1
DRUG ADMINISTRATION AND SITE OF ACTION
_ different routes of administration are chosen depending on
• Desired onset of action
• Systemic or local effects
• Patient characteristics
• Properties of the drug
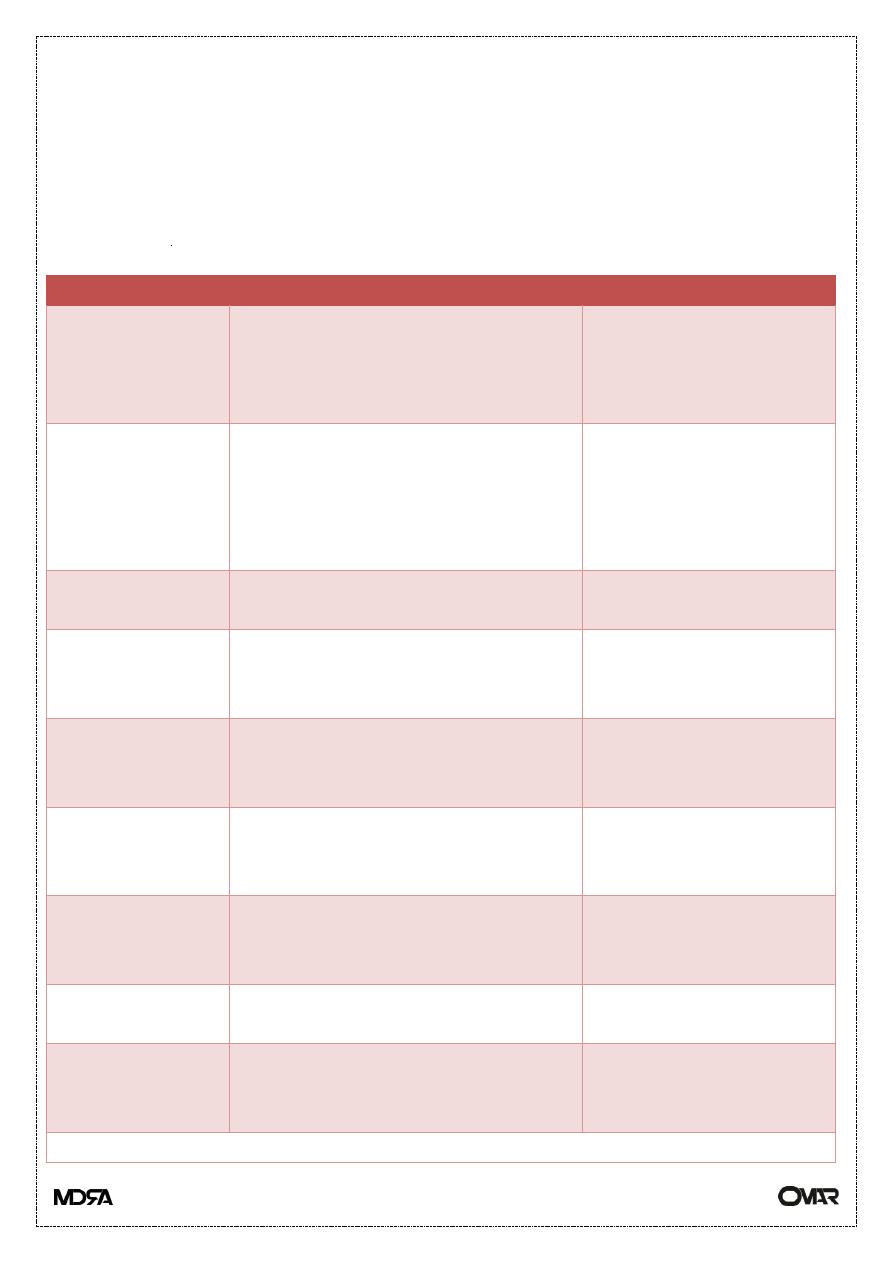
Routes of Drug Administration
Route
Advantage
Disadvantage
Oral (PO)
Convenient Drug metabolism
Large surface area for absorption
Incomplete absorption
First pass effect
Gastrointestinal (GI)
upset
Intravenous (IV)
Direct
No first pass effect
Slow infusions or rapid onset of
action
Easier to titrate dose
Requires IV access
Hard to remove
Vascular injury,
extravasation
Intra-arterial
To specific organs, e.g. brain,
heart
Intramuscular
(IM)
Good for depot storage (if oil
based)
Rapid onset of action
Pain at site of injection
Subcutaneous
(SC)
Non irritating small volumes
Even slow absorption
Adrenaline in local anesthetics
Pain at site of injection
Topical
Convenient
Localized
Limited systemic absorption
Effects are limited to
area of application
Inhalation
Immediate action in lungs
Rapid delivery to blood
Local or systemic action
Must be in gas, vapor or
aerosol form
Buccal
Rapid onset of action
No first pass effect
Must be lipid soluble
Transdermal
Direct application
Rapid onset of action
Irritation at site of
application
Delayed onset of action
Others: Intrathecal, Intraperitoneal, RectalpppkkPHARMACOKINETICS

2
PppppppppppppppppppppppppppDME)
_Pharmacokinetics
Definition: the manner in which the body handles a drug
“ADME”: absorption, distribution, metabolism, excretion
• Absorption: movement of drug into the body from the site of administration
• Output processes: responsible for drug delivery and removal from the body
• Distribution: movement of drug from intravascular to extravascular
compartment
• Metabolism: chemical transformation of drug
• Elimination: removal of drug from the body
ABSORPTION
PRINCIPLE: The amount of drug that reaches the systemic circulation
(bioavailability) is highly
Dependent on absorption. Properties of the drug, route of administration and
patient factors
Should be considered to ensure clinical effectiveness.
_ Most drugs are absorbed into the systemic circulation via passive diffusion
_ Other mechanisms of absorption include: active transport, facilitated diffusion,
pinocytosis/phagocytosis
_ Absorption rate and amount depends on
1• local blood flow at administration site (e.g. sublingual vessels provide
significant blood flow therefore rapid absorption)
2• lipid solubility: greater lipid solubility = increased rate of diffusion through
membranes
E.g. anesthetics are very lipid soluble therefore have a rapid onset of action

3
3• molecular size: small size, water soluble drugs can pass through channels in
membranes,
Large molecules cannot e.g. aminoglycosides are large molecules and are not
absorbed
Through intestinal mucosa and are therefore not orally active
4• local pH and drug ionization: charged molecules do not cross membranes e.g.
lactulose ionizes ammonia to ammonium and keeps it in the bowel
5• total surface area for absorption: the small intestine has villi which
Increase the surface area for absorption, and hence is the primary site of
absorption for most oral drugs
Bioavailability
_ the percentage of dose given that reaches the systemic circulation in unchanged
form
_ the administered dose does not equal active dose
_ Drugs with a low bioavailability may be ineffective orally
• E.g. penicillin G is destroyed by gastric enzymes and needs to be administered
IV
_ Fate of oral drug: GI Tract ––> portal vein ––> liver (metabolism) ––>
systemic circulation
First Pass Effect
_ Metabolism of orally administered drug in the liver before it reaches the
systemic circulation
_ Significant first pass metabolism limits a drug’s bioavailability
_ Drugs with a high first-pass effect include: chlorpromazine, levodopa,
morphine, propranolol,
Lidocaine, hydralazine, nortriptyline, and organic nitrates
_ Drugs with low hepatic extraction (little or no first pass effect) include:

4
Diazepam, digoxin, phenylbutazone, phenytoin, theophylline, tolbutamide,
warfarin
Volume of Distribution (Vd)
Definition: process by which drugs are carried throughout the body to reach
target sites of action
PRINCIPLE: Some drugs have uncommon distribution parameter (e.g. Vd,
protein binding,
Storage in fat depots) and have to be dosed carefully to avoid toxicity while
ensuring
Therapeutic efficacy.
_ Actual volume of distribution (Vd): the anatomic volume that is accessible to
drug,
e.g. total body water of 40 L
_ Apparent volume of distribution (Vd) is a calculated value that does not
correspond to an anatomical space,
a drug with a large Vd (larger than 40 L) must distribute in other tissues besides
body water
• e.g. Amiodarone; Vd=400L in a 70kg person
(ADME). . .
Protein Binding
Drug molecules in the blood are in two forms:
1• bound to plasma proteins, mainly albumin
2• free
Principles of protein binding
• Only free drug can distribute into tissues and exert its action, and is subject to
metabolism and elimination
• Affinity of a protein binding site for a drug determines bound/unbound
concentrations, and reversibility of interaction

5
• Saturation of binding sites may result in a large increase in unbound drug
concentration, which could cause toxicity
• If albumin concentration is decreased (liver failure or nephrotic syndrome),
dose of highly bound drug must be lowered to avoid toxicity
• Competition for binding sites between drugs and endogenous substrates can
result in interactions and toxicity
• Significant drug interactions can occur due to competitive protein binding
• E.g. ASA displaces several drugs which are highly bound to plasma proteins
such as phenytoin, increasing risk of toxicity
• In general, only drugs that are highly protein bound, e.g. > 90%, are involved in
drug interactions due to competitive binding
Depots
_ a part of the body (e.g. a type of tissue) where drug molecules tend to be stored
fat tends to be a depot for very lipid soluble drugs (e.g. diazepam)
Barriers
_ Body structures that limit/prevent diffusion of drug molecules,
_ E.g. blood-brain barrier (BBB), placenta
METABOLISM (BIOTRANSFORMATION)
PRINCIPLE: Drugs that are metabolized by similar enzymes, e.g. the same
cytochrome P450
isoenzymes, have the potential to interfere with each other’s metabolism.
_ Conversion of a drug into another form may result in
1• activation of pro-drug: e.g. Codeine to morphine, nitroglycerine to NO
2• maintenance of activity, e.g. Diazepam is metabolized to an active metabolite
3• inactivation, e.g. Procaine to PABA
_ Main site of biotransformation in the body is the LIVER.

6
_ Drug metabolizing enzyme pathways generally mediate 2 types of reactions
a• Phase I reactions
• Oxidation-reduction and hydrolysis
• introduce or unmask polar chemical groups therefore increase water solubility
• mediated by cytochrome P450 enzymes
• P450’s are found in the endoplasmic reticulum or cell cystoplasm
b• phase II reactions
• Conjugation with polar endogenous substrates e.g. glucoronic acid, glutathione
• increases water solubility and renal elimination
_ Cytochrome P450 isoenzyme CYP 3A4 metabolizes about 50% of all drugs,
hence if a drug
Which is metabolized by 3A4 is prescribed, double check for possible
interactions if any
Other drug is added to the regimen.
Examples of Important Highly Protein Bound Drugs
Name % Bound
Salicylic Acid 82
Phenytoin 90
Propranolol 93
Diazepam 99
Warfarin 99.5
Organ Distribution of Drug Metabolizing Enzymes
Site Relative Activity
Liver 100
Lung 20-30
Kidney 8
Intestine 6
Term Placenta 5
Adrenal Glands 2
Skin 1

7
PHARMACOKINETICS (ADME). . . CONT.
Drug Interactions are Often Due to Interactions in Biotransformation Pathways
_ Phase I (Cytochrome P450 enzymes)
1. Erythromycin inhibits the CYP3A4 enzyme, and predisposes to cisapride
toxicity and possible fatality
2. Cimetidine inhibits P450 enzymes, leading to increased levels of theophylline,
diazepam,
Warfarin, phenytoin
3. Phenobarbital induces P450 enzymes, which could decrease levels of other
drugs
(See below: Enzyme Induction)
4. The SSRIs could inhibit CYP 2D6 (and 3A4), and therefore increase serum
levels of other
Drugs metabolized by these enzymes, e.g. benzodiazepines, carbamazepine,
phenytoin
5. The new HIV drugs, the protease inhibitors, are metabolized by cytochrome
P450 enzymes, (e.g. indinavir is metabolized by CYP 3A4), and hence could
interact with other drugs metabolized by this route
_ Phase II (Conjugation reactions):
1. Acetaminophen is 95% metabolized to inactive glucuronic acid and sulfate
conjugates,
And 5% oxidized by P450, generating a reactive metabolite which is then
conjugated with glutathione.
If glutathione stores are depleted, e.g. Massive dose of acetaminophen, the
reactive metabolite
Remains unconjugated and causes hepatocellular damage. In concurrent
ingestion of alcohol and large
Doses of acetaminophen, a double whammy situation occurs. Alcohol induces
the P450 enzymes,
And hence the generation of the reactive metabolite; alcoholics tend to be
deficient in nutrients,
Notably glutathione, hence depletion occurs more readily, resulting in massive
hepatocellular
Damage in this situation
_ Enzyme Induction
• Over 200 unrelated drugs have the ability to increase the activity of drug bio
transforming
Enzymes generally reducing activity/intensity of drug action
• reflects de novo synthesis of P450 and other bio transforming enzymes
• Induction of P450 can stimulate multiple iso-enzymes specifically or non-
specifically

8
Examples of Inducing Agents
Inducing Agent Substance whose metabolism is increased
Phenobarbital Bilirubin
DDT Chlorpromazine
Phenytoin Codeine
Glutethamide Cortisol
Phenylbutazone Estradiol, meperidine, morphine, testosterone,
thyroxin
_ other factors affecting drug metabolism
• Age
• Early in fetal life drug metabolizing enzyme levels are low
• Elderly have reduced rates of metabolism due to reduced hepatic function
• Nutrition
• Inhibition or drug metabolizing enzymes with decreased protein,
decreased fatty acids
• Alcohol, vitamin deficiency states
• Induction of P450 with chronic ingestion
• Inhibition of P450 with acute ingestion
• Radiation
• Sex
• Race
ELIMINATION
PRINCIPLE: Dosing of drugs needs to be adjusted according to the elimination
characteristics
Of the patient (e.g. in renal impairment) in order to avoid toxicity from drug or
metabolite
Accumulation.
_ Routes of elimination include
1• stool (e.g. corticosteroids from biliary system)
2• lungs (e.g. general anesthetics eliminated by expiration)
3• skin and mucous membranes (e.g. rifampin in tears)
4_ KIDNEYS are the main organ of drug excretion through
a• glomerular filtration: passive, pore size about 400-600 Angstroms
b• tubular secretion: active, against concentraion gradient, saturable,
Two distinct transport mechanisms for weak acids and weak bases
• E.g. acids: penicillin, salicyclic acid, probenecid, chlorothiazide

9
• E.g. bases: quinine, quaternary ammonium compounds (e.g. choline)
• Tubular reabsorption: can be active or passive (depending on charge)
_ Elimination rate depends on renal function (assessed clinically, using serum
creatinine levels)
• The Cockroft-Gault equation can estimate creatinine clearance (CrCl) for males
as:
CrCl (mL/min) = (140-patient's age in yrs) x IBW (kg)
50 x SCr (μmol/L)
• For females above equation x 0.85
_ Drug interactions due to interference with filtration, secretion, reabsorption
• probenecid significantly reduces renal excretion of penicillin by competing
For the weak acid transport
• Lithium is renally eliminated through glomerular filtration, much of the filtered
load is reabsorbed at the proximal renal tubule. Sodium competes for the
reabsorption site with lithium. Hence, thiazide diuretics, which can cause
hyponatremia and reduced sodium load in the renal tubule, increase the
Reabsorption of lithium and can predispose to increased serum lithium levels and
lithium toxicity
CALCULATION. CONT.
Half-Life (t1/2)
_ defined as the time it takes for blood level of a drug to fall to one-half (50%) of
the level
Measured at some prior time
_ For most drugs, half-life correlates with the elimination phase
_ In general it takes 5 half-lives to reach steady state with repeated dosing or for
drug elimination
Once dosing is stopped
Steady State
_ The concentration at which the same amount of drug entering the system is
eliminated from the system
_ Time is important for therapeutic monitoring as drug levels are only reliable
when the drug has
Reached this steady state
_ Any change in drug dose and interval will change the steady state level _
special situations

11
• Drug with long half-life and the need to rapidly increase blood levels –
Give a loading dose (e.g. phenytoin)
• Drugs with a very short half-life and the need for a long term effect – multiple,
frequent repeated doses
Are too inconvenient thus use a continuous infusion (e.g. nitroprusside)
Elimination Kinetics
_ first-order kinetics (the most common type)
• A constant fraction of drug is eliminated per unit time
• The amount of drug eliminated is based on the concentration of drug present
• This relationship is linear and predictable
_ zero-order kinetics (less common, associated with toxicities)
• Non-linear kinetics
• A constant amount (number of molecules) of drug is eliminated per unit time
• Clearance slows as drug concentration rises
• Some drugs can follow first order kinetics until elimination is saturated (usually
at large doses)
And the clearance decreases
• Some drugs follow non-linear kinetics at therapeutic levels e.g. phenytoin
Pharmacodynamics
Definition: the relationship between the drug concentration and effect (what the
drug does to the body)
Agonists Have Two Main Properties
_ Affinity: the ability of the agonist to “bind to” the receptor
_ Efficacy: the ability to cause a response via the receptor interaction
_ e.g. the ß2-agonist (salbutamol) bind to ß2-receptors (i.e. has affinity) and
result in activation of
Smooth muscle relaxation (i.e. has efficacy)
Antagonists
_ have affinity (can bind to a receptor) but no efficacy
_ Chemical antagonism: direct chemical interaction between agonist and
antagonist
Prevents agonist binding to receptor
• E.g. chelator agents for removal of heavy metals

11
_ Functional (physiological) antagonism: interaction of 2 agonists that act at
different receptors
Independent of each other but have opposite physiological effects
• E.g. acetylcholine at the muscurinic receptor decreases HR, constricts pupil,
stimulates intestinal motility
• Epinephrine at the adrenergic receptor increases HR, dilates pupil, decreases
intestinal motility
_ Competitive antagonism (most common in clinical practice)
• Antagonist acts at same receptor (i.e. binds) displacing agonist
• Antagonist binding is reversible and can be overcome
_ Non-competitive antagonism
• Irreversible binding of antagonist to receptor
• Allosteric effect: changes ability of the agonist to bind to the receptor through
various
Mechanisms such as changing the conformation of the receptor
• increasing concentrations of agonist cannot reverse the antagonism
Dose-response relationship_ pharmacodynamics principles measuring efficacy
and potency can be quantified using dose-response curves
_ With gradual dose response relationships the response of the drug reflects the
number of receptors that are effectively occupied
_ Efficacy
• The maximum intensity of response to a drug, e.g. If Drug A causes a
greater maximum intensity of response than Drug B (regardless of dose), then
Drug A is more efficacious than Drug B
• ED50 (effective dose-50%) the dose or drug that gives rise to the designated
response in
50% of the subjects
• ED50 is easier to measure than maximum effect and is used to determine
efficacy
_ Potency
• A comparison of the ED50 of two or more drugs that have parallel log dose-
response curves
• The drug that reaches the ED50 at the lower dose is the more potent
• Potency is a term that is often misused (confused with efficacy)

12
• Potency is not important if you can increase the dose of the less potent drug
Without causing side effects
Effectiveness and Safety
_ The two most clinically relevant properties of any drug are effectiveness and
safety
_ Effectiveness
• Similar to efficacy but in real populations (i.e. not experimental)
_ Safety
• LD50 (lethal dose-50%): defined as the dose of a drug needed to cause death in
50% of a
Test population of subjects (e.g. usually rodents)
• TD50 (Toxic Dose - 50%): defined as the dose needed to cause a
Harmful effect in 50% of the subjects
CONT.
Therapeutic Index (TI)
_ defined as TD50/ED50
_ reflects the “margin of safety” for a drug - the likelihood of a high dose causing
serious toxicity/death
_ The larger the TI, the safer a drug
_ Factors can change the ED50, LD50 or the TD50
• Presence of interacting drugs
• Changes in drug absorption, distribution, metabolism, elimination
• E.g. amoxicillin has a large TI, therefore therapeutic monitoring is not needed,
Whereas warfarin has a small TI and must have accurate therapeutic monitoring.
Variability in Drug Action
PRINCIPLE: not everyone experiences the same response to the same dose
(Route of administration, dosage interval, etc. may need to be adjusted in some
cases).
_ Some common causes of variable responses to a drug
1• age
2• gastric pH and gut motility (affects absorption of certain drugs)
3• body composition (changes in fat, muscle, water content)
4• plasma protein levels (affects various aspects of pharmacokinetics)
5• renal, liver function (affects excretion and metabolism respectively)

13
6• gender: mainly due to presence or absence of certain enzymes, hormones, etc.
7• genetics: presence/absence of one or more genes needed to form enzymes,
Other proteins, hormones, etc.
8• overall health - presence/absence of other diseases
9• use of other drugs (i.e. interactions)
10• nutritional status - excess or deficiency of key vitamins, minerals, etc.
11• compliance
ADVERSE DRUG REACTIONS (ADRs)
_ Classification of adverse drug reactions
• Type A: predictable
• Type B: unpredictable
Type A
_ Side effects: excessive but characteristic pharmacological effect from usual
dose of a drug
_ Overdose / toxicity: exaggerated but characteristic pharmacological effect from
supratherapeutic dose
_ Teratogen: drug may produce developmental defects in fetus
_ Characteristics
• Account for 80% + of all ADRs
• Extension of pharmacological effect
• Dose-related and generally not severe
• Usually do not require discontinuation
• Dose reduction or titration may help minimize effect
• E.g. a common side effect of beta-blockers is bradycardia (an extension of its
therapeutic effect)
Type B
_ Idiosyncratic: uncharacteristic response to drug, unrelated to pharmacology
_ Pseudo-allergenic: mimics immune-mediated reaction
_ Allergic / immune-mediated: does not occur on first exposure (up to 7d),
immediate with subsequent
Exposure, may occur with low dose, resolves within 3-4 days of discontinuation
• Characteristics
• Usually more severe
• Usually require discontinuation

14
• Not dose-related
• E.g. sulpha based drugs (such as septra) can cause an idiosyncratic Stevens
Johnson Syndrome (SJS)
Approach to Suspected ADRs
_ History and physical examination: symptoms, timing, risk factors, medication
related, dechallenge
And rechallenge information is needed, look up previous reports in the literature
_ Differential diagnosis: therapy or disease pathophysiology
_ treat the adverse drug reaction: stop the drug, supportive care, symptomatic
relief
