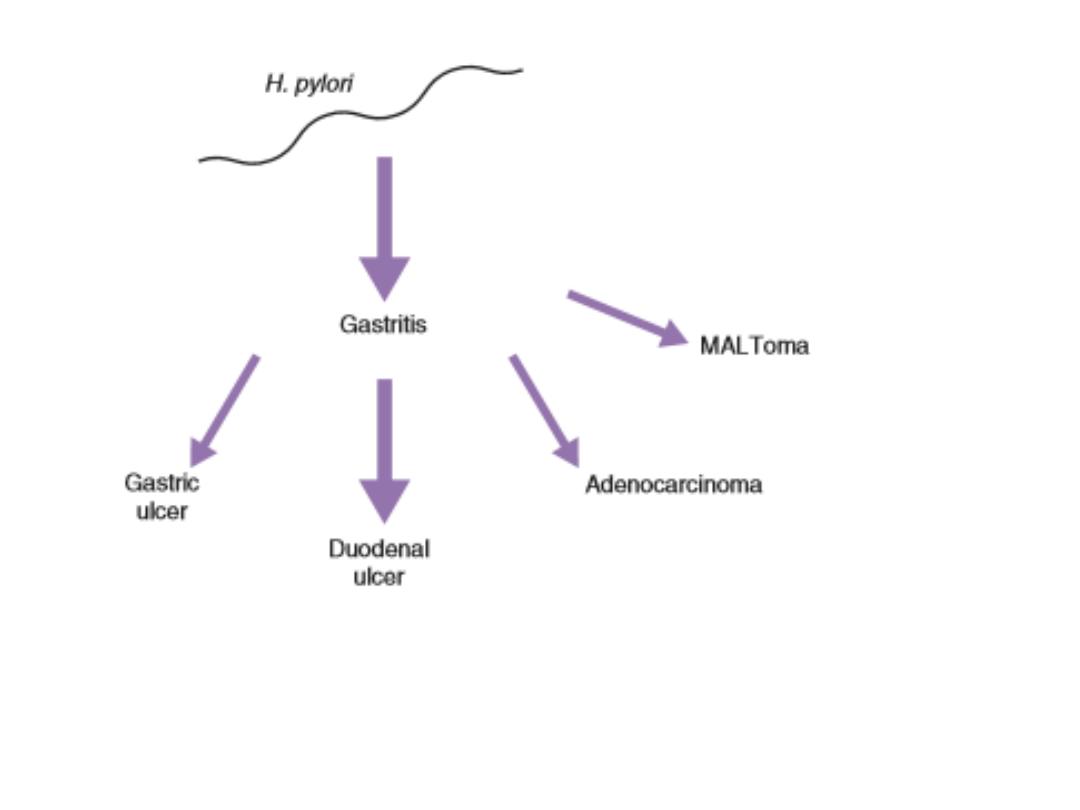
Helicobacterpylori disease associations. The size of the arrow
is a rough indication of the clinical magnitude of the
association. MALToma = mucosal associated lymphoid
tumours
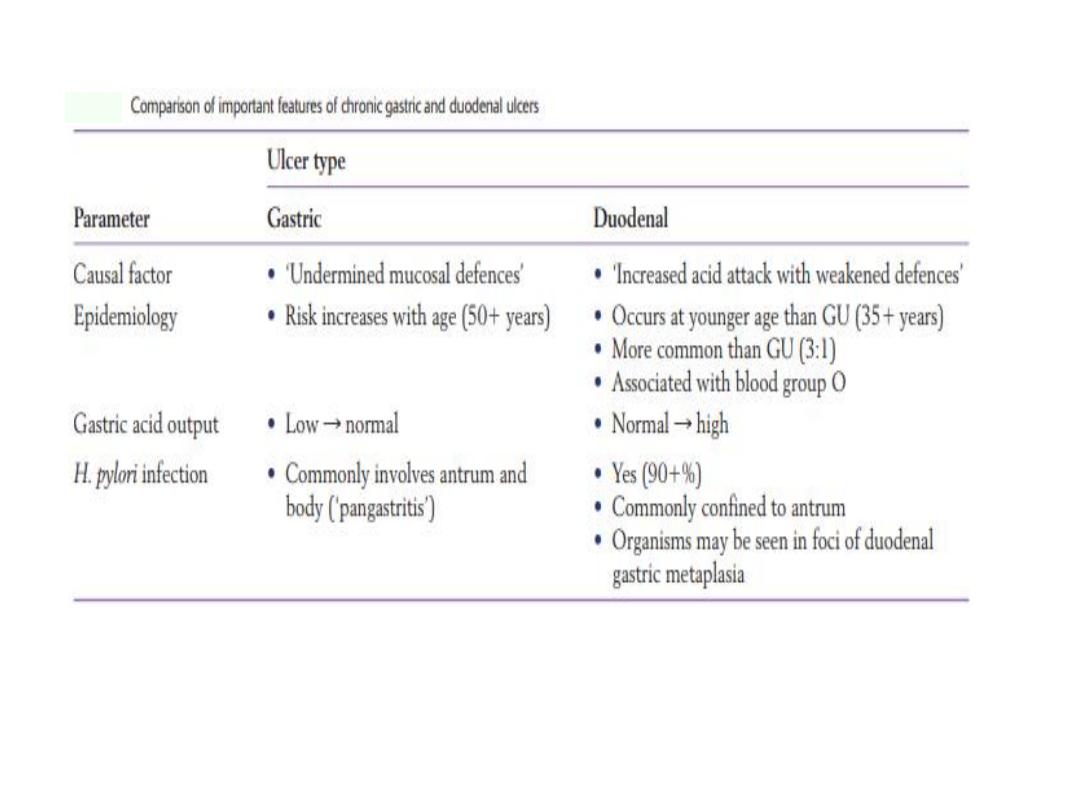
PEPTIC ULCER DISEASE
• Ulcer: focal destruction of mucosa or deeper (necrosis)
• Erosion: less than full thickness focal loss of mucosa, can
progress to ulcer or heal without scarring.
• Peptic : primary autodigestive lesion caused by the action of
gastric juice.
• Sites: Esophagus, stomach, duodenum and ectopic mucosa
Hyperacidity.
Decreased mucosal protection.
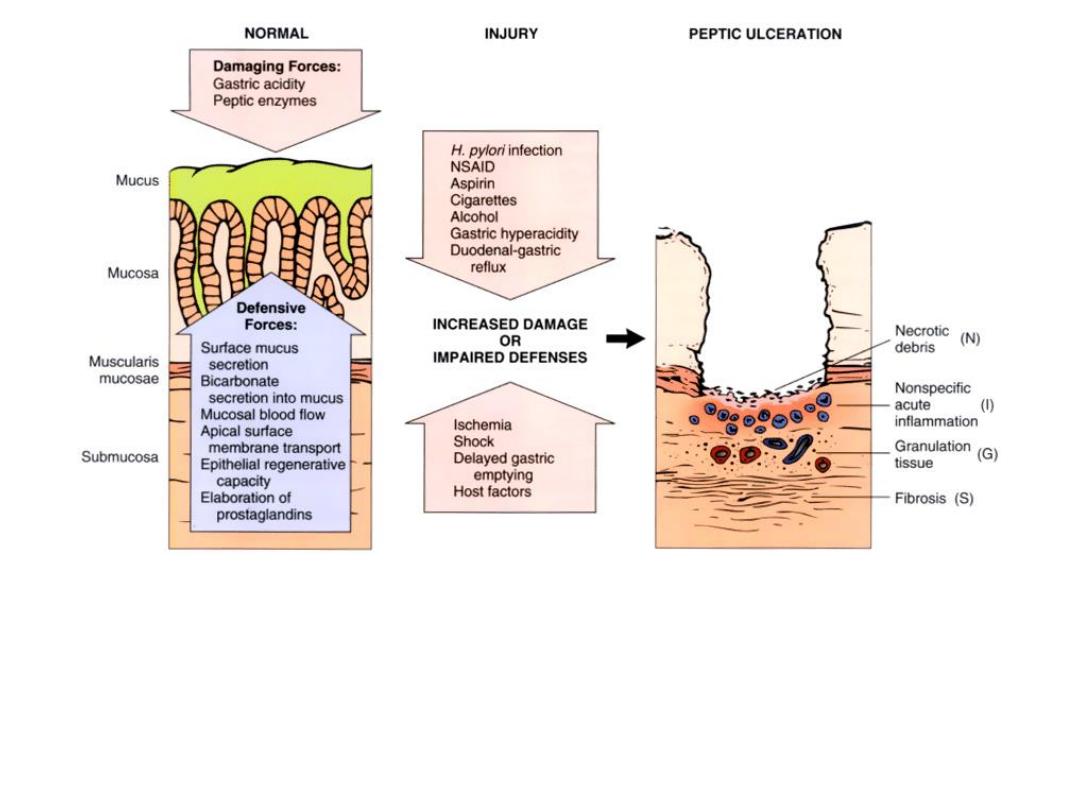
Diagram of causes of, and defense mechanisms against,
peptic ulceration.
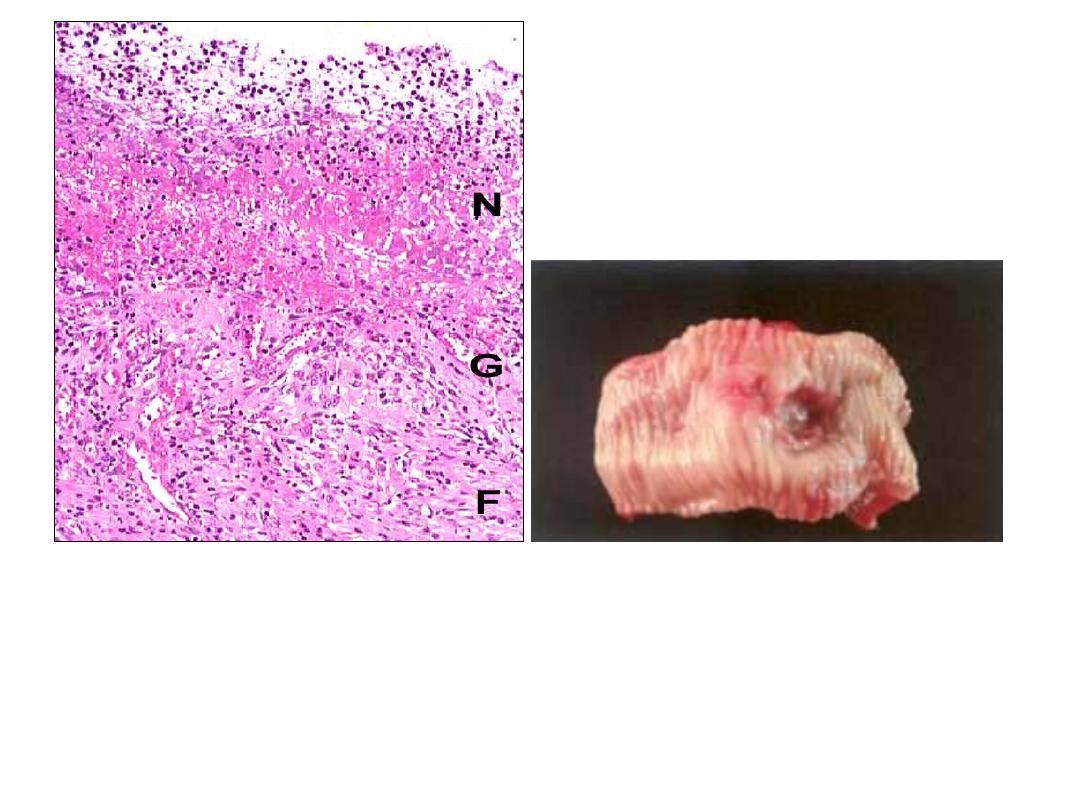
Diagram of the base of a non perforated peptic ulcer, demonstrating the layers
of necrosis (N), inflammation (I), granulation tissue (G), and scar (S), moving
from the luminal surface at the top to the muscle wall at the bottom.

Complications of ulcer
• Bleeding
• Perforation
• Obstruction
• Penetration
• Pain
Complications of gastric ulcer:
Bleeding
1.
Occurs in 15% to 20 % of patients.
2.
Most frequent complication.
3.
May be life-threatening.
4.
Accounts for 25% of ulcer deaths.
5.
May be the first indication of an ulcer.
Perforation
1.
Occurs in up to 5% of patients.
2.
Accounts for two thirds of ulcer deaths.
3.
It is rarely the first indication of an ulcer.
Obstruction
1.
Mostly in chronic ulcers.
2.
Secondary to edema or scarring.
3.
Occurs in about 2% of patients.
4.
Most often associated with pyloric channel ulcers.
5.
May occur with duodenal ulcers.
6.
Causes incapacitating crampy abdominal pain.
7.
Can rarely cause total obstruction and intractable vomiting.
Predisposing factors for peptic ulcer of
stomach and duodenum
• Helicobacter pyIori
– direct mucosal injury
– increased acid secretion
– inflammatory reaction
• Trauma: surgery, fractures, burns (Curling ulcer)
• Stress.
• Cerebral Lesions.
– Vagus nerve stimulation (hypothalamus)
• Smoking.
• Zollinger-Ellison syndrome.
– gastrin overproduction, tumor (gastrinoma)
• Genetic Factors.
• Steroid hormons and Nonsteroidal anti-inflammatory drugs.
– blocks prostaglandin synthesis.
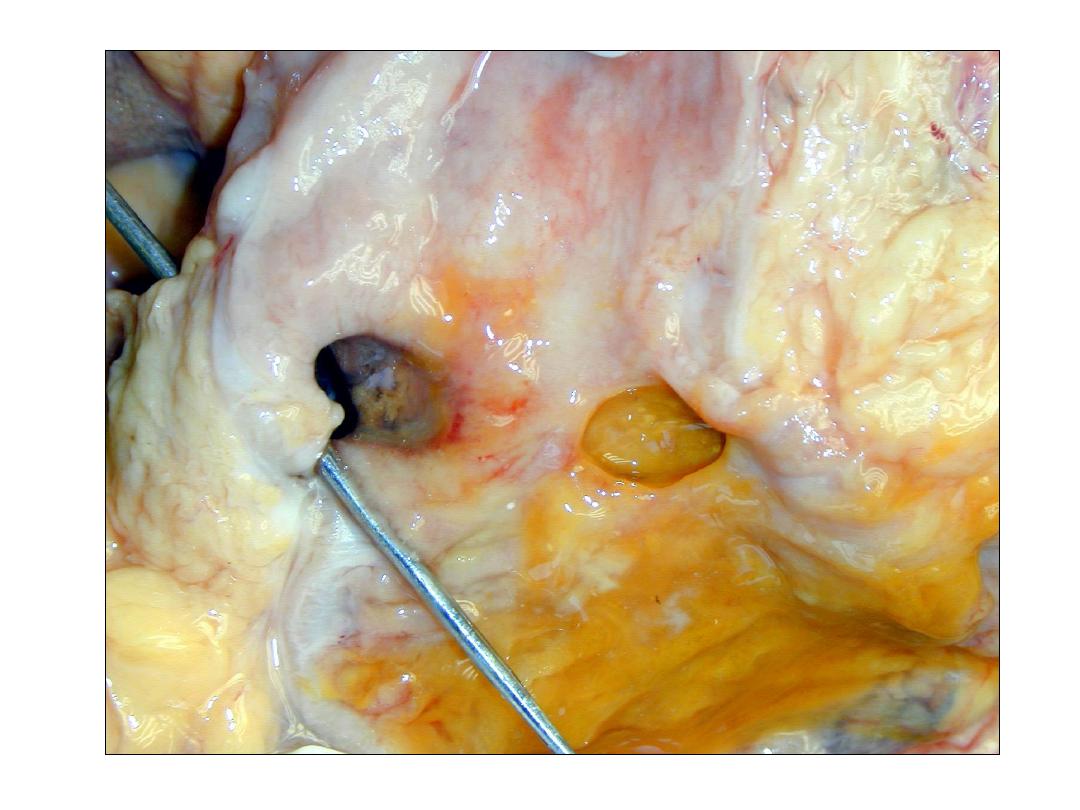
Peptic ulcer of the duodenum.
Note that the ulcer is small (2 cm) with a sharply
punched-out appearance. Unlike cancerous ulcers, the
margins are not elevated.
The ulcer base is clean.
TUMORS
• Benign Epithelial Tumors:
• Hyperplastic and inflammatory polyps
• True adenomas rare and not the leading cause of carcinoma
• Malignant Epithelial Tumors:
– Adenocarcinoma
• Malignant Non - Epithelial Tumors:
– Lymphoma
• Potentially Malignant Tumors:
– Endocrine tumors (carcinoid)
– GIST
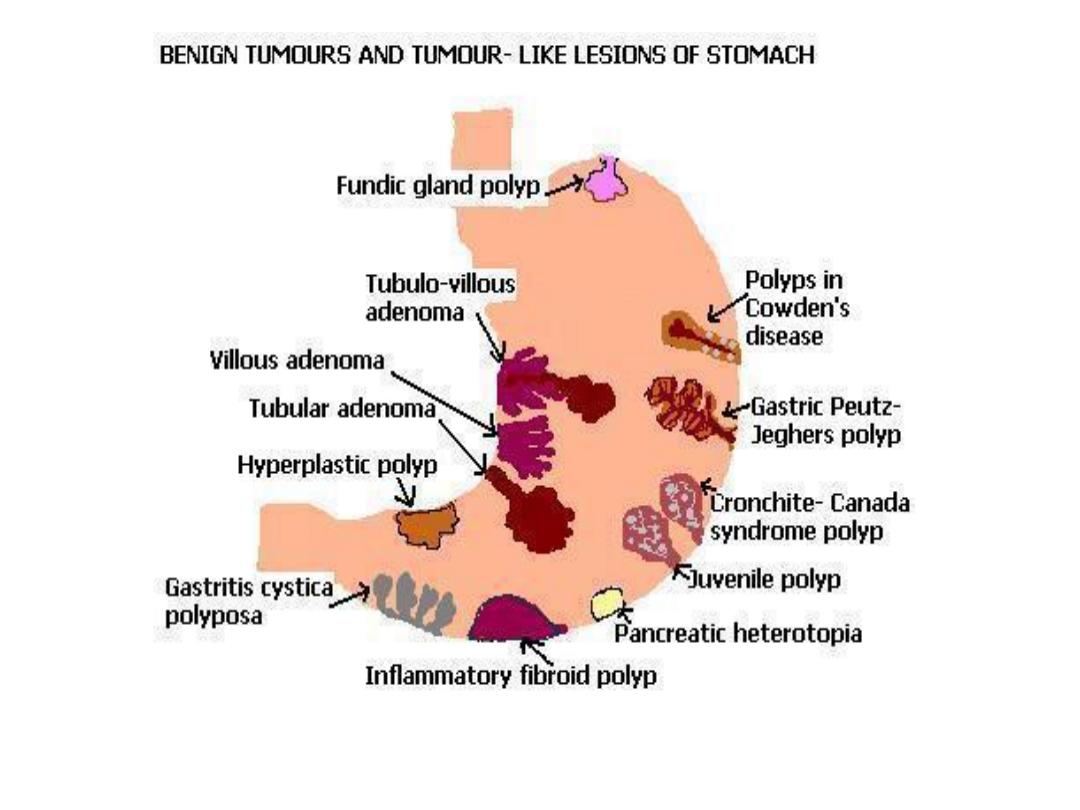
Gastric tumors
• Polyps
Uncommon 0.4% of adults at autopsy as compared
to colon polyps in 25-50% of adults at autopsy.
Many types
Hyperplastic- response to damage.
Fundic gland polyp: small hamartoma.
Note:
(hyperplastic and fundic gland polyps have no malignant
potential)
Adenomatous polyp have malignant potential!
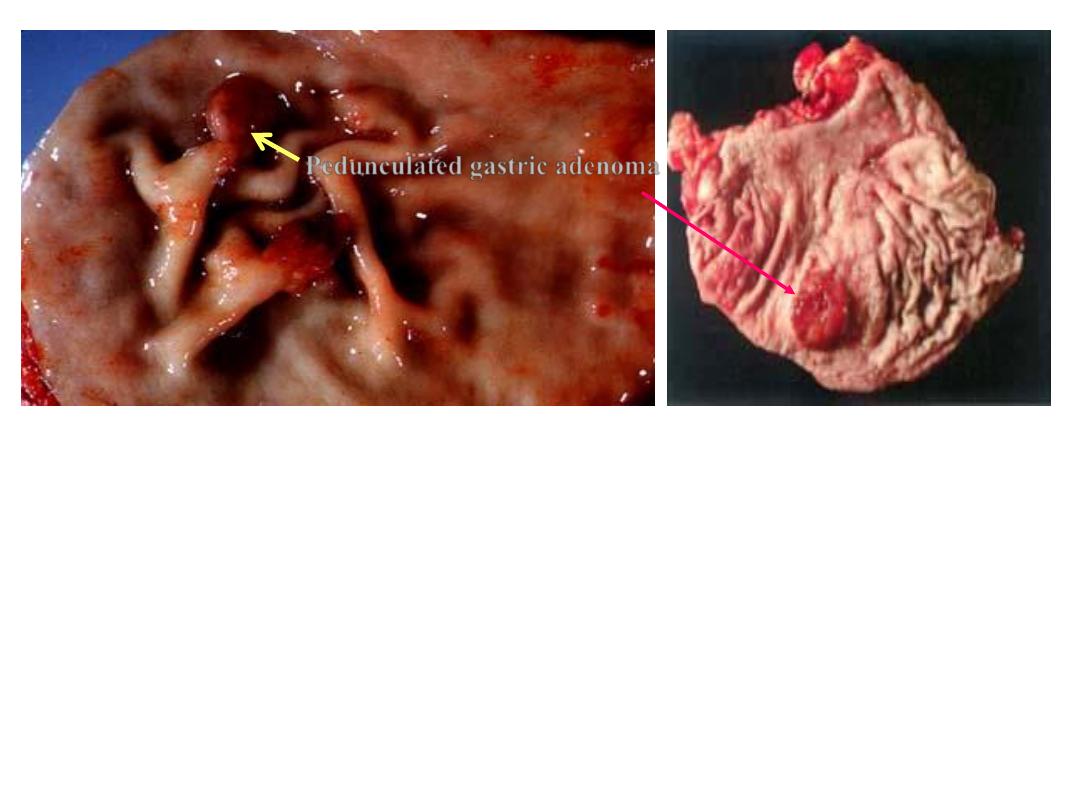
Pedunculated gastric adenoma
Gastric adenoma
Gross photograph showing a large polyp in the stomach.

Etiologic factors of stomach carcinoma
1. Related to socioeconomic level and diet.
2. High incidence in Japan, actually is decreasing in the
Western world, continue to be high in Asia and Russia.
3. Smoked, salted foods.
4. Low fresh vegetable diet.
5. Nitrosamines.
6. Chronic gastritis with atrophy and metaplasia.
7. H. pylori infection.
8. Intestinal reflux.
9. Blood group A.
10. Relatives with stomach cancer.
Precancerous lesions
• Dysplasia
– Enlarged, hyperchromatic, irregularly outlined, crowded
nuclei.
– Irregular glands, mitoses.
High grade dysplasia = in situ carcinoma =
intraepithelial neoplasia.
• Adenoma

Malignant neoplasms of the stomach:
• Gastric Adenocarcinoma (~ 95%).
• Squamous Cell Carcinoma.
• Adenoacanthoma.
• Carcinoid.
• Gastrointestinal stromal tumors.
• Lymphoma.
Carcinoma of the stomach
• Sites: classically prepyloric, antral and lesser curvature.
• Macroscopic types (Borrmann I-IV):
polypoid, ulcerative, ulcerating and infiltrating,
infiltrating.
• Microscopic types (Laurens):
Intestinal:
Gland forming, distal, better differentiated, usually old.
Diffuse:
Proximal, intracellular mucus, signet ring cells, less
differentiated, usually young patient.

Lauren classification System for gastric adenocarcinoma
Intestinal
• Environmental.
• Gastric atrophy with intestinal metaplasia.
• Men > women.
• Increasing incidence with age.
• Gland formation.
• Hematogenous Spread.
• Microsatellite instability.
• APC gene mutations.
• p53, p16 inactivation.
• APC, adenomatous polyposis coli.

Diffuse
• Blood group type A.
• Women > men.
• Younger age group.
• Poorly differentiated,signet ring cells.
• Transmural /lymphatic spread.
• Decreased E-cadhedrin.
• p53, p16 inactivation.

Clinical Presentation
Asymptomatic
Early:
Vague epigastric discomfort / indigestion
Pain is constant, nonradiating, unrelieved by food
digestion
More advanced disease:
Weight loss
Anorexia
Fatigue
Emesis
Symptoms dependent on location
Proximal
Distal
Diffuse gastric ca. presented with GI bleeding, obstruction
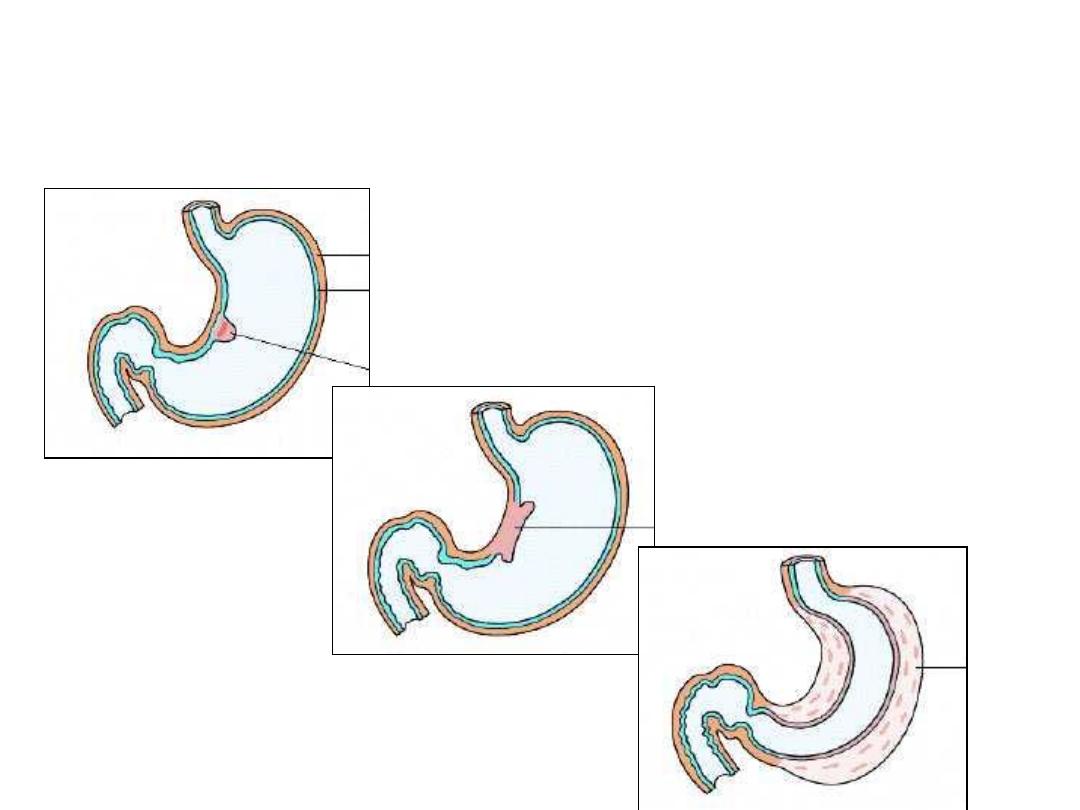
Morphologic types of
carcinoma of the stomach
Fungating
Ulcerating
Diffuse
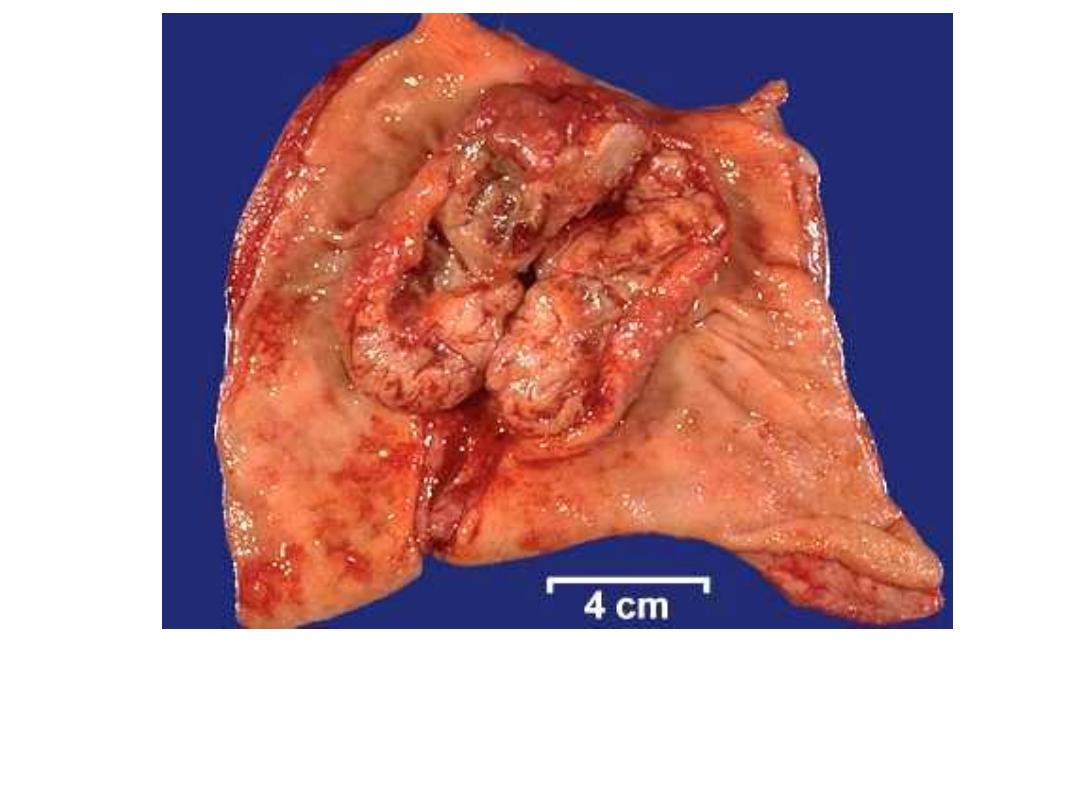
Fungating Carcinoma Stomach
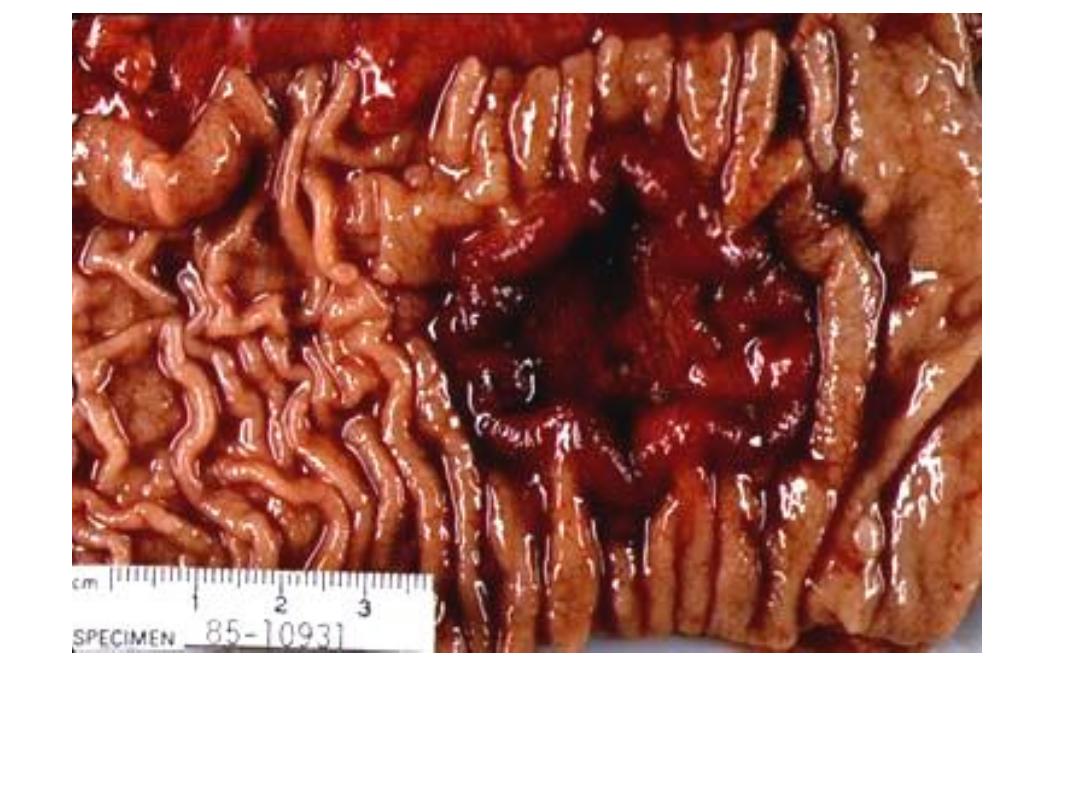
Ulcerating Gastric Carcinoma
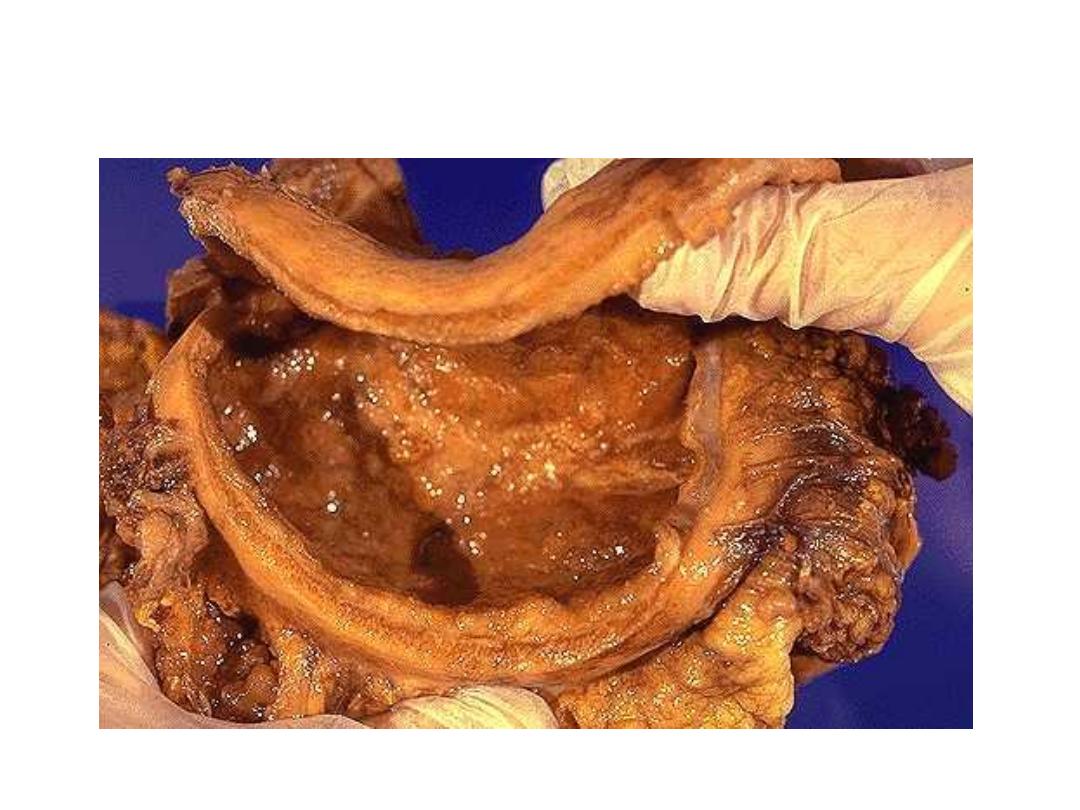
Linitis Plastica – Schirrhous
Carcinoma (diffuse ca.).
