
Diagnosis and Treatment
of Furcation‐Involved Teeth

Diagnosis and Treatment
of Furcation‐Involved Teeth
Edited by Luigi Nibali
Senior Clinical Lecturer
Centre for Immunobiology and Regenerative Medicine
Centre for Oral Clinical Research, Institute of Dentistry
Barts and the London School of Medicine and Dentistry
Queen Mary University of London (QMUL), London, UK
Honorary Associate Professor, University of Hong Kong

This edition first published 2018
© 2018 John Wiley & Sons Ltd
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted,
in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as
permitted by law. Advice on how to obtain permission to reuse material from this title is available at
http://www.wiley.com/go/permissions.
The right of Luigi Nibali to be identified as the author of the editorial material in this work has been asserted
in accordance with law.
Registered Offices
John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, USA
John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK
Editorial Office
9600 Garsington Road, Oxford, OX4 2DQ, UK
For details of our global editorial offices, customer services, and more information about Wiley products visit us
at www.wiley.com.
Wiley also publishes its books in a variety of electronic formats and by print‐on‐demand. Some content that
appears in standard print versions of this book may not be available in other formats.
Limit of Liability/Disclaimer of Warranty
The contents of this work are intended to further general scientific research, understanding, and discussion only
and are not intended and should not be relied upon as recommending or promoting scientific method, diagnosis, or
treatment by physicians for any particular patient. In view of ongoing research, equipment modifications, changes
in governmental regulations, and the constant flow of information relating to the use of medicines, equipment, and
devices, the reader is urged to review and evaluate the information provided in the package insert or instructions
for each medicine, equipment, or device for, among other things, any changes in the instructions or indication of
usage and for added warnings and precautions. While the publisher and authors have used their best efforts in
preparing this work, they make no representations or warranties with respect to the accuracy or completeness of the
contents of this work and specifically disclaim all warranties, including without limitation any implied warranties of
merchantability or fitness for a particular purpose. No warranty may be created or extended by sales representatives,
written sales materials or promotional statements for this work. The fact that an organization, website, or product
is referred to in this work as a citation and/or potential source of further information does not mean that the
publisher and authors endorse the information or services the organization, website, or product may provide or
recommendations it may make. This work is sold with the understanding that the publisher is not engaged in
rendering professional services. The advice and strategies contained herein may not be suitable for your situation.
You should consult with a specialist where appropriate. Further, readers should be aware that websites listed in
this work may have changed or disappeared between when this work was written and when it is read. Neither the
publisher nor authors shall be liable for any loss of profit or any other commercial damages, including but not limited
to special, incidental, consequential, or other damages.
Library of Congress Cataloging‐in‐Publication Data
Names: Nibali, Luigi, 1978– editor.
Title: Diagnosis and treatment of furcation-involved teeth / edited by Luigi Nibali.
Description: Hoboken, NJ : Wiley, [2018] | Includes bibliographical references and index. |
Identifiers: LCCN 2018010570 (print) | LCCN 2018011380 (ebook) | ISBN 9781119270669 (pdf) |
ISBN 9781119270676 (epub) | ISBN 9781119270652 (hardback)
Subjects: | MESH: Furcation Defects–diagnosis | Furcation Defects–therapy | Models, Animal
Classification: LCC RK450.P4 (ebook) | LCC RK450.P4 (print) | NLM WU 242 | DDC 617.6/32–dc23
LC record available at https://lccn.loc.gov/2018010570
Cover image: (Main, top left and middle images) © Luigi Nibali; (Top right image) © Roberto Rotundo
Cover design: Wiley
Set in 10/12pt Warnock by SPi Global, Pondicherry, India
10 9 8 7 6 5 4 3 2 1

Chapter No.: 1 Title Name: <TITLENAME>
ftoc.indd
Comp. by: R. RAMESH Date: 14 May 2018 Time: 04:18:23 PM Stage: Revises1 WorkFlow:
<WORKFLOW>
Page Number: v
v
List of Contributors
vii
Foreword
ix
Preface
xi
About the Companion Website
xiii
1 Anatomy of Multi‐rooted Teeth and Aetiopathogenesis of the Furcation Defect
1
Bernadette Pretzl
2 Clinical and Radiographic Diagnosis and Epidemiology of Furcation Involvement
15
Peter Eickholz and Clemens Walter
3 How Good are We at Cleaning Furcations? Non‐surgical and Surgical Studies
33
Jia‐Hui Fu and Hom‐Lay Wang
4
Furcation: The Endodontist’s View
55
Federica Fonzar and Riccardo Fabian Fonzar
5 Why do We Really Care About Furcations? Long‐term Tooth Loss Data
91
Luigi Nibali
6 Regenerative Therapy of Furcation Involvements in
Preclinical Models: What is Feasible?
105
Nikolaos Donos, Iro Palaska, Elena Calciolari, Yoshinori Shirakata, and Anton Sculean
7 Regenerative Therapy of Furcations in Human Clinical Studies: What has been Achieved
So Far?
137
Søren Jepsen and Karin Jepsen
8 Furcation Therapy: Resective Approach and Restorative Options
161
Roberto Rotundo and Alberto Fonzar
9
Furcation Tunnelling
177
Stefan G. Rüdiger
10 Innovative and Adjunctive Furcation Therapy: Evidence of Success
and Future Perspective
191
Luigi Nibali and Elena Calciolari
Contents

Contents
vi
11 Furcation: Why Bother? Treat the Tooth or Extract and Place an Implant?
209
Nikos Mardas and Stephen Barter
12 Is it Worth it? Health Economics of Furcation Involvement
229
Falk Schwendicke and Christian Graetz
13 Deep Gaps between the Roots of the Molars: A Patient’s Point of View
249
Luigi Nibali
14 Assessment of Two Example Cases Based on a Review of the Literature
257
Luigi Nibali
15 Furcations: A Treatment Algorithm
269
Luigi Nibali
Index
285

Chapter No.: 1 Title Name: <TITLENAME>
fbetw.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:18:26 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: vii
vii
Dr Stephen Barter
Private practice, Eastbourne, UK
Dr Elena Calciolari
Centre for Immunobiology and Regenerative
Medicine
Centre for Oral Clinical Research
Institute of Dentistry
Barts and the London School of Medicine
and Dentistry
Queen Mary University of London (QMUL)
London, UK
Prof. Nikolaos Donos
Centre for Immunobiology and Regenerative
Medicine
Centre for Oral Clinical Research
Institute of Dentistry
Barts and the London School of Medicine
and Dentistry
Queen Mary University of London (QMUL)
London, UK
Prof. Peter Eickholz
Poliklinik für Parodontologie
Zentrum der Zahn‐ Mund‐ und
Kieferheilkunde (Carolinum)
Johann Wolfgang Goethe‐Universität
Frankfurt
Frankfurt am Main
Germany
Dr Federica Fonzar
Private practice
Udine
Italy
Dr Alberto Fonzar
Private practice
Udine
Italy
Dr Riccardo Fabian Fonzar
Private practice
Udine
Italy
Dr Jia‐Hui Fu
Discipline of Periodontics
Faculty of Dentistry
National University of Singapore
Singapore
Dr Christian Graetz
Clinic for Conservative Dentistry and
Periodontology
Christian‐Albrechts‐University
Kiel
Germany
Dr Karin Jepsen
Department of Periodontology
Operative and Preventive Dentistry
University of Bonn
Germany
Prof. Søren Jepsen
Department of Periodontology
Operative and Preventive Dentistry
University of Bonn
Germany
Dr Nikos Mardas
Centre for Immunobiology and Regenerative
Medicine
Centre for Oral Clinical Research
Institute of Dentistry
Barts and the London School of Medicine
and Dentistry
Queen Mary University of London (QMUL)
London
UK
List of Contributors

List of Contributors
viii
Dr Luigi Nibali
Centre for Immunobiology and Regenerative
Medicine
Centre for Oral Clinical Research
Institute of Dentistry
Barts and the London School of Medicine
and Dentistry
Queen Mary University of London (QMUL)
London
UK
Dr Iro Palaska
Centre for Immunobiology and Regenerative
Medicine
Centre for Oral Clinical Research
Institute of Dentistry
Barts and the London School of Medicine
and Dentistry
Queen Mary University of London (QMUL)
London
UK
Dr Bernadette Pretzl
Section of Periodontology
Department of Operative Dentistry
University Clinic Heidelberg
Heidelberg
Germany
Dr Roberto Rotundo
Periodontology Unit
UCL Eastman Dental Institute
London
UK
Dr Stefan G. Rüdiger
Department of Periodontology
Public Dental Service/Malmö University
Malmö
Sweden
Dr Falk Schwendicke
Department of Operative and Preventive
Dentistry
Charité‐Universitätsmedizin Berlin
Berlin
Germany
Prof. Anton Sculean
Department of Periodontology
School of Dental Medicine
University of Bern
Bern
Switzerland
Dr Yoshinori Shirakata
Department of Periodontology
Kagoshima
University Graduate School of Medical and
Dental Sciences
Kagoshima
Japan
Prof. Clemens Walter
Klinik für Parodontologie
Endodontologie und Kariologie
Universitätszahnkliniken,
Universitäres Zentrum für
Zahnmedizin Basel
Basel
Switzerland
Prof. Hom‐Lay Wang
Department of Periodontics and Oral
Medicine
School of Dentistry
University of Michigan
Ann Arbor
MI
USA

Chapter No.: 1 Title Name: <TITLENAME>
fbetw.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:18:26 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: ix
ix
Foreword
The preservation of tissues and structures
that support the dentition is a major goal of
conservative dentistry for the benefit of our
patients. As dental practitioners, we are
trained to maintain and restore function,
aesthetics, and phonetics for the promotion
of oral health. In the development of this
book, Diagnosis and Treatment of Furcation‐
Involved Teeth, Luigi Nibali and his
co‐authors have assembled an excellent text
that comprehensively examines the manage-
ment of the most challenging‐to‐treat teeth
in the jaws – the molar and premolar teeth
with furcation involvement. It is clear that
clinicians are continually tested on which are
the best approaches to handle these clinical
scenarios that include furcated teeth. The
education, skill, and training required to
manage furcations are significant given the
anatomy, location, and functional biome-
chanical occlusal forces associated with
posterior teeth that make for complex clinical
decision‐making.
In this text, Dr Nibali has convened inter-
national experts providing chapters ranging
from the diagnosis of disease to clinical out-
comes from the health policy expert’s, cari-
ologist’s, periodontist’s, and endodontist’s
perspectives on periodontal‐endodontic‐
restorative dilemmas in patient care. It is
important to recognize that there is a large
evidence base that was initiated from the
‘pre–dental implant era’ on the long‐term
success in the maintenance of compromised
teeth affected by extensive restorative care,
periodontal involvement, and/or pulpal
pathology. This text not only focuses on
diagnosis and treatment, but includes valua-
ble information from a health economics and
treatment algorithms perspectives on long‐
term tooth preservation.
In the first part of the book, a thorough
background on the unique anatomy of multi‐
rooted teeth and corresponding diagnostic,
prognostic, and therapeutic intricacies is
presented. The next section provides a strong
rationale regarding the concept of tooth
preservation from the restorative, periodon-
tal, and endodontic perspectives, which
highlights the strong evidence base of
treatment success of tooth furcations. This
background is important to examine criti-
cally, since many oral implantologists in the
field are not adequately versed on the ramifi-
cations of premature tooth removal versus
those teeth that can be predictably retained
for the long‐term success of the patient. The
application in clinical practice by those with-
out adequate training occasionally errs on
the expedience of tooth extraction, without
pausing to weigh methodically the advan-
tages and disadvantages of embarking on
comprehensive therapy for furcated teeth.
Those without access to this text on the many
options available to increase the lifespan of
molar and premolar teeth may not be pre-
pared to treatment plan the complex dental
patient appropriately for the comprehensive
assessment of restorative, periodontal, endo-
dontic, functional, and aesthetic needs.
Given practice trends of more common
extractions of furcated periodontally and
endodontically compromised teeth, it sug-
gests that ‘the time is right’ to emphasize the

orreorr
x
great potential available in the proper assess-
ment and treatment of furcated teeth. This
section highlights the long‐term success with
proper therapy in maintaining furcated teeth.
The next part of the text highlights the many
different therapeutic modalities that are clini-
cally available to treat multi‐rooted teeth,
including non‐surgical maintenance, resective
procedures (including tunnelling, root resec-
tion, and bicuspidization), and reconstructive
regenerative therapy using biologics or bioma-
terials. Other chapters in the book build on
our existing evidence base to examine the
cases that can genuinely be retained versus
those teeth too compromised as ‘hopeless’
that may benefit from extraction, implant site
development (bone grafting and alveolar ridge
preservation), followed by dental implant
reconstruction. Indeed, dental implant ther-
apy has revolutionized oral care and clinical
treatment decision‐making paradigms for
advanced reconstructive procedures. It is also
crucial for the advanced clinician to under-
stand when and when not to attempt to retain
advanced disease cases. Large epidemiologi-
cal studies have demonstrated that dental
implant therapy is not a ‘panacea’ and that,
given the significant incidence and prevalence
of peri‐implant biological complications in
the molar regions, we should re‐examine the
opportunities for maintaining and treating
furcated teeth more diligently and more fully.
The concluding chapters scrutinize the health
economics opportunities at the patient and
clinician levels in terms of tooth preservation
of furcated molars, and in which types of cases
which treatment planning approach is indi-
cated for such advanced clinical scenarios.
Stimulated by the comprehensive approach
in this book, this can be a renaissance period
in reconstructive dentistry when we firmly
consider the many options available to us as
clinicians to better preserve the dentition in
treating furcation‐involved teeth. This text
lays out a contemporary and exciting oppor-
tunity for us as clinicians to provide our
patients with state‐of‐the‐art therapy for the
betterment of oral health!
William V. Giannobile, DDS, MS, DMedSc
Najjar Endowed Professor of Dentistry &
Biomedical Engineering
Departments of Periodontics and Oral
Medicine & Biomedical Engineering,
University of Michigan School of Dentistry and
College of Engineering, Ann Arbor, MI, USA

Chapter No.: 1 Title Name: <TITLENAME>
fpref.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:18:30 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: xi
xi
Declare the past, diagnose the present,
foretell the future.
Hippocrates
Doubt is not a pleasant condition, but
certainty is an absurd one.
Voltaire
Young and new to a periodontal clinic, I
remember looking at cases of extensive peri-
odontal and bone loss in multi‐rooted teeth
and wondering how the problem could be
solved, and if and how the tooth could be
retained. The fascination with the spaces
created by inter‐radicular bone resorption,
called ‘furcations’, and the struggle over how
to manage them in the clinic, continues to
occupy large parts of my days and has
prompted me to write this book. Here, with
the help of several expert colleagues, I have
tried to:
●
Critically appraise the evidence.
●
Present expert opinions.
●
Show treated cases.
●
Present useful clinical guidelines, step‐by
step procedures, and treatment algorithms.
The emphasis of the book is to try and
maintain molars affected by furcation involve-
ment and regenerate the lost support, when
possible, accepting that this is not always pos-
sible in the long term. It goes without saying
that primary prevention of periodontitis
remains the best way to prevent tooth loss.
I hope that periodontists, dental/postgraduate
students, hygienists, and
general dentists
might find this book useful for the treatment
of molars already affected by periodontitis
and furcation involvement.
Immense thanks go to all the expert collab-
orators and friends, Will Giannobile,
Bernadette Pretzl, Peter Eickholz, Clemens
Walter, Jia‐Hui Fu, Hom‐Lay Wang, Federica,
Riccardo, and Alberto Fonzar, Roberto
Rotundo, Stefan Rüdiger, Nikos Donos, Toni
Sculean, Elena Calciolari, Iro Palaska,
Yoshinori Shirakata, Søren and Karin Jepsen,
Nikos Mardas, Steve Barter, Christian Graetz,
and Falk Schwendicke, who all contributed
chapters to this book, and to Paul Kletz for
kindly proofreading some of the chapters and
for his support throughout my career. Special
thanks go to the patients who over the years
have been a big source of inspiration with
their interest and commitment, and who
every day make me want to be a better perio-
dontist. I also need to thank my teachers at the
University of Catania and at the UCL Eastman
Dental Institute, who have all contributed,
some with small and some with larger
ingredients, to the cauldron of periodontal
knowledge from which I drew for the plan-
ning and editing of this book. The students
and staff at Barts and the London School of
Medicine and Dentistry, Queen Mary
University of London (QMUL), are gratefully
acknowledged. But most of all, I would like to
thank my family, Daniela, Domenico, Lorenzo,
Delia, and my parents and in‐laws, for their
continued support of my work.
Luigi Nibali
Preface

Chapter No.: 1 Title Name: <TITLENAME>
flast.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:18:33 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: xiii
xiii
Don’t forget to visit the companion website for this book:
www.wiley.com/go/nibali/diagnosis
There you will find valuable material designed to enhance your learning, including:
●
video clips
●
additional treated cases
Scan this QR code to visit the companion website
About the Companion Website

Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
Chapter No.: 1 Title Name: <TITLENAME>
c01.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:18:39 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 1
1
1.1 Introduction: Why
Focus on Molars?
Dentists generally agree on three statements
about molars:
●
They play an important role in the
dentition.
●
They are difficult to reach for self‐performed
as well as professional cleaning due to their
posterior position in the mouth.
●
They pose some challenges due to their
unique anatomy.
The important role of molar teeth in the den-
tition mainly consists in their contribution to
mastication, because they carry a considera-
ble part of the occlusal load. Hiiemäe (1967)
focused on the masticatory function in mam-
mals and molars grinding the food, and in
1975 Bates et al. reviewed the literature on
the masticatory cycle in natural and artificial
dentitions of men, attributing a fundamental
role to our posterior teeth regarding the
intake and preparation of nutrition. Thus, a
focus on molars and the endeavour to retain
our posterior teeth in a healthy functional
state seems justified.
This chapter will reveal how the posterior
position of molars makes them less accessi-
ble for cleaning, whether it may be self‐
performed or carried out by a dental
professional. This fact, combined with the
unique anatomy of molars, poses a challenge
for all dentists focusing on molar retention.
1.2 The ‘Special’ Anatomy
of Molar Teeth
The essential knowledge of molar root anat-
omy for every periodontist is stressed in a
review by Al‐Shammari et al. (2001). Due to
the higher mortality and compromised
diagnoses of furcation‐involved molars, and
likewise to the reduced efficacy of perio-
dontal therapy in multi‐rooted teeth, the
authors suggest a thorough engagement
with possibly decisive tooth factors such as
furcation entrance area, (bi)furcation
ridges, root surface area, root separation,
and root trunk length, because they may
critically affect the diagnosis and therapy
of multi‐rooted teeth (Leknes 1997; Al‐
Shammari et al. 2001).
For centuries, scientists have concerned
themselves with the human teeth, their anat-
omy, evolution, function, histology, and
histogenesis. Almost 3000 years ago, the
Etruscans populating the northern and cen-
tral part of what is now Italy from 900 to 100
bc recognized the importance of teeth and
fabricated quite delicate dental prostheses,
which Loevy and Kowitz (1997) compared to
prostheses from the mid‐twentieth century.
Chapter 1
Anatomy of Multi‐rooted Teeth and Aetiopathogenesis
of the Furcation Defect
Bernadette Pretzl
Section of Periodontology, Department of Operative Dentistry, University Clinic Heidelberg, Heidelberg, Germany

Chapter 1
2
The formation and genesis of teeth have
been studied in more detail during the last
three and a half centuries, starting with the
works of the so‐called father of microscopic
anatomy and histology, Marcello Malpighi
(1628–1694) from Italy (Rifkin and
Ackerman 2011), who referred to an ‘invo-
lucrum externum’ describing the outer part
of the tooth, which is today known as
enamel. More than a century later the
formation of cementum (1798–1801) and
dentine (1835–1839) was described (e.g.
Blake 1801; Bell 1835). Written in 1935,
Meyer’s Normal Histology and Histogenesis
of the Human Teeth and Associated Parts
(Churchill 1935) builds the foundation of
our understanding regarding the anatomy
of teeth. Orban and Mueller (1929), who
studied the development of furcations in
multi‐rooted teeth, set a focus on molars
using graphic reconstructions as early as
1929. Their three‐dimensional illustrations
allow a detailed impression of the root area
comparable to those documented by
Svärdström and Wennström (1988). In later
years, scientists focused more and more on
micro‐anatomical and histological research.
Based on the knowledge thus created, the
sequence of molar development can be
divided into three phases analogous to the
development of all teeth (Thesleff and
Hurmerinta 1981): initiation, morphogene-
sis, and cell differentiation. The evolution of
more than one root sets molars apart from
the rest of the dentition: in multi‐rooted
teeth the enamel organ expands with
projections of Hertwig’s root sheath (an epi-
thelial diaphragm). These expansions were
described as lobular growing inwards
between the lobes. Depending on the num-
ber of lobes, two to three (in rarer cases four)
roots develop as soon as the projections have
fused (Bhussry 1980). In an investigation by
Bower (1983) of furcation development,
evolving mandibular molars from 13 foetuses
between 17 and 38 weeks of gestation were
fixed, sectioned, and stained, giving a unique
and detailed impression of furcation devel-
opment. The author measured the base of
the dental papilla as well as the buccal and
lingual epithelial elements and described the
development as follows: The first epithelial
elements, which later evolve into the bifurca-
tion, appear at the 24‐week stage of gesta-
tional age. At that time, the crown formation
of the molar is not complete and Hertwig’s
root sheath has not developed yet (Bhussry
1980; Bower 1983). Thus, the author suggests
that the epithelial elements form extensions
of the epithelium of the developing crown
rather than the root (Bower 1983).
Additionally, he detected stellate reticulum
(which is essential for the formation of
ameloblasts) in the furcation area. The
author speculated about a possible mecha-
nism of enamel formation due to the presence
of stellate reticulum in the region of the
furcation, which develops into ameloblasts,
for example resulting in cervical projections
of enamel.
1.3 Anatomical Factors
in Molar Teeth
In 1988, Svärdström and Wennström plotted
three‐dimensional contour maps in order to
describe the topography of the furcation area
and compared drawings of maxillary and
mandibular molars. These show a complex
area with small ridges, peaks, and pits, and
the authors summarize that the complexity
of the furcation topography evidently
increases the difficulties with respect to
proper debridement once the periodontal
pocket reaches the furcation entrance and
runs into the furcation area. Thus, in addi-
tion to the aforementioned potentially deci-
sive factors – furcation entrance area,
bifurcation ridges, root surface area, and
root trunk length – it has to be kept in mind
that the complexity of the furcation area
itself poses a challenge to the dental practi-
tioner (Svärdström and Wennström 1988).
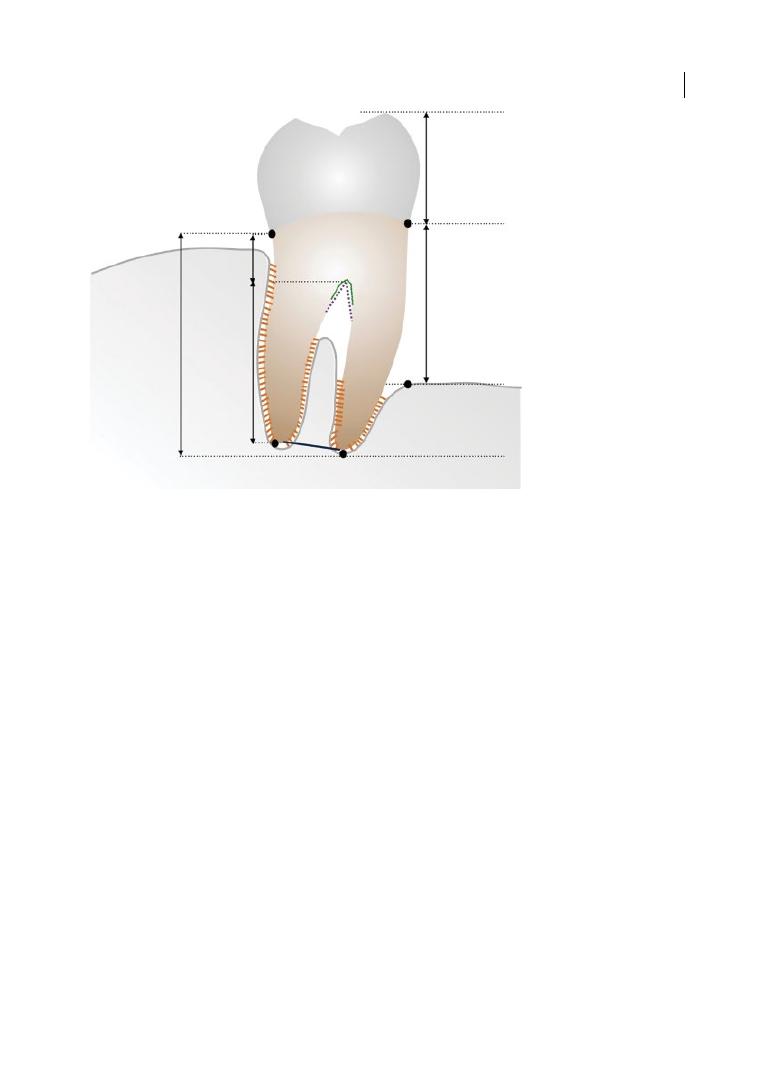
Figure 1.1 shows a diagram of a mandibular
molar, highlighting the main anatomical
features.
Anatomy of Multi-rooted Teeth 3
1.3.1 Furcation Entrance Area
The furcation entrance area was measured
by Bower (1979a) in 114 maxillary and 103
mandibular first molars. The diameter of
the entrance area was smaller than a curette
blade in more than 50% of the examined fur-
cations, with the smallest average diameter
in buccal (b) sites of maxillary as well as
mandibular first molars. No correlation
between the size of the tooth and its furca-
tion entrance area could be detected (Bower
1979a). Hou et al. (1994) studied 89
extracted maxillary and 93 extracted man-
dibular first and second molars microscopi-
cally. In their Chinese population sample,
they concurred with the results presented
by Bower (1979a) in the maxilla and found a
larger diameter in mesio‐ (mp) and disto‐
palatal (dp) furcation entrances for first and
second molars (mp: 1.04 mm and 0.90 mm;
dp: 0.99 mm and 0.67 mm; b: 0.74 mm and
0.63 mm, respectively), which was con-
firmed by Svärdström and Wennström
(1988) and dos Santos et al. (2009).
In mandibular molars the results differed,
with wider entrance areas in buccal furca-
tions of first and second molars (b: 0.88 mm
and 0.73 mm; l: 0.81 mm and 0.71 mm,
respectively). Nonetheless, the furcation
entrance area was < 1 mm in the majority of
molars and < 0.75 mm in 58%, 49%, and 52%
of molars, respectively (Bower 1979a; Chiu
et al. 1991; Hou et al. 1994). Thus, the stand-
ard width of curettes (0.75–1.0 mm) is
mostly too large to access, let alone properly
clean, a furcation entrance. Hou et al. (1994)
concluded that in order to achieve complete
debridement of root surfaces within furca-
tions, an appropriate selection and combi-
nation of ultrasonic tips (diameter 0.56 mm)
and periodontal curettes should be consid-
ered. A recent study by dos Santos et al.
Fornix
Divergence
Root trunk
Root complex
Root cone
Crown
Bone loss
Degree of
separation
Figure 1.1
Drawing of mandibular molar with furcation involvement, showing the main anatomical features,
including root trunk (part of the root from the cemento‐enamel junction [CEJ] to the furcation entrance)
and root cones, and pointing at root divergence and degree of separation between roots. The ‘bone loss’ is
schematically indicated as the distance between the CEJ and the most apical part of the bone. Source:
Courtesy of Dr Aliye Akcali.
Chapter 1
4
(2009) analysed 50 maxillary and 50 man-
dibular molars and confirmed the afore-
mentioned findings, concluding that some
molar furcation entrances could not be
adequately instrumented with curettes and
suggesting the use of alternative hand
instruments. In a review, Matthews and
Tabesh (2004) stressed the importance of
the diameter of the furcation entrance in
order to judge the effect of professional
cleaning, and thus the probable success of
periodontal therapy. The challenges of fur-
cation cleaning are discussed by Fu and
Wang in Chapter 3.
1.3.2 (Bi)furcation Ridges
In early morphological studies of extracted
first molar teeth, cementum was found in the
furcation area in a ridge, building the furca-
tion region in mandibular molars, and was
called an intermediate bifurcation ridge
(IBR), with a high presence of cementum
adjacent to the furcation entrance (Everett
et al. 1958; Bower 1979a, b; see Figure 1.2).
In a study on developing first mandibular
molars sectioned at different gestational
ages, the lingual element was found to be
wider in a mesio‐distal dimension comparable
to studies in extracted molars (Bower 1983,
1979b). Secondly, the exclusion of ectomes-
enchyme between the lobes described by
Bhussry (1980) may explain the large quanti-
ties of cementum in the furcation area of the
mature tooth corresponding to bifurcation
ridges (Bower 1983). In general, two types of
bifurcation ridge are known: one in the
bucco‐lingual direction, the other in the
mesio‐distal direction (intermediate = IBR).
Everett et al. (1958) detected buccal and lin-
gual ridges, mainly constituting of dentine, in
63% of mandibular first molars and IBRs,
mainly composed of cementum, in 73%. The
findings of Burch and Hulen (1974), Dunlap
and Gher (1985), and Hou and Tsai (1997a)
concur, with a prevalence of 76.3%, 70%, and
67.9%, respectively, in mandibular first
molars.
Gher and Vernino (1980) suggest a connec-
tion between the presence of an IBR and the
progression of the furcation defect due to the
morphology and location of IBRs. Hou and
Tsai (1997a) confirmed this correlation.
Additionally, they stated that an even higher
significant correlation exists between the
simultaneous presence of IBRs combined
with cemento‐enamel projections and furca-
tion involvement (FI).
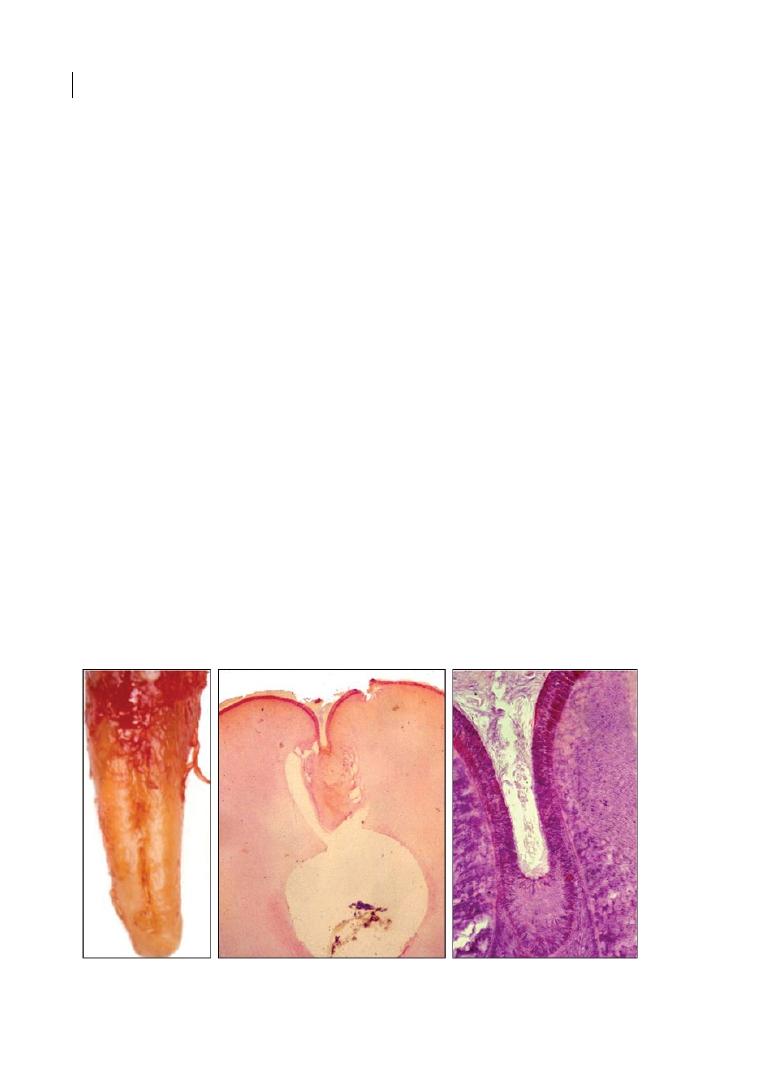
Figure 1.2
Furcation ridge. Source: Courtesy of Dr Nicola Perrini.

Anatomy of Multi-rooted Teeth 5
1.3.3 Root Surface Area
A team of researchers (Hermann et al. 1983;
Dunlap and Gher 1985; Gher and Dunlap
1985) focused on the topic of root surface
area (RSA) in maxillary and mandibular first
molars. In a meta‐analysis derived of data
from 22 original articles, Hujoel (1994) com-
puted a total RSA (corresponding to the
periodontal surface area) for the complete
dentition of 65–86
cm
2
, excluding third
molars. In maxillary first molars a mean of
4.5 cm
2
(second: 4.0 cm
2
) and in mandibular
first molars a mean of 4.2 cm
2
(second:
3.4 cm
2
) were calculated. In molars, it is often
difficult to judge the extent of FI clinically
(Bower 1979b) and thus to determine the
RSA exactly.
1.3.3.1 RSA in the Maxilla
Hermann et al. (1983) as well as Gher and
Dunlap (1985) dissected 20 extracted first
maxillary molars and cross‐sectioned them in
1 mm increments. Molars with fused roots
were excluded. They observed that the disto‐
buccal root had a significantly smaller RSA
than either the mesio‐buccal or palatal root,
confirming the results of Bower (1979b). The
root trunk surface area was significantly larger
than any surface of the three individual roots,
and averaged 32% of the total RSA of the max-
illary first molar (Hermann et al. 1983). Gher
and Dunlap (1985) measured a mean root
length of 13.6 mm (ranging from 10.5 to
16 mm) and a total RSA of 4.77 cm
2
(ranging
from 3.36 to 5.84 cm
2
). Additionally, a ‘bal-
looning’ of the RSA percentage in the furca-
tion area of maxillary molars was described,
which could not be detected in other teeth.
Accordingly, the importance of periodontal
support in the furcation area of maxillary
molars was stressed, concluding that a rela-
tively small attachment gain or loss may have
a significant impact on the stability of the
maxillary first molar (Gher and Dunlap 1985).
1.3.3.2 RSA in the Mandible
For a study on mandibular first molars,
10 teeth were hemisected and measured by
Anderson et al. (1983). They concluded that
the mesial root showed a statistically signifi-
cant greater RSA than the distal root, which
should be taken into consideration when
planning treatment, especially regarding
resective approaches. Dunlap and Gher
(1985) dissected 20 extracted mandibulary
first molars and cross‐sectioned them in
1 mm increments. They too observed that
the distal root had a significantly smaller
RSA than the mesial one, but stressed that
the shapes of the roots (conical for the distal
one; hour‐glass shaped for the mesial one)
should be taken into consideration as well. In
contrast to their findings in the maxilla, the
root trunk surface area was not larger than
the surface of the individual roots, and aver-
aged 30.5% of the total RSA of the mandibu-
lary first molar. They found a mean root
length of 14.4 ± 1.1 mm and a total RSA of
4.37 ± 0.64 cm
2
. In other studies (Jepsen 1963;
Anderson et al. 1983), the total RSA varied
from 4.31 to 4.7 cm
2
.
1.3.4 Root Trunk Length
The portion of multi‐rooted teeth located api-
cal to the cemento‐enamel junction (CEJ) is
called the ‘root complex’ and is divided into
root trunk and root cones. The root trunk is
generally defined as the area of the tooth from
the CEJ to the furcation fornix. In a study by
Gher and Dunlap (1985), the distance between
the CEJ and the furcation entrance in maxil-
lary molars differed considerably between the
mesial (3.6 ± 0.8 mm) and the distal entrance
(4.8 ± 0.8 mm), whereas the buccal entrance
was detected 4.2 ± 1.0 mm apical to the CEJ.
These findings led to the conclusion that the
clinician should suspect a through‐and‐
through furcation (degree III according to
Hamp et al. 1975) in maxillary molars once a
loss of 6 mm in vertical attachment occurred.
In more than 50% of the dissected maxillary
molars, the furcation roof was found coronal
of the root separations and formed a concave
dome between the three roots.
It should be emphasized that the dome‐like
anatomy further complicates therapy and

Chapter 1
6
maintenance of maxillary first molars (Gher
and Dunlap 1985). Hou and Tsai (1997b)
measured the root trunk in 166 extracted
first and second maxillary and 200 extracted
first and second mandibular molars of a
Taiwanese tooth sample. In the maxilla, short
root trunks were more commonly found
buccally, whereas long root trunks were more
commonly found mesially (Hou and Tsai
1997b). The authors found generally longer
root trunks in second molars than in first
molars in both jaws, and additionally stated
that long root trunks are associated with
short root cone length (Hou and Tsai 1997b).
In 134 extracted first and second mandibu-
lar molars, Mandelaris et al. (1998) detected
longer root trunks in lingual molar surfaces
when compared to buccal surfaces (mean:
4.17 mm and 3.14 mm, respectively), confirm-
ing the results of Hou and Tsai (1997b). The
mean distance between the CEJ and the furca-
tion entrance was 4.0 ± 0.7 mm in mandibular
molars (4.6 ± 0.6 mm in maxillary first molars;
Dunlap and Gher 1985; Gher and Dunlap
1985), whereas no root trunk of > 6 mm could
be found (Dunlap and Gher 1985; Mandelaris
et al. 1998). Like in maxillary molars, it can be
concluded that a through‐and‐through furca-
tion (Hamp et al. 1975) should be expected in
the mandible once a loss of 6 mm in vertical
attachment was reached on both sides (buccal
and lingual). On the other hand, it has to be
kept in mind that a furcation defect has a
horizontal component as well. Santana et al.
(2004) measured 100 extracted first and sec-
ond mandibular molars and their findings
suggest that a horizontal attachment loss of
4.3–6.9 mm is essential in order to allow com-
munication between the buccal and lingual
furcation entrance. Complete or partial fusion
of roots is also not unusual in multi‐rooted
teeth. Some 40% of maxillary premolars are
two‐rooted and the entrance to the furcation
is located an average 8 mm from the CEJ, well
into the middle third of the root complex
(Bower 1979a).
A clinically evident FI correlates with the
vertical length and type of the root trunk
(Carnevale 1995; Hou and Tsai 1997b,
Al‐Shammari et al. 2001). Thus, Al‐
Shammari et al. (2001) summarized that the
root trunk length significantly relates to the
prognosis and treatment of molars. A short
root trunk worsens the prognosis with regard
to a more likely FI, but once periodontal
destruction has occurred, it improves the
chances of a successful treatment (Horwitz
et al. 2004).
1.4 Anatomical Aetiological
Factors
1.4.1 Cervical Enamel Projections
Enamel surfaces do not allow for the attach-
ment of connective tissue and represent an
anatomical abnormality in the root area.
Thus, cervical enamel projections (CEP) may
contribute to the development of a furcation
defect (Al‐Shammari et al. 2001). The first to
report a possible connection between CEPs
and periodontal destruction in molars was
Atkinson in 1949. According to Masters and
Hoskins (1964), CEPs can be classified in
three grades (Table 1.1).
Different prevalences of CEPs have been
documented so far. Masters and Hoskins
(1964) found CEPs in 29% of mandibular and
17% of maxillary molars. In Egyptian skulls,
Bissada and Abdelmalek (1973) detected a
CEP prevalence of 8.6%. In the 1138 molars
studied, a higher incidence of CEPs in the
Table 1.1
Classification of cervical enamel
projections.
Grade I
The enamel projection extends from
the cemento‐enamel junction of the
tooth towards the furcation entrance
(<1/3 of the root trunk).
Grade II The enamel projection approaches
the furcation entrance but does not
enter it. No horizontal component is
present (>1/3 of the root trunk).
See Figure 1.3a.
Grade III The enamel projection extends
horizontally into the furcation.
Compare Figures 1.3b and 1.3c.
Anatomy of Multi-rooted Teeth 7
mandible could be confirmed. A study in 200
East Indian skulls with 2000 molars reported a
32.6% incidence rate of CEPs (Swan and Hurt
1976). They were most often reported in man-
dibular second molars (51.0%), followed by
maxillary second molars (45.6%), mandibular
first and maxillary first molars (13.6%). Grade
I enamel projections (Masters and Hoskins
1964) were detected most frequently. These
could not be significantly related to furcation
involvement, as could grade II and III CEPs
(Swan and Hurt 1976). An observation in 78
Taiwanese individuals reported detection of
CEPs in 49.3% of second and 62.3% of first
maxillary and 51.2% of second and 73.9% of
first mandibular molars (Hou and Tsai 1987).
A study by the same authors in furcation‐
involved mandibular molars reported even
higher CEP percentages: 71% of second and
92.9% of first mandibular molars showed
enamel projections (Hou and Tsai 1997b).
Mandelaris et al. (1998) documented CEPs in
66.4% of mandibular molars (61.9% of buccal
and 50.8% of lingual surfaces) ranging from
0.98 to 1.33 mm in diameter. Current research
on CEPs was published in 2013 and 2016.
Bhusari et al. (2013) investigated their inci-
dence on the buccal surface of 944 upper and
lower first, second and third permanent
molars from 89 Indian dry human skulls, and
additionally measured FI. Again, it could be
confirmed that CEPs are found more fre-
quently in the mandible and are significantly
associated with the occurrence of FI. The
incidence ranged from 14.7% in mandibular
second molars to 5.5% in wisdom teeth. The
most recent study was performed using cone‐
beam computed tomography data in a Korean
population analysing 982 mandibular molars
(Lim et al. 2016) and reported an overall
prevalence rate of CEP of 76%. Grade I CEPs
were the most common, followed by CEPs of
grades II and III (Lim et al. 2016).
The huge variations can partly be explained
by different study objects: in human skulls
healthier periodontal conditions can be
assumed, while extracted molars most prob-
ably show worse conditions, and Hou and
Tsai (1987, 1997a) as well as Mandelaris et al.
(1998) studied furcation‐involved molars in
periodontal patients. Additionally, a higher
prevalence of CEPs in Oriental subjects than
in Caucasians is suspected (Hou and Tsai
1987; Lim et al. 2016).
Nonetheless, it can be concluded that CEPs
are a common problem which must be
addressed by clinicians when treating molar
teeth. They are more prevalent than enamel
pearls and prevent connective tissue attach-
ment, thus contributing to the aetiology of
furcation defects, possibly resulting in localized
chronic periodontitis and FI in molars (Leknes
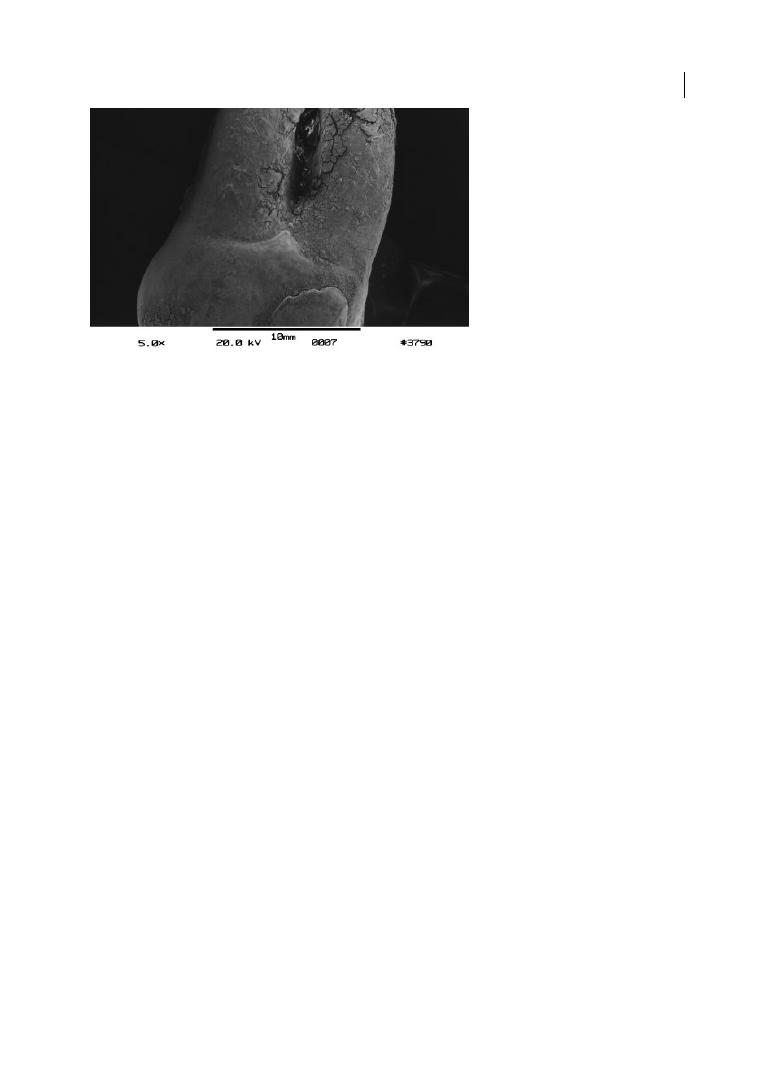
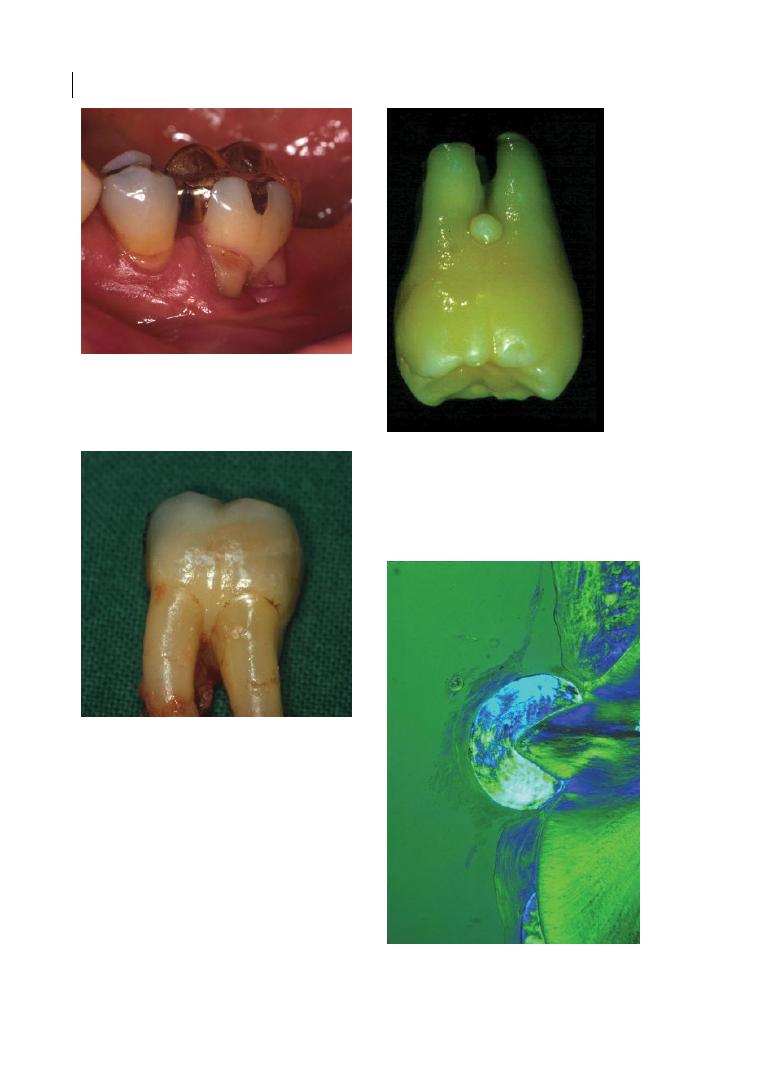
Figure 1.3a
Cervical enamel projection grade II (>1/3 of root trunk; Masters and Hoskins 1964) on upper right
first molar (REM microscope). Source: Eickholz and Hausmann 1998.
Chapter 1
8
1997; Al‐Shammari et al. 2001; Bhusari et al.
2013). Additionally, significantly higher plaque
and gingivitis index values have been reported
in the presence of CEPs (Carnevale et al. 1995).
1.4.2 Enamel Pearls
Enamel pearls (see Figure 1.4) were first
described in an article in the American
Journal of Dental Science in 1841 (Moskow
Figure 1.3b
Cervical enamel projection on lower
left first molar; grade III (reaching furcation
entrance area; Masters and Hoskins 1964). Source:
Eickholz 2005.
Figure 1.3c
Cervical enamel projection on extracted
lower right first molar; grade III (reaching furcation
entrance area; Masters and Hoskins 1964). Source:
Eickholz and Hausmann 1998.
Figure 1.4a
Macroscopic image of an enamel pearl
on an extracted molar. Source: Courtesy of Prof.
Dr. H.-K. Albers.
Figure 1.4b
Microscopic image of an enamel pearl.
Source: Courtesy of Prof. Dr. H.-K. Albers.

Anatomy of Multi-rooted Teeth 9
and Canut 1990). They are ectopic globules
consisting mostly of enamel, often contain-
ing a core of dentine, and they adhere to the
tooth root surface, with a distinct predilec-
tion for the furcation areas of molar teeth,
particularly maxillary third and second
molars. In a review from 1990, an incidence
of 2.6% (ranging from 1.1 to 9.7%) was
reported, with differences among racial
groups and a greater incidence in histological
studies (Moskow and Canut 1990). Like
CEPs, enamel projections prevent connec-
tive tissue attachment and thus contribute to
the aetiology of periodontal destruction.
They usually occur singularly, but up to four
enamel pearls have been observed on the
same tooth (Moskow and Canut 1990).
More recent research demonstrates an
incidence within the range documented by
Moskow and Canut (1990). Darwazeh and
Hamasha (2000) evaluated the presence of
enamel pearls in a Jordanian patient sample,
studying 1032 periapical radiographs. An
incidence of 1.6% of enamel pearls in molars
and 4.76% per subject with no gender differences
was reported. Chrcanovic et al. (2010) evalu-
ated the prevalence of enamel pearls in
45 539 permanent teeth (20 218 molars) from
a human tooth bank in Brazil. They con-
firmed the predominant presence in the
maxilla and reported an incidence of 1.71%
in molars. Akgül et al. (2012) evaluated the
presence of enamel pearls using cone‐beam
computed tomography in 15 185 teeth (4334
molars). An incidence of enamel pearls of
0.83% in molars and 4.69% per subject with no
gender differences was reported. Again, the
incidence was significantly higher in the max-
illa. Colak et al. (2014) studied the prevalence
of enamel pearls in Turkish dental patients
and detected them in 0.85% of teeth and 5.1%
of subjects, with a contradictory higher inci-
dence in the mandible and in male patients.
Although lower in incidence than enamel
projections, it can be summarized that
enamel pearls play an important role in the
aetiology of furcation defects, and it is con-
sidered essential to diagnose enamel pearls
early on to allow for an adequate prognosis of
molar retention and probably alter the thera-
peutic approach.
1.5 Periodontal Aetiological
Factors in Molar Teeth
Aetiological factors interact with the previ-
ously described anatomical factors and may
lead to periodontal destruction and attach-
ment loss in molars, and thus result in a fur-
cation defect. According to Al‐Shammari
et al. (2001), plaque‐associated inflammation,
Figure 1.4c
Orthopantomogram showing enamel pearls on upper right and left second molars. Source:
Eickholz and Hausmann 1998.

Chapter 1
10
trauma from occlusion, pulpal pathology,
vertical root fractures, and iatrogenic factors
need to be taken into consideration.
1.5.1 Plaque‐associated
Inflammation
The reader of this book will surely be well
accustomed to plaque formation and the
inflammatory component of gingivitis and
periodontitis. What is special about molars
in this context? In general, it can be stated
that furcations are more prone to plaque
adhesion and less likely to stay plaque free.
The anatomy of the furcation favours reten-
tion of bacterial deposits and renders hygiene
procedures difficult (Matthews and Tabesh
2004). In 1987, Nordland et al. monitored
2472 sites in 19 periodontal patients for
24 months after periodontal therapy, and
reported that furcation sites responded less
favourably to therapy and were more likely to
exhibit higher plaque and gingivitis scores.
Apart from that, it is assumed that furcation
areas are an extension of periodontal pock-
ets, because unique histological features are
lacking (Glickman 1950; Al‐Shammari et al.
2001). Thus, plaque formation follows the
same process in molars and their furcations
as in the remaining dentition (Leknes 1997).
1.5.2 Occlusal Trauma
Trauma from occlusion is suspected to
be another aetiological factor contributing
to periodontal destruction in molars.
Two groups of researchers, Glickman and
co‐workers as well as Lindhe and co‐workers,
focused on this topic in animal studies apply-
ing excessive occlusal forces on molars. In
their classic studies on beagle dogs, Lindhe
and Svanberg (1974) and Nyman et al. (1978)
reported significant alterations in tooth
mobility combined with angular bony defects
and loss of periodontal support in artificially
created, gingivally inflamed multi‐rooted
teeth carrying splints, compared to teeth
with inflammation but carrying no addi-
tional occlusal load. Even before that,
Glickman et al. (1961) compared the effect of
occlusal force on splinted and non‐splinted
teeth in rhesus monkeys, and suggested that
the fibre orientation in the furcation area
makes multi‐rooted teeth more susceptible
to increased functional forces. More recently,
Nakatsu et al. (2014) confirmed the afore-
mentioned findings in an observation in rats.
On the other hand, Waerhaug (1980) con-
cluded from his observations of 46 human
molars (extracted because of advanced peri-
odontal destruction) that increased mobility
and occlusal trauma are not involved in the
aetiology of the FI and are instead a late
symptom of periodontal disease. Thus, the
impact of occlusal forces in the aetiology of
periodontitis in general and FI in particular
remains controversial (Al‐Shammari et al.
2001; Reinhardt and Killeen 2015). In a
review, Harrel (2003) suggest that occlusal
interferences should be regarded as a poten-
tial risk factor comparable to smoking, rather
than a causative or aetiological factor.
1.5.3 Vertical Root Fractures
It is generally agreed that vertical root frac-
tures, which can occur in a longitudinal
direction on any surface of the root, are dif-
ficult to diagnose because they share symp-
toms with other dental conditions (Matthews
and Tabesh 2004). Additionally, in most cases
mild pain or a dull discomfort is the only
clinical symptom of a vertical root fracture
(Meister at al. 1980). They result in rapid
localized loss of attachment and bone
(Walton et al. 1984) and can lead to FI
depending on their position. Mostly, a poor
prognosis is assigned to teeth exhibiting ver-
tical root fractures (Al‐Shammari et al. 2001;
Matthews and Tabesh 2004).
1.5.4 Endodontic Origin
and Pulpal Pathology
Accessory canals are quite common in molar
teeth. A study of 46 extracted molars of both

Anatomy of Multi-rooted Teeth 11
jaws found accessory canals in 59% of exam-
ined teeth (Lowman et al. 1973). Burch and
Hulen (1974) reported ‘openings’ in 76% of
the furcations of maxillary and mandibular
molars. These canals allow for products of
pulpal necrosis to enter the furcation area
and cause an inflammatory lesion (Carnevale
et al. 1995). Thus, a pulpal pathosis can result
in FI. Carnevale et al. (1995) reported that
proximal and inter‐radicular bone destruc-
tion of endodontic origin is reversible after
root canal treatment. Periodontal therapy
only becomes necessary in the case of a
persistent lesion after the endodontic treat-
ment. A more detailed description of the
associations between FI and endodontic
pathology is provided in Chapter 4.
1.5.5 Iatrogenic Factors
Generally, overhanging dental restorations
or discrepancies of the subgingival margin in
any kind of restoration or even orthodontic
bands allow for adhesion of plaque and show
detrimental effects on adjacent gingival tis-
sues; additionally, the fit of prosthetic resto-
rations is mostly less than perfect (Leknes
1997) and builds a niche, where plaque
formation is facilitated and cleansing diffi-
cult. According to a study by Lang et al.
(1983) in dental students with healthy gingi-
vae who received proximal inlays with 1 mm
overhangs, the microbial composition of the
subgingival biofilm shifted from healthy to a
composition characteristically found in peri-
odontitis. Thus, the authors concluded that
the changes observed in the subgingival
microflora document a potential mechanism
for the initiation of periodontal disease asso-
ciated with iatrogenic factors. Wang et al.
(1993) focused on molars and assessed the
correlation between FI and the presence of a
crown or proximal restoration in 134 perio-
dontal patients during maintenance therapy.
Their results showed a significant associa-
tion between FI as well as periodontal attach-
ment loss and the presence of a crown or
restoration.
Additionally, Matthews and Tabesh (2004)
commented that overhangs not only build a
plaque retention niche, but also impinge on
the biological width (between the depth of a
healthy sulcus and the alveolar crest) and
thus cause damage. They report ranges of
overhangs in restored teeth from 18 to 87%
(Matthews and Tabesh 2004). In general, the
placement of restorative margins subgingi-
vally results in more plaque, more gingival
inflammation and deeper periodontal
pockets.
It can be concluded that special care needs to
be taken when placing restorations, and over-
hangs need to be diagnosed and removed as
early as possible. Should a restoration margin
need to be placed subgingivally, the biological
width has to be kept in mind and crown length-
ening considered. Thus, a dento‐gingival attach-
ment may be achieved (Herrero et al. 1995).
Summary of Evidence
●
Numerous anatomical factors like furca-
tion entrance area, bifurcation ridges,
root surface area, and root trunk length
need to be considered in the diagnosis and
periodontal treatment of molars. The
periodontist should be aware of these fac-
tors because they may have a significant
impact on the prognosis and therapeutic
outcome of multi‐rooted teeth.
●
Iatrogenic factors should be tackled early
on (at the beginning of periodontal ther-
apy), thus allowing for improvement of
gingival and periodontal conditions.

Chapter 1
12
References
Akgül, N., Caglayan, F., Durna, N. et al. (2012).
Evaluation of enamel pearls by cone‐beam
computed tomography (CBCT). Medicina
Oral Patologica Oral y Cirurgia Bucal 17,
e218–e222.
Al‐Shammari, K.F., Kazor, C.E., and Wang,
H.‐L. (2001). Molar root anatomy and
management of furcation defects. Journal of
Clinical Periodontology 28, 730–740.
Anderson, R.W., McGarrah, H.E., Lamb, R.D.,
and Eick, J.D. (1983). Root surface
measurements of mandibular molars using
stereophotogrammetry. Journal of the
American Dental Association 107, 613–615.
Atkinson, S.R. (1949). Changing dynamics of
the growing face. American Journal of
Orthodontics 35, 815–836.
Bates, J.F., Stafford, G.D., and Harrison, A.
(1975). Masticatory function – a review of
the literature: 1. The form of the masticatory
cycle. Journal of Oral Rehabilitation 2 (3),
281–301.
Bell, T. (1835). The Anatomy, Physiology, and
Diseases of the Teeth. London: S. Highley.
Bhusari, P., Sugandhi, A., Belludi, S.A., and
Shoyab Khan, S. (2013). Prevalence of
enamel projections and its co‐relation with
furcation involvement in maxillary and
mandibular molars: A study on dry skull.
Journal of the Indian Society of
Periodontology 17, 601–604.
Bhussry, B.R. (1980). Development and growth
of teeth. In: Orban’s Oral Histology and
Embryology (ed. G.S. Kumar), 23–44. St
Louis, MO: C.V. Mosby.
Bissada, N.F., and Abdelmalek, R.G. (1973).
Incidence of cervical enamel projections and
its relationship to furcation involvement in
Egyptian skulls. Journal of Periodontology
44, 583–585.
Blake, R. (1801). An Essay on the Structure and
Formation of the Teeth in Man and Various
Animals. Dublin: Porter.
Bower, R.C. (1979a). Furcation morphology
relative to periodontal treatment: Furcation
entrance architecture. Journal of
Periodontology 50, 23–27.
Bower, R.C. (1979b). Furcation morphology
relative to periodontal treatment: Furcation
root surface anatomy. Journal of
Periodontology 50, 366–374.
Bower, R.C. (1983). Furcation development of
human mandibular first molar teeth: A
histologic graphic reconstructional study.
Journal of Periodontal Research 18, 412–419.
Burch, J.G., and Hulen, S. (1974). A study of
the presence of accessory foramina and the
topography of molar furcations. Oral
Surgery, Oral Medicine, Oral Pathology 38,
451–455.
Carnevale, G., Pontoriero, R., and Hürzeler,
M.B. (1995). Management of furcation
involvement. Periodontology 2000 9, 69–89.
Chiu, B.M., Zee, K.Y., Corbet, E.F., and
Holmgren, C.J. (1991). Periodontal
implications of furcation entrance dimensions
in Chinese first permanent molars. Journal
of Periodontology 62, 308–311.
Chrcanovic, B.R., Abreu, M.H.N.G., and
Custódio A.L.N. (2010). Prevalence of
enamel pearls in teeth from a human teeth
bank. Journal of Oral Science 52, 257–260.
Churchill, H.R. (1935). Meyer’s Normal
Histology and Histogenesis of the Human
Teeth and Associated Parts (trans. and ed.
H.R. Churchill). Philadelphia, PA: J.B.
Lippincott.
Çolak, H., Hamidi, M.M., Uzgur, R. et al.
(2014). Radiographic evaluation of the
prevalence of enamel pearls in a sample
adult dental population. European Review
for Medical and Pharmacological Sciences
18, 440–444.
Darwazeh, A., and Hamasha, A.A. (2002).
Radiographic evidence of enamel pearls in
Jordanian dental patients. Oral Surgery, Oral
Medicine, Oral Pathology, Oral Radiology
Endodontology 89, 255–258.
dos Santos, K.M., Pinto, S.C., Pochapski, M.T.
et al. (2009). Molar furcation entrance and
its relation to the width of curette blades
used in periodontal mechanical therapy.
International Journal of Dental Hygiene 7,
263–269.

Anatomy of Multi-rooted Teeth 13
Dunlap, R.M., and Gher, M.E. (1985). Root
surface measurements of the mandibular
first molar. Journal of Periodontology 56 (4),
234–248.
Eickholz, P. (2005). Clinical and radiographic
diagnosis and epidemiology of furcation
involvement. In: Parodontologie: Praxis der
Zahnheilkunde Band 4 (ed. D. Heidemann),
Chapter 2. Munich: Urban & Fischer/
Elsevier.
Eickholz, P., and Hausmann, E. (1998).
Diagnostik der Furkationsbeteiligung: Eine
Übersicht. Quintessenz 49 (1), 59–67.
Everett, F.G., Jump, E.B., Holder, T.D., and
Williams, G.C. (1958). The intermediate
bifurcational ridge: A study of the
morphology of the bifurcation of the lower
first molar. Journal of Dental Research 37,
162–169.
Gher, M.W. Jr, and Dunlap, R.M. (1985). Linear
variation of the root surface area of the
maxillary first molar. Journal of
Periodontology 56, 39–43.
Gher, M.E., and Vernino, A.R. (1980). Root
morphology: Clinical significance in
pathogenesis and treatment of periodontal
disease. Journal of the American Dental
Association 101, 627–633.
Glickman, I. (1950). Bifurcation involvement
in periodontal disease. Journal of the
American Dental Association 40, 528–538.
Glickman, I., Stein, R.S., and Smulow, J.B.
(1961). The effect of increased functional
forces upon the periodontium of splinted
and non‐splinted teeth. Journal of
Periodontology 32, 290–300.
Hamp, S.‐E., Nyman, S., and Lindhe, J. (1975).
Periodontal treatment of multirooted teeth:
Results after 5 years. Journal of Clinical
Periodontology 2, 126–135.
Harrel, S.K. (2003). Occlusal forces as a risk
factor for periodontal disease.
Periodontology 2000 32, 111–117.
Hermann, D.W., Gher, M.E., Jr, Dunlap, R.M.,
and Pelleu, G.B., Jr (1983). The potential
attachment area of the maxillary first molar.
Journal of Periodontology 54, 431–434.
Herrero, F., Scott, J.B., Maropis, P.S., and
Yukna R.A. (1995). Clinical comparison of
desired versus actual amount of surgical
crown lengthening. Journal of
Periodontology 66, 568–571.
Hiiemäe, K.M. (1967). Masticatory function in
the mammals. Journal of Dental Research
46, 883–893.
Horwitz, J., Machtei, E.E., Reitmeir, P. et al.
(2004). Radiographic parameters as
prognostic indicators for healing of class II
furcation defects. Journal of Clinical
Periodontology 31, 105–111.
Hou, G.L., and Tsai, C.C. (1987).
Relationship between periodontal furcation
involvement and molar cervical enamel
projections. Journal of Periodontology 58,
715–721.
Hou, G.L., and Tsai, C.C. (1997a). Cervical
enamel projections and intermediate
bifurcational ridge correlated with molar
furcation involvements. Journal of
Periodontology 68, 687–693.
Hou, G.L., and Tsai, C.C. (1997b). Types and
dimensions of root trunk correlating with
diagnosis of molar furcation involvements.
Journal of Clinical Periodontology 24,
129–135.
Hou, G.L., Chen, S.F., Wu, Y.M., and Tsai, C.C.
(1994). The topography of the furcation
entrance in Chinese molars: Furcation
entrance dimensions. Journal of Clinical
Periodontology 21, 451–456.
Hujoel, P.P. (1994). A meta‐analysis of normal
ranges for root surface areas of the
permanent dentition. Journal of Clinical
Periodontology 21, 225–229.
Jepsen, A. (1963). Root surface measurement
and a method for x‐ray determination of
root surface area. Acta Odontologica
Scandinavica 21, 35–46.
Lang, N.P., Kiel, R.A., and Anderhalden, K.
(1983). Clinical and microbiological effects
of subgingival restorations with overhanging
or clinically perfect margins. Journal of
Clinical Periodontology 10, 563–578.
Leknes, K.N. (1997). The influence of anatomic
and iatrogenic root surface characteristics
on bacterial colonization and periodontal
destruction: A review. Journal of
Periodontology 68, 507–516.

Chapter 1
14
Lim, H.‐C., Jeon, S.‐K., Cha, J.‐K. et al. (2016).
Prevalence of cervical enamel projection
and its impact on furcation involvement in
mandibular molars: A cone‐beam computed
tomography study in Koreans. The
Anatomical Record 299, 379–384.
Lindhe, J., and Svanberg, G. (1974). Influence
of trauma from occlusion on progression of
experimental periodontitis in the beagle dog.
Journal of Clinical Periodontology 1, 3–14.
Loevy, H.T., and Kowitz, A.A. (1997). The
dawn of dentistry: Dentistry among the
Etruscans. International Dental Journal 47,
279–284.
Lowman, J.V., Burke, R.S., and Pelleu, G.B.
(1973). Patent accessory canals: Incidence in
molar furcation region. Oral Surgery Oral
Medicine Oral Pathology 38, 451–455.
Mandelaris, G.A., Wang, H.L., and MacNeil,
R.L. (1998). A morphometric analysis of the
furcation region of mandibular molars.
Compendium of Continuing Education in
Dentistry 19, 113–120.
Masters, D.H., and Hoskins, S.W. (1964).
Projection of cervical enamel into molar
furcations. Journal of Periodontology 35,
49–53.
Matthews, D., and Tabesh, M. (2004).
Detection of localized tooth‐related factors
that predispose to periodontal infections.
Periodontology 2000 34, 136–150.
Meister, F., Lommel, T.J., and Gerstein, H.
(1980). Diagnosis and possible causes of
vertical root fractures. Oral Surgery, Oral
Medicine, Oral Pathology 49, 243–253.
Moskow, B.S., and Canut, P.M. (1990). Studies
on root enamel. Journal of Clinical
Periodontology 17, 275–281.
Nakatsu, S., Yoshinaga, Y., Kuramoto, A. et al.
(2014). Occlusal trauma accelerates
attachment loss at the onset of experimental
periodontitis in rats. Journal of Periodontal
Research 49, 314–322.
Nordland, P., Garrett, S., Kiger, R.D. et al.
(1987). The effect of plaque control and root
debridement in molar teeth. Journal of
Clinical Periodontology 14, 231–236.
Nyman, S., Lindhe, J., and Ericsson, I. (1978).
The effect of progressive tooth mobility on
destructive periodontitis in the dog. Journal
of Clinical Periodontology 5, 213–225.
Orban, B., and Mueller, E. (1929). The
development of the bifurcation of
multirooted teeth. Journal of the American
Dental Association 16, 297–319.
Reinhardt, R.A., and Killeen, A.C. (2015). Do
mobility and occlusal trauma impact
periodontal longevity? Dental Clinics of
North America 59, 873–883.
Rifkin, B.A., and Ackerman, M.J. (2011).
Human Anatomy: A Visual History from the
Renaissance to the Digital Age. New York,
NY: Abrams Books.
Santana, R.B., Uzel, I.M., Gusman, H. et al.
(2004). Morphometric analysis of the
furcation anatomy of mandibular molars.
Journal of Periodontology 75, 824–829.
Svärdström, G., and Wennström, J.L. (1988).
Furcation topography of the maxillary and
mandibular first molars. Journal of Clinical
Periodontology 15, 271–275.
Swan, R.H., and Hurt, W.C. (1976). Cervical
enamel projections as an etiologic factor in
furcation involvement. Journal of the
American Dental Association 93, 342–345.
Thesleff, I., and Hurmerinta, K. (1981). Tissue
interactions in tooth development.
Differentiation 18, 75–88.
Waerhaug, J. (1980). The furcation problem:
Etiology, pathogenesis, diagnosis, therapy
and prognosis. Journal of Clinical
Periodontology 7, 73–95.
Walton, R.E., Michelich, R.J., and Smith, G.N.
(1984). The histopathogenesis of vertical
root fractures. Journal of Endodontics 10,
48–56.
Wang, H.L., Burgett, F.G., and Shyr, Y. (1993).
The relationship between restoration and
furcation involvement on molar teeth.
Journal of Periodontology 64, 302–305.

Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
Chapter No.: 1 Title Name: <TITLENAME>
c02.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:18:51 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 15
15
2.1 Introduction
In single‐rooted teeth, periodontal destruc-
tion proceeds from the cemento‐enamel
junction (CEJ) apically, predominantly in a
vertical direction. The vertical attachment
loss is assessed as vertical probing attach-
ment loss (PAL‐V) from the CEJ, or if the CEJ
is destroyed by a restoration from the resto-
ration margin (RM) to the bottom of the per-
iodontal pocket. Vertical bone loss is assessed
radiographically or by vertical probing bone
level (PBL‐V) from the CEJ or RM to the
alveolar crest. If periodontitis affects multi‐
rooted teeth, the tissues are not only
destroyed vertically but also horizontally
between the roots, creating furcation involve-
ment. This dimension of periodontal
destruction (horizontal attachment and bone
loss) may be assessed as horizontal probing
attachment loss (PAL‐H) or horizontal
probing bone level (PBL‐H).
Horizontal probing attachment loss and
bone loss in the furcation area create a niche
(furcation involvement), which impedes
accessibility for individual oral hygiene in the
molar region (Lang et al. 1973) and profes-
sional root debridement (Fleischer et al.
1989). This adds to the factors contributing
to more severe disease progression in
furcation‐involved molars, recurrent perio-
dontal infection, and as a result an inferior
long‐term prognosis of these teeth (McGuire
and Nunn 1996; Dannewitz et al. 2006, 2016;
Pretzl et al. 2008; Salvi et al. 2014; Graetz
et al. 2015). Furcation‐involved molars
respond less favourably to periodontal ther-
apy than molars without furcation involve-
ment or single‐rooted teeth, and are at
greater risk for further attachment loss
(Nordland et al. 1987; Loos et al. 1989; Wang
et al. 1994) than other teeth. Addressing this
issue, Kalkwarf et al. (1988) reported the suc-
cess of different surgical and non‐surgical
treatment modalities in 158 molars.
Irrespective of the therapy performed, the
horizontal defect in the furcation area
increased during the two‐year follow‐up.
Thus, reliable diagnosis of incidence and
extent of furcation involvement is decisive
for prognosis and treatment planning.
2.2 Clinical Furcation
Diagnosis
Furcation involvement can only be found in
multi‐rooted teeth (Table 2.1). More than
one root is regularly found in maxillary and
mandibular molars as well as in first maxillary
Chapter 2
Clinical and Radiographic Diagnosis and Epidemiology
of Furcation Involvement
Peter Eickholz
1
and Clemens Walter
2
1
Poliklinik für Parodontologie, Zentrum der Zahn‐ Mund‐ und Kieferheilkunde (Carolinum), Johann Wolfgang Goethe‐Universität
Frankfurt, Frankfurt am Main, Germany
2
Klinik für Parodontologie, Endodontologie und Kariologie, Universitätszahnkliniken, Universitäres Zentrum für Zahnmedizin Basel,
Basel, Switzerland
Chapter 2
16
premolars (see Chapter 1). However, two‐
rooted variants may be found in second max-
illary premolars and mandibular anteriors.
Rarely, three‐rooted variants may be found in
mandibular molars and maxillary premolars
(Mohammadi et al. 2013). Those sites at
which furcation entrances are regularly
expected have to be examined for furcation
involvement on a regular basis in the course
of periodontal examination. Search for and
scoring of furcation involvement are funda-
mental elements of periodontal examination.
Particularly in untreated periodontal
patients, furcation entrances do not lie open.
In most cases they are covered by gingiva.
Thus, furcation involvement cannot be seen
simply with the naked eye, but has to be
probed below the gingival margin. The
bizarre anatomy of furcations (Schroeder
and Scherle 1987), their curved course, and
the fact that the furcation entrances of maxil-
lary premolars and molars open into inter-
proximal spaces require the use of particular
curved furcation probes in furcation diagno-
sis (e.g. Nabers probe; Figure 2.1). The probe
is placed onto the tooth surface coronally of
the gingival margin at the site where a furca-
tion entrance is expected (e.g. lingual of a
mandibular molar). Then the probe is pushed
apically, gently displacing the gingiva in
zigzag movements until the bottom of the
sulcus or pocket is reached. If the probe falls
into a pit horizontally, in most cases furca-
tion involvement has been detected.
Straight rigid periodontal probes (e.g.
PCPUNC15) are inappropriate for furcation
Table 2.1
Regularly multi‐rooted teeth with location of roots and location
of furcation entrances.
Tooth type
Location of
roots
Location of furcation
entrance
Maxillary molars
Mesio‐buccal
Disto‐buccal
Palatal
Buccal
Mesio‐palatal
Disto‐palatal
Maxillary premolars
Buccal
Palatal
Mesial
Distal
Mandibular molars
Mesial
Distal
Buccal
Lingual
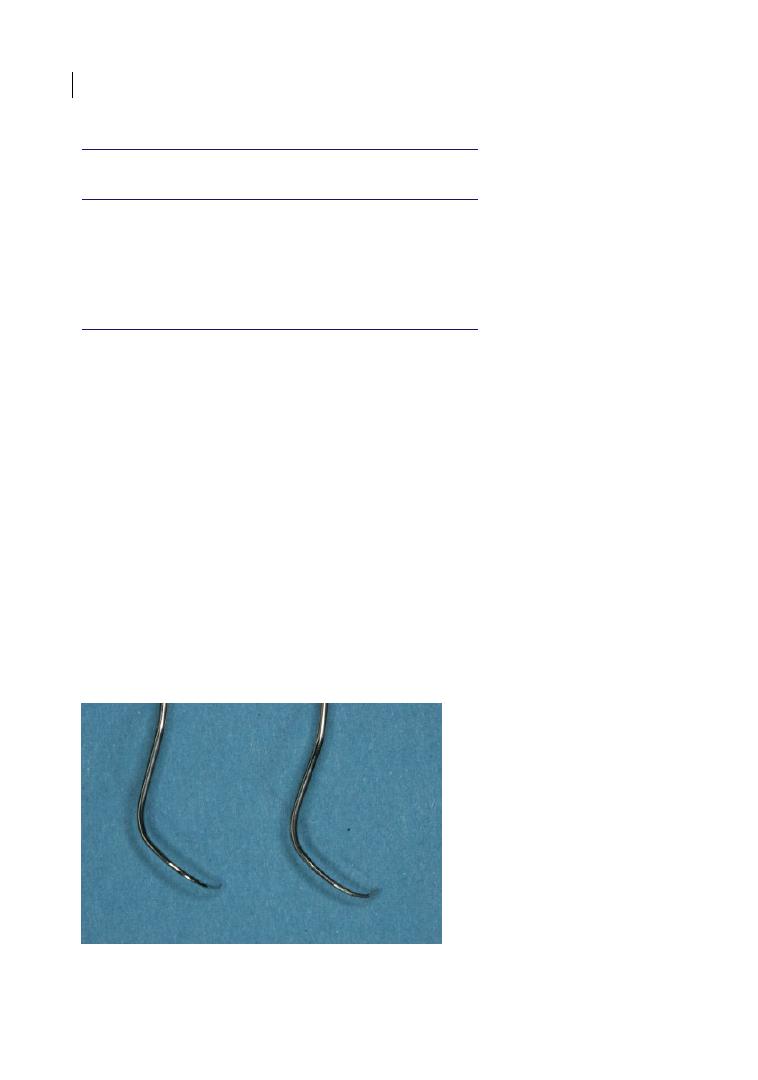
Figure 2.1
Curved furcation probes: Nabers probes (left: without markings; right: marked in 3 mm steps up to
12 mm).
Clinical and Radiographic Diagnosis and Epidemiology 17
diagnosis because they fail to follow the
curved course of most furcations. Their use
bears a high risk of underestimating the
extent of the furcation involvement (Eickholz
and Kim 1998).
2.2.1 Classification of Furcation
Involvement
Besides the simple fact of the existence of a
furcation involvement and its location, the
severity of furcation involvement is of major
significance. Severity of furcation involve-
ment is assessed by probing the respective
furcation in a horizontal direction using a
rigid curved probe (e.g. Nabers probe) and
measuring the distance from the probe tip to
a virtual tangent to the root convexities adja-
cent to the furcation (Figure 2.2). Measuring
this distance allows assessment of different
degrees of furcation involvement or the
amount of horizontal attachment loss in mil-
limetres (horizontal probing/clinical attach-
ment level: PAL‐H/CAL‐H; Figures 2.2–2.4).
Whereas assessment of the continuous vari-
able horizontal attachment loss provides
information on small changes of inter‐radicular
tissues (since they are relevant after regener-
ative therapy), the categorical classification
of inter‐radicular t issue destruction as degree
(a)
(b)
(c)
(d)
Figure 2.2
Furcation involvement degree I (Eickholz and Staehle 1994; Table 2.4): horizontal loss of periodontal
tissue support up to 3 mm: (a) schematic (maxillary molar, buccal furcation entrance): horizontal probing/clinical
attachment level 2.5 mm; (b) mesial tooth 24 with neighbouring tooth; (c) buccal tooth 46: the probe does not
penetrate more than 3 mm between the two buccal roots; (d) disto‐palatal tooth 16 with neighbouring tooth.
Chapter 2
18
of furcation involvement provides suffi-
ciently relevant information for prognosis
and decision regarding therapy of the respec-
tive multi‐rooted tooth.
The different classifications of furcation
involvement basically exhibit differences
only in the details (Tables 2.2–2‐3). The clas-
sification by Glickman (1953) provides some-
what vague criteria to distinguish classes of
furcation involvement, and also considers
radiographic information which is known to
be of low reliability (Table 2.2; Ammons and
Harrington 2006). The criteria for the Hamp
et al. (1975) classification are based on meas-
urements (threshold: PAL‐H = 3 mm). The
colour‐coded version of the Nabers probe,
marked in 3 mm steps (PQ2N; Figure 2.1), is
particularly suitable for scoring degrees of
furcation involvement, according to Hamp
et al. (1975; Eickholz and Kim 1998).
However, there exist also furcation probes
with 2 mm markings (Zappa probe ZA 2).
The distinction between degrees I and II of
the Glickman classification is not as clear or
definite as the distinction between degrees I
and II according to Hamp et al. (1975); that
is, horizontal loss of periodontal tissue sup-
port less than 3 mm (degree I) or exceeding
3 mm (degree II). Degrees III and IV of the
Glickman classification describe two severity
grades of the situation where the desmodon-
tal fibres are detached from the furcation
fornix/dome throughout the diameter of
the tooth; that is, horizontal ‘through‐
and‐through’ destruction of the periodontal
tissue in the furcation (degree III according
to Hamp et al. 1975).
The criteria for assigning a degree III
(Hamp et al. 1975) to a furcation have also
been modified. For Graetz et al. (2014), it was
required to see the tip of the furcation probe
(Nabers) at the opposite furcation opening to
assign a degree III. For all other cases of deep
but not completely penetrating horizontal
probing, a degree II was assigned (Graetz
et al. 2014). Walter et al. (2009) created a
degree II–III for the situation of horizontal
probing of more than 6 mm, but not com-
pletely penetrating to the opposite furcation
entrance (Table 2.3). This at least partially
(a)
(b)
Figure 2.3
Furcation involvement degree II (Hamp et al. 1975; Tables 2.3 and 2.4): horizontal loss of support
exceeding 3 mm, but not encompassing the total width of the furcation area: (a) schematic (maxillary molar,
buccal furcation entrance): horizontal probing/clinical attachment level 5 mm; (b) tooth 47: the 9 mm marking
is at the gingival margin. However, the 6 mm marking is at the height of the virtual tangent placed to the roots
adjacent to the furcation. Source: Eickholz (2010).
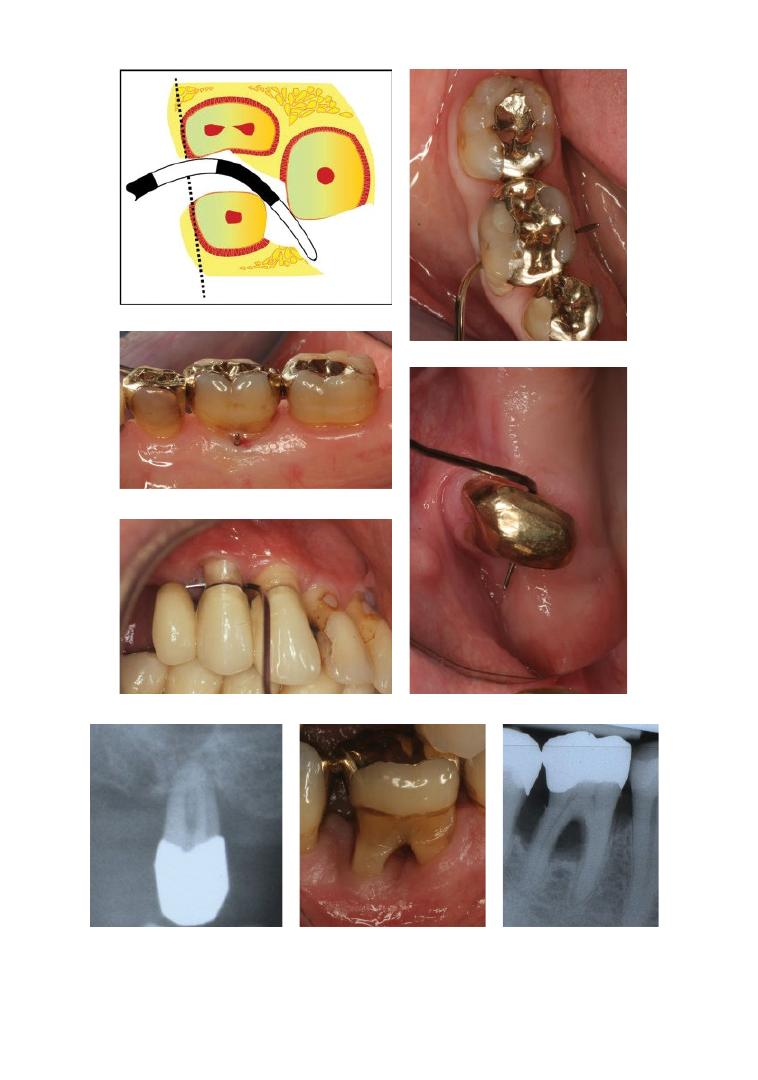
Chapter No.: 1 Title Name: <TITLENAME>
c02.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:18:51 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 19
(a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
Figure 2.4
Furcation involvement degree III (Ammons and Harrington 2006): horizontal ‘through‐and‐through’
destruction of the periodontal tissue in the furcation: (a) schematic (maxillary molar, buccal to interproximal
furcation entrance); (b) tooth 46 (occlusal view); (c) lingual view; (d) tooth 14; (e) tooth 16 without
neighbouring tooth from mesio‐palatal to disto‐palatal; (f) respective radiograph; (g) tooth 46: the interdental
bone is destroyed, and the soft tissues have receded apically so that the furcation opening is clinically visible.
A tunnel therefore exists between the roots of such an affected tooth (Glickman degree IV); (h) respective
radiograph. Source: d and e, Eickholz (2010).

Chapter No.: 1 Title Name: <TITLENAME>
c02.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:18:51 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 20
Table 2.2
Classification of furcation involvement according to Glickman (1953).
Degree 0
No furcation involvement.
Degree I
Early/incipient stage of furcation involvement.
The pocket is suprabony and primarily affects the soft tissue.
Early bone loss may have occurred with an increase in probing depth.
Radiographic changes are not usually found.
Degree II
Can affect one or more of the furcations of the same tooth.
The furcation lesion is essentially a cul‐de‐sac with a definite horizontal component.
If multiple defects are present, they do not communicate with each other because a portion
of alveolar bone remains attached to the tooth.
The extent of the horizontal probing of the furcation determines whether the defect is early
or advanced.
Vertical bone loss may be present and represents a therapeutic complication.
Radiographs may or may not depict the furcation involvement, particularly with maxillary
molars because of the radiographic overlap of the roots. In some views, however, the
presence of furcation ‘arrows’ indicates possible furcation involvement.
Degree III
The bone is not attached to the dome of the furcation.
In early degree III involvement, the opening may be filled with soft tissue and may not be
visible. The clinician may not even be able to pass a periodontal probe completely through
the furcation because of interference with the bifurcational ridges or facial/lingual bony
margins. However, if the clinician adds the buccal and lingual probing dimensions and
obtains a cumulative probing measurement that is equal to or greater than the buccal/
lingual dimension of the tooth at the furcation orifice, the clinician must conclude that a
degree III furcation exists (Figure 2.5).
Properly exposed and angled radiographs of early degree III furcations display the defect as
a radiolucent area in the crotch of the tooth.
Degree IV
The interdental bone is destroyed, and the soft tissues have receded apically so that the
furcation opening is clinically visible.
A tunnel therefore exists between the roots of such an affected tooth.
The periodontal probe passes readily from one aspect of the tooth to another.
Source: A mmons and Harrington (2006).
Table 2.3
Classification of furcation involvement according to Hamp et al. (1975).
Degree 0
No furcation involvement.
Degree I
Horizontal loss of periodontal tissue support less than 3 mm (Figure 2.2).
Modifications by:
●
Eickholz and Staehle (1994): horizontal loss of periodontal tissue support up to 3 mm.
●
Carnevale et al. (1995): horizontal loss of periodontal support not exceeding
one‐third of the width of the tooth.
Degree II
Degree II–III
Horizontal loss of support exceeding 3 mm, but not encompassing the total width of
the furcation area (Figure 2.3).
Modifications by:
●
Carnevale et al. (1995): horizontal loss of periodontal support exceeding one‐
third of the width of the tooth, but not encompassing the total width of the
furcation area.
●
Walter et al. (2009): degree II – horizontal loss of support exceeding 3 mm, but
no more than 6 mm.
●
Walter et al. (2009): horizontal loss of support exceeding 6 mm, but no detectable
‘through‐and‐through’ destruction.
Degree III
Horizontal ‘through‐and‐through’ destruction of the periodontal tissue in the
furcation (Figure 2.4).
Modification by:
●
Graetz et al. (2014): through‐and‐through furcation (requiring seeing the tip of
the Nabers probe at the contralateral furcation opening).
Source: Hamp et al. (1975).

Clinical and Radiographic Diagnosis and Epidemiology 21
explains the low validity of detecting degree
III furcations accurately by clinical probing
compared to cone‐beam computer tomography
(CBCT; Walter et al. 2009) or intrasurgical
assessments (Graetz et al. 2014).
Svärdström and Wennström (1996) pro-
posed another classification that does not
count millimetres but estimates horizontal
probing: degree 0 = the furcation site not pro-
beable; degree 1 = the root trunk coronal to
the furcation entrance probeable; degree
2 = the tip of the probe passes horizontally
into the furcation but does not reach the cen-
tre of the furcation area; degree 3 = the tip of
the probe reaches to or beyond the centre of
the furcation area (Svärdström & Wennström
1996). The definition of degree 3 is quite sim-
ilar to Walter et al.’s (2009) degree II–III.
However, this classification does not con-
sider the case of a clearly probeable through‐
and‐through furcation.
2.2.2 Distinction Between Degree II
and Degree III Furcation Involvement
The distinction between degree II (Hamp
et al. 1975; Figure 2.3) and through‐and‐
through furcation (degree III; Figure 2.4) is of
decisive significance for either prognosis as
well as choice of therapy:
●
Molars with degree III furcation defects
have a worse long‐term prognosis than
degree II lesions (McGuire and Nunn 1996;
Dannewitz et al. 2006, 2016; Salvi et al.
2014; Graetz et al. 2015).
●
Whereas buccal and lingual degree II
lesions at least can be improved by regen-
erative therapy, there is no clinical evidence
for any benefit of regenerative treatment
in through‐and‐through furcations (Sanz
et al. 2015; see Chapters 6 and 7).
Particularly from interproximally located
furcation entrances in the presence of
adjacent teeth, a furcation probe cannot be
completely pushed through the whole furca-
tion area involved. Nevertheless, hard and
soft tissue may be detached from the furca-
tion fornix; that is, furcation involvement
degree III. In the definition of degree III by
Graetz et al. (2014), this situation would be
rated degree II. Walter et al. (2009) would
rate this situation degree II–III. In these
cases, it is recommended to follow Ammons
and Harrington (2006): in cases where the
clinician may not even be able to pass a peri-
odontal probe completely through the furca-
tion because of interference with the
bifurcational ridges or facial/lingual bony
margins, they may add the buccal and lin-
gual probing dimensions. If a cumulative
probing measurement is obtained that is
equal to or greater than the buccal/lingual
dimension of the tooth at the furcation ori-
fice, the furcation is rated degree III
Table 2.4
Recommended classification of furcation involvement.
Degree 0
No furcation involvement.
Degree I
Horizontal loss of periodontal tissue support up to 3 mm (Eickholz and Staehle 1994).
Degree II
Horizontal loss of support exceeding 3 mm, but not encompassing the total width of the
furcation area (Hamp et al. 1975).
Degree III
Horizontal ‘through‐and‐through’ destruction of the periodontal tissue in the furcation.
In early degree III involvement, the opening may be filled with soft tissue and may not be
visible. The clinician may not even be able to pass a periodontal probe completely through
the furcation because of interference with the bifurcational ridges or facial/lingual bony
margins. However, if the clinician adds the buccal and lingual probing dimensions and
obtains a cumulative probing measurement that is equal to or greater than the buccal/
lingual dimension of the tooth at the furcation orifice, the clinician must conclude that a
degree III furcation exists (Ammons and Harrington 2006).
Sources: Hamp et al. (1975); Eickholz and Staehle (1994); Ammons and Harrington (2006).

Chapter 2
22
(Tables 2.2 and 2.4). Thus, underestimation
of furcation involvement as observed by
Walter et al. (2009) and Graetz et al. (2014)
can be avoided.
2.2.3 The Vertical Dimension
of Furcation Involvement
The central problem about furcation involve-
ment is the difficult‐to‐access horizontal
niche between the roots of multi‐rooted
teeth. Thus, the classifications referred to
consider mainly the horizontal component of
attachment/bone loss. However, it is plausi-
ble that in addition to horizontal attachment/
bone loss, vertical attachment/bone loss in
the furcation area plays a role. It has been
demonstrated that survival of molars after
furcation therapy does not only depend on
baseline furcation involvement, but also on
baseline bone loss (Dannewitz et al. 2006;
Park et al. 2009). Thus, a subclassification
has been proposed that measures the probe-
able vertical depth from the roof of the furca-
tion apically. Subclass A indicates a probeable
vertical depth of 1–3 mm, B 4–6 mm, and C
7 mm or more of probeable depth from the
roof of the furcation apically. Furcations
would thus be classified as IA, IB, IC, IIA,
IIB, IIC, and IIIA, IIIB, IIIC (Tarnow and
Fletcher 1984). The more severe the vertical
component the worse is long-term prognosis
of molars with degree II furcation involve-
ment (Tonetti et al. 2017). Prognosis also
depends on the remaining circular attach-
ment of each root (Walter et al. 2009).
2.2.4 Reproducibility and Validity
of the Assessment of Furcation
Involvement
Furcation involvement is difficult to access for
hygiene. How reliably can furcation lesions be
diagnosed; that is, scored? Whereas for buccal,
lingual, and mesio‐ lingual scoring of furcation
degrees excellent intrarater reproducibility is
reported, disto‐lingual furcation lesions pro-
vide only moderate reproducibility. Similar
results are reported for PAL‐H measurements
(excluding degree III furcation involvement).
Intrarater reproducibility in disto‐lingual
furcations is significantly worse than for all
other locations. In mesio‐buccal furcations, a
neighbouring tooth is associated with higher
variability (Eickholz and Staehle 1994; Eickholz
and Kim 1998). Interproximal furcations, in
particular the disto‐lingual site and in the pres-
ence of a neighbouring tooth, are more difficult
to access and to measure than the other loca-
tions. This fact has to be kept in mind when
the clinical examiner scores maxillary molars
in particular.
How accurately does the clinical measure-
ment assess the intrasurgically measured fur-
cation involvement (PBL‐H)? Disto‐ lingual
location and a neighbouring tooth are also
associated with less accuracy. Furthermore, a
curved rigid furcation probe (Nabers probe)
demonstrated better accuracy than a straight
rigid (PCPUNC 15) and flexible plastic (TPS)
probe (Eickholz and Kim 1998). Interestingly,
clinical PAL‐H measurements on average
overestimated intrasurgically measured
PBL‐H. However, the difference was only sig-
nificant for measurements with a Nabers
probe in degree I furcation lesions (Eickholz
1995; Eickholz and Kim 1998).
2.2.5 Documentation
of Furcation Involvement
As documented in Chapter 5, differentiated
documentation of furcation involvement
according to extent (degree) and location is a
prerequisite for proper prognosis and treat-
ment planning (Figure 2.5). In the meantime,
many computer programs for dental patient
charting provide the necessary differentiated
digital documentation (Florida probe chart;
Figure 2.6).
2.3 Radiographic Diagnosis
of Furcation Involvement
In general, radiographs provide information
on the translucency to X‐rays of different tis-
sues. The denser a tissue (e.g. compact
bone) is, the less translucent it is for X‐rays.
Thus, both two‐ and three‐dimensional radi-
ographic images primarily provide information
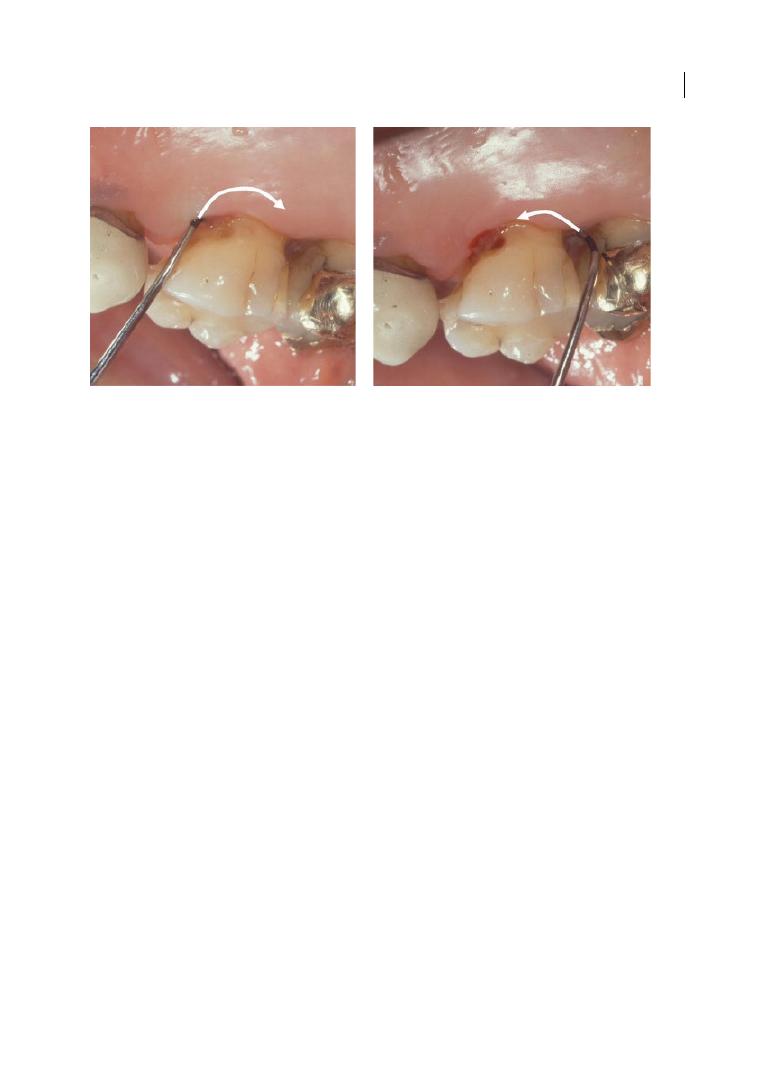
Clinical and Radiographic Diagnosis and Epidemiology 23
on bone in contrast to soft tissue. However,
furcation involvement is not only a matter of
bone, but also of connective tissue attach-
ment. Therefore, radiographs tell a substan-
tial part of but not the whole story about
furcation involvement. This is particularly
true after regenerative treatment, where
there may be a new connective tissue attach-
ment without new bone formation within a
furcation.
Using two‐dimensional radiographic tech-
niques (projection radiography: periapical
and panoramic radiographs), reliable diagno-
sis of furcation involvement is not provided
(Topoll et al. 1988). For maxillary premolars,
the furcation channel is oriented perpendic-
ularly to the central beam. Thus, furcation
involvement in maxillary premolars cannot
be visualized using projection radiography. In
three‐rooted maxillary molars, the furcation
channel between mesio‐ and disto‐palatal
furcation entrances also runs parallel to the
plane of the radiographic film or sensor and
perpendicular to the central beam. The buc-
cal furcation entrance is in most cases over-
lapped by the palatal root. Thus, in maxillary
molars inter‐radicular bone can be judged
only to a very limited extent. Only in man-
dibular molars is the
furcation channel
located perpendicularly to the plane of the
film/sensor and parallel to the central beam.
Therefore, under conditions of orthoradial
projection, inter‐radicular bone may be
assessed in mandibular molars. However,
radiographs only provide information on
resorption or density of bone. Reduced bone
density may be due to periodontal destruc-
tion or reduced bone density caused by loose
spongeous structure. Thus, conventional
radiographs may only provide hints for a sus-
picion of furcation involvement; this suspi-
cion has to be confirmed or rejected by
furcation probing using a curved probe.
Additional to degree of furcation involve-
ment, radiographs may provide information
to judge whether a buccal or lingual degree II
furcation may benefit from regenerative
therapy. In molars with class II furcation
involvement, a long root trunk, a furcation
fornix located coronally of the adjacent inter-
proximal alveolar crest, and a wide furcation
are associated with less favourable horizontal
attachment gain after regenerative therapy
(Horwitz et al. 2004).
(a)
(b)
Figure 2.5
Furcation probing at tooth 16: (a) from mesio‐palatal – probing (PAL‐H)/clinical horizontal
attachment loss (CAL‐H) = 9 mm; (b) from disto‐palatal – probing (PAL‐H)/clinical horizontal attachment loss
(CAL‐H) = 6 mm. In tooth 16 the PAL‐H/CAL‐H measurements add up to 15 mm. At the furcation entrances
tooth 16 has a width less than 15 mm. Thus the furcation is through and through (degree III; Table 2.4). Source:
Eickholz (2010).
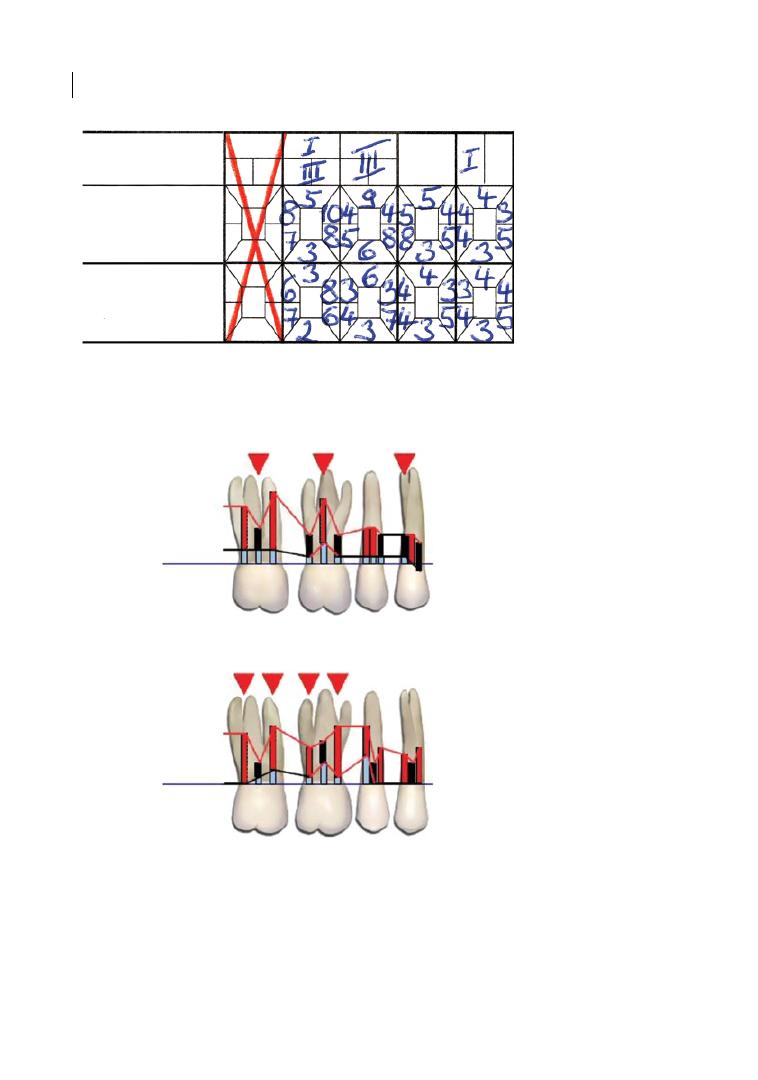
Chapter 2
24
Figure 2.6
Differentiated documentation of furcation scores: (a) periodontal chart from the Department of
Periodontology of the Johann Wolfgang Goethe‐Universität, Frankfurt am Main: tooth 17 – buccal degree I
furcation; through‐and‐through furcation from mesio‐palatal to disto‐palatal (Grade III); tooth 16 – through‐
and‐through furcation at all furcation entrances; tooth 14 – distal degree I furcation. (b) Florida Probe: tooth
17 – buccal degree I furcation; through‐and‐through furcation from mesio‐palatal to disto‐palatal (Grade III);
tooth 16 – through‐and‐through furcation at all furcation entrances; tooth 14 – distal degree I furcation.
(a)
Furkation
b
0
AL
ST/LG
b
0
b
0
(b)
2 2 2
6
3
8
1
1
111
44
3
3
4
4
10
–1
Tooth #
1
GM
GM
3
3
3
3
3
3
3
1
1
3
6
3
7
2
6
0 1
0
2
4
400
0
4
3
5
4
3
5
000
0
3
7
1 3
0
1
Recession
Mobility
Depth
Recession
Right
Depth
2
3
4
5
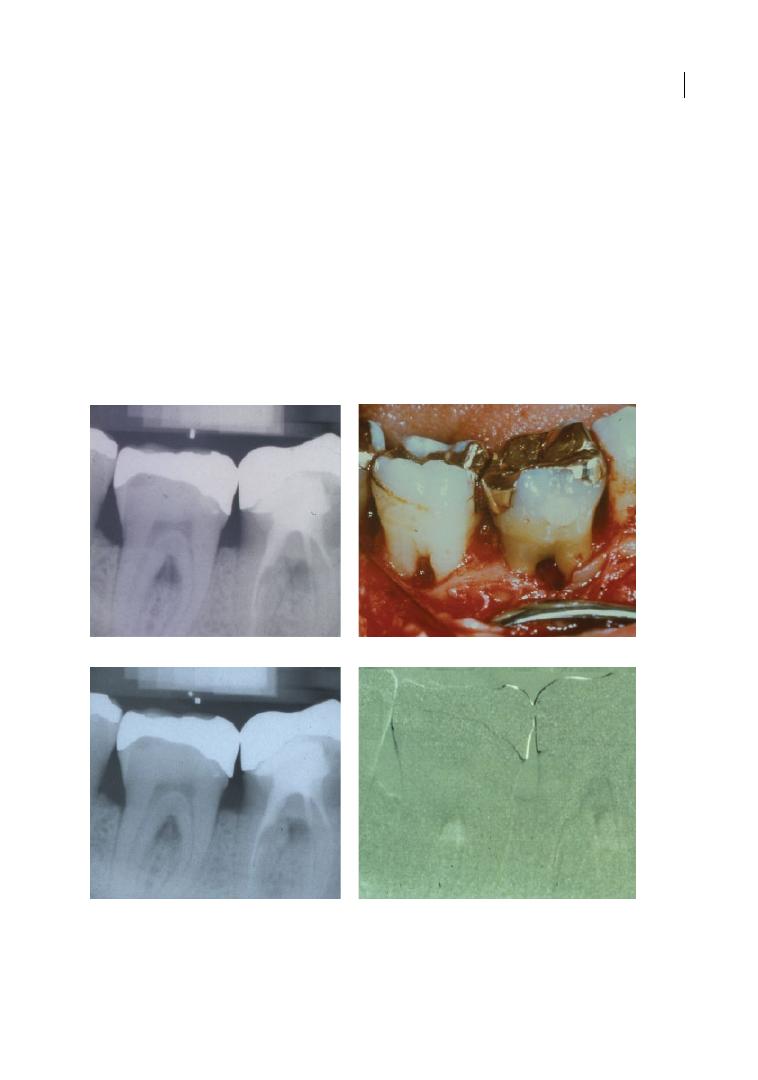
Clinical and Radiographic Diagnosis and Epidemiology 25
2.3.1 Digital Subtraction
Radiography
A highly specialized and technically sensitive
radiographic method may be used to follow
up changes of inter‐radicular bone in molar
furcations: digital subtraction radiography
(DSR; (Eickholz and Hausmann 1997). Two
consecutively obtained radiographs (e.g. prior
to and 12 months after therapy) of the same
tooth are overlapped in such a way that cor-
responding structures are positioned exactly
over one another. The grey values of the
baseline radiograph are inverted (white to
black, black to white) and added to those of
the follow‐up radiograph. In two completely
identical radiographs that overlap perfectly, a
middle grey value will result. An increase of
bone density (bony fill) results in lighter grey
values, a decrease of bone density (bone loss) in
darker grey values (Eickholz and Hausmann
1997; Figure 2.7). However, DSR requires strict
standardization of projection geometry and is
highly sensitive to misalignment. Thus, the
technique is rarely applied in clinical practice.
(a)
(b)
(c)
(d)
Figure 2.7
Follow‐up of inter‐radicular bone at teeth 46 and 47 using digital subtraction radiography (DSR):
(a) standardized radiograph of teeth 46 and 47 prior to regenerative therapy; (b) intrasurgical view – buccal
degree II furcation involvement at both teeth; (c) standardized radiograph six months after regenerative
therapy; (d) subtraction image – increase of bone density within the furcations of 46 and 47. Source:
Eickholz (2010).
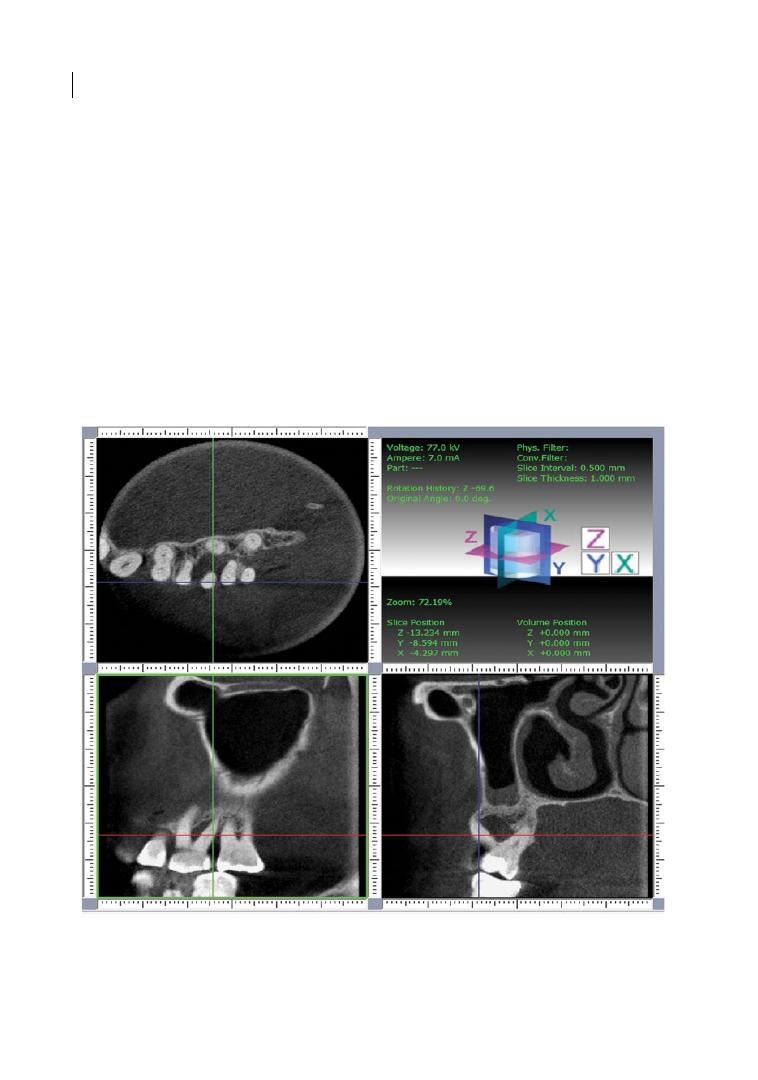
Chapter 2
26
2.3.2 Three‐dimensional
Radiography
Since conventional two‐dimensional radio-
graphic imaging may have some clinically rel-
evant drawbacks, it might be useful to analyse
distinct clinical situations, particularly in
maxillary molar teeth, with a suitable three‐
dimensional diagnostic approach with appro-
priate exposure to radiation (Laky et al. 2013;
Walter et al. 2016). Cone Beam Computed
Tomography (CBCT) has been validated in
vivo for the assessment of furcation‐involved
maxillary molars (Walter et al. 2016). CBCT
data were found to be accurate in assessing
the amount of periodontal tissue loss and in
classifying the degree of furcation involve-
ment in maxillary molars (Walter et al. 2009,
2010, 2016). In addition, the three‐dimensional
images revealed several findings, such as the
surrounding bony support of each maxillary
molar root, fusion or proximity of roots,
periapical lesions, root perforations, and/or
missing bony walls (Walter et al. 2009). The
clinical relevance of these radiographic data
was analysed regarding the decision‐making
process for resective or non‐resective thera-
pies (Figures 2.8 and 2.9). These treatment
options were classified according to their
graduation of invasiveness (GoI), ranging
from minimally invasive SPT to maximally
invasive extraction and implant restoration:
GoI 0 = supportive periodontal treatment
Figure 2.8
Diagnosis and treatment planning using cone‐beam computed tomography (CBCT). CBCT images
with horizontal, sagittal, and transversal sections of first and second left maxillary molars. According to the
bone loss around the disto‐buccal root and the remaining periodontal attachment around the mesio‐buccal
and palatal root, it was decided to extract the distobuccal root. Source: Walter et al. (2010).
Clinical and Radiographic Diagnosis and Epidemiology 27
(SPT); GoI 1 = open flap debridement with or
without gingivectomy or apically repositioned
flap and/or tunnelling; GoI 2 = root separa-
tion; GoI 3 = amputation/trisection of one
root (with or without root separation or tun-
nel preparation; GoI 4 = amputation/trisec-
tion of two roots; and GoI 5 = extraction of
the entire tooth. Significant discrepancies
between conventional and CBCT‐based
treatment approaches were found in most
situations, which possibly necessitates intras-
urgical changes in the treatment plan in those
cases where no CBCT is available (Walter
et al. 2009).
(a)
(b)
(c)
(d)
(e)
Figure 2.9
Root resection in a maxillary first molar: (a) pre‐surgical view; (b) tri‐section of the distobuccal root;
(c) the flap is fixed with monofil synthetic sutures 5 × 0; (d) four months post‐operation, the wound healing was
uneventful; (e) a crown with an extended metal margin is placed and the patient is introduced to meticulous
oral hygiene.

Chapter 2
28
However, the findings from a cost-benefit
analysis indicate the need for a critical
appraisal of CBCT applications in upper
molars (Walter et al. 2012). In most cases
with clinically based GoI ≤ 1, CBCT imaging
seems to have no or only minor impact on
economic benefit and to reduce treatment
time only slightly, if at all. With more inva-
sive clinically based treatment decisions
(GoI > 1), however, the benefits of using
CBCT were greater, probably because the
indication for tooth extraction is clarified.
On the one hand, a straightforward tooth
extraction followed by implant placement
and restoration is feasible, thereby avoiding
explorative periodontal surgeries when the
tooth is not maintainable. On the other
hand, unnecessary tooth extractions and
implant placement in sites where teeth
would be maintainable may be avoided.
Moreover, root canal treatments in sites
planned for GoI degrees 2, 3, or 4 may be
prevented, when CBCT reveals morphologi-
cal variations such as root proximities or
root fusions, which preclude clinically based
resective treatment planning.
The main goal of diagnostic radiology is to
keep the radiation dose as low as reasonably
achievable (ALARA), and this should also be
a prerequisite for CBCT application in den-
tistry, since increased radiation in the dental
office may potentially cause malignancies,
including thyroid cancer or intracranial
meningioma (Hallquist and Näsman 2001;
Longstreth et al. 2004; Hujoel et al. 2006).
The potential risks associated with additional
radiation exposure are only justified in single
cases and have to be evaluated in each indi-
vidual situation.
2.4 Epidemiology of
Furcation Involvement
How frequent is furcation involvement?
There exists only one population‐representative
study from the USA on the frequency of
furcation involvement. Even in periodontitis
patients, studies reporting the frequency of
furcation involvement differentiated accord-
ing to degree are rare and relatively small.
For the third National Health and Nutrition
Examination Survey (NHANES III), 9689
individuals representative of the US popula-
tion received periodontal examinations
including furcation scores. Partial furcation
involvement was scored in sites where the
explorer was definitely catching into but did
not pass through the furcation. This repre-
sents degrees I and II of the Hamp et al.
(1975) classification (Table 2.3). Total furca-
tion involvement was assigned when the
explorer could be passed between the roots
and through the entire furcation. This repre-
sents degree III of the Hamp et al. (1975)
classification (Table 2.3). The prevalence of
furcation involvement for all age groups was
13.7%, and the extent was 6.8% of posterior
teeth per person. The prevalence of through‐
and‐through furcation involvement was 0.9%
(extent: 0.5%). The prevalence of furcation‐
involved teeth (all/through‐and‐through)
increased with age (60–69 years: 27.6/2.1%;
70–79: 31.7/3.2%; 80–89: 37.9/3.4%) and was
higher in males (17.8/1.2%) than in females
(11.3/0.7%; Albandar et al. 1999).
For a sample of 71 periodontally diseased
patients in Germany, Dannewitz et al. (2006)
reported tooth‐based furcation involvement;
that is, they assigned to each molar the most
severe furcation involvement that was
observed within the particular tooth. Using
this mode, the information relevant for prog-
nosis is given for each molar. However, the
frequency of less severe furcation degrees is
underestimated. They observed degree I
furcation lesions in 23%, and degree II and III
furcation lesions in 24% and 13% of all
molars, respectively. No furcation involve-
ment at all was exhibited in 40% of all molars.
Premolars were not scored (Dannewitz et al.
2006).
In a sample of 345 periodontitis patients
(Eickholz et al. 2016), the degree of furcation
involvement of all sites was reported (site
based). This enlarges the proportion of less
severe furcation lesions in comparison to
reporting tooth‐based furcation involvement.

Clinical and Radiographic Diagnosis and Epidemiology 29
There was no furcation involvement in 45%
of all furcation sites, which approximately
confirms the frequency reported for molars
(Dannewitz et al. 2006). The study observed
degree I furcation lesions in 36%, and degree
II and III furcation lesions in 13.5% and 5.5%
of all molars and first maxillary premolars,
respectively. Considering this and the fact
that Dannewitz et al. (2006) did not report
premolars, the data on frequency of furcation
lesions roughly confirm the earlier findings in
a much larger sample (Eickholz et al. 2016).
In individuals aged 40 years or older, every
second molar was affected by advanced peri-
odontal destruction (score 2–3) in at least one
furcation site (Svärdström and Wennström
1996). Furcation involvement was found
more frequently in the maxilla than in the
mandible (Svärdström and Wennström 1996;
Dannewitz et al. 2006). However, this may be
due simply to the fact that maxillary molars
have more sites at risk than mandibular
molars (maxillary molars with three, man-
dibular with two furcation entrances).
At least in periodontitis patients, furcation
involvement is a frequent finding. In perio-
dontally diseased patients, roughly one‐third
of all molars and almost one‐fifth of all
furcation sites exhibit degree II and III furca-
tion involvement, which affects prognosis
and choice of therapy for the respective
multi‐rooted teeth.
References
Albandar, J.M., Brunelle, J.A., and Kingman, A.
(1999). Destructive periodontal disease in
adults 30 years of age and older in the
United States, 1988–1994. Journal of
Periodontology 70, 13–29.
Ammons, W.F., and Harrington G.W. (2006).
Furcation: Involvement and treatment. In:
Carranza’s Clinical Periodontology (ed. M.G.
Newman, H.H. Takei, P.R. Klokkevold, and
F.A. Carranza), 991–1004. St. Louis, MO:
Saunders Elsevier.
Carnevale, G., Pontoriero, R., and Hürzeler,
M.B. (1995) Management of furcation
involvement. Periodontology 2000 9, 69–89.
Dannewitz, B., Krieger, J.K., Hüsing, J., and
Eickholz, P. (2006). Loss of molars in
periodontally treated patients: A
retrospective analysis five years or more
after active periodontal treatment. Journal
of Clinical Periodontology 33, 53–61.
Dannewitz, B., Zeidler, A., Hüsing, J. et al.
(2016). Loss of molars in periodontally
Summary of Evidence
●
Reliable clinical furcation diagnosis
requires a rigid curved furcation probe
(e.g. colour‐coded Nabers probe).
●
The distinction between degree II (Hamp
et al. 1975) and through‐and‐through fur-
cation (degree III) is as difficult as it is of
decisive significance for either prognosis
as well as choice of therapy.
●
A modified classification of furcation
involvement is recommended.
●
Conventional radiographs may only pro-
vide hints for a suspicion of furcation
involvement. However, this suspicion has
to be confirmed or rejected by furcation
probing using a curved probe. CBCT may
provide additional information for deci-
sion-making particularly in maxillary
molars if periodontal surgery is required.
●
In periodontally diseased patients, roughly
one‐third of all molars and almost one‐
fifth of all furcation sites exhibit degree II
and III furcation involvement.

Chapter 2
30
treated patients: Results ten years and
more after active periodontal therapy.
Journal of Clinical Periodontology 43,
53–62.
Eickholz, P. (1995). Reproducibility and
validity of furcation measurements as
related to class of furcation invasion. Journal
of Periodontology 66, 984–989.
Eickholz, P. (2010). Glossar der Grundbegriffe
für die Praxis: Parodontologische Diagnostik
6: Furkationsdiagnostik. Parodontologie 21,
261–266.
Eickholz, P., and Hausmann, E. (1997).
Evidence for healing of class II and III
furcations after GTR‐therapy: Digital
subtraction and clinical measurements.
Journal of Periodontology 68, 636–644.
Eickholz, P., and Kim, T.‐S. (1998).
Reproducibility and validity of the
assessment of clinical furcation parameters
as related to different probes. Journal of
Periodontology 69, 328–336.
Eickholz, P., and Staehle, H.J. (1994). The
reliability of furcation measurements.
Journal of Clinical Periodontology 21,
611–614.
Eickholz, P., Nickles, K., Koch, R. et al. (2016).
Is furcation class involvement affected by
adjunctive systemic amoxicillin plus
metronidazole? A clinical trial’s exploratory
subanalysis. Journal of Clinical
Periodontology 43 (10), 839–848.
Fleischer, H.C., Mellonig, J.T., Brayer, W.K.
et al. (1989). Scaling and root planing
efficacy in multirooted teeth. Journal of
Periodontology 60, 402–409.
Glickman, I. (1953). Clinical Periodontology.
Philadelphia, PA: Saunders.
Graetz, C., Plaumann, A., Wiebe, J.F. et al.
(2014). Periodontal probing versus
radiographs for the diagnosis of furcation
involvement. Journal of Periodontology 85,
1371–1379.
Graetz, C., Schützhold, S., Plaumann, A. et al.
(2015). Prognostic factors for the loss of
molars – an 18‐years retrospective cohort
study. Journal of Clinical Periodontology 42,
943–950.
Hallquist, A., and Näsman, A. (2001). Medical
diagnostic X‐ray radiation: An evaluation
from medical records and dentist cards in a
case‐control study of thyroid cancer in the
northern medical region of Sweden.
European Journal of Cancer Prevention 10,
147–152.
Hamp, S.‐E., Nyman, S., and Lindhe, J. (1975).
Periodontal treatment of multirooted teeth:
Results after 5 years. Journal of Clinical
Periodontology 2, 126–135.
Horwitz, J., Machtei, E.E., Reitmeir, P. et al.
(2004). Radiographic parameters as
prognostic indicators for healing of class II
furcation defects. Journal of Clinical
Periodontology 31, 105–111.
Hujoel, P., Hollender, L., Bollen, A.M. et al.
(2006). Radiographs associated with one
episode of orthodontic therapy. Journal of
Dental Education 70, 1061–1065.
Kalkwarf, K.L., Kaldahl, W.B., and Patil, K.D.
(1988). Evaluation of furcation region
response to periodontal therapy. Journal of
Periodontology 59, 794–804.
Laky, M., Majdalani, S., Kapferer, I., et al.
(2013). Periodontal probing of dental
furcations compared with diagnosis by
low‐dose computed tomography: A case
series. Journal of Periodontology 84,
1740–1746.
Lang, N.P., Cumming, B., and Löe, H. (1973).
Toothbrushing frequency as it relates to
plaque development and gingival health.
Journal of Periodontology 44, 396–405.
Longstreth, W.T., Jr, Phillips, L.E., Drangsholt,
M. et al. (2004). Dental X‐rays and the risk of
intracranial meningioma: A population‐based
case‐control study. Cancer 100, 1026–1034.
Loos, B., Nylund, K., Claffey, N., and Egelberg,
J. (1989). Clinical effects of root
debridement in molar and non‐molar teeth:
A 2‐year follow‐up. Journal of Clinical
Periodontology 16, 498–504.
McGuire, M.K., and Nunn, M.E. (1996).
Prognosis versus actual outcome. III: The
effectiveness of clinical parameters in
developing an accurate prognosis. Journal of
Periodontology 67, 666–674.

Clinical and Radiographic Diagnosis and Epidemiology 31
Mohammadi, Z., Shalavi, S., and Jafarzadeh, H.
(2013). Extra roots and root canals in
premolar and molar teeth: Review of an
endodontic challenge. Journal of
Contemporary Dental Practice 14, 980–986.
Nordland, P., Garrett, S., Kriger, R. et al.
(1987). The effect of plaque control and root
debridement in molar teeth. Journal of
Clinical Periodontology 14, 231–236.
Park, S.Y., Shin, S.Y., Yang, S.M., and Kye, S.B.
(2009). Factors influencing the outcome of
root‐resection therapy in molars: A 10‐year
retrospective study. Journal of
Periodontology 80, 32–40.
Pretzl, B., Kaltschmitt, J., Kim, T.‐S. et al.
(2008). Tooth loss after active periodontal
therapy. 2: Tooth‐related factors. Journal of
Clinical Periodontology 35, 175–182.
Salvi, G.E., Mischler, D.C., Schmidlin, K. et al.
(2014). Risk factors associated with the
longevity of multi‐rooted teeth: Long‐term
outcomes after active and supportive
periodontal therapy. Journal of Clinical
Periodontology 41, 701–707.
Sanz, M., Jepsen, K., Eickholz, P., and Jepsen,
S. (2015). Clinical concepts for regenerative
therapy in furcations. Periodontology 2000
68, 308–332.
Schroeder, H.E., and Scherle, W.F. (1987).
Warum die Furkation menschlicher Zähne
so unvorhersehbar bizarr gestaltet ist.
Schweizerische Monatsschrift für
Zahnmedizin 97, 1495–1508.
Svärdström, G., and Wennström, J.L. (1996).
Prevalence of furcation involvements in
patients referred for periodontal treatment.
Journal of Clinical Periodontology 23,
1093–1099.
Tarnow, D., and Fletcher, P. (1984).
Classification of the vertical component of
furcation involvement. Journal of
Periodontology 55, 283–284.
Tonetti, M.S., Christiansen A.L., and
Cortellini, P. (2017). Vertical
subclassification predicts survival of molars
with class II furcation involvement during
supportive periodontal care. J Clin
Periodontol 44, 1140–1144.
Topoll, H.H., Streletz, E., Hucke, H.P., and
Lange, D.E. (1988). Furkationsdiagnostik:
Ein Vergleich der Aussagekraft von OPG,
Röntgenstatus und intraoperativem Befund.
Deutsche Zahnärztliche Zeitschrift 43,
705–708.
Walter, C., Kaner, D., Berndt, D.C. et al. (2009).
Three‐dimensional imaging as a pre‐
operative tool in decision making for
furcation surgery. Journal of Clinical
Periodontology 36, 250–257.
Walter, C., Schmidt, J.C., Dula, K. et al. (2016).
Cone beam computed tomography (CBCT)
for diagnosis and treatment planning in
periodontology: A systematic review.
Quintessence International 47, 25–37.
Walter, C., Weiger, R., Dietrich, T. et al. (2012).
Does three‐dimensional imaging offer a
financial benefit for the treatment of
maxillary molars with furcation involvement?
A pilot clinical case series. Clinical Oral
Implants Research 23, 351–358.
Walter, C., Weiger, R., and Zitzmann, N.U.
(2010). Accuracy of three‐dimensional
imaging in assessing maxillary molar
furcation involvement. Journal of Clinical
Periodontology 37, 436–441.
Wang, H.L., Burgett, F.G., Shyr, Y., and
Ramfjord, S. (1994). The influence of molar
furcation involvement and mobility on
future clinical periodontal attachment loss.
Journal of Periodontology 65, 25–29.

Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
Chapter No.: 1 Title Name: <TITLENAME>
c03.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:19:19 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 33
33
3.1 Introduction
An experimental gingivitis model in humans
established that microbial plaque is the aetio-
logical factor of gingivitis (Loe et al. 1965).
A 26‐year longitudinal study on well‐maintained
Norwegian males found that sites with per-
sistent plaque‐induced gingival inflamma-
tion had 70% more clinical attachment loss
(odds ratio [OR] = 3.22) compared to sites
that were always healthy, thereby supporting
the concept that gingivitis is a prerequisite
for the inception of periodontitis (Schatzle
et al. 2003). Microbial plaque exists in the
oral cavity as biofilms, which are consortia of
micro‐organisms interacting with the sur-
rounding environment in a dynamic manner.
Subgingival plaque samples from 588 patients
with chronic periodontitis demonstrated
that with increasing probing depth, there was
a significant increase in the ‘orange’ and ‘red
complex’ microbes (Socransky and Haffajee
2005). These Gram‐negative bacteria release
molecules such as lipopolysaccharide and
extracellular proteolytic enzymes, which
interact with the innate host inflammatory
surveillance system to mount an immune
response against the invading bacteria
(Darveau et al. 1997). The inflammatory
response results in breakdown of the con-
nective tissue attachment and supporting
bone, leading to established periodontitis
lesions (Page and Kornman 1997). The
microbial nature of periodontitis is very
complex; it is thought that alterations in the
composition of the subgingival biofilm (dys-
biosis) involving ‘accessory’ and ‘keystone’
pathogens and pathobionts drive periodonti-
tis in a susceptible host (Hajishengallis and
Lamont 2012).
In order to arrest the initiation and pro-
gression of periodontitis, its management is
predominantly focused on removing micro-
bial plaque and its retentive factors from root
surfaces and gingival sulci. This is primarily
achieved by professional supra‐ and subgin-
gival mechanical debridement, with the aim
of disrupting the microbial biofilm growing
on the root surface. Subgingival tooth
debridement has traditionally been referred
to as ‘scaling and root planing’, although the
importance of necessarily planing or smooth-
ening the root surface has been questioned
(Checchi and Pelliccioni 1988; Smart et al.
1990). Microbial biofilm disruption leads to a
reduction of the host response cascade,
which halts periodontal destruction and thus
results in improvement of the clinical signs of
disease. Immediately after scaling and root
planing, the denuded root surface will be
partially covered by fibrin and polymorpho-
nuclear leukocytes. The junctional epithelium
Chapter 3
How Good are We at Cleaning Furcations? Non‐surgical
and Surgical Studies
Jia‐Hui Fu
1
and Hom‐Lay Wang
2
1
Discipline of Periodontics, Faculty of Dentistry, National University of Singapore, Singapore
2
Department of Periodontics and Oral Medicine, School of Dentistry, University of Michigan, Ann Arbor, MI, USA

Chapter 3
34
will start to migrate apically towards the per-
iodontal ligament. Granulation tissue will
form in the transseptal fibre region. By the
third week, the apical migration of the junc-
tional epithelium will terminate at the apical
end of the root instrumentation, with the
periodontal ligament fibres oriented parallel
to the root surface. Some root resorption or
even crestal bone loss may occur along the
root surface at areas that are not covered by
the long junctional epithelium. As a result,
the healing after scaling and root planing is
therefore mainly by periodontal repair, with
the formation of the long junctional epithe-
lium and gingival recession (Tagge et al.
1975; Biagini et al. 1988).
It is relatively straightforward to debride
single‐rooted teeth; however, in multi‐rooted
teeth the furcal areas are anatomically chal-
lenging to access because of their configura-
tions. Several anatomical factors related to
furcations and roots, covered by Pretzl in
Chapter 1, contribute to the aetiology and
compromised prognoses of furcation‐
involved teeth. These factors include furca-
tion entrance width, root trunk length, and
the presence of root concavities, cervical
enamel projections, bifurcation ridges, and
enamel pearls. An evaluation of 50 mandibu-
lar molars revealed variations in the furca-
tion area, with 48%, 34%, and 18% having flat,
convex, and concave domes, respectively
(Matia et al. 1986). Therefore, it has been
reported that complete plaque and calculus
removal in the furcation is highly unlikely
(Matia et al. 1986; Parashis et al. 1993a, b;
Kocher et al. 1998a, b). The imperfect
debridement can be attributed to the pres-
ence of difficult‐to‐reach root concavities,
which are commonly found in the furcal
areas, with an incidence of 100% in maxillary
premolars, 17–94% in maxillary molars, and
99–100% in mandibular molars (Bower
1979a, b; Booker and Loughlin 1985). In
addition, furcation entrances are generally
narrower (less than 0.75 mm) than the blade
of conventional curettes (0.75–1.10
mm;
Bower 1979a, b; Chiu et al. 1991; dos Santos
et al. 2009). Therefore, this chapter attempts
to provide an overview of the efficacy of
non‐surgical and surgical debridement of
furcal areas, using instruments such as
curettes, ultrasonic scalers, lasers, photody-
namic therapy, and interdental brushes.
3.2 Longitudinal Studies
on Management of
Furcation‐involved Teeth
Ramfjord and colleagues first introduced
the concept of longitudinal studies in 1968,
where they compared different treatment
modalities in a large subject population over
time using the split‐mouth design. This
approach allowed clinicians to better appre-
ciate the possible treatment outcomes that
will arise over time with minimal host varia-
bility. Subsequently, several research groups
have adopted this approach to evaluate the
treatment outcomes of non‐surgical and
surgical debridement of single‐ and multi‐
rooted teeth. These studies are generally
described based on geographical locations
and treatment modalities performed,
including scaling and root planing, subgingi-
val curettage, modified Widman flap, modi-
fied Kirkland’s flap, pocket elimination, and
apically positioned flap with and without
osseous resection. Clinical parameters, such
as clinical attachment level gain, probing
depth reduction, bleeding on probing, and
plaque index, were used to determine the
outcome of the treatment rendered. Table 3.1
shows a summary of longitudinal studies that
reported data on multi‐rooted teeth. The
results described the treatment outcomes
specific to multi‐rooted teeth.
Results from the Michigan longitudinal
studies reported more tooth loss in teeth
with baseline furcation involvement (FI)
despite surgical interventions, thus imply-
ing that the quality of surgical debridement
or post‐surgical home or professional care
did not deter disease progression in the long
term (Ramfjord et al. 1968, 1987; Wang
et al. 1994). The authors believed that
although flap elevation would improve
access to the furcation areas, complete
Chapter No.: 1 Title Name: <TITLENAME>
c03.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:19:19 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 35
Table 3.1
Summary of some longitudinal studies that evaluated multi‐rooted teeth.
Group
Author/
Year
Sample
Size
No. of
Teeth
Treatment Modalities
Follow‐Up
Years
SPT Protocol Results
Michigan Ramfjord et al. 1968 32
729
Initial SRP with curettes.
Subgingival curettage vs
gingivectomy/APF with
osseous resection as needed.
2
3 months
Baseline FI did not significantly affect CAL in molars in
the short term.
Ramfjord et al. 1987 72
1881
Initial SRP with curettes.
Surgical pocket elimination
vs MWF vs subgingival
curettage vs SRP
5
3 months
Out of 17 teeth that were lost due to periodontal reasons:
●
16 had FI at baseline.
●
15 were treated with surgical interventions and 2 were
treated with SRP.
Wang et al. 1994
24
165
Initial SRP with curettes.
Surgical pocket elimination
vs MWF vs subgingival
curettage vs SRP.
8
3 months
FI molars had 2.54 times greater risk of being lost.
Minnesota Pihlstrom et al. 1984 10
266
SRP alone vs SRP with
MWF vs subgingival
curettage vs SRP
6.5
3–4 months Compared to non‐molars:
●
Molars with baseline PPD 4–6 mm: significantly deeper
residual PPD (1.05 mm) and greater apical CAL
(0.54 mm) after SRP alone.
●
Molars with baseline PPD of 4–6 mm: significantly
deeper residual PPD (1.02 mm) and greater apical CAL
(1.27 mm) after SRP with MWF.
●
Molars with baseline PPD ≥ 7 mm: no significant
difference in PPD or CAL, for both treatment
modalities.
9/11 teeth lost after completion of treatment were molars.
Loma Linda Nordland et al. 1987 19
Initial SRP with curettes
and ultrasonic scaler
2
3 months Compared to non‐molar or non‐furcation sites, molar FI
sites had:
●
More bleeding on probing.
●
Higher bleeding scores of 60% to 70% and > attachment
loss (1 out of 5 molars) when PPDs were 7 mm or more.
●
Lowest post‐treatment reduction was in PPD (1.0 mm).
●
0.5 mm loss of CAL instead of attachment gain.
(Continued)
Chapter No.: 1 Title Name: <TITLENAME>
c03.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:19:19 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 36
Table 3.1
(Continued)
Group
Author/
Year
Sample
Size
No. of
Teeth
Treatment Modalities
Follow‐Up
Years
SPT Protocol Results
Loos et al. 1988
11
43
Initial SRP with ultrasonic
scaler.
1.1
3 months
Compared to non‐molar sites, molar FI sites had:
●
Greater tendency to rebound after treatment.
●
Mean probing attachment level gain of 0.1 mm (0.7 mm
at non‐molar sites).
●
Significantly higher microbial count.
Loos et al. 1989
12
1682
Initial SRP with ultrasonic
or sonic scaler.
2
3 months
Molar FI sites:
●
Similar PPD and CAL pre‐ and post‐treatment.
●
With at least 7.0 mm PPD had lower PPD reduction
after treatment.
●
Did not have significant changes in CAL.
●
Had greater percentage of sites worsening over time
(38.5%).
Nebraska Kalkwarf et al. 1988 82
1394
Initial scaling with curettes
and ultrasonic scaler.
Coronal scaling only vs SRP
vs SRP with MWF vs SRP
with flap and osseous
resection.
2
3 months
●
FI sites tended to progress with horizontal probing
attachment loss irrespective of treatment.
●
Periodontal breakdown rate was 2.6% for sites that had
osseous resection, 5.9% for sites that had MWF, 8.4%
for sites that had SRP, and 8.3% for sites that had
coronal scaling only.
North
Carolina
Hirschfeld and
Wasserman 1978
600
15 666 SRP, gingivectomy,
gingivoplasty, and APF
with osseous surgery.
15
4–6 months
●
19.3% of FI molars were lost compared to 1.7% of
incisors in a well‐maintained population.
McFall 1982
100
2627
SRP, curettage, gingivectomy,
gingivoplasty, and APF with
osseous surgery.
15
3–6 months
●
27.3% of FI molars were lost compared to 0.6% of
incisors in a well‐maintained population.
Wood et al. 1989
63
1607
SRP.
13.6
6–9 months
●
23.2% of FI molars were lost compared to 0.8% of
incisors in a well‐maintained population.
Chapter No.: 1 Title Name: <TITLENAME>
c03.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:19:19 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 37
Table 3.1
(Continued)
Group
Author/
Year
Sample
Size
No. of
Teeth
Treatment Modalities
Follow‐Up
Years
SPT Protocol Results
Loos et al. 1988
11
43
Initial SRP with ultrasonic
scaler.
1.1
3 months
Compared to non‐molar sites, molar FI sites had:
●
Greater tendency to rebound after treatment.
●
Mean probing attachment level gain of 0.1 mm (0.7 mm
at non‐molar sites).
●
Significantly higher microbial count.
Loos et al. 1989
12
1682
Initial SRP with ultrasonic
or sonic scaler.
2
3 months
Molar FI sites:
●
Similar PPD and CAL pre‐ and post‐treatment.
●
With at least 7.0 mm PPD had lower PPD reduction
after treatment.
●
Did not have significant changes in CAL.
●
Had greater percentage of sites worsening over time
(38.5%).
Nebraska Kalkwarf et al. 1988 82
1394
Initial scaling with curettes
and ultrasonic scaler.
Coronal scaling only vs SRP
vs SRP with MWF vs SRP
with flap and osseous
resection.
2
3 months
●
FI sites tended to progress with horizontal probing
attachment loss irrespective of treatment.
●
Periodontal breakdown rate was 2.6% for sites that had
osseous resection, 5.9% for sites that had MWF, 8.4%
for sites that had SRP, and 8.3% for sites that had
coronal scaling only.
North
Carolina
Hirschfeld and
Wasserman 1978
600
15 666 SRP, gingivectomy,
gingivoplasty, and APF
with osseous surgery.
15
4–6 months
●
19.3% of FI molars were lost compared to 1.7% of
incisors in a well‐maintained population.
McFall 1982
100
2627
SRP, curettage, gingivectomy,
gingivoplasty, and APF with
osseous surgery.
15
3–6 months
●
27.3% of FI molars were lost compared to 0.6% of
incisors in a well‐maintained population.
Wood et al. 1989
63
1607
SRP.
13.6
6–9 months
●
23.2% of FI molars were lost compared to 0.8% of
incisors in a well‐maintained population.
Group
Author/
Year
Sample
Size
No. of
Teeth
Treatment Modalities
Follow‐Up
Years
SPT Protocol Results
New
Jersey
Ross and
Thompson 1978
100
387
Scaling, curettage,
gingivectomy, gingivoplasty,
and APF
5–24
–
●
12% of FI maxillary molars were extracted, of which
22% had been present for at least 6 years and 33% for
11–18 years.
●
Changes in bone support of FI maxillary molars at
5–24 years post‐treatment:
– 75% had no significant change.
– 11% had bone loss.
– 2% had slight improvement.
– 12% were extracted.
Sweden
Lindhe et al. 1982
15
–
SRP vs MWF.
2
2 weeks for
6 months,
followed by
once every
3 months
●
Reduction in mean plaque index scores was greater in
non‐molars.
●
In surgically treated sites, greater PPD reduction in
non‐molars, but in non‐surgically treated sites it was
comparable between non‐molars and molars.
Germany Dannewitz
et al. 2006
71
505
Initial SRP, followed by OFD,
GTR, root resection or
separation, or tunnelling
procedure.
>5 years
–
●
3.8% of FI molars lost after active therapy.
●
31.8% of FI molars lost over time after SRP.
●
34.6% of FI molars lost over time after SRP and flap
surgery.
●
Molars with class III FI tended to deteriorate
significantly over time.
Dannewitz
et al. 2016
136
–
Initial SRP
13.2
●
Molars with class III furcations had 4.68 times higher
risk of being lost compared to non‐FI molars.
APF = apically positioned flap; CAL = clinical attachment level; FI = furcation involvement; GTR = guided tissue regeneration; MWF = modified Widman flap; OFD = open‐flap
debridement; PPD = probing pocket depth; SRP = scaling and root planning; SPT = supportive periodontal therapy.

Chapter 3
38
debridement was difficult to achieve and
maintain post‐surgically due to the complex
configurations of the furcal area; therefore,
furcation‐involved teeth had a poorer long‐
term prognosis. In addition, it was hard to
maintain furcated molars, thus they had a
2.54 times greater susceptibility of being
lost during the periodontal maintenance
phase (Wang et al. 1994). A longitudinal
study by the Minnesota group demonstrated
that molars compared to non‐molars had
significantly greater post‐treatment pocket
depth and clinical attachment level changes
at sites with baseline pocket depths of
4–6 mm, regardless of the treatment ren-
dered. However, at deeper sites (pocket
depth of 7 mm or more), no significant dif-
ferences were detected between molars and
non‐molars in the long term. Although the
authors did not report the severity of FI in
the molars in their study, 9 out of the 11
teeth that were extracted at the end of treat-
ment were molars (Pihlstrom et al. 1984).
The Loma Linda group evaluated the
effectiveness of plaque control and subgin-
gival root debridement of non‐molar, molar
non‐furcation, and molar furcation sites
over a two‐year period and found that
molar furcation sites had persistent inflam-
mation, the poorest response to treatment
in terms of pocket depth reduction and
clinical attachment gain, and a significant
tendency to rebound to baseline status after
treatment (Nordland et al. 1987; Loos et al.
1988, 1989). The Nebraska group compared
coronal scaling alone, complete scaling
and root planing alone and followed by
modified Widman flap or osseous‐respec-
tive surgery in the management of molars
with FI. Their results showed that osseous
resection had the greatest probing depth
reduction with the least tendency for fur-
ther breakdown (Kalkwarf et al. 1988). The
North Carolina group evaluated patients
over 13–15 years and found that molars
with FI were lost 10–27 times more fre-
quently compared to incisors, despite hav-
ing non‐surgical and surgical periodontal
treatment with regular maintenance
(Hirschfeld and Wasserman 1978; McFall
1982; Wood et al. 1989).
A group in New Jersey evaluated 384
maxillary molars with varying degrees of FI
over 5–24 years. They used a combination
of scaling, curettage, gingivectomy and/or
gingivoplasty, and apically positioned flap
to manage the furcation‐involved maxillary
molars. Their results showed that the ther-
apies rendered were able to maintain maxil-
lary molars with FI over that period, with
only 12% of molars being lost over time.
However, the therapies did not have any
effect on the bone support, as 75% of the
molars had no significant changes in bone
levels. On the contrary, 11% had detectable
bone loss and 12% were extracted (Ross and
Thompson 1978). A German group evalu-
ated only molars in 136 patients over a
mean follow‐up period of 13 years. They
reported that similar percentages of molars
were lost when treated with both closed‐
and open‐flap scaling and root planing
(Dannewitz et al. 2006, 2016). A group in
Sweden too found that reduction in the
mean plaque index score was greater in
non‐molars compared to molars (Lindhe
et al. 1982).
A review of the longitudinal studies seemed
to show that debridement of the furcation
areas during root planing or with a surgical
procedure, for instance apically positioned
flap with osseous surgery, did not signifi-
cantly improve the long‐term prognosis of
these teeth. Although furcation‐involved
teeth might survive in the long term, their
survival rates were substantially lower than
single‐rooted teeth such as incisors. The
authors alluded that the anatomy of the fur-
cation area complicated both professional
debridement and patient home care, imply-
ing that the quality of debridement might not
be ideal and thereby indicating that molar
teeth are tougher to maintain successfully
over time. A summary on the long‐term sur-
vival of molars with FI is provided in
Chapter 5.

How Good are We at Cleaning Furcations? 39
3.3 Professional
Debridement
Conventionally, scaling and root planing are
carried out using manual instruments, such
as curettes, sickles, chisels, hoes, and files, or
power‐driven scalers, for example sonic or
ultrasonic scalers. These instruments can be
used in both the non‐surgical and surgical
phases of periodontal therapy. Curettes, for
instance universal and Gracey, are double‐
ended instruments with customized cutting
edges, shank lengths, blade lengths, and
angulations. Therefore, each one of the nine
standard Gracey curettes is designed to scale
and root plane a specific area in the mouth.
These curettes have a blade width of at least
0.76 mm and a blade length of 5 mm (Oda
et al. 2004).
Powered scalers are either sonic or ultra-
sonic. In sonic scalers, compressed air causes
the working tip to vibrate in an elliptical fash-
ion at frequencies of 2000 to 6000 Hz under a
water spray. Ultrasonic scalers, on the other
hand, are subclassified into magnetostrictive
and piezoelectric scalers. The magnetostric-
tive scalers, such as Cavitron® (Dentsply,
USA), work by creating a magnetic field
where an expanding and contracting coil,
together with an alternating current, results
in vibrations that are transmitted to the
working tip. The tip moves in an elliptical
motion, thus all sides of the tip are active. In
the piezoelectric scalers, such as Piezon
Master® (EMS, Switzerland), reactive ceramic
crystals undergo dimensional changes when
subjected to an alternating electrical current.
The expansion and contraction result in
vibrations that are transmitted to the work-
ing tip, which moves in a linear manner, thus
only the lateral sides of the tip act as the
active sides. The average ultrasonic scaler tip
width is 0.55 mm (Oda et al. 2004).
A comparison between the use of hand
instruments and ultrasonic scalers showed
that the latter were better suited for the
debridement of narrow furcation areas, as
their tips were narrower than curettes and
thus could debride the hard‐to‐reach areas
(Matia et al. 1986; Sugaya et al. 2002). In
addition, they were able to significantly
reduce the bacterial counts for all degrees of
FI and were more effective in debriding the
class II and III furcations compared to
curettes (Leon and Vogel 1987). A study that
evaluated the efficacy of four ultrasonic
sharp tips (Cavitron TFI 10 tip, Cavitron
EWPP [Probe] tip, Titan‐S Universal tip, and
Titan‐S Sickle tip) showed that there were no
significant differences in calculus removal
when different tips were used (Patterson
et al. 1989). When sharp tips were compared
to ball tips, the Titan sonic scaler with uni-
versal tip and Cavitron with ball tip were the
most efficient at debriding both maxillary
and mandibular molars, especially the furca-
tion roofs (Takacs et al. 1993). Other similar
studies evaluated modified sonic tips with
different angulations, and found that angu-
lated tips provided a more thorough debride-
ment because the tips could better access the
furcations (Kocher et al. 1996, 1998a, b). In
addition, some of the sonic tips had an ellip-
soidal terminal end of 0.8 mm in diameter,
which would provide more intimate contact
with the root concavities and the furcation
dome, thus improving the quality of the
instrumentation. Diamond‐coated ultra-
sonic and sonic scaler tips were found to
remove calculus 2–3.3 times faster than
manual curettes, but they were prone to
remove cementum and dentine during
debridement (Kocher and Plagmann 1999;
Scott et al. 1999).
A study that investigated the quality of the
mechanical and chemical debridement of root
surfaces on 90 periodontally involved
extracted teeth found that with unlimited
access to the root surfaces, all mechanical
debridement methods – that is, curettes, and
ultrasonic with regular or diamond‐coated
P‐10 tip – were equally effective. Thus, this
study suggested that access was the main criti-
cal factor that affected the quality of root sur-
face debridement (Eschler and Rapley 1991).
In addition, it was reported that interproximal
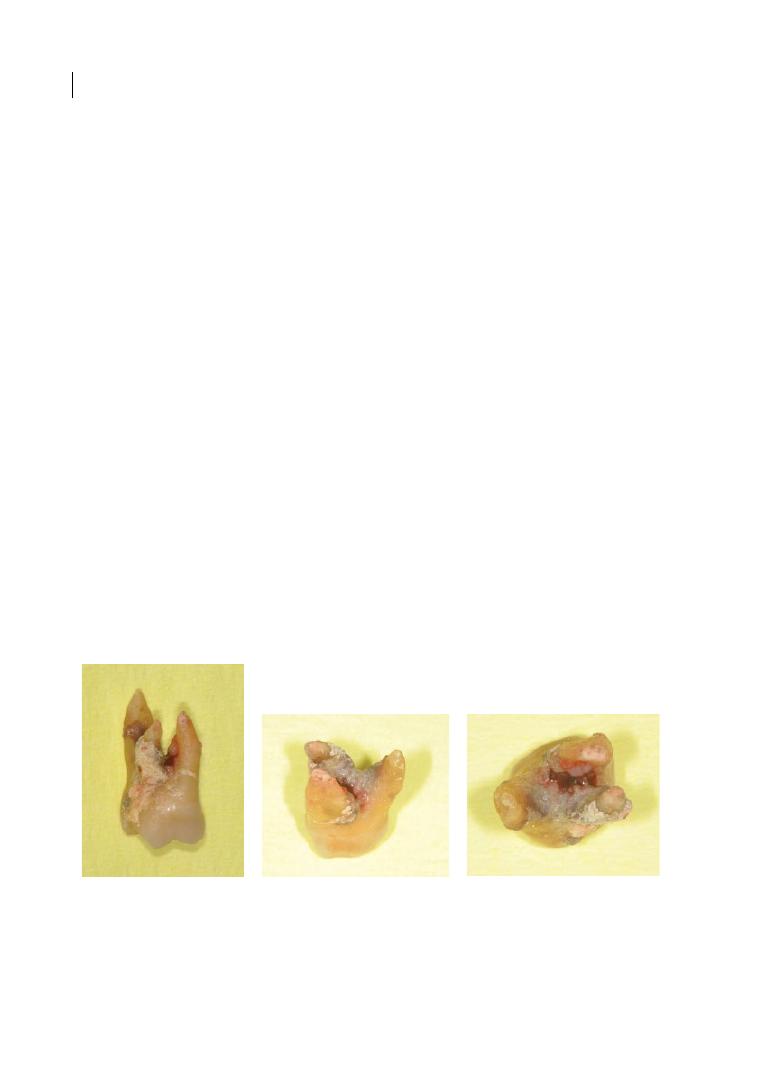
Chapter 3
40
FIs responded less favourably to mechanical
debridement compared to their buccal and
lingual counterparts. This phenomenon
could be due to the increased difficulty in
accessing the interproximal furcations for
debridement (Del Peloso Ribeiro et al.
2007).
Besides modifying the scaler tips, root
surface debridement can be performed
through a non‐surgical (closed) or surgical
(open) approach. It was reported that sig-
nificantly more residual calculus was found
in groups that had the closed approach
(34.1–37.0%) compared to the open
approach (1.0–2.7%; Matia et al. 1986). In
addition, clinical experience and proficiency
did significantly affect the quality of debride-
ment at the furcations. Less experienced
residents left significantly more calculus‐
free surfaces with the open approach (43%)
compared to the closed approach (8%). This
percentage of only 8% calculus‐free furca-
tion surfaces for less experienced operators
with a closed approach was particularly
striking. On the other hand, there was no
significant difference between the open and
closed approaches for experienced perio-
dontists (Fleischer et al. 1989). Using the
open approach, experienced periodontists
had 68% calculus‐free furcation surfaces
compared to 44% with a closed approach.
Although the percentages were not statisti-
cally significant, they were clinically impor-
tant, because almost a quarter of the root
surfaces had residual calculus when the
closed approach was used. However, despite
the increased visibility with the open
approach, the use of hand instruments at the
furcal areas was ineffective (Fleischer et al.
1989; Wylam et al., 1993).
Figure 3.1 shows an extracted maxillary
first molar which had received what was
defined as ‘deep cleaning’ by a general dental
practitioner. The tooth was extracted, since it
was deemed hopeless due to bone loss to the
apex and mobility degree III. The images
show very extensive deposits of calculus in
the furcation region.
Table 3.2 shows a summary of the studies
that evaluated the effectiveness of mechanical
and chemical debridement at multi‐rooted
teeth. It is obvious that there is a paucity of
available literature on the effectiveness of
mechanical debridement in furcation areas.
A systematic review of 13 randomized
controlled clinical trials revealed that there
(a)
(b)
(c)
Figure 3.1
The upper right maxillary molar (UR6) of a 53‐year‐old female patient affected by generalized
advanced chronic periodontitis, extracted as deemed hopeless due to bone loss to the apex and mobility
degree III. This tooth had received what was defined as ‘deep cleaning’ by a general dental practitioner.
However, the images show very extensive deposits of calculus in the furcation region, testifying to the
difficulty of achieving good subgingival debridement in furcation regions.

Table 3.2
Summary of studies that evaluated efficacy of mechanical debridement.
Author/
Year
Sample
Size
No. of Teeth
Study Design
Results
Matia et al.
1986
48
50 mandibular
molars with class II
or III FI
Curette (closed approach) vs curette
(open approach) vs ultrasonic (closed
approach) vs ultrasonic (open approach)
vs no treatment.
●
Closed approach: ultrasonic scaler and curette had
37.7% and 34.1% of residual calculus, respectively.
●
Open approach: ultrasonic scaler and curette had
1.0% and 2.7% of residual calculus, respectively.
●
More calculus was removed with the open
approach.
●
Ultrasonic scaling removed more calculus in
narrow furcations (<2.3 mm) compared to curette.
Leon and
Vogel 1987
6
33 maxillary and
mandibular molars
(class I, II, and III FI)
Gracey curettes vs ultrasonic scaler
(Cavitron® with P‐10 tip) vs no
treatment.
●
Ultrasonic scaling was significantly more effective
than curettes in reducing the bacterial counts for all
degrees of FI.
●
Ultrasonic scaling was more effective in the class II
and III FI.
Oda and
Ishikawa
1989
–
120 extracted
maxillary and
mandibular molars
Gracey curettes #11/12 and #13/14 vs
standard tip for ultrasonic scaler (ST‐08)
vs newly designed spherical tip (0.8 mm
diameter).
●
Mean % of residual marks that represents calculus:
15.1%, 50.3%, and 61.1% for newly designed tip,
conventional ultrasonic scaler tip, and Gracey
curettes, respectively, in maxillary molars (16.7%,
44.1%, and 39.5%, respectively, in mandibular molars).
●
The newly designed tip produced surfaces that
were as smooth as those produced by the Gracey
curettes.
●
The newly designed tip was more effective in
debriding the furcation area.
Patterson
et al. 1989
–
24 extracted
mandibular molars
mounted on a
typodont
Cavitron TFI 10 tip vs Cavitron EWPP
(Probe) tip vs Titan‐S Universal tip vs
Titan‐S Sickle tip.
●
Mean % of residual calculus: 13 mm
2
, 11 mm
2
,
9 mm
2
, and 8 mm
2
for Cavitron TFI 10 tip, Cavitron
EWPP (Probe) tip, Titan‐S Universal tip, and
Titan‐S Sickle tip (no significant differences).
●
On average 25–30% of calculus remained after
debridement.
●
No significant differences between the efficacy of
the four ultrasonic tips.
(Continued)
Chapter No.: 1 Title Name: <TITLENAME>
c03.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:19:19 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 42
Table 3.2
(Continued)
Author/
Year
Sample
Size
No. of Teeth
Study Design
Results
Scott et al.
1999
–
60 extracted
mandibular molars
Cavitron TFI‐10 tip vs Gracey curettes
vs fine‐ or medium‐grit diamond‐coated
ultrasonic tips.
●
Ultrasonic scaling was significantly faster than
hand curettes in calculus removal.
Parashis
et al. 1993b
23
30 mandibular
molars (60
furcations)
SRP with closed approach vs SRP with
open approach vs SRP with open
approach + rotary diamond
instrumentation.
●
The use of the rotary diamond tip significantly
removed more calculus in the furcation area.
Kocher
et al. 1996
–
24 acrylic molars
Universal curettes vs sonic scaler vs
modified sonic scaler with bud‐shaped
tips and different angulations vs
modified sonic scaler tips with plastic
coating.
●
Ultrasonic scaling was more effective than hand
instrumentation.
●
Specific angulations of the scaler tips were more
suited for distal furcations or the root of the
furcations.
●
Sonic scaler tips with plastic coatings appeared to
remove only plaque and thus were not suitable for
furcation debridement.
Kocher
et al. 1998a
–
15 extracted
maxillary and
mandibular molars
placed in a dummy
model
Curettes vs diamond bur and curettes vs
ultrasonic scaler vs sonic scaler vs
diamond‐coated sonic scaler tips
(angulated like Gracey curette #13/14
with 1.5 mm diameter and 45 microns
diamond grit size).
●
Ultrasonic and sonic scalers cleaned only 70% of
the root surfaces compared to the other groups
(85%).
●
Only the diamond‐coated sonic scaler managed to
effectively clean the maxillary molars (85% of the
root surfaces were clean compared to 75% by
curettes).
Kocher
et al. 1998b
–
15 extracted
maxillary and
mandibular molars
placed in a dummy
model
Curettes vs diamond‐coated sonic scaler
tips (angulated like Gracey curette
#13/14 and universal curette, with 1 mm
diameter and 15 microns diamond grit
size).
●
Mandibular molars were better debrided compared
to maxillary molars.
●
Diamond‐coated sonic scalers do damage the root
surfaces to the same degree as hand curettes (more
definitive notches were seen on the palatal roots).
●
Diamond‐coated sonic scaler tips with varying
angulations did improve root surface debridement.

Chapter No.: 1 Title Name: <TITLENAME>
c03.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:19:19 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 43
Table 3.2
(Continued)
Author/
Year
Sample
Size
No. of Teeth
Study Design
Results
Scott et al.
1999
–
60 extracted
mandibular molars
Cavitron TFI‐10 tip vs Gracey curettes
vs fine‐ or medium‐grit diamond‐coated
ultrasonic tips.
●
Ultrasonic scaling was significantly faster than
hand curettes in calculus removal.
Parashis
et al. 1993b
23
30 mandibular
molars (60
furcations)
SRP with closed approach vs SRP with
open approach vs SRP with open
approach + rotary diamond
instrumentation.
●
The use of the rotary diamond tip significantly
removed more calculus in the furcation area.
Kocher
et al. 1996
–
24 acrylic molars
Universal curettes vs sonic scaler vs
modified sonic scaler with bud‐shaped
tips and different angulations vs
modified sonic scaler tips with plastic
coating.
●
Ultrasonic scaling was more effective than hand
instrumentation.
●
Specific angulations of the scaler tips were more
suited for distal furcations or the root of the
furcations.
●
Sonic scaler tips with plastic coatings appeared to
remove only plaque and thus were not suitable for
furcation debridement.
Kocher
et al. 1998a
–
15 extracted
maxillary and
mandibular molars
placed in a dummy
model
Curettes vs diamond bur and curettes vs
ultrasonic scaler vs sonic scaler vs
diamond‐coated sonic scaler tips
(angulated like Gracey curette #13/14
with 1.5 mm diameter and 45 microns
diamond grit size).
●
Ultrasonic and sonic scalers cleaned only 70% of
the root surfaces compared to the other groups
(85%).
●
Only the diamond‐coated sonic scaler managed to
effectively clean the maxillary molars (85% of the
root surfaces were clean compared to 75% by
curettes).
Kocher
et al. 1998b
–
15 extracted
maxillary and
mandibular molars
placed in a dummy
model
Curettes vs diamond‐coated sonic scaler
tips (angulated like Gracey curette
#13/14 and universal curette, with 1 mm
diameter and 15 microns diamond grit
size).
●
Mandibular molars were better debrided compared
to maxillary molars.
●
Diamond‐coated sonic scalers do damage the root
surfaces to the same degree as hand curettes (more
definitive notches were seen on the palatal roots).
●
Diamond‐coated sonic scaler tips with varying
angulations did improve root surface debridement.
Author/
Year
Sample
Size
No. of Teeth
Study Design
Results
Kocher
and
Plagmann
1999
15
45 maxillary and
mandibular molars
OFD with hand curettes (Barnhart
curette #5/6 and Gracey curettes #7/8,
#11/12, and #13/14) vs diamond‐coated
sonic scaler.
●
Diamond‐coated sonic scalers cleaned two times
faster than hand curettes.
●
The initial reduction in probing depths at the
furcations was not maintained over time in both
groups.
Auplish
et al. 2000
–
Acrylic molars in
dummy head
Curettes (Gracey #11/12 and #13/14) vs
diamond‐coated sonic scaler vs sonic
scaler.
●
Diamond‐coated sonic scaler took the least time to
complete the debridement.
●
Diamond‐coated sonic scaler significantly removed
more calculus compared to the sonic scalers or
curettes.
Fleischer
et al. 1989
36
61
Curette (closed approach) by
experienced periodontist vs curette
(open approach) by experienced
periodontist vs ultrasonic (closed
approach) by less experienced residents
vs ultrasonic (open approach) by less
experienced residents vs no treatment.
●
Experienced periodontists: significantly greater
calculus‐free area (78%) in open approach vs closed
approach (36%).
●
Less experienced residents: significantly greater
calculus‐free area (45%) in open approach vs closed
approach (18%).
●
At the furcal areas, less experienced residents:
significantly greater calculus‐free surface with open
approach (43%) vs closed approach (8%).
●
At the furcal areas, no significant difference
between the open (68%) and closed (44%)
approaches for experienced periodontists.
Eschler and
Rapley 1991
–
90 extracted teeth
Grouping (1) curette (Columbia #13/14)
vs (2) curette (Columbia #13/14),
antiformin‐citric acid vs (3) ultrasonic
with P‐10 tip vs (4) ultrasonic with
diamond‐coated P‐10 tip vs (5) ultrasonic
with diamond‐coated P‐10
tip + antiformin‐citric acid vs (6)
ultrasonic with diamond‐coated P‐10 tip
and curette (Columbia #13/14) vs (7)
ultrasonic with diamond‐coated P‐10 tip
and curette (Columbia
#13/14) + antiformin‐citric acid vs (8)
antiformin‐citric acid vs (9) no treatment.
●
All groups that had mechanical debridement had
significantly less residual stains compared to groups
that had no treatment and antiformin‐citric acid
treatment (chemical root preparation did not
improve stain removal).
●
Debridement with ultrasonic with P‐10 tip:
significantly greater residual stains vs debridement
with diamond‐coated P‐10 tip and curette.
●
With unlimited access, all mechanical debridement
methods appeared to be equally effective.
(Continued)
Chapter No.: 1 Title Name: <TITLENAME>
c03.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:19:19 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 44
Table 3.2
(Continued)
Author/
Year
Sample
Size
No. of Teeth
Study Design
Results
Takacs
et al. 1993
–
100 extracted molars
with FI
Cavitron® ultrasonic with 0.8 mm ball tip
vs Cavitron ultrasonic with
EWP12R/12 L pointed tip vs ENAC
ultrasonic with furcation 0.8 mm ball tip
vs EVA contra‐angle reciprocating
handpiece with Per‐Io‐Tor #1 and #2 tips
(similar to threaded fissure bur) vs
Titan‐S sonic scaler with universal tip.
●
On average 74.2% of calculus was left behind at the
furca roof of mandibular molars after debridement
(the pointed tip and universal tip removed the most
calculus).
●
On average 76.4% of calculus was left behind at the
furca roof of maxillary molars after debridement
(Cavitron ball tip and universal tip removed the
most calculus).
●
Titan sonic scaler with universal tip and Cavitron
with ball tip were the most efficient at debriding
molars.
Wylam
et al. 1993
26
60 molars with class
II or III FI
Curettes (closed approach) vs curettes
(open approach) vs no treatment.
●
Molars after non‐surgical SRP: significantly greater
residual calculus (54.3%) vs open approach (33.0%).
●
At the furcal area, molars after non‐surgical SRP:
slightly greater residual calculus (93.2%) vs open
approach (91.1%).
●
Hand instrumentation was unable to effectively
clean the furcal area.
Otero‐
Cagide and
Lang
1997
–
100 artificial teeth
Curettes (Vision curvettes #11/12 and
#13/14) vs ultrasonic with EWP‐12 L‐R
scaler tip.
●
Debridement with curettes had significantly less
residual calculus compared to ultrasonics.
FI = furcation involvement; OFD = open‐flap debridement; SRP = scaling and root planing.

How Good are We at Cleaning Furcations? 45
was no evidence on the efficacy of powered
scalers in debriding multi‐rooted teeth. This
outcome needs to be interpreted with caution,
because out of the 13 studies, 7 evaluated
only single‐rooted teeth, and the remaining
6 studies focused on patient healing out-
comes. In addition, the instruments used in
the studies were conventional Gracey and
Columbia curettes and Cavitron ultrasonic
tips, which have thicker blade widths than the
instruments that are available today. The
evidence, however, did show that powered
scalers debride faster than hand curettes
(Tunkel et al. 2002).
In recent years, advancements in material
science have allowed curettes and ultrasonic
tips to be stronger and thinner than in past
decades. Gracey curettes are available in
different angulations, for example Gracey
curette #17/#18, or blade widths, for instance
Micro Mini Five® Gracey curettes. The newer
Micro Mini Five curettes have a shorter blade
length (2 mm) and thinner blade width
(0.6
mm) compared to regular Gracey
curettes, with a blade length and width of
5mm and 0.9mm respectively. A study of 100
artificial teeth used Vision curvettes that had
50% shorter blades and increased blade cur-
vature to debride the furcation of mandibular
molars. The authors reported that the modi-
fied blades were more effective than ultra-
sonic scalers in the debridement of the
furcation area (Otero‐Cagide and Long
1997). Besides curettes, ultrasonic tips are
now slimmer with improved angulations.
For example, the Cavitron ultrasonic tips
THINserts® are 47% thinner than the
Slimline® inserts (diameter 0.5 mm), with an
additional 9° backbend to gain better access
to the subgingival areas. The EMS Piezon
Master universal ultrasonic tips are 0.6 mm
in diameter, and are thus able to access most
furcation areas (<0.75 mm). Certain systems,
such as Kavo®, have an inbuilt light‐emitting
diode (LED) to improve visibility in the more
posterior areas of the oral cavity. All these
modifications aim to improve the access
of instruments into the furcation area
(Figures 3.2–3.5).
Other authors have also proposed the use
of chemotherapeutics, such as chlorhexidine,
essential oils, locally delivered tetracycline,
and doxycycline, to facilitate microbial
biofilm removal at the furcation areas, with
contradictory results. A more detailed review
of this topic is provided in Chapter 10.
Figures 3.6 and 3.7 show two cases of FI
with pre‐, intra‐, and post‐treatment views,
showing limitations in non‐surgical debride-
ment of furcation defects and tools used for
intrasurgical furcation debridement.
3.4 Patient Home Care
It has been shown that poor plaque control
results in suboptimal treatment outcome
(Rosling et al. 1976). The clinician can
perform effective instrumentation, but the
patient must be able to maintain the root
surface free of microbial biofilm. Numerous
tools are available for the patient to clean
their furcation‐involved multi‐rooted teeth.
These are manual and powered toothbrushes,
interspace brushes, interdental brushes, and
the WaterPik®. A recent Cochrane systematic
review conducted a meta‐analysis on 51 clini-
cal trials concluded that powered tooth-
brushes, compared to manual ones, were
more effective in reducing plaque and gingi-
val inflammation in both the short and long
term (Yaacob et al. 2014). Powered tooth-
brushes have different modes of action,
namely side to side, counter‐oscillation, rota-
tion oscillation, multi‐dimensional, and cir-
cular. The rotating oscillating powered
toothbrushes were found to significantly
reduce plaque and gingivitis in the short and
long term, with the greatest benefit at the lin-
gual surfaces (Klukowska et al. 2014a, b).
Another Cochrane review evaluated 17 rand-
omized controlled trials comparing the effi-
cacy of powered toothbrushes with different
modes of action (Deacon et al. 2010). It was
reported that powered toothbrushes with
the rotation oscillation motion performed
better than those with the side‐to‐side action.
However, limited studies with short follow‐up
Table 3.2
(Continued)
Author/
Year
Sample
Size
No. of Teeth
Study Design
Results
Takacs
et al. 1993
–
100 extracted molars
with FI
Cavitron® ultrasonic with 0.8 mm ball tip
vs Cavitron ultrasonic with
EWP12R/12 L pointed tip vs ENAC
ultrasonic with furcation 0.8 mm ball tip
vs EVA contra‐angle reciprocating
handpiece with Per‐Io‐Tor #1 and #2 tips
(similar to threaded fissure bur) vs
Titan‐S sonic scaler with universal tip.
●
On average 74.2% of calculus was left behind at the
furca roof of mandibular molars after debridement
(the pointed tip and universal tip removed the most
calculus).
●
On average 76.4% of calculus was left behind at the
furca roof of maxillary molars after debridement
(Cavitron ball tip and universal tip removed the
most calculus).
●
Titan sonic scaler with universal tip and Cavitron
with ball tip were the most efficient at debriding
molars.
Wylam
et al. 1993
26
60 molars with class
II or III FI
Curettes (closed approach) vs curettes
(open approach) vs no treatment.
●
Molars after non‐surgical SRP: significantly greater
residual calculus (54.3%) vs open approach (33.0%).
●
At the furcal area, molars after non‐surgical SRP:
slightly greater residual calculus (93.2%) vs open
approach (91.1%).
●
Hand instrumentation was unable to effectively
clean the furcal area.
Otero‐
Cagide and
Lang
1997
–
100 artificial teeth
Curettes (Vision curvettes #11/12 and
#13/14) vs ultrasonic with EWP‐12 L‐R
scaler tip.
●
Debridement with curettes had significantly less
residual calculus compared to ultrasonics.
FI = furcation involvement; OFD = open‐flap debridement; SRP = scaling and root planing.
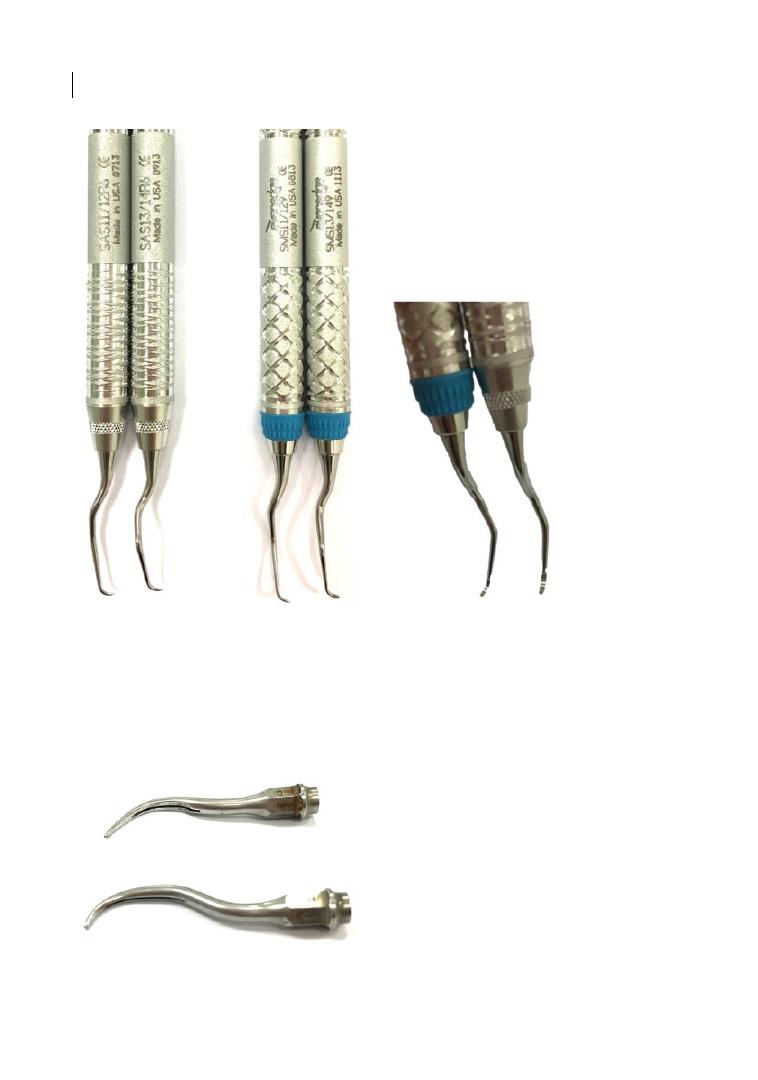
Chapter 3
46
periods (three months or less) and unclear
risk of bias were included. Therefore, the
results of the review should be interpreted
with caution.
There is only one study that evaluated the
efficacy of brushing instruments at the furcal
areas (Bader and Williams 1997). The authors
compared a pointed‐end tufted powered brush
and a small‐head powered toothbrush, and
found that the former was more effective in
removing plaque at the furcal area. The pointed
tip is able to fit into the furcal area to remove
the plaque. As the end‐tufted brush looks simi-
lar to the interspace brush, it might be inferred
that the interspace brush will be effective in
maintaining the furcation area free of biofilm.
Interdental brushes come in various sizes
and shapes. They can be conical or cylindrical
in shape, have angled or straight handles,
have regular nylon bristles or rubber bristles,
and be of different sizes. They are used to
(a)
(b)
(c)
Figure 3.2
(a) Mini Five® Gracey curettes #11/12 and #13/14 with blade width of 0.76 mm; (b) Micro Mini Five®
Gracey curettes #11/12 and #13/14 with blade width of 0.6 mm; (c) difference in the blade widths of the Micro
Mini Five Gracey curette #11/12 (left) and Mini Five Gracey curette #11/12 (right).
Figure 3.3
Traditional piezoelectric ultrasonic scaler
tips with a diameter of 0.7–0.8 mm.

How Good are We at Cleaning Furcations? 47
clean the interproximal surfaces or furcation
areas. A comparison between dental floss
and the interdental brush showed that the
latter was significantly more effective in
removing plaque (mean proximal plaque
score: 1.22 for interdental brush and 1.71 for
dental floss; Kiger et al. 1991), hence it is
better suited for cleaning proximal surfaces.
As evidenced by the significantly higher
bleeding and plaque scores, a short‐term
study found that conical interdental brushes
did not clean the lingual proximal surface as
well as their cylindrical counterparts (Larsen
et al. 2017). Also, straight brushes can clean
better than angled ones (Jordan et al. 2014).
It seemed that rubber‐bristled interdental
brushes were as effective as regular interden-
tal brushes in terms of plaque removal and
patients found them more comfortable to use
(Abouassi et al. 2014). Therefore, in patients
with periodontitis, and possibly interproxi-
mal FI, interdental brushes remove more
plaque than flossing or brushing alone
(Christou et al. 1998).
The WaterPik is an oral irrigator introduced
in 1962 that uses pulsating hydrodynamic
force to remove food debris from the tooth
surface. Its clinical benefits include removal of
subgingival bacteria, clinical and histological
reduction in gingival inflammation, down‐
regulation of pro‐inflammatory cytolines,
reduction of probing depths, improvement of
clinical attachment loss, being safe for gingival
tissues, and minimal bacteremia (Jolkovsky
and Lyle 2015; Cutler et al. 2000). Therefore,
in patients with less than ideal oral hygiene,
supragingival irrigation will flush out the sub-
gingival bacteria, reducing gingival inflamma-
tion to a degree greater than toothbrushing
alone (Research, Science and Therapy
Committee of the American Academy of
Periodontology 2001). In comparison to the
Sonicare Airfloss®, which uses a fluid spray of
micro‐bubbles to disrupt the plaque, the
WaterPik may be more effective in terms of
removing plaque and consequently reducing
the bleeding score (Goyal et al. 2015).
Evidence on home care of furcations is
scarce. As such, results from studies that
assessed interproximal cleaning was used to
infer the effectiveness of these brushes in
removing plaque from the tooth surfaces. It
appears that powered toothbrushes with the
rotation oscillation action, interspace
brushes, straight cylindrical interdental
brushes with rubber or nylon bristles, and
the WaterPik are effective in cleaning inter-
proximal areas and potentially furcations.
(a)
(b)
(c)
Figure 3.4
EMS Piezon® Master
ultrasonic scaler and tips: (a) PL1 tip
with a diameter of 0.5 mm for
debridement of hard‐to‐reach
interproximal areas; (b) PL5 tip with a
ball end of diameter 0.8 mm for
debridement of furcations and
concavities; (c) PS universal tip with a
diameter of 0.6 mm for debridement of
deep pockets.
Figure 3.5
Cavitron® Slimline™ insert with a
diameter of 0.5 mm.
Chapter No.: 1 Title Name: <TITLENAME>
c03.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:19:19 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 48
(a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
Figure 3.6
(a) The maxillary right first molar presented with a Grade I cervical enamel projection, 6 mm
pockets, and a class II furcation. (b) Periapical radiograph showed radiolucency in the furcation area.
(c) Cervical enamel projection was evident and seen protruding into the furcation. (d) Furcal area was debrided
with After‐5 Gracey curettes (Hu‐Friedy, USA) and Piezo ultrasonic scalers (EMS, Switzerland). (e) Newmeyer’s
rotary bur was used to remove the cervical enamel projection and any remaining granulomatous tissue.
(f) Clinical photo showed the defect after cleaning. (g) Defect was grafted with human cancellous allograft
(LifeNet, USA). (h) The graft was then protected with a well‐trimmed collagen membrane (2–3 mm beyond the
defect margin all around).
How Good are We at Cleaning Furcations? 49
(i)
(j)
(k)
(l)
(m)
Figure 3.6 (Continued)
(i) The flap was then coronally advanced and sutured. (j) At the two‐year post‐surgery
follow‐up visit, the probing pocket depth at the furcation area was reduced to 3 mm and (k) radiographic bone fill
was observed. (l) The furcation area remained stable clinically and (m) radiographically even at the four‐year post‐
surgery follow‐up visit. Further details and indications of furcation regenerative therapy are given in Chapter 7.
Chapter 3
50
(a)
(b)
(c)
(d)
Figure 3.7
The mandibular left second molar presented with a grade III cervical enamel projection, 6–8 mm
pockets, and a class III furcation. (a) Residual calculus observed on flap elevation, reflecting the ineffectiveness
of non‐surgical debridement. (b) Surgical open‐flap scaling and root planing with fine ultrasonic inserts
(Cavitron®, Dentsply, USA) and Gracey curettes (Hu‐Friedy, USA) were performed to achieve a smooth root
surface. The flap was repositioned and sutured apically, exposing the class III furcation involvement. (c) At the
two‐year post‐surgery follow‐up visit, there was recurrence of the pocket to 5 mm at the midlingual site (d)
with bleeding on probing, despite the patient using an interdental brush of the appropriate size and being on
a strict three‐monthly periodontal maintenance regimen. This therefore concurs with the literature that plaque
removal at the furcation area is unpredictable, even when exposed and visible.
Summary of Evidence
●
Longitudinal studies show that, compared
to single‐rooted teeth, furcation‐involved
teeth respond poorly to non‐surgical and
surgical treatment. Improvements in the
furcation area, if any, are also less sustain-
able over time.
●
Finer and angulated ultrasonic tips can
better debride the furcation area than
hand instruments, e.g. Gracey curettes.
●
There is limited evidence on home care of
furcations, however tools such as pow-
ered toothbrushes, interspace brushes,
interdental brushes, and the WaterPik
may be useful in removing plaque from
the furcation area.

How Good are We at Cleaning Furcations? 51
References
Abouassi, T., Woelber, J.P., Holst, K. et al.
(2014). Clinical efficacy and patients’
acceptance of a rubber interdental bristle:
A randomized controlled trial. Clinical Oral
Investigations 18, 1873–1880. doi: 10.1007/
s00784‐013‐1164‐3.
Auplish, G., Needleman, I.G., Moles, D.R., and
Newman, H.N. (2000). Diamond‐coated
sonic tips are more efficient for open
debridement of molar furcations: A
comparative manikin study. Journal of
Clinical Periodontology 27, 302–307.
Bader, H., and Williams, R. (1997). Clinical and
laboratory evaluation of powered electric
toothbrushes: Comparative efficacy of two
powered brushing instruments in furcations
and interproximal areas. Journal of Clinical
Dentistry 8, 91–94.
Biagini, G., Checchi, L., Miccoli, M.C. et al.
(1988). Root curettage and gingival repair in
periodontitis. Journal of Periodontology 59,
124–129. doi: 10.1902/jop.1988.59.2.124.
Booker, B.W., III, and Loughlin, D.M. (1985).
A morphologic study of the mesial root
surface of the adolescent maxillary first
bicuspid. Journal of Periodontology 56,
666–670. doi: 10.1902/jop.1985.56.11.666.
Bower, R.C. (1979a). Furcation morphology
relative to periodontal treatment: Furcation
entrance architecture. Journal of
Periodontology 50, 23–27. doi: 10.1902/
jop.1979.50.1.23.
Bower, R.C. (1979b). Furcation morphology
relative to periodontal treatment: Furcation
root surface anatomy. Journal of
Periodontology 50, 366–374. doi: 10.1902/
jop.1979.50.7.366.
Checchi, L., and Pelliccioni, G.A. (1988). Hand
versus ultrasonic instrumentation in the
removal of endotoxins from root surfaces in
vitro. Journal of Periodontology 59, 398–402.
doi: 10.1902/jop.1988.59.6.398.
Chiu, B.M., Zee, K.Y., Corbet, E.F., and
Holmgren, C.J. (1991). Periodontal
implications of furcation entrance
dimensions in Chinese first permanent
molars. Journal of Periodontology 62,
308–311. doi: 10.1902/jop.1991.62.5.308.
Christou, V., Timmerman, M.F., Van der
Velden, U., and Van der Weijden, F.A.
(1998). Comparison of different approaches
of interdental oral hygiene: Interdental
brushes versus dental floss. Journal of
Periodontology 69, 759–764. doi: 10.1902/
jop.1998.69.7.759.
Cutler, C.W., Stanford, T.W., Abraham, C. et al.
(2000). Clinical benefits of oral irrigation for
periodontitis are related to reduction of
pro‐inflammatory cytokine levels and
plaque. Journal of Clinical Periodontology
27, 134–143.
Dannewitz, B., Krieger, J.K., Husing, J., and
Eickholz, P. (2006). Loss of molars in
periodontally treated patients: A
retrospective analysis five years or more
after active periodontal treatment. Journal
of Clinical Periodontology 33, 53–61. doi:
10.1111/j.1600‐051X.2005.00858.x.
Dannewitz, B., Zeidler, A., Husing, J. et al.
(2016) Loss of molars in periodontally
treated patients: Results 10 years and more
after active periodontal therapy. Journal of
Clinical Periodontology 43, 53–62. doi:
10.1111/jcpe.12488.
Darveau, R.P., Tanner, A., and Page, R.C.
(1997). The microbial challenge in
periodontitis. Periodontology 2000 14,
12–32.
Deacon, S.A., Glenny, A.M., Deery, C. et al.
(2010). Different powered toothbrushes for
plaque control and gingival health. Cochrane
Database of Systematic Reviews 12 (Art. No.
CD004971). doi: 10.1002/14651858.
CD004971.pub2.
Del Peloso Ribeiro, E., Bittencourt, S., Nociti,
F.H., Jr. et al. (2007). Comparative study of
ultrasonic instrumentation for the non‐
surgical treatment of interproximal and
non‐interproximal furcation involvements.
Journal of Periodontology 78, 224–230. doi:
10.1902/jop.2007.060312.
dos Santos, K.M., Pinto, S.C., Pochapski, M.T.
et al. (2009). Molar furcation entrance and
its relation to the width of curette blades
used in periodontal mechanical therapy.
International Journal of Dental Hygiene 7,

Chapter 3
52
263–269. doi: 10.1111/j.1601‐5037.
2009.00371.x.
Eschler, B.M., and Rapley, J.W. (1991).
Mechanical and chemical root preparation
in vitro: Efficiency of plaque and calculus
removal. Journal of Periodontology 62,
755–760. doi: 10.1902/jop.1991.62.12.755.
Fleischer, H.C., Mellonig, J.T., Brayer, W.K.
et al. (1989). Scaling and root planing
efficacy in multirooted teeth. Journal of
Periodontology 60, 402–409. doi: 10.1902/
jop.1989.60.7.402.
Goyal, C.R., Lyle, D.M., Qaqish, J.G., and
Schuller, R. (2015). Efficacy of two
interdental cleaning devices on clinical signs
of inflammation: A four‐week randomized
controlled trial. Journal of Clinical Dentistry
26, 55–60.
Hajishengallis, G., and Lamont, R.J. (2012).
Beyond the red complex and into more
complexity: The polymicrobial synergy and
dysbiosis (PSD) model of periodontal
disease etiology. Molecular and Oral
Microbiology 27, 409–419.
Hirschfeld, L., and Wasserman, B. (1978). A
long‐term survey of tooth loss in 600 treated
periodontal patients. Journal of
Periodontology 49, 225–237. doi: 10.1902/
jop.1978.49.5.225.
Jolkovsky, D.L., and Lyle, D.M. (2015). Safety of
a water flosser: A literature review.
Compendium of Continuing Education in
Dentistry 36, 146–149.
Jordan, R.A., Hong, H.M., Lucaciu, A., and
Zimmer, S. (2014). Efficacy of straight versus
angled interdental brushes on interproximal
tooth cleaning: A randomized controlled
trial. International Journal of Dental Hygiene
12, 152–157. doi: 10.1111/idh.12042.
Kalkwarf, K.L., Kaldahl, W.B., and Patil, K.D.
(1988). Evaluation of furcation region
response to periodontal therapy. Journal of
Periodontology 59, 794–804. doi: 10.1902/
jop.1988.59.12.794.
Kiger, R.D., Nylund, K., and Feller, R.P.
(1991). A comparison of proximal plaque
removal using floss and interdental
brushes. Journal of Clinical Periodontology
18, 681–684.
Klukowska, M., Grender, J.M., Conde, E. et al.
(2014a). A randomized 12‐week clinical
comparison of an oscillating‐rotating
toothbrush to a new sonic brush in the
reduction of gingivitis and plaque. Journal of
Clinical Dentistry 25, 26–31.
Klukowska, M., Grender, J.M., Conde, E. et al.
(2014b). A six‐week clinical evaluation of
the plaque and gingivitis efficacy of an
oscillating‐rotating power toothbrush with a
novel brush head utilizing angled CrissCross
bristles versus a sonic toothbrush. Journal of
Clinical Dentistry 25, 6–12.
Kocher, T., and Plagmann, H.C. (1999). Root
debridement of molars with furcation
involvement using diamond‐coated sonic
scaler inserts during flap surgery: A pilot study.
Journal of Clinical Periodontology 26, 525–530.
Kocher, T., Gutsche, C., and Plagmann, H.C.
(1998a). Instrumentation of furcation with
modified sonic scaler inserts: Study on
manikins, part I. Journal of Clinical
Periodontology 25, 388–393.
Kocher, T., Ruhling, A., Herweg, M., and
Plagman, H.C. (1996). Proof of efficacy of
different modified sonic scaler inserts used
for debridement in furcations: A dummy
head trial. Journal of Clinical Periodontology
23, 662–669.
Kocher, T., Tersic‐Orth, B., and Plagmann,
H.C. (1998b). Instrumentation of furcation
with modified sonic scaler inserts: A study
on manikins, part II. Journal of Clinical
Periodontology 25, 451–456.
Larsen, H.C., Slot, D.E., Van Zoelen, C. et al.
(2017). The effectiveness of conically shaped
compared with cylindrically shaped
interdental brushes: A randomized
controlled clinical trial. International
Journal of Dental Hygiene 13 (3), 211–218.
doi: 10.1111/idh.12189.
Leon, L.E., and Vogel, R.I. (1987). A
comparison of the effectiveness of hand
scaling and ultrasonic debridement in
furcations as evaluated by differential dark‐
field microscopy. Journal of Periodontology
58, 86–94. doi: 10.1902/jop.1987.58.2.86.
Lindhe, J., Westfelt, E., Nyman, S. et al. (1982).
Healing following surgical/non‐surgical

How Good are We at Cleaning Furcations? 53
treatment of periodontal disease: A clinical
study. Journal of Clinical Periodontology 9,
115–128.
Loe, H., Theilade, E., and Jensen, S.B. (1965).
Experimental gingivitis in man. Journal of
Periodontology 36, 177–187. doi: 10.1902/
jop.1965.36.3.177.
Loos, B., Claffey, N., and Egelberg, J. (1988).
Clinical and microbiological effects of root
debridement in periodontal furcation pockets.
Journal of Clinical Periodontology 15, 453–463.
Loos, B., Nylund, K., Claffey, N., and Egelberg,
J. (1989). Clinical effects of root
debridement in molar and non‐molar teeth:
A 2‐year follow‐up. Journal of Clinical
Periodontology 16, 498–504.
Matia, J.I., Bissada, N.F., Maybury, J.E., and
Ricchetti, P. (1986). Efficiency of scaling of the
molar furcation area with and without surgical
access. International Journal of Periodontics
and Restorative Dentitry 6, 24–35.
McFall, W.T., Jr (1982). Tooth loss in 100
treated patients with periodontal disease: A
long‐term study. Journal of Periodontology
53, 539–549. doi: 10.1902/jop.1982.53.9.539.
Nordland, P., Garrett, S., Kiger, R. et al. (1987).
The effect of plaque control and root
debridement in molar teeth. Journal of
Clinical Periodontology 14, 231–236.
Oda, S., and Ishikawa, I. (1989). In vitro
effectiveness of a newly‐designed ultrasonic
scaler tip for furcation areas. Journal of
Periodontology 60, 634–639. doi: 10.1902/
jop.1989.60.11.634.
Oda, S., Nitta, H., Setoguchi, T. et al. (2004).
Current concepts and advances in manual
and power‐driven instrumentation.
Periodontology 2000 36, 45–58. doi:
10.1111/j.1600‐0757.2004.03674.x.
Otero‐Cagide, F.J., and Long, B.A. (1997).
Comparative in vitro effectiveness of closed
root debridement with fine instruments on
specific areas of mandibular first molar
furcations. II. Furcation area. Journal of
Periodontology 68, 1098–1101. doi: 10.1902/
jop.1997.68.11.1098.
Page, R.C., and Kornman, K.S. (1997). The
pathogenesis of human periodontitis: An
introduction. Periodontology 2000 14, 9–11.
Parashis, A.O., Anagnou‐Vareltzides, A., and
Demetriou, N. (1993a). Calculus removal
from multirooted teeth with and without
surgical access. I: Efficacy on external and
furcation surfaces in relation to probing
depth. Journal of Clinical Periodontology 20,
63–68.
Parashis, A.O., Anagnou‐Vareltzides, A., and
Demetriou, N. (1993b). Calculus removal
from multirooted teeth with and without
surgical access. II: Comparison between
external and furcation surfaces and effect of
furcation entrance width. Journal of Clinical
Periodontology 20, 294–298.
Patterson, M., Eick, J.D., Eberhart, A.B. et al.
(1989). The effectiveness of two sonic and
two ultrasonic scaler tips in furcations.
Journal of Periodontology 60, 325–329. doi:
10.1902/jop.1989.60.6.325.
Pihlstrom, B.L., Oliphant, T.H., and McHugh,
R.B. (1984). Molar and nonmolar teeth
compared over 6½ years following two
methods of periodontal therapy. Journal of
Periodontology 55, 499–504. doi: 10.1902/
jop.1984.55.9.499.
Ramfjord, S.P., Caffesse, R.G., Morrison, E.C.
et al. (1987). 4 modalities of
periodontal treatment compared over 5
years. Journal of Clinical Periodontology
14, 445–452.
Ramfjord, S.P., Nissle, R.R., Shick, R.A., and
Cooper, H., Jr (1968). Subgingival curettage
versus surgical elimination of periodontal
pockets. Journal of Periodontology 39,
167–175.
Research, Science and Therapy Committee of
the American Academy of Periodontology
(2001). Treatment of plaque‐induced
gingivitis, chronic periodontitis, and other
clinical conditions. Journal of Periodontology
72, 1790–1800. doi: 10.1902/
jop.2001.72.12.1790.
Research, Science and Therapy Committee of
the American Academy of Periodontology
(2005). Position paper: The role of supra‐
and subgingival irrigation in the treatment
of periodontal diseases. Journal of
Periodontology 76, 2015–2027. doi: 10.1902/
jop.2005.76.11.2015.

Chapter 3
54
Rosling, B., Nyman, S., and Lindhe, J. (1976).
The effect of systematic plaque control on
bone regeneration in infrabony pockets.
Journal of Clinical Periodontology 3, 38–53.
Ross, I.F., and Thompson, R.H., Jr (1978).
A long term study of root retention in the
treatment of maxillary molars with furcation
involvement. Journal of Periodontology 49,
238–244. doi: 10.1902/jop.1978.49.5.238.
Schatzle, M., Loe, H., Burgin, W. et al. (2003).
Clinical course of chronic periodontitis.
I: Role of gingivitis. Journal of Clinical
Periodontology 30, 887–901.
Scott, J.B., Steed‐Veilands, A.M., and Yukna,
R.A. (1999). Improved efficacy of calculus
removal in furcations using ultrasonic
diamond‐coated inserts. International
Journal of Periodontics and Restorative
Dentistry 19, 355–361.
Smart, G.J., Wilson, M., Davies, E.H., and
Kieser, J.B. (1990). The assessment of
ultrasonic root surface debridement by
determination of residual endotoxin levels.
Journal of Clinical Periodontology 17,
174–178.
Socransky, S.S., and Haffajee, A.D. (2005).
Periodontal microbial ecology.
Periodontology 2000 38, 135–187. doi:
10.1111/j.1600‐0757.2005.00107.x.
Sugaya, T., Kawanami, M., and Kato, H. (2002).
Effects of debridement with an ultrasonic
furcation tip in degree II furcation
involvement of mandibular molars. Journal
of the International Academy of
Periodontology 4, 138–142.
Tagge, D.L., O’Leary, T.J., and El‐Kafrawy, A.H.
(1975). The clinical and histological
response of periodontal pockets to root
planing and oral hygiene. Journal of
Periodontology 46, 527–533. doi: 10.1902/
jop.1975.46.9.527.
Takacs, V.J., Lie, T., Perala, D.G., and Adams,
D.F. (1993). Efficacy of 5 machining
instruments in scaling of molar furcations.
Journal of Periodontology 64, 228–236. doi:
10.1902/jop.1993.64.3.228.
Tunkel, J., Heinecke, A., and Flemmig, T.F.
(2002). A systematic review of efficacy of
machine‐driven and manual subgingival
debridement in the treatment of chronic
periodontitis. Journal of Clinical
Periodontology 29 (Suppl. 3), 72–81;
discussion 90–91.
Wang, H.L., Burgett, F.G., Shyr, Y., and
Ramfjord, S. (1994). The influence of molar
furcation involvement and mobility on
future clinical periodontal attachment loss.
Journal of Periodontology 65, 25–29. doi:
10.1902/jop.1994.65.1.25.
Wood, W.R., Greco, G.W., and McFall, W.T., Jr
(1989). Tooth loss in patients with moderate
periodontitis after treatment and long‐term
maintenance care. Journal of Periodontology
60, 516–520. doi: 10.1902/jop.1989.60.9.516.
Wylam, J.M., Mealey, B.L., Mills, M.P. et al.
(1993). The clinical effectiveness of open
versus closed scaling and root planing on
multi‐rooted teeth. Journal of
Periodontology 64, 1023–1028. doi: 10.1902/
jop.1993.64.11.1023.
Yaacob, M., Worthington, H.V., Deacon, S.A.
et al. (2014). Powered versus manual
toothbrushing for oral health. Cochrane
Database of Systematic Reviews 6 (Art. No.:
CD002281). doi:10.1002/14651858.
CD002281.pub3.

Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
Chapter No.: 1 Title Name: <TITLENAME>
c04.indd
Comp. by: KJAYASEELAN Date: 14 May 2018 Time: 04:19:46 PM Stage: Proof WorkFlow:
CSW
Page Number: 55
55
4.1 Introduction
To avoid contact with the exogenous sub-
stances of the oral cavity, pulp and dentine are
genetically protected by the overlying enamel
and cementum. Despite these defensive phys-
ical barriers, the pulp can be threatened by
manifold insults, such as caries, restorative
procedures, and mechanical, chemical, and
thermal trauma. In periodontitis‐affected
patients, periodontal pathogens can induce
pulp infection because of the vascular inter-
connections between periodontium and
endodontium. Through the accessory canals
or exposed dentinal tubules, bacteria and tox-
ins might gain the access to the pulp. The
effect is generally atrophy and is comparable
with ‘pulp aging’. Likewise, endodontic infec-
tions can influence periodontal health. When
the noxae of degenerated pulp involve the
supporting periodontium, rapid inflamma-
tory responses, characterized by bone loss,
tooth mobility, and/or sinus tract formation,
might develop. These clinical conditions
where both endodontium and periodontium
are simultaneously affected in what appears
to be a single periodontal lesion are known as
endodontic‐periodontal lesions.
Despite the topic of the book regarding
exclusively furcation pathology, the ethi-
opathogenesis of endodontic‐periodontal
disease cannot be described by merely
considering the inter‐radicular space only.
Therefore, the present chapter aims to com-
prehensively detail the aetiology and devel-
opment of endodontic‐periodontal lesions,
with particular emphasis on the diagnosis,
management, and long‐term prognoses of
the affected teeth, especially when the furca-
tion region is involved.
4.2 Pathways Between
Endodontium and
Periodontium: Anatomical
Considerations
Because of the anatomical and vascular inter-
connections, periodontium and endodon-
tium can influence each other during
function and should therefore be considered
as one biological unit. The main pathways
involved in the development of endodontic‐
periodontal lesions are the dentinal tubules,
the lateral and accessory canals, and the
apical foramen or foramina (Seltzer et al. 1963).
4.2.1 Dentinal Tubules
Crown and root dentinal tubules, which
extend from the pulp to the amelodentinal
and dentinocemental junctions, respectively,
are permeable structures. Their permeability
varies with regard to the dentine type, tooth
Chapter 4
Furcation: The Endodontist’s View
Federica Fonzar and Riccardo Fabian Fonzar
Private practice, Udine, Italy

Chapter 4
56
area, and functional tubular diameter
(Pashley 1990).
The root dentine is less patent than the
coronal. The number of tubules generally
ranges from approximately 42 000/mm
2
in
the cervical area to about 8000/mm
2
in the
radicular. By the lower permeability, roots
and furcation dentine act as a real protective
barrier (Rapp et al. 1992). From the outer to
the closer surfaces to the pulp, tubules, which
follow an S‐shaped path, are denser, wider,
more patent, and therefore greater in flow
rate (Ghazali 2003). With ageing or as a
response to continuous low‐grade stimuli,
diameters and patency might decrease
through the apposition of highly mineralized
peritubular dentine.
On healthy teeth, enamel and cementum
usually prevent the pulpo‐dentinal complex
from contact with the oral cavity micro‐
organisms. Owing to developmental defects,
caries, trauma, restorative procedures, or
periodontal disease, cementum might not
cover the underlying dentine any longer, and
the exposed dentinal tubules might serve as
communication pathways between the endo-
dontium and periodontium (Adriaens et al.
1988; Love and Jenkinson 2002). Bacteria and
bacterial products can therefore induce
pulpal reactions by migrating towards the
pulp (Langeland et al. 1974; Bergenholtz
1981; Adriaens et al. 1988). By colonizing the
root dentine tubules of periodontally diseased
teeth, pathogens might act as a reservoir for
pocket recolonization after debridement
(Adriaens et al. 1987). As proof of this, micro-
biological investigations revealed the presence
of Gram‐negative and Gram‐positive species
in the root dentine (Adriaens et al. 1988;
Guiliana et al. 1997).
While it has been proved that bacteria are
able to invade radicular dentine from the
periodontal pocket, it remains unclear
whether bacteria invade the healthy cemen-
tum before penetrating the dentine, or reach
the root dentine through breaches in the
cementum layer (Adriaens et al. 1987, 1988;
Guiliana et al. 1997; Love and Jenkinson
2002). Cementum is a thin, often discontinuous
layer that commonly shows surface defects,
for instance in the sites where Sharpey’s
fibres attach to its matrix (Adriaens et al.
1987). Its exposure to crevicular fluid, bacte-
rial enzymes, or acidic metabolites might
induce physicochemical and structural alter-
ations, such as localized resorptive lacunae
or demineralization (Daly et al. 1982;
Adriaens et al. 1987). It can be speculated,
therefore, that cementum might be structur-
ally damaged by physiological, bacterial, and
environmental factors, and that this altera-
tion might facilitate bacterial penetration
within the exposed root of periodontally
diseased teeth.
The vitality of the tooth is another variable
that might play an important role in hinder-
ing bacteria migration towards the pulp. By
exposing the dentine surface to the oral envi-
ronment for 150 days, bacterial invasion
occurs faster in non‐vital rather than vital
teeth (Nagaoka et al. 1995). A possible expla-
nation of this finding might be sought in the
resistance offered by outward dentinal fluid
movement and the presence of odontoblast
processes in the tubules of vital teeth
(Vongsavan and Matthews 1991, 1992;
Pashley et al. 2002). In addition, antibodies
and anti‐microbial components contained
within the dentinal fluid might also help the
vital teeth to be more properly defended
(Hahn and Overton 1997).
4.2.2 Lateral and Accessory
Canals
An accessory canal is any branch starting
from the pulp chamber or the main root
canal that communicates with the external
surface of the root. When the location is
the coronal or middle third of the root,
and the orientation is horizontal with respect
to the main canal, accessory canals are named
lateral canals (American Association of
Endodontists 2015).
It is estimated that 30–40% of teeth exhibit
accessory canals, most of which are found in
the apical third of the root (De Deus 1975).
Their prevalence might vary within the teeth,
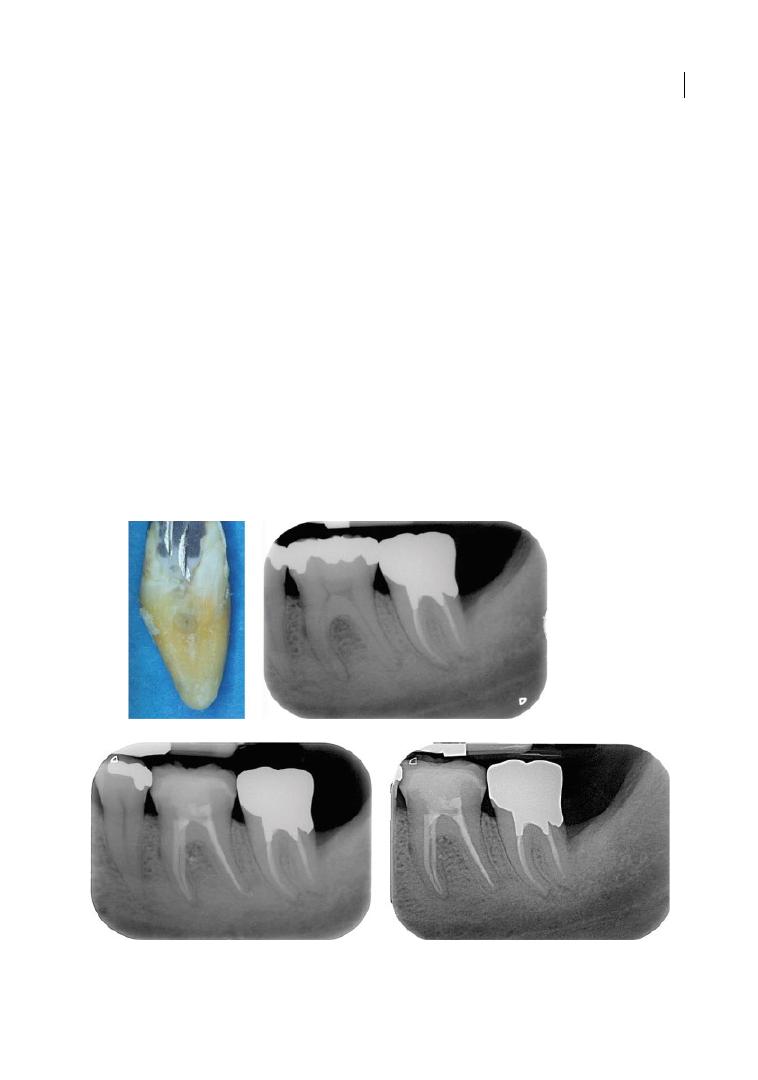
The Endodontist’s View 57
and was seen to be greater in mandibular
molars and premolars than in maxillary
molars and lateral incisors (Kirkham 1975).
In addition, it can also change according to
the root third analysed. In fact, De Deus
(1975) found that 17%, 9%, and less than 2%
of teeth showed accessory canals in the api-
cal, middle, and coronal third, respectively.
With regard to the number of accessory
canals per tooth, 17% and 6% of teeth have
one or two accessory canals (Kirkham 1975).
Despite these anatomical considerations, the
prevalence of periodontal disease associated
with accessory canals seems to be relatively
low (Rotstein and Simon 2004).
In multi‐rooted teeth, furcation dentine
might represent a communication pathway
between endodontium and periodontium. In
fact, the vascular system of the pulp is con-
nected to that of periodontium through the
accessory canals. At the furcation, their
prevalence generally ranges from 23 to 76%
(Lowman et al. 1973; Burch and Hulen 1974;
Goldberg et al. 1987), but the extension rarely
covers the entire distance between the pulp
chamber and the furcation floor (Goldberg
et al. 1987) and only 30–60% of molars have
patent canals connecting the main root canal
system and the periodontal ligament (see
Figure 4.1). In particular, mandibular molars
have a higher incidence (56%) than maxillary
(48%) (Lowman et al. 1973; Gutmann 1978;
Vertucci 2005). Because of these interconnec-
tions, pulp inflammation might have detrimental
consequences on the furcation by inducing
inflammatory responses on the inter‐radicular
periodontal tissues (Seltzer et al. 1963).
4.2.3 Apical Foramen or Foramina
The major connections between periodontal
and pulp tissues are the apical foramina. The
(a)
(b)
(c)
(d)
Figure 4.1
Accessory canal located distally to the mesial root on 4.6 (LR6) (a). Interradicular radiolucency on
3.6 (LL6) (b). After root canal therapy, the accessory canal located distally to the mesial root was filled with
cement (c). Complete remineralization after four‐year follow‐up (d).
Chapter 4
58
morphology of the apex might be quite varia-
ble. All the teeth have at least one accessory
foramen. Generally, there are fewer primary
dentinal tubules in the apical root third than
in the coronal dentine. Their direction and
density might be somewhat irregular, and
some areas can be completely free of tubules
(Mjör et al. 2001). Maxillary premolars have
the most complicated apical morphology with
the largest accessory foramina, followed by
maxillary and mandibular molars (Marroquin
et al. 2004), and this makes the prognosis of
endodontic therapy in premolars and molars
more uncertain than for other teeth.
4.2.4 Non‐physiological
Communications
Root perforation, vertical root fracture, and
inflammatory root resorption are artificial
pathways between periodontal and pulpal
tissues.
Iatrogenic root canal perforations are
serious complications that originate from
manual/rotatory instrumentation or post‐
space preparation, and can threaten the
prognosis of the tooth (Tsesis et al. 2010;
Gorni et al. 2016). The treatment outcome
depends on several factors that should be
evaluated early, such as the size and position
of the root perforation, the time elapsed
before making the diagnosis and treatment,
the degree of periodontal involvement, and
the sealing ability and biocompatibility of the
sealant. The faster the sealing, the more con-
trolled the infection. Hence, time elapsed
before treatment seems to be crucial for
success. Among sealants, reinforced zinc
oxide‐eugenol cements and bioceramic mate-
rials, such as mineral trioxide aggregate, are
used most for this purpose (Weldon et al.
2002; Parirokh and Torabinejad 2010;
Haapasalo et al. 2015; Gorni et al. 2016).
Vertical root fractures (see Figures 4.2 and
4.3) are generally caused by loading trauma
and occur more frequently in non‐vital teeth
(Chan et al. 1999; Sugaya et al. 2015). In vital
teeth, vertical fractures can initiate coronally
in ‘cracked tooth syndrome’ (Cameron 1964)
or can involve the root only (Chan et al. 1999;
Sugaya et al. 2015). While in the past endo-
dontically treated teeth were considered to
be weaker due to structural changes in den-
tine composition, such as water and collagen
cross‐linking loss, currently it is believed that
the greater brittleness is linked to the loss of
structural integrity. In fact, the extension
of cavity access might influence the degree of
cuspal deflection during function, increasing
the risk of fracture. Furthermore, a history of
extensive restorations, especially in mandib-
ular posterior teeth, might make the tooth
even more susceptible to fracture, especially
in elderly patients (Lewinstein and Grajower
1981; Huang et al. 1992; Cheron et al. 2011;
Faria et al. 2011).
Root resorption is a pathological process
associated with dentine, cementum, and/or
(a)
(b)
Figure 4.2
Vertical root fracture starting from the apex on 2.7 (UL7). The lesion resembled an endodontic
infection (a). Complete failure after two years follow‐up (b). Root resection was not possible due to
unfavourable anatomy.

The Endodontist’s View 59
bone loss. It can be external or internal,
depending on whether the origin is the peri-
odontium or the pulp. The aetiopathogenesis
is far from being completely understood.
Mechanical and infective factors such as
orthodontic treatment, trauma, intracoronal
bleaching, periodontitis, and thermal stimuli
might be considered as predisposing factors.
The presence of profuse bleeding on prob-
ing, granulation tissue, and hard cavity
bottom might confirm the diagnosis of exter-
nal inflammatory root resorption. Electric
and cold pulp tests might be positive.
However, sensitivity tests alone do not differ-
entiate this pathological process from dental
caries or internal resorption. Radiographic
evaluation reveals that the canal profile is
well defined in internal resorption, and
rather undefined and faded in external.
External resorption progression (see
Figure 4.4) can lead to the invasion of the
pulp space as a last resort. Likewise, untreated
internal resorption can establish a communi-
cation between endodontium and periodon-
tium by breaking the external root surface
(Tronstad 1988; Trope 1998; Andreasen and
Andersson 2007; Patel et al. 2010).
4.3 Bacteria Involved in
Endodontic‐periodontal Disease
Periodontal diseases are mixed anaerobic
infections, modulated by a complex interplay
between local and host factors (Page 1999).
Similarly, endodontic infection has an anaer-
obic nature. Most of the species found in
infected root canals are also present in perio-
dontal pockets. However, the endodontic
biofilm seems to be less complex than the
periodontal biofilm (Trope et al. 1988;
Kobayashi et al. 1990; Sundqvist 1994;
Kurihara et al. 1995; Zehnder et al. 2002).
Root canal infection is a dynamic process,
and different bacterial species can apparently
prevail at different stages. The most prevalent
named species detected in primary endodon-
tic infections, including abscessed cases,
belong to diverse genera of Gram‐negative
(Fusobacterium, Dialister, Porphyromonas,
Prevotella,
Tannerella,
Treponema,
Campylobacter, and Veillonella) and Gram‐
positive bacteria (Parvimonas, Filifactor,
Pseudoramibacter, Olsenella, Actinomyces,
Peptostreptococcus, Streptococcus, Propioni
bacterium, and Eubacterium). Conversely,
the microflora changes if endodontic therapy
fails. Several culture and molecular biology
studies revealed that Enterococcus faecalis is
the most frequent species in root canal–
treated teeth, with a prevalence of up to 90%
of cases and a strong association with persis-
tent infections (Rôças et al. 2004; Mohammadi
et al. 2013). Canals that are apparently well
treated might contain from 1 to 5 bacterial
species; however, in those not properly
treated, the number might vary from 10 to 30,
which is very similar to that of untreated
canals (Sundqvist et al. 1998; Pinheiro et al.
2003; Sakamoto et al. 2008).
(a)
(b)
(c)
Figure 4.3
Vertical fracture of the distal root on 3.6 (LL6) (a). Rizectomy of the distal root (b) and after healing (c).
Chapter 4
60
4.4 Relationship Between
Periodontal Disease and
Histological Pulp Changes
Many controversies still exist about the rela-
tionship between periodontal inflammation
and pulp health (see Table 4.1). However, it
seems to be widely accepted that periodontitis
and periodontal therapy can induce pathologi-
cal changes in the pulp. Periodontal disease
progression can lead to the exposure and bacte-
rial contamination of the accessory canals,
more frequent in the apical third of the tooth
and at the furcation (Seltzer et al. 1963; Rubach
and Mitchell 1965), or it can reach the root
apex with subsequent neuro‐vascular bundle
damage (Langeland et al. 1974; De Deus 1975).
Cementum removal, resulting from scaling and
root planing, can expose the dentinal tubules
and accessory canals (Adriaens et al. 1988),
therefore the micro‐organisms can migrate
towards the pulp, inducing hystological
changes (Rubach and Mitchell 1965; see
Figure 4.5). However, the deposition of repara-
tive dentine (Bergenholtz and Lindhe 1978;
Nilvéus and Selvig 1983; Hattler and Listgarten
1984), the outward movement of the dentinal
fluid (Vongsavan and Matthews 1991, 1992;
Pashley et al. 2002), the presence of odonto-
blast processes in the tubules (Nagaoka et al.
1995; Pasley et al. 2002), and the presence of
antibodies and anti‐microbial components
within the dentinal fluid (Hahn and Overton
1997) can act as a defense system, preventing
the bacteria from reaching the pulp. In refer-
ence to this, we should stress that, as mentioned
in Chapter 3, subgingival debridement is now
moving away from the concept of ‘root planing’
and ‘removal of all diseased cementum’ (Aleo
et al. 1974) towards an emphasis on disruption
of the subgingival biofilm, with minimal altera-
tion to the cementum (Nibali et al. 2015).
Figure 4.4
Disto‐lingual external progressive root resorption on 4.7 (LR7). Initally, the invisible resorption
induced pulpitis and root canal therapy was performed.
Chapter No.: 1 Title Name: <TITLENAME>
c04.indd
Comp. by: KJAYASEELAN Date: 14 May 2018 Time: 04:19:46 PM Stage: Proof WorkFlow:
CSW
Page Number: 61
Table 4.1
Effect of periodontal disease on pulp tissue.
Do progressive periodontitis and periodontal
treatment affect the pulp?
Pulp damage
Pulp response
Progressive
periodontal
disease
Yes
Apex
Apical foramen
(Langeland et al. 1974 ;
Harrington et al. 2002 ;
Sheykhrezaee et al. 2007 ;
Aguiar et al. 2014 ; Rathod
et al. 2014 )
Neuro‐vascular
bundle damage
Main canal involved
Irreversible
Inflammatory
Degenerative
(Rubach and Mitchell 1965 ; Zehnder 2001 ;
Sheykhrezaee et al. 2007 ; Aguiar et al. 2014 ;
Rathod et al. 2014 )
Complete necrosis
Apical root third
(Rubach and Mitchell 1965 ;
Adriaens et al. 1988 ;
Sheykhrezaee et al. 2007 ;
Zuza et al. 2012 )
Bacteria and toxins
migrate towards the
pulp through the
accessory canals
Irreversible
or
Reversible
Inflammatory
Degenerative
(Rubach and Mitchell 1965 ; Sheykhrezaee et al.
2007 ; Rathod et al. 2014 )
Fibrosis
Calcification
Inflammation
Odontoblast integrity loss
Neuro‐vascular alteration
Partial/complete necrosis
Furcation
(Rubach and Mitchell 1965 ;
Bender and Seltzer 1972 ;
Adriaens et al. 1988 ; Zuza
et al. 2012 )
Reparative
(Mazur and Massler 1964 ; Bender and Seltzer
1972 ; Langeland et al. 1974 ; Lantelme et al.
1976 ; Bergenholtz and Lindhe 1978 ; Ross and
Thompson 1978 ; Czarnecki and Schilder 1979 ;
Torabinejad and Kiger 1985 ; Cortellini and
Tonetti 2001 ; Harrington et al. 2002 ; Aguiar
et al. 2014)
Calcification
Fibrosis
Vascular
alteration
Nerve
alteration
≈ pulp ageing
Periodontal
treatment
(scaling and
root planing)
Yes
(Rubach and Mitchell 1965 ;
Adriaens et al. 1988 )
Neuro‐vascular
bundle damage
Main canal involved
Irreversible
Inflammatory
Degenerative
(Rubach and Mitchell 1965 ; Sheykhrezaee et al.
2007 ; Rathod et al. 2014 )
Complete necrosis
(Continued )
Chapter No.: 1 Title Name: <TITLENAME>
c04.indd
Comp. by: KJAYASEELAN Date: 14 May 2018 Time: 04:19:46 PM Stage: Proof WorkFlow:
CSW
Page Number: 62
Do progressive periodontitis and periodontal
treatment affect the pulp?
Pulp damage
Pulp response
Cementum removal:
bacteria and toxins
migrate towards the
pulp through the
accessory canals and
the exposed dentinal
tubules
Irreversible
or
Reversible
Inflammatory
Degenerative
(Rubach and Mitchell 1965 ; Sheykhrezaee et al.
2007 ; Rathod et al. 2014 )
Fibrosis
Calcification
Inflammation
Odontoblast integrity loss
Vascular alteration
Partial/complete necrosis
Reparative
(Mazur and Massler 1964 ; Bender and Seltzer
1972 ; Langeland et al. 1974 ; Lantelme et al. 1976 ;
Bergenholtz and Lindhe 1978 ; Ross and
Thompson 1978 ; Czarnecki and Schilder 1979 ;
Torabinejad and Kiger 1985 ; Cortellini and
Tonetti 2001 ; Harrington et al. 2002 ; Aguiar et al.
2014 )
Calcification
Fibrosis
Vascular
alteration
Nerve
alteration
≈ pulp ageing
No
(Bergenholtz and Lindhe
1978 ; Nilvéus and Selvig
1983 ; Hattler and
Listgarten 1984 ; Nagaoka
et al. 1995 ; Hahn and
Overton 1997 ; Pashley
et al. 2002 )
No bacteria and
toxins migrate
towards the pulp
through the
accessory canals and
the exposed dentinal
tubules
Reversible
Reparative dentine (Bergenholtz and Lindhe 1978 ; Hattler and Listgarten 1984 ;
Vongsavan and Matthews 1991 )
+
Dentinal fluid (Vongsaven and Matthews 1991, 1992; Pashley et al. 2002 )
+
Odontoblast processes (Nagaoka et al. 1995 ; Pashley et al. 2002 )
Antibodies/antimicrobial components within the dentinal fluids
(Hahn and Overton 1997 )
Table 4.1
(Continued)
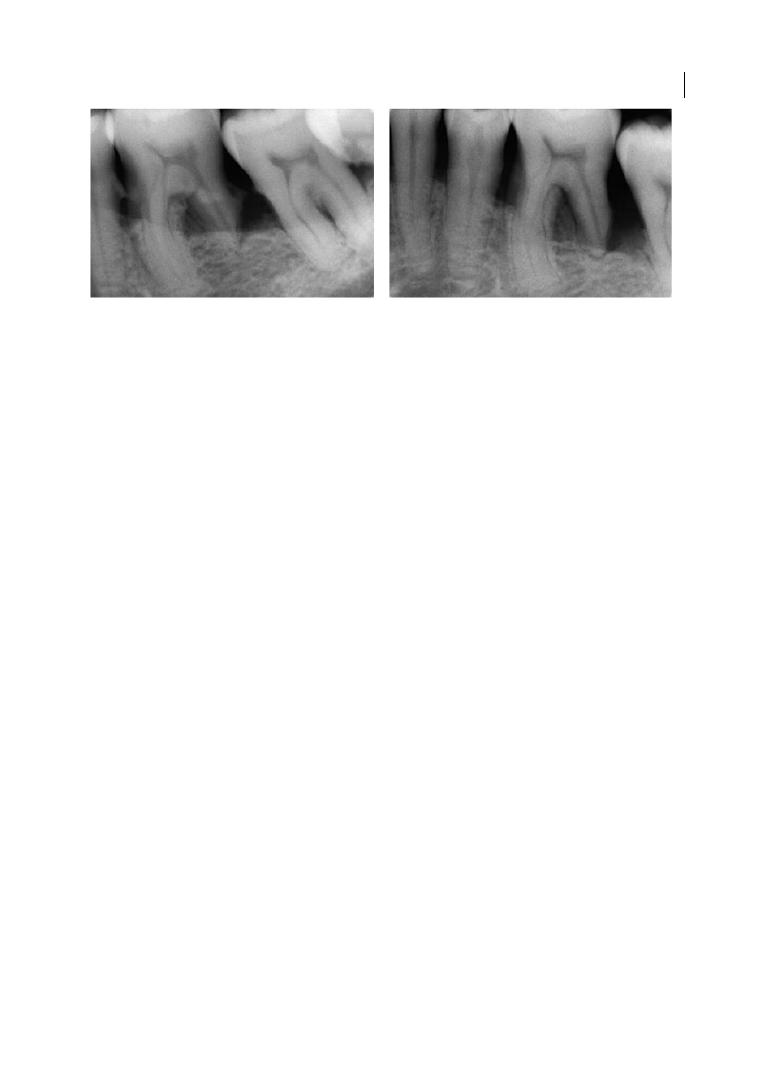
The Endodontist’s View 63
Pulp response might vary, from normal
(Mazur and Massler 1964; Smukler and
Tagger 1976; Czarnecki and Schilder 1979;
Torabinejad and Kiger 1985) to reparative
(Mazur and Massler 1964; Langeland et al.
1974; Czarnecki and Schilder 1979;
Torabinejad and Kiger 1985; Harrington
et al. 2002) or degenerative (Rubach and
Mitchell 1965; Sheykhrezaee et al. 2007;
Zuza et al. 2012; Aguiar et al. 2014; Rathod
et al. 2014). Repaired vital pulp is calcificated,
fibrotic, and has fewer blood vessels and
nerve fibres (Mazur and Massler 1964;
Langeland et al. 1974; Czarnecki and Schilder
1979; Torabinejad and Kiger 1985; Harrington
et al. 2002). Conversely, degenerated pulp
exhibits fibrosis, calcification, inflammation,
vascular alteration, loss of odontoblast integ-
rity, and partial necrosis (Rubach and
Mitchell 1965; Aguiar et al. 2014; Rathod
et al. 2014). Complete necrosis seems to only
occur if the apical neuro‐vascular bundle is
involved (Langeland et al. 1974; Zehnder
2001; Harrington et al. 2002; Sheykhrezaee
et al. 2007; Aguiar et al. 2014; Rathod et al.
2014).
The lack of randomized clinical trials with
test and control groups prevents a clear asso-
ciation between progressive periodontal
disease and pulp alterations being established.
Data have to be carefully interpreted, since
pulpal alterations might be the result of multi-
ple factors, such as periodontal disease, history
of caries, physiological pulp ageing, previous
ignored trauma, or pulp tissue fixation. Indeed,
pulp fixation is challenging and artifacts result-
ing from improper specimen preparation
might lead to misjudgements (Harrington
et al. 2002; Sheykhrezaee et al. 2007).
At the current stage of scientific knowl-
edge, we could summarize that periodontal
disease might induce reparative or degenera-
tive pulp changes. Pulp necrosis is rather rare
and occurs when the defect is up to the apical
third of the tooth and the neuro‐vascular
bundle is involved. If the blood supply
through the apical foramen remains intact,
the pulp is usually able to withstand the
physiological insults induced by both perio-
dontal disease and therapy.
4.5 Endodontic‐periodontal
Disease
Because of their anatomical and functional
interconnection, pulp and periodontium can
be simultaneously affected in what appears
to be a single periodontal lesion. This clinical
scenario, known as ‘endodontic‐periodontal
lesion’ (Bergenholtz and Hasselgreen 2008)
was first described by Simring and Goldberg
in 1964.
4.5.1 Classification
Despite the many attempts to classify endo-
dontic‐periodontal lesions, the Simon, Glick,
and Frank (1972) classification remains the
most widely accepted point of reference (see
Figure 4.5
Pulp necrosis on 3.6 (LL6) after deep root debridement. Source: Courtesy Dr Cristiano Luciano.
Do progressive periodontitis and periodontal
treatment affect the pulp?
Pulp damage
Pulp response
Cementum removal:
bacteria and toxins
migrate towards the
pulp through the
accessory canals and
the exposed dentinal
tubules
Irreversible
or
Reversible
Inflammatory
Degenerative
(Rubach and Mitchell 1965 ; Sheykhrezaee et al.
2007 ; Rathod et al. 2014 )
Fibrosis
Calcification
Inflammation
Odontoblast integrity loss
Vascular alteration
Partial/complete necrosis
Reparative
(Mazur and Massler 1964 ; Bender and Seltzer
1972 ; Langeland et al. 1974 ; Lantelme et al. 1976 ;
Bergenholtz and Lindhe 1978 ; Ross and
Thompson 1978 ; Czarnecki and Schilder 1979 ;
Torabinejad and Kiger 1985 ; Cortellini and
Tonetti 2001 ; Harrington et al. 2002 ; Aguiar et al.
2014 )
Calcification
Fibrosis
Vascular
alteration
Nerve
alteration
≈ pulp ageing
No
(Bergenholtz and Lindhe
1978 ; Nilvéus and Selvig
1983 ; Hattler and
Listgarten 1984 ; Nagaoka
et al. 1995 ; Hahn and
Overton 1997 ; Pashley
et al. 2002 )
No bacteria and
toxins migrate
towards the pulp
through the
accessory canals and
the exposed dentinal
tubules
Reversible
Reparative dentine (Bergenholtz and Lindhe 1978 ; Hattler and Listgarten 1984 ;
Vongsavan and Matthews 1991 )
+
Dentinal fluid (Vongsaven and Matthews 1991, 1992; Pashley et al. 2002 )
+
Odontoblast processes (Nagaoka et al. 1995 ; Pashley et al. 2002 )
Antibodies/antimicrobial components within the dentinal fluids
(Hahn and Overton 1997 )
Table 4.1
(Continued)
Chapter 4
64
also Gargiulo 1984; Guldener 1985; Abbott
and Salgado 2009; Kerns and Glickman
2011). According to Simon et al., endodon-
tic‐periodontal lesions are classified as:
●
Primary endodontic lesion.
●
Primary periodontal lesion.
●
Primary endodontic lesion with secondary
periodontal involvement.
●
Primary periodontal lesion with secondary
endodontic involvement.
●
True combined lesion.
4.5.1.1 Primary Endodontic Lesion
Primary endodontic lesions (Box 4.1 and
Table 4.2) mostly involve decayed, restored, or
traumatized teeth. As a result of the endodon-
tic inflammatory process, bone resorption
occurs at the apex, along the lateral aspect of
the root, or at the furcation area when multi‐
rooted teeth are affected (see Figures 4.6 and 4.7).
Because of the suppurative process, the sinus
tract can develop through the periodontal lig-
ament space or the cortical bone in accord-
ance with the locus minoris resistentiae (place
of least resistance) principle. For multi‐rooted
teeth, the tract can drain off into the furcation
area, resembling a class III periodontal defect.
Pain, tooth mobility, tenderness to pres-
sure and percussion, and periodontal
abscess‐like swelling can be the related clini-
cal inflammatory signs.
Sensitivity tests show necrotic pulp, even
though in multi‐rooted teeth the response
can be positive because of the partial necrosis.
As the lesion has an endodontic origin, root
canal treatment (European Society of
Endodontology 2006) is mandatory for sinus
tract resolution without any associated peri-
odontal therapy (Zehnder et al. 2002;
Bergenholtz and Hasselgreen 2008; Shenoy
and Shenoy 2010; Kerns and Glickman 2011).
Sometimes, a period of up to four or five
years might be required for the complete
radiographic healing of the periapical lesion
(Ng et al. 2007; Zitzmann et al. 2009).
Incongruous root canal treatment or
untreated canals (i.e. MB2 in first maxillary
molars or D2 in lower molars) might prevent
the lesion from healing, because of the
high residual bacterial load within the
endodontium.
4.5.1.2 Primary Periodontal Lesion
Periodontitis (Box 4.2 and Table 4.3) is a pro-
gressive inflammatory process that starts in
the sulcus and moves towards the apex due
to the accumulation of plaque and calculus
on the root surface. The final effect is the loss
of alveolar bone and supporting tissues
around the teeth. This process can also be
accompanied by periodontal abscess in the
acute phases of the disease (Toto and
Gargiulo 1970; Hoffman and Gold 1971).
Clinical examination reveals soft‐tissue
inflammation, tooth mobility, bleeding on
probing, and the presence of wide pockets
(i.e. accessible at different points on the
Box 4.1 Primary endodontic lesion.
●
Tooth history: caries, cracks, extensive restoration, crown or bridge abutment, incongruous
root canal treatment, dental trauma,* root resorption.**
●
Root surface: smooth on probing. No presence of subgingival calculus.
●
Pocket conformation: narrow if the pocket is present.
●
Sensitivity pulp test response: generally negative. However, it might be positive in multi‐rooted
teeth.
●
Radiographic sign: lateral, apical and/or inter‐radicular radiolucency.
●
Treatment: root canal treatment.
* Post‐traumatic pulp healing, especially after luxation injuries, is characterized by temporary loss of sensitivity.
All sensitivity tests show low reliability right after the trauma (Bastos et al. 2014).
** Root resorption might affect the external surfaces of the tooth (external resorption) or the internal dentine (inter-
nal resorption) after being started in the periodontium or within the pulp space, respectively.
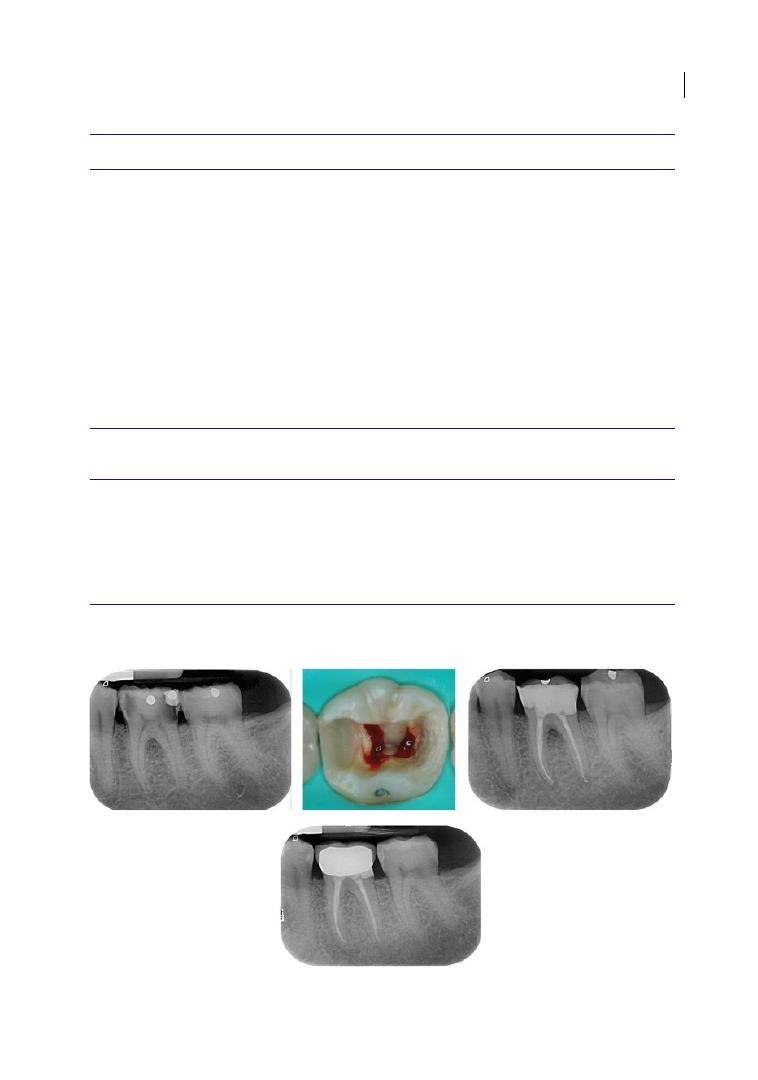
The Endodontist’s View 65
Table 4.2
Primary endodontic lesions.
Diagnostic Elements
Findings
Clinical Management
Presence of caries/restorations/cracks
+
Anamnesis
Clinical examination
Periodontal probing
Radiographic examination
Sensitivity tests
Endodontic treatment
Re‐evaluation after a few months
Subgingival calculus
−
History of trauma
+/−
Abscess
+*
Narrow deep pocket
+/−
Bleeding on probing
−
Thermal test
−/+**
Electric test
−/+**
Radiolucency
+
Mobility
+/−
Palpation test
+
Percussion test
+
* In the acute phase.
** The test is negative if all the pulp tissue is necrotic, except in case of gases‐related thermal expansion.
The test could be positive in case of partial necrosis.
Differential Diagnosis
Vertical root fractures
Primary periodontal lesions
Favourable Endodontic
Prognostic Factors
Congruous endodontic treatment
No symptoms
No probing within 30 days
Fistular track closure within 30 days
Radiolucency improvement within 6 months
Source: Adapted from AIE – Collana di Monografie Piccin Nuova Libraria S.p.A 2014.
Figure 4.6
Pulpitis on 3.6 (LL6). Inter‐radicular and periapical radiolucencies due to bacterial toxins. Five‐year
follow‐up after root canal therapy.
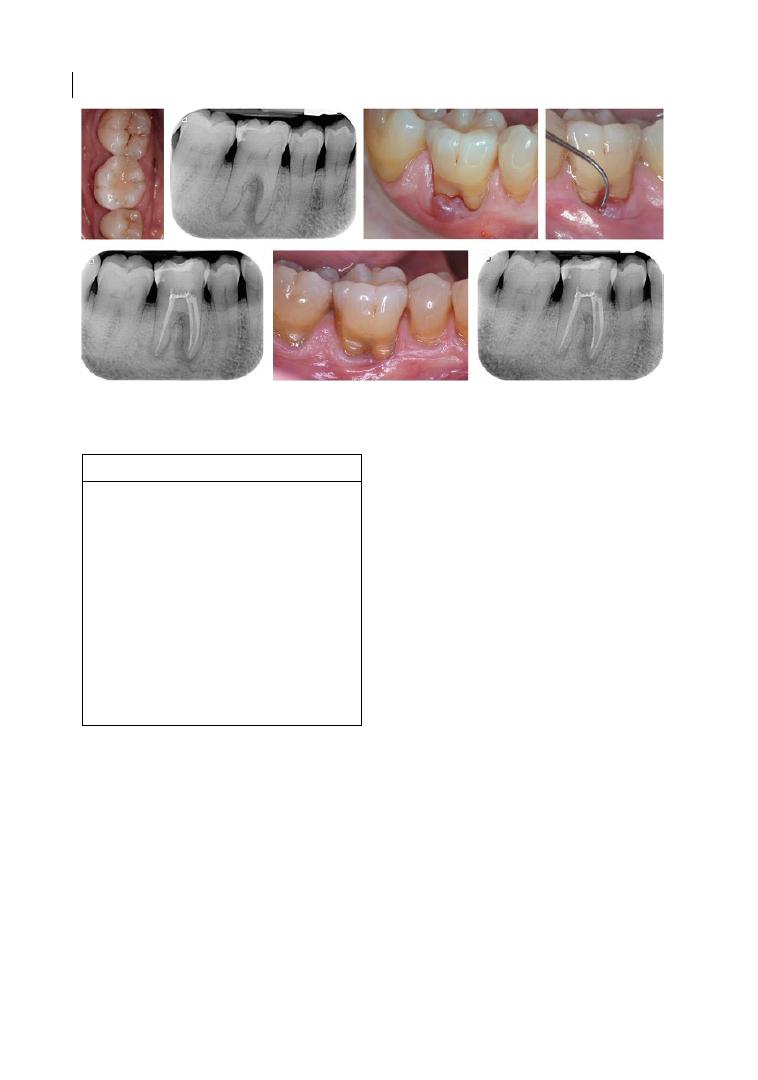
Chapter 4
66
tooth) supported by plaque and calculus
along the root. It should be noted that perio-
dontal pockets might also originate from
anomalies in the root development (Rotstein
and Simon 2004). During the diagnostic
phase, pulp sensitivity tests generally reveal
positive responses. However, negative
responses can also be recorded and do not
necessarily account for pulp necrosis. Owing
to dystrophic calcifications, the pulp space
might be reduced and the tooth might not
respond to the sensitivity tests despite its
vitality (Abou‐Rass 1982).
On the basis of the previous considera-
tions, the prognosis would mainly depend on
the extension of the periodontal disease, the
outcome of the periodontal therapy, and the
patient’s ability to comply with potential
long‐term maintenance (Bergenholtz and
Hasselgreen 2008; Kerns and Glickman
2011).
Primary Endodontic Lesion with Secondary
Periodontal Involvement
Periodontal pathogens might induce perio-
dontitis by migrating apically into the patent
sinus tract when the endodontic infection is
not treated or persists after root canal ther-
apy. At the radiographic evaluation, angular
intrabony or inter‐radicular osseous defects
are appreciated. Plaque and calculus are
detected on probing, therefore healing
requires both endodontic and periodontal
therapies for necrotic pulp removal and root
debridement. Since root canal treatment
resolves only a part of the defect (European
Society of Endodontology 2006; Shenoy and
Shenoy 2010), dental prognosis relies on the
extent and severity of the osseous defect, and
on the efficacy of the periodontal therapy.
For suppurative processes fistulizing
through the cortical bone, bacteria from the
oral cavity might colonize first the fistula and
Box 4.2 Primary periodontal lesion.
●
Tooth history: periodontal pocket, attach-
ment loss, bleeding on probing.
●
Root surface: rough on probing because
of subgingival plaque and calculus.
●
Pocket conformation: wide, often multiple
pockets.
●
Sensitivity pulp test response: generally
positive.
●
Radiographic sign: lateral and/or inter‐
radicular radiolucency, apical radiolu-
cency in advanced disease.
●
Treatment: oral hygiene instructions (OHI)
and root debridement.
Figure 4.7
Primary endodontic lesion with inter‐radicular and apical involvement on 4.6 (LR6). No furcation
probing after three months and partial healing after one‐year follow‐up.

The Endodontist’s View 67
then the apex, affecting the tooth prognosis.
Primary endodontic lesion with secondary
periodontal involvement (Box 4.3 and
Table 4.4) might also occur in endodontically
treated teeth because of root perforation
and/or fracture. Once the communication
between endodontium and periodontium is
established, the secondary periodontal lesion
can develop as a result of the micro‐organ-
isms’ migration from the root canal to the
periodontium.
The clinical signs may range from local
periodontal pocket deepening to abscess for-
mation associated with pain, exudate, and
tooth mobility. In single‐rooted teeth, the
prognosis is usually poor if the defect is close
to the apex. In multi‐rooted teeth, the prog-
nosis might be better, since the tooth can be
maintained by resecting the affected root, if
the anatomy is indicated for this procedure
Table 4.3
Primary periodontal lesions.
Diagnostic Elements
Findings
Clinical Management
Presence of caries/restorations/cracks
+/−
Anamnesis
Clinical examination
Periodontal probing
Radiographic examination
Sensitivity tests
Splinting of mobile teeth (if needed)
Oral hygiene instructions and periodontal
non‐surgical therapy
Periodontal re‐evaluation after a few months
Periodontal surgical therapy (if needed)
Subgingival calculus
+
History of trauma
+/−
Abscess
+*
Wide, not isolated, deep pocket/furcation
+
Bleeding on probing
+
Thermal test
+
Electric test
+
Lateral/interradicular radiolucency
+
Mobility
+
Palpation test
+*
Percussion test
+*
* In the acute phase.
Differential Diagnosis
Endodontic‐periodontal lesions
Favourable
Prognostic Factors
No symptoms
Pulpal vitality
No bleeding on probing
Probing pocket depth reduction
Mobility reduction
Radiographic bone remineralization in the apical part
of the defect
Source: Adapted from AIE – Collana di Monografie Piccin Nuova Libraria S.p.A 2014.
Box 4.3 Primary endodontic lesion
with secondary periodontal involvement.
●
Tooth history: caries, cracks, extensive
restoration, crown or bridge abutment,
incongruous root canal treatment, dental
trauma.
●
Root surface: rough on probing because
of subgingival plaque and calculus.
●
Pocket conformation: narrow to wide,
depending on the exposition time
of the sinus tract to periodontal
pathogens.
●
Sensitivity pulp test response: negative.
●
Radiographic sign: lateral, apical, and/or
inter‐radicular radiolucency.
●
Treatment: root canal treatment, oral
hygiene instructions (OHI), and root
debridement.

Chapter 4
68
(Cameron 1964; Chan et al. 1999; Zehnder
et al. 2002; Sunitha et al. 2008; Kerns and
Glickman 2011; Sugaya et al. 2015; see also
Chapter 8).
Primary Periodontal Lesion with Secondary
Endodontic Involvement
Primary periodontal lesion with secondary
endodontic involvement (Box 4.4 and
Table 4.5) differs from primary endodontic
lesion with secondary periodontal involve-
ment only in the temporal sequence of the
disease processes. If periodontitis remains
untreated, periodontal pathogens can reach
the pulp through the accessory canals or
Table 4.4
Primary endodontic lesions with secondary periodontal involvement.
Diagnostic Elements
Findings
Clinical Management
Presence of caries/restorations/cracks
+
Anamnesis
Clinical examination
Periodontal probing
Radiographic examination
Sensitivity tests
Splinting of mobile teeth (if
needed)
Oral hygiene instructions and
non‐surgical periodontal therapy
§
Endodontic treatment
Re‐evaluation after a few months
Surgical periodontal therapy (if
needed)
Subgingival calculus
+
History of trauma
+/−
Abscess
+*
Narrow to wide deep pocket
+
Bleeding on probing
+
Thermal test
−
Electric test
−
Radiolucency
+
Mobility
+
Palpation test
+
Percussion test
+
* In the acute phase
§
Deep Root Debridement Should Be Avoided Before Determining The Endodontic
Component Of The Defect (See Section 4.5.3)
Differential Diagnosis
Primary periodontal lesion with secondary endodontic
involvement
Vertical root fracture
Root/furcation perforation in endodontically treated
teeth
Favourable Endodontic
Prognostic Factors
Congruous endodontic treatment
No symptoms
Partial probing depth reduction within 30 days
Fistular track closure within 30 days
Mobility reduction
Partial radiolucency improvement within 6 months
Source: Adapted from AIE – Collana di Monografie Piccin Nuova Libraria S.p.A 2014.
Box 4.4 Primary periodontal lesion with
secondary endodontic involvement.
●
Tooth history: probing pocket depth
deepening, bleeding on probing.
●
Root surface: rough on probing because
of subgingival plaque and calculus.
●
Pocket conformation: wide, often multiple
pockets.
●
Sensitivity pulp test response: generally
negative.
●
Radiographic sign: lateral, apical, and/or
inter‐radicular radiolucency.
●
Treatment: root canal treatment, oral hygiene
instructions (OHI), and root debridement.
The Endodontist’s View 69
apical foramina. Pulp necrosis occurs and an
endodontic‐periodontal lesion develops
(Rubach and Mitchell 1965; Aguiar et al.
2014). Periodontal therapy can also lead to
secondary pulp involvement. By exposing
lateral canals and dentine during scaling and
root planing or surgical flap procedures,
blood supply might be interrupted and
micro‐organisms might penetrate into the
tubules, resulting in pulp inflammation and/
or necrosis (Adriaens et al. 1988).
To differentiate between endodontic‐
periodontal lesions with primary periodontal
and endodontic origin, anamnesis and
clinical examination have to be exhaustively
performed (see Figure 4.8). A history of
generalized periodontitis might suggest a
primary periodontal origin. The number and
conformation of pockets can help in the
diagnosis. Wider or narrower defects gener-
ally suggest a periodontal or endodontic
origin of the lesions, respectively (Zehnder
et al. 2002; Sunitha et al. 2008; Kerns and
Glickman 2011). In addition, periodontal
probing may reveal the presence of calculus
on the root surface. Pulp sensitivity tests are
generally negative when the endodontium is
involved, whereas the radiographic evalua-
tion does not prove the primary origin of the
lesion.
Once the endodontic therapy is properly per-
formed (European Society of Endodontology
Table 4.5
Primary periodontal lesions with secondary endodontic involvement.
Diagnostic Elements
Findings
Clinical Management
Presence of caries/restorations/cracks
+/−
Anamnesis
Clinical examination
Periodontal probing
Radiographic examination
Sensitivity tests
Splinting of mobile teeth (if
needed)
Oral hygiene instructions and
non‐surgical periodontal therapy
§
Endodontic treatment
Periodontal re‐evaluation after a
few months
Surgical periodontal therapy (if
needed)
Subgingival calculus
+
History of trauma
+/−
Abscess
+*
Not isolated, wide deep pocket/furcation
+
Bleeding on probing
+
Thermal test
−**
Electric test
−**
Lateral/apical/inter‐radicular radiolucency
+
Mobility
+
Palpation test
+
Percussion test
+
* In the acute phase.
** The test is negative if all the pulp tissue is necrotic, except in case of gases‐related thermal expansion.
The test could be positive in the case of partial necrosis.
§
Deep Root Debridement Should Be Avoided Before Determining The Endodontic Component of
The Defect (See Section 4.5.3)
Differential Diagnosis
Primary endodontic lesions with secondary periodontal
involvement
Favourable Endodontic
Prognostic Factors
Congruous endodontic treatment
No symptoms
Partial probing depth reduction within 30 days
Fistular track closure within 30 days
Mobility reduction
Partial radiolucency improvement within 6 months
Source: Adapted from AIE – Collana di Monografie Piccin Nuova Libraria S.p.A 2014.
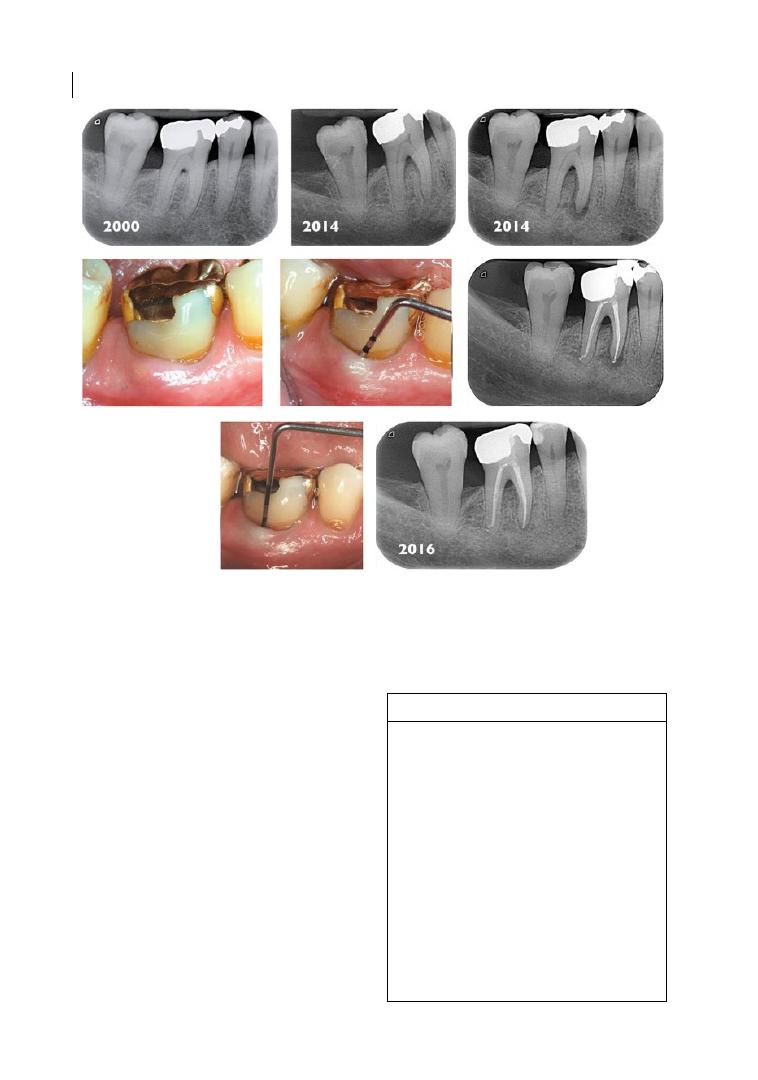
Chapter 4
70
2006; Shenoy and Shenoy 2010), clinical success
basically depends on the outcome of the
periodontal therapy and the patient’s ability to
comply with potential long‐term maintenance.
As previously mentioned, multi‐rooted teeth
might have a better prognosis than single‐
rooted teeth, since root resection represents an
alternative for tooth survival.
True Combined Lesion
A true combined lesion (Box 4.5 and
Table 4.6) means that the endodontic and
periodontal infections simultaneously exist
as independent, separated, or merging
lesions. When the periodontal pocket deep-
ens up to the periapical lesion, the endodon-
tic and periodontal components of the defect
are unidentifiable. Symptoms are similar to
Figure 4.8
Degree III furcation involvement on 4.6 (LR6) with progressive deepening of the defect and
secondary pulp necrosis after 14 years. Root canal therapy led to resolution of the endodontic component of
the defect. Besides scaling and root planing and oral hygiene instructions, no further periodontal treatment
was performed.
Box 4.5 True combined lesions.
●
Tooth history: caries, cracks, extensive
restoration, crown or bridge abutment,
incongruous root canal treatment, dental
trauma, probing pocket depth deepen-
ing, bleeding on probing.
●
Root surface: rough on probing because
of subgingival plaque and calculus.
●
Pocket conformation: wide and conical
pocket.
●
Sensitivity pulp test response: negative.
●
Radiographic signs: communicating or
non‐communicating extensive apical, lat-
eral, and/or inter‐radicular radiolucencies.
●
Treatment: root canal treatment, oral hygiene
instructions (OHI), and root debridement.
The Endodontist’s View 71
those previously mentioned for the com-
bined lesions with primary endodontic or
periodontal origin. The radiographic evalua-
tion shows extensive osseous radiolucencies,
communicating or not, similar to those of
vertically fractured teeth. Indeed, pulp space
invasion through vertical root fracture might
also be considered as a true combined lesion.
Prior to endodontic therapy, mobile teeth
should be splinted and carefully debrided.
Once the root canal treatment is properly per-
formed (European Society of Endodontology
2006), the endodontic component of the
defect is expected to heal within a couple of
months (Shenoy and Shenoy 2010; see
Figure 4.9). Tooth prognosis would entirely
depend on both the periodontal pocket depth
and the related periodontal therapy. Uncertain
prognosis concerns more single‐rooted that
multi‐rooted teeth, since root resection might
be a treatment option if not all the roots are
severely involved and tooth anatomy is indica-
tive for this procedure (Zehnder et al. 2002;
Rotstein and Simon 2004; Sunitha et al. 2008).
4.5.2 Diagnosis of Endodontic‐
periodontal Disease
Diagnosis of endodontic‐periodontal lesions
can be easily performed when the patient has
been monitored over time. Similar clinical
and radiographic findings might make the
differential diagnosis somewhat challenging.
To avoid any misinterpretation, comprehen-
sive information can be obtained through
detailed anamnesis and clinical examination,
and by the use of specific tests aimed to assess
the vitality of the pulp. Primary endodontic
Table 4.6
True periodontal‐endodontic combined lesions.
Diagnostic Elements
Findings
Clinical Management
Presence of caries/restorations/cracks
+
Anamnesis
Clinical examination
Periodontal probing
Radiographic examination
Sensitivity tests
Splinting of mobile teeth (if needed)
Oral hygiene instructions and
non‐surgical periodontal therapy
§
Endodontic treatment
Periodontal re‐evaluation after a
few months
Surgical periodontal therapy (if
needed)
Subgingival calculus
+
History of trauma
+/−
Abscess
+*
Not isolated, wide deep pocket/furcation
+
Bleeding on probing
+
Thermal test
−
Electric test
−
Lateral/apical/inter‐radicular radiolucency
+
Mobility
+
Palpation test
+
Percussion test
+
* It depends on whether the phase is acute or chronic.
§
Deep Root Debridement Should Be Avoided Before Determining The Endodontic
Component Of The Defect (see Section 4.5.3)
Differential Diagnosis
Vertical root fracture
Favourable Endodontic
Prognostic Factors
Congruous endodontic treatment
No symptoms
Partial probing depth reduction within 30 days
Fistular track closure within 30 days
Mobility reduction
Partial radiolucency improvement within 6 months
Source: Adapted from AIE – Collana di Monografie Piccin Nuova Libraria S.p.A 2014.
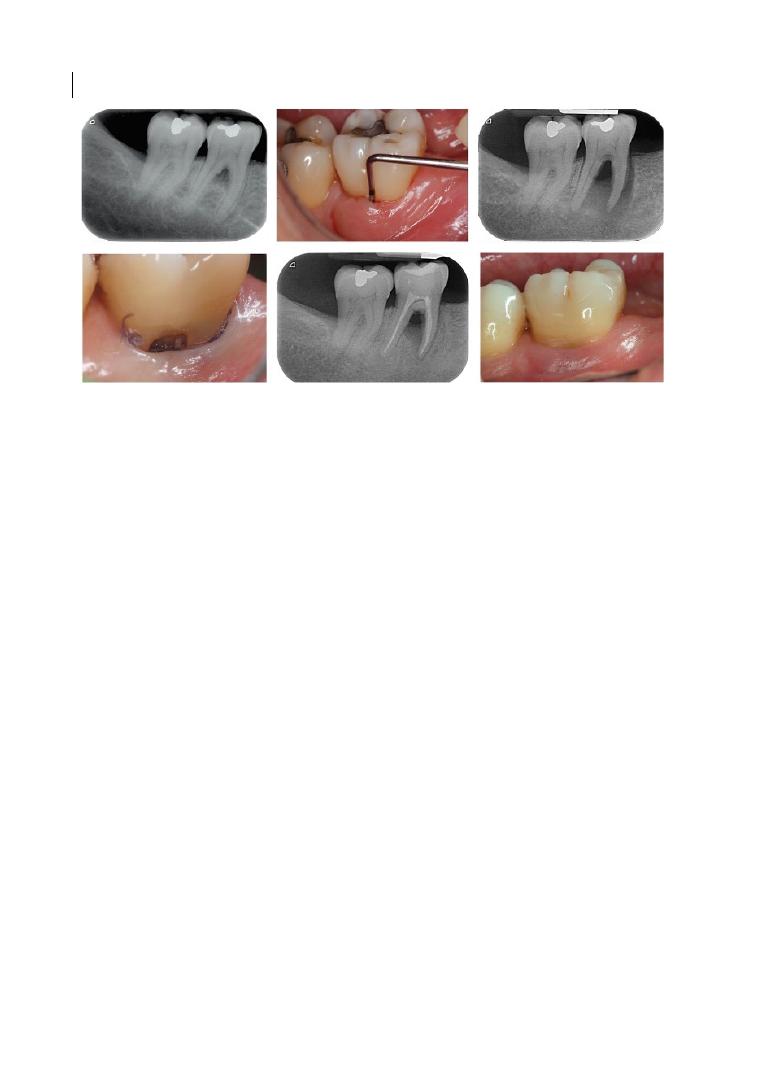
Chapter 4
72
lesions generally originate from infected and
non‐vital pulp, whereas vital teeth are more
characteristic of primary periodontal disease
(Rotstein and Simon 2004; Bergenholtz and
Hasselgreen 2008; Sunitha et al. 2008; Parolia
et al. 2013).
4.5.2.1 Clinical Examination
Pulpal and periodontal diseases might have
many clinical signs in common, such as gin-
gival swelling, pus discharge, probing, tooth
mobility, and tenderness to percussion. Teeth
have to be evaluated for caries, incongruous
under‐ or over‐contoured restorations, loss
of marginal seal, erosions, abrasions, cracks,
and fractures. All these situations are more
related to endodontic disease.
4.5.2.2 Palpation
Palpation is performed by applying firm dig-
ital pressure in correspondence with the
root and the apex, with the index finger
pressing the mucosa against the underlying
cortical bone. A positive response might
indicate an active periradicular inflamma-
tory process. However, this test does not
indicate whether the origin is endodontic or
periodontal. The test should be compared to
control teeth.
4.5.2.3 Percussion
This test indicates the presence of periradic-
ular inflammation without revealing the
status of the pulp. An abnormal positive
response shows inflammation of the perio-
dontal ligament, but it does not indicate
whether the origin is endodontic or perio-
dontal. The test should be compared to
control teeth.
4.5.2.4 Bite Test
This test does not disclose the condition of
the pulp. However, it might be positive in
vital teeth affected by cracked tooth syn-
drome (Cameron 1964) and in non‐vital
teeth with periradicular inflammation.
4.5.2.5 Mobility
This clinical sign does not prove whether the
origin of the lesion is primarily periodontal
or endodontic. It might be speculated that its
primary cause is periodontitis. In fact, tooth
mobility depends on the amount and inflam-
mation of the residual supporting tissues.
The greater the bone loss, the higher the
mobility. However, periradicular oedema or
trauma, with or without tooth fracture, can
also lead to similar mobility (Biancu et al.
1995; Séguier et al. 2000).
Figure 4.9
Class 3 inter‐radicular defect and concomitant decay of the furcation roof on 4.7 (LR7). The caries
progression led to pulp necrosis. Tunnelling spontaneously occurred after non‐surgical periodontal treatment.
One‐year follow‐up after root canal therapy.
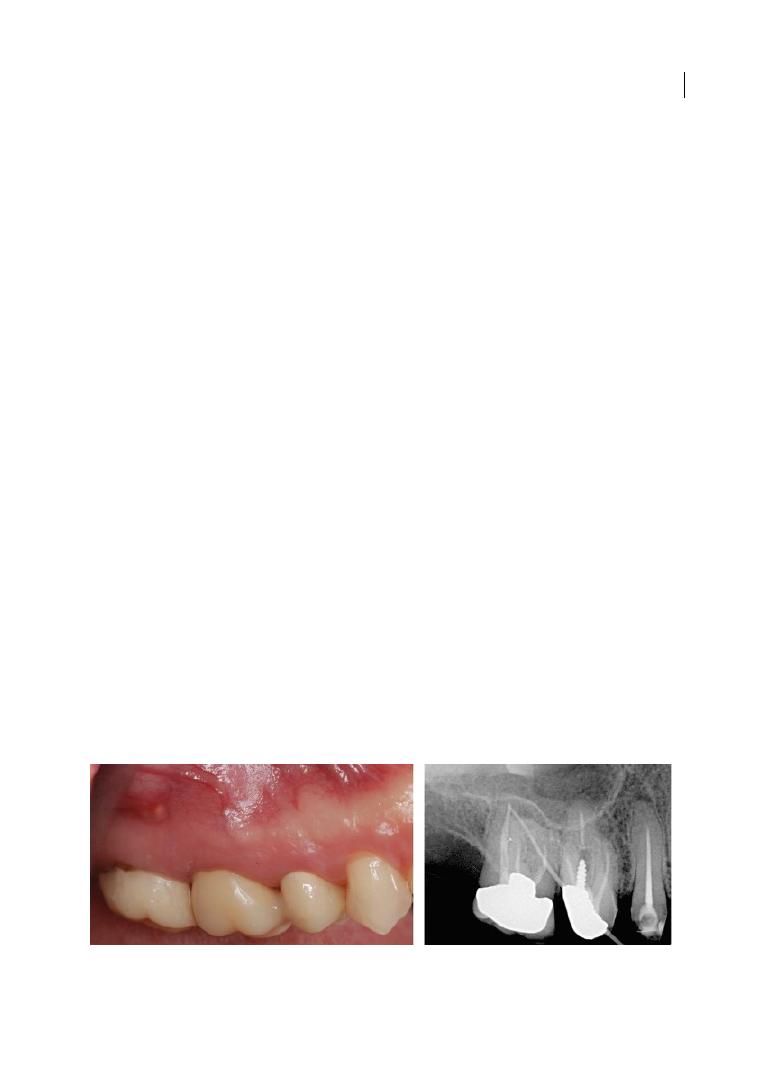
The Endodontist’s View 73
4.5.2.6 Fistula Tracking
Endodontic and periodontal diseases can
lead to the formation of a fistulous sinus
track. Following a minor resistance path,
inflammatory exudate drains off into the oral
mucosa through the attached buccal gingiva
or the vestibule. The track is generally more
representative of the endodontic infection
rather than the periodontal disease, which
often drains through the periodontal pocket
without any fistulous sinus track formation.
Fistula tracking is performed by inserting a
semirigid radiopaque material, commonly a
gutta‐percha cone, into the sinus track until
resistance is met (see Figure 4.10). A radio-
graph is taken to identify the course of the
sinus tract and, therefore, the tooth involved.
4.5.2.7 Cracked Tooth Testing
Cracked teeth (Cameron 1964) or vertical
root fractures can be diagnosed through the
observation of incomplete or complete
cracks by transillumination. The fibre‐optic
light source is directly placed on the cleaned
tooth. Cracks can be appreciated with a mag-
nification source by evaluating the disrup-
tion of the light transmission (Liewehr 2001;
Liewehr et al. 2010). Unlike a vertical root
fracture with a ‘tear‐shaped’ radiographic
radiolucency, cracked teeth do not generally
show any pathognomonic radiographic signs.
4.5.2.8 Radiographs
Despite the benefits of radiographs, consist-
ing in the detection of caries, over‐ or
under‐
contoured restorations, pulp caps,
periradicular radiolucencies, periodontal
ligament widening, calculus, alveolar bone
loss, and root fractures, this examination
alone does not indicate whether the radiolu-
cency has an endodontic, periodontal, or any
additional origin. It is important to consider
that some other pathologies, such as cysts
and neoplasia, can resemble periodontal or
endodontic lesions in radiographic
appearance.
Occlusal trauma may also lead to radio-
graphic radiolucencies on the lateral, apical,
or inter‐radicular aspect of the root. In
periodontally involved teeth suffering from
occlusal trauma, the amount of deminerali-
zation is not quantitatively reflected in the
probing pocket depth, which is less deep
than could be guessed radiographically.
Occlusal adjustment might be necessary and
must always precede any endodontic or
periodontal therapy. Demineralization
resolves within a few months when the
occlusal interferences and the mobility are
eliminated by grinding and splinting, respec-
tively [75,81,96,97] (Bergenholtz and
Hasselgreen 2008; Carnevale et al. 2008;
Lindhe et al. 2008; Kerns and Glickman 2011;
see Figures 4.11 and 4.12).
4.5.2.9 Pocket Probing
To assess whether the origin of the lesion is
endodontic or periodontal, probing can be
crucial in the diagnosis (Harrington and
Steiner 2002). Defects are evaluated for
(a)
(b)
Figure 4.10
Fistulous sinus track between 1.6 (UR6) and 1.7 (UR7) (a). Fistula tracking revealed the origin of the
endodontic infection on 1.7 (b).
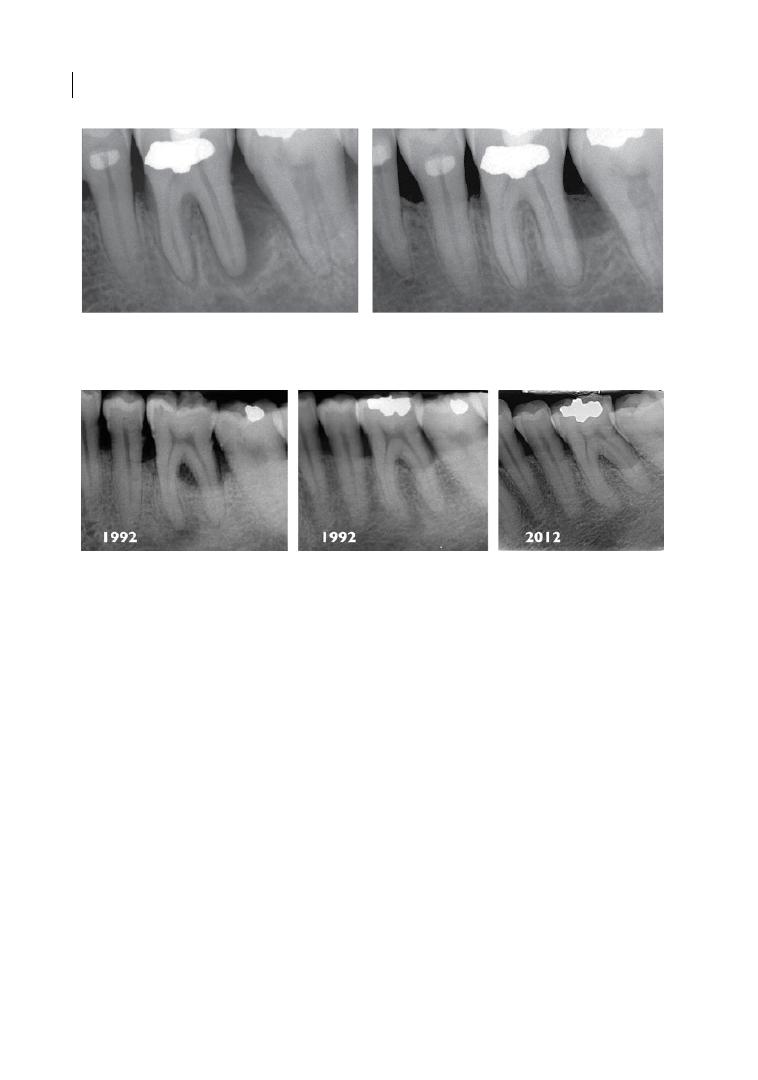
Chapter 4
74
extent, severity, and shape by means of a
calibrated periodontal probe. The presence
of plaque and calculus, detected by sounding
the root surface with the tip of the probe,
explains the periodontal involvement,
although this may not be necessarily easy to
detect. Primary periodontal lesions are fre-
quently characterized by wide calculus‐
induced defects in patients with further
periodontal pockets, whereas primary endo-
dontic lesions typically show narrow solitary
calculus‐free defects. Inter‐radicular involve-
ment without further signs of periodontal
disease might indicate the endodontic origin
of the lesion.
Periodontal probing can be considered as a
prognostic indicator in the short term. In
fact, early fistulous sinus track resolution
after root canal therapy (Shenoy and Shenoy
2010) might confirm the endodontic origin
of the defect without any further concomi-
tant causes, such as vertical root fracture or
periodontal involvement. On the contrary, a
persisting sinus track might imply periodon-
tal involvement or unsolved endodontic
infection (Harrington and Steiner 2002;
Walton and Torabinejad 2002; Rotstein and
Simon 2004).
4.5.2.10 Pulp Vitality Tests
Pulp vitality tests are very important to eval-
uate whether the lesion has an endodontic or
periodontal origin (Walton and Torabinejad
2002). The sensitivity rather than the vitality
of the pulp is assessed through sensory nerve
stimulation. Two different stimuli, electric
and/or thermal (cold or hot), can be applied,
and complaints and painful sensations are
recorded. Figure 4.13 summarizes the diag-
nostic tests for pulp health assessment, and
(a)
(b)
Figure 4.11
Radiographic radiolucency on 3.6 (LL6) affected by secondary occlusal trauma without furcation
involvement (a). Six‐month follow‐up after root debridement and occlusal adjustment (b). Source: AIE—Collana
di Monografie Piccin Nuova Libraria S.p.A 2014, p. 139.
Figure 4.12
Degree II furcation defect (lingual) on 3.6 (LL6) affected by secondary occlusal trauma. Being the
possible result of jiggling movements, the apical radiolucency present on the mesial root disappeared after
the occlusal adjustment. Twenty‐year follow‐up after non‐surgical periodontal therapy and oral hygiene
instructions. Source: AIE—Collana di Monografie Piccin Nuova Libraria S.p.A 2014, p. 140.
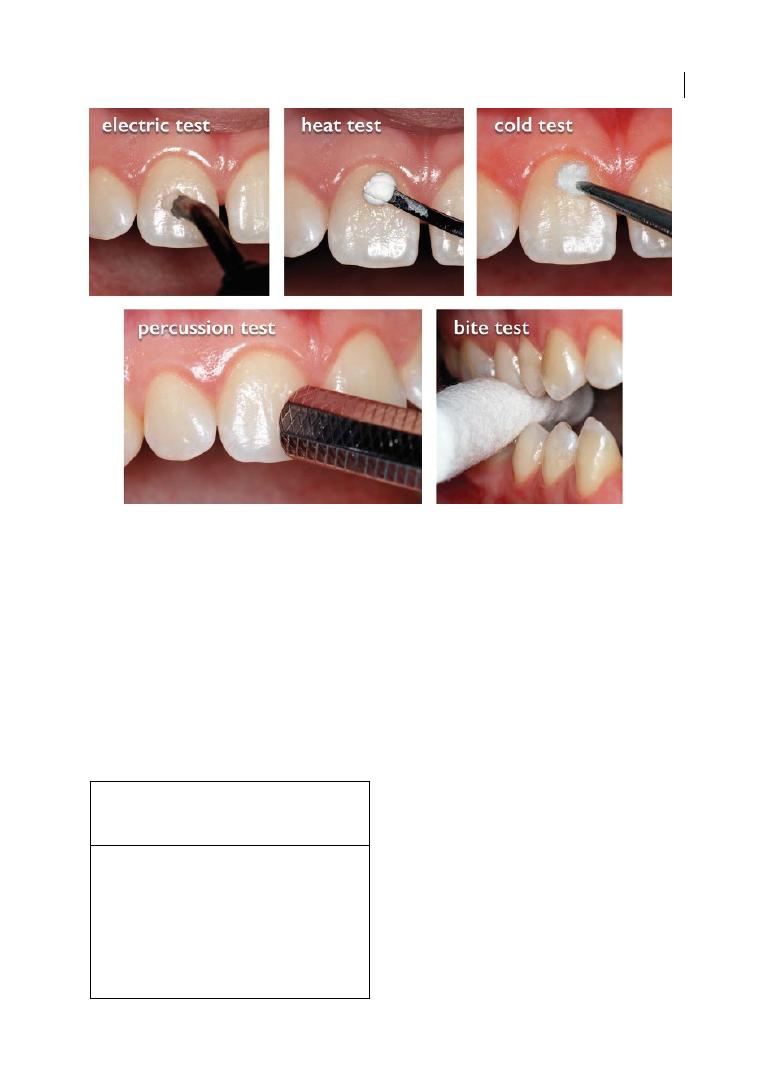
The Endodontist’s View 75
Box 4.6 considers how sensitivity tests might
be misinterpreted.
Vital teeth react to cold and hot stimuli by
exhibiting short‐lasting sharp pain or mild
heat sensation, respectively. Intense and
long‐lasting painful reactions might indicate
irreversible pulp changes. In molars, tissue
degeneration might be limited to part of the
pulp only (see Figure 4.14) and the reliability
of the tests might be questioned, as false
negatives can be wrongly recorded (Abou‐
Rass 1982; Mejàre et al. 2012; Levin 2013). A
lack of response is often associated with pulp
necrosis (Rowe and Pitt Ford 1990; Peters
et al. 1994).
Vital teeth react to an electric test by exhib-
iting tingling, slight discomfort, or a burning
sensation. Scored values per se do not mean
the presence or absence of pathology, since
no general threshold for pulpal disease has
been established so far. As a general rule, the
higher the scored values, the higher the prob-
ability of irreversible pulpal alterations. To
better assess the response, healthy teeth
should be taken as controls. By comparing the
values obtained at different follow‐up stages,
more clinical information is provided for the
diagnosis. However, false negatives and posi-
tives might make clinical evaluation some-
what challenging (Rotstein and Simon 2004;
Gopikrishna et al. 2007; Chen and Abbott
2009; Jafarzadeh and Abbott 2010; Mejàre
et al. 2012; Alghaithy and Qualtrough 2017).
Figure 4.13
Diagnostic tests for pulp health assessment. Source: AIE—Collana di Monografie Piccin Nuova
Libraria S.p.A 2014, pp. 266–268, 271.
Box 4.6 Clinical situations where pulp
response to sensitivity tests might be
misinterpreted.
●
Teeth with calcified root canals.
●
Multi‐rooted teeth with partially affected
pulp.
●
Teeth with partial‐ or full‐coverage
restorations.
●
Traumatized teeth.
●
Endodontically treated teeth with untreated
canals.
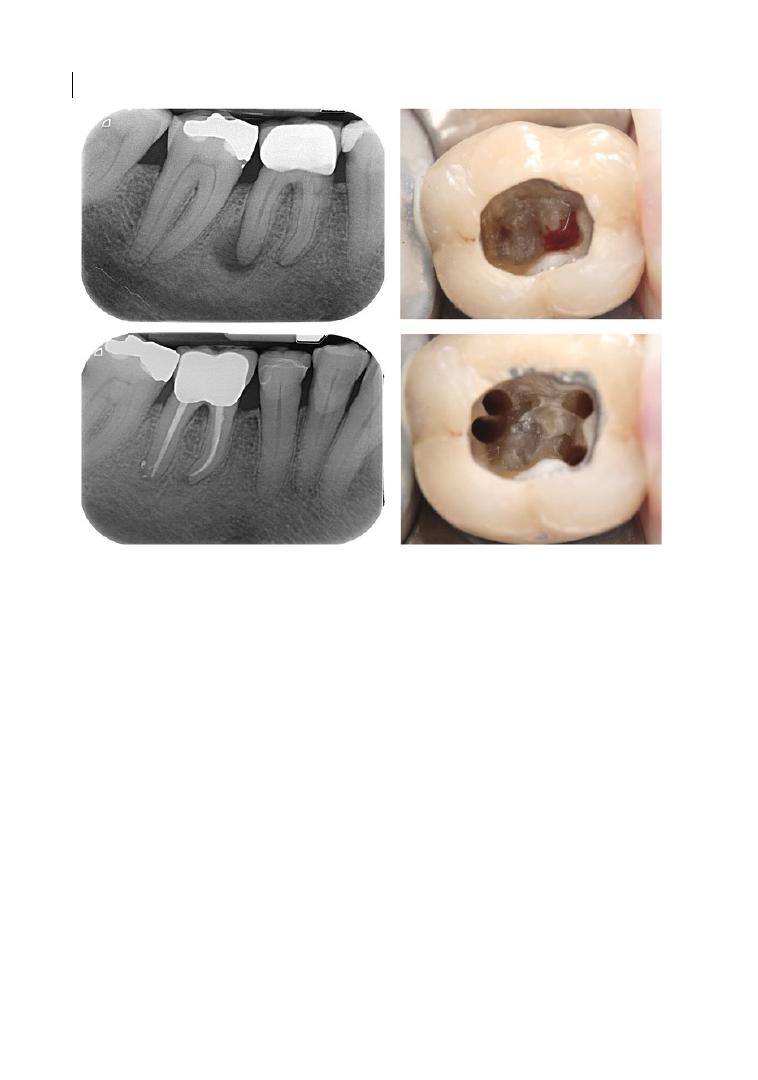
Chapter 4
76
While a lack of response can be associated
with pulp necrosis, exaggerated or misleading
responses following cold or electric tests can
be the result of pulpitis, patient anxiety,
dentinal hypersensitivity, trauma, or enamel‐
to‐dentine cracks (Abou‐Rass 1982; Eli 1993;
Peters et al. 1994; Bastos et al. 2014). Cold and
hot stimuli can respectively mitigate or exacer-
bate the symptoms in partially necrotic teeth.
Vital teeth with a history of deep caries,
periodontitis, bruxism, or trauma may not
respond to thermal or electrical stimuli
because of the reparative changes in the pulp
tissues (Bastos et al. 2014). Partial‐ or full‐
coverage restorations can also act as a barrier
to thermal and, to a lesser degree, electrical
stimuli, preventing the pulp from being
properly evaluated (Rowe and Pitt Ford 1990;
Peters et al. 1994; Myers 1998; Petersson
et al. 1999).
The vitality rather than the sensitivity of
the pulp can be assessed by measuring the
pulp blood flow through laser doppler flow-
metry or similar procedures. Many investiga-
tions have been conducted to validate the
efficacy of these tests. However, their clinical
applicability is still questioned (Gopikrishna
et al. 2007; Mejàre et al. 2012; Alghaithy and
Qualtrough 2017).
4.5.2.11 Cavity Test
By drilling the cavity without anaesthetic, the
pulp status can be objectively evaluated
through patient‐referred symptoms. The so‐
called cavity test can be performed when all
the aforementioned tests have failed to give
comprehensive information about the vitality
of the pulp. Positive and negative responses
indicate vital and necrotic pulp, respectively.
If no symptoms are reported by extending
the cavity towards the pulp chamber, partial
or complete pulp necrosis is confirmed and
the endodontic treatment can be started
(Kerns and Glickman 2011).
Figure 4.14
Pulp necrosis limited to the distal root on 4.6 (LR6) and result after root canal therapy.
The Endodontist’s View 77
4.5.2.12 Selective Anaesthesia Test
To determine the origin of pain, teeth might
be selectively anaesthetized by carefully
injecting the anaesthetic through the perio-
dontal ligament. Periodontal intraligament
injection is limited to a single tooth without
involving the adjacent teeth. The test is use-
ful to identify the origin of pulpitis‐related
radiating pain (D’Souza et al. 1987; Rotstein
and Simon 2004).
4.5.3 Management of
Endodontic‐periodontal Disease
To properly manage endodontic‐periodontal
pathology (Box 4.7; Berner and Graber 2008),
prognosis and treatment decision‐making
should be based on scrupulous diagnosis.
The pulp should be assessed for sensitivity,
whereas bone defects should be assessed for
severity, extension, and shape.
Primary endodontic disease is character-
ized by necrotic pulp and narrower calculus‐
free defect, thus the prognosis would mainly
depend on the outcome of root canal therapy.
Once calculus‐related pockets are excluded
and root canal treatment is properly per-
formed, the diagnosis of primary endodontic
lesions is confirmed by the disappearance of
symptoms, physiological values on soft tissue
probing, and bony remineralization on recall
radiographs.
Primary periodontal lesions show vital
pulp and a wide calculus‐associated pocket.
In this case, the prognosis depends on peri-
odontal disease severity, treatment execu-
tion, and patient response, motivation, and
compliance.
Despite the similar clinical and radio-
graphic findings, the presence of plaque and
calculus is crucial for the diagnosis and prog-
nosis of combined or true endodontic‐
periodontal lesions. From a treatment
viewpoint, the calculus, if present, might be
useful to detect the limit between the perio-
dontal (rough surfaces due to calculus) and
endodontic component of the defect (smooth
surfaces without calculus). When this differ-
ential diagnosis is not possible, deep and
heavy debridement should be avoided before
root canal therapy, since healthy cementum
might be wrongly removed, and a second re‐
evaluation of the site should be made two to
three months after the endodontic treatment
(Zehnder 2001; Parolia et al. 2013; Paul and
Hutter 1997). This time is required for the
initial bone remineralization, thus the extent
of the periodontal component can be more
precisely assessed.
Tooth maintainability should be deeply
questioned once the extent of the defect is
seen to depend more on periodontal than
endodontic disease. The prognosis depends
on periodontal disease severity, overall treat-
ment execution, and patient response,
motivation, and compliance. Cases of true
combined disease might have more a guarded
prognosis than the combined endodontic‐
periodontal lesions (Paul and Hutter 1997;
Rotstein and Simon 2004; Bergenholtz and
Hasselgreen 2008; Kerns and Glickman 2011;
Schmidt et al. 2014).
4.5.4 Endodontic‐periodontal Disease
in Endodontically Treated Teeth
Sensitivity tests cannot be used for diagnostic
purposes in endodontically treated teeth.
Improper root canal treatment (see Figure 4.15)
or iatrogenic injuries (see Figure 4.16), such as
stripping or perforation, should be radio-
graphically detected to determine whether the
origin of the lesion is endodontic, particularly
if there are no signs of periodontal disease. The
diagnostic dilemma can only be solved through
proper endodontic retreatment. By controlling
the infection, clinical and radiographic healing
Box 4.7 Proper management of
endodontic‐periodontal pathology.
●
Collect all the information referred to by
the patient (i.e. previous trauma, pulp
capping).
●
Perform all the tests mentioned.
●
Match and interprete the data collected.
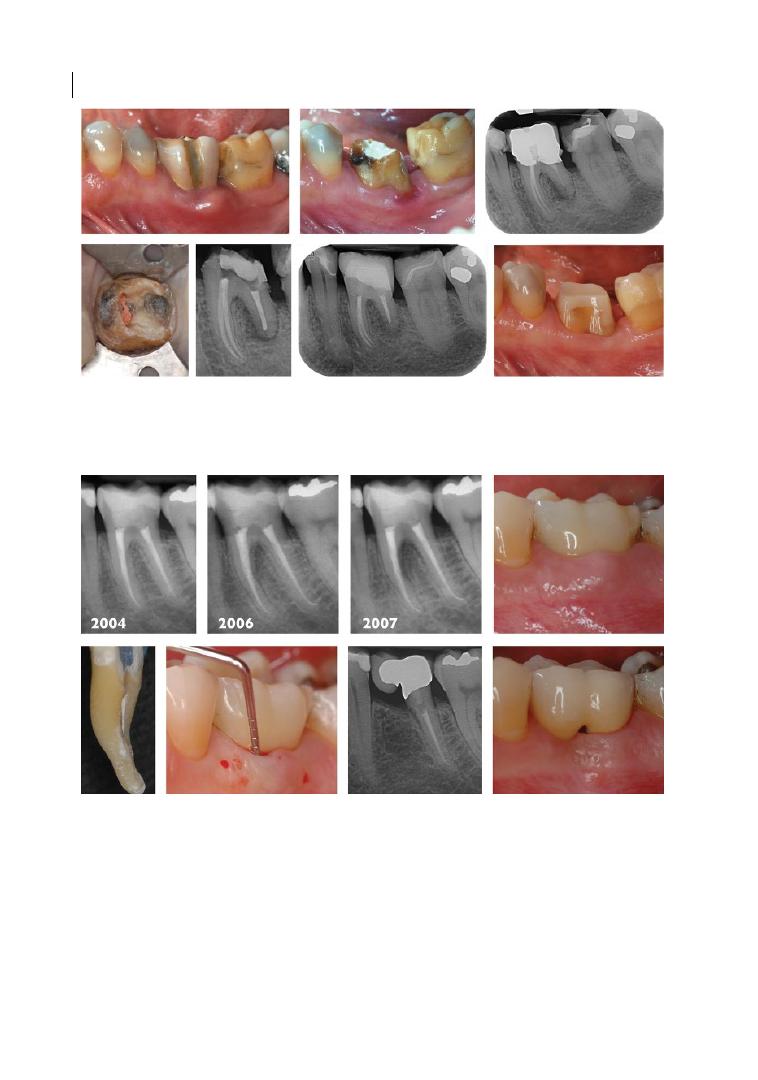
Chapter 4
78
can be expected within two to three months
after the retreatment (European Society of
Endodontology 2006; Shenoy and Shenoy
2010). If resolution does not occur, periodontal
disease, vertical root fracture, or persisting
endodontic infection can be individually con-
sidered as possible causes of the disease
(Rotstein and Simon 2004).
4.6 Relationship Between
Pulp and Periodontal
Furcation Therapies
4.6.1 Non‐surgical Periodontal Therapy
The previous chapter discussed how, since
bacteria are the primary aetiological factor in
Figure 4.15
True combined lesion on 3.6 (LL6) with an incongruous root canal therapy. The endodontic
infection led to the apical resorption of the distal root. Despite the inter‐radicular radiolucency, furcation
probing was negative. A periodontal defect was present on the distal aspect of the tooth. Two‐year follow‐up
after non‐surgical periodontal therapy, oral hygiene instructions, and root canal retreatment.
Figure 4.16
Inter‐radicular defect on 3.6 (LL6) due to stripping of the mesial root and results after rizectomy.

The Endodontist’s View 79
periodontal disease, periodontal furcation
therapy aims to subgingivally remove plaque
and calculus from the contaminated root
surfaces (Wennström et al. 2005; Tomasi and
Wennström 2009). Despite the benefits for
periodontal health (Löe et al. 1965), mechan-
ical instrumentation might have some side
effects on root integrity and, therefore, on
the endodontium. Following root cementum
and superficial dentine removal, bacteria can
more easily penetrate into the tubules and
induce a localized inflammatory response on
the pulp (Adriaens et al. 1988; Bergenholtz
and Ricucci 2008). Nevertheless, some
authors have reported that cementum and
dentine removal do not appear to have con-
sequences for pulp health (Bergenholtz and
Lindhe 1978), even when the exposed root is
in contact with plaque (Nilvéus and Selvig
1983; Hattler and Listgarten 1984). In fact,
the incidence of pulp lesions was seen to be
similar between scaling and root planing–
treated and untreated teeth (Bergenholtz and
Lindhe 1978).
Among the instrumentation‐related side
effects, root dentine hypersensitivity is
widely reported as a complaint by patients.
In fact, half of cases usually report sensitivity
after subgingival scaling and root planning
(von Troil et al. 2002). Painful symptoms,
which affect the upper premolars and first
molars more than the rest of the teeth
(Bartold 2006), are normally evoked by evap-
orative, tactile, thermal, or osmotic stimuli,
and can prevent the patient from undertak-
ing daily oral hygiene procedures. In accord-
ance with the most accredited hydrodynamic
theory, fluid shift across the exposed tubules
can be responsible for the painful sensation
(Pashley et al. 1996).
Generally, root dentine hypersensitivity
disappears within a couple of weeks after
subgingival debridement because of the nat-
ural occlusion of the tubules. Mineral crystal
deposition on the tubular lumen inactivates
the hydrodynamic mechanism for dentinal
pain and limits the potential for an inward
diffusion of bacterial elements towards the
pulp (Yoshiyama et al. 1989; 1990).
Besides the patient’s pain perception and
threshold, eating habits, such as consumption
of citrus fruit, fruit juice, yogurt, and wine,
can promote the onset of root dentine hyper-
sensitivity. Acid nourishment can act as
conditioners for mineralized tissues, prevent-
ing the tubules from occluding (Bergenholtz
and Ricucci 2008; Addy et al. 1987).
A wide number of treatment options seem
to be effective in the management of dentinal
hypersensitivity. Chemical or physical agents
are professionally or domestically applied, to
either desensitize the nerve or cover the
exposed dentinal tubules (Gillam and
Orchardson 2006). Sometimes, for stressed
patients with poor eating habits and a low
pain threshold, dentinal hypersensitivity can
persist for months or years after mechanical
instrumentation, and root canal treatment
might be required to improve their daily oral
hygiene and the related quality of life (Bartold
2006; Gillam and Orchardson 2006).
4.6.2 Regenerative Furcation
Therapy
Chapters 6 and 7 will cover the regenerative
options for periodontal furcation involve-
ment (FI). Despite the effort to establish
whether a negative effect of guided tissue
regeneration (GTR) on the pulp exists, clear
evidence is still lacking (Chen et al. 1997).
According to Cortellini and Tonetti (2001),
GTR of deep intrabony defects extended to
the apical third of the root does not nega-
tively influence the vitality of the tooth. This
is particularly evident when the neuro‐vas-
cular bundle is not damaged by debridement.
Clinical attachment level (CAL) gain follow-
ing GTR appears to be quite similar between
vital and endodontically treated teeth. In
fact, the healing process does not seem to be
influenced by root canal therapy successfully
performed prior to the regeneration
(Cortellini and Tonetti 2001).
As reported by other authors (Lasho et al.
1983; Polson et al. 1984; Gkranias et al. 2012;
Garg et al. 2015), conditioners, such as citric
acid and ethylenediaminetetraacetic acid
Chapter 4
80
(EDTA), are effective in smear layer, endo-
toxins, and anaerobic bacteria removal. Root
conditioning improves the attractiveness of
the surface as a substrate to which cells/
blood components can adhere (Boyko et al.
1980), therefore the exposed collagen fibres
can act as a matrix for a new connective tissue
attachment to cementum (Pitaru and Melcher
1987). Conversely, smear layer dissolution
can threaten pulp health. By removing this
protective barrier, dentine permeability
increases and the pulp might be more likely to
be injured (Ryan et al. 1984; McInnes‐Ledoux
et al. 1985). As observed by Cotton and Siegel
(1977), citric acid application on freshly cut
dentine may have a detrimental toxic effect
on human pulp. However, several studies do
not endorse this finding (Nilvéus and Selvig
1983; Lambrianidis et al. 1988).
Without evidence‐based operating proto-
cols, the recommendations in Box 4.8 should
be followed.
4.6.3 Resective Therapy
As discussed by Rotundo and Fonzar in
Chapter 8, endodontic treatment is manda-
tory before resective therapy whenever the
tooth is vital or the previous endodontic
treatment is incongruous (see Figures 4.17
and 4.18). Rubber dam is required for optimal
working conditions (Ahmad 2009; Lin et al.
2014). During cleaning and shaping, root
integrity has to be preserved as much as pos-
sible by minimally removing the dentine
along the canals. To avoid resection‐related
gutta‐percha exposure, the canal space has to
be filled 2–3 mm apical to the furcation
(Marin et al. 1989). Prior to the resective
therapy, resin composites can be used to
adhesively build up the abutment. Endodontic
posts or screws might be necessary whenever
the retention for the build‐up material is
poor. When the endodontic and restorative
protocols are properly followed, retention‐
related complications such as build‐up
debonding or breaking are generally avoided
(Carnevale et al. 2008).
Occasionally, the FI might be preoperatively
or intraoperatively underestimated and its
resolution might not be obtained by barrelling
only (Jameson and Malone 1982). The exposed
root canal entrances have to be carefully
sealed after the resective therapy, since the
incidence of pulp failures increases over time
(Smukler and Tagger 1976). In particular, 41%,
Box 4.8 Recommendations for regenerative furcation therapy.
●
For deep periodontal defects, with or without furcation involvement, on impairment‐free vital
teeth, regenerative therapy may be performed without endodontic pre‐treatment, since pulp
vitality is likely to be preserved.
●
For up‐to‐the‐apex periodontal defects, scaling and root planing (SRP) procedures might dam-
age the neuro‐vascular bundle of the tooth. Since pulp necrosis might occur during periodon-
tal healing, according to some authors root canal treatment could be preventively performed
to avoid any interference with the regeneration process (Cortellini and Tonetti 2001).
●
For deep periodontal defects on asymptomatic congruously root‐filled teeth with periapical
radiolucency, root canal retreatment should be delayed, since a periapical lesion might require
up to five years for comprehensive radiographic healing (Molven et al. 2002; Zitzmann et al.
2009; Abbott 2011).
●
For deep periodontal defects on symptomatic incongruously root‐filled teeth with periapical
radiolucency, root canal retreatment is mandatory before proceeding with guided tissue
regeneration therapy.
●
For deep periodontal defects on symptom‐free incongruously root‐filled teeth without peria-
pical translucency, no evidence‐based endodontic protocol has been defined so far, thus root
canal retreatment may be performed or not, depending on restorative purposes.
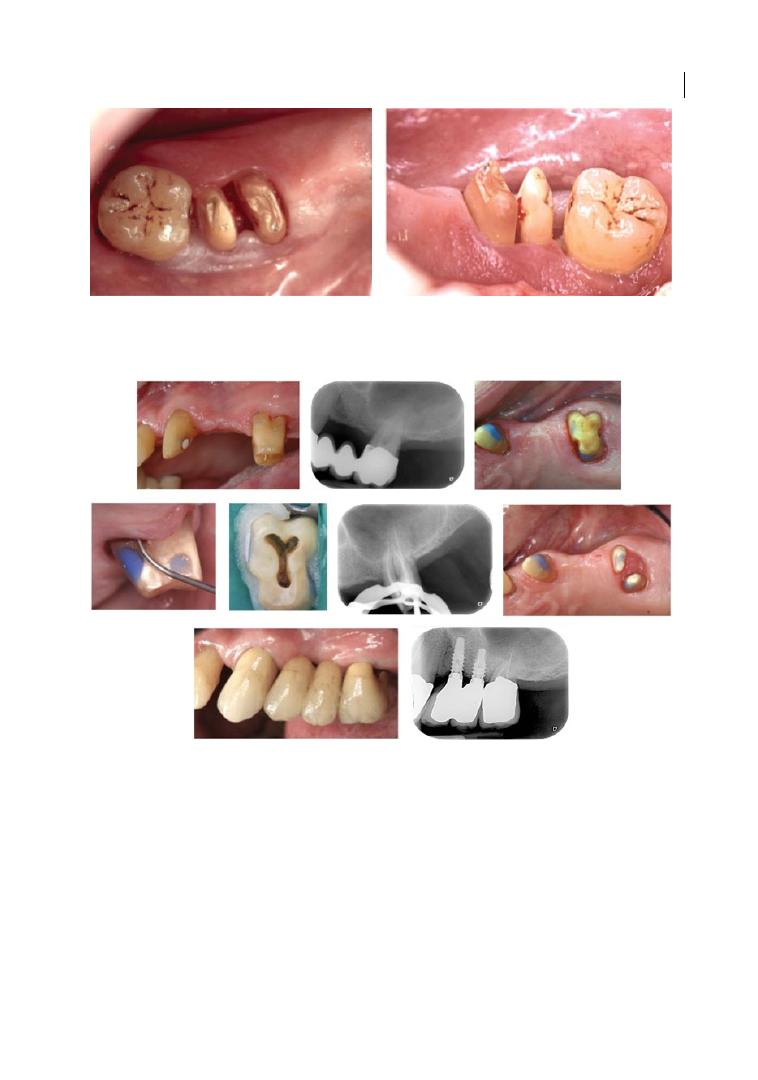
The Endodontist’s View 81
62%, and 87% of resected teeth show pulp
necrosis after six months, one year, and five
years, respectively (Filipowicz et al. 1984).
Because of the poor short‐term endodontic
prognosis, vital teeth should be devitalized
before the resective therapy, or at least
within two weeks afterwards (Smukler and
Tagger 1976).
The operating recommendations in
Box 4.9 should be followed.
Figure 4.17
Pulp exposure after resective therapy on vital 4.6 (LR6). Root canal treatment was performed one
week later.
Figure 4.18
Degree II furcation defect (mesial and distal) on 2.6 (UL6). Minimally invasive access to the
endodontic space was obtained after isolation with rubber dam. Canals were conservatively shaped and filled
with gutta‐percha apical to the furcation floor. Resin composite was used to fill the root canal entrances and to
build up the cavity access. The rizotomy (root separation) of the mesio-buccal and palatal roots and the rizectomy
(root amputation) of the disto-buccal root were performed after endodontic treatment. All the root canals
were endodontically treated, since there was no pre‐operative certainty of the extraction of the distal root.

Chapter 4
82
References
Abbott, P.V. (2011). Diagnosis and
management planning for root‐filled teeth
with persisting or new apical pathosis.
Endodontic Topics 19, 1–21.
Abbott, P.V., and Salgado, J.C. (2009).
Strategies for the endodontic management
of concurrent endodontic and periodontal
diseases. Australian Dental Journal 54,
70–85.
Abou‐Rass, M. (1982). The stressed pulp
condition: An endodontic‐restorative
diagnostic concept. Journal of Prosthetic
Dentistry 48, 264–267.
Addy, M., Mostafa, P., and Newcombe, R.G.
(1987). Dentine hypersensitivity: The
distribution of recession, sensitivity and
plaque. British Dental Journal 162, 253–256.
Adriaens, P.A., De Boever, J.A., and Loesche,
W.J. (1987). Bacterial invasion in root
cementum and radicular dentin of
periodontally diseased teeth in humans:
A reservoir of periodontopathic bacteria.
Journal of Periodontology 59, 222–230.
Adriaens, P.A., Edwards, C.A., De Boever, J.A.,
and Loesche, W.J. (1988). Ultrastructural
observations on bacterial invasion in
Box 4.9 Recommendations for resective therapy.
●
Cleaning and shaping should be conservatively performed, and residual dentine thickness
should be preserved as much as possible to avoid root weakening.
●
For canal filling, gutta‐percha should be extended 2–3 mm apical to the furcation.
●
For the build‐up, resin composite should be used to adhesively restore the abutment.
●
Endodontic posts or screws should be placed only in low‐retention teeth.
●
Vital resected teeth should be endodontically treated within two weeks after the resective
therapy.
Summary of Evidence
●
Endodontium and periodontium influ-
ence each other during health, function,
and disease.
●
The histological pulp changes induced by
periodontal disease can be reparative or
degenerative. Pulp necrosis generally
occurs when the apical neuro‐vascular
bundle is involved.
●
Accessory canals in the furcation
region are frequent and might repre-
sent a communication pathway between
endodontic and periodontal patholo-
gies through the induction of inflam-
matory responses.
●
Primary endodontic lesions might resem-
ble a furcation class III periodontal defect,
when the fistulous sinus tract drains
through the periodontal ligament in the
inter‐radicular space of multi‐rooted teeth.
●
Pulp sensitivity tests and periodontal
probing are essential for the differential
diagnosis of endodontic and periodontal
diseases.
●
When combined endodontic and perio-
dontal lesions merge, root canal therapy
should be carried out a few months before
any further surgical periodontal treat-
ment, in order to evaluate the part of the
defect originating from the endodontic
disease.
●
The vitality of the pulp seems to hinder
bacteria migration from the periodontal
pocket to the endodontium.
●
Except for root resective therapy, non‐
surgical, surgical, and regenerative perio-
dontal therapies for furcation lesions do
not benefit from any preventive root canal
treatment.

The Endodontist’s View 83
cementum and radicular dentin of
periodontally diseased human teeth. Journal
of Periodontology 59, 493–503.
Aguiar, T.R., Tristao, G.C., Mandarino, D. et al.
(2014). Histopathologic changes in dental
pulp of teeth with chronic periodontitis.
Compendium of Continuing Education in
Dentistry 35, 344–351.
Ahmad, I.A. (2009). Rubber dam usage for
endodontic treatment: A review. International
Endodontic Journal 42, 963–972.
AIE Accademia Italiana di Endodonzia (2014).
Patologia da carico e sovraccarico dentale.
In: Elementi di anatomia, fisiologia e
patologia del complesso pulpo‐dentinale: La
diagnosi (ed. F. Fonzar and M. Venturi), 139.
Padova: Piccin Nuova Libraria.
Aleo, J,J., De Renzis, F.A., Farber, P.A., and
Varboncoeur, A.P. (1974), The presence and
biologic activity of cementum‐bound
endotoxin. Journal of Periodontology 45,
672–675.
Alghaithy, R.A., and Qualtrough, A.J. (2017).
Pulp sensibility and vitality tests for
diagnosing pulpal health in permanent
teeth: A critical review. International
Endodontic Journal 50, 135–142.
American Association of Endodontists (2015).
Glossary of Endodontic Terms, 9th edn.
Chicago, IL: American Association of
Endodontists.
Andreasen, F.M., and Andersson, L. (2007).
Textbook and Color Atlas of Traumatic
Injuries to the Teeth, 4th edn. Oxford:
Blackwell.
Bartold, P.M. (2006). Dentinal hypersensitivity:
A review. Australian Dental Journal 51,
212–218.
Bastos, J.V., Goulart, E.M., and de Souza
Côrtes, M.I. (2014). Pulpal response to
sensibility tests after traumatic dental
injuries in permanent teeth. Dental
Traumatology 30, 188–192.
Bender, I.B., and Seltzer, S. (1972). The effect
of periodontal disease on the pulp. Oral
Surgery, Oral Medicine, Oral Pathology 33,
458–474.
Bergenholtz, G. (1981). Inflammatory
response of the dental pulp to bacterial
irritation. Journal of Endodontics 7,
100–104.
Bergenholtz, G., and Hasselgreen, G. (2008).
Endodontics and periodontics. In: Clinical
Periodontology and Implant Dentistry, 5th
edn (ed. J. Lindhe, N.P. Lang, and T.
Karring), 848–874. Oxford: Blackwell
Munksgaard.
Bergenholtz, G., and Lindhe, J. (1978). Effect of
experimentally induced marginal
periodontitis and periodontal scaling on the
dental pulp. Journal of Clinical
Periodontology 5, 59–73.
Bergenholtz, G., and Ricucci, D. (2008).
Lesions of endodontic origin. In: Clinical
Periodontology and Implant Dentistry, 5th
edn (ed. J. Lindhe, N.P. Lang, and T.
Karring), 518–519. Oxford: Blackwell
Munksgaard.
Berner, E.S., and Graber, M.L. (2008).
Overconfidence as a cause of diagnostic
error in medicine. American Journal of
Medicine 121, 2–23.
Biancu, S., Ericsson, I., and Lindhe, J. (1995).
Periodontal ligament tissue reactions to
trauma and gingival inflammation: An
experimental study in the beagle dog.
Journal of Clinical Periodontology 22,
772–779.
Boyko, G.A., Brunette, D.M., and Melcher,
A.H. (1980). Cell attachment to
demineralized root surfaces in vitro. Journal
of Periodontal Research 15, 297–303.
Burch, J.G., and Hulen, S. (1974). A study of
the presence of accessory foramina and the
topography of molar furcations. Oral
Surgery, Oral Medicine, Oral Pathology 38,
451–455.
Cameron, C.E. (1964). Cracked‐tooth
syndrome. Journal of the American Dental
Association 68, 405–411.
Carnevale, G., Pontoriero, R., and Lindhe, J.
(2008). Treatment of furcation‐involved
teeth. In: Clinical Periodontology and
Implant Dentistry, 5th edn (ed. J. Lindhe,
N.P. Lang, and T. Karring), 349–374.
Oxford: Blackwell Munksgaard.
Chan, C.P., Lin, C.P., Tseng, S.C., and Jeng, J.H.
(1999). Vertical root fracture in

Chapter 4
84
endodontically versus non endodontically
treated teeth: A survey of 315 cases in
Chinese patients. Oral Surgery, Oral
Medicine, Oral Pathology, Oral Radiology,
Endodontics 87, 504–507.
Chen, E., and Abbott, P.V. (2009). Dental pulp
testing: A review. International Journal of
Dentistry 2009, 1–12.
Chen, S.Y., Wang, H.L., and Glickman, G.N.
(1997). The influence of endodontic
treatment upon periodontal wound healing.
Journal of Clinical Periodontology 24,
449–456.
Cheron, R.A., Marshall, S.J., Goodis, H.E., and
Peters, O.A. (2011). Nanomechanical
properties of endodontically treated teeth.
Journal of Endodontics 37, 1562–1565.
Cortellini, P., and Tonetti, M.S. (2001).
Evaluation of the effect of tooth vitality on
regenerative outcomes in infrabony defects.
Journal of Clinical Periodontology 28,
672–679.
Cotton, W.R., and Siegel, R.L. (1977). Pulp
response to citric acid cavity cleanser. US
Navy Medicine 68, 27–29.
Czarnecki, R.T., and Schilder, H. (1979). A
histological evaluation of the human pulp in
teeth with varying degrees of periodontal
disease. Journal of Endodontics 5, 242–253.
Daly, C.G., Seymour, G.J., Kieser, J.B., and
Corbet, E.F. (1982). Histological assessment
of periodontally involved cementum.
Journal of Clinical Periodontology 9,
266–274.
De Deus, Q.D. (1975). Frequency, location, and
direction of the lateral, secondary, and
accessory canals. Journal of Endodontics 1,
361–366.
D’Souza, J.E., Walton, R.E., and Peterson, L.C.
(1987). Periodontal ligament injection: An
evaluation of the extent of anaesthesia and
postinjection discomfort. Journal of the
American Dental Association 114, 341–344.
Eli, I. (1993). Dental anxiety: A cause for
possible misdiagnosis of tooth vitality.
International Endodontic Journal 26,
251–253.
European Society of Endodontology (2006).
Quality guidelines for endodontic treatment:
Consensus report of the European Society of
Endodontology. International Endodontic
Journal 39, 921–930.
Faria, A.C., Rodrigues, R.C., de Almeida
Antunes, R.P. et al. (2011). Endodontically
treated teeth: Characteristics and
considerations to restore them. Journal of
Prosthodontic Research 55, 69–74.
Filipowicz, F., Umstott, P., and England, M.
(1984). Vital root resection in maxillary
molar teeth: A longitudinal study. Journal of
Endodontics 10, 264–268.
Garg, J., Maurya, R., Gupta, A. et al. (2015). An
in vitro scanning electron microscope study
to evaluate the efficacy of various root
conditioning agents. Journal of Indian
Society of Periodontology 19, 520–524.
Gargiulo, A.V., Jr (1984). Endodontic‐
periodontic interrelationships: Diagnosis
and treatment. Dental Clinics of North
America 28, 767–781.
Ghazali, F.B. (2003). Permeability of dentine.
Malaysian Journal of Medical Sciences 10,
27–36.
Gillam, D.G., and Orchardson, R. (2006).
Advances in the treatment of root dentine
sensitivity: Mechanisms and treatment
principles. Endodontic Topics 13, 13–33.
Giuliana, G., Ammatuna, P., Pizzo, G. et al.
(1997). Occurrence of invading bacteria in
radicular dentin of periodontally diseased
teeth: Microbiological findings. Journal of
Clinical Periodontology 24, 478–485.
Gkranias, N.D., Graziani, F., Sculean, A., and
Donos, N. (2012). Wound healing following
regenerative procedures in furcation degree
III defects: Histomorphometric outcomes.
Clinical Oral Investigation 16, 239–249.
Goldberg, F., Massone, E.J., Soares, I., and
Bittencourt, A.Z. (1987). Accessory orifices:
Anatomical relationship between the pulp
chamber floor and the furcation. Journal of
Endodontics 13, 176–181.
Gopikrishna, V., Tinagupta, K., and
Kandaswamy, D. (2007). Comparison of
electrical, thermal, and pulse oximetry
methods for assessing pulp vitality in
recently traumatized teeth. Journal of
Endodontics 33, 531–535.

The Endodontist’s View 85
Gorni, F.G., Andreano, A., Ambrogi, F. et al.
(2016). Patient and clinical characteristics
associated with primary healing of
iatrogenic perforations after root canal
treatment: Results of a long‐term Italian
study. Journal of Endodontics 42, 211–215.
Guldener, P.H. (1985). The relationship
between periodontal and pulpal disease.
International Endodontic Journal 18, 41–54.
Gutmann, J.L. (1978). Prevalence, location, and
patency of accessory canals in the furcation
region of permanent molars. Journal of
Periodontology 49, 21–26.
Haapasalo, M., Parhar, M., Huang, X. et al.
(2015). Clinical use of bioceramic materials.
Endodontic Topics 32, 97–117.
Hahn, C.L., and Overton, B. (1997). The effects
of immunoglobulins on the convective
permeability of human dentine in vitro.
Archives of Oral Biology 42, 835–843.
Harrington, G.W., and Steiner, D.R. (2002).
Periodontal‐endodontic considerations. In:
Principles and Practice of Endodontics, 3rd
edn (ed. R.E. Walton and M. Torabinejad),
466–484. Philadelphia, PA: W.B. Saunders.
Harrington, G.W., Steiner, D.R., and Ammons,
W.F. (2002). The periodontal‐endodontic
controversy. Periodontology 2000 30,
123–130.
Hattler, A.B., and Listgarten, M.A. (1984).
Pulpal response to root planing in a rat
model. Journal of Endodontics 10,
471–476.
Hoffman, I.D., and Gold, W. (1971). Distances
between plaque and remnants of attached
periodontal tissues on extracted teeth.
Journal of Periodontology 42, 29–30.
Huang, T.J., Schilder, H., and Nathanson, D.
(1992). Effects of moisture content and
endodontic treatment on some mechanical
properties of human dentin. Journal of
Endodontics 18, 209–215.
Jafarzadeh, H., and Abbott, P.V. (2010). Review
of pulp sensibility tests. Part II: Electric pulp
tests and test cavities. International
Endodontic Journal 43, 945–958.
Jameson, L.M., and Malone, W.F. (1982).
Crown contours and gingival response.
Journal of Prosthetic Dentistry 47, 620–624.
Kerns, D.G., and Glickman G.N. (2011).
Endodontic and periodontal
interrelationships. In: Cohen’s Pathways of
the Pulp, 10th edn (ed. K.M. Hargraves and
S. Cohen), 655–670. St Louis, MO:
Elsevier.
Kirkham, D.B. (1975). The location and
incidence of accessory pulpal canals in
periodontal pockets. Journal of the
American Dental Association 91, 353–356.
Kobayashi, T., Hayashi, A., Yoshikawa, R. et al.
(1990). The microbial flora from root canals
and periodontal pockets of non‐vital teeth
associated with advanced periodontitis.
International Endodontic Journal 23,
100–106.
Kurihara, H., Kobayashi, Y., Francisco, I.A. et al.
(1995). A microbiological and immunological
study of endodontic‐periodontic lesions.
Journal of Endodontics 21, 617–621.
Lambrianidis, T., Tziafas, D., and Kolokuris, I.
(1988). Pulpal response to topical
application of citric acid to root dentin.
Endodontics and Dental Traumatology 4,
12–15.
Langeland, K., Rodrigues, H., and Dowden, W.
(1974). Periodontal disease, bacteria, and
pulpal histopathology. Oral Surgery, Oral
Medicine, Oral Pathology 37, 257–270.
Lantelme, R.L., Handelman, S.L., and
Herbison, R.J. (1976). Dentin formation in
periodontally diseased teeth. Journal of
Dental Research 55, 48–51.
Lasho, D.J., O’Leary, T.J., and Kafrawy, A.H.
(1983). A scanning electron microscope
study of the effects of various agents on
instrumented periodontally involved root
surfaces. Journal of Periodontology 54,
210–220.
Levin, L.G. (2013). Pulp and periradicular
testing. Journal of Endodontics 39, 13–19.
Lewinstein, I., and Grajower, R. (1981). Root
dentin hardness of endodontically treated
teeth. Journal of Endodontics 7, 421–422.
Liewehr, F.R. (2001). An inexpensive device for
transillumination. Journal of Endodontics 27,
130–131.
Lin, P.Y., Huang, S.H., Chang, H.J., and Chi,
L.Y. (2014). The effect of rubber dam usage

Chapter 4
86
on the survival rate of teeth receiving initial
root canal treatment: A nationwide
population‐based study. Journal of
Endodontics 40, 1733–1737.
Lindhe, J., Nyman S., and Ericsson I. (2008).
Trauma from occlusion: Periodontal tissues.
In: Clinical Periodontology and Implant
Dentistry, 5th edn (ed. J. Lindhe, N.P. Lang,
and T. Karring), 349–374. Oxford: Blackwell
Munksgaard.
Löe H., Theilade, E., and Jensen, S.B. (1965).
Experimental gingivitis in man. Journal of
Periodontology 36, 177–187.
Love, R.M., and Jenkinson, H.F. (2002).
Invasion of dentinal tubules by oral bacteria.
Critical Reviews in Oral Biology and
Medicine 13, 171–183.
Lowman, J.V., Burke, R.S., and Pelleu, G.B.
(1973). Patent accessory canals: Incidence in
molar furcation region. Oral Surgery, Oral
Medicine, Oral Pathology 36, 580–584.
Lubisich, E.B., Hilton, T.J., and Ferracane, J.
(2010). Cracked teeth: A review of the
literature. Journal of Esthetic and Restorative
Dentistry 22, 158–167.
Marin, C., Carnevale, G., Di Febo, G., and
Fuzzi, M. (1989). Restoration of
endodontically treated teeth with
interradicular lesions before root removal
and/or root separation. International
Journal of Periodontics and Restorative
Dentistry 9, 42–57.
Marroquin, B.B., El‐Sayed, M.A., and
Willershausen‐Zönnchen, B. (2004).
Morphology of the physiological foramen: I.
Maxillary and mandibular molars. Journal
of Endodontics 30, 321–328.
Mazur, B., and Massler, M. (1964). Influence of
periodontal disease on the dental pulp. Oral
Surgery, Oral Medicine, Oral Pathology 17,
592–603.
McInnes‐Ledoux, P., Cleaton‐Jones, P.E., and
Austin, J.C. (1985). The pulpal response to
dilute citric acid smear removers. Journal of
Oral Rehabilitation 12, 215–228.
Mejàre, I.A., Axelsson, S., Davidson, T. et al.
(2012). Diagnosis of the condition of the
dental pulp: A systematic review.
International Endodontic Journal 45,
597–613.
Mjör, I.A., Smith, M.R., Ferrari, M., and
Mannocci, F. (2001). The structure of dentin
in the apical region of human teeth.
International Endodontic Journal 34,
346–353.
Mohammadi, Z., Palazzi, F., Giardino, L., and
Shalavi, S. (2013). Microbial biofilms in
endodontic infections: An update review.
Biomedical Journal 36, 59–70.
Molven, O., Halse, A., Fristad, I., and
MacDonald‐Jankowski, D. (2002). Periapical
changes following root‐canal treatment
observed 20–27 years postoperatively.
International Endodontic Journal 35,
784–790.
Myers, J.W. (1998). Demonstration of a
possible source of error with an electric pulp
tester. Journal of Endodontics 24, 199–200.
Nagaoka, S., Miyazaki, Y., Liu, H.J. et al. (1995).
Bacterial invasion into dentinal tubules of
human vital and nonvital teeth. Journal of
Endodontics 21, 70–73.
Ng, Y.L., Mann, V., Rahbaran, S. et al. (2007).
Outcome of primary root canal treatment:
Systematic review of the literature. Part 1:
Effects of study characteristics on
probability of success. International
Endodontic Journal 40, 921–939.
Nibali, L., Pometti, D., Chen, T.T., and Tu, Y.K.
(2015). Minimally invasive non‐surgical
approach for the treatment of periodontal
intrabony defects: A retrospective analysis.
Journal of Clinical Periodontology 42,
853–859.
Nilvéus, R., and Selvig, K.A. (1983). Pulpal
reactions to the application of citric acid to
root‐planed dentin in beagles. Journal of
Periodontal Research 18, 420–428.
Page, R.C. (1999). Milestones in periodontal
research and the remaining critical issues.
Journal of Periodontal Research 34,
331–339.
Parirokh, M., and Torabinejad, M. (2010).
Mineral trioxide aggregate:
A comprehensive literature review. Part III:
Clinical applications, drawbacks, and

The Endodontist’s View 87
mechanism of action. Journal of Endodontics
36, 400–413.
Parolia, A., Gait, T.C., Porto, I.C.C.M., and
Mala, K. (2013). Endo‐perio lesion: A
dilemma from 19th until 21st century.
Journal of Interdisciplinary Dentistry 3, 2–11.
Pashley, D.H. (1990). Mechanisms of dentin
sensitivity. Dental Clinics of North America
34, 449–473.
Pashley, D.H., Matthews, W.G., Zhang Y., and
Johnson, M. (1996). Fluid shifts across
human dentine in vitro in response to
hydrodynamic stimuli. Archives of Oral
Biology 41, 1065–1072.
Pashley, D.H., Pashley, E.L., Carvalho, R.M.,
and Tay, F.R. (2002). The effects of dentin
permeability on restorative dentistry. Dental
Clinics of North America 46, 211–245.
Patel, S., Ricucci, D., Durak, C., and Tay, F.
(2010). Internal root resorption: A review.
Journal of Endodontics 36, 1107–1121.
Paul, B.F., and Hutter, J.W. (1997). The
endodontic‐periodontal continuum revisited:
New insights into aetiology, diagnosis and
treatment. Journal of the American Dental
Association 128, 1541–1548.
Peters, D.D., Baumgartner, J.C., and Lorton, L.
(1994). Adult pulpal diagnosis. I: Evaluation
of the positive and negative responses to
cold and electrical pulp tests. Journal of
Endodontics 20, 506–511.
Petersson, K., Söderström, C., Kiani‐Anaraki,
M., and Lévy, G. (1999). Evaluation of the
ability of thermal and electrical tests to
register pulp vitality. Endodontics and
Dental Traumatology 15, 127–131.
Pinheiro, E.T., Gomes, B.P., Ferraz, C.C. et al.
(2003). Microorganisms from canals of
root‐filled teeth with periapical lesions.
International Endodontic Journal 36, 1–11.
Pitaru, S., and Melcher, A.H. (1987).
Organization of an oriented fiber system in
vitro by human gingival fibroblasts attached
to dental tissue: Relationship between cells
and mineralized and demineralized tissue.
Journal of Periodontal Research 22, 6–13.
Polson, A.M., Frederick, G.T., Ladenheim, S.,
and Hanes, P.J. (1984). The production of a
root surface smear layer by instrumentation
and its removal by citric acid. Journal of
Periodontology 55, 443–446.
Rapp, R., Matthews, G., Simpson, M., and
Pashley, D.H. (1992). In vitro permeability of
furcation dentin in permanent teeth. Journal
of Endodontics 18, 444–447.
Rathod, S.R., Fande P., and Sarda, T.S. (2014).
The effect of chronic periodontitis on dental
pulp: A clinical and histopathological study.
Journal of the International Clinical Dental
Research Organization 6, 107–111.
Rôças, I.N., Siqueira, J.F., Jr, and Santos, K.R.
(2004). Association of Enterococcus faecalis
with different forms of periradicular
diseases. Journal of Endodontics 30,
315–320.
Ross, I.F., and Thompson, R.H. (1978). A long
term study of root retention in the
treatment of maxillary molars with furcation
involvement. Journal of Periodontology 49,
238–244.
Rotstein, I., and Simon, J.H.S. (2004).
Diagnosis, prognosis and decision‐making
in the treatment of combined periodontal‐
endodontic lesions. Periodontology 2000 34,
165–203.
Rowe, A.H., and Pitt Ford, T.R. (1990). The
assessment of pulpal vitality. International
Endodontic Journal 23, 77–83.
Rubach, W.C., and Mitchell, D.F. (1965).
Periodontal disease, age, and pulp status.
Oral Surgery, Oral Medicine, Oral Pathology
19, 482–493.
Ryan, P.C., Newcomb, G.M., Seymour, G.J.,
and Powell, R.N. (1984). The pulpal
response to citric acid in cats. Journal of
Clinical Periodontology 11, 633–643.
Sakamoto, M., Siqueira, J.F., Jr, Rôças, I.N., and
Benno, Y. (2008). Molecular analysis of the
root canal microbiota associated with
endodontic treatment failures. Oral
Microbiology and Immunology 23, 275–281.
Schmidt, J.C., Walter, C., Amato, M., and
Weiger, R. (2014). Treatment of periodontal‐
endodontic lesions: A systematic review.
Journal of Clinical Periodontology 41,
779–790.

Chapter 4
88
Séguier, S., Godeau, G., and Brousse, N. (2000).
Collagen fibers and inflammatory cells in
healthy and diseased human gingival tissues:
A comparative and quantitative study by
immunohistochemistry and automated
image analysis. Journal of Periodontology 71,
1079–1085.
Seltzer, S., Bender, I.B., and Ziontz, M. (1963).
The interrelationship of pulp and
periodontal disease. Oral Surgery, Oral
Medicine, Oral Pathology 16, 1474–1490.
Shenoy, N., and Shenoy, A. (2010). Endo‐perio
lesions: Diagnosis and clinical
considerations. Indian Journal of Dental
Research 21, 579–585.
Sheykhrezaee, M.S., Eshghyar, N.,
Khoshkhounejad, A.A., and
Khoshkhounejad, M. (2007). Evaluation of
histopathologic changes of dental pulp in
advanced periodontal diseases. Acta Medica
Iranica 45, 51–57.
Simon, J.H., Glick, D.H., and Frank, A.L.
(1972). The relationship of endodontic‐
periodontic lesions. Journal of
Periodontology 43, 202–208.
Simring, M., and Goldberg, M. (1964). The
pulpal pocket approach: Retrograde
periodontitis. Journal of Periodontology 35,
22–48.
Smukler, H., and Tagger, M. (1976). Vital root
amputation: A clinical and histological
study. Journal of Periodontology 47,
324–330.
Sugaya, T., Nakatsuka, M., Inoue, K. et al.
(2015). Comparison of fracture sites and
post lengths in longitudinal root fractures.
Journal of Endodontics 41, 159–163.
Sundqvist, G. (1994). Taxonomy, ecology, and
pathogenicity of the root canal flora. Oral
Surgery, Oral Medicine, Oral Pathology 78,
522–530.
Sundqvist, G., Figdor, D., Persson, S., and
Sjögren, U. (1998). Microbiologic analysis of
teeth with failed endodontic treatment and
the outcome of conservative re‐treatment.
Oral Surgery, Oral Medicine, Oral Pathology,
Oral Radiology, Endodontics 85, 86–93.
Sunitha, V.R., Emmadi, P., Namasivayam, A.
et al. (2008). The periodontal‐endodontic
continuum: A review. Journal of
Conservative Dentistry 11, 54–62.
Tomasi, C., and Wennström, J.L. (2009).
Full‐mouth treatment vs. the conventional
staged approach for periodontal infection
control. Periodontology 2000 51, 45–62.
Torabinejad, M., and Kiger, R.D. (1985). A
histologic evaluation of dental pulp tissue of
a patient with periodontal disease. Oral
Surgery, Oral Medicine, Oral Pathology 59,
198–200.
Toto, P.D., and Gargiulo, A.W. (1970). Epithelial
and connective tissue changes in periodontitis.
Journal of Periodontology 41, 587–590.
Tronstad, L. (1988). Root resorption:
Aetiology, terminology and clinical
manifestations. Endodontics and Dental
Traumatology 4, 241–252.
Trope, M. (1998). Subattachment
inflammatory root resorption: Treatment
strategies. Practical Periodontics and
Aesthetic Dentistry 10, 1005–1010.
Trope, M., Tronstad, L., Rosenberg, E.S., and
Listgarten, M. (1988). Darkfield microscopy
as a diagnostic aid in differentiating
exudates from endodontic and periodontal
abscesses. Journal of Endodontics 14, 35–38.
Tsesis, I., Rosenberg, E., Faivishevsky, V. et al.
(2010). Prevalence and associated
periodontal status of teeth with root
perforation: A retrospective study of 2,002
patients’ medical records. Journal of
Endodontics 36, 797–800.
Vertucci, F.J. (2005). Root canal morphology
and its relationship to endodontic
procedures. Endodontic Topics 10, 3–29.
von Troil, B., Needleman, I., and Sanz, M.
(2002). A systematic review of the
prevalence of root sensitivity following
periodontal therapy. Journal of Clinical
Periodontology 29, 173–177.
Vongsavan, N., and Matthews, B. (1991). The
permeability of cat dentine in vivo and in
vitro. Archives of Oral Biology 36, 641–646.
Vongsavan, N., and Matthews, B. (1992). Fluid
flow through cat dentine in vivo. Archives of
Oral Biology 37, 175–185.
Walton, R.E., and Torabinejad, M. (2002).
Diagnosis and treatment planning. In:

The Endodontist’s View 89
Principles and Practice of Endodontics, 3rd
edn (ed. R.E. Walton and M. Torabinejad),
49–70. Philadelphia, PA: W.B. Saunders.
Weldon, J.K., Jr, Pashley, D.H., Loushine, R.J.
et al. (2002). Sealing ability of mineral
trioxide aggregate and super‐EBA when
used as furcation repair materials: A
longitudinal study. Journal of Endodontics
28, 467–470.
Wennström, J.L., Tomasi, C., Bertelle, A., and
Dellasega, E. (2005). Full‐mouth ultrasonic
debridement versus quadrant scaling and
root planing as an initial approach in the
treatment of chronic periodontitis. Journal
of Clinical Periodontology 32, 851–859.
Yoshiyama, M., Masada, J., Uchida, A., and
Ishida, H. (1989). Scanning electron
microscopic characterization of sensitive
vs. insensitive human radicular dentin.
Journal of Dental Research 68,
1498–1502.
Yoshiyama, M., Noiri, Y., Ozaki, K. et al.
(1990). Transmission electron microscopic
characterization of hypersensitive human
radicular dentin. Journal of Dental Research
69, 1293–1297.
Zehnder, M. (2001). Endodontic infection
caused by localized aggressive periodontitis:
A case report and bacteriologic evaluation.
Oral Surgery, Oral Medicine, Oral Pathology,
Oral Radiology, Endodontics 92, 440–445.
Zehnder, M., Gold, S.I., and Hasselgren, G.
(2002). Pathologic interactions in pulpal and
periodontal tissues. Journal of Clinical
Periodontology 29, 663–671.
Zitzmann, N.U., Krastl, G., Hecker, H. et al.
(2009). Endodontics or implants? A review
of decisive criteria and guidelines for single
tooth restorations and full arch
reconstructions. International Endodontic
Journal 42, 757–774.
Zuza, E.P., Carrareto, A.L., Lia, R.C. et al.
(2012). Histopathological features of dental
pulp in teeth with different levels of chronic
periodontitis severity. International Scholarly
Research Notices Dentistry 2012, 1–6.

Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
Chapter No.: 1 Title Name: <TITLENAME>
c05.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:20:17 PM Stage: <STAGE> WorkFlow:
CSW
Page Number: 91
91
5.1 Introduction
Chapter 1 highlighted how the anatomy of
multi‐rooted teeth favours microbial accu-
mulation, leading to periodontal breakdown
inside the root separation area. Furthermore,
we have now learned (see Chapter 3) that
plaque removal inside the furcation area is a
rather daunting and difficult task, both for
the clinician and for patients themselves. It is
therefore natural to assume that teeth
affected by furcation involvement (FI), being
more exposed to the microbial challenge, will
develop periodontal progression more rap-
idly and will have a higher risk of tooth loss.
This chapter reviews the evidence for this
and aims to provide long‐term data on tooth
loss in teeth with FI. This answers the ques-
tion ‘Why do really we care about furca-
tions?’ and perhaps provides the rationale for
the whole book, justifying the interest in
furcations as a therapeutic challenge for peri-
odontists, general dentists, and hygienists.
5.2 Measures of Disease
Progression
The reader of this book will be well aware that
periodontitis causes inflammatory resorption
of the attachment apparatus of the tooth,
which results in gingival bleeding, discomfort,
and eventually tooth mobility and exfoliation.
This is also potentially associated with onset
of systemic diseases like diabetes mellitus,
rheumatoid arthritis, and cardiovascular dis-
ease (EFP 2014). Therefore, the ‘effects’ or
end‐points of periodontitis could be meas-
ured as tooth loss, decreased patient quality of
life (QOL), and perhaps systemic effects of the
periodontal inflammatory reaction. It is logi-
cal to assume that these would be the out-
comes measured by any study assessing the
impact of periodontitis. However, reality tells
us that, since periodontitis is a chronic disease
that usually occurs over a long time span, most
periodontal studies focus on other, shorter‐
term measures of disease as main outcomes,
such as probing pocket depth (PPD), clinical
attachment levels (CAL), and bleeding on
probing (BOP). This is done with the under-
standing that these are surrogate markers of
the really relevant outcomes just described.
Recent systematic reviews on furcations
followed this approach and focused on short‐
term outcomes after regenerative surgery
(Graziani et al. 2015; Reddy et al. 2015; see
Chapters 6 and 7). This clearly represents a
limitation as, although an association exists
between these clinical parameters and disease
progression and tooth loss (Claffey and
Egelberg 1995; Chambrone et al. 2010), it is a
Chapter 5
Why do We Really Care About Furcations? Long‐term
Tooth Loss Data
Luigi Nibali
Centre for Immunobiology and Regenerative Medicine, Centre for Oral Clinical Research, Institute of Dentistry, Barts and the London
School of Medicine and Dentistry, Queen Mary University of London (QMUL), London, UK
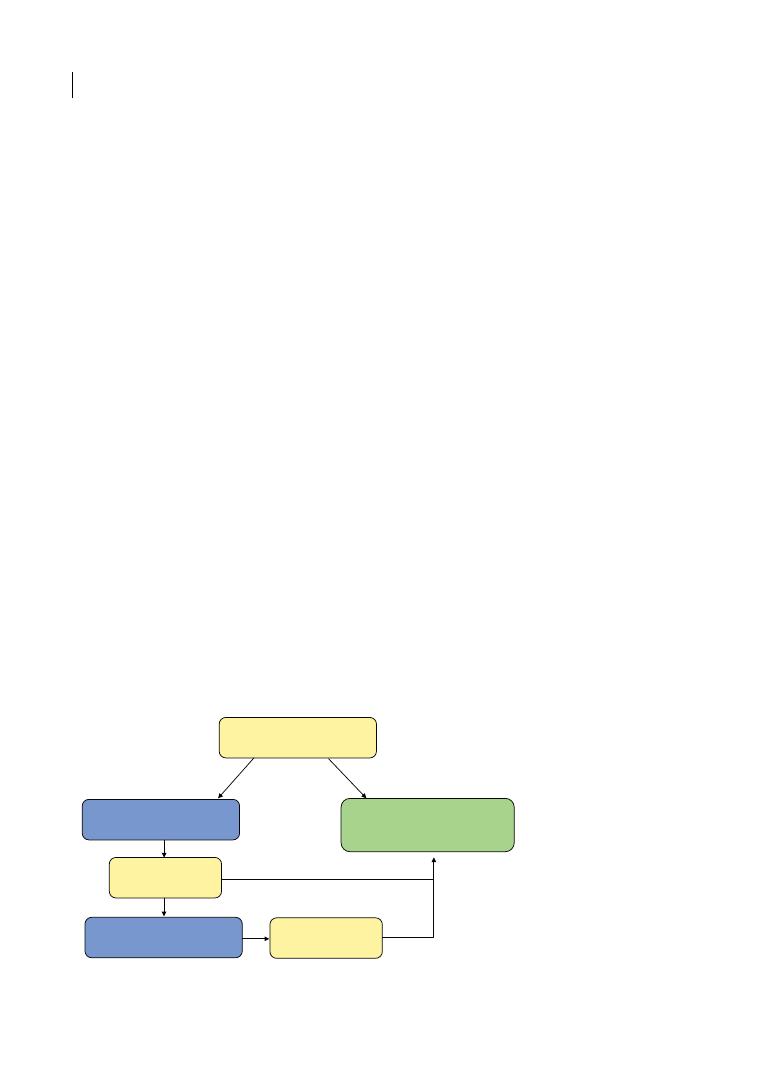
Chapter 5
92
far from ideal approach. On the other end, the
outcome ‘tooth loss’ is severely influenced by
the treating clinician, and by their treatment
philosophy and inclination to be more or less
conservative. With this in mind, in the view of
this author studies on FI should ideally meas-
ure ‘tooth loss’, QOL measures, and measures
of systemic burden of periodontitis as out-
comes. In reality, what emerges from the peri-
odontal literature is that only the outcome
‘tooth loss’ has been assessed by a large
enough number of studies to allow for making
conclusions on how it can be influenced by FI.
5.3 Tooth Loss
Although natural tooth exfoliation can still
occur in the general population, it is assumed
that ‘tooth loss’ usually occurs as tooth extrac-
tion performed by a dentist, at least in indus-
trialized countries. Severe periodontitis is
estimated to be the sixth most prevalent
chronic disease in humans (Kassebaum et al.
2014) and it is considered one of the main
causes of tooth loss (Hull et al. 1997; Al‐
Shammari et al. 2005; Akhter et al. 2008).
Periodontal treatment classically consists of
oral hygiene instructions, supra‐ and subgin-
gival tooth debridement (with or without
adjunctive therapy such as antimicrobials),
followed by a re‐evaluation. At this stage,
cases deemed to have reached stability will
enter a phase named ‘maintenance care’ or
‘supportive periodontal therapy’ (SPT)
directly or after the provision of surgical ther-
apy, depending on the case (see Figure 5.1).
Provision of regular SPT, consisting of oral
hygiene reinforcement and motivation, peri-
odontal charting, and supra‐ and subgingival
debridement, is associated with a reduced risk
of tooth loss (Lee et al. 2015). Long‐term lon-
gitudinal studies in unspecified periodontitis
cohorts or chronic periodontitis in SPT
reported tooth loss of approximately 0.10
(Hirschfeld and Wasserman 1978), 0.13
(McGuire and Nunn 1996), 0.15 (Eickholz
et al. 2008), 0.18 (McFall 1982), and up to 0.30
teeth per patient per year (Tsami et al. 2009).
A systematic review of studies including peri-
odontal maintenance care following compre-
hensive periodontal treatment showed that in
the studies included, 3919 teeth from a total of
41 404 were lost during the maintenance
period. From 36 to 88.5% of patients did not
experience tooth loss during the follow‐up
period in the different studies. The percent-
ages of tooth loss due to periodontal reasons
varied from 1.5 to 9.8%. Patient‐related fac-
tors (i.e. age and smoking) and tooth‐related
factors (tooth type and location, and the initial
tooth prognosis) were associated with tooth
loss (Chambrone et al. 2010). In a more recent
systematic review, Trombelli and co‐workers
Corrective
(surgical) therapy
Supportive
periodontal therapy
(Maintenance)
Cause-related
therapy
Periodontal visit
Periodontitis
Re-evaluation
Re-evaluation
Health
Figure 5.1
The different steps of periodontal therapy.

Long-term Tooth Loss Data 93
observed a weighted mean yearly tooth loss
rate during SPT of 0.15 and 0.09 teeth/patient/
year for follow‐up of 5 years and 12–14 years,
respectively (Trombelli et al. 2015). Another
systematic review in aggressive periodontitis
(AgP) cases, including 16 longitudinal studies,
revealed that the average tooth loss for all AgP
cases was 0.09 teeth/patient/year (95% confi-
dence interval [CI] = 0.06–0.16), therefore in
line with chronic periodontitis (CP) studies
(Nibali et al. 2013).
But what is the relative contribution of FI
to the tooth loss outcome? The following
paragraphs will review the evidence from the
periodontal literature for tooth loss in molars
with FI.
5.4 Tooth Loss for Untreated
Furcation‐involved Teeth
Although it seems almost obvious from what
has been discussed so far that molars with FI
have a greater risk of being extracted com-
pared with molars with no FI, very few stud-
ies have systematically assessed this question
and the magnitude of such a risk, especially
in untreated populations. Bjorn and Hjort
(1982) published the results of a longitudinal
study on a sample of 221 staff members of a
Swedish industrial company, originally
examined in 1965 and then re‐examined in
1978. These subjects were not receiving a
specific treatment protocol. Radiographic
mandibular molar inter‐radicular bone
destruction was used for furcation diagnosis,
in the absence of clinical data. Only 1.1–2.7%
of the molars had bone loss affecting more
than 50% of the distance vertex to apex, and
bone loss in furcation increased from 18 to
32% in the 13‐year follow‐up period. During
this time, 9% of furcated molars were lost,
but only 2.5% were estimated to have been
lost due to progressive FI. Although these
percentages are relatively low, we should
highlight that this was a general population
(not specifically subjects selected for hav-
ing periodontitis) and it is not clear what
treatment if any they received during the
follow‐up period.
Similarly, data were recently published on a
total of 3267 molars of 1897 subjects partici-
pating in the 11‐year follow‐up of the Study
of Health in Pomerania (SHIP; Nibali et al.
2017). All subjects had half‐mouth periodon-
tal examinations, including FI measurements
with a straight probe in one upper and one
lower molar at baseline. Only 28% of subjects
reported having had some form of unspeci-
fied ‘gum treatment’ throughout the course
of the observational period. In total, 375 sub-
jects (19.8%) lost molars during the follow‐
up period. Respectively 5.6%, 12.7%, 34.0%,
and 55.6% of molars without FI, degree I FI,
degree II FI, and degree III FI were lost. As
well as initial PPD and CAL and diagnosis of
periodontitis (p < 0.001), FI was associated
with molar loss in the 11‐year follow‐up. The
calculated incidence rate ratios (IRR) for
molar loss were 1.73 (95% CI = 1.34–2.23,
p < 0.001) for degree I FI and 3.88 (95%
CI = 2.94–5.11, p < 0.001) for degree II–III,
compared with without FI at baseline. These
results were confirmed in subanalysis of the
72% of subjects who had no periodontal
treatment during the course of the study
(who could more genuinely be considered
‘untreated’; Nibali et al. 2017).
5.5 Tooth Loss for Treated
Furcation‐involved Teeth
Fu and Wang summarized in Table 3.1 some
longitudinal studies reporting tooth loss by
FI by research groups in the USA and Europe.
The classic study by Hirschfeld and
Wasserman (1978) was perhaps the first large
published study assessing long‐term tooth
prognosis in patients with periodontitis.
Following up 600 patients during SPT for at
least 15 years retrospectively (average
22 years), the authors observed that 300
patients had lost no teeth from periodontal
disease, 199 had lost 1–3 teeth, 76 had lost
4–9 teeth, and 25 had lost 10–23 teeth. These

Chapter 5
94
figures helped identify three different groups
of patients based on progression pattern:
‘well‐maintained’ (the great majority), ‘down-
hill’, and ‘extreme downhill’. Of 1464 teeth
which originally had FI, 460 were lost after
the average 22 years follow‐up, 240 of them
by one‐sixth of the patients who deteriorated
the most.
A systematic review on long‐term tooth
loss related to FI revealed that the survival
rate of molars treated non‐surgically was
more than 90% after 5–9 years, with different
breakdowns according to treatment proto-
cols and varying degrees of disease severity
(Huynh‐Ba et al. 2009). Although no meta‐
analysis could be produced, the authors con-
cluded that initial FI (degree I) could be
successfully managed by non‐surgical
mechanical debridement, and that vertical
root fractures and endodontic failures were
the most frequent complications observed
following resective procedures of molars
with FI.
A more recent systematic review tried to
answer the focused question: ‘What is the
risk of tooth loss in teeth with furcation
involvement and which factors affect the
outcome?’ (Nibali et al. 2016). Longitudinal
human studies in patients with CP present-
ing data on furcation diagnosis and tooth loss
were considered eligible. In order to be
included, studies had to have ‘secure’ furca-
tion diagnosis (clinical with Nabers probe or
equivalent), treatment of FI provided, a fol-
low‐up of at least three years, and had to
report tooth loss data by furcation diagnosis.
The literature search was conducted at Ovid
Medline, Embase, LILACS, and Cochrane
Library and complemented by a hand search.
Studies were selected in two‐stage screening
carried out by two independent reviewers.
Following an initial screening of 1207 arti-
cles, full‐text review resulted in 21 articles
which met the defined inclusion criteria.
Table 5.1 reports the characteristics of the
sample included in the reviewed studies,
which had been carried out in the USA
(n = 11), Germany (n = 6), Sweden (n = 2),
Switzerland (n = 1), and Italy (n = 1) across
five decades from the 1970s to the 2010s, and
the interventions of these studies (divided
into active and supportive periodontal ther-
apy). Five of the included papers focused on
specific treatment for a specific group of
furcation‐involved teeth (Haney et al. 1997;
Yukna and Yukna 1997; Eickholz and
Hausmann 2002; Little et al. 1995;
Zafiropoulos et al. 2009), while fourteen
papers assessed long‐term tooth loss in
cohorts of periodontitis patients during
maintenance care and were suitable for
meta‐analysis. SPT protocols (when speci-
fied) generally included periodic (3‐ to 6‐ to
12‐monthly) periodontal clinical measure-
ments, oral hygiene instructions, and subgin-
gival debridement and a range of different
periodontal surgeries if considered neces-
sary. The risk of bias analyses performed
using the Newcastle Ottawa scale showed
that study quality scores ranged from a total
of 3 to a total of 5 (out of a maximum total of
9 stars). The asymmetrical results of funnel
plots of meta‐analysis of relative risk for
tooth loss based on follow‐up periods
revealed potential publication bias (see
Nibali et al. 2016).
Data on tooth loss by furcation diagnosis
was obtained, when possible with a break-
down on first, second, and third molars.
Although studies focusing only on AgP had
been excluded, some of the included studies
incorporated a small subset of AgP cases
(Dannewitz et al. 2006; Pretzl et al. 2008;
Salvi et al. 2014; Graetz et al. 2015) and only
in one of these papers was it possible to
obtain separate data on CP from the authors
(Dannewitz et al. 2006). Only data on tooth
loss following initial therapy (during mainte-
nance care) were analysed.
5.5.1 Tooth Loss for FI vs No FI
Grouping studies reporting data on tooth loss
for molars with and without FI, a total of 8143
molars without FI and a total of 5772 molars
with FI were included. Tooth survival ranged
from 94 to 100% after 4–7.5 years in regenera-
tion studies (Haney et al. 1997; Yukna and

Chapter No.: 1 Title Name: <TITLENAME>
c05.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:20:17 PM Stage: <STAGE> WorkFlow:
CSW
Page Number: 95
Table 5.1
Summary of study procedures for all included studies.
Author/ Year
Sample no.
Follow‐up years
(range)
Inclusion/disease classification
Active periodontal therapy (APT)
Supportive periodontal therapy
(SPT)
Lindhe and
Nyman 1975
75
5
≥50% loss of periodontal support and
optimal oral hygiene
OHI, SRP, restorative therapy if needed,
periodontal surgery in PPDs > 4 mm
(gingivectomy, Widman flaps, bone
recontouring, furcation plasty, tunnelling,
root resection as indicated)
3–6 monthly OHI and
prophylaxis by hygienist, yearly
periodontal examinations and
radiographs
Hirschfeld and
Wasserman 1978
600
22 (15–53)
‘Early’: PPD of 4 mm or less, with
gingival inflammation and subgingival
calculus; ‘intermediate’: PPD of
4–7 mm; ‘advanced’: PPD > 7 mm,
furcation involvement
Subgingival scaling with or without
surgery (additional surgical procedure or
non‐surgical procedure performed
depending on tooth diagnosis)
Deep scaling + ‘problem areas’
retreated when necessary,
occlusion
checked and adjusted as
indicated, OHI
McFall 1982
100
19 (15–29)
‘Early’: PPD ≤ 4 mm (n = 11);
‘intermediate’: PPD 4–7 mm (n = 53);
‘advanced’ PPD > 7 mm (n = 36)
Supragingival and subgingival scaling,
polishing, OHI, occlusal adjustment and
biteguards if needed, gingival curettage,
gingivectomy, gingivoplasty, ostectomy,
osteoplasty
Generally every 3–4 to 6 months
(including curettage, muco‐
periosteal flaps, osseous surgery,
root resection if needed)
Goldman et al.
1986
211
22.2 (15–34)
CP
Oral physiotherapy, supragingival and
subgingival scaling, OHI
3–6‐month recalls (selective
grinding and coronal reshaping,
adjunct restorative treatment if
needed)
Wood et al. 1989 63
13.6 (10–34)
Patients with moderate periodontitis
treated and maintained by SRP for 10
years or longer
OHI, non‐surgical (SRP, curettage,
occlusal adjustment) and surgical
treatment (gingivectomy, flap surgery, flap
curettage, osseous contouring, osseous
grafting, root amputation)
Not reported
Kuhrau et al. 1990 59
5.8 (4–8)
Patients with periodontitis with
furcation‐involved teeth treated
surgically
Surgical therapy (modified Widman flap,
root resection, tunnelling)
‘Regular’
Wang et al. 1994
24
8
Patients with CP who had completed
an 8‐year clinical trial and had no
more than 2 first or second molars
missing at baseline
SRP followed by one of three procedures:
pocket elimination
surgery, modified Widman flap surgery, or
gingival
curettage
3‐month recall interval for
maintenance prophylaxis and
yearly examinations
(Continued)
Chapter No.: 1 Title Name: <TITLENAME>
c05.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:20:17 PM Stage: <STAGE> WorkFlow:
CSW
Page Number: 96
Table 5.1
(Continued)
Author/ Year
Sample no.
Follow‐up years
(range)
Inclusion/disease classification
Active periodontal therapy (APT)
Supportive periodontal therapy
(SPT)
Little et al. 1995 18
4.6
Patient with periodontal disease with
deep class Il or III molar furcation
invasion
Surgical therapy consisting of osseous
resectioning and/or recontouring to the
adjacent mesial tooth and tunnelling
3‐monthly following surgery to
control plaque and potential
bacterial
pathogens
McGuire and
Nunn 1996
100
10
Chronic generalized moderate to
severe adult periodontitis
SRP, OHI, removal of fremitus, surgery if
indicated (osseous surgery, open SRP,
rarely bone grafts)
2‐ or 3‐month intervals (majority
under a 3‐month interval) – SRP,
polishing, minor occlusal
adjustments
Haney et al. 1997 13
4–5
CP
Coronally advanced flap procedures and
citric acid root treatment with or without
adjunctive implantation of freeze‐dried,
demineralized, allogeneic bone
6‐monthly for 5 years
Yukna and Yukna
1997
13
6.7 (6–7.5)
Grade II molar furcation
defects, with adjacent bone crest
height > 75% of the root length and
coronal to the furcation bone level
Regenerative surgery with bone grafts and
coronally advanced flaps
Weekly, then monthly deplaquing
until surgical re‐entry at 6–12
months, then 3‐month recalls
McLeod et al.
1998
114
12.5 (5–29)
Moderate to advanced
periodontitis with 4–7 mm or greater
AL
Non‐surgical therapy (OHI, SRP, occlusal
adjustment,
occasional use of systemic AB) followed by
surgical treatment (pocket reduction,
pocket elimination, occasional
regeneration)
6‐monthly
Eickholz and
Hausmann 2002
9
5
Advanced periodontal disease
Guided tissue regeneration
3‐monthly for the first 2 years
(OHI and professional tooth
cleaning), then 3–6‐monthly
according to individual risk
Checchi et al.
2002
92
6.7 (3–12)
Chronic adult periodontitis who
completed APT and have been on a
recall SPT schedule
OHI, SRP, re‐evaluation, and periodontal
surgery
3–4‐monthly hygienist
appointment recall

Chapter No.: 1 Title Name: <TITLENAME>
c05.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:20:17 PM Stage: <STAGE> WorkFlow:
CSW
Page Number: 97
Table 5.1
(Continued)
Author/ Year
Sample no.
Follow‐up years
(range)
Inclusion/disease classification
Active periodontal therapy (APT)
Supportive periodontal therapy
(SPT)
Little et al. 1995 18
4.6
Patient with periodontal disease with
deep class Il or III molar furcation
invasion
Surgical therapy consisting of osseous
resectioning and/or recontouring to the
adjacent mesial tooth and tunnelling
3‐monthly following surgery to
control plaque and potential
bacterial
pathogens
McGuire and
Nunn 1996
100
10
Chronic generalized moderate to
severe adult periodontitis
SRP, OHI, removal of fremitus, surgery if
indicated (osseous surgery, open SRP,
rarely bone grafts)
2‐ or 3‐month intervals (majority
under a 3‐month interval) – SRP,
polishing, minor occlusal
adjustments
Haney et al. 1997 13
4–5
CP
Coronally advanced flap procedures and
citric acid root treatment with or without
adjunctive implantation of freeze‐dried,
demineralized, allogeneic bone
6‐monthly for 5 years
Yukna and Yukna
1997
13
6.7 (6–7.5)
Grade II molar furcation
defects, with adjacent bone crest
height > 75% of the root length and
coronal to the furcation bone level
Regenerative surgery with bone grafts and
coronally advanced flaps
Weekly, then monthly deplaquing
until surgical re‐entry at 6–12
months, then 3‐month recalls
McLeod et al.
1998
114
12.5 (5–29)
Moderate to advanced
periodontitis with 4–7 mm or greater
AL
Non‐surgical therapy (OHI, SRP, occlusal
adjustment,
occasional use of systemic AB) followed by
surgical treatment (pocket reduction,
pocket elimination, occasional
regeneration)
6‐monthly
Eickholz and
Hausmann 2002
9
5
Advanced periodontal disease
Guided tissue regeneration
3‐monthly for the first 2 years
(OHI and professional tooth
cleaning), then 3–6‐monthly
according to individual risk
Checchi et al.
2002
92
6.7 (3–12)
Chronic adult periodontitis who
completed APT and have been on a
recall SPT schedule
OHI, SRP, re‐evaluation, and periodontal
surgery
3–4‐monthly hygienist
appointment recall
Author/ Year
Sample no.
Follow‐up years
(range)
Inclusion/disease classification
Active periodontal therapy (APT)
Supportive periodontal therapy
(SPT)
Dannewitz et al.
2006
71
5
CP or AgP
(≥50% bone loss in at least 2
permanent teeth)
OHI, professional tooth cleaning, SRP,
surgical intervention included access flap
surgery, GTR, tunnelling, resective
procedures, or tooth extraction
3–6‐ or 12‐monthly (clinical
measurements, plaque score, and
if needed re‐instrumentation of
PPD ≥ 4 mm and BOP, or ≥ 5 mm
Pretzl. et al. 2008 100
10
Generalized moderate CP and
generalized severe or aggressive
periodontitis
Subgingival debridement under local
anaesthesia and periodontal surgery if
required
Patients with and without SPT
(3–6‐monthly including OHI,
professional tooth cleaning,
polishing, application of a
fluoride gel)
Zafiropoulos et al.
2009
60
Min. 4
CP with a minimum of 4 sites with
CAL loss < 4 mm, radiographic
evidence of bone loss, and BOP in
4 sites
56 mandibular first and second molars
treated by hemisection (Group H, n = 32);
36 implants in the mandible to replace
periodontally involved first and second
molars (Group I, n = 28).
6‐monthly (OHI, supra‐ and
subgingival debridement,
polishing)
Johansson et al.
2013
64
14.8 (13–16)
Patients referred to the Department
of Periodontology
OH, supra‐ and subgingival scaling,
selective periodontal surgeries
(occasionally regenerative)
3–4‐monthly for 2 years by dental
hygienists (then referred back to
general dentist/hygienist for
supportive care)
Miller et al. 2014 106
15
Moderate to severe CP
Non‐surgical and surgical periodontal
treatment
Lasted for as long as the patient
continued to be seen (periodontal
health and oral hygiene
assessment, retreatment and
surgery when necessary)
Salvi et al. 2014
199
11.5
CP or AgP (Level 1: proximal
AL ≥ 3 mm at ≥ 2 non‐adjacent teeth;
level 2: proximal AL ≥ 5 mm in ≥ 30%
of teeth)
OHI, SRP, surgery if needed (OFD,
regeneration, tunnelling, or resective
surgery)
SPT at Department of
Periodontology or private
practice according to needs
(some ‘non‐compliers’)
Graetz et al. 2015 379
18.3
Chronic or aggressive periodontitis
with at least one first or second molar
present, regular SPT, and complete
radiological documentation at
baseline and last visit
SRP, OFD in case of PPD ≥ 5 mm with
BOP, or PPD ≥ 6 mm (tunnelling or root
resection when needed)
3–12‐monthly (non‐surgical or
surgical subgingival debridement
with or without AB)
AB = antibiotic; AgP = aggressive periodontitis); AL = attachment loss: APT = active periodontal treatment; BOP = bleeding on probing; CP = chronic periodontitis; OFD = Open
flap debridement; OHI = oral hygiene instructions; PPD = probing pocket depth; SPT = supportive periodontal therapy; SRP = scaling and root planing.
Source : Adapted from Nibali et al. ( 2016 ).

Chapter 5
98
Yukna 1997; Eickholz and Hausmann 2002),
89% after 5.8 years in a tunnelling study (Little
et al. 1995), 79% after a minimum of 4 years in
a root resection study (Zafiropoulos et al.
2009), and 43–100% after 5–53 years for stud-
ies including combined therapies (Hirschfeld
and Wasserman 1978; McFall 1982; Goldman
et al. 1986; Wood et al. 1989; Kuhrau et al.
1990; Wang et al. 1994; McGuire and Nunn
1996; Checchi et al. 2002; Dannewitz et al.
2006; Pretzl et al. 2008; Johansson et al. 2013;
Miller et al. 2014; Salvi et al. 2014; Graetz et al.
2015). Among teeth reported in these studies,
the average tooth loss/patient/year was 0.01
and 0.02 for molars without and with FI,
respectively. Periodontal progression, endo-
dontic complications, caries, and fractures
were reported as main causes of tooth loss
(Kuhrau et al. 1990; McLeod et al. 1998; Haney
et al. 1997; Yukna and Yukna 1997; Dannewitz
et al. 2006).
In studies reporting data for only first and
second molars (Hirschfeld and Wasserman
1978; McFall 1982; Goldman et al. 1986;
Wood et al. 1989; Dannewitz et al. 2006;
Pretzl et al. 2008; Johansson et al. 2013;
Miller et al. 2014; Graetz et al. 2015), the
following relative risks (RR) of tooth loss
were detected (see Figure 5.2):
●
RR = 2.90 (95% CI = 2.01–4.18) for molars
with FI vs no FI (p < 0.0001; various lengths
of follow‐up).
●
RR = 1.46 (95% CI = 0.99–2.15, p = 0.06) for
molars with FI vs no FI (p < 0.0001; 5–10
years follow‐up).
Total events
Heterogeneity: Tau
2
= 0.00; Chi
2
= 0.75, df = 1 (P = 0.39); I
2
= 0%
Test for overall effect: Z = 1.90 (P = 0.06)
74
35
2006
2.13 [0.83, 5.45]
6.9%
111
5
240
23
2008
1.35 [0.88, 2.06]
11.0%
309
30
390
51
Dannewitz et al. 2006
Pretzl et al. 2008
Total events
Heterogeneity: Tau
2
= 0.00; Chi
2
= 0.92, df = 2 (P = 0.63); I
2
= 0%
Test for overall effect: Z = 7.30 (P < 0.0001)
192
116
Miller et al. 2014
127
419
Subtotal (95% Cl)
664
50
397
879
2014
11.9%
2.41 [1.79, 3.24]
Johansson et al. 2013
30
94
37
267
2013
11.1%
2.18 [1.44, 3.31]
Wood et al. 1989
35
151
27
215
1989
10.7%
1.86 [1.17, 2.91]
33.7%
2.21 [1.79, 2.74]
Subtotal (95% Cl)
630
420 17.9%
1.67 [1.14, 2.43]
Study or Subgroup
5–10 years
10–15 years
Total events
Heterogeneity: Tau
2
= 0.28; Chi
2
= 67.73, df = 3 (P = 0.00001); I
2
= 96%
Test for overall effect: Z = 5.49 (P < 0.0001)
192
116
Goldman et al. 1986
235
562
Subtotal (95% Cl)
3177
110
835
4599
1986
12.4%
3.17 [2.60, 3.88]
Graetz et al. 2015
315 1183
118 1022
2015
0.01
0.1
Favors FI
Favors No FI
1
10
100
12.4%
2.31 [1.90, 2.80]
McFall 1982
82
147
25
492
1982
11.1% 10.98 [7.30, 16.51]
Hirchfeld & Wasserman 1978
384 1285
124 2250
1978
12.5%
5.42 [4.48, 6.56]
38.4%
4.46 [2.62, 7.62]
>15 years
With FI
Events Total
No FI
Events Total Weight IV. Random, 95% Cl Year
Risk Ratio
IV. Random, 95% Cl
Risk Ratio
Total events
Heterogeneity: Tau
2
= 0.27; Chi
2
= 104.40, df = 8 (P < 0.00001); I
2
= 92%
Test for overall effect: Z = 5.72 (P < 0.0001)
1282
528
Total (95% Cl)
4471
5898 100.0%
2.90 [2.01, 4.18]
Figure 5.2
Forest plot presenting relative risk (RR) of tooth loss based on follow‐up periods (excluding third
molars). Meta‐analysis for the comparison of tooth loss among selected studies presented an overall odds ratio
of 2.90 (95% confidence interval [CI] = 2.01–4.18, p < 0.0001). For studies with a follow‐up period of 5–10 years,
10–15 years, and > 15 years, the RR of tooth loss between teeth with and without furcation involvement was
1.46 (95% CI = 0.99–2.15, p = 0.06), 2.21 (95% CI = 1.79–2.74, p < 0.0001), and 4.46 (95% CI = 2.62–7.62,
p < 0.0001), respectively. Source: Nibali et al. (2016).

Long-term Tooth Loss Data 99
●
RR = 2.21 (95% CI = 1.79–2.74, p < 0.0001)
for molars with FI vs no FI (p < 0.0001;
10–15 years follow‐up).
●
RR = 4.46 (95% CI = 2.62–7.62, p < 0.0001)
for molars with FI vs no FI (p < 0.0001; > 15
years follow‐up).
Only the comparison for studies over more
than 15 years had a high degree of heteroge-
neity (p value for chi‐square test < 0.0001 and
I
2
test = 96%), hence it needs to be interpreted
cautiously. When third molars were included,
only small changes to the summary estimate
for risk of tooth loss by FI were detected (see
Nibali et al. 2016 for details).
5.5.2 Tooth loss by Different
Furcation Degree
An important question with clinical relevance
is whether the degree of FI affects the risk of
tooth loss. We previously (Section 5.4) dis-
cussed an increased risk of tooth loss by
increased degree of FI in a largely untreated
population participating in the SHIP in
Pomerania (Nibali et al. 2017). When studies
included in Nibali et al.’s (2016) systematic
review of treated patients reporting tooth loss
by degree of FI were considered (McGuire and
Nunn 1996; Dannewitz et al. 2006; Johansson
et al. 2013; Salvi et al. 2014, Graetz et al. 2015),
8%, 18%, and 30% of the total of teeth with fur-
cation degrees I, II, and III, respectively, were
lost in the follow‐up period (0.01, 0.02, and 0.03
teeth/patient/year). Meta‐analysis for tooth
loss among the included studies (see Figure 5.3)
presented a relative risk of tooth loss of:
●
RR = 1.67 (95% CI = 1.14–2.43, p = 0.008)
for FI degree II vs I.
●
RR = 1.83 (95% CI = 1.37–2.45, p < 0.0001)
for FI degree III vs II.
●
RR = 3.13 (95% CI = 2.30–4.24, p < 0.0001)
for FI degree III vs I.
The comparisons presented a low to moder-
ate degree of heterogeneity among the
selected studies (p value for chi‐square
test = 0.04, 0.20, and 0.26, and I
2
test = 61%,
33%,and 25%, for degree II vs I, III vs II, and
III vs I comparisons, respectively).
5.5.3 Tooth Loss by Vertical
Furcation Component
The previous discussion points to the fact that
the great majority of long‐term follow‐up stud-
ies of molars with FI have focused on the hori-
zontal component, as measured by the Hamp
classification (Hamp et al. 1975). Chapter 2
also introduced the concept of ‘vertical’ FI and
its relative subclassification into A, B, and C
(Tarnow and Fletcher 1984), which could be
associated with the measure of horizontal
involvement. A recent paper hypothesized that
different levels of vertical FI may have a bear-
ing on the risk of tooth loss of horizontal
degree II FI (Tonetti et al. 2017). The authors
retrospectively assessed 200 molars followed
up for 10 years of supportive therapy after con-
servative periodontal surgery with limited
osseous surgery. Vertical furcation subclassifi-
cation was established according to a modifi-
cation of the classification proposed by Tarnow
and Fletcher (1984) using bone loss observed
in a periapical radiograph and clinical probing
depths/CAL. A gradually higher incidence of
tooth loss was observed for degree II FI with
bone loss up to the apical third, middle third,
or coronal third of the root (respectively 77%,
33%, and 9% tooth loss at 10 years). The
authors advocated that the vertical component
might be an important predictor of tooth loss
in molars with degree II horizontal FI. They
also suggested that vertical involvement of the
apical third is often associated with the
presence of an intrabony defect within the fur-
cation defect. Treating such intrabony defects
may reduce the level of vertical FI, thus poten-
tially reducing the future risk of molar loss
(Tonetti et al. 2017).
5.6 Conclusions on Risk of
Tooth Loss by Furcation
Involvement
Based on the review presented here (Nibali
et al. 2016), FI approximately doubles the risk
of tooth loss for molars in supportive perio-
dontal therapy for up to 10–15 years. In
Chapter 5
100
particular, first and second molars with FI had
an RR of tooth loss of 1.46 (p = 0.06) up to 10
years and of 2.21 from 10 to 15 years
(p < 0.0001), compared with molars with no FI
(RR 1.69 and 2.06, respectively, including third
molars). Studies up to 15 years of follow‐up
had consistent results and reported similar
relative risk for tooth loss. This may be attrib-
utable to the similar designs of these studies,
consisting of initial periodontal therapy, surgi-
cal therapy when needed (including access
flaps, osseous resective surgery, root resec-
tion, tunnelling, or occasionally regenerative
surgery), and then supportive periodontal
therapy (at regular intervals for most studies,
generally every 3, 4, 6, or up to 12 months).
A three to four times higher risk of tooth loss
was observed for studies with longer follow‐
ups (>15 years, up to 53 years), although data
relative to this outcome have to be interpreted
cautiously due to high heterogeneity. A similar
and perhaps higher risk of tooth loss could be
attributed to molars with furcation not under-
going regular periodontal treatment (Nibali
et al. 2017). Furthermore, there is enough evi-
dence that the degree of FI (Hamp et al. 1975)
is significantly associated with risk of tooth
loss during supportive periodontal therapy,
increasing from furcation degree I to II to III
(Nibali et al. 2016, 2017). The vertical furca-
tion subclassification and potential intrabony
defects associated with one or multiple roots
within the furcation may also affect the long‐
term tooth loss risk (Tonetti et al. 2017).
Based on the available evidence, it is
worth mentioning that it is not possible to
discriminate the relative contribution of FI
and PPD on molar loss. In other words, we
cannot be certain that the higher risk of
tooth loss in molars with FI is due to them
having FI rather than to them having a deep
pocket. The relative contribution of FI to
Study or Subgroup
McGuire & Nunn 1996
Total events
Heterogeneity: Tau
2
= 0.10; Chi
2
= 10.50, df = 4 (P = 0.03); I
2
= 62%
Test for overall effect: Z = 2.66 (P = 0.008)
27
176
198
153
Dannewitz et al. 2006
Johansson et al. 2013
3
10
98
25
Salvi et al. 2014
28
118
Graetz et al. 2015
108
357
Subtotal (95% Cl)
751
14
9
18
36
121
178
95
67
376
638
1354
6.1%
2.24 [1.22, 4.12]
1996
2006
2013
2014
2015
2.4%
6.0%
7.7%
10.2%
0.32 [0.09, 1.16]
1.49 [0.80, 2.77]
2.48 [1.58, 3.88]
1.60 [1.27, 2.00]
32.3%
1.67 [1.14, 2.43]
McGuire & Nunn 1996
Total events
Heterogeneity: Tau
2
= 0.05; Chi
2
= 7.21, df = 4 (P = 0.12); I
2
= 45%
Test for overall effect: Z = 4.05 (P < 0.0001)
19
148
176
64
Dannewitz et al. 2006
Johansson et al. 2013
11
2
47
2
Salvi et al. 2014
30
69
Graetz et al. 2015
86
188
Subtotal (95% Cl)
370
27
3
10
28
108
153
98
25
118
357
751
7.1%
1.68 [1.01, 2.80]
1996
2006
2013
2014
2015
2.5%
5.4%
8.0%
10.2%
7.65 [2.24, 26.11]
2.06 [1.04, 4.11]
1.83 [1.20, 2.79]
1.51 [1.27, 1.89]
33.3%
1.83 [1.37, 2.45]
Larger degree
Events Total
Lesser degree
Events Total Weight IV. Random, 95% Cl Year
Risk Ratio
IV. Random, 95% Cl
Risk Ratio
III vs. II
McGuire & Nunn 1996
Total events
Heterogeneity: Tau
2
= 0.06; Chi
2
= 8.01, df = 4 (P = 0.09); I
2
= 50%
Test for overall effect: Z = 7.32 (P < 0.0001)
19
148
198
64
Dannewitz et al. 2006
Johansson et al. 2013
11
2
47
2
Salvi et al. 2014
30
69
Graetz et al. 2015
86
188
Subtotal (95% Cl)
370
14
9
18
36
212
178
95
67
376
357
1354
5.9%
3.77 [2.01, 7.08]
1996
0.01
0.1
1
Favors larger degree
Favors lesser degree
10
100
2006
2013
2014
2015
4.5%
5.8%
8.1%
10.2%
2.47 [1.10, 5.55]
3.06 [1.62, 5.80]
4.54 [3.01, 6.85]
2.41[1.93, 3.02]
34.5%
3.13 [2.30, 4.24]
III vs. I
II vs. I
Figure 5.3
Forest plot presenting relative risk (RR) of tooth loss based on degrees of furcation involvement
(excluding third molars). Meta‐analysis for the comparison of tooth loss among selected studies presented an
RR of 1.67 (95% confidence interval [CI] = 1.14–2.43, p = 0.008), 1.83 (95% CI = 1.37–2.45, p < 0.0001), and 3.13
(95% CI = 2.30–4.24, p < 0.0001) when comparing degree II to I, degree III to II, and degree III to I furcation
involvement, respectively. Source: Nibali et al. (2016).
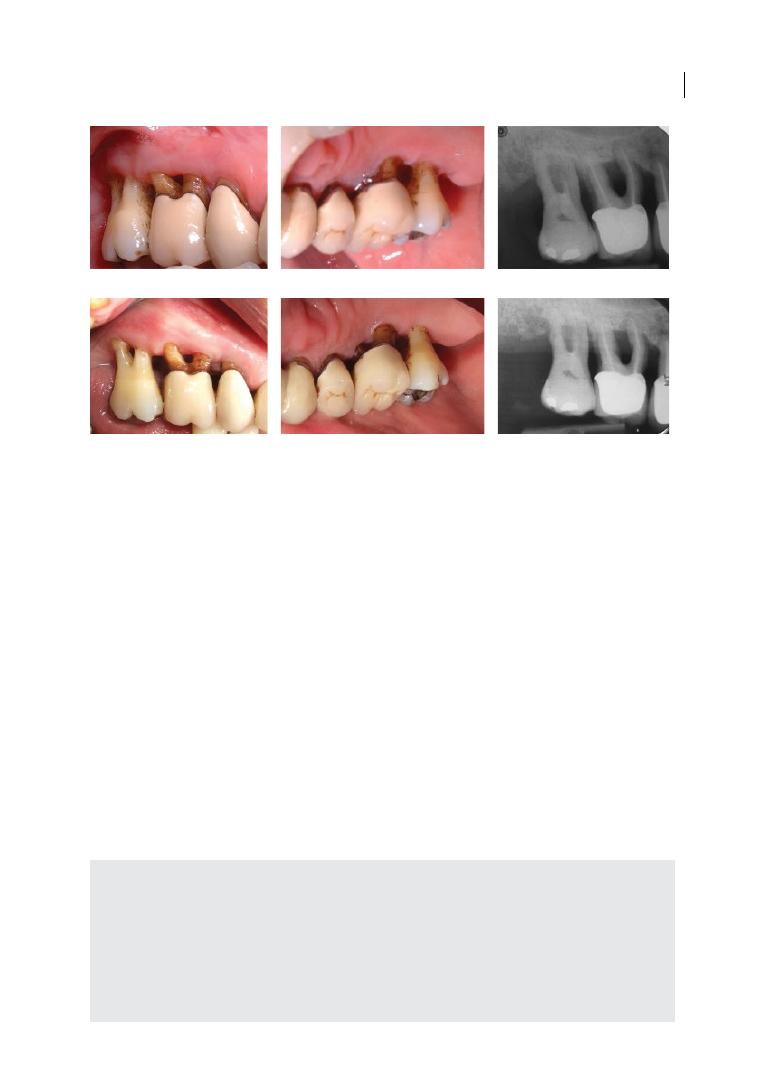
Long-term Tooth Loss Data 101
molar loss could be tested by assessing risk
of tooth loss prospectively in molars with
similar pocket depth differing only by FI (for
example, a 6 mm vertical PPD in a buccal
surface of a lower molar with no FI vs a 6 mm
vertical PPD of a lower molar with degree II
FI). However, we are not aware of any studies
testing this hypothesis. An indirect compar-
ison of the risk of tooth loss attributable only
to residual PPD is difficult, as limited data
are available and merely on single‐rooted
teeth, since most studies present short‐term
disease progression data only (Badersten
et al. 1984) or tooth loss data for all teeth
combined (Matuliene et al. 2008).
In conclusion, in patients undergoing com-
prehensive periodontal treatment (cause‐
related, surgical therapy if needed, and SPT),
most molars affected by FI respond well to
periodontal treatment, judging by the fact
that even in the presence of degree III FI,
only 30% of molars were lost with up to
15 years of follow‐up (see Figure 5.4).
The risk of tooth loss (for FI vs non‐FI molars)
is in the region of 1.5–2.2 up to 15 years in
maintenance. Such risk seems to increase
sharply after the 15‐year time point, although
study heterogeneity does not allow clear con-
clusions on this. Similarly, an increased risk of
tooth loss by FI exists for cases not undergoing
periodontal maintenance. Among the relevant
long‐term outcomes introduced in Section 5.2,
we are not aware of any studies specifically
assessing the effect of FI on the systemic inflam-
matory burden, while patient‐reported out-
comes are covered in Chapter 13.
(a)
(b)
(c)
(d)
(e)
(f)
Figure 5.4
(a, b) Clinical photographs of 55‐year‐old male patient affected by chronic periodontitis; (c)
periapical radiographs of upper right molars showing triple degree III furcation involvement on UR6 and 7; (d, e)
clinical photographs taken 10 years after tunnelling surgery; (f) Periapical photographs at 10‐year follow‐up.
Summary of Evidence
●
Periodontal treatment and maintenance
care lead to low tooth loss rates of molars
with furcation involvement (FI).
●
The tooth loss of molars with FI is approx-
imately double that of molars without FI
up to 15 years of follow‐up.
●
Degree of horizontal FI affects risk of
tooth loss (increasing from degree I to II
to III).
●
Degree of vertical FI may also affect the
risk of tooth loss (increasing from subclas-
sification A to B to C).

Chapter 5
102
References
Akhter, R., Hassan, N.M., Aida, J. et al. (2008).
Risk indicators for tooth loss due to caries
and periodontal disease in recipients of free
dental treatment in an adult population in
Bangladesh. Oral Health & Preventive
Dentistry 6, 199–207.
Al‐Shammari, K.F., Al‐Khabbaz, A.K., Al‐
Ansari, J.M. et al. (2005). Risk indicators for
tooth loss due to periodontal disease.
Journal of Periodontology 76, 1910–1918.
Badersten, A., Nilveus, R., and Egelberg, J.
(1984). Effect of nonsurgical periodontal
therapy. II. Severely advanced periodontitis.
Journal of Clinical Periodontology 11,
63–76.
Bjorn, A.L., and Hjort, P. (1982). Bone loss of
furcated mandibular molars: A longitudinal
study. Journal of Clinical Periodontology 9,
402–408.
Chambrone, L., Chambrone, D., Lima, L.A.,
and Chambrone, L.A. (2010). Predictors of
tooth loss during long‐term periodontal
maintenance: A systematic review of
observational studies. Journal of Clinical
Periodontology 37, 675–684.
Checchi, L., Montevecchi, M., Gatto, M.R., and
Trombelli, L. (2002). Retrospective study of
tooth loss in 92 treated periodontal patients.
Journal of Clinical Periodontology 29,
651–656.
Claffey, N., and Egelberg, J. (1995). Clinical
indicators of probing attachment loss
following initial periodontal treatment in
advanced periodontitis patients. Journal of
Clinical Periodontology 22, 690–696.
Dannewitz, B., Krieger, J.K., Husing, J., and
Eickholz, P. (2006). Loss of molars in
periodontally treated patients: A
retrospective analysis five years or more
after active periodontal treatment. Journal
of Clinical Periodontology 33, 53–61.
Eickholz, P., and Hausmann, E. (2002).
Evidence for healing of periodontal defects
5 years after conventional and regenerative
therapy: Digital subtraction and bone level
measurements. Journal of Clinical
Periodontology 29, 922–928.
Eickholz, P., Kaltschmitt, J., Berbig, J. et al.
(2008). Tooth loss after active periodontal
therapy. 1: Patient‐related factors for risk,
prognosis, and quality of outcome. Journal
of Clinical Periodontology 35, 165–174.
EFP European Federation of Periodontology
(2004). EFP Manifesto: Perio and General
Health. http://www.efp.org/efp‐manifesto/
index.html (accessed 6 February 2018).
Goldman, M.J., Ross, I.F., and Goteiner, D.
(1986). Effect of periodontal therapy on
patients maintained for 15 years or longer:
A retrospective study. Journal of
Periodontology 57, 347–353.
Graetz, C., Schutzhold, S., Plaumann, A. et al.
(2015). Prognostic factors for the loss of
molars: An 18‐years retrospective cohort
study. Journal of Clinical Periodontology 42,
943–950.
Graziani, F., Gennai, S., Karapetsa, D. et al.
(2015). Clinical performance of access flap
in the treatment of class II furcation defects:
A systematic review and meta‐analysis of
randomized clinical trials. Journal of
Clinical Periodontology 42, 169–181.
Hamp, S.E., Nyman, S., and Lindhe, J. (1975).
Periodontal treatment of multirooted teeth:
Results after 5 years. Journal of Clinical
Periodontology 2, 126–135.
Haney, J.M., Leknes, K.N., and Wikesjo, U.M.
(1997). Recurrence of mandibular molar
furcation defects following citric acid root
treatment and coronally advanced flap
procedures. International Journal of
Periodontics and Restorative Dentistry 17,
528–535.
Hirschfeld, L., and Wasserman, B. (1978).
A long‐term survey of tooth loss in 600
treated periodontal patients. Journal of
Periodontology 49, 225–237.
Hull, P.S., Worthington, H.V., Clerehugh, V.
et al. (1997). The reasons for tooth
extractions in adults and their validation.
Journal of Dentistry 25, 233–237.
Huynh‐Ba, G., Kuonen, P., Hofer, D. et al.
(2009). The effect of periodontal therapy on
the survival rate and incidence of

Long-term Tooth Loss Data 103
complications of multirooted teeth with
furcation involvement after an observation
period of at least 5 years: A systematic
review. Journal of Clinical Periodontology
36, 164–176.
Johansson, K.J., Johansson, C.S., and Ravald, N.
(2013). The prevalence and alterations of
furcation involvements 13 to 16 years after
periodontal treatment. Swedish Dental
Journal 37, 87–95.
Kassebaum, N.J., Bernabe, E., Dahiya, M. et al.
(2014). Global burden of severe
periodontitis in 1990–2010: A systematic
review and meta‐regression. Journal of
Dental Research 93, 1045–1053.
Kuhrau, N., Kocher, T., and Plagmann, H.C.
(1990). [Periodontal treatment of furcally
involved teeth: With or without root
resection?] Deutsche Zahnarztliche
Zeitschrift 45, 455–457.
Lee, C.T., Huang, H.Y., Sun, T.C., and
Karimbux, N. (2015). Impact of patient
compliance on tooth loss during supportive
periodontal therapy: A systematic review
and meta‐analysis. Dental Research 94,
777–786.
Lindhe, J., and Nyman, S. (1975). The effect of
plaque control and surgical pocket
elimination on the establishment and
maintnance of periodontal health: A
longitudinal study of periodontal therapy in
cases of advanced disease. Journal of
Clinical Periodontology 2, 67–79.
Little, L.A., Beck, F.M., Bagci, B., and Horton,
J.E. (1995). Lack of furcal bone loss
following the tunnelling procedure. Journal
of Clinical Periodontology 22, 637–641.
Matuliene, G., Pjetursson, B.E., Salvi, G.E. et al.
(2008). Influence of residual pockets on
progression of periodontitis and tooth loss:
Results after 11 years of maintenance.
Journal of Clinical Periodontology 35,
685–695.
McFall, W.T., Jr (1982). Tooth loss in 100
treated patients with periodontal disease:
A long‐term study. Journal of Periodotology
53, 539–549.
McGuire, M.K., and Nunn, M.E. (1996).
Prognosis versus actual outcome. III: The
effectiveness of clinical parameters in
accurately predicting tooth survival. Journal
of Periodontology 67, 666–674.
McLeod, D.E., Lainson, P.A., and Spivey, J.D.
(1998). The predictability of periodontal
treatment as measured by tooth loss:
A retrospective study. Quintessence
International 29, 631–635.
Miller, P.D., Jr, McEntire, M.L., Marlow, N.M.,
and Gellin, R.G. (2014). An evidenced‐based
scoring index to determine the periodontal
prognosis on molars. Journal of
Periodontology 85, 214–225.
Nibali, L., Farias, B.C., Vajgel, A. et al. (2013).
Tooth loss in aggressive periodontitis:
A systematic review. Journal of Dental
Research 92, 868–875.
Nibali, L., Krajewski, A. Donos, N. et al.
(2017). The effect of furcation involvement
on tooth loss in a population without
regular periodontal therapy. Journal of
Clinical Periodontology 44, 813–821.
Nibali, L., Zavattini, A., Nagata, K. et al.
(2016). Tooth loss in molars with and
without furcation involvement: A systematic
review and meta‐analysis. Journal of Clinical
Periodontology 43, 156–166.
Pretzl, B., Kaltschmitt, J., Kim, T.S. et al.
(2008). Tooth loss after active periodontal
therapy. 2: Tooth‐related factors. Journal of
Clinical Periodontology 35, 175–182.
Reddy, M.S., Aichelmann‐Reidy, M.E.,
Avila‐Ortiz, G. et al. (2015). Periodontal
regeneration – furcation defects:
A consensus report from the AAP
Regeneration Workshop. Journal of
Periodontology 86, S131–S133.
Salvi, G.E., Mischler, D.C., Schmidlin, K. et al.
(2014). Risk factors associated with the
longevity of multi‐rooted teeth: Long‐term
outcomes after active and supportive
periodontal therapy. Journal of Clinical
Periodontology 41, 701–707.
Tarnow, D., and Fletcher, P. (1984).
Classification of the vertical component of
furcation involvement. Journal of
Periodontology 55, 283–284.
Tonetti, M., Christianes, A., and Cortellini, P.
(2017). Vertical sub‐classification predicts

Chapter 5
104
survival of molars with class II furcation
involvement during supportive periodontal
care. Journal of Clinical Periodontology 44,
1140–1144.
Trombelli, L., Franceschetti, G., and Farina, R.
(2015). Effect of professional mechanical
plaque removal performed on a long‐term,
routine basis in the secondary prevention of
periodontitis: A systematic review. Journal
of Clinical Periodontology 42 (Suppl. 16),
S221–S236.
Tsami, A., Pepelassi, E., Kodovazenitis, G., and
Komboli, M. (2009). Parameters affecting
tooth loss during periodontal maintenance in
a Greek population. Journal of the American
Dental Association 140, 1100–1107.
Wang, H.L., Burgett, F.G., Shyr, Y., and
Ramfjord, S. (1994). The influence of molar
furcation involvement and mobility on
future clinical periodontal attachment loss.
Journal of Periodontology 65, 25–29.
Wood, W.R., Greco, G.W., and McFall, W.T., Jr
(1989). Tooth loss in patients with moderate
periodontitis after treatment and long‐term
maintenance care. Journal of Periodontology
60, 516–520.
Yukna, R.A., and Yukna, C.N. (1997). Six‐year
clinical evaluation of HTR synthetic bone
grafts in human grade II molar furcations.
Journal of Periodontal Research 32,
627–633.
Zafiropoulos, G.G., Hoffmann, O., Kasaj, A.
et al. (2009). Mandibular molar root
resection versus implant therapy: A
retrospective nonrandomized study. Journal
of Oral Implantology 35, 52–62.

Chapter No.: 1 Title Name: <TITLENAME>
c06.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:20:29 PM Stage: <STAGE> WorkFlow:
CSW
Page Number: 105
105
Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
6.1 Introduction
In everyday clinical practice, the presence
of furcation involvement constitutes a sig
nificant challenge (de Santana et al. 1999;
Avila‐Ortiz et al. 2015). Regeneration of the
furcation defect with restitution ad integrum
is a highly desirable outcome in these cases.
This chapter and the next will review the
current evidence on pre‐clinical and clinical
human studies on the potential to regenerate
furcation defects.
The regeneration of furcation involve
ments following the use of various bio
logical materials, extracellular matrix
proteins, growth factors, and cell therapy is a
complex biological process involving various
tissue components, including epithelium,
connective tissue, cementum, and alveolar
bone (Ivanovic et al. 2014). Historically,
animal models have been used as proof‐of‐
principle models and for providing first‐
level in vivo evidence in potential translation
of different regenerative materials to the
clinical setting.
6.2 Available Preclinical
Models
Different animal species have been used
to evaluate the regeneration of furcation
defects, with non‐human primates, dogs,
rabbits, and pigs being the most commonly
employed (Struillou et al. 2010; Kantarci
et al. 2015). Variations between species
include anatomy, dimensions of teeth and
alveolar process, gingival biotype, local phys
iological environment, animal behaviour,
and healing rate (Caton et al. 1994).
Small animals (especially mice and rats)
have generated substantial data on the patho
genetic mechanisms of systemic inflamma
tion and their correlation with periodontal
disease in transgenic and knockout animal
models (Graves et al. 2008). The major draw
back of small animals is the limited similarity
of their dentition to the human dentition,
which limits the possibility of translating the
results to the clinical situation. Conversely,
the dental anatomy of large animals
(mainly non‐human primates and dogs) may
Chapter 6
Regenerative Therapy of Furcation Involvements in
Preclinical Models: What is Feasible?
Nikolaos Donos
1
, Iro Palaska
1
, Elena Calciolari
1
, Yoshinori Shirakata
2
, and
Anton Sculean
3
1
Centre for Immunobiology and Regenerative Medicine, Centre for Oral Clinical Research, Institute of Dentistry, Barts and the London
School of Medicine and Dentistry, Queen Mary University of London (QMUL), London, UK
2
Department of Periodontology, Kagoshima, University Graduate School of Medical and Dental Sciences, Kagoshima, Japan
3
Department of Periodontology, School of Dental Medicine, University of Bern, Bern, Switzerland

Chapter 6
106
resemble better the human dento‐alveolar
architecture. In these animals, it has been
suggested that it is feasible to study wound
healing/regeneration in periodontal defects
of clinically relevant size and configuration
(Selvig 1994).
6.2.1 Non‐human Primates
Non‐human primates have naturally occur
ring dental plaque, calculus, oral microbial
pathogens, and periodontal disease, although
this occurs late in life and the lesions tend
to be asymmetrical (Schou et al. 1993; Oz
and Puleo 2011). Therefore, if osseous lesions
are investigated, they are usually experimen
tally induced. Similar dental anatomy and
periodontal wound healing (Caton and
Kowalski 1976), suitability of furcation sites
(Giannobile et al. 1994), and experimentally
induced defects that do not spontaneously
regenerate indicated that mature, adult,
Macaca mulatta and Macaca fascicularis
species could be used as models for evaluat
ing periodontal regenerative procedures
(Schou et al. 1993). However, it should be
emphasized that their use is controversial, as
this model shares some structural and func
tional features with humans, thus raising sig
nificant ethical concerns. Besides the ethical
issues related to the close phylogenetic rela
tionship with humans, primate research also
requires expensive facilities, with dedicated
and trained personnel and environmental
enrichment.
6.2.2 Dog Model
Dogs are among the most widely used exper
imental animals for studying naturally occur
ring gingivitis and periodontitis, wound
healing, and tissue regeneration (Wikesjö
et al. 1994). The Beagle dog (Canis lupus
familiaris) is commonly used because of its
size and its cooperative nature, but several
studies have also used mongrel dogs
(Struillou et al. 2010). The characteristics of
periodontal tissues and the size of teeth in
dogs present, to a certain extent, some
similarity to those of humans. However, dogs
lack lateral jaw movements and premolar
occlusal contacts (Kantarci et al. 2015). In
dogs, the severity of periodontal disease
increases with age and can result in tooth loss
(Berglundh et al. 1991). In regenerative peri
odontal medicine, the dog model has been
used for the histological demonstration of
guided tissue regeneration (GTR) in various
defect types, such as furcations, supracrestal,
and infrabony defects (Caffesse et al. 1990). In
addition, the dog model has been employed
in studies which have led to our current
understanding of the limitations of regener
ative approaches, including membrane‐
associated properties (Araujo et al. 1998).
6.2.3 Miniature Pig Model
The miniature pig model has emerged as an
alternative to the dog model. Varieties of
miniature pig have been extensively used in
biomedical research (Polejaeva et al. 2000).
These animals have oral and maxillofacial
structures similar to those of humans in
terms of anatomy, physiology, and disease
development (Wang et al. 2007). Natural
gingivitis can be observed at 6 months of age.
The pattern of disease progression follows
the same stages as that in humans: gingival
swelling, plaque accumulation, calculus
formation, and bleeding on probing (Lang
et al. 1998). Histologically, these clinical
features are accompanied by inflammatory
cell infiltration and vasodilatation. Starting
at 16 months of age, miniature pigs may
develop advanced periodontitis, with pocket
depths up to 5 mm and alveolar bone resorp
tion (Kantarci et al. 2015).
6.3 Defect Types
Four types of experimentally induced furca
tion defects have been used for testing the
effects of different therapeutic modalities in
pre‐clinical studies. These include defects

Regenerative Therapy in Animal Models 107
resulting from naturally occurring periodon
titis and three types of experimentally
produced defects: the acute defect, the
chronic defect, and the combined acute/
chronic defect.
6.3.1 Naturally Occurring
Periodontitis Defects
Historically, periodontal defects caused by
natural periodontal disease have been
considered as a necessity in the study of peri
odontal regeneration (Haney et al. 1995).
These defects occur late in the animal’s life
and the lesions are usually asymmetrical,
as they result from a gradual and variable
destruction of the periodontium, and include
deposition of calculus and endotoxins on
the root surface (Haney et al. 1995). In addi
tion, they feature compromised mucogingi
val dimensions, which is a confounding
factor when these models are used to study
the biological potential of regeneration under
optimized conditions of wound healing
(Wikesjö et al. 1994). Taking into account the
aforementioned limitations, the rationale
for using these types of defects to study
periodontal wound healing/regeneration
appears to be limited (Caton et al. 1994).
6.3.2 Experimentally
Induced Defects
6.3.2.1 The Acute Defect Model
In this model, mucoperiosteal flaps are
elevated and the bone, the periodontal liga
ment, and cementum are surgically removed
to create the defect type of the desired shape
and dimension. Reference notches are usu
ally created by a round bur on the roots, at
the level of the reduced alveolar bone. These
notches start on the buccal aspect of the root
and extend into the furcation area, and they
are used as a reference point for the histo
logical analysis. The major drawback of the
acute defect model is that approximately
50–70% of spontaneous regeneration can be
expected, thus creating an important source
of bias when studying the effect of different
surgical techniques and biomaterials on
tissue regeneration (Caton et al. 1994;
Mardas et al. 2012).
6.3.2.2 The Chronic Defect Model
These defects are created by placing ortho
dontic elastics or ligatures around the cir
cumference of teeth, or slightly apical to
the gingival margin. The elastics/ligatures
gradually migrate apically, as plaque‐induced
inflammation destroys the periodontal liga
ment and supporting bone in 3–6 months
(Caton and Kowalski 1976). Then, the root
surfaces are carefully scaled and planed and a
notch is placed on the roots, at the base of
the defect, as a reference for the histological
analysis. The advantage of this model is that
spontaneous healing is not observed, but the
disadvantages include the considerable time
needed for the creation of the defects and
the asymmetrical nature of the defects.
In addition, conventional root debridement
produces root surface conditions similar to
those of surgically induced (acute) defects.
Taking into account these limitations, the rel
evance for using this type of defect to study
periodontal wound healing/regeneration
appears to be limited (Caton et al. 1994).
6.3.2.3 The Acute/Chronic
Defect Model
The majority of the available pre‐clinical
studies use this model to study wound
healing/periodontal regeneration in class II
or III furcation defects. This defect is devel
oped by surgically removing alveolar bone,
periodontal ligament, and cementum in the
experimental site. Prior to flap closure, the
defect is placed into a chronically inflamed
state to reduce spontaneous regeneration by
placing a foreign body, such as metal strips,
orthodontic wires and bands, impression
material, or gutta‐percha for 1–3 months
(Caton et al. 1994). At surgical re‐entry, the
foreign bodies are removed, and the lesions
are debrided from granulation tissue, plaque
biofilm, and calculus. After the root surface

Chapter 6
108
is scaled, the tested biomaterial/active prin
ciple can eventually be delivered into the
defect (Araújo and Lindhe 1997; Takayama
et al. 2001; Donos et al. 2003b). The advan
tages of this model are that defects are pro
duced rapidly, they do not heal spontaneously,
and bilateral symmetrical periodontal tissue
loss can be predictably induced.
6.3.3 The Critical Size Defect
Concept in Furcation Studies
A critical size defect (CSD) is defined as
‘the smallest size intra‐osseous wound in
a particular bone and species that will not
heal spontaneously during the lifetime of
the animal’ (Schmitz and Hollinger 1986).
Even if a certain biological variability exists
between different animal models, it is of the
outmost importance that the experimental
bone defect created is of a critical size for
the animal model used, thus avoiding
the occurrence of spontaneous periodontal
regeneration and allowing testing of the
true regenerative potential of the biomateri
als and surgical techniques investigated.
However, because most pre‐clinical studies
have an evaluation time limit, Gosain et al.
(2000) state that the CSD in animal research
refers to the size of a defect that will not heal
over the duration of the study.
6.3.3.1 Furcation Degree II CSD
In order to test the regenerative potential of
different regenerative treatments in class II
furcation defects, the majority of studies
have used surgically created CSDs measuring
5 mm in height (from the roof of the bifurca
tion) and 2 mm in depth (Lekovic and Kenney
1993; Hürzeler 1997; Deliberador et al. 2006).
After intrasulcular incisions were made from
the mesial side of the involved teeth (mainly
premolars) to the distal side of the molars, a
mucoperiosteal flap was elevated to expose
both buccal and lingual alveolar bone plates.
The defects were then created by using
carbide round burs under abundant saline
irrigation. The interproximal bone remained
intact. Then, the surfaces were carefully
scaled and root planed. Following removal of
granulation tissue and complete root instru
mentation, reference notches were placed in
the roots at the level of the alveolar bone
crest, using a number 1/2 round bur. These
notches were positioned on the buccal
aspects of the roots and extended interproxi
mally and into the furcation areas, as deep as
the involvement of the class II furcation
permits. After the root surface is scaled,
the tested biomaterial/active principle can
be delivered into the defect.
6.3.3.2 Furcation Degree III CSD
The first attempts to create defects that
would not heal spontaneously were made by
the team of Ellegaard et al. (1974), who
induced through‐and‐through bifurcation
defects in posterior teeth of Rhesus monkeys
by passing a round bur through surgically
denuded bifurcations. The size of the open
ings was approximately 2 mm in diameter.
To avoid spontaneous healing, a steel wire
of periodontal dressing was placed in the
defects for four weeks. This study has shown
that following surgical removal of the inter‐
radicular septum and the promotion of
plaque retention in the monkey’s posterior
teeth, bifurcation defects may develop into
lesions, which after six weeks of healing
display features similar to those of inter‐
radicular pockets in humans. Six weeks post‐
operatively the height of the bone in the
lesions produced was essentially maintained
at the level determined at the time of surgery.
The lesions were characterized by chroni
cally inflamed connective tissue covered
with epithelium of varying thickness, and
they showed no tendency for spontaneous
healing.
Based on this concept, Klinge et al. (1981),
by using two different animal models (dog
model and non‐human primate model),
examined the influence of different defect
sizes and different flap management
techniques on the healing potential after
reconstructive surgery using citric acid
conditioning. Chronic through‐and‐through
defects of different sizes were created.

Regenerative Therapy in Animal Models 109
Specifically, bone was surgically removed
from the furcation area and around the cir
cumference of the tooth, including the proxi
mal bone (horizontal defect). Three different
levels of bone reduction were studied (2 mm,
3.5 mm, and 5 mm high). In addition, two
different positions of the flap were studied
(coronally positioned flap and replaced flap).
It was demonstrated that new attachment
occurred for teeth treated with coronally
positioned flaps regardless of the defect size.
This implied that variations in the dimension
of the defects played a secondary role in the
healing potential, and that the adequate post‐
operative flap coverage of the furcation was
the critical step for the successful healing of
through‐and‐through defects.
In a subsequent study by Klinge et al.
(1985a), it was shown that healing was
accomplished in even larger defects (~9 mm)
when a coronal flap placement was used. It is
possible that the magnitude of coronal flap
positioning is of limited importance, pro
vided a certain amount of coverage of the
furcation is accomplished. It was also noticed
that one important reason for the failure at
larger defects was found to be recession of
the flap, which resulted in early exposure of
the furcation site (Klinge et al. 1985a). To
counteract such soft tissue recession, the
authors developed a technique which
involved the utilization of ‘crown attached’
sutures and reported that, provided flap
recession was prevented, new attachment
occurred at both large and small defects
(Klinge et al. 1985a). Similar results were
obtained by Lindhe et al. (1995), who showed
that comparatively large furcation defects
(‘key‐hole’ defects with cross‐section dimen
sions of > 11 mm
2
) could be successfully
regenerated by GTR therapy provided that
the soft‐tissue flaps covering the membranes
are prevented from receding apical of the
furcation fornix during healing, and that
the clot in the furcation defect remains
non‐infected.
The potential for new attachment forma
tion after GTR was examined by Pontoriero
et al. (1992) in three differently shaped
furcation defects: (i) a small, 2 × 2 mm key‐
hole furcation defect, where the removal of
the supporting bone and root cementum was
confined to the furcation area, thus leaving
intact the interdental alveolar bone; (ii) a
large key‐hole defect about 3 mm in the
apico‐coronal direction and 4 mm in the
mesio‐distal direction; and (iii) a large
‘ horizontal’ defect about 5 mm in the apico‐
coronal direction and about 4 mm in the
mesio‐distal direction, where the removal of
bone and root cementum was performed
within the furcation area and extended at the
buccal/lingual and interproximal surfaces. It
was observed that small key‐hole, degree III
furcation defects healed following GTR
treatment with complete new attachment.
At control sites, only minor amounts of new
attachment formation occurred. In larger
defects, it was observed that GTR therapy
failed to generate new attachment to a degree
where the furcation defects became closed. It
was also observed clinically that at such sites,
extensive flap recession occurred following
suture removal. The study revealed that the
size of the furcation defect and the degree of
bone loss adjacent to the defect were deter
mining factors for the outcome of this kind of
treatment. Thus, if the furcation defect was
associated with circumferential bone loss, or
if the defect was more than 3 mm in the
apico‐coronal direction, complete new
attachment failed to occur. Thus, while small
key‐hole defects – that is, small vertical
defects – healed, large key‐hole defects and
horizontal defects were consistently associ
ated with flap recession and failure. Taking
into consideration the CSD concept (smallest
bone defect created), the key‐hole defect was
optimal for testing the regenerative potential
of different materials, and the approach
based on this study was adopted in clinical
studies as well.
The most widely used model in regenera
tive studies remains the critical size supra‐
alveolar periodontal defect model, which was
developed in beagle dogs by Wikesjö and his
team (Wikesjö et al. 1999). Supra‐alveolar
critical size periodontal defects are produced

Chapter 6
110
by the resection of the buccal and lingual/
palatal bone of premolar teeth. Osseous
resection can be restricted to the interdental
area, which measures approximately 4–5 mm
(height) and 3 mm (width; Araújo et al. 1998),
or extended to create a horizontal circumfer
ential defect up to 5–6 mm below the fornix
of furcation (Giannobile et al. 1998; Wikesjö
et al. 2003a, b). In this model, innate regen
eration of alveolar bone and cementum does
not exceed 25% of the defect height over a
three‐week healing interval following wound
closure or primary intention healing.
Extending the healing interval to eight weeks
does not result in additional regeneration
(Wikesjö et al. 1994, 2003a, b; Koo et al.
2004). This defect is a valuable model to test
the regenerative potential of candidate
treatments on class III furcation defects,
as it allows evaluation of the regenerative
potential of experimental treatments under
optimal conditions and in a biologically
controlled environment. By applying this
model, different studies have evaluated
root‐conditioning protocols, bone grafts
and bone substitutes, biological substances,
and different barrier membranes (GTR) as
stand‐alone protocols or in combination
(Sanz et al. 2015).
6.4 Regeneration
Treatments of Class II
Furcation Defects
6.4.1 Guided Tissue Regeneration
The potential to regenerate furcation defects
in a predictable manner emerged with the
development of the concept of GTR more
than 30 years ago (Karring and Warrer 1992;
Karring et al. 1993). The use of a barrier
membrane prevents both gingival epithelial
cells and connective tissue cells from repop
ulating the root surface during healing, and
allows repopulation only by cells from the
periodontal ligament or the alveolar bone
marrow, thus inducing the formation of new
cementum and connective tissue attachment
(Nyman et al. 1980; Karring et al. 1980, 1993;
Sander and Karring 1995; Sanz et al. 2015).
A variety of biocompatible barriers, non‐
absorbable (Caffesse at al. 1990, 1994;
Danesh‐Meyer et al. 1997; Bogle et al. 1997;
Lekovic et al. 1998; Macedo et al. 2006) or
bioabsorbable (Cirelli et al. 1997; Hürzeler
et al. 1997; de Andrade et al. 2007; Wang
et al. 2014) has been used for the regenera
tion of class II furcation defects. From a
clinical and histological point of view, similar
results can be achieved in GTR, whether bio
absorbable (such as collagen‐based) or non‐
absorbable (such as polytetrafluoroethylene,
ePTFE) membranes are applied (Murphy and
Gunsolley 2003). However, the bioabsorba
ble membranes do not require a second
surgery for their retrieval, thus reducing
the patient’s discomfort and morbidity.
The studies applying non‐resorbable and
resorbable membranes in critical size (5 mm
in height × 2 mm in depth), surgically created
furcation II defects showed a mean percent
age of bone fill and new cementum forma
tion ranging between 60 and 80% (Caffesse at
al. 1990, 1994; Cirelli et al. 1997; Danesh‐
Meyer et al. 1997; Bogle et al. 1997; Hürzeler
et al. 1997; Lekovic et al. 1998; Macedo et al.
2006; de Andrade et al. 2007; Wang et al.
2014). The results of these studies are
presented in detail in Table 6.1.
6.4.2 Bone Grafts and GTR
The placement of bone grafts or alloplastic
materials in furcation defects in association
with flap surgery or GTR has been exten
sively evaluated in animal experiments.
The biological rationale behind the use of
grafts is the assumption that the material
may contain bone‐forming cells (osteogene
sis) or serve as a scaffold for bone formation
(osteoconduction), or that the matrix may
contain bone‐inductive substances (osteoin
duction), which could stimulate both the
regrowth of bone and the formation of new
attachment (Karring and Cortellini 1999).
Limited studies in naturally occurring
defects showed that bone grafts combined
with different barrier membranes did
not enhance the regeneration process of

Regenerative Therapy in Animal Models 111
Table 6.1
Guided tissue regeneration (GTR) in class II furcation defects.
Study
Model
Tooth
Type of membrane
Healing
time
(months)
Histomorphometric results
NATURALLY OCCURING PERIODONTITIS
Bogle
et al. 1997
Dog
Mandibular
premolars
Control: Open‐flap
debridement
Experimental group:
Bioabsorbable
polylactic acid–based
membrane
6
Cementum formation (%)
Control: 71
Test: 17*
Bone filling (%)
Control: 74
Test: 14*
ACUTE DEFECT MODEL
Caffesse
et al. 1994
Dog
Mandibular
premolars
Control: PTFE non‐
resorbable membrane
Test 1: Bioabsorbable
type I barrier
Test 2: Bioabsorbable
type II barrier
6
Notch to new
cementum (mm)
Control: 4.42 ± 1.40
Test 1: 4.42 ± 1.08
Test 2: 5.80 ± 0.68
Notch to bone crest (mm)
Control: 2.72 ± 1.43
Test 1: 2.95 ± 1.24
Test 2: 4.55 ± 1.05
Danesh‐
Meyer
et al. 1997
Sheep
Mandibular
premolars
Control: Open‐flap
debridement
Test 1: PTFE non‐
resorbable membrane
Test 2: PTFE non‐
resorbable soft‐tissue
patch
2
Alveolar bone height (%)
Control: 61.5 ± 7.13
Test 1: 78.4 ± 6.89*
Test 2: 71.7 ± 6.73
Cementum height (%)
Control: 93.0 ± 4.34
Test 1: 98.5 ± 1.01*
Test 2: 98.4 ± 1.03*
ACUTE/CHRONIC DEFECT MODEL
Cirelli
et al. 1997
Mongrel
dogs
Mandibular
premolars
Control: Open‐flap
debridement
Test: GTR with
anionic collagen
membrane
3
Cementum formation (%)
Control: 59.34
Test: 92.35*
Bone filling (%)
Control: 48.58
Test: 56.33
Macedo
et al. 2006
Dog
Mandibular
bicuspids
Control: PTFE non‐
resorbable (Gore‐Tex
+
)
removed at 2 weeks
Test: PTFE non‐
resorbable (Gore‐Tex
+
)
removed at 4 weeks
3
New tissue (mm
2
)
Control: 12.45 ± 3.54
Test: 14.32 ± 4.01
Bone height (mm)
Control: 3.56 ± 1.21
Test: 4.03 ± 0.94
de Andrade
et al. 2007
Dog
Mandibular
premolars
Control: Bioabsorbable
membrane
(polyglycolic acid)
Test: Acellular dermal
matrix
3
New tissue (mm
2
)
Control: 8.01 ± 2.69
Test: 7.21 ± 1.33
Bone height (mm)
Control: 2.56 ± 0.84
Test: 2.86 ± 0.32
(Continued )

Chapter 6
112
Table 6.1
(Continued)
Study
Model
Tooth
Type of membrane
Healing
time
(months)
Histomorphometric results
Wang
et al. 2014
Dog
Mandibular
premolars
Control: Open‐flap
debridement
Test 1: Open‐flap
debridement + LIPUS
Test 2: Resorbable
bovine collagen
membrane (BioGide
†
)
Test 3: Resorbable
bovine collagen
membrane
(BioGide
†
) + LIPUS
2
Micro‐CT scanning
New alveolar bone
surface (mm
2
)
Control: 82.84 ± 16.67
Test 1: 98.44 ± 18.57
Test 2: 132.11 ± 22.76*
Test 3: 150 ± 21.20*
Hürzeler
et al. 1997
Monkey
Mandibular
molars
Control: Open‐flap
debridement
Test: Synthetic
resorbable membrane
Resolute
‡
(glycolide/
lactic copolymer)
5
Cementum
deposition (mm)
Initial defect size: 5.05 ± 0.45
Control: 0.83 ± 0.19
Test: 2.88 ± 0.63*
Bone formation (mm)
Initial defect size: 2.81 ± 0.65
Control: 1.14 ± 0.35
Test: 2.78 ± 0.53*
CHRONIC DEFECT MODEL
Caffesse
et al. 1990
Dog
Mandibular
premolars,
molars
Control: Open‐flap
debridement
Test: PTFE non‐
resorbable membrane
(Gore‐Tex
+
)
3
Furcation fill (mm
2
)
Control: Ep + CT + B = 1.94
Experimental: Ep + CT+
B = 3.38
(no statistical analysis)
Lekovic
et al. 1998
Dog
Mandibular
premolars,
molars
Control: Open‐flap
debridement
Test 1: PTFE non‐
resorbable membrane
Test 2: Silicone rubber
barrier material
Test 3: Polycarbonate
filter with pore size of
0.45 barrier material
Test 4:
Polycarpolactone
barrier material
6
Notch to new
cementum (mm)
Control: 0.24 ± 0.007
Test 1: 1.96 ± 0.031*
Test 2: 2.16 ± 0.011*
Test 3: 2.18 ± 0.015*
Test 4: 2.04 ± 0.037*
Notch to bone crest (mm)
Control: 0.32 ± 0.017
Test 1: 1.18 ± 0.019*
Test 2: 1.44 ± 0.014*
Test 3: 1.32 ± 0.015*
Test 4: 1.2 ± 0.010*
*Statistically significant difference from control;
+
Gore‐Tex, W.L. Gore and Assoc., Flagstaff, AZ, USA;
†
Biogide, Geistlich Biomaterials, Wolhusen, Switzerland;
‡
Resolute, W.L. Gore and Assoc., Flagstaff, AZ, USA.
CT = computed tomography; LIPUS = low‐intensity pulsed ultrasound; PTFE = polytetrafluoroethylene.

Regenerative Therapy in Animal Models 113
furcation class II defects (Caffesse et al.
1993; Lekovic and Kenney 1993). Likewise,
in critical size acute defects in the dog
model, Deliberador et al. (2006) compared
the results of autogenous bone (AB) alone
and in combination with a calcium sulphate
paste as a barrier to an empty defect (con
trol). At three months, most specimens
failed to show complete bone fill of the fur
cation. New bone formation was moderate
and restricted between the notch areas to
the mid portion of the defect. The amount
of periodontal regeneration in the three
groups was approximately 50% of the root
length, without differences between the
groups. Areas of ankyloses were also pre
sent in some sections, but no active root
resorption was observed. More recently,
Struillou et al. (2011) investigated the
regenerative capacity of injectable biphasic
calcium phosphate (BCP) in combination
with injectable polymer (Si‐HPMC) in crit
ical size (5 mm high, 3 mm deep), acute sur
gically created defects in premolar furcation
of beagle dogs. Three months after treat
ment, a bone in‐growth of 23% ± 10%
and 35.5% ± 13.9% was observed in the
empty and biomaterial‐filled defects,
respectively. Although a tendency for higher
bone in‐growth was observed in the defects
filled with the biomaterial, this difference
did not reach statistical significance.
Deproteinized bovine bone mineral
(DBBM) is an extensively used bone substi
tute with documented reconstructive poten
tial (Baldini et al. 2011). In a mini‐pig animal
model, the potential of porous titanium
granules (PTGs) and DBBM in the recon
structive treatment of surgically created
acute buccal degree II furcation defects was
tested. Six weeks after treatment, the histo
logical analysis showed significantly
increased vertical bone formation in both the
PTG (62.5%) and control (empty; 64.3%)
groups compared to the DBBM‐treated
defects (41.9%, p < 0.01), which, on the con
trary, had a reduced regenerative response.
The micro‐computed tomography (CT)
analysis showed significantly more buccal‐
palatal defect fill in furcation defects treated
with PTG (96.8%) compared to the DBBM
(62%) and control groups (72.2%, p < 0.05;
Wohlfart et al. 2012). It should be noted that
in this study, degree II furcation defects
larger than what was typically used were sur
gically created (buccal bone was removed to
half of the root width) and this might have
affected their regenerative potential.
6.4.3 Enamel Matrix Proteins
Biomimetic substances have been tested in
experimental studies to assess their regener
ative capability in furcation lesions. This
group of proteins, which play a key role in
root formation during odontogenesis, have
demonstrated in both in vitro and in vivo
studies a capacity to attract and increase the
migration and proliferation of undifferenti
ated mesenchymal cells, which then form
acellular cementum, periodontal ligament,
and alveolar bone (Zetterström et al. 1997;
Amin et al. 2012, 2013, 2014, 2016).
Remarkably, not only did regenerative tech
niques using enamel matrix derivatives show
enhanced hard‐tissue formation, they also
reported increased gingival tissue thickness
using the beagle dog model (Al Hezaimi et al.
2012). As class II furcation defects are non‐
containing defects, it has been suggested that
the use of biological substances may be clini
cally applied in combination with different
bone graft materials, although this combina
tion therapy has not yet been experimentally
evaluated to provide histological outcomes
(Sculean et al. 2007; Trombelli and Farina
2008). In an experimental study in mongrel
dogs, the use of enamel matrix derivative
(EMD) alone was associated with better
regenerative outcomes in comparison to its
combined use with ePTFE membranes in
critical size acute/chronic surgically created
buccal class II furcation defects. Following
eight weeks of healing, the EMD group
resulted in 67% new bone formation and 94%
new cementum. The combined approach

Chapter 6
114
resulted in compromised healing due to
membrane exposure (28% new bone, 80%
new cementum; Regazzini et al. 2004).
6.4.4 Growth Factors
Growth factors form a class of natural
polypeptides that act as biological mediators
regulating cell proliferation, chemotaxis,
differentiation, and synthesis. These factors
also play a major role during tissue regenera
tion, by binding to specific receptors on the
cell surface. Growth and differentiation fac
tor technologies have been evaluated for
their potential to enhance periodontal wound
healing/regeneration in healthy and systemi
cally compromised conditions (Stavropoulos
and Wikesjö 2012; Bizenjima et al. 2015).
Such biologically active substances, used
either alone or in combination with GTR,
have also been tested for their efficacy in
improving regeneration outcomes in critical
size furcation lesions. In particular, fibroblast
growth factor (FGF; Murakami et al. 1999,
2003; Takayama et al. 2001), bone morpho
genetic proteins (BMPs; Ripamonti et al.
1996, 2001), transforming growth factor‐β
(TGF‐β; Teares et al. 2008, 2012), insulin
growth factor (IGF‐1), and platelet‐derived
growth factor (PDGF; Soares et al. 2005) are
the most studied. The results of the applica
tion of growth factors in class II furcation
defects are presented in Table 6.2.
6.4.5 Cell Therapy
The most common cells applied for regener
ation are mesenchymal stem cells (MSCs), as
these pluripotent cells can differentiate into a
significant number of cell types, including
osteoblasts, fibroblasts, and cementoblasts
(Risbud and Shapiro 2005). MSCs have been
isolated from periodontal ligament (PDL;
Seo et al. 2004; Trubiani et al. 2005) and it
was demonstrated that, when they are cul
tured in contact with native PDL cells, MSCs
acquire the characteristics of PDL cells,
making them suitable for periodontal regen
eration purposes (Kramer et al. 2004). Dogan
and co‐workers retrieved cells from regenerated
periodontal defects in dogs, which were then
expanded in culture and transplanted into
critical size surgically created class II furca
tion defects in the same animals. At 42 days
post‐surgery, a trend towards better bone
formation (control 32.9% vs test 51.2%) and
less cementum formation (control 71.7% vs
test 75.5%) in the test group was reported,
but no statistical analysis was performed in
the study (Dogan et al. 2002).
The successful use of PDL cells to regener
ate class II furcation defects was documented
by Suaid and co‐workers in a dog model. PDL
cells obtained from extracted teeth were cul
tured in vitro and phenotypically character
ized with regard to their biological properties.
Acute CSDs were then surgically created and
treated with either GTR (control group) or
GTR associated to a cell‐seeded collagen
sponge (test group). Three months after treat
ment, the histomorphometric analysis showed
that the cell‐treated group presented a supe
rior length of new cementum (8.08 ± 1.08 mm
vs 6.00 ± 1.5), a greater extension of periodon
tal regeneration (7.28 ± 1.00 mm vs 3.94 ± 1.20,
p < 0.05), a lower formation of connective tis
sue/epithelium (0.60 ± 0.99 mm vs 2.15 ± 1.92,
p
<
0.05), a larger area of new bone
(9.02 ± 2.30 mm
2
vs 7.01 ± 0.61, p < 0.05), and a
smaller area of connective tissue/epithelium
(4.22 ± 0.95 mm
2
vs 5.90 ± 1.67, p > 0.05), com
pared with the control group (Suaid et al.
2011). More recently, Chantarawaratit et al.
(2014) employed primary human PDL cells
treated with acemannan, a polysaccharide
extracted from aloe vera gel, for the regenera
tion of critical size acute class II furcation
defects in mongrel dogs. It was observed that
acemannan significantly increased the per
centage of new bone formation at 30 and
60 days post‐operatively, as well as the percent
age of new cementum formation at 60 days
post‐operatively (Chantarawaratit et al. 2013).
Simsek et al. (2012) compared the effec
tiveness of MSCs with platelet‐rich plasma
(PRP) as a scaffold to PRP alone, autogenous
cortical bone (ACB) graft alone, and the
combination of ACB with PRP in the
treatment of acute/chronic class II furcation
defects versus open flap debridement

Regenerative Therapy in Animal Models 115
Table 6.2
Growth factors in class II furcation defects.
Study
Model
Tooth
Type of membrane
Healing
time
(months)
Histomorhometric
results
ACUTE/CHRONIC DEFECT MODEL
Murakami
et al. 1999
Dog
Mandibular
premolars,
molars
Test 1: Gelatinous carrier
(fibrin gel) alone
Test 2: b‐FGF + carrier
1.5
New bone formation
rate (%)
Test 1: 42.8 ± 10.7
Test 2: 79.6 ± 16.8*
New cementum
formation rate (%)
Test 1: 34.3 ± 14.5
Test 2: 75.8 ± 22.7*
Murakami
et al. 1999
Non‐human
primates
Mandibular
molars
Test 1: Carrier alone
Test 2: b‐FGF + carrier
2
New bone formation
rate (%)
Test 1: 54.3 ± 8.0
Test 2: 71.3 ± 13.5*
New cementum
formation rate (%)
Test 1: 38.9 ± 9.2
Test 2: 71.2. ± 15.2*
Murakami
et al. 2003
Dog
Mandibular
molars
Control: Gelatinous carrier
(fibrin gel) alone
Test 1: 0.1% b‐
FGF + gelatinous carrier
1.5
New bone formation
rate (%)
Test 1: 35.4 ± 8.9
Test 2: 83.6 ± 14.3*
New cementum
formation rate (%)
Test 1: 37.2 ± 15.1
Test 2: 97.7 ± 7.5*
Takayama
et al. 2001
Non‐human
primates
Maxillary,
mandibular
molars
Control: Open‐flap
debridement
Test 1: Gelatinous carrier
Test 2: 0.1% FGF‐2
Test 3: 0.4% FGF‐2
2
New bone formation
rate (%)
Control: 44.7 ± 6.2
Test 1: 54.3 ± 8.0
Test 2: 58.0 ± 21.9
Test 3: 71.3 ± 13.5*
New cementum
formation rate (%)
Control: 46.7 ± 12.1
Test 1: 38.9 ± 9.2
Test 2: 79.1 ± 23.9*
Test 3: 72.2 ± 14.4*
Keles et al.
2009
Dog
Mandibular
premolars
Control: Open‐flap
debridement
Test 1: Platelet pellet
Test 2: Platelet
pellet + resorbable
membrane of polylactic acid
(Atrisorb
+
)
3
New cementum (%)
Control: 45.60 ± 11.92
Test 1: 83.99 ± 7.70*
Test 2: 81.63 ± 8.17*
New bone (%)
Control: 42.44 ± 6.07
Test 1: 62.64 ± 7.89
Test 2: 61.06 ± 7.90
(Continued )

Chapter 6
116
Study
Model
Tooth
Type of membrane
Healing
time
(months)
Histomorhometric
results
Suaid
et al. 2010
Dog
Mandibular
premolars
Control: Synthetic
resorbable membrane
Resolute† (glycolide/lactic
copolymer) + bioactive glass
(Perioglas
‡
)
Test: Synthetic resorbable
membrane Resolute
(glycolide/lactic
copolymer) + bioactive glass
(Perioglas) + PRP
3
New bone (mm)
Control:4.33 ± 0.62
Test: 5.01 ± 0.63
New cementum (mm)
Control: 9.20 ± 3.21
Test: 12.45 ± 1.73*
Teares
et al. 2008
Baboon
Mandibular
molars
Control: Carrier alone:
Basement membrane matrix
(Matrigel
¶
)
Test 1: TGF‐β3 with carrier
Test 2: TGF‐β3 with carrier
and minced muscle tissue
2
New cementum (mm)
Control: 3.7 ± 0.7
Test 1: 3.5 ± 0.6
Test 2: 6.1 ± 0.4*
New bone (mm):
Control: 2.3 ± 0.4
Test 1: 2.8 ± 0.8
Test 2: 4.7 ± 0.3*
Teares
et al. 2012
Baboon
Mandibular
molars
Test 1: Matrigel containing
25 µg of recombinant hOP‐1
Test 2: Matrigel containing
75 µg TGF‐β3
Test 3: Matrigel with 25 µg
hOP‐1 and 25 µg TGF‐
β3
(20:1 ratio)
Test 4: Matrigel with 25 µg
hOP‐1 and 1.25 µg TGF‐β3
(20:1 ratio) plus morcellated
autogenous muscle
2
New cementum (mm):
Test 1: 6.18 ± 0.33*
Test 2: 3.65 ± 0.88
Test 3: 5.45 ± 0.89*
Test 4: 2.69 ± 1.06
New bone (mm):
Test 1: 5.93 ± 0.92
Test 2: 5.67 ± 1.17
Test 3: 7.07 ± 0.57*
Test 4: 4.73 ± 1.08
ACUTE DEFECT MODEL
Ripamonti
et al. 1996
Baboon
Mandibular
molars
Control: Carrier
Test 1: Carrier + 0.1 µg/ml
hOP‐1
Test 2: Carrier + 0.5 µg/ml
hOP‐1
2
No bone formation
was detected
New cementum
formation (distal
root)
Control: 2.6 ± 0.2
Test 1: 6.2 ± 0.5*
Test 2: 6.7 ± 0.3*
Ripamonti
et al. 2001
Baboon
Mandibular
molars
Test 1: BMP
2
(100 µg/ml)
Test 2: hOP‐1 (100 µg/ml)
Test 3: hOP‐1 + BMP
2
(100 µg/ml)
2
New bone formation
Test 1: 4.2 ± 0.2
Test 2: 3.7 ± 0.4
Test 3: 3.1 ± 0.2
New cementum
formation (distal
root)
Test 1: 3.7 ± 0.4
Test 2: 5.7 ± 0.3*
Test 3: 3.6 ± 0.2
Table 6.2
(Continued)

Regenerative Therapy in Animal Models 117
( control) in the dog model. At eight weeks,
cementum formation was significantly
higher in the ACB, combination of ACB/PRP,
and combination of MSCs/PRP groups com
pared to the control group (p < 0.05). It was
concluded that periodontal regeneration
with complete filling of class II furcation
defects can be obtained with the use of GTR,
PPR, MSCs, and their combinations. Finally,
it was shown that a collagen matrix overlaid
with embryonic stem cells (ES) is able to
improve the regeneration of class II furcation
defects (4 mm wide, 5 mm deep) in mini‐pigs
(Yang et al. 2013).
6.5 Regeneration
Treatments of Class III
Furcation Defects
6.5.1 Guided Tissue Regeneration
Starting from the late 1980s, the effects of
placing non‐bioresorbable or bioabsorbable
membranes on degree III furcation CSD
defects (3 mm wide and 4 mm high) as
compared with those in control sites was
evaluated in dogs (Niederman et al. 1989;
Pontoriero et al. 1992; White et al. 1994;
Lindhe et al. 1995; Araújo et al. 1997, 1998).
The results of the different studies are
presented in Table 6.3. GTR resulted in
significantly more gain of connective tissue
attachment and regrowth of alveolar
bone than control therapy, where no mem
brane was used. Complete closure of class III
furcation defects with the formation of peri
odontal ligament and regrowth of the bone
was achieved. It was shown that the size of
the defect and the shape of the surrounding
bone determined the outcome of regenera
tion, as already mentioned. The treatment
failures were consistently associated with
recession of the covering flaps and exposure
of the defect. In addition, the results also
demonstrated that bioabsorbable mem
branes provided a barrier that was equally
effective to that of non‐bioabsorbable mem
branes (Lindhe et al. 1995; Araújo et al. 1998).
6.5.2 Bone Grafts
The first attempts to treat class III furcation
defects with the use of bone grafts (fresh
autogenous hip marrow grafts, autogenous
cancellous bone grafts) were made by
Ellegaard et al. (1974, 1975) in the monkey
model and Nilvéus et al. (1978) in the dog
model. Although a higher frequency of furca
tion closure occurred with the use of the
Study
Model
Tooth
Type of membrane
Healing
time
(months)
Histomorhometric
results
Soares
et al. 2005
Dog
Mandibular
molars
Control: No graft
Test: Reparative tissue of
extraction socket enhanced
with PDGF‐BB + IGF
45 days
New cementum
Control: 2.49 ± 0.82
Test: 2.48 ± 0.47
New bone
Control: 2.73 ± 0.42
Test: 2.49 ± 0.71
*Statistically significant difference from control;
+
Atrisorb, Atrix Laboratories, Fort Collins, CO, USA;
†
Perioglas, US Biomaterials, Alachua, FL, USA;
‡
Resolute, W.L. Gore and Assoc., Flagstaff, AZ, USA;
¶
Matrigel™, BD Biosciences, San Jose, CA, USA; b‐FGF = basic fibroblast growth factor; BMP
2
= bone morphogenetic
protein‐2; hOP‐1 = human osteogenic protein‐1; IGF = insulin growth factor; PDGF = platelet‐derived growth factor;
PRP = platelet‐rich plasma; TGF = transforming growth factor.
Table 6.2
(Continued)

Chapter 6
118
Table 6.3
Guided tissue regeneration (GTR) in class III furcation defects.
Study
Model
Tooth
Type of membrane
Healing
time
(months)
Histomorhometric
results
ACUTE DEFECT MODEL
White et al.
1994
Dog
Mandibular
premolars
Control: Open‐flap
debridement
Test: PTFE non‐resorbable
membrane
3
Distance from notch
to coronal extent of
bone (mm)
Control: ‐0.21 ± 7.27
Test: 1.50 ± 4.31
ACUTE/CHRONIC DEFECT MODEL
Pontoriero
et al. 1992
Dog
Mandibular
premolars
Control: No membrane
Test: PTFE non‐
resorbable membrane
(Gore‐Tex
+
)
●
Small key‐hole defect
(2 × 2 mm)
●
Large key‐hole defect
(3 × 4 mm)
●
Furcation defect part of
circumferential loss of
attachment
4
Surface area (mm
2
)
Small key‐hole
defect
Control: 4.8 ± 1.3
Test: 4.1 ± 1.4
Large key‐hole
defect:
Control: 13.6 ± 1
Test: 12.8 ± 2.8
Furcation defect part
of circumferential
loss
Control: 22.3 ± 2.8
Test: 20.9 ± 2.4
Lindhe et al.
1995
Dog
Mandibular
premolars
Study 1:
Control: No membrane
Test: PTFE non‐resorbable
membrane
Study 2:
Control: PTFE non‐
resorbable membrane
Test: Synthetic resorbable
membrane made of
glycolide/lactic copolymer
(Resolute†)
5
Study 1
Amount of new
cementum
Control: 43% (±12)
Test: 74% (±11)*
Height of new
bone (mm)
Control: 0.7 ± 0.6
Test: 1.7 ± 0.5
Study 2
Amount of new
cementum
Control: 82% (±13)
Test: 86% (±11)
Height of new
bone (mm)
Control: 2.8 ± 1.4
Test: 3.1 ± 1.1
Araújo et al.
1997
Dog
Mandibular
premolars
Synthetic resorbable
membrane made of
glycolide/lactic copolymer
(Resolute)
5
Mineralized
bone (%)
36 (±12.6)
PDL (%)
20 (±11.1)

Regenerative Therapy in Animal Models 119
grafts, ankyloses and root resorption were
documented as well. By using acute/chronic
surgically created class III defects (larger
than CSD) in dogs, Roriz et al. (2006) studied
the regenerative potential of bovine‐derived
bone matrix with or without ePTFE mem
brane. Twelve weeks after treatment, it was
concluded that both treatments had similar
results and were not able to result in closure
of class III furcation defects. It should be
noted that in this study, the size of the defect
(more than 4 mm in height) may have con
tributed to the lack of complete fill of the fur
cation in both groups. Another pre‐clinical
study using the dog model showed that
implantation of beta tricalcium phosphate
(β‐TCP) with a tunnel pipe structure resulted
in new bone formation and new cementum
in acute surgically created lesions (defect
height was 4 mm) after eight weeks of
treatment, but total closure of the furcation
was not achieved (Saito et al. 2012).
6.5.3 Enamel Matrix Proteins
The first study evaluating the effect of EMD
in combination with GTR versus GTR alone
in class III furcation defects was performed
by Araújo and Lindhe (1997) in surgically
created acute/chronic lesions in the dog
model. At four months, the central portion of
both control and test furcation defects (4 mm
high, 3 mm wide) was closed and the relative
amounts of mineralized bone, bone marrow,
Table 6.3
(Continued)
Study
Model
Tooth
Type of membrane
Healing
time
(months)
Histomorhometric
results
Araújo et al.
1998
Dog
Mandibular
premolars
Test 1: Synthetic resorbable
membrane made of
glycolide/lactic copolymer
(Resolute)
Test 2: Absorbable
membrane made of
polylactic acid (Guidor
‡
)
6
Mineralized bone (%)
Test 1: 35 (±3)
Test 2: 12 (±7)*
PDL (%)
Test 1: 19 (±4)*
Test 2: 6 (±2)
Araújo et al.
1999
Dog
Mandibular
premolars
Synthetic resorbable
membrane made of
glycolide/lactic copolymer
(Resolute)
6
Bone tissue (%)
78 (±4.8)
CHRONIC DEFECT MODEL
Gonçalves
et al. 2006
Dog
Mandibular
premolars
Control: Synthetic
resorbable membrane
made of glycolide/lactic
copolymer
(Resolute) + removal of
cementum (scaling and
root planing)
Test: Synthetic resorbable
membrane made of
glycolide/lactic copolymer
(Resolute) + preservation of
cementum (polishing)
4
New cementum (mm)
Control: 3.59 ± 1.67
Test: 6.20 ± 2.26 mm*
New bone (mm)
Control: 1.86 ± 1.76
Test: 4.62 ± 3.01 mm*
*Statistically significant difference from control;
+
Gore‐Tex, W.L. Gore and Assoc., Flagstaff, AZ, USA;
†
Resolute, W.L. Gore and Assoc., Flagstaff, AZ, USA;
‡
Guidor, Sunstar Americas Inc., Schaumburg, IL, USA; PDL = periodontal ligament; PTFE = polytetrafluoroethylene.

Chapter 6
120
and periodontal ligament were similar in
both groups.
In a subsequent study, Donos et al. (2003b)
evaluated the healing of mandibular degree
III furcation treated with GTR, EMD, or a
combination in monkeys. The dimensions of
the furcation defects were approximately
4 mm wide and 3 mm high, corresponding to
the CSD. The alveolar bone on the buccal
and lingual aspect of the mesial and distal
root of each molar was also removed, creat
ing a ‘horizontal’ pattern of bone loss.
However, the height of alveolar bone on the
mesial and distal interproximal aspect of
each experimental tooth was maintained. At
five months of healing, similar results in
terms of quality of the regenerated periodon
tal tissues were reported in the sites treated
with GTR alone or in combination with
EMD. Remarkably, the application of EMD
alone resulted in unpredictable amounts of
regenerated periodontal tissues and defect
closure by newly formed bone (Figure 6.1).
The same group also showed that only when
GTR or GTR associated with EMD was
applied was it possible to obtain complete
defect regeneration (up to the fornix; Donos
et al. 2003b; Gkranias et al. 2012). Moreover,
they demonstrated different histological fea
tures in relation to the different treatment
modalities. The sites treated according to the
GTR principle showed a predominance of
cellular cementum with extrinsic fibres or
mixed fibres, while in the sites that were
treated with EMD or a combination of GTR
and EMD, the cementum was characterized
apically as acellular and with extrinsic fibres
and coronally as mixed with stratified fibres.
In a recent investigation in dogs, the com
bined use of EMD with a synthetic bone graft
(Emdogain Plus, Institut Straumann AG,
Basel, Switzerland) was compared to coro
nally repositioned flap for the treatment of
surgically created acute class III furcation in
dogs. After two months of healing, in the
experimental group, a significant amount of
new attachment and bone formation was
observed in the majority of the specimens
(Mardas et al. 2012).
A new liquid carrier for EMD, Osteogain
(Institut Straumann AG, Basel, Switzerland),
specifically designed for mixing with differ
ent biomaterials, has also been for the first
time tested in the regeneration of acute/
chronic class III furcation defects in mon
keys (Shirakata et al. 2017). The dimensions
of the exposed furcation defects were 5 mm
wide and 5 mm high (Figure 6.2). When com
paring the histological outcome of defects
treated with open‐flap debridement (OFD;
control), OFD and a collagen sponge satu
rated with EMD (OFD/EMD), and OFD and
a collagen sponge saturated with Osteogain
(OFD/Osteogain), higher amounts of con
nective tissue were observed in both test
groups. Furthermore, the OFD/Osteogain
group showed higher new attachment
formation, cementum, and new bone area.
None of the treatments achieved complete
regeneration; that is, class III furcation
persisted after treatment (Figure 6.3).
6.5.4 Growth Factors
These biologically active substances, used
either alone or in combination with GTR,
have been tested for their efficacy in improv
ing regenerative outcomes in class III furca
tion lesions. Rossa et al. (2000) combined the
use of b‐FGF with GTR for the treatment of
acute/chronic surgically created defects in
dogs. The defects had a vertical height of
5 mm and a horizontal width of 7 mm. The
proximal bone crest was not removed, result
ing in an angular‐type defect rather than a
horizontal one. The study showed improve
ments in histological outcomes, such as
newly formed cementum, and lower extent
of epithelial migration when b‐FGF was asso
ciated with GTR in comparison to GTR
alone. However, differences between experi
mental and control groups did not reach sta
tistical significance (this might be due to the
large size of the created defects) and full clo
sure of the furcation was not achieved in any
of the specimens. Conversely, the combina
tion of b‐FGF with β‐TCP was shown to
enhance connective tissue attachment and to
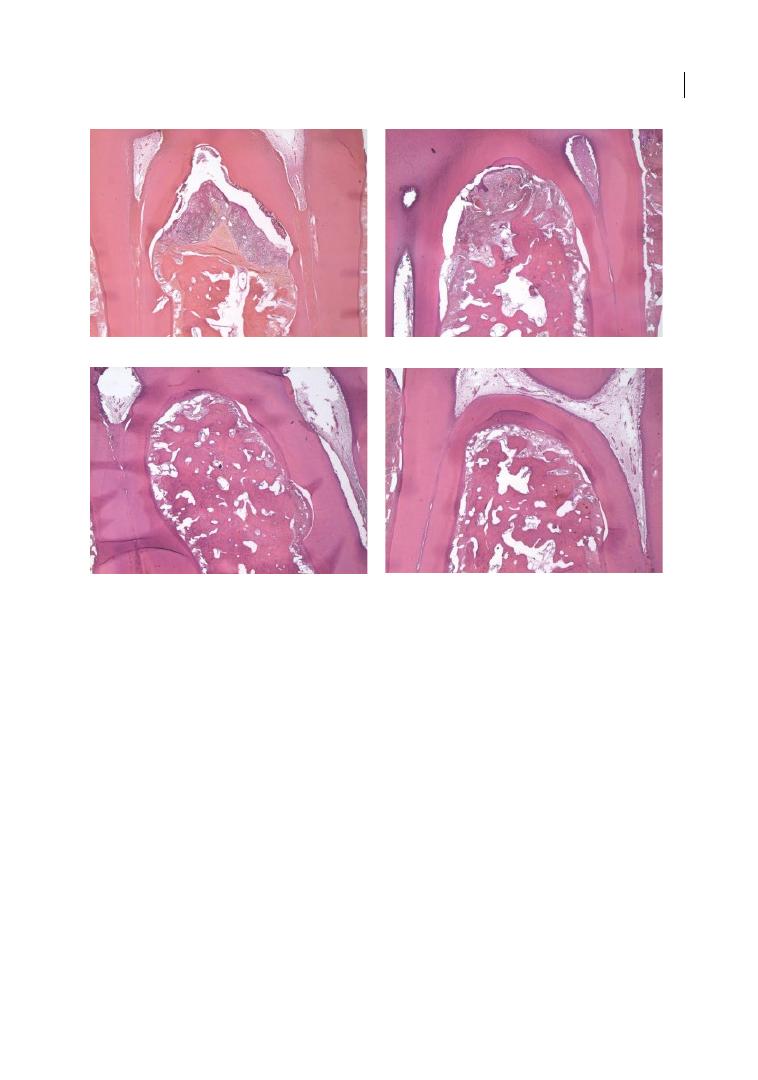
Regenerative Therapy in Animal Models 121
induce higher bone formation (up to the
fornix) compared to b‐FGF alone in class III
furcation acute smaller defects (4 mm high)
created in dogs (Saito et al. 2013).
By using the supra‐alveolar, critical size
model, the effect of recombinant human
bone morphogenetic protein (rhBMP
2
) was
tested and substantial regeneration of alveo
lar bone and cementum was demonstrated
(Wikesjö et al. 1994). However, treatment
with rhBMP
2
did not appear to induce a
functionally oriented periodontal ligament,
and resulted in ankyloses and root resorption
(Wikesjö et al. 1999, 2003a; Takahashi et al.
2005). The use of PDGF in combination with
GTR has been successfully tested by Park
et al. (1995) in acute/chronic supra‐alveolar,
critical size surgically created defects in bea
gle dogs. They reported almost complete clo
sure of the lesions without the occurrence of
resorption or ankyloses. Using the supra‐
alveolar, critical size model, different con
centrations of osteogenic protein‐1 (OP‐1)
were evaluated in acute lesions in the dog.
(a)
(b)
(c)
(d)
Figure 6.1
Overview photomicrographs of all class III furcation defects in different groups. (a) Furcation
treated with ethylenediaminetetraacetic acid (EDTA). Regeneration was observed only at the level of the notch,
with connective tissue and granulation tissue plus epithelium covering the rest of the space. (b) Furcation
treated with enamel matrix derivative (EMD). The furcation was partially closed and a layer of new cementum
with inserting fibres identified coronal to the notch. Regenerated bone filled most of the furcation area.
(c) Guided tissue regeneration (GTR)‐treated furcation where the membrane remained covered. The entire
circumference of the defect is covered with a layer of new cementum. Regenerated alveolar bone fills the
furcation defect completely. (d) Furcation treated with GTR + EMD. The furcation was closed and new
cementum can be seen on most of the circumference of the defect. The defect was filled with new bone up
to the fornix.
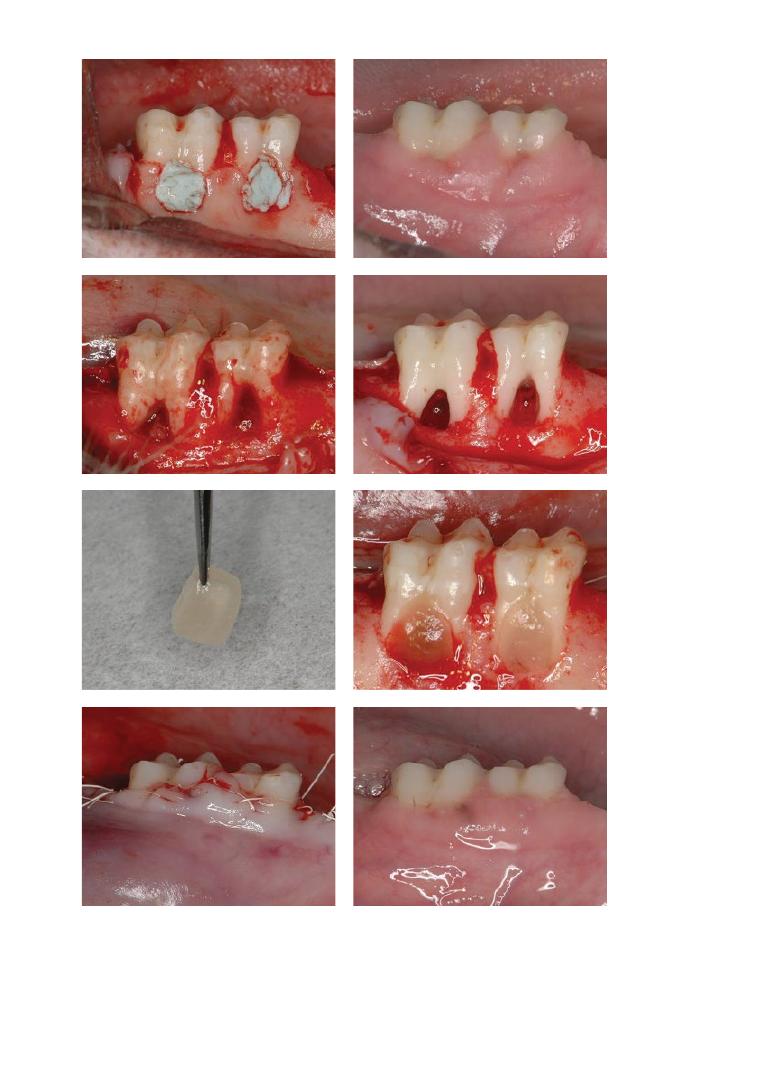
Chapter No.: 1 Title Name: <TITLENAME>
c06.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:20:29 PM Stage: <STAGE> WorkFlow:
CSW
Page Number: 122
(a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
Figure 6.2
Clinical appearance of the mandibular buccal aspect of Macaca fascicularis. (a) Induction of chronic
inflammation. After fabrication of class III furcation defects, impression materials were placed to encourage
growth of oral microflora along the exposed root surfaces. (b) Prior to reconstructive surgery. (c) Immediately
after flap reflection. Note the excessive granulation tissue in the chronic defects. (d) Defects were exposed and
debrided again at the time of reconstructive surgery. (e) Osteogain/absorbable collagen sponge (ACS)
construct before surgical implantation. (f) Left (second molar): ACS alone; right (first molar): placement of
Osteogain/ACS. (g) Flaps coronally repositioned and sutured. (h) 16 weeks after reconstructive surgery.
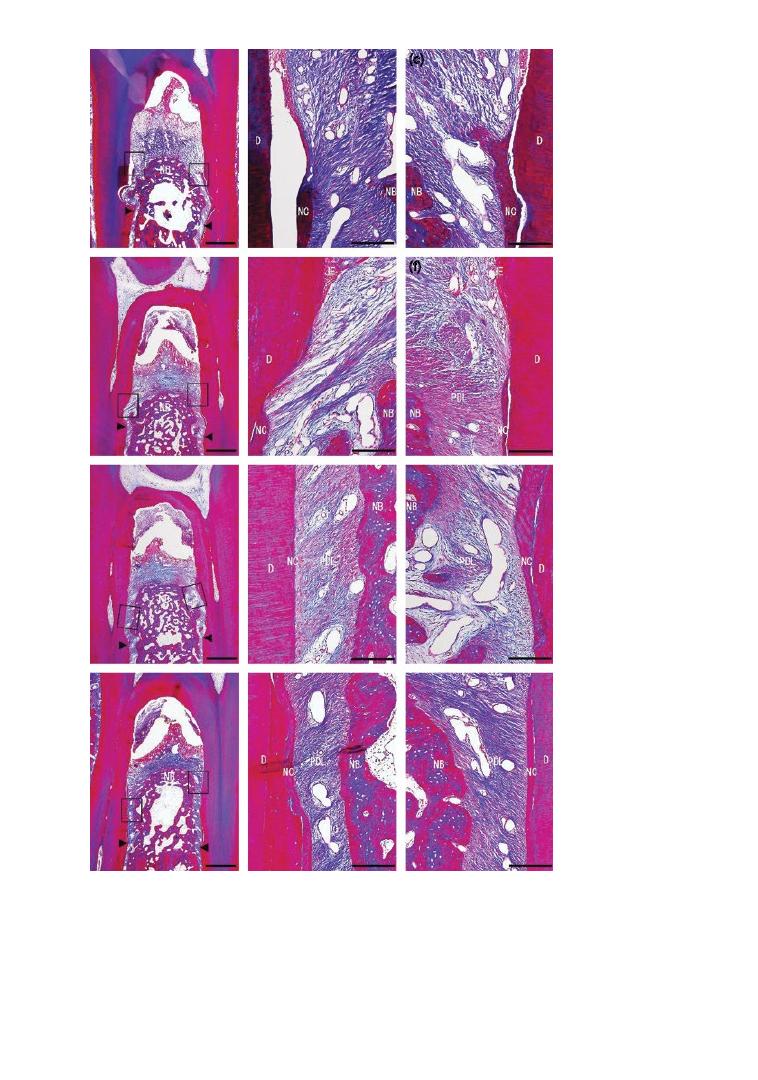
Chapter No.: 1 Title Name: <TITLENAME>
c06.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:20:29 PM Stage: <STAGE> WorkFlow:
CSW
Page Number: 123
(a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
(i)
(j)
(k)
(l)
Figure 6.3
Overview photomicrographs of all class III furcation defects in different groups (Azan‐Mallory
staining). (a) OFD (open‐flap debridement) group overview (scale bar: 1 mm). (b) Higher magnification of framed
area (left) in (a) (scale bar: 200 µm). (c) Higher magnification of framed area (right) in (a) (scale bar: 200 µm).
(d) Absorbable collagen sponge (ACS) group overview (scale bar: 1 mm). (e) Higher magnification of framed area
(left) in (d) (scale bar: 200 µm). (f) Higher magnification of framed area (right) in (d) (scale bar: 200 µm).
(g) Emdogain/ACS group overview (scale bar: 1 mm). (h) Higher magnification of apical framed area (left) in (g)
(scale bar: 200 µm). (i) Higher magnification of framed area (right) in (g) (scale bar: 200 µm). (j) Osteogain/ACS
group overview (scale bar: 1 mm). Arrowhead: notch (apical extent of root planing). (k) Higher magnification of
framed area (left) in (j) (scale bar: 200 µm). (l) Higher magnification of framed area (right) in (j) (scale bar: 200 µm).
JE = junctional epithelium, NB = new bone; NC = new cementum; PDL = periodontal ligament.

Chapter 6
124
At a dose of 7.5 µg/ml of OP‐1 in a collagen
carrier 3.9 ± 1.7 mm and 6.1 ± 3.4 mm
2
of lin
ear bone height and bone area were achieved.
These outcomes were significantly improved
in comparison to the outcomes achieved in
the defects treated with surgery only or with
carrier only (Giannobile et al. 1998).
6.5.5 Cell Therapy
Only limited studies have investigated the
use of cell therapy in the treatment of class III
furcation defects. Autogenous periosteal
cells combined with the application of β‐TCP
have shown improved periodontal tissue
regeneration in acute/chronic surgically cre
ated defects compared to β‐TCP‐treated and
empty defects in a dog model. The furcation
defects were approximately 3 mm wide and
4 mm high (Jiang et al. 2010). More recently,
Nagahara et al. (2015), using the same defect
size, also confirmed that applying a β‐TCP
scaffold to bone marrow mesenchymal stem
cells helps enhance new bone formation in
class III furcation defects exposed to inflam
mation in beagle dogs.
Using the supra‐alveolar, critical size
model, autologous periodontal ligament cells
were isolated from extracted teeth, cultured
and phenotypically characterized, and even
tually applied on a collagen sponge carrier
alone or in combination with GTR in surgi
cally created acute/chronic defects in the
beagle dog model (Murano et al. 2006; Suaid
et al. 2012). After three months of healing,
both groups resulted in additional new
cementum and new periodontal ligament
production, together with a larger area of
new bone formation.
6.6 Discussion
It is well accepted that there is no single
animal model that represents all aspects of
periodontal human disease, tissue architec
ture, and the healing and ageing processes.
However, human studies cannot always be
coupled with tissue harvesting, which is
however necessary for microscopic and his
tological analyses that define the biological
impact of the regenerative methods and
materials applied (Kantarci et al. 2015).
Therefore, it has been suggested that animal
studies are still an important step for estab
lishing cause‐and‐effect relationships and for
the initial evaluation of principles in the
development of new regenerative devices
and advanced therapeutics.
Regenerative therapy of advanced furca
tion involvement (classes II and III) has been
extensively studied in pre‐clinical models,
but the question remains whether this pre‐
clinical evidence is enough to support the
clinical use of the different techniques and
materials investigated in regenerative perio
dontal therapy. The clinical challenge in fur
cation involvement is the destruction of the
horizontal component, but also the com
bined need for vertical periodontal regenera
tion within the same area. There is extensive
evidence that, independent of the defect type
and animal model adopted, regenerative
periodontal surgery using a combination of
barrier membranes and grafting materials
may result in periodontal regeneration to a
varying extent (Sculean et al. 2008). GTR is
more predictable in class II furcation than in
class III furcation defects. Some studies
reported failures in the closure of the furca
tion, often associated with recession of the
covering tissue flaps, which subsequently
resulted in exposure of the membrane. These
results suggested that regenerative outcomes
in the treatment of furcation defects are only
possible if the healing environment under
the membrane is well protected by the flaps
during healing, and the barrier membranes
are not exposed and hence contaminated by
the oral micro‐environment (Klinge et al.
1981, 1985a, b; Lindhe et al. 1995). Superior
histological outcomes, predominantly bone
repair, following the use of a combination of
grafting materials and barrier membranes,
compared with grafting materials alone or
membranes alone, were only found in non‐
contained periodontal defects (class III
furcation defects). However, in contained

Regenerative Therapy in Animal Models 125
defects, such as class II furcation lesions, no
additional advantage of a combined treat
ment was suggested overall. This implies
that the principal mechanism by which a
graft material supports regeneration may
not be its osteoconductivity, but rather its
space provision capacity, a question which
warrants further investigation (Polimeni
at al. 2004).
Biological and biomimetic substances,
such as EMD, have also been tested in exper
imental studies to assess their regenerative
capability in furcation lesions. As class III
furcation defects are non‐contained defects,
the use of biologics is associated with impor
tant limitations. Owing to their liquid/gel‐
like consistency, any space‐making effect is
in fact prevented, and therefore the regenera
tive potential of such materials may be lim
ited in furcation defects. Remarkably, in
some studies, the use of EMD in combina
tion with GTR in class III furcation defects
resulted in some periodontal regeneration.
However, there should always be caution in
extrapolating results from experimental
studies in animals to the clinical scenario,
where it has been shown that the same treat
ment principles may not apply in class III
clinical cases (Donos et al. 2003a, 2004).
Growth and differentiation factor technol
ogies have also been evaluated for their
potential to enhance periodontal wound
healing/regeneration in furcation lesions
(Stavropoulos and Wikesjö 2012). Such bio
logically active substances, used either alone
or in combination with GTR, seem to be
promising in enhancing the regenerative out
comes in class II and III furcation defects,
but further pre‐clinical and clinical research
is needed to adequately evaluate the efficacy
of these novel treatments in periodontal
wound healing/regeneration. Cell‐based
therapies have also received considerable
attention in regenerative medicine, but their
experimental evaluation in the treatment of
periodontal furcation lesions is still at a very
early stage of development.
In recent years, due to the low rate of cell
survival after cell implantation, the paracrine
functions of mesenchymal stem cells have
received increasing attention as a regenera
tive mechanism (Nagata et al. 2017). The
possibility of enhancing the regeneration
of furcation defects with the help of trans
planted conditioned medium obtained from
cultured periodontal stem cells is certainly
an interesting and stimulating area for future
research.
6.6.1 Limitations of Pre‐clinical
Studies
It is important to recognize the limitations of
animal studies, as answers obtained from
experiments performed under standardized
conditions are specific to the questions
posed, and do not necessarily translate into
the clinical setting (Donos et al. 2003a). In
addition, ‘biological variability’ is still a con
cern and is often resorted to as an explana
tion for divergent experimental results. This
variability may be due to the erratic behav
iour of genetic, biochemical, physiological,
or immunological host factors, or to the
microbial flora associated with the individual
animal. The lack of genetically defined stocks
within dogs and primates (the animals mainly
used in this field of research), together with
the ethical concerns, may represent an
important limiting factor in our attempts to
reduce the effect of biological variability.
As mentioned previously, regenerative
therapies require a histological demonstra
tion of the actual outcome of periodontal
regeneration by measuring the tooth‐
supporting tissues (i.e. cementum, periodon
tal ligament, and alveolar bone) over a
previously diseased root surface (Sanz et al.
2015). The heterogeneity of the available
studies in terms of species, study design,
observation period, and materials makes
them difficult to compare. The evaluation of
the results may also be impaired by the diffi
culty in standardizing the defect morphology
and the extent of bone loss (horizontal and
non‐horizontal), as well as by the different
nature of the defects (naturally occurring vs
ligature induced, or acute vs chronic).

Chapter 6
126
The different anatomy and dimensions of
teeth and alveolar processes in the available
experimental animal models may reduce the
clinical value of the outcomes. For example,
experimental procedures performed in nar
row and shallow two‐rooted canine mandib
ular and maxillary premolar furcation defects
with short trunks might translate poorly to
clinical furcation defects in humans, espe
cially when considering the large three‐
rooted maxillary molars.
Another important factor affecting the
outcome of pre‐clinical studies is the concept
of the CSD. It was shown that degree III fur
cation involvements with a cross‐section
dimension larger than 4 mm are more diffi
cult to regenerate than smaller ones
(Pontoriero et al. 1989, 1992). On the other
hand, complete healing was reported in
larger degree III furcation involvements
when the membrane was kept completely
covered by the flap during healing (Lindhe
et al. 1995; Araújo et al. 1997, 1998; Araújo
and Lindhe 1997). These results indicate that
defect morphology might be less important
than post‐surgical flap dehiscence and con
sequent exposure of the membrane. The
complete closure of degree III furcation is
unpredictable and depends on the size of the
entrance of the defect (Pontoriero et al. 1989,
1992), the height of the defect, and the com
plete flap coverage of the membrane during
the healing period (Lindhe et al. 1995). Key‐
hole furcation defects without concomitant
horizontal bone reduction seemed to provide
better post‐operative support to the flaps
and prevent recession. In addition, taking
into consideration that the healing process in
dogs is more rapid compared to humans
(Cardarapoli et al. 2003; Mardas et al. 2012),
the development of new, more challenging
CSDs (width, height) should be considered to
evaluate the regenerative potential of differ
ent materials.
The lack of standardization in terms of
sample orientation for histological evalua
tion and quantitative assessment is another
potential source of bias. In the early studies,
the mesial‐distal section plane was the one
most commonly employed (Crigger et al.
1978). The true value of the histological anal
ysis of mesial‐distal sections in class II and III
furcation has been questioned by Selvig
(1994). This plane might impair interpreta
tion, since it is very difficult to determine at
which point in the buccal‐lingual direction
the section was obtained. When the section
is obtained at the most proximal point from
the intact attachment apparatus (e.g. from
the lingual wall of the buccal furcation
defect), analysis might provide a false idea of
a greater regenerative response than a sec
tion obtained at a more distant point from
the remaining periodontium, simply because
of a smaller distance from precursor cells in
the first case. According to Bogle et al. (1997),
for a more precise histomorphometric evalu
ation of the whole regenerative process of
furcation lesions, buccal‐lingual histological
sections must be obtained, because they
allow for an analysis of the healing response
from the lingual limits of the defect to the
buccal cemento‐enamel junction.
Another difficulty when trying to compare
pre‐clinical studies is the different ways of
calculating the results. In some studies the
attachment gain is measured in millimetres,
while in others it is calculated as a percentage
of the original defect height. The use of per
centages compensates for differences in size
between different experimental teeth and
defects, but tends to mask the fact that a
large percentage change may reflect a very
small change in real units of measurement
(Selvig 1994).
Furthermore, it is still unclear what is the
necessary minimum observation period to
ensure that the observed result is, in fact, the
endpoint of the healing process. The obser
vation period in most pre‐clinical studies
varies from a couple of weeks up to three or
six months. If the aim is to record the maxi
mum extent of repair, including cementum
and bone regeneration, a longer observation
period should be recorded. Cementum does
not form on root‐planed surfaces in the dog
before approximately three weeks after sur
gery. At six weeks, considerable amounts of

Regenerative Therapy in Animal Models 127
new cementum may have formed. Connective
tissue attachment may be well established at
its final level six months after surgery, but the
final picture of mature cementum and bone
formation may not be expressed until a
later stage. It has been reported that regen
erated tissues continue their formation and/
or remodelling even after three months of
wound healing, and that this process
can continue for up to six months (Araújo
et al. 1997).
6.6.2 Ethical Codes for Animal
Experimentation
There is increasing concern in society and
the medical profession regarding animal wel
fare, with significant controversy surround
ing the use of animals in research and testing
(Biller‐Andorno et al. 2015). It has been sug
gested that animal experiments can be sanc
tioned if there is no alternative means of
achieving the same scientific or educational
objective, and if the benefits to society out
weigh the costs in terms of animal harm
(Rusche 2003). Harming animals is highly
undesirable and experiments can only be jus
tified if the social good derived from this type
of use actively outweighs the negative aspect
of harming a sensitive creature (Kolar 2006).
Whenever possible, alternative methods
should be sought. The three Rs (3Rs) princi
ple, which should be applied as a guide when
conducting animal research, includes
replacement, reduction, and refinement:
●
Replacement: Using an experimental sub
ject that is phylogenetically lower or using
non‐animal systems. A few promising
alternative methods put forward recently
are in vitro techniques; tissue culture
methods; use of lower organisms including
microbes, tissues from slaughter, and
autopsy embryos; and non‐animal systems
such as computers or mathematical
modelling.
●
Reduction: Before proposing to conduct
animal experimentation, efforts should
be made to ascertain that the proposed
animal experiment has not been done pre
viously. Also, the minimum possible num
ber of animals required should be used to
yield meaningful data and not maximum
precision.
●
Refinement: A multitude of refinements of
technique that would reduce animal harm
are ready for immediate application in bio
medical research.
Since 1986, the European Union (EU) has
had specific legislation covering the use of
animals for scientific purposes.
The Council for International Organiz
ations of Medical Sciences (COIMS) is an
international non‐governmental representa
tive of many branches of medicine and cog
nate disciplines, which has laid down the
guiding principles to provide a conceptual
ethical framework acceptable to both the
international biomedical community and
animal welfare groups. COIMS set the fol
lowing international guiding principles
(Howard‐Jones 1985):
●
The use of animals for scientific purposes
is innately undesirable.
●
Another method should be used whenever
possible.
●
The use of animals in the present state of
knowledge is unavoidable.
●
Scientists should have a moral obligation
in designing the plan for the minimal num
ber of animals to be employed.
The guiding principles are the product of
consultation with a large and representative
sample of the biomedical community,
including experts from the World Health
Organization (WHO) and representatives of
animal welfare groups.
6.7 Conclusions
Furcation involvement poses one of the most
difficult challenges in periodontal therapy.
Based on the available data, if one considers
closure of the furcation defect as the main
endpoint of therapy, then the results of

Chapter 6
128
regeneration have to be regarded as satisfy
ing and predictable only for class II furcation
involvements. Conversely, class III furcation
defects are still considered a great challenge
in terms of periodontal regeneration and,
although the efficacy of different treatments
has been demonstrated in some pre‐clinical
studies, the effectiveness and relevance for
clinical practice may be questioned. In the
future, new regenerative treatment modali
ties and the development of more challeng
ing CSDs in pre‐clinical studies are clearly
needed to improve the predictability of com
plete resolution of class III furcation defects.
References
Al‐Hezaimi, K., Al‐Fahad, H., O’Neill, R. et al.
(2012). The effect of enamel matrix protein
on gingival tissue thickness in vivo.
Odontology 100, 61–66.
Amin, H.D., Olsen, I., Knowles, J.C. and
Donos, N. (2012). Differential effect of
amelogenin peptides on osteogenic
differentiation in vitro: Identification of
possible new drugs for bone repair and
regeneration. Tissue Engineering Part A 18,
1193–1202.
Amin, H.D., Olsen, I., Knowles, J.C. et al.
(2013). Effects of enamel matrix proteins on
multi‐lineage differentiation of periodontal
ligament cells in vitro. Acta Biomaterialia 9,
4796–4805.
Amin, H.D., Olsen, I., Knowles, J. et al. (2014).
A tyrosine‐rich amelogenin peptide
promotes neovasculogenesis in vitro and ex
vivo. Acta Biomaterialia 10, 1930–1939.
Amin, H.D., Olsen, I., Knowles, J. et al. (2016).
Interaction of enamel matrix proteins with
human periodontal ligament cells. Clinical
Oral Investigation 20, 339–347.
Araújo, M.G., Berglundh, T., Albrekstsson, T.,
and Lindhe, J. (1999). Bone formation in
furcation defects: An experimental study in
the dog. Journal of Clinical Periodontology
26, 643–652.
Araújo, M.G., Berglundh, T., and Lindhe, J.
(1997). On the dynamics of periodontal
tissue formation in degree III furcation
Summary of Evidence
●
Animal studies, despite their limitations,
are an important step for establishing
cause‐and‐effect relationships and for the
initial evaluation of principles in the
development of new regenerative devices
and advanced therapeutics.
●
Independent of the defect type and animal
model adopted, regenerative periodontal
surgery using a combination of barrier
membranes and grafting materials may
result in periodontal regeneration to a
variable extent in furcation involvements.
●
The results of regeneration have to be
regarded as satisfying and predictable
only for class II furcation involvements, as
class III furcation defects are still consid
ered a great challenge in terms of perio
dontal regeneration, although the efficacy
of different treatments has been demon
strated in some pre‐clinical studies.
●
The concept of the critical size defect (CSD)
is an important factor affecting the outcome
in pre‐clinical studies, and new, more chal
lenging CSDs (in width and height) should
be developed to evaluate the regenerative
potential of different materials in furcation
involvement. This is in particular consider
ation of the higher regeneration ability of
animals in comparisons to humans.
●
Growth and differentiation factor tech
nologies and cell‐based therapies have
also received considerable attention in
regenerative medicine, but their experi
mental evaluation in the treatment of per
iodontal furcation lesions is still at a very
early stage of development.

Regenerative Therapy in Animal Models 129
defects: An experimental study in dogs.
Journal of Clinical Periodontology 24,
738–746.
Araújo, M.G., Berglundh, T., and Lindhe, J.
(1998). GTR treatment of degree III
furcation defects with 2 different resorbable
barriers: An experimental study in dogs.
Journal of Clinical Periodontology 25,
253–259.
Araújo, M.G., and Lindhe, J. (1997). GTR
treatment of degree III furcation defects
following application of enamel matrix
proteins: An experimental study in dogs.
Journal of Clinical Periodontology 25,
524–530.
Avila‐Ortiz, G., De Buitrago, J.G., and Reddy,
M.S. (2015). Periodontal
regeneration – furcation defects: A
systematic review from the AAP
Regeneration Workshop. Journal of
Periodontology 86 (Suppl. 2), 69–77.
Baldini, N., De Sanctis, M., and Ferrari, M.
(2011). Deproteinized bovine bone in
periodontal and implant surgery. Dental
Materials 27, 61–70.
Berglundh, T., Lindhe, J., and Sterrett, J.D.
(1991). Clinical and structural
characteristics of periodontal tissues in
young and old dogs. Journal of Clinical
Periodontology 18, 616–623.
Biller‐Andorno, N., Grimm, H., and Walker,
R.L. (2015). Professionalism and ethics in
animal research. Natural Biotechnology 33,
1027–1028.
Bizenjima, T., Seshima, F., Ishizuka, Y. et al.
(2015). Fibroblast growth factor‐2 promotes
healing of surgically created periodontal
defects in rats with early, streptozotocin‐
induced diabetes via increasing cell
proliferation and regulating angiogenesis.
Journal of Clinical Periodontology 42, 62–71.
Bogle, G., Garrett, S., Stoller, N.H. et al. (1997).
Periodontal regeneration in naturally
occurring Class II furcation defects in
beagle dogs after guided tissue regeneration
with bioabsorbable barriers. Journal of
Periodontology 68, 536–544.
Caffesse, R.G., Dominguez, L.E., Nasjleti, C.E.,
et al. (1990). Furcation defects in dogs
treated by guided tissue regeneration (GTR).
Journal of Periodontology 61, 45–50.
Caffesse, R.G., Nasjleti, C.E., Morrison, E.C.,
and Sanchez, R. (1994). Guided tissue
regeneration: Comparison of bioabsorbable
and non‐bioabsorbable membranes.
Histologic and histometric study in dogs.
Journal of Periodontology 65, 583–591.
Caffesse, R.G., Nasjleti, C.E., Plotzke, A.E.,
et al. (1993). Guided tissue regeneration and
bone grafts in the treatment of furcation
defects. Journal of Periodontology 64 (Suppl.
11), 1145–1153.
Cardaropoli, G., Araújo, M., and Lindhe, J.
(2003). Dynamics of bone tissue formation
in tooth extraction sites: An experimental
study in dogs. Journal of Clinical
Periodontology 30, 809–818.
Caton, J.G., and Kowalski, C.J. (1976). Primate
model for testing periodontal treatment
procedures: II. Production of contralaterally
similar lesions. Journal of Periodontology 47,
506–510.
Caton, J., Mota, L., Gandini, L., and Laskaris,
B. (1994). Non‐human primate models for
testing the efficacy and safety of periodontal
regeneration procedures. Journal of
Periodontology 65, 1143–1150.
Chantarawaratit, P., Sangvanich, P., Banlunara, W.
et al. (2014). Acemannan sponges stimulate
alveolar bone, cementum and periodontal
ligament regeneration in a canine class II
furcation defect model. Journal of
Periodontal Research 49, 164–178.
Cirelli, J.A., Marcantonio, E., Jr, Adriana, R.
et al. (1997). Evaluation of anionic collagen
membranes in the treatment of class II
furcation lesions: A histometric analysis in
dogs. Biomaterials 18, 1227–1234.
Crigger, M., Bogle, G., Nilvéus, R. et al. (1978).
The effect of topical citric acid application
on the healing of experimental furcation
defects in dogs. Journal of Periodontal
Research 13, 538–549.
Danesh‐Meyer, M.J., Pack, A.R., and
McMillan, M.D. (1997). A comparison of 2
polytetrafluoroethylene membranes in
guided tissue regeneration in sheep. Journal
of Periodontal Research 32, 20–30.

Chapter 6
130
de Andrade, P.F., de Souza, S.L., de Oliveira,
M.G. et al. (2007). Acellular dermal matrix
as a membrane for guided tissue
regeneration in the treatment of Class II
furcation lesions: A histometric and clinical
study in dogs. Journal of Periodontology 78,
1288–1299.
Deliberador, T.M., Nagata, M.J., Furlaneto, F.A.
et al. (2006). Autogenous bone graft with or
without a calcium sulfate barrier in the
treatment of Class II furcation defects: A
histologic and histometric study in dogs.
Journal of Periodontology 77, 780–789.
De Santana, R.B., Gusman, H.C., and Van
Dyke, T.E. (1999). The response of human
buccal maxillary furcation defects to
combined regenerative techniques: Two
controlled clinical studies. Journal of the
International Academy of Periodontology 1,
69–77.
Dogan, A., Ozdemir, A., Kubar, A., and Oygür,
T. (2002). Assessment of periodontal healing
by seeding of fibroblast‐like cells derived
from regenerated periodontal ligament in
artificial furcation defects in a dog: aA pilot
study. Tissue Engineering 8, 273–282.
Donos, N., Glavind, L., Karring, T., and
Sculean, A. (2003a). Clinical evaluation of
an enamel matrix derivative in the treatment
of mandibular degree II furcation
involvement: A 36‐month case series.
International Journal of Periodontics and
Restorative Dentistry 23, 507–512.
Donos, N., Glavind, L, Karring T., and Sculean
A. (2004). Clinical evaluation of an enamel
matrix derivative and a bioresorbable
membrane in the treatment of degree III
mandibular furcation involvement: A series
of nine patients. International Journal of
Periodontics and Restorative Dentistry 200,
362–369.
Donos, N., Sculean, A., Glavind, L. et al.
(2003b). Wound healing of degree III
furcation involvements following guided
tissue regeneration and/or Emdogain: A
histologic study. Journal of Clinical
Periodontology 30, 1061–1068.
Ellegaard, B., Karring, T., Davies, R., and Löe,
H. (1974). New attachment after treatment
of intrabony defects in monkeys. Journal of
Periodontology 45, 368–377.
Ellegaard, B., Karring, T., and Löe, H. (1975).
The fate of vital and devitalized bone grafts
in the healing of interradicular lesions.
Journal of Periodontal Research 10, 88–97.
Giannobile, W.V., Finkelman, R.D., and Lynch,
S.E. (1994). Comparison of canine and
non‐human primate animal models for
periodontal regenerative therapy: Results
following a single administration of PDGF/
IGF‐I. Journal of Periodontology 65,
1158–1168.
Giannobile, W.V., Ryan, S., Shih, M.S. et al.
(1998). Recombinant human osteogenic
protein‐1 (OP‐1) stimulates periodontal
wound healing in class III furcation defects.
Journal of Periodontology 69, 129–137.
Gkranias, N.D., Graziani, F., Sculean, A., and
Donos, N. (2012). Wound healing following
regenerative procedures in furcation degree
III defects: Histomorphometric outcomes.
Clinical Oral Investigation 16, 239–249.
Gonçalves, P.F., Gurgel, B.C., Pimentel, S.P.
et al. (1996). Root cementum modulates
periodontal regeneration in Class III
furcation defects treated by the guided
tissue regeneration technique: A histometric
study in dogs. Journal of Periodontology 77,
976–982.
Gosain, A.K., Song, L., Yu, P. et al. (2000).
Osteogenesis in cranial defects:
Reassessment of the concept of critical size
and the expression of TGF‐beta isoforms.
Plastic Reconstructive Surgery 106,
360–371.
Graves, D.T., Fine D., Teng, Y.T. et al. (2008).
The use of rodent models to investigate
host–bacteria interactions related to
periodontal diseases. Journal of Clinical
Periodontology 35, 89–105.
Haney, J.M., Zimmerman, G.J., and Wikesjö,
U.M. (1995). Periodontal repair in dogs:
Evaluation of the natural disease model.
Journal of Clinical Periodontology 22,
208–213.
Howard‐Jones, N.A. (1985). CIOMS ethical
code for animal experimentation. WHO
Chronicles 39, 51–56.

Regenerative Therapy in Animal Models 131
Hürzeler, M.B., Quiñones, C.R., Caffesse, R.G.
et al. (1997). Guided periodontal tissue
regeneration in Class II furcation defects
following treatment with a synthetic
bioabsorbable barrier. Journal of
Periodontology 68, 498–505.
Ivanovic, A., Nikou, G., Miron, R.J. et al.
(2014). Which biomaterials may promote
periodontal regeneration in intrabony
periodontal defects? A systematic review of
preclinical studies. Quintessence
International 45, 385–395.
Jiang, J., Wu, X., Lin, M. et al. (2010).
Application of autologous periosteal cells for
the regeneration of class III furcation defects
in beagle dogs. Cytotechnology 62, 235–243.
Kantarci, A., Hasturk, H., and Van Dyke, T.E.
(2015). Animal models for periodontal
regeneration and peri‐implant responses.
Periodontology 2000 68, 66–82.
Karring, T., and Cortellini, P. (1999).
Regenerative therapy: Furcation defects.
Periodontology 2000 19, 115–137.
Karring, T., Nyman, S., Gottlow, J., and Laurell,
L. (1993). Development of the biological
concept of guided tissue regeneration:
Animal and human studies. Periodontology
2000 1, 26–35.
Karring, T., Nyman, S., and Lindhe, J. (1980).
Healing following implantation of
periodontitis affected roots into bone tissue.
Journal of Clinical Periodontology 7, 96–105.
Karring, T., and Warrer, K. (1992).
Development of the principle of guided
tissue regeneration. Alpha Omegan 85,
19–24.
Keles, G.C., Cetinkaya, B.O., Baris, S. et al.
(2009). Comparison of platelet pellet with or
without guided tissue regeneration in the
treatment of class II furcation defects in
dogs. Clinical Oral Investigation 13,
393–400.
Klinge, B., Nilvéus, R., and Egelberg, J. (1985a).
Effect of crown‐attached sutures on healing
of experimental furcation defects in dogs.
Journal of Clinical Periodontology 12,
369–373.
Klinge, B., Nilvéus, R., and Egelberg, J. (1985b).
Bone regeneration pattern and ankylosis in
experimental furcation defects in dogs.
Journal of Clinical Periodontology 12,
456–464.
Klinge, B., Nilvéus, R., Kiger, R.D., and
Egelberg, J. (1981). Effect of flap placement
and defect size on healing of experimental
furcation defects. Journal of Periodontal
Research 16, 236–248.
Kolar, R. (2006). Animal experimentation.
Science and Engineer Ethics 12, 111–122.
Koo, K.T., Polimeni, G., Albandar, J.M., and
Wikesjö, U.M. (2004). Periodontal repair in
dogs: Analysis of histometric assessments in
the supraalveolar periodontal defect model.
Journal of Periodontology 75, 1688–1693.
Kramer, P.R., Nares, S., Kramer, S.F. et al.
(2004). Mesenchymal stem cells acquire
characteristics of cells in the periodontal
ligament in vitro. Journal of Dental Research
83, 27–34.
Lang, H., Schuler, N., and Nolden, R. (1998).
Attachment formation following
replantation of cultured cells into
periodontal defects: A study in minipigs.
Journal of Dental Research 77, 393–405.
Lekovic, V., and Kenney, E.B. (1993). Guided
tissue regeneration using calcium phosphate
implants together with 4 different
membranes: A study on furcations in dogs.
Journal of Periodontology 64, 1154–1156.
Lekovic, V., Klokkevold, P.R., Kenney, E.B.
et al. (1998). Histologic evaluation of guided
tissue regeneration using 4 barrier
membranes: A comparative furcation study
in dogs. Journal of Periodontology 69, 54–61.
Lindhe, J., Pontoriero, R., Berglundh, T., and
Araujo, M. (1995). The effect of flap
management and bioresorbable occlusive
devices in GTR treatment of degree III
furcation defects: An experimental study in
dogs. Journal of Clinical Periodontology 22,
276–283.
Macedo, G.O., Souza, S.L., Novaes, A.B., Jr
et al. (2006). Effect of early membrane
removal on regeneration of Class II
furcation defects in dogs. Journal of
Periodontology 77, 46–53.
Mardas, N., Kraehenmann, M., and Dard, M.
(2012). Regenerative wound healing in acute

Chapter 6
132
degree III mandibular defects in dogs.
Quintessence International 43, e48–e59.
Murakami, S., Takayama, S., Ikezawa, K. et al.
(1999). Regeneration of periodontal tissues
by basic fibroblast growth factor. Journal of
Periodontal Research 34, 425–430.
Murakami, S., Takayama, S., Kitamura, M.
et al. (2003). Recombinant human basic
fibroblast growth factor (bFGF) stimulates
periodontal regeneration in class II furcation
defects created in beagle dogs. Journal of
Periodontal Research 38, 97–103.
Murano, Y., Ota, M., Katayama, A. et al.
(2006). Periodontal regeneration following
transplantation of proliferating tissue
derived from periodontal ligament into class
III furcation defects in dogs. Biomedical
Research 27, 139–147.
Murphy, K.G., and Gunsolley, J.C. (2003).
Guided tissue regeneration for the treatment
of periodontal intrabony and furcation
defects: A systematic review. Annals of
Periodontology 8, 266–302.
Nagahara, T., Yoshimatsu, S., Shiba, H. et al.
(2015). Introduction of a mixture of
β‐tricalcium phosphate into a complex of
bone marrow mesenchymal stem cells and
type I collagen can augment the volume of
alveolar bone without impairing cementum
regeneration. Journal of Periodontology 86,
456–464.
Nagata, M., Iwasaki, K., Akazawa, K. et al.
(2017). Conditioned medium from
periodontal ligament stem cells enhances
periodontal regeneration. Tissue
Engineering, Part A 23, 367–377.
Niederman, R., Savitt, E.D., Heeley, J.D., and
Duckworth, J.E. (1989). Regeneration of
furca bone using Gore‐Tex periodontal
material. International Journal of
Periodontics and Restorative Dentistry 9,
468–480.
Nilvéus, R., Johansson, O., and Egelberg, J.
(1978). The effect of autogenous cancellous
bone grafts on healing of experimental
furcation defects in dogs. Journal of
Periodontal Research 13, 532–537.
Nyman, S., Karring, T., Lindhe, J., and Plantén,
S. (1980). Healing following implantation of
periodontitis‐affected roots into gingival
connective tissue. Journal of Clinical
Periodontology 7, 394–401.
Oz, H.S., and Puleo, D.A. (2011). Animal
models for periodontal disease. Journal of
Biomedical Biotechnology 2011, 754857.
Park, J.B., Matsuura, M., Han, K.Y. et al.
(1995). Periodontal regeneration in class III
furcation defects of beagle dogs using
guided tissue regenerative therapy with
platelet‐derived growth factor. Journal of
Periodontology 66, 462–477.
Polejaeva, I.A., Chen, S.H., Vaught, T.D. et al.
(2000). Cloned pigs produced by nuclear
transfer from adult somatic cells. Nature
407, 86–90.
Polimeni, G., Koo, K.T., Qahash, M. et al.
(2004). Prognostic factors for alveolar
regeneration: Effect of a space‐providing
biomaterial on guided tissue regeneration.
Journal of Clinical Periodontology 31,
725–729.
Pontoriero, R., Lindhe, J., Nyman, S., et al.
(1989). Guided tissue regeneration in the
treatment of furcation defects in mandibular
molars: A clinical study of degree III
involvements. Journal of Clinical
Periodontology 16, 170–174.
Pontoriero, R., Nyman, S., Ericsson, I., and
Lindhe, J. (1992). Guided tissue regeneration
in surgically‐produced furcation defects: An
experimental study in the beagle dog.
Journal of Clinical Periodontology 19,
159–163.
Regazzini, P.F., Novaes, A.B. Jr, de Oliveira, P.T.
et al. (2004). Comparative study of enamel
matrix derivative with or without GTR in
the treatment of class II furcation lesions in
dogs. International Journal of Periodontics
and Restorative Dentistry 24, 476–487.
Ripamonti, U., Crooks, J., Petit, J.C., and
Rueger, D.C. (2001). Tissue regeneration by
combined applications of recombinant
human osteogenic protein‐1 and bone
morphogenetic protein‐2: A pilot study in
Chacma baboons (Papio ursinus). European
Journal of Oral Sciences 109, 241–248.
Ripamonti, U., Heliotis, M., Rueger, D.C., and
Sampath, T.K. (1996). Induction of

Regenerative Therapy in Animal Models 133
cementogenesis by recombinant human
osteogenic protein‐1 (hop‐1/bmp‐7) in the
baboon (Papio ursinus). Archive of Oral
Biology 41, 121–126.
Risbud, M.V., and Shapiro, I.M. (2005). The
effect of brain‐derived neurotrophic factor
on periodontal furcation defects: Stem cells
in craniofacial and dental tissue engineering.
Orthodontic and Craniofacial Research 8, 54.
Roriz, V.M., Souza, S.L., Taba, M., Jr, et al.
(2006). Treatment of Class III furcation
defects with expanded
polytetrafluoroethylene membrane
associated or not with anorganic bone
matrix/synthetic cell‐binding peptide: A
histologic and histomorphometric study in
dogs. Journal of Periodontology 77, 490–497.
Rossa, C., Marcantonio, E., Jr, Cirelli, J.A. et al.
(2000). Regeneration of Class III furcation
defects with basic fibroblast growth factor
(b‐FGF) associated with GTR: A descriptive
and histometric study in dogs. Journal of
Periodontology 71, 775–784.
Rusche, B. (2003). The 3Rs and animal welfare:
Conflict or the way forward? ALTEX 20,
63–76.
Saito, A., Saito, E., Kuboki, Y. et al. (2013).
Periodontal regeneration following
application of basic fibroblast growth
factor‐2 in combination with beta tricalcium
phosphate in class III furcation defects in
dogs. Dental Materials Journal 232,
256–262.
Saito, E., Saito, A., Kuboki, Y. et al. (2012).
Periodontal repair following implantation of
beta‐tricalcium phosphate with different
pore structures in Class III furcation defects
in dogs. Dental Materials Journal 31,
681–688.
Sander, L., and Karring, T. (1995). New
attachment and bone formation in
periodontal defects following treatment of
submerged roots with guided tissue
regeneration. Journal of Clinical
Periodontology 22, 295–299.
Sanz, M., Jepsen, K., Eickholz, P., and Jepsen,
S. (2015). Clinical concepts for regenerative
therapy in furcations. Periodontology 2000
68, 308–332.
Schmitz, J.P., and Hollinger, J.O. (1986). The
critical size defect as an experimental model
for craniomandibulofacial nonunions.
Clinical Orthopaedics and Related Research
205, 299–308.
Schou, S., Holmstrup, P., and Kornman, K.S.
(1993). Non‐human primates used in studies
of periodontal disease pathogenesis: A
review of the literature. Journal of
Periodontology 64, 497–508.
Sculean, A., Nikolidakis, D., and Schwarz, F.
(2008). Regeneration of periodontal tissues:
Combinations of barrier membranes and
grafting materials – biological foundation
and preclinical evidence: A systematic
review. Journal of Clinical Periodontology
35, 106–116.
Sculean, A., Windisch, P., Döri, F. et al. (2007).
Emdogain in regenerative periodontal
therapy: A review of the literature. Fogorvosi
Szemle 100, 220–232.
Selvig, K.A. (1994). Discussion: Animal models
in reconstructive therapy. Journal of
Periodontology 65, 1169–1172.
Seo, B.M., Miura, M., Gronthos, S. et al.
(2004). Investigation of multipotent
postnatal stem cells from human
periodontal ligament. Lancet 10–16,
149–155.
Shirakata, Y., Miron, R.J., Nakamura, T. et al.
(2017). Effects of EMD liquid (Osteogain)
on periodontal healing in class III furcation
defects in monkeys. Journal of Clinical
Periodontology 44, 298–307.
Simsek, S.B., Keles, G.C., Baris, S., and
Cetinkaya, B.O. (2012). Comparison of
mesenchymal stem cells and autogenous
cortical bone graft in the treatment of class
II furcation defects in dogs. Clinical Oral
Investigation 16, 251–258.
Soares, F.P., Hayashi, F., Yorioka, C.W. et al.
(2005). Repair of Class II furcation defects
after a reparative tissue graft obtained from
extraction sockets treated with growth
factors: A histologic and histometric study
in dogs. Journal of Periodontology 76,
1681–1689.
Stavropoulos, A., and Wikesjö, U.M. (2012).
Growth and differentiation factors for

Chapter 6
134
periodontal regeneration: A review on
factors with clinical testing. Journal of
Periodontal Research 47, 545–553.
Struillou, X., Boutigny, H., Badran, Z. et al.
(2011). Treatment of periodontal defects in
dogs using an injectable composite
hydrogel/biphasic calcium phosphate.
Journal of Material Sciences Materials in
Medicine 22, 1707–1717.
Struillou, X., Boutigny, H., Soueidan, A., and
Layrolle, P. (2010). Experimental animal
models in periodontology: A review. Open
Dental Journal 4, 37–47.
Suaid, F.A., Macedo, G.O., Novaes, A.B. et al.
(2010). The bone formation capabilities of
the anorganic bone matrix‐synthetic cell‐
binding peptide 15 grafts in an animal
periodontal model: A histologic and
histomorphometric study in dogs. Journal of
Periodontology 81, 594–603.
Suaid, F.F., Ribeiro, F.V., Gomes, T.R. et al.
(2012). Autologous periodontal ligament
cells in the treatment of Class III furcation
defects: A study in dogs. Journal of Clinical
Periodontology 39, 377–384.
Suaid, F.F., Ribeiro, F.V., Rodrigues, T.L. et al.
(2011). Autologous periodontal ligament
cells in the treatment of class II furcation
defects: A study in dogs. Journal of Clinical
Periodontology 38, 491–498.
Takahashi, D., Odajima, T., Morita, M. et al.
(2005). Formation and resolution of
ankylosis under application of recombinant
human bone morphogenetic protein‐2
(rhBMP‐2) to class III furcation defects in
cats. Journal of Periodontal Research 40,
299–305.
Takayama, S., Murakami, S., Shimabukuro, Y.
et al. (2001). Periodontal regeneration by
FGF‐2 (bFGF) in primate models. Journal of
Dental Research 80, 2075–2079.
Teares, J.A., Petit, J.C., and Ripamonti, U.
(2012). Synergistic induction of periodontal
tissue regeneration by binary application of
human osteogenic protein‐1 and human
transforming growth factor‐β3 in Class II
furcation defects of Papio ursinus. Journal of
Periodontal Research 47, 336–344.
Teares, J.A., Ramoshebi, L.N., and Ripamonti,
U. (2008). Periodontal tissue regeneration by
recombinant human transforming growth
factor‐beta 3 in Papio ursinus. Journal of
Periodontal Research 43, 1–8.
Trombelli, L., and Farina, R (2008). Clinical
outcomes with bioactive agents alone or in
combination with grafting or guided tissue
regeneration. Journal of Clinical
Periodontology 35, 117–135.
Trubiani, O., Di Primio, R., Traini, T. et al.
(2005). Morphological and cytofluorimetric
analysis of adult mesenchymal stem cells
expanded ex vivo from periodontal
ligament. International Journal of
Immunopathology and Pharmacology 18,
213–221.
Wang, S., Liu, Y., Fang, D., and Shi, S. (2007).
The miniature pig: A useful large animal
model for dental and orofacial research.
Oral Disease 13, 530–537.
Wang, Y., Chai, Z., Zhang, Y. et al. (2014).
Influence of low‐intensity pulsed ultrasound
on osteogenic tissue regeneration in a
periodontal injury model: X‐ray image
alterations assessed by micro‐computed
tomography. Ultrasonics 54, 1581–1584.
White, C., Jr., Hancock, E.B., Garetto, L.P., and
Kafrawy, A.A. (1994). A histomorphometric
study on the healing of class III furcations
utilizing bone labelling in beagle dogs.
Journal of Periodontology 65, 84–92.
Wikesjö, U.M., Guglielmoni, P., Promsudthi, A.
et al. (1999). Periodontal repair in dogs:
Effect of rhBMP‐2 concentration on
regeneration of alveolar bone and
periodontal attachment. Journal of Clinical
Periodontology 26, 392–400.
Wikesjö, U.M., Kean, C.J., and Zimmerman,
G.J. (1994). Periodontal repair in dogs:
Supraalveolar defect models for evaluation
of safety and efficacy of periodontal
reconstructive therapy. Journal of
Periodontology 65, 1151–1157.
Wikesjö, U.M., Lim, W.H., Thomson, R.C.
et al. (2003a). Periodontal repair in dogs:
Evaluation of a bioabsorbable space‐
providing macroporous membrane with

Regenerative Therapy in Animal Models 135
recombinant human bone morphogenetic
protein‐2. Journal of Periodontology 74,
635–647.
Wikesjö, U.M., Xiropaidis, A.V., Thomson,
R.C. et al. (2003b). Periodontal repair in
dogs: Space‐providing ePTFE devices
increase rhBMP‐2/ACS‐induced bone
formation. Journal of Clinical Periodontology
30, 715–725.
Wohlfahrt, J.C., Aass, A.M., Rønold, H.J. et al.
(2012). Microcomputed tomographic and
histologic analysis of animal experimental
degree II furcation defects treated with
porous titanium granules or deproteinized
bovine bone. Journal of Periodontology 83,
211–221.
Yang, J.R., Hsu, C.W., Liao, S.C. et al. (2013).
Transplantation of embryonic stem cells
improves the regeneration of periodontal
furcation defects in a porcine model. Journal
of Clinical Periodontology 40, 364–371.
Zetterström, O., Andersson, C., Eriksson, L.
et al. (1997). Clinical safety of enamel matrix
derivative (EMDOGAIN) in the treatment
of periodontal defects. Journal of Clinical
Periodontology 24, 697–704.

Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
Chapter No.: 1 Title Name: <TITLENAME>
c07.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:20:50 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 137
137
7.1 Introduction
Different strategies are available to address
the problem of furcation involvement (FI).
One option is the elimination of the furcation
defect. This can be achieved by removal of the
involved root(s) using resective approaches
(see Chapter 8). Alternatively, periodontal tis-
sues that have been destroyed by periodonti-
tis can be regenerated, thereby decreasing the
lesion. Regenerative periodontal therapy of
furcation defects has proven successful in
many experimental pre‐clinical studies (see
Chapter 6).
This chapter reviews the evidence for the
effectiveness of regenerative therapy for the
treatment of furcation defects in different
clinical scenarios, in order to address the
question: ‘What has been achieved so far?’
7.2 Outcome Measures
for Regenerative Therapy
in Furcation Defects
A variety of outcome measures can be
considered to assess the effectiveness of
regenerative furcation therapies.
7.2.1 Human Histology
Evidence for periodontal regeneration requires
the histological demonstration of restored
tooth‐supporting tissues, including cemen-
tum, periodontal ligament, and alveolar bone,
over a previously diseased root surface. Even
though such outcomes have been demon-
strated in well‐controlled experimental animal
studies for a variety of treatment modalities
(see Chapter 6), information derived from
human histology is scarce. Four histological
studies investigated human degree II furcation
defects (Harris 2002; Stoller et al. 2001; Camelo
et al. 2003; Nevins et al. 2003), one studied
degree III defects (Mellonig et al. 2009), while
one presented data from degree II and III fur-
cation defects (Gottlow et al. 1986).
7.2.1.1 Degree II Furcation Defects
Regarding degree II defects, all five studies
reported partial regeneration of the perio-
dontal tissues. Two studies applied deminer-
alized freeze‐dried bone allograft (DFDBA)
combined with recombinant human platelet‐
derived growth factor‐BB (rhPDGF‐BB) and
reported formation of bone, cementum, and
periodontal ligament coronally to the notch
(Camelo et al. 2003; Nevins et al. 2003). Two
other studies used barrier membranes
(guided tissue regeneration, GTR) and
described formation of cementum, perio-
dontal ligament, and bone (Gottlow et al.
1986; Stoller et al. 2001). Harris (2002) used
a combination (DFDBA + polyhydroxyal-
kanoate
[PHA] + tetracycline + resorbable
Chapter 7
Regenerative Therapy of Furcations in Human Clinical Studies:
What has been Achieved So Far?
Søren Jepsen and Karin Jepsen
Department of Periodontology, Operative and Preventive Dentistry, University of Bonn, Germany

Chapter 7
138
membrane) and observed partial defect
closure with new bone, cementum, and con-
nective tissue attachment coronal or limited
to the notch area.
7.2.1.2 Degree III Furcation Defects
The two studies evaluating degree III furcation
defects reported only partial regeneration.
Gottlow et al. (1986), using barrier membranes
(GTR), demonstrated 2.8 mm new cementum
with inserting collagen fibres in a 7 mm furca-
tion defect. Mellonig et al. (2009), using a com-
bined technique (rhPDGF + beta‐tricalcium
phosphate [β‐TCP] + collagen membrane)
reported partial closure in three out of the four
defects. The histomorphometric data revealed
new cementum ranging from 0.0 to 5.5 mm,
while the length of new bone and new collagen
fibres ranged from 0.0 to 2.0 mm.
7.2.2 Clinical Outcomes
From a clinical point of view, complete elimi-
nation of the inter‐radicular defect appears to
be the most important outcome. Decreasing
furcation degree is associated with a
decreased long‐term tooth loss risk (see
Chapter 5). Thus, the main outcome variables
for studies evaluating the efficacy of regener-
ative techniques in furcations are change of
furcation status (conversion into class I or
complete closure) and horizontal hard‐tissue
fill. As histological evidence for successful
furcation regeneration is not a practical out-
come variable for controlled clinical trials,
changes in direct bone measurements (hori-
zontal probing bone level, at surgery and
during re‐entry) serve as primary outcome
variables for evaluating clinical success, while
clinical attachment level gain (horizontal/
vertical probing attachment level), probing
depth reduction (horizontal/vertical), and
radiographic assessments may serve as sec-
ondary outcomes (Machtei 1997). Bone fill
during a re‐entry procedure is the only com-
ponent of a regenerated periodontium that
can be accurately assessed clinically. In fact, it
was stated at a European consensus confer-
ence that it would be desirable for all future
GTR studies to report the reduction in hori-
zontal probing during re‐entry, and also the
frequency (predictability) of complete furca-
tion closure (Jepsen et al. 2002).
As an alternative to open probing bone
level assessments during a re‐entry proce-
dure, probing bone measurements were pro-
posed and evaluated (Suh et al. 2002). In
some clinical trials, horizontal probing bone
level was assessed after only six months, and
it may be speculated that this is too early for
a final evaluation of bone fill in furcation
defects. Patient‐reported outcomes follow-
ing regenerative furcation surgery may
include postoperative pain, the rate of com-
plications, perceived benefit, and change in
quality of life (see Chapter 13).
7.3 Clinical Scenarios
Most of the currently available clinical stud-
ies to date have been devoted to mandibular
molars with buccal/lingual degree II furca-
tion defects and maxillary molars with buc-
cal/interproximal degree II furcation defects.
More limited information is available on
mandibular degree III furcation defects and
maxillary degree III furcation defects,
whereas there is a paucity of data on regen-
erative treatment in degree I furcations and
in maxillary premolars (Avila‐Ortiz et al.
2015; Reddy et al. 2015).
The efficacy of various regenerative
approaches in furcation defects has been
evaluated by several systematic reviews with
or without meta‐analyses (Jepsen et al. 2002;
Murphy and Gunsolley 2003; Reynolds et al.
2003; Kinaia et al. 2011; Chen et al. 2013;
Avila‐Ortiz et al. 2015) and has also been
addressed in a recent comprehensive narra-
tive review (Sanz et al. 2015), which served as
a basis for this chapter.
7.3.1 Degree II Furcation Defects
7.3.1.1 Barrier Membranes (GTR)
After clinical case series (Becker et al. 1988)
had demonstrated promising results for GTR

Regenerative Therapy in Human Clinical Studies 139
therapy in furcation defects using expanded
PTFE barriers (Gore‐Tex Periodontal
Membrane, W.L. Gore and Assoc., Flagstaff,
AZ, USA), several randomized controlled
clinical trials compared GTR therapy with
open‐flap debridement (OFD, representing
standard control treatment) in human degree
II furcation defects. Several studies observed
more favourable horizontal probing attach-
ment level gain and horizontal probing bone
level gain after GTR than after OFD in degree
II furcation defects of mandibular molars
(Pontoriero et al. 1988; Lekovic et al. 1989,
1991; Mellonig et al. 1994; Wang et al. 1994;
Mombelli et al. 1996; Prathibha et al. 2002;
Cury et al. 2003; Bremm et al. 2004; see also
Table 7.1), of maxillary molars (Metzler et al.
1991; Mellonig et al. 1994; Pontoriero and
Lindhe 1995a; Avera et al. 1998; see also
Table 7.2), and of maxillary and mandibular
molars (Flanary et al. 1991; Paul et al. 1992;
Twohey et al. 1992; Caton et al. 1994; Yukna
and Yukna 1996; see also Table 7.3). Whereas
some authors observed more favourable
results six months after GTR therapy in
maxillary degree II furcations only in buccal
sites (Pontoriero and Lindhe 1995a), others
reported statistically better horizontal prob-
ing bone level gain also in mesiopalatal
degree II furcations nine months following
GTR (Avera et al. 1998).
A systematic review with meta‐analyses
assessed the efficacy of membrane therapy in
the treatment of periodontal furcation
defects measured against standard surgical
periodontal treatment (i.e. OFD; Jepsen et al.
2002), and confirmed the superiority of GTR
over OFD in class II furcation defects;
however, the results also showed significant
heterogeneity, indicating high variability.
These results were subsequently also con-
firmed by other systematic reviews (Murphy
and Gunsolley 2003; Kinaia et al. 2011). This
variability may be explained by prognostic
factors (e.g. smoking, peri‐surgical antibiot-
ics, or defect morphology; Bowers et al. 2003;
Horwitz et al. 2004). Deep pockets at base-
line facilitate more favourable results after
regenerative therapy (Machtei et al. 1994;
Horwitz et al. 2004). However, other authors
have found deep baseline pockets to be asso-
ciated with significant reductions in the
number of complete furcation closures
(Bowers et al. 2003). This discrepancy may
be a result of differences in bone morphol-
ogy. Wide furcations respond less favourably
and in deep degree II furcations (≥5 mm)
complete closure is less likely (Bowers et al.
2003). If the fornix of the furcation is located
apically to the interproximal alveolar crest
(key‐hole defect), more horizontal attach-
ment gain may be expected than in teeth
with a furcation fornix located coronally of
the interproximal bone level. If there is bone
coronal of the furcation fornix adjacent to
the tooth, coverage and stabilization of the
membrane may be achieved by a coronal
positioning of the flap. Under such condi-
tions, the surface of the periodontal ligament
to provide cells to colonize the blood clot
within the defect is larger than in a tooth
where the fornix is located coronal of the
alveolar crest (Bowers et al. 2003; Horwitz
et al. 2004).
When comparing the use of non‐resorbable
and biodegradable barrier membranes in the
treatment of mandibular degree II furcation
defects, similar horizontal defect fill has
been reported (Blumenthal 1993; Bouchard
et al. 1993; Christgau et al. 1995; Hugoson
et al. 1995; Yukna and Yukna 1996;
Caffesse et al. 1997; Eickholz et al. 1997,
1998; Garrett et al. 1997; Scott et al. 1997;
Dos Anjos et al. 1998; Pruthi et al. 2002; see
also Table 7.4). Only a few studies have com-
pared the clinical efficacy of different bioab-
sorbable barrier membranes for treatment
of Class II furcations; none found one bioab-
sorbable material to be superior to another
(Vernino et al. 1999; Eickholz et al. 2000).
7.3.1.2 Combination Therapy (GTR
and Bone Grafts)
The combination of a barrier membrane
with a filler material may enhance the hori-
zontal fill of molars with degree II FI as
shown in a systematic review with meta‐
analysis (Chen et al. 2013). Out of four studies

Chapter No.: 1 Title Name: <TITLENAME>
c07.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:20:50 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 140
Table 7.2
Compar
ison of clinical r
esults af
ter open‐flap debr
idemen
t and guided tissue r
egener
ation in deg
ree II fur
ca
tion def
ec
ts of maxillar
y molars
.
A
uthors
Study
type
Par
amet
er
O
pen‐flap
debridemen
t
baseline
(mm)
G
ain
(mm)
n
G
uided tissue
regener
ation
baseline
(mm)
G
ain
(mm)
n
Barrier
ma
terial
Obser
va
tion
period
M
etzler e
t al. 1991
RC
T
H
or
izon
tal pr
obing
bone le
ve
l
Bucc
al a
nd
inter
pro
xi
ma
l
3.7
0.3
17
3.7
0.9*
17
a
Ex
pande
d
poly
te
traf
luor
oe
th
ylene
6 mon
ths
M
ellonig e
t al. 1994
RC
T
H
or
izon
tal pr
obing
bone le
ve
l
4.5
0.3
8
4.9
1.0*
8
a
Ex
pande
d
poly
te
traf
luor
oe
th
ylene
6 mon
ths
Pon
tor
ier
o and
Lindhe 1995a
RC
T
H
or
izon
tal pr
obing
bone le
ve
l
Bucc
al
M
es
io
lin
gu
al
D
istol
in
gu
al
3.2 3.4 3.2
0.3 0.2 0.2
10
10 8 8
3.2 3.5 3.4
1.1* 0.4 0.2
10
a
10
a
8
a
8
a
Ex
pande
d
poly
te
traf
luor
oe
th
ylene
6 mon
ths
Avera e
t al. 1998
RC
T
H
or
izon
tal pr
obing
bone le
ve
l
M
es
io
lin
gu
al
No d
at
a
‐0.69
No d
at
a
1.19*
Ex
pande
d
poly
te
traf
luor
oe
th
ylene
9 mon
ths
*S
ta
tistic
ally sig
nif
ic
an
t dif
fer
enc
e b
etwe
en op
en‐f
la
p de
br
idemen
t and g
uide
d ti
ssue r
egenera
tion;
Table 7.1
Comparison of clinical results after open‐flap debridement and guided tissue regeneration in degree II furcation defects of mandibular molars.
Authors
Study
type
Parameter
Open‐flap
debridement
baseline
(mm)
Gain
(mm)
n
Guided tissue
regeneration
baseline
(mm)
Gain
(mm)
n
Barrier
material/
filler
Observation
period
Lekovic et al. 1989 RCT
Horizontal probing
bone level
Buccal
No data
‐0.14
12
No data
0.18
12
a
Expanded
polytetrafluoroethylene
6 months
Lekovic et al. 1991 RCT
Horizontal probing
bone level
Buccal
4.2
‐0.2
15
4.2
1.6 *
15
a
Connective tissue graft
including periosteum
6 months
Mellonig et al.
1994
RCT
Horizontal probing
bone level
7.6
1.0
11
8.4
4.5 *
11
a
Expanded
polytetrafluoroethylene
6 months
Wang et al. 1994
RCT
Horizontal probing
bone level
5.58
1.08
12
6.00
2.04 *
12
a
BioMend®
b
12 months
Prathibha et al.
2002
RCT
Horizontal probing
bone level
4.7
0.64
10
4.79
2.38 *
10
a
TefGen®
c
6 months
Comparison of open‐flap debridement with guided tissue regeneration in combination with fillers
Houser et al. 2001
RCT
Horizontal probing
bone level
6.2
0.9
13
5.7
3.0 *
18
BioGide®
d
and BioOss®
Tsao et al. 2006
RCT
Horizontal probing
bone level
4.7
0.2
9
4.3
4.4
1.1 *
1.1 *
9
9
Puros®
e
BioMend
and Puros
6 months
* Statistically significant difference between open‐flap debridement and guided tissue regeneration;
a
split‐mouth design;
b
bovine type 1 collagen;
c
polytetrafluoroethylene;
d
deproteinized bovine bone mineral/porcine collagen;
e
mineralized solvent‐dehydrated bone allograft;
RCT = randomized controlled trial.
Chapter No.: 1 Title Name: <TITLENAME>
c07.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:20:50 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 141
Table 7.2
Comparison of clinical results after open‐flap debridement and guided tissue regeneration in degree II furcation defects of maxillary molars.
Authors
Study
type
Parameter
Open‐flap
debridement
baseline
(mm)
Gain
(mm)
n
Guided tissue
regeneration
baseline
(mm)
Gain
(mm)
n
Barrier
material
Observation
period
Metzler et al. 1991
RCT
Horizontal probing
bone level
Buccal and
interproximal
3.7
0.3
17
3.7
0.9 *
17
a
Expanded
polytetrafluoroethylene
6 months
Mellonig et al. 1994
RCT
Horizontal probing
bone level
4.5
0.3
8
4.9
1.0 *
8
a
Expanded
polytetrafluoroethylene
6 months
Pontoriero and
Lindhe 1995a
RCT
Horizontal probing
bone level
Buccal
Mesiolingual
Distolingual
3.2
3.4
3.2
0.3
0.2
0.2
10
10
8
8
3.2
3.5
3.4
1.1 *
0.4
0.2
10
a
10
a
8
a
8
a
Expanded
polytetrafluoroethylene
6 months
Avera et al. 1998
RCT
Horizontal probing
bone level
Mesiolingual
No data
‐0.69
No data
1.19 *
Expanded
polytetrafluoroethylene
9 months
* Statistically significant difference between open‐flap debridement and guided tissue regeneration;
a
split‐mouth design;
RCT = randomized controlled trial.
Table 7.1
Comparison of clinical results after open‐flap debridement and guided tissue regeneration in degree II furcation defects of mandibular molars.
Authors
Study
type
Parameter
Open‐flap
debridement
baseline
(mm)
Gain
(mm)
n
Guided tissue
regeneration
baseline
(mm)
Gain
(mm)
n
Barrier
material/
filler
Observation
period
Lekovic et al. 1989 RCT
Horizontal probing
bone level
Buccal
No data
‐0.14
12
No data
0.18
12
a
Expanded
polytetrafluoroethylene
6 months
Lekovic et al. 1991 RCT
Horizontal probing
bone level
Buccal
4.2
‐0.2
15
4.2
1.6 *
15
a
Connective tissue graft
including periosteum
6 months
Mellonig et al.
1994
RCT
Horizontal probing
bone level
7.6
1.0
11
8.4
4.5 *
11
a
Expanded
polytetrafluoroethylene
6 months
Wang et al. 1994
RCT
Horizontal probing
bone level
5.58
1.08
12
6.00
2.04 *
12
a
BioMend®
b
12 months
Prathibha et al.
2002
RCT
Horizontal probing
bone level
4.7
0.64
10
4.79
2.38 *
10
a
TefGen®
c
6 months
Comparison of open‐flap debridement with guided tissue regeneration in combination with fillers
Houser et al. 2001
RCT
Horizontal probing
bone level
6.2
0.9
13
5.7
3.0 *
18
BioGide®
d
and BioOss®
Tsao et al. 2006
RCT
Horizontal probing
bone level
4.7
0.2
9
4.3
4.4
1.1 *
1.1 *
9
9
Puros®
e
BioMend
and Puros
6 months
* Statistically significant difference between open‐flap debridement and guided tissue regeneration;
a
split‐mouth design;
b
bovine type 1 collagen;
c
polytetrafluoroethylene;
d
deproteinized bovine bone mineral/porcine collagen;
e
mineralized solvent‐dehydrated bone allograft;
RCT = randomized controlled trial.
a
split‐mouth design;
RCT = randomized controlled trial.
Chapter No.: 1 Title Name: <TITLENAME>
c07.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:20:50 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 142
Table 7.3
Compar
ison of clinical r
esults af
ter open‐flap debr
idemen
t and guided tissue r
egener
ation in deg
ree II fur
ca
tion def
ec
ts of maxillar
y and mandibular
molars
.
A
uthors
Study
type
Par
amet
er
O
pen‐flap
debridemen
t
baseline
(mm)
G
ain
(mm)
n
G
uided tissue
regener
ation
baseline
(mm)
G
ain
(mm)
n
Barrier
ma
terial
Obser
va
tion period
Fl
anar
y e
t al. 1991
RC
T
H
or
izon
tal pr
obing
bone le
ve
l
2.9
0.8
19
3.3
1.5*
19
a
Biobrane®
b
6 mon
ths
Pa
ul e
t al. 1992 (132)
RC
T
H
or
izon
tal pr
obing
bone le
ve
l
3.86
0
7
4.71
0.86*
7
a
Colli
st
ar®
b
6 mon
ths
Twohe
y e
t al. 1992
RC
T
H
or
izon
tal pr
obing
bone le
ve
l
Bucc
al
2.6
0.3
8
3.3
1.4*
8
a
Biobrane
6 mon
ths
Yuk
na and Y
uk
na
1996
RC
T
H
or
izon
tal pr
obing
bone le
ve
l
5.3
1.1
27
5.0
2.0*
27
a
BioM
end®
c
6–12 mon
ths
(me
an
=
11.1
mon
ths)
*S
ta
tistic
ally sig
nif
ic
an
t dif
fer
enc
e b
etwe
en op
en‐f
la
p de
br
idemen
t and g
uide
d ti
ssue r
egenera
tion;
a
split‐mout
h de
sig
n;
Table 7.3
Comparison of clinical results after open‐flap debridement and guided tissue regeneration in degree II furcation defects of maxillary and mandibular
molars.
Authors
Study
type
Parameter
Open‐flap
debridement
baseline
(mm)
Gain
(mm)
n
Guided tissue
regeneration
baseline
(mm)
Gain
(mm)
n
Barrier
material
Observation period
Flanary et al. 1991
RCT
Horizontal probing
bone level
2.9
0.8
19
3.3
1.5 *
19
a
Biobrane®
b
6 months
Paul et al. 1992 (132)
RCT
Horizontal probing
bone level
3.86
0
7
4.71
0.86 *
7
a
Collistar®
b
6 months
Twohey et al. 1992
RCT
Horizontal probing
bone level
Buccal
2.6
0.3
8
3.3
1.4 *
8
a
Biobrane
6 months
Yukna and Yukna
1996
RCT
Horizontal probing
bone level
5.3
1.1
27
5.0
2.0 *
27
a
BioMend®
c
6–12 months
(mean = 11.1 months)
* Statistically significant difference between open‐flap debridement and guided tissue regeneration;
a
split‐mouth design;
b
poly‐dimethyl‐siloxane mechanically bonded to a fine‐knit, flexible nylon fabric;
c
bovine type 1 collagen;
RCT = randomized controlled trial.
Chapter No.: 1 Title Name: <TITLENAME>
c07.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:20:50 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 143
Table 7.4
Comparison of clinical results after guided tissue regeneration using expanded polytetrafluoroethylene and biodegradable barriers in degree II furcation
defects of maxillary and/or mandibular molars.
Authors
Defect type
Parameter
Expanded
polytetrafluoroethylene
baseline
(mm)
Gain
(mm) n
Biodegradable
baseline
(mm)
Gain
(mm)
n
Barrier
material
Observation period
Bouchard
et al. 1993
Mandibular
molars
*
Connective
tissue graft
12 months
Buccal
Horizontal
probing bone level
No data
2.2
12
No data
1.5
12
a
Yukna and
Yukna 1996
Maxillary and
mandibular
molars
Horizontal
probing bone level
4.3
1.7
32
4.7
1.7
32
a
BioMend®
b
6–12 months
(mean = 11.1 months)
Scott et al.
1997
Mandibular
molars
Horizontal
probing bone level
5.0
2.2
12
5.4
2.0
12
a
LamBone
c
6 months
Dos Anjos
et al. 1998
Mandibular
molars
Horizontal
probing bone level
3.8
2.87
15
4.0
2.93
15
a
Gengiflex
d
6 months
Pruthi et al.
2002
Mandibular
molars
Horizontal
probing bone level
2.00
0.41
17
2.00
0.41
17
a
BioMend
12 months
* Statistically significant difference between expanded polytetrafluoroethylene and biodegradable barriers;
a
split‐mouth design;
b
bovine type 1 collagen;
c
laminar bone membrane and particulate decalcified freeze‐dried bone;
d
cellulose.
Table 7.3
Comparison of clinical results after open‐flap debridement and guided tissue regeneration in degree II furcation defects of maxillary and mandibular
molars.
Authors
Study
type
Parameter
Open‐flap
debridement
baseline
(mm)
Gain
(mm)
n
Guided tissue
regeneration
baseline
(mm)
Gain
(mm)
n
Barrier
material
Observation period
Flanary et al. 1991
RCT
Horizontal probing
bone level
2.9
0.8
19
3.3
1.5 *
19
a
Biobrane®
b
6 months
Paul et al. 1992 (132)
RCT
Horizontal probing
bone level
3.86
0
7
4.71
0.86 *
7
a
Collistar®
b
6 months
Twohey et al. 1992
RCT
Horizontal probing
bone level
Buccal
2.6
0.3
8
3.3
1.4 *
8
a
Biobrane
6 months
Yukna and Yukna
1996
RCT
Horizontal probing
bone level
5.3
1.1
27
5.0
2.0 *
27
a
BioMend®
c
6–12 months
(mean = 11.1 months)
* Statistically significant difference between open‐flap debridement and guided tissue regeneration;
a
split‐mouth design;
b
poly‐dimethyl‐siloxane mechanically bonded to a fine‐knit, flexible nylon fabric;
c
bovine type 1 collagen;
RCT = randomized controlled trial.

Chapter 7
144
on mandibular molars, two showed statisti-
cally significantly more horizontal bone fill
following the combination therapy (Wallace
et al. 1994; Luepke et al. 1997; Simonpietri
et al. 2000; Maragos et al. 2002; see also
Table 7.5).
7.3.1.3 Long‐term Results
Long‐term data following GTR therapy in
furcation defects are sparse (Figueira et al.
2014). Using GTR, horizontal probing attach-
ment level gains from 0.75 to 4.1 mm and
horizontal probing bone level gains from
0.2 to 4.5 mm may be achieved, and degree II
furcation defects may be closed or converted
to degree I. Molars with degree I FI have a
better long‐term prognosis than molars with
degree II defects (McGuire and Nunn 1996),
whereas a gradual increase in the risk of tooth
loss was observed for molars with degree II
and III FI (Nibali et al. 2016). To date there are
only limited data on the long‐term results
(≥4 years) after GTR therapy in degree II fur-
cations. Significant gains in horizontal attach-
ment (2.59 mm) were obtained one year post
surgery for GTR‐treated sites. These changes
were maintained over four years with a slight
decline at the end of year 3 (Machtei et al.
1996). Mean horizontal probing attachment
level gains after the use of non‐resorbable and
biodegradable barriers could be maintained
for five years (Eickholz et al. 2001). A 10‐year
follow‐up of 18 teeth in 9 patients revealed
further stability of horizontal probing attach-
ment level gains between 12 and 120 months.
However, two molars were lost in one patient,
and another molar lost more than 2 mm of
horizontal probing attachment level (Eickholz
et al. 2006).
7.3.1.4 Enamel Matrix Derivative (EMD)
Only a limited number of clinical studies
have evaluated enamel matrix derivative
(Emdogain, Straumann, Basel, Switzerland)
for the treatment of FI, either alone or in
combination with another regenerative
therapy (for review Donos et al. 2010; Koop
et al. 2012; Miron et al. 2014, 2016), and no
meta‐analyses have been performed.
Mandibular Molars
In a case series study with 36 months of fol-
low‐up on 10 patients with 8 buccal and 8
lingual degree II FI, the use of EMD was eval-
uated (Donos et al. 2003a). The follow‐up
periods were 6, 12, and 36 months. At the
buccal furcation defects, the horizontal prob-
ing attachment level measurements were
reduced from 4.0 mm at baseline to 2.6 mm
at 6 months, demonstrating a mean horizon-
tal probing attachment level change of
1.4 mm. However, at 12 and 36 months the
change was reduced to 0.8 mm and 0.6 mm,
respectively, and, as such, the horizontal
probing attachment level changes were not
adequate to transform the degree II FI to
degree I. At the lingual sites, the horizontal
probing attachment level changes were mini-
mal. In all cases, following the 12‐month
healing period the furcation defects remained
as degree II. This study was performed in a
small number of mandibular molars, and it
did not have a control group in which either
OFD or another established regenerative
procedure, such as GTR, was performed.
When investigating the adjunctive use of
EMD with OFD in 10 patients with 20 degree
II furcation defects on contralateral molars
by re‐entry after 6 months, a significantly
enhanced horizontal bone gain (2 mm in the
EMD vs 0.8 mm in the OFD group) of the
bony defects was found in EMD‐treated
furcations (Chitsazi et al. 2007). Complete
furcation closure was reported in 1 of the
10 defects treated with EMD. However, a re‐
entry at 6 months post‐operatively may be
too early to evaluate bone fill of a furcation
lesion.
A multi‐centre randomized controlled
clinical trial compared EMD with GTR in
the treatment of degree II buccal furcation
defects in mandibular molars (Jepsen et al.
2004). In this study, the investigators treated
45 patients with a total of 90 similar degree
II furcation defects on contralateral molars,
either with EMD or with a bioresorbable
membrane. The clinical measurements
performed at baseline, 8 months, and
14 months following surgery included
Chapter No.: 1 Title Name: <TITLENAME>
c07.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:20:50 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 145
Table 7.5
Comparison of clinical results after guided tissue regeneration using a barrier membrane alone and in combination with osseous grafts in degree II
furcation defects of mandibular molars (all randomized controlled trials).
Authors
Defect type
Parameter
Guided tissue
regeneration
barrier material
alone
(baseline)
(mm)
Gain
(mm) n
Guided tissue
regeneration barrier
material + osseous
graft
baseline
(mm)
Gain
(mm) n
Osseous graft
material
Observation
period
Wallace et al.
1994
Mandibular
molars
Horizontal
probing bone
level
Expanded
polytetrafluoroethylene
7
10
Decalcified
freeze‐dried bone
allograft
b
12 months
Buccal
6.0
2.3
6.5
2.4
Luepke et al. 1997 Mandibular
molars
Horizontal
probing bone
level
Guidor®
c
6.03
1.80 14 5.90
2.1
14
a
Decalcified
freeze‐dried bone
allograft
b
6 months
Simonpietri‐C
et al. 2000
Mandibular
molars
Horizontal
probing bone
level
Gengiflex®
d
5.0
2.47 15 5.53
3.27 * 15
a
Bon‐Apatite®
e
6 months
Maragos et al.
2002
Mandibular
molars
Horizontal
probing bone
level
CaSO
4
3.8
0.9
11 3.5
3.7
1.2
2.2
11
14
CaSO
4
/
doxycycline
CaSO
4
/
decalcified
freeze‐dried bone
allograft
b
12 months
* Statistically significant difference between guided tissue regeneration and guided tissue regeneration + osseous grafts;
a
split‐mouth design;
b
decalcified freeze‐dried bone allograft;
c
synthetic biodegradable polymer;
d
cellulose;
e
anorganic bovine bone.

Chapter 7
146
gingival margin levels, pocket probing
depth, bleeding on probing, vertical attach-
ment levels, vertical bone sounding from a
stent at five buccal sites per tooth, and hori-
zontal bone sounding at the furcation area.
Similar defect measurements were per-
formed during a re‐entry procedure on all
defects at 14 months post surgery. Change
of horizontal furcation depth (comparing
intrasurgical baseline and re‐entry meas-
urements) served as the primary outcome
variable. The results indicated that both
regenerative procedures produced clinical
improvement. More specifically, EMD dem-
onstrated a mean reduction of horizontal
probing bone level of 2.6 mm, whereas the
GTR‐treated sites showed a horizontal
probing bone level reduction of 1.9 mm
(Table 7.6). Complete furcation closure was
achieved in 8 of the 45 furcation defects
treated with EMD and in 3 of the 45 defects
treated with GTR. Partial closure (resulting
in a change from degree II to degree I) was
the same (27 of 45) in both groups. No
change in furcation status was observed in 9
of 45 and in 11 of 45 defects, respectively,
and deterioration was observed in one of 45
EMD‐treated sites and in four of 45 GTR‐
treated sites. Furthermore, less post‐opera-
tive pain and swelling was reported
following the use of EMD, which could be
explained by the antibacterial (Sculean et al.
2001) or anti‐inflammatory potential that
EMD might possess (Myhre et al. 2006;
Nokhbehsaim et al. 2012). The study con-
cluded that the use of EMD not only has a
similar effect to GTR in transforming degree
II buccal furcation defects to degree I in a
predictable manner, but it may also achieve
complete closure of the furcation defects to
a greater extent than GTR. Furthermore, for
furcation defects at mid‐buccal sites, the
EMD‐treated sites presented less gingival
recession than the GTR‐treated sites (Meyle
et al. 2004). This could be attributed to the
fact that no measurable bone resorption
occurred in the EMD‐treated sites, whereas
slight bone resorption occurred in the GTR‐
treated sites.
From the same sample of patients, it was
also observed that the best clinical outcome
in buccal degree II furcation defects follow-
ing treatment with EMD was in male patients
over 54 years of age who were non‐smokers
(Hoffmann et al. 2006), which is in agree-
ment with observations in previous studies
with GTR (Machtei et al. 1994). However,
these results need to be interpreted with cau-
tion, because the number of patients in each
subgroup (age, gender, smoking habit, etc.)
was relatively low. Furthermore, in this study
patient selection was of paramount clinical
importance, because all selected teeth pre-
sented with proximal levels at or above the
fornix of the furcation and there was always a
zone of keratinized tissue of at least 2 mm
present, for covering the furcation following
the application of EMD. Similar results, with
regard to the treatment with EMD in man-
dibular degree II FI, were reported in another
randomized controlled trial with re‐entry
after 12 months (Barros et al. 2005). In
10 patients with 20 paired furcation defects,
GTR therapy using an expanded PTFE mem-
brane led to a mean horizontal defect fill of
3.3 mm, whereas EMD application resulted
in a mean horizontal defect fill of 2.2 mm,
with no significant difference between the
modalities (Table 7.6).
Maxillary Molars
A randomized controlled trial with a split‐
mouth design in 15 patients with one pair of
contralateral degree II proximal FI compared
the use of EMD with OFD in conjunction
with conditioning of the root surfaces with
ethylenediaminetetraacetic acid (EDTA) gel
(Casarin et al. 2008). At 6 months, a mean
horizontal bone gain was 1.0 mm for the con-
trol group and 1.1 mm for the test group.
However, there was a statistically significant
difference in the number of remaining degree
II FI, in favour of EMD. Of 15 proximal
degree II furcations, 2 were completely
closed and 9 were converted into degree I, 6
months following EMD application. In con-
trast, following OFD, only 5 furcations were
converted into degree I, with the other

Regenerative Therapy in Human Clinical Studies 147
10 defects remaining degree II. Of the
15 patients, 12 were followed up for 24 months
(Casarin et al. 2010) and at this time point
the test group presented with 5 remaining
degree II furcations versus 10 degree II furca-
tions in the control group (p < 0.05). Overall,
the treatment response of proximal furca-
tions in maxillary molars to EMD application
was not as favourable as that of mandibular
furcations. The authors attributed this to
more difficult access and higher plaque
retention during follow up.
7.3.1.5 Combination Therapy (EMD
and Bone Grafts)
Only a few clinical studies have evaluated the
combination of EMD with bone grafts or
bone substitutes in furcation defects (Miron
et al. 2014).
Mandibular Molars
In a case series of 11 patients, each contribut-
ing one buccal mandibular degree II furcation
defect, a combination therapy of EMD and
autologous bone grafts was evaluated (Aimetti
et al. 2007). After two years, complete clinical
closure was achieved in four sites and all resid-
ual defects were reduced to degree I.
A comparative study tested the effective-
ness of EMD in combination with DFDBA
and a resorbable membrane (GTR; Jaiswal
and Deo 2013). Using a parallel design,
30 buccal or lingual mandibular degree II
furcations in 30 patients received either
EMD + DFDBA + GTR, DFDBA + GTR, or
OFD. After 12 months, mean reductions in
horizontal probing depths were 2.1 mm for
the EMD + DFDBA + GTR group and 1.5 mm
for the DFDBA + GTR group (p > 0.05). The
number of degree II furcations that were
closed or converted to class I was greater for
EMD + DFDBA + GTR.
A recently published parallel group rand-
omized controlled trial with 40 patients
compared EMD, beta‐tricalcium phosphate
coated hydroxyapatite (ß‐TCP/HA), and
EMD + ß‐TCP/HA in buccal mandibular
degree II furcation defects (Queiroz et al.
2016). After 12 months, the mean horizontal
clinical attachment level gain was 2.7 mm for
EMD, 2.6 mm for β‐TCP/HA, and 2.9 mm
for EMD + β‐TCP/HA, with no significant
differences among the groups. After
12 months, 13 of 13 furcations in the EMD
group, 10 of 14 furcations in the β‐TCP/HA
group, and 12 of 14 furcations in the β‐TCP/
HA + EMD group improved their diagnoses
to degree I. However, complete furcation
closure was not detectable during the
study period.
Table 7.6
Comparison of clinical results after guided tissue regeneration or enamel matrix derivative
(Emdogain) application in degree II furcation defects of mandibular molars.
Authors
Study
type Parameter
Guided tissue
regeneration
baseline
(mm)
Gain
(mm) n
EMD
baseline
(mm)
Gain
(mm) n
Barrier
material/
filler
Observation
period
Jepsen et al.
2004
RCT Horizontal
probing
bone level
Buccal
No data
1.9
45 No data 2.6* 45
a
Resolut®
b
14 months
Barros et al.
2005
RCT Horizontal
probing
bone level
No data
3.3
15 No data 2.2
15
a
Expanded
polytetrafluoro-
ethylene
6 months
*Statistically significant difference between guided tissue regeneration and enamel matrix derivative;
a
split‐mouth design;
b
synthetic biodegradable polymer;
RCT = randomized controlled trial.

Chapter 7
148
Maxillary Molars
A randomized controlled trial evaluated the
combination of EMD + ß‐TCP/HA com-
pared with ß‐TCP/HA alone in 30 patients
with 30 proximal class II furcation defects in
maxillary molars (Peres et al. 2013). Mean
horizontal bone level gains after 6 months
were 1.7 mm for both treatment modalities.
The EMD + ß‐TCP/HA group showed 7
closed furcations and 7 converted to degree
I, versus 4 closed furcations and 10 con-
verted to degree I in the ß‐TCP/HA group
(p > 0.05).
At present, no long‐term data (>3 years) are
available for the effects of EMD application in
the regenerative therapy of furcation defects.
7.3.1.6 Platelet Concentrates
Growth and differentiation factor technolo-
gies have been evaluated for their potential
to enhance periodontal wound healing/
regeneration (Stavropoulos and Wikesjö
2012). Autologous platelet concentrates,
such as platelet‐rich plasma (PRP) and plate-
let‐rich fibrin (PRF), are a source for growth
factors that can be applied to the periodontal
wound (Dohan Ehrenfest et al. 2009; Del
Fabbro et al. 2011). Very recently, systematic
reviews with meta‐analyses have evaluated
the regenerative potential of these approaches
for furcation defects (Troiano et al. 2016;
Castro et al. 2017). While three original stud-
ies were included in one systematic review
(Troiano et al. 2016), the other included only
two of them (Castro et al. 2017). These stud-
ies are presented in more detail in what
follows.
Mandibular Molars
In a randomized clinical trial of six months’
duration using a split‐mouth design (Pradeep
et al. 2009), the effectiveness of autologous
PRP was compared with OFD in the treat-
ment of 20 patients with a total of 40 man-
dibular degree II furcation defects. Although
there was significantly more horizontal clini-
cal attachment level gain (2.5 mm vs 0.8 mm)
and radiographic bone fill following the
application of PRP, all furcation defects
retained their degree II status.
Another randomized controlled trial of
nine months’ duration evaluated in a split‐
mouth design the use of autologous PRF in
the treatment of mandibular degree II furca-
tion defects in comparison with OFD, in 18
patients with 36 furcations (Sharma and
Pradeep 2011). Complete clinical closure was
achieved in 12 of 18 test defects, whereas
another 5 were reduced to degree I. Change
in horizontal clinical attachment level
amounted to 2.7 mm following PRF versus
1.9 mm following OFD (p < 0.05).
A randomized controlled trial compared
PRP, PRF, and OFD in the treatment of 72
mandibular degree II furcations in 42 patients
after nine months (Bajaj et al. 2013). In this
study, both forms of autologous platelet con-
centrates led to significantly better outcomes
in all clinical and radiographic parameters
compared with the OFD control, with no dif-
ferences between PRP and PRF. Horizontal
clinical attachment gain amounted to
2.75 mm (PRF) and 2.5 mm (PRP).
It should be noted that all these studies are
from the same centre. More recently the
authors have published modified PRF proto-
cols using the addition of synthetic statins
and hydroxyapatite (HA) bone grafts (Pradeep
et al. 2016), or the addition of alendronate gel
(Kanoriya et al. 2017), thereby further
enhancing the outcomes of PRF therapy.
Finally, another group of authors (Siddiqui
et al. 2016) evaluated in a six‐month study the
efficacy of PRF compared to ß‐TCP and to
OFD alone in the treatment of degree II man-
dibular furcation defects. Horizontal probing
bone level changes amounted to 2.1 mm,
2.2 mm, and 1.0 mm, respectively.
7.3.2 Degree III Furcation Defects
7.3.2.1 Barrier Membranes (GTR)
Only two randomized controlled clinical
trials have compared OFD and GTR in
molars with degree III FI (Pontoriero et al.
1989; Pontoriero and Lindhe 1995b).

Regenerative Therapy in Human Clinical Studies 149
Mandibular Molars
The earlier study reported therapy in man-
dibular molars (Pontoriero et al. 1989). After
assessing FI clinically, only 1 of 42 furcations
was scored as ‘through‐and‐through’ (degree
III). After flap elevation, but before debride-
ment, all 42 furcations were scored as degree
III. Six months after treatment, furcation
involvement was assessed clinically (i.e. with-
out elevation of a flap). In the GTR group, 3
molars remained as degree III, whereas in the
OFD group, 11 remained as degree III, indi-
cating better results with GTR.
Maxillary Molars
OFD and GTR were also compared in the
treatment of maxillary interproximal degree
III furcation defects (Pontoriero and Lindhe
1995b). Baseline and six‐month examinations
were performed by re‐entry after flap eleva-
tion. Neither OFD nor GTR led to even partial
closure of the 22 degree III furcations.
These results are supported by other clini-
cal trials, which also demonstrated very low
frequency and predictability of closure in
degree III furcation defects after GTR ther-
apy: no complete and 3 partial closures of 10
degree III furcations, 12 and 24 months
following GTR (Eickholz et al. 1998); and 6
partial closures of 10 degree III furcations, 24
months after GTR (Eickholz and Hausmann).
Complete closure of degree III furcations
(as evaluated during re‐entry) was never
reported (Jepsen et al. 2002).
7.3.2.2 Enamel Matrix Derivative
One case series study evaluated the treat-
ment of degree III mandibular furcation
defects by the use of EMD alone or in combi-
nation with a bioresorbable membrane
(Donos et al. 2004). Nine patients with a
total of 14 degree III mandibular furcation
defects were assigned to one of three groups:
EMD in four defects; GTR in three defects;
and EMD + GTR in seven defects. None of
the treatments resulted predictably in com-
plete healing of the defects, and there was
no obvious difference between the various
treatment modalities. At 6 and 12 months,
partial closure of the degree III involvements
had occurred in 6 of the 14 treated furca-
tions. The remaining teeth still presented
through‐and‐through furcation defects.
Within the limits of this case series, and tak-
ing into account the small number of patients
and furcations included in each treatment
group, it was concluded that the use of EMD
alone or in combination with GTR did not
result in predictable regeneration of degree
III mandibular defects.
7.4 Furcation Regeneration:
Step‐by‐step Procedure
The suggested treatment sequence is as
follows:
1)
Patient selection. Systemic factors that
limit the success of periodontal surgery,
such as uncontrolled diabetes and immu-
nocompromised status, must be consid-
ered. Poor patient compliance, inadequate
oral hygiene, and smoking are the most
frequent patient factors limiting the selec-
tion of this procedure. Treatment options
and alternatives must be presented to the
patient and the potential problems and
the additional costs should be discussed.
Regenerative furcation surgery should be
part of a comprehensive treatment plan
aiming at complete periodontal and func-
tional rehabilitation.
2)
Tooth selection. Adequate access to the
surgical site and also for future mainte-
nance is extremely important. Molars
with degree II furcations (mandibular and
buccal maxillary FI) are the best candi-
dates to be considered for a regenerative
procedure. Based on the available evi-
dence, interproximal maxillary degree II
furcation defects are significantly less
suited, most likely due to limited access.
Degree III mandibular and maxillary
furcations have shown various treatment
responses and in general there are no

Chapter 7
150
significant differences in treatment out-
comes comparing regenerative therapy
with conventional surgery. Defect and site
characteristics have been identified that
have impacts on the outcomes of regen-
erative furcation surgery (Reddy et al.
2015). For example, a thicker biotype and
the absence of soft‐tissue recession can
positively influence healing following
GTR procedures. More favourable out-
comes can be expected in sites in which
the remaining interproximal bone height
is coronal to the entrance of the furcation
defect, compared to those in which the
bone is at or apical to the furcation
entrance (Figure 7.1). Interdental root
proximity may impair proper defect
debridement. Presence of a root canal fill-
ing is not a contraindication to furcation
regeneration per se, provided there are no
signs of apical pathology.
3)
Regenerative periodontal surgery. The goal
is to obtain sufficient access to the defect
for meticulous debridement and applica-
tion of the regenerative device. In the case
of isolated defects, vertical releasing
incisions may be used (Figure 7.2).
Alternatively, the flap can be extended lat-
erally (Figure 7.1). Keratinized tissues
should be preserved by intrasulcular inci-
sion and the elevation of a full‐thickness
mucoperiostal flap. Granulation tissue
will be removed and the exposed root
surfaces carefully cleaned by hand instru-
ments, power‐driven scalers (optionally
with diamond‐coated tips), or rotary
instruments. Root anomalies such as
enamel projections/pearls should be
removed. If EMD is part of the regenera-
tive strategy, it is usually applied following
two minutes of root conditioning with
EDTA and rinsing with sterile saline.
Subsequently a bone graft/substitute can
be used to fill the furcation defect.
Alternatively, a GTR barrier membrane
can be applied, with or without an addi-
tional defect filler (Figures 7.1 and 7.2).
The barrier membrane is secured by a
resorbable sling suture to cover the furcation
entrance and to promote wound and clot
stabilization. In order to facilitate com-
plete coverage of the barrier, the perios-
teum can be cut to allow for a coronal
advancement of the flap. The flap is
secured in a coronal position by a sling
suture and interrupted sutures over the
vertical releasing incisions (Figure 7.2), or
interdental sutures in the case of a laterally
extended flap (Figure 7.1). The patient is
instructed to abstain from mechanical
plaque removal in the surgical area for a
period of up to four weeks. During this
time, chlorhexidine rinses or topical gel
application are used. The patient returns
for monitoring of healing after one and
two weeks, when sutures are removed.
Interdental hygiene and mechanical
plaque removal are started again after four
weeks, and the personalized maintenance
recall programme will be determined.
7.5 Furcation Regeneration:
How to Take the Next Step?
It emerges clearly from this chapter that the
main challenge for regenerating furcation
defects is presented by improving the pre-
dictability in degree II FI (in particular maxil-
lary interproximal furcations) and even more
by achieving regeneration in degree III furca-
tion defects (maxillary or mandibular).
However, the previous chapter produced
clinical and histological evidence for regen-
eration of degree III furcations in animal
models, including complete closure with the
formation of periodontal ligament and
regrowth of the bone with GTR (Lindhe et al.
1995; Araújo et al. 1998) or GTR associated
with EMD (Donos et al. 2003a, 2003b;
Gkranias et al. 2012) in animal models. So
how can we take the decisive step towards
predictable furcation regeneration based on
pre‐clinical studies?
Complete flap coverage of the membrane
during healing seems to be crucial, probably
more than defect morphology (Lindhe et al.
1995; Araújo et al. 1997, 1998; Araújo and
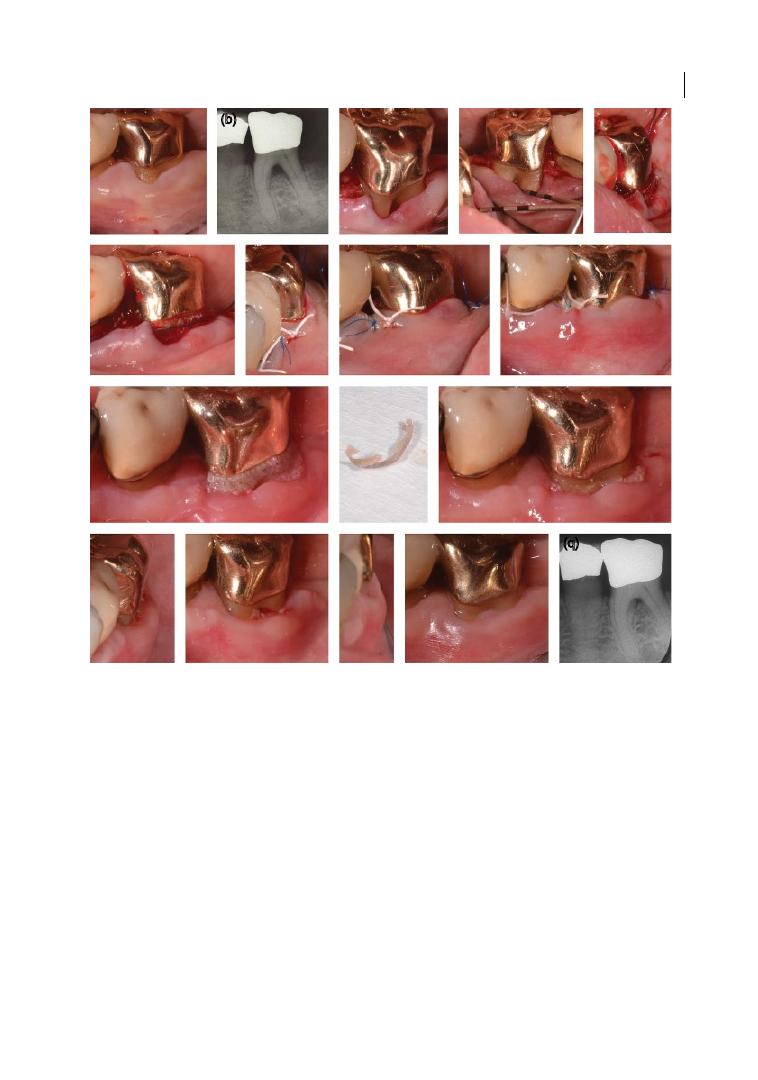
Regenerative Therapy in Human Clinical Studies 151
Lindhe, 1998). Unfortunately, the different
anatomy and dimensions of teeth and alveo-
lar processes in the experimental animal
models reduce their external validity to
human cases. Furthermore, experimentally
induced furcation defects in animal models
may not reproduce the chronic lesions
encountered in humans. Therefore, more
progress in techniques and materials is
needed in order to predictably achieve in
humans the same results observed in animal
models, before implementing regenerative
surgery as the treatment of choice for every
deep furcation defect.
(a)
(f)
(j)
(l)
(m)
(n)
(o)
(p)
(q)
(k)
(g)
(h)
(i)
(c)
(d)
(e)
(b)
Figure 7.1
(a) Periodontal measurements at baseline, tooth no. 36 (LL6). Probing depth mesial and distal:
2 mm, furcation degree II buccally, horizontal probing: 4 mm, recession 3 mm. (b) Radiograph of tooth no.36
with visible furcation defect, adjacent bone level at forcation fornix. (c) Flap elevation: intrasulcular incision/
horizonal release, mucoperiostal flap, papillae de‐epithelialized, periosteal split in the vestibule. Root surface
debridement. (d) Horizontal probing bone level: 4 mm. (e, f) Placement of a bioresorbable matrix barrier
(Guidor™ MSL‐configuration, Sunstar Americas, Inc., Schaumburg, IL, USA) to facilitate guided tissue
regeneration. Fixation of the barrier with integrated sling sutures. (g, h) Coronally advanced flap secured with
sling and interrupted sutures. (i) One day after periodontal regenerative surgery. (j) Clinical view 3 weeks after
surgery with matrix exposure. (k, l) Exposed matrix partially removed. (m, n) 5 weeks after surgery. (o, p)
12 months after surgery. Horizontal and vertical probing depths: 2 mm, recession 3 mm. (q) Radiograph taken
12 months after surgery. Almost complete radiographic bone fill in furcation area.
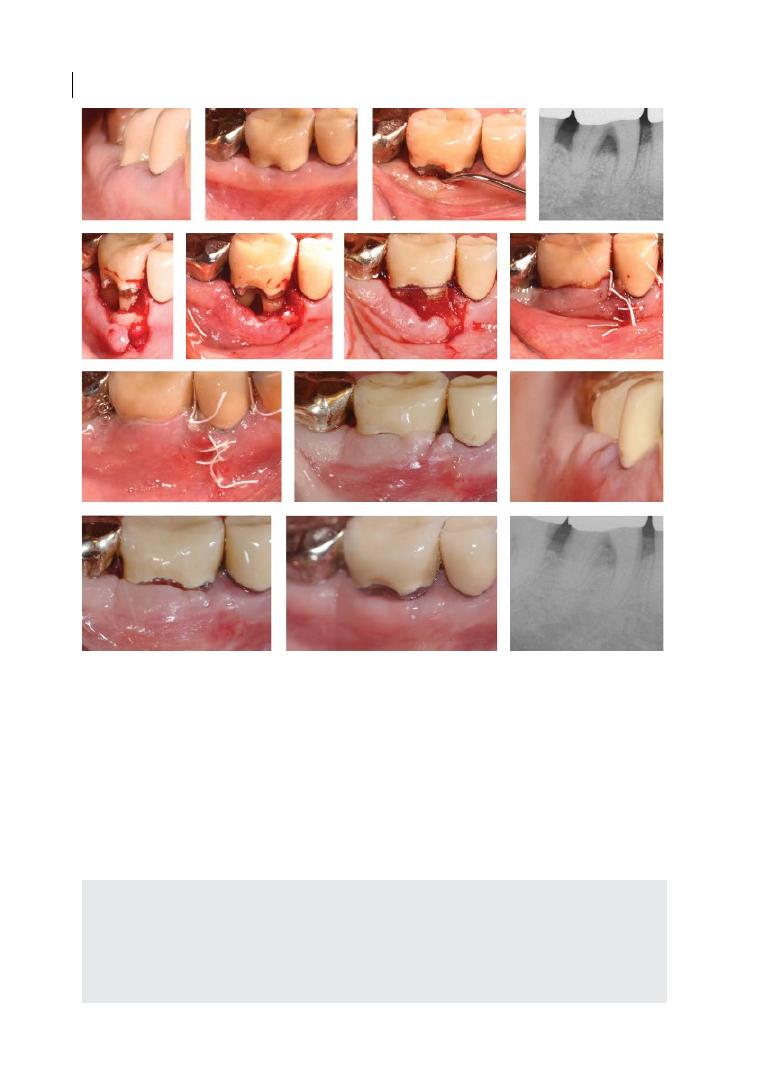
Chapter 7
152
(a)
(e)
(i)
(l)
(m)
(n)
(j)
(k)
(f)
(g)
(h)
(c)
(d)
(b)
Figure 7.2
(a, b) Periodontal measurements at baseline, tooth no. 46 (LR6). Probing depth mesial and distal: 3 mm,
furcation degree II. Situation 2 months after an acute abscess and mobility grade 2 treated with debridement of
the accessible root surfaces and local antimicrobials. (c) Radiograph of tooth no. 46 with visible furcation defect,
proximal bone loss to the level of the furcation, and a very short distal root. (d) Horizontal probing bone level:
7 mm, crown margin reduced and polished. (e, f) Debrided root surfaces. Flap design: intrasulcular incision/
vertical release mesial, mucoperiostal flap, papilla mesial de‐epithelialized, periosteal split in the vestibule. The
distal papilla was left intact, but mobilized and slightly elevated by a tunnelling procedure. (g) Placement of a
bioresorbable matrix barrier (Guidor™ MSL‐configuration, Sunstar Americas, Inc., Schaumburg, IL, USA) after
application of a xenogeneic bone mineral into the furcation defect (Bio‐oss collagen™, Geistlich Biomaterials,
Wollhusen, Switzerland) to facilitate guided tissue regeneration. (h) Coronally advanced minimally rotated flap
secured with sling and interrupted sutures. (i) Clinical view one day after periodontal regenerative surgery. (j, k)
Clinical view 2 weeks after surgery. (l) Clinical view 3 months after surgery. (m) 9 months, vertical and horizontal
probing depths: 2 mm. (n) 9 months, radiographic fill of the furcation defect.
Summary of Evidence
●
Various regenerative approaches have
shown to be effective in the treatment of
degree II furcation involvement (FI) com-
pared with access flap surgery.
●
Complete furcation closure in degree II FI
is not a predictable outcome
●
Degree III FI cannot be improved predict-
ably by regenerative therapy.

Regenerative Therapy in Human Clinical Studies 153
References
Aimetti, M., Romano, F., Pigella, E., and
Piemontese, M. (2007). Clinical evaluation
of the effectiveness of enamel matrix
proteins and autologous bone graft in the
treatment of mandibular class II furcation
defects: A series of 11 patients. International
Journal of Periodontics and Restorative
Dentistry 27, 441–447.
Araújo, M.G., Berglundh, T., and Lindhe, J.
(1997). On the dynamics of periodontal
tissue formation in degree III furcation
defects: An experimental study in dogs.
Journal of Clinical Periodontology 24,
738–746.
Araújo, M.G., Berglundh, T., and Lindhe, J.
(1998). GTR treatment of degree III
furcation defects with 2 different resorbable
barriers: An experimental study in dogs.
Journal of Clinical Periodontology 25,
253–259.
Araújo, M.G., and Lindhe, J. (1998). GTR
treatment of degree III furcation defects
following application of enamel matrix
proteins: An experimental study in dogs.
Journal of Clinical Periodontology 25,
524–530.
Avera, J.B., Camargo, P.M., Klokkevold, P.R.
et al. (1998). Guided tissue regeneration in
class II furcation involved maxillary molars:
A controlled study of 8 split‐mouth cases.
Journal of Periodontology 69, 1020–1026.
Avila‐Ortiz, G., De Buitrago, J.G., and Reddy,
M.S. (2015). Periodontal
regeneration – furcation defects: A
systematic review from the AAP
regeneration workshop. Journal of
Periodontology 86 (Suppl.), S108–S130.
Bajaj, P., Pradeep, A.R., Agarwal, E. et al.
(2013). Comparative evaluation of
autologous platelet‐rich fibrin and platelet‐
rich plasma in the treatment of mandibular
degree II furcation defects: A randomized
controlled clinical trial. Journal of
Periodontal Research 48, 573–581.
Barros, R.R.M., Oliveira, R.R., Novaes, A.B., Jr
et al. (2005). Treatment of class II furcation
defects with guided tissue regeneration or
enamel matrix derivative proteins: A 12‐
month comparative clinical study. Perio 2,
275–284.
Becker, W., Becker, B.E., Berg, L. et al. (1988).
New attachment after treatment with root
isolation procedures: Report for treated
class III and class II furcations and vertical
osseous defects. International Journal of
Periodontics and Restorative Dentistry 3,
2–16.
Blumenthal, N.M. (1993). A clinical
comparison of collagen membranes with
ePTFE membranes in the treatment of
human mandibular buccal Class II furcation
defects. Journal of Periodontology 64,
454–459.
Bouchard, P., Ouhayoun, J.‐P., and Nilvéus,
R.E. (1993). Expanded
polytetrafluoroethylene membranes and
connective tissue grafts support bone
regeneration for closing mandibular class II
furcations. Journal of Periodontology 64,
1193–1198.
Bowers, G.M., Schallhorn, R.G., McClain, P.K.
et al. (2003). Factors influencing the
outcome of regenerative therapy in
mandibular class II furcations: Part I.
Journal of Periodontology 74, 1255–1268.
Bremm, L.L., Sallum, A.W., Casati, M.Z. et al.
(2004). Guided tissue regeneration in class II
furcation defects using a resorbable
polylactic acid barrier. American Journal of
Dentistry 17, 443–446.
Caffesse, R.G., Mota, L., Quinones, C., and
Morrison, E. (1997). Clinical comparison of
resorbable and non‐resorbable barriers for
guided tissue regeneration. Journal of
Clinical Periodontology 24, 747–752.
Camelo, M., Nevins, M.L., Schenk, R.K. et al.
(2003). Periodontal regeneration in human
class II furcations using purified
recombinant human platelet‐derived growth
factor‐BB (rhPDGF‐BB) with bone allograft.
International Journal of Periodontics and
Restorative Dentistry 23, 213–225.
Casarin, R.C., Del Peloso, R.E., Nociti, F.H., Jr
et al. (2008). A double‐blind randomized

Chapter 7
154
clinical evaluation of enamel matrix
derivative proteins for the treatment of
proximal class‐II furcation involvements.
Journal of Clinical Periodontology 35,
429–437.
Casarin, R.C., Ribeiro Edel, P., Nociti, F.H. Jr
et al. (2010). Enamel matrix derivative
proteins for the treatment of proximal class
II furcation involvements: A prospective
24‐month randomized clinical trial. Journal
of Clinical Periodontology 37, 1100–1109.
Castro, A.B., Meschi, N., Temmerman, A. et al.
(2017). Regenerative potential of leucocyte‐
and platelet‐rich fibrin. Part A: Intra‐bony
defects, furcation defects and periodontal
plastic surgery: A systematic review and
meta‐analysis. Journal of Clinical
Periodontology 44, 67–82.
Caton, J., Greenstein, G., and Zappa, U. (1994).
Synthetic bioabsorbable barrier for
regeneration in human periodontal defects.
Journal of Periodontology 65, 1037–1045.
Chen, T.H., Tu, Y.K., Yen, C.C., and Lu, H.K.
(2013). A systematic review and meta‐
analysis of guided tissue regeneration/
osseous grafting for the treatment of class II
furcation defects. Journal of Dental Science
8, 209–224.
Chitsazi, M.T., Farahani, R.M.Z., Pourabbas,
M., and Bahaeddin, N. (2007). Efficacy of
open flap debridement with and without
enamel matrix derivatives in the treatment
of mandibular degree II furcation
involvement. Clinical Oral Investigations 11,
385–389.
Christgau, M., Schmalz, G., Reich, E., and
Wenzel, A. (1995). Clinical and
radiographical split‐mouth‐study on
resorbable versus non‐resorbable GTR‐
membranes. Journal of Clinical
Periodontology 22, 306–315.
Cury, P.R., Sallum, E.A., Nociti, F.H. et al.
(2003). Long‐term results of guided tissue
regeneration therapy in the treatment of
class II furcation defects: A randomised
clinical trial. Journal of Periodontology 74,
3–9.
Del Fabbro, M., Bortolin, M., Taschieri, S., and
Weinstein, R. (2011). Is platelet concentrate
advantageous for the surgical treatment of
periodontal diseases? A systematic review
and meta‐analysis. Journal of Periodontology
82, 1100–1111.
Dohan Ehrenfest, D.M., Rasmusson, L., and
Albrektsson, T. (2009). Classification of
platelet concentrates: From pure platelet‐
rich plasma (P‐PRP) to leucocyte‐ and
platelet‐rich fibrin (L‐PRF). Trends in
Biotechnology 27, 158–167.
Donos, N., Glavind, L., Karring, T., and
Sculean, A. (2003a). Clinical evaluation of
an enamel matrix derivative in the treatment
of mandibular degree II furcation
involvement: A 36‐month case series.
International Journal of Periodontics and
Restorative Dentistry 23, 507–512.
Donos, N., Sculean, A., Glavind, L., Reich, E.,
and Karring, T. (2003b). Wound healing of
degree III furcation involvements following
guided tissue regeneration and/or
Emdogain. A histologic study. Journal of
Clinical Periodontology 30, 1061–1068.
Donos, N., Glavind, L., Karring, T., and
Sculean, A. (2004). Clinical evaluation of an
enamel matrix derivative and a
bioresorbable membrane in the treatment of
degree III mandibular furcation
involvement: A series of nine patients.
International Journal of Periodontics and
Restorative Dentistry 24, 362–369.
Donos, N., Heijl, L., and Jepsen, S. (2010).
Application of enamel matrix proteins in
furcation defects. In: Periodontal
Regenerative Therapy (ed. A. Sculean),
103–117. Berlin: Quintessence.
Dos Anjos, B., Novaes, A.B., Jr, Meffert, R., and
Porto Barboza, E. (1998). Clinical
comparison of cellulose and expanded
polytetrafluoroethylene membranes in the
treatment of class II furcations in
mandibular molars with 6‐month re‐entry.
Journal of Periodontology 69, 454–459.
Eickholz, P., and Hausmann, E. (1999).
Evidence for healing of class II and III
furcations 24 months after GTR therapy:
Digital subtraction and clinical
measurements. Journal of Periodontology 70,
1490–1500.

Regenerative Therapy in Human Clinical Studies 155
Eickholz, P., Kim, T.‐S., and Holle R. (1997).
Guided tissue regeneration with non‐
resorbable and biodegradable barriers: 6
months results. Journal of Clinical
Periodontology 24, 92–101.
Eickholz, P., Kim, T.‐S., and Holle R. (1998).
Regenerative periodontal surgery with non‐
resorbable and biodegradable barriers:
Results after 24 months. Journal of Clinical
Periodontology 25, 666–676.
Eickholz, P., Kim, T.S., Holle, R., and
Hausmann, E. (2001). Long‐term results of
guided tissue regeneration therapy with
non‐resorbable and bioabsorbable barriers.
I. Class II furcations. Journal of
Periodontology 72, 35–42.
Eickholz, P., Kim, T.‐S., Steinbrenner, H. et al.
(2000). Guided tissue regeneration with
bioabsorbable barriers: Intrabony defects
and class II furcations. Journal of
Periodontology 71, 999–1008.
Eickholz, P., Pretzl, B., Holle, R., and Kim, T.‐S.
(2006). Long‐term results of guided tissue
regeneration therapy with non‐resorbable
and bioabsorbable barriers. III. Class II
furcations after 10 years. Journal of
Periodontology 77, 88–94.
Figueira, E.A., de Assis, A.O., Montenegro,
S.C. et al. (2014). Long‐term periodontal
tissue outcome in regenerated infrabony and
furcation defects: A systematic review.
Clinical Oral Investigations 18,
1881‐–1892.
Flanary, D.B., Twohey, S.M., Gray, J.L. et al.
(1991). The use of synthetic skin substitute
as a physical barrier to enhance healing in
human periodontal furcation defects: A
follow up report. Journal of Periodontology
62, 684–689.
Garrett, S., Polson, A.M., Stoller, N.H. et al.
(1997). Comparison of a bioabsorbable GTR
barrier to a non‐absorbable barrier in
treating human class II furcation defects: A
multi‐center parallel design randomized
single‐blind trial. Journal of Periodontology
68, 667–675.
Gkranias, N.D., Graziani, F., Sculean, A., and
Donos, N. (2012). Wound healing following
regenerative procedures in furcation degree
III defects: Histomorphometric
outcomes. Clinical Oral Investigation 16,
239–249.
Gottlow, J., Nyman, S., Lindhe, J. et al. (1986).
New attachment formation in the human
periodontium by guided tissue regeneration:
Case reports. Journal of Clinical
Periodontology 13, 604–616.
Harris, R.J. (2002). Treatment of furcation
defects with an allograft‐alloplast‐
tetracycline composite bone graft combined
with GTR: Human histologic evaluation of a
case report. International Journal of
Periodontology and Restorative Dentistry 22,
381–387.
Hoffmann, T., Richter, S., Meyle, J. et al.
(2006). A randomized clinical multicentre
trial comparing enamel matrix derivative
and membrane treatment of buccal class II
furcation involvement in mandibular
molars. Part III: Patient factors and
treatment outcome. Journal of Clinical
Periodontology 33, 575–583.
Horwitz, J., Machtei, E.E., Reitmeir, P. et al.
(2004). Radiographic parameters as
prognostic indicators for healing of class II
furcation defects. Journal of Clinical
Periodontology 31, 105–111.
Houser, B.E., Mellonig, J.T., Brunsvold, M.A.
et al. (2001). Clinical evaluation of anorganic
bovine bone xenograft with a bioabsorbable
collagen barrier in the treatment of molar
furcation defects. International Journal of
Periodontics and Restorative Dentistry 21,
161–169.
Hugoson, A., Ravald, N., Fornell, J. et al.
(1995). Treatment of class II furcation
involvements in humans with bioresorbable
and nonresorbable guided tissue
regeneration barriers: A randomized
multi‐center study. Journal of Periodontology
66, 624–634.
Jaiswal, R., and Deo, V. (2013). Evaluation of
the effectiveness of enamel matrix
derivative, bone grafts, and membrane in
the treatment of mandibular class II
furcation defects. International Journal of
Periodontics and Restorative Dentistry 33,
e58–e64.

Chapter 7
156
Jepsen, S., Eberhard, J., Herrera, D., and
Needleman, I. (2002). A systematic review of
guided tissue regeneration for periodontal
furcation defects: What is the effect of
guided tissue regeneration compared with
surgical debridement in the treatment of
furcation defects? Journal of Clinical
Periodontology 29 (Suppl. 3), 103–116.
Jepsen, S., Heinz, B., Jepsen, K. et al. (2004). A
randomized clinical trial comparing enamel
matrix derivative and membrane treatment
of buccal Class II furcation involvement in
mandibular molars. Part I: Study design and
results for primary outcomes. Journal of
Periodontology 75, 1150–1160.
Kanoriya, D., Pradeep, A.R., Garg, V., and
Singhal S. (2017). Mandibular degree II
furcation defects treatment with platelet‐
rich fibrin and 1% alendronate gel
combination: A randomized controlled
clinical trial. Journal of Periodontology 88,
250–258.
Kinaia, B.M., Steiger, J., Neely, A.L. et al.
(2011). Treatment of class II molar furcation
involvement: Meta‐analyses of re‐entry
results. Journal of Periodontology 82,
413–428.
Koop, R., Merheb, J., and Quirynen, M. (2012).
Periodontal regeneration with enamel
matrix derivative in reconstructive
periodontal therapy: A systematic review.
Journal of Periodontology 83, 707–720.
Lekovic, V., Kenney, E.B., Carranza, F.A., and
Martignoni, M. (1991). The use of
autogenous periosteal grafts as barriers for
the treatment of class II furcation
involvements in lower molars. Journal of
Periodontology 62, 775–780.
Lekovic, V., Kenney, E.B., Kovacevic, K., and
Carranza, F.A. (1989). Evaluation of guided
tissue regeneration in class II furcation
defects: A clinical re‐entry study. Journal of
Periodontology 60, 694–698.
Lindhe, J., Pontoriero, R., Berglundh, T., and
Araujo, M. (1995). The effect of flap
management and bioresorbable occlusive
devices in GTR treatment of degree III
furcation defects: An experimental study in
dogs. Journal of Clinical Periodontology 22,
276–283.
Luepke, P.G., Mellonig, J.T., and Brunsvold,
M.A. (1997). A clinical evaluation of a
bioabsorbable barrier with and without
decalcified freeze‐dried bone allograft in the
treatment of molar furcations. Journal of
Clinical Periodontology 24, 440–446.
Machtei, E.E. (1997). Outcome variables in the
study of periodontal regeneration. Annals of
Periodontology 2, 229–239.
Machtei, E.E., Cho, M.I., Dunford, R. et al.
(1994). Clinical, microbiological, and
histological factors which influence the
success of regenerative periodontal therapy.
Journal of Periodontology 65, 154–161.
Machtei, E.E., Grossi, S.G., Dunford, R. et al.
(1996). Long‐term stability of class II
furcation defects treated with barrier
membranes. Journal of Periodontology 67,
523–527.
Maragos, P., Bissada, N.F., Wang, R., and Cole,
B.P. (2002). Comparison of three methods
using calcium sulfate as a graft/barrier
material for the treatment of class II
mandibular molar furcation defects.
International Journal of Periodontics and
Restorative Dentistry 22, 493–501.
McGuire, M.K., and Nunn, M.E. (1996).
Prognosis versus actual outcome. III. The
effectiveness of clinical parameters in
accurately predicting tooth survival. Journal
of Periodontology 67, 666–674.
Mellonig, J.T., Seamons, B.C., Gray, J.L., and
Towle, H.J. (1994). Clinical evaluation of
guided tissue regeneration in the treatment
of grade II molar furcation invasions.
International Journal of Periodontics and
Restorative Dentistry 14, 255–271.
Mellonig, J.T., Valderrama Mdel, P., and
Cochran, D.L. (2009). Histological and
clinical evaluation of recombinant human
platelet‐derived growth factor combined
with beta tricalcium phosphate for the
treatment of human class III furcation
defects. International Journal of
Periodontics and Restorative Dentistry 29,
169–177.

Regenerative Therapy in Human Clinical Studies 157
Metzler, D.G., Seamons, B.C., Mellonig, J.T.
et al. (1991). Clinical evaluation of guided
tissue regeneration in the treatment of
maxillary class II molar furcation invasions.
Journal of Periodontology 62, 353–360.
Meyle, J., Gonzales, J.R., Bodeker, R.H. et al.
(2004). A randomized clinical trial
comparing enamel matrix derivative and
membrane treatment of buccal class II
furcation involvement in mandibular
molars. Part II: Secondary outcomes. Journal
of Periodontology 75, 1188–1195.
Miron, R.J., Guillemette, V., Zhang, Y. et al.
(2014). Enamel matrix derivative in
combination with bone grafts: A review of
the literature. Quintessence International 45,
475–487.
Miron, R.J., Sculean, A., Cochran, D.L. et al.
(2016). Twenty years of enamel matrix
derivative: The past, the present and the
future. Journal of Clinical Periodontology 43,
668–683.
Mombelli, A., Zappa, U., Brägger, U., and
Lang, N.P. (1996). Systemic antimicrobial
treatment and guided tissue regeneration:
Clinical and microbiological effects in
furcation defects. Journal of Clinical
Periodontology 23, 386–396.
Murphy, K.G., and Gunsolley, J.C. (2003).
Guided tissue regeneration for the treatment
of periodontal intrabony and furcation
defects: A systematic review. Annals of
Periodontology 8, 266–302.
Myhre, A.E., Lyngstadaas, S.P., Dahle, M.K.
et al. (2006). Anti‐inflammatory properties
of enamel matrix derivative in human blood.
Journal of Periodontal Research 41, 208–213.
Nevins, M., Camelo, M., Nevins, M.L. et al.
(2003). Periodontal regeneration in humans
using recombinant human platelet‐derived
growth factor‐BB (rhPDGF‐BB) and
allogenic bone. Journal of Periodontology 74,
1282–1292.
Nibali, L., Zavattini, A., Nagata, K. et al.
(2016). Tooth loss in molars with and
without furcation involvement: A systematic
review and meta‐analysis. Journal of Clinical
Periodontology 43, 156–166.
Nokhbehsaim, M., Deschner, B., Winter, J.
et al. (2012). Anti‐inflammatory effects of
EMD in the presence of biomechanical
loading and interleukin‐1β in vitro. Clinical
Oral Investigations 16, 275–283.
Paul, B.F., Mellonig, J.T., Towle, H.J., III, and
Gray, J.L. (1992). Use of a collagen barrier to
enhance healing in human periodontal
furcation defects. International Journal of
Periodontics and Restorative Dentistry 12,
123–131.
Peres, M.F.S., Ribeiro, E.D.P., Casarin, R.C.V.
et al. (2013). Hydroxyapatite/β‐tricalcium
phosphate and enamel matrix derivative for
treatment of proximal class II furcation
defects: A randomized clinical trial. Journal
of Clinical Periodontology 40, 252–259.
Pontoriero, R., and Lindhe, J. (1995a). Guided
tissue regeneration in the treatment of
degree II furcations in maxillary molars.
Journal of Clinical Periodontology 22,
756–763.
Pontoriero, R., and Lindhe, J. (1995b). Guided
tissue regeneration in the treatment of
degree III furcation defects in maxillary
molars. Journal of Clinical Periodontology
22, 810–812.
Pontoriero, R., Lindhe, J., Karring, T. et al.
(1988). Guided tissue regeneration in degree
II furcation‐involved mandibular molars.
Journal of Clinical Periodontology 15, 247–254.
Pontoriero, R., Lindhe, J., Nyman, S. et al. (1989).
Guided tissue regeneration in the treatment
of defects in mandibular molars: A clinical
study of degree III involvements. Journal of
Clinical Periodontology 16, 170–174.
Pradeep, A.R., Karvekar, S., Nagpal, K. et al.
(2016). Rosuvastatin 1.2 mg in situ gel
combined with 1:1 mixture of autologous
platelet‐rich fibrin and porous
hydroxyapatite bone graft in surgical
treatment of mandibular class II furcation
defects: A randomized clinical control trial.
Journal of Periodontology 87, 5–13.
Pradeep, A.R., Pai, S., Garg, G. et al. (2009). A
randomized clinical trial of autologous
platelet‐rich plasma in the treatment of
mandibular degree II furcation defects.

Chapter 7
158
Journal of Clinical Periodontology 36,
581–588.
Prathibha, P.K., Faizuddin, M., and Pradeep,
A.R. (2002). Clinical evaluation of guided
tissue regeneration procedure in the
treatment of grade II mandibular molar
furcations. Indian Journal of Dental
Research 13, 37–47.
Pruthi, V.K., Gelskey, S.C., and Mirbod, S.M.
(2002). Furcation therapy with
bioabsorbable collagen membrane: A
clinical trial. Journal of the Canadian Dental
Association 68, 610–615.
Queiroz, L.A., Santamaria, M.P., Casati, M.Z.
et al. (2016). Enamel matrix derivative and/
or synthetic bone substitute for the
treatment of manibular class II buccal
furcation defects: A 12‐months randomized
clinical trial. Clinical Oral Investigations 20,
1597–1606.
Reddy, M.S., Aichelmann‐Reddy, M.E.,
Avila‐Ortiz, G. et al. (2015). A consensus
report from the AAP regeneration
workshop. Journal of Periodontology 86
(Suppl.), S131–S133.
Reynolds, M.A., Aichelmann‐Reidy, M.E.,
Branch‐Mays, G.L., and Gunsolley, J.C.
(2003). The efficacy of bone replacement
grafts in the treatment of periodontal
osseous defects: A systematic review. Annals
of Periodontology 8, 227–265.
Sanz, M., Jepsen, K., Eickholz, P., and Jepsen,
S. (2015). Clinical concepts for regenerative
therapy in furcations. Periodontology 2000
68, 308–332.
Scott, T.A., Towle, H.J., Assad, D.A., and
Nicoll, B.K. (1997). Comparison of
bioabsorbable laminar bone membrane and
non‐resorbable ePTFE membrane in
mandibular furcations. Journal of
Periodontology 68, 679–686.
Sculean, A., Ausschill, T.M., Donos, N. et al.
(2001). Effects of an enamel matrix protein
derivative (Emdogain) on ex vivo dental
plaque vitality. Journal of Clinical
Periodontology 28, 1074–1078.
Sharma, A., and Pradeep, A.R. (2011).
Autologous platelet‐rich fibrin in the
treatment of mandibular degree II furcation
defects: A randomized clinical trial. Journal
of Periodontology 82, 1396–1403.
Siddiqui, Z.R., Jhingram, R., Bains, V.K. et al.
(2016). Comparative evaluation of platelet‐
rich fibrin versus beta‐tri‐calcium
phosphate in the treatment of Grade II
mandibular furcation defects using cone‐
beam computed tomography. European
Journal of Dentistry 10, 496–506.
Simonpietri‐C, J.J., Novaes, E.L., Jr, Batista,
E.L., Jr, and Filho, E.J. (2000). Guided tissue
regeneration associated with bovine‐derived
anorganic bone in mandibular class II
furcation defects: 6 month results at re‐
entry. Journal of Periodontology 71,
904–911.
Stavropoulos, A., and Wikesjö, U.M. (2012).
Growth and differentiation factors for
periodontal regeneration: A review on
factors with clinical testing. Journal of
Periodontal Research 47, 545–553.
Stoller, N.H., Johnson, L.R., and Garrett, S.
(2001). Periodontal regeneration of a class II
furcation defect utilizing a bioabsorbable
barrier in a human: A case study with
histology. Journal of Periodontology 72,
238–242.
Suh, Y.I., Lundgren, T., Sigurdsson, T. et al.
(2002). Probing bone level measurements
for determination of the depths of Class II
furcation defects. Journal of Periodontology
73, 637–642.
Troiano, G., Laino, L., Dioguardi, M. et al.
(2016). Mandibular class II furcation defect
treatment: Effects of the addition of platelet
concentrates to open flap: A systematic
review and meta‐analysis of randomized
cinical trials. Journal of Periodontology 87,
1030–1038.
Tsao, Y.‐.P, Neiva, R., Al‐Shammari, K. et al.
(2006). Effects of a mineralized human
cancellous bone allograft in regeneration of
mandibular class II furcation defects.
Journal of Periodontology 77, 416–425.
Twohey, S.M., Mellonig, J.T., Towle, H.J., and
Gray, J.L. (1992). Use of a synthetic skin
substitute as a physical barrier to enhance

Regenerative Therapy in Human Clinical Studies 159
healing in human periodontal furcation
defects. International Journal of Periodontics
and Restorative Dentistry 12, 383–393.
Vernino, A.R., Wang, H.L., Rapley, J. et al.
(1999). The use of biodegradable polylactic
acid barrier materials in the treatment of
grade II periodontal furcation defects in
humans. Part II: A multicenter investigative
surgical study. International Journal of
Periodontics and Restorative Dentistry 19,
56–65.
Wallace, S.C., Gellin, R.G., Miller, M.C., and
Mishkin, D.J. (1994). Guided tissue
regeneration with and without decalcified
freeze‐dried bone in mandibular class II
furcation invasions. Journal of
Periodontology 65, 244–254.
Wang, H.L., O’Neal, R.B., Thomas, C.L. et al.
(1994). Evaluation of an absorbable collagen
membrane in treating class II furcation defects.
Journal of Periodontology 65, 1029–1036.
Yukna, C.N., and Yukna, R.A. (1996). Multi‐
center evaluation of bioabsorbable collagen
membrane for guided tissue regeneration in
human class II furcations. Journal of
Periodontology 67, 650–657.

Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
Chapter No.: 1 Title Name: <TITLENAME>
c08.indd
Comp. by: RKarthikeyan Date: 14 May 2018 Time: 04:21:05 PM Stage: Proof WorkFlow:
<WORKFLOW>
Page Number: 161
161
8.1 Anatomical Considerations
for Treatment Planning
Furcation defects present the greatest chal-
lenges to the success of periodontal therapy,
as a reduced efficacy of periodontal therapy
and higher risk of tooth loss have been con-
sistently observed in multi‐rooted teeth with
furcation involvement, regardless of the
treatment modality employed. Regenerative
therapy might be considered the ideal treat-
ment for furcations. However, indications for
regenerative periodontal therapy (discussed
in Chapter 7) are still very limited. Maxillary
molars with degree II interproximal furca-
tions, as well as all degree III furcation‐
involved molars, are generally not suitable
for regenerative therapy. Therefore, different
treatment strategies (such as resective) have
to be employed to eliminate or manage fur-
cation defects.
The survival of molars with furcation
involvement in longitudinal studies following
different treatment procedures was discussed
in Chapter 5. It is interesting to notice how a
systematic review observed that the most fre-
quent complications occurring during the
follow‐up period were caries in the furcation
area after tunnelling procedures, and root
fractures after root‐resective procedures
(Huynh‐Ba G et al. 2009). From an anatomi-
cal point of view, the poorer prognosis of
furcation‐involved teeth may be due to the
fact that the persistence of a defect within the
inter‐radicular space creates an anatomical
environment that interferes with professional
and domiciliary oral hygiene. Numerous
morphological factors may explain the aetiol-
ogy, the more severe disease progression, and
the less favourable response to periodontal
treatment of furcation‐involved molars (De
Sanctis and Murphey 2000). These factors
(extensively covered in Chapter 1) are:
●
Furcation access diameter.
●
Root irregularities and roughness.
●
Anatomical complexity of the root complex.
●
Cervical enamel projections.
●
Enamel pearls.
●
Accessory pulp canals.
8.2 Pre‐surgical Diagnosis
8.2.1 Pre‐surgical Clinical Diagnosis
An accurate and precise pre‐surgical diagno-
sis is essential in order to approach correctly
patients affected by periodontal disease
complicated by the invasion of molar furca-
tion areas. Before planning the definitive
Chapter 8
Furcation Therapy: Resective Approach and Restorative Options
Roberto Rotundo
1
and Alberto Fonzar
2
1
Periodontology Unit, UCL Eastman Dental Institute, London, UK
2
Private practice, Udine, Italy

Chapter 8
162
treatment plan, clinicians should carefully
evaluate the following:
●
Patient’s periodontal and caries risk profile.
●
Horizontal (Hamp et al. 1975) and vertical
(Tarnow and Fletcher 1984) amount of per-
iodontal tissue loss in the inter‐radicular
areas.
●
Anatomy and morphology of the root
complex: the length of the root trunk, the
degree of separation, and the divergence
between the roots, as well as their shape
and length.
●
Amount of residual attachment and prob-
ing pocket depth (PPD) of each single root.
●
Access for oral hygiene procedures.
●
Endodontic prognosis of each single root
(endodontically treated teeth).
●
Need for endodontic treatment (endodon-
tically untreated teeth).
●
Need for restorative treatment and restor-
ative deficiencies (i.e. insufficient residual
healthy tooth structure).
●
Single tooth or multiple molars with furca-
tion involvement.
This information should be obtained by
carefully combining the data acquired from
both clinical and radiological analysis.
8.2.2 Pre‐surgical Radiological
Diagnosis
It has been observed that clinical examination
alone detected furcation involvement in only
3% of maxillary and 9% of mandibular molars.
The combination of radiographic and clinical
examinations improved detection to 65% in
maxillary molars, but only 23% in mandibular
molars (Ross and Thompson 1978). Parallel
periapical and/or vertical bite‐wing radio-
graphs should always be taken after the clinical
examination in order to confirm the informa-
tion obtained through the periodontal probing
(Horwitz et al. 2004). It is important to know
that the bone density (especially in mandibular
molars) and the superimposition of the palatal
root (maxillary molars) could partially hide
the root complex, and so make it difficult or
impossible to confirm the defect previously
detected by probing. Therefore, it appears
fundamental to combine the clinical and radi-
ological data in order to perform an accurate
diagnosis of multi‐rooted affected teeth.
As discussed in Chapter 2, cone‐beam
computed tomography (CBCT) may improve
diagnostic accuracy and optimize treatment
planning in periodontal defects, particularly
in maxillary molars with furcation involve-
ment. However, the higher irradiation doses
and cost–benefit ratio should be carefully
analysed before using CBCT for periodontal
lesions (including tooth furcation; Walter
et al. 2016). The radiographic analysis should
allow the clinician to evaluate the following:
●
Horizontal and vertical amount of hard
tissue loss in inter‐radicular areas.
●
Length of root trunk.
●
Length, divergence, and shape of roots.
●
Presence/absence of fusion between roots.
●
Amount of residual support.
●
Endodontic diagnosis and prognosis.
●
Presence of post and core build‐up restoration.
●
Presence of caries in furcation‐involved
molars.
We should also remember that radiolucency
in the inter‐radicular area does not always
indicate the presence of a furcation involve-
ment. Trauma from occlusion (occlusal
interferences, bruxism, clenching) with the
consequent increased tooth mobility may
produce vascular changes along the whole
periodontal space, involving also the inter‐
radicular area, which leads to periodontal
ligament space remodelling and bone demin-
eralization (Svanberg and Lindhe 1973;
Polson et al. 1976a, b). In such a case the
radiolucency is not confirmed by the clinical
examination (probing fails to detect an
involvement of the furcation) and the defect
usually disappears some weeks following the
elimination of the occlusal overload.
8.3 Treatment of Furcation
Defects
The objectives of periodontal therapy in
multi‐rooted teeth with furcation involve-
ment are no different from the objective of

Resective Approach and Restorative Options 163
single‐rooted teeth therapy: arresting disease
progression and maintaining the teeth in
health and function with proper aesthetics.
These goals can be met first by eliminating
the microbial plaque from the surfaces of the
root complex, and then by establishing an
anatomy that facilitates proper self‐performed
plaque removal.
Treatment options for molars with furca-
tion involvement could be divided into three
different modalities:
●
Conservative procedures: subgingival
debridement, access‐flap surgeries, tunnel
preparation. The main aim of these proce-
dures is to remove the residual bacterial
infection and improve self‐performed
plaque control.
●
Regenerative procedures (already discussed
in Chapters 6 and 7): guided tissue regen-
eration, induced periodontal regeneration,
bone grafting. The goal of these proce-
dures is not only the removal of the resid-
ual infection, but also the elimination of
the furcation defect through reconstruc-
tion of the lost inter‐radicular periodontal
tissues.
●
Resective procedures: root separation, root
resection, root amputation. The objective
of these procedures is to eliminate the
inter‐radicular lesion by completely remov-
ing both the dental and osseous structures
that make up the defect. The tooth and
root complex morphology is deeply
changed by this therapeutic modality, in
order to open the furcation completely and
create an area conducive to performing
easier and better plaque removal.
The choice of the appropriate treatment
modality for a given clinical situation
depends on a wide variety of factors that
should be carefully evaluated before initiat-
ing treatment:
●
Degree of furcation involvement.
●
Patient expectations.
●
Patient compliance.
●
Patient susceptibility to periodontal disease.
●
Patient susceptibility to caries.
●
Amount of residual attachment.
●
Strategic value of the tooth.
●
Root complex anatomy and morphology.
●
Periodontal condition of adjacent teeth.
●
Need for prosthetic rehabilitation.
●
Need for endodontic treatment.
●
Bone volume/quality.
●
Financial considerations for the patient.
8.4 Resective Procedures and
Restorative Approaches
8.4.1 Indications
Resective techniques have been developed
especially for deep class II and class III furca-
tion‐involved molars to overcome these ana-
tomical limitations by physically removing
both the dental (the roof of the involved fur-
cation) and osseous (pocket elimination)
structures that make up the defect.
Several definitions for resective proce-
dures have been proposed by different
authors and therefore there is no uniformity
in the terms used in the literature. According
to Carnevale et al. (1995), the terms are
defined as follows:
8.4.1.1 Root Separation
This indicates the sectioning of the multi‐
rooted tooth with the maintenance of all the
roots (Figure 8.1). This procedure can be
used for treating the following clinical
situations:
●
Deep class II and class III furcated molars.
●
Root‐trunk fracture or decay.
●
Perforation of the middle of the furcation
trunk.
Root separation is usually indicated in the
following clinical situations:
●
Mandibular molars for separating the mesial
from the distal root (premolariza tion).
●
Maxillary molars for separating the mesial
root from the undivided distal and palatal
roots.
●
Maxillary molars for dividing the palatal
root from the undivided mesial and distal
roots. The separation of the three roots of a
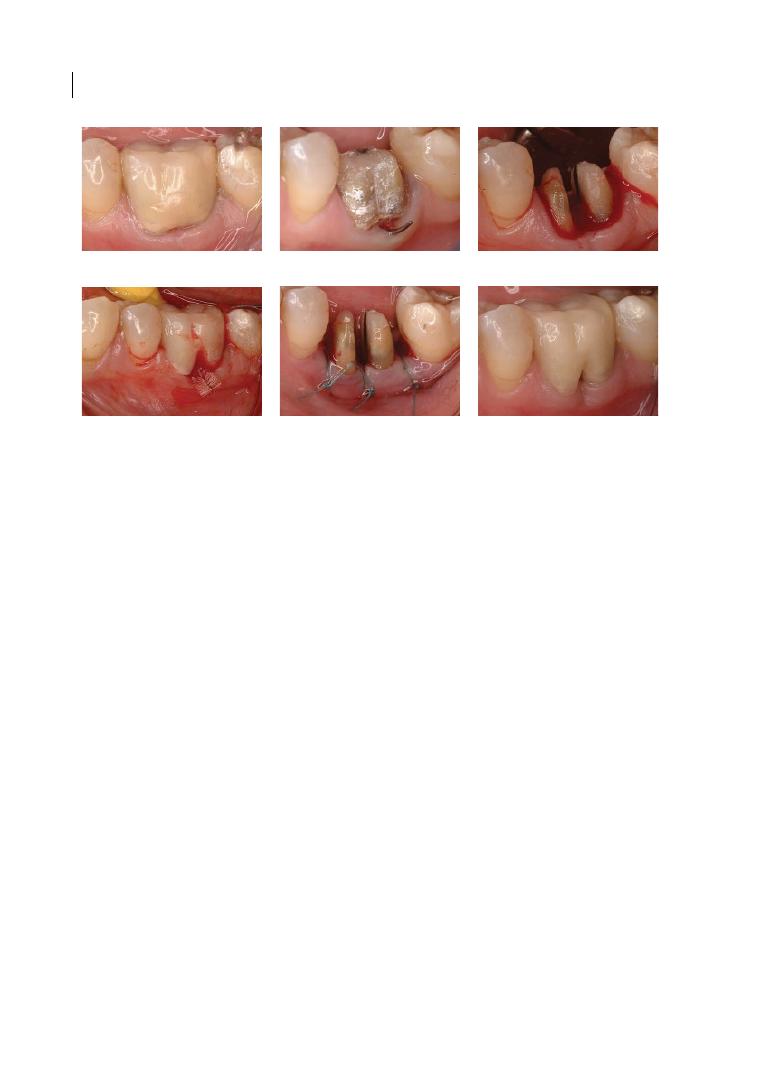
Chapter 8
164
maxillary molar should be considered quite
exceptional, because the presence of all the
roots would make the passage of plaque
removal devices too difficult or impossible.
8.4.1.2 Root Resection
This indicates the sectioning of the multi‐
rooted tooth and the removal of one or two
roots and the associated portion of the crown.
Even if in the literature this term is often used
regardless of how the crown is treated, it is
opportune to distinguish it from the term
‘root amputation’, which refers to the removal
of one root without removal of the overhang-
ing portion of the crown. Root amputation
can usually be indicated in the maxillary
molars for removing the distal root, thus
avoiding the need to restore the tooth with a
crown in a conservative treatment plan.
The root resection procedure can be used
for treating the following clinical situations:
●
Deep class II and class III furcated molars
with severe root proximity or a long root
trunk.
●
Severe bone loss affecting one or more
roots of molars with and without furcation
involvement.
●
Root or root trunk fracture or perforation.
●
Untreatable apical endodontic lesion
affecting one root.
●
Severe root decay or resorption.
●
Severe recession or dehiscence affecting
one root.
When considering root resection for treating
molars with furcation involvement, the clini-
cian has to choose between different
alternatives.
Mandibular Molars
These have only one furcation and therefore
there are just two possibilities (Figure 8.2):
●
Resection of the mesial root.
●
Resection of the distal root.
Assuming that the two roots do not present
significant differences in terms of periodontal,
endodontic, and restorative prognosis, the fol-
lowing morphological characteristics should
be considered before deciding which root has
to be extracted (Majzooub and Kon 1992):
●
The mesial root usually presents a greater
root surface, but also quite often a deep
concavity, making it difficult first to prop-
erly prepare and restore it, and for the
(a)
(b)
(c)
(d)
(e)
(f)
Figure 8.1
Root separation (rizotomy: sectioning of the multi‐rooted tooth with the maintenance of all the
roots) of a mandibular first molar affected by degree III furcation involvement (a–d), followed by an apical
positioned flap (e) and final restoration (f), allowing self‐performed oral hygiene.
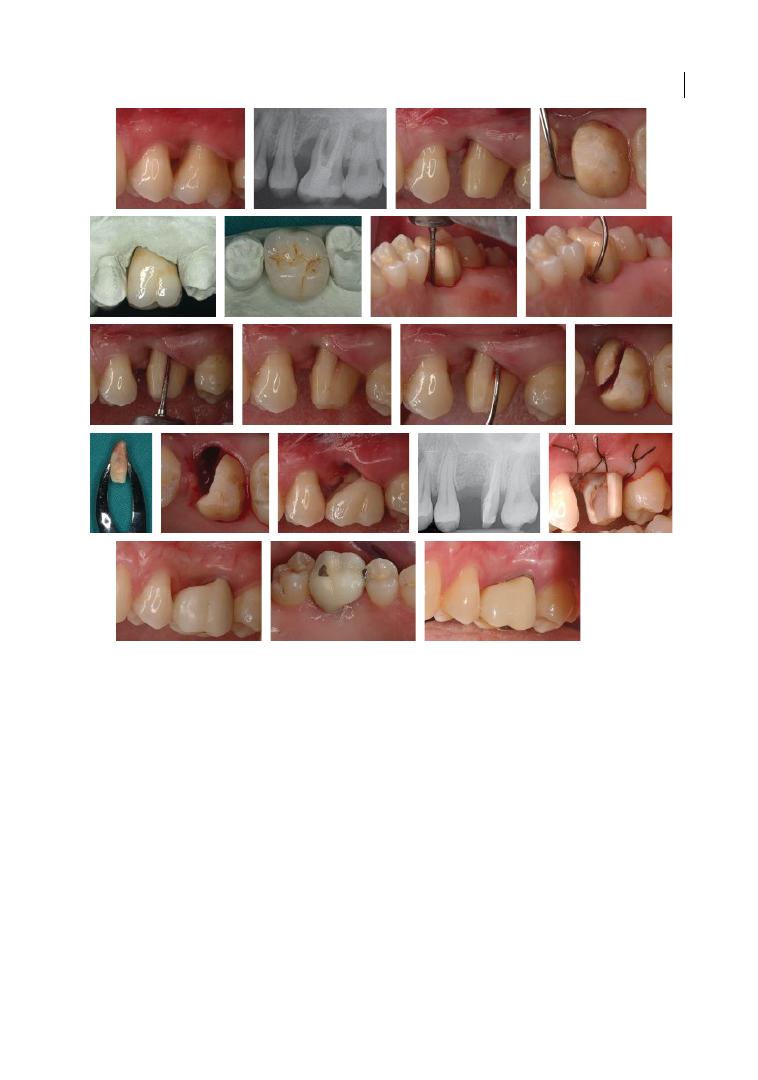
Resective Approach and Restorative Options 165
patient to keep clean with standard plaque‐
control procedures. It is important to
stress the risk of producing a fenestration
in the distal surface of the root, during
both the tooth preparation and the endo-
dontic treatment/retreatment of the two
narrow and superficial root canals.
●
The distal is a comparatively large root,
with usually one wide root canal and an
oval convex cross‐section with a greater
bulk of dentine. These characteristics make
the distal root less prone to root fracture;
easier to treat endodontically, to prepare,
and to restore; and finally for the patient to
keep clean through self‐performed plaque
removal.
Maxillary Molars
These have three furcations and therefore
several root resection possibilities (Figure 8.3):
●
Resection of the mesio‐buccal root with-
out separating the other two roots. This
Figure 8.2
Root resection (rizectomy) of the mesio‐buccal root of an upper maxillary molar, without
separation of the other two roots, performed due to the severe loss of bone support caused by an endodontic‐
periodontal lesion. Degree III furcation involvement on the mesial and buccal furcations was present, with no
distal furcation involvement. This procedure was carried out after appropriate endodontic therapy. Three
months after the root resection, an apically positioned flap was performed and a provisional prosthetic crown
was positioned. Three months later, a final metal‐ceramic crown was cemented, with a good long‐term (five
years) clinical result (last image).
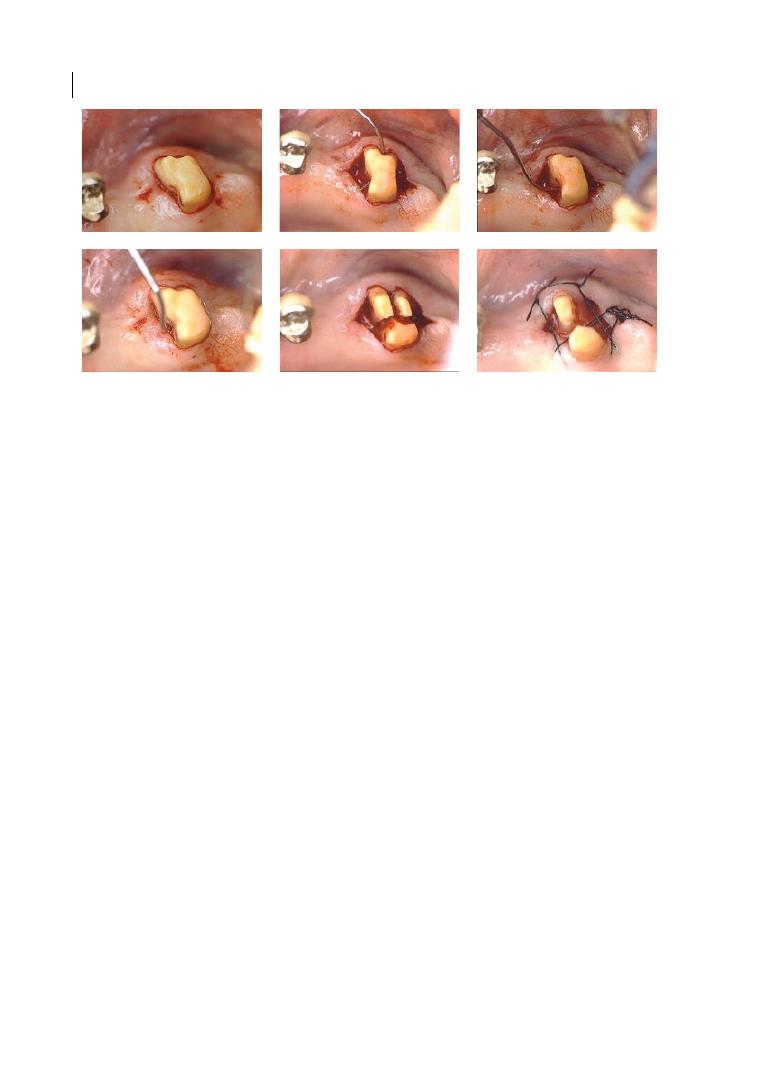
Chapter 8
166
procedure can be performed in case of
involvement of the buccal and/or mesial
furcation (in such a case root separation is
also possible), and when the mesio‐buccal
root is affected by a deep infrabony defect
or an untreatable endodontic or restorative
problem. After the mesio‐buccal root
removal, the residual root trunk shows a
flat or convex mesial surface that facilitates
both prosthetic procedures and self‐
performed plaque control.
●
Resection of the disto‐buccal root without
separating the other two roots. This proce-
dure can be performed in case of involve-
ment of the buccal and/or distal furcation,
and when the disto‐buccal root is affected
by a deep infrabony defect or by an untreat-
able endodontic or restorative problem.
Following the extraction of the disto‐buccal
root, the residual root trunk often exhibits
a deep distal concavity, which should be
flattened during tooth preparation in order
to improve the quality of the prosthetic
restoration and the patient’s self‐performed
plaque removal.
●
Resection of the palatal root without sepa-
rating the other two roots. This procedure
can be performed in case of involvement of
the mesial and/or distal furcation (in such
a case root separation is also possible), or
when the palatal root is affected by a deep
infrabony defect or an endodontic or
restorative untreatable problem.
When all three furcations of a maxillary
molar are involved, following root separation
one or two roots can be extracted. In such
clinical situations, if two roots are maintain-
able (a careful evaluation of periodontal,
endodontic, and prosthetic prognosis is an
essential prerequisite), the following options
are possible (Figure 8.4):
●
Root separation of all three roots and
extraction of the disto‐buccal one. The
disto‐buccal root is statistically the most
frequently extracted (Rosenberg 1978;
Ross and Thompson 1978), because it is
usually the shortest with a long root trunk
and therefore has a smaller amount of bone
support. The anatomy and morphology of
the root trunk usually make access between
the divided mesial and palatal roots easy
for the patient’s self‐performed oral
hygiene.
●
Root separation of all three furcations and
extraction of the mesio‐buccal root. This
option is less frequent than the previous
one, because often the disto‐buccal root is
too thin, and also because proper self‐
performed plaque removal in the distal
Figure 8.3
Root separation of all three roots of a maxillary upper second molar and extraction (rizectomy) of
the disto‐buccal root.
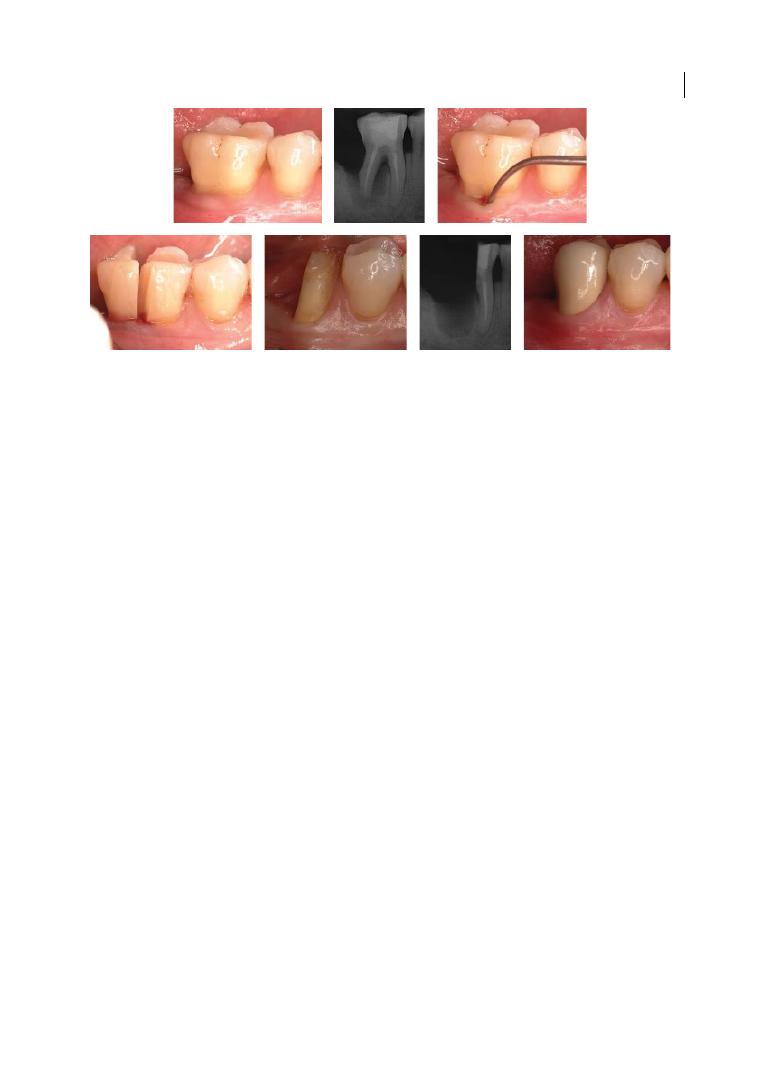
Resective Approach and Restorative Options 167
furcation is nearly impossible if distally
the neighbouring molar is present.
●
Root separation of all three furcations and
extraction of the palatal root. This option
is less frequent too because of the anatomy
of the palatal root (large root surfaces and
an oval convex cross‐section with thick
dentine), but also because the thickness of
the palatal bone makes this root particu-
larly stable and firm. When the clinician
has to choose between the mesial and the
palatal root, they should consider that the
mesial root has a root surface area that is
equal to or even larger than the palatal one,
but it presents two or even three narrow
root canals instead of the sole and wide
canal of the palatal root.
8.4.2 Scientific Evidence
Ten studies (only one prospective) reporting
the results of root resection with at least five‐
year follow‐up were found in the literature
(see Table 8.1). The survival rates reported in
these studies range from about 60 to 100%
after a mean observation period of 5–10 years.
Bergenholtz (1972) retrospectively
reported the results of 45 teeth treated with
root resection up to 11 years before. Out of
the 20 teeth with a 5–10‐year follow‐up, 17
teeth were still present, 2 teeth were lost
because of periodontal complications, and 1
because of root perforation, meaning a
survival rate of 85%. Hamp et al. (1975)
reported a survival rate of 100% after 5 years
in 87 molars treated by means of root resec-
tion and/or separation. The authors attrib-
uted their success to the elimination of
plaque‐retentive areas in the furcations,
meticulous patient oral hygiene, and regular
maintenance care.
Langer et al. (1981) reported a survival rate
of 62% in a 10‐year retrospective evaluation
of 100 molars (50 maxillary and 50 mandibu-
lar) treated with resection. The main causes
of tooth loss were root fracture in 18 teeth
(47.4%), periodontal complications in 10
teeth (26.3%), endodontic failures in 7 teeth
(18.4%), and cement washouts leading to car-
ies in 3 teeth (7.9%). It is interesting to point
out that the ratio of maxillary to mandibular
failures was approximately 2:1 and only
15.8% of the tooth loss occurred within the
first five years after surgery, whereas
the vast majority – that is, 55.3% of the
losses – occurred between the fifth and sev-
enth years of function. The remaining losses
took place between the eighth and tenth
years of observation. Buhler (1988) presented
a 10‐year follow‐up of 28 root‐resected
Figure 8.4
Root separation in a mandibular first molar affected by degree III furcation involvement associated
with periodontal‐endodontic pathology on the distal root. Distal root resection (rizectomy) was carried out,
maintaining the mesial root in situ. A prosthetic metal‐ceramic crown was carried out after the three‐month
healing period.
Chapter No.: 1 Title Name: <TITLENAME>
c08.indd
Comp. by: RKarthikeyan Date: 14 May 2018 Time: 04:21:05 PM Stage: Proof WorkFlow:
<WORKFLOW>
Page Number: 168
Table 8.1
Clinical studies on the tr
ea
tmen
t of fur
ca
tion‐in
volv
ed molars with r
oot separ
ation/r
esec
tion.
A
uthor
Year
Study desig
n
Number
of t
eeth
Follo
w‐up (mean)
Complica
tions
Sur
viv
al
Ber
genholtz
1972
Re
tr
osp
ec
tive
45
5–10 ye
ars
66.6% p
er
io
don
tal 33.3% r
oot p
er
fora
tion
85%
H
am
p e
t al.
1975
Re
tr
osp
ec
tive
87
5 ye
ars
100%
Langer e
t al.
1981
Re
tr
osp
ec
tive
100
10 ye
ars
47.4% r
oot f
rac
tur
e 26.3% p
er
io
don
tal
18.4% endo
don
tic 7.9% w
as
h‐out/c
ar
ie
s
62%
Buhler
1988
Re
tr
osp
ec
tive
28
10 ye
ars
33.3% endo
don
tic 22.2% p
er
io‐endo 22.2%
per
io
don
tal 11.1% r
oot f
rac
tur
e 111%
w
as
h‐out/c
ane
s
67.9%
C
ar
ne
vale e
t al.
1991
Re
tr
osp
ec
tive
488
Gr
oup I 303 t
ee
th: 3–6 ye
ars
Gr
oup I
I 185 t
ee
th:7–11 ye
ars
33.3% c
ar
ie
s 33.3% r
oot f
rac
tur
e 33.3% PPD
> 5mm (
gr
oup I
I)
Gr
oup I
I 98.4%
Blomlof e
t al.
1997
Re
tr
osp
ec
tive
78
5–10 ye
ars
81.3% p
er
io
don
tal 25% p
er
io‐endo 28.1
endo
don
tic
5 ye
ars
: 83% 10
ye
ars
: 68%
C
ar
ne
vale e
t al.
1998
Pr
osp
ec
tive
175
10ye
ars
33.3% endo
don
tic 25% r
oot c
ar
ie
s 25%
per
io
don
tal 16.7 r
oot f
rac
tur
e
5 ye
ars
: 98.9%
10 ye
ars
: 93.1%
H
ou e
t al.
1999
Re
tr
osp
ec
tive
52
6.7 (5–13 ye
ars)
100%
Sv
ar
dstr
om &.
W
ennstr
om
2000
Re
tr
osp
ec
tive
47
9.5 (8–12 ye
ars)
80% r
oot f
rac
tur
e
89.4%
D
anne
w
itz e
t al.
2006
Re
tr
osp
ec
tive
19
~9 (~5–12 ye
ars)
Not r
ep
or
te
d
92.9%
Table 8.1
Clinical studies on the treatment of furcation‐involved molars with root separation/resection.
Author
Year
Study design
Number
of teeth
Follow‐up (mean)
Complications
Survival
Bergenholtz
1972
Retrospective
45
5–10 years
66.6% periodontal 33.3% root perforation
85%
Hamp et al.
1975
Retrospective
87
5 years
100%
Langer et al.
1981
Retrospective
100
10 years
47.4% root fracture 26.3% periodontal
18.4% endodontic 7.9% wash‐out/caries
62%
Buhler
1988
Retrospective
28
10 years
33.3% endodontic 22.2% perio‐endo 22.2%
periodontal 11.1% root fracture 111%
wash‐out/canes
67.9%
Carnevale et al.
1991
Retrospective
488
Group I 303 teeth: 3–6 years
Group II 185 teeth:7–11 years
33.3% caries 33.3% root fracture 33.3% PPD
> 5mm (group II)
Group II 98.4%
Blomlof et al.
1997
Retrospective
78
5–10 years
81.3% periodontal 25% perio‐endo 28.1
endodontic
5 years: 83% 10
years: 68%
Carnevale et al.
1998
Prospective
175
10years
33.3% endodontic 25% root caries 25%
periodontal 16.7 root fracture
5 years: 98.9%
10 years: 93.1%
Hou et al.
1999
Retrospective
52
6.7 (5–13 years)
100%
Svardstrom &.
Wennstrom
2000
Retrospective
47
9.5 (8–12 years)
80% root fracture
89.4%
Dannewitz et al.
2006
Retrospective
19
~9 (~5–12 years)
Not reported
92.9%
PPD = probing pocket depth.

Resective Approach and Restorative Options 169
molars, mainly used as bridge abutments.
The calculated survival rate was 67.9% and
the reasons for tooth loss were as follows:
endodontic failures 33.3%, combined perio-
dontal and endodontic lesions 22.2%, perio-
dontal reasons 22.2%, root fracture 11.1%,
and loss of retention leading to secondary
caries 11.1%. Curiously similar to the find-
ings of Langer and co‐workers, no tooth loss
could be observed during the first four years
following therapy.
Carnevale et al. (1991) published a retro-
spective analysis of 488 molars after tooth
resection and/or separation and prosthetic
reconstruction. The follow‐up period was
3–6 years for 303 teeth (62%) and 7–11 years
for 175 teeth (38%). Considering only the
group with the longer follow‐up, 3 teeth were
lost (1 for caries, 1 for root fracture, and 1 for
PPD > 5 mm), yielding a survival rate of 98.4%.
In contrast to Langer et al. (1981) and Bühler
(1998), most failures occurred early (3–6‐year
group) rather than later (7–11‐year group).
The authors attributed the high success rate
to an optimal hygiene regimen and frequent
maintenance recall. A more recent 10‐year
follow‐up prospective investigation by
Carnevale et al. (1998) reported the success
rate of root‐resective therapy in 175 molars
used as abutments for single‐unit crowns or
fixed dental prostheses to be 98.9% after
5 years and 93% at the end of the study. Only
12 of 175 teeth (7%) were extracted, 4 for
endodontic reasons, 3 for root caries, 3 for
periodontal reasons, and 2 for root fracture.
Hou et al. (1999) reported a survival rate of
100% of 52 root‐separated molars in a case
series including 25 patients followed up for a
mean observation period of 6.7 years (range
5–13 years). Svardström and Wennström
(2000) reported a survival rate of 89.4% of 47
molars 8–12 years following root‐resective
procedures (mean observation period
9.5 years). Five teeth (10.6%) had to be
extracted during the follow‐up period and
root fracture was the main reason for extrac-
tion (80.0%). Dannewitz et al. (2006) per-
formed 19 root resections while treating 305
furcation‐involved molars. Following a mean
observation period of about 9 years, 8 resected
teeth were lost, yielding a survival rate of
57.9%. The complications – that is, the reason
for the tooth loss – were not reported.
It is fitting to consider that a true com-
parison among these studies is almost
impossible: different pre‐therapeutic condi-
tions of the involved molars, no uniformity
in the terminology used, different reasons
for performing these procedures (periodon-
tal? endodontic? root fracture? caries?),
different techniques used in each step of
both root separation and resections (endo-
dontic treatment, tooth build‐up, prepara-
tion, provisional and definitive crown
morphology), different recall intervals, and
smoking habits (Mullally and Linden 1996)
make drawing indisputable conclusions
about the efficacy of this therapeutic modal-
ity an impossible task. In spite of that, it
must be pointed out that with the exception
of three studies, the average survival rate of
the molars treated with root separation/
resection was very high (close to 90%) and
comparable to that of implants inserted in
the posterior areas of the mouth, and that
the reasons for tooth extraction were mainly
related to endodontic complications and
root fractures, and not to periodontal
disease recurrence.
8.4.3 Contraindications
Root‐separation/resection procedures present
some important anatomical and technical
contraindications:
●
Poor compliance with oral hygiene.
●
Patients with high caries susceptibility.
●
Patients with severe parafunctional habits.
●
Inadequate residual attachment on the
remaining roots.
●
Serious discrepancies in adjacent inter-
proximal bone level.
●
Unfavourable anatomical factors (long root
trunk, short divergence between roots,
fused roots, presence of inter‐radicular
septa).
●
Retained roots endodontically untreatable.
Table 8.1
Clinical studies on the treatment of furcation‐involved molars with root separation/resection.
Author
Year
Study design
Number
of teeth
Follow‐up (mean)
Complications
Survival
Bergenholtz
1972
Retrospective
45
5–10 years
66.6% periodontal 33.3% root perforation
85%
Hamp et al.
1975
Retrospective
87
5 years
100%
Langer et al.
1981
Retrospective
100
10 years
47.4% root fracture 26.3% periodontal
18.4% endodontic 7.9% wash‐out/caries
62%
Buhler
1988
Retrospective
28
10 years
33.3% endodontic 22.2% perio‐endo 22.2%
periodontal 11.1% root fracture 111%
wash‐out/canes
67.9%
Carnevale et al.
1991
Retrospective
488
Group I 303 teeth: 3–6 years
Group II 185 teeth:7–11 years
33.3% caries 33.3% root fracture 33.3% PPD
> 5mm (group II)
Group II 98.4%
Blomlof et al.
1997
Retrospective
78
5–10 years
81.3% periodontal 25% perio‐endo 28.1
endodontic
5 years: 83% 10
years: 68%
Carnevale et al.
1998
Prospective
175
10years
33.3% endodontic 25% root caries 25%
periodontal 16.7 root fracture
5 years: 98.9%
10 years: 93.1%
Hou et al.
1999
Retrospective
52
6.7 (5–13 years)
100%
Svardstrom &.
Wennstrom
2000
Retrospective
47
9.5 (8–12 years)
80% root fracture
89.4%
Dannewitz et al.
2006
Retrospective
19
~9 (~5–12 years)
Not reported
92.9%
PPD = probing pocket depth.

Chapter 8
170
●
Excessive endodontic instrumentation of
retained roots.
●
Severe root decay/resorption.
We should also bear in mind that these are
sensitive techniques that require a careful
interdisciplinary approach, a widespread
knowledge of prosthodontics, endodontics,
and periodontology, and an accurate evalua-
tion of the cost–benefit ratio with respect to
the treatment alternatives. The need for
endodontic treatment, prosthetic rehabilita-
tion, and periodontal surgery actually makes
this therapeutic modality a demanding treat-
ment, in terms of both economic cost and
biological tissue loss.
8.4.4 Step‐by‐step Procedure
Considering that root separation/resection is
an interdisciplinary procedure and that most
of the failures reported in the literature are
basically generated by reasons other than
new periodontal breakdown, first careful
patient selection and then precise sequenc-
ing and correct execution of each phase of
the therapeutic protocol are crucial to the
long‐term success of the procedure. The sug-
gested therapeutic sequence is as follows.
8.4.4.1 Patient Selection
This is the first, fundamental step of the
sequence, because not all patients are equally
suitable for root separation/resection. Poor
patient compliance, high caries susceptibil-
ity, and limited financial resources are the
most frequent factors limiting the use of this
procedure. Treatment options must be
presented to the patient and the potential
problems should be discussed. Root separa-
tion/resection should be considered as part
of an overall treatment plan aiming at
complete periodontal, functional, and
aesthetic rehabilitation.
8.4.4.2 Tooth Selection
As already mentioned, long root trunk, short
divergences between the roots, fused roots,
and presence of inter‐radicular septa represent
contraindications for a root‐ separation/resec-
tion procedure. Particular caution should be
used when the multi‐rooted teeth are intact,
because this is an invasive procedure involving
a considerable biological cost that must always
be carefully evaluated.
8.4.4.3 Endodontic Treatment
Since root and/or build‐up fractures have
often been reported as one of the most
frequent reasons for the failure of a root‐
separation/resection procedure, correct
endodontic treatments must preserve as
much tooth structure as possible at both cor-
onal (access opening should be kept as small
as possible) and radicular levels (conserva-
tive instrumentation). Excessive instrumen-
tation of radicular canals and/or immoderate
pressure during gutta‐percha condensation
should be avoided.
Although a vital root resection is possible
without any initial post‐surgical discomfort
for the patient (Smukler and Tagger 1976),
when the tooth to be treated is vital or the
root filling is suboptimal, endodontic treat-
ment/retreatment should be always the first
procedure performed. The reasons for a
preliminary root canal treatment are:
●
Easier rubber dam isolation and easier
access for the endodontist.
●
Evaluation of tooth/root endodontic
prognosis before separation/resection
procedure.
●
Crown build‐up before separation/resec-
tion procedure.
If from a clinical and radiological point of
view it is not possible to identify the root(s)
to be resected with certainty, each root has to
be endodontically treated/retreated. In order
to avoid useless treatments and costs, when
the clinical periodontal evaluation of the
tooth is deeply doubtful, endodontic treat-
ment can be exceptionally postponed until
after the root separation/resection. In these
cases, root canal treatment should be per-
formed within two weeks (Smukler and
Tagger 1976).

Resective Approach and Restorative Options 171
8.4.4.4 Crown Build‐up
After completion of the endodontic therapy,
the crown of the molar, the pulp chamber,
and almost 2–3 mm of the canals apical to
the furcation entrance are prepared, etched,
and filled with light or chemically cured
composite by using a dentine adhesive to
improve the retention of the material. This
step is of the outmost importance, because
the replacement of the missing coronal and
radicular tooth structure should provide to
the abutments a complete marginal seal and
proper retention and resistance for the sub-
sequent full‐coverage restoration.
8.4.4.5 Root separation/resection During
Preliminary Prosthetic Preparation
Root separation/resection may be per-
formed as part of the initial tooth prepara-
tion for the prosthetic rehabilitation
(‘prosthetic preparation’), when a prefabri-
cated shell provisional restoration is relined
and temporarily cemented. Performing root
separation/resection prior to and not dur-
ing periodontal surgery (Carnevale et al.
1981, 1997) presents several important
clinical advantages:
●
Accurate evaluation of the periodontal
condition of the molar and thus the possi-
bility to change the treatment plan at an
early stage. In molars with furcation
involvement, it is often impossible to pre-
cisely assess the inter‐radicular attachment
loss before root separation, and therefore
no conclusive clinical decision about the
prognosis of the tooth can be made prior
to this procedure.
●
Earlier elimination/reduction of the inter‐
radicular periodontal infection and earlier
extraction of hopeless roots. This can
enhance the healing of the infrabony
lesions that might be present in the inter‐
radicular area at the extraction site, and
therefore generate an osseous morphology
more favourable for being corrected at the
time of resective bone surgery.
●
Creating access for plaque removal in an
otherwise inaccessible area.
●
Possibility of reducing tooth mobility
before surgery by splinting the roots with
the provisional restoration.
●
If the root trunk is short and infrabony
defects are not present, periodontal sur-
gery can often be avoided.
Root separation or resection can otherwise
be performed following the same technique
during the periodontal surgical phase, if
there is a diagnostic problem or difficult
access (Carnevale et al. 1990).
Before starting with tooth preparation/
resection, it is of the utmost importance to
carefully probe each root and especially the
furcation area in order to identify three‐
dimensionally the position of furcation
entrances, the anatomy of the root trunk, and
the potential presence of infrabony defects
affecting one or more roots (Zappa et al.
1993). Considering the reduced diameter
and thickness of the roots, the preparation
must be as conservative as possible. For this
reason, tooth structure saving a knife‐edge
finishing line should be preferred. Usually
local anaesthesia is not necessary during the
root‐separation phase and therefore should
not be used, because the patient’s feeling of
pain can help the clinician to avoid moving
the bur too deeply in the tissue and therefore
reducing the risk of damaging the preserved
inter‐radicular attachment.
In order to get access to the subgingival
root surfaces with the precise root axis and
to limit soft‐tissue damage, a small‐diameter
flame‐shape diamond bur can be used, and
the buccal and lingual enamel prominence
should be eliminated first. The access to each
involved furcation (buccal and lingual in
mandibular molars; buccal, mesial, and distal
in the maxillary) should be initially ‘marked’
with vertical grooves that can be used as ref-
erence points during the root‐separation/
resection procedure. In every passage the
flame‐shape diamond bur has to be moved
first forwards into the furcation and then
backwards, working in both interproximal
line angles of each root in order to widen the
space previously created between the roots.

Chapter 8
172
Once the furcation has been separated, the
whole roof of the involved furcation must be
eliminated and an adequate space for plaque‐
removal devices should be created between
the roots.
It is important to remember that the distal
aspect of the molar mesial root (especially
the mandibular one) often presents a deep
concavity. In order to avoid weakening the
mesial root or creating a root fenestration,
the desired distance within the distal root
should be created by preparing the mesial
aspect of the distal root rather than the distal
of the mesial one. The mesial and distal sur-
faces of mesial and distal mandibular roots
and the buccal and palatal surface of respec-
tively mesio‐buccal and palatal roots must be
parallel to each other (if both the roots are
maintained) and to the neighbouring abut-
ments to ensure a proper insertion axis of the
prosthetic rehabilitation, while the inter-
proximal surfaces can be divergent in order
to widen the space between the roots.
In order to be sure that the whole roof of the
involved furcation has been eliminated, a
curved periodontal probe (Nabers probe)
should be moved in the apico‐coronal direction
to detect the potential presence of furcation
lips or ledges. With the aim of facilitating provi-
sional relining and domiciliary plaque removal
in the exposed root surfaces, at the end of tooth
preparation/resection, the root surfaces should
be made smooth and even by using fine and
extra‐fine diamond burs, and the line angles of
the abutment should be rounded.
8.4.4.6 Provisional Restoration
At the end of tooth separation/resection, a
prefabricated shell provisional restoration is
relined and temporarily cemented. In order
not to disturb both soft and hard interproxi-
mal tissue healing, it is important to shorten
at about the gingival level and to precisely
refine the margins of the relined provisional
restoration. The excess of temporary cement
must be carefully removed and the
patient should be taught to clean the new
interproximal spaces properly with appro-
priate plaque‐removal devices. The provi-
sional restoration can be strengthened with
specific commercially available reinforcing
fibres or, in the case of long‐span bridges or
in patients with parafunctional habits,
replaced after an impression with a custom‐
made metal‐ reinforced temporary restoration.
8.4.4.7 Periodontal Surgery
The objectives of this phase of the root‐sepa-
ration/resection procedure are the following:
●
To eliminate possible angular bone defects
around the maintained roots and recreate
a positive bone architecture in order to
obtain an environment favourable to good
hygiene and easy dental care. Bone resec-
tion may also be performed to reduce the
bucco‐lingual dimension of the alveolar
process in the extraction sites. Soft‐ and
hard‐tissue management and post‐surgical
care are the same as used for pocket elimi-
nation with resective osseous surgery.
●
To facilitate both soft adaptation and dom-
iciliary plaque removal by modifying the
root contour through intrasurgical prepa-
ration of prosthetic abutments.
Following flap elevation, the maintained
roots and the other non‐vital abutments are
newly prepared with the purpose of remov-
ing the residual plaque and calculus, improving
the space between the roots, eliminating any
residual undercuts, and reducing the natural
anatomical concavities present on the root
surfaces. As proposed by Di Febo (1985),
concavities can be reduced by preparing a
chamfer on only the portions of the roots
that are convex, without touching the con-
cave portions, which thereby present a knife‐
edge finishing line (‘combined preparation’).
The intra‐operative preparation does not
always extend to the alveolar crest. On the
contrary, the operator should prepare the
tooth to the level of connective tissue attach-
ment and avoid, wherever possible, injuring
intact fibres and removing healthy cemen-
tum. Following intra‐surgical abutment
preparation and before suturing, the tempo-
rary restoration must be relined with a
self‐curing acrylic resin and the margins
trimmed 3 mm short of the alveolar crest so
as not to disturb the healing process.

Resective Approach and Restorative Options 173
Throughout the tissue maturation period,
patients are maintained on a plaque‐control
programme that includes professional tooth
cleaning and oral hygiene instruction once a
month. In order to reduce the risk of cement
washout during these recall appointments,
the provisional restoration should be
removed and re‐cemented and, where neces-
sary, new interdental plaque‐removal devices
are recommended (Walter et al. 2011).
8.4.4.8 Final Prosthesis
Once the healing period is complete and
before the impression for the definitive
prosthesis, the endodontic, periodontal, and
provisional prosthetic treatments have to be
clinically and radiographically re‐evaluated.
If the treatments could achieve successful
outcomes, the abutments can be refined and
polished and the final impression can be
taken, with or without extra‐thin retraction
cords. The design and construction of the
metal framework in combination with a good
crown fitting and sitting play a fundamental
role in the long‐term success of fixed bridges
using root‐separated or root‐resected abut-
ments (Carnevale et al. 1991; Newell 1991).
The strength and stability of the metal frame-
work should compensate for the structural
weakness of the abutments, and for the high
tooth mobility often present in severely
involved periodontal cases (Wang et al.
1994). For the same mechanical reasons,
occlusion should be designed and set to min-
imize occlusal lateral forces. Interproximal
spaces should be created in order to facilitate
oral hygiene as much as possible, and the
patient should be taught to use self‐per-
formed plaque‐removal devices correctly. At
the completion of therapy, patients are then
enrolled in a personalized maintenance recall
programme that generally includes three‐
monthly appointments.
8.5 Conclusions
The long‐term prognosis of teeth with
furcation involvement treated with conven-
tional therapy demonstrates a higher fre-
quency of tooth loss than non‐furcated
molars. The reduced success rate may be due
to the fact that the persistence of a defect
within the inter‐radicular space creates an
anatomical environment that interferes with
oral hygiene efforts. In fact, partial gain of
clinical attachment levels within the defect,
although statistically or clinically significant,
may not effectively improve the outcome dur-
ing the maintenance phase of therapy. It is
important to point out that comparative stud-
ies between the different procedures are lack-
ing, and therefore in treatment decisions for
furcation‐involved molars there is no scien-
tific evidence that a given treatment modality
is superior to the others. Patient‐related fac-
tors such age and health conditions, compli-
ance, susceptibility to caries, strategic value of
the tooth in relation to the overall treatment
plan, functional and aesthetic demands, and,
last but not least, financial resources should
guide clinicians in their choice of treatment.
Summary of Evidence
An accurate and precise diagnosis is essen-
tial in order to correctly approach affected
molar furcation areas. In particular, clini-
cians must initially evaluate all the patient‐
and tooth/site‐related factors that are able
to determine indications and contraindica-
tions for the treatment of the defects.
Afterwards, this step‐by‐step approach
should be followed:
●
Endodontic treatment and tooth
restoration.
●
Root separation/resection.
●
Provisional restoration.
●
Periodontal surgery.
●
Final prosthesis.

Chapter 8
174
References
Bergenholtz, A. (1972). Radectomy of multi‐
rooted teeth. Journal of the American Dental
Association 85, 870–875.
Blomlof, L., Jansson, L., Appelgren, R. et al.
(1997). Prognosis and mortality of root
resected molars. International Journal of
Periodontics and Restorative Dentistry 17,
191–201.
Buhler, H. (1988). Evaluation of root‐resected
teeth: Results after 10 years. Journal of
Periodontology 59, 805–810.
Carnevale, G., Di Febo, G., and Fuzzi, M.
(1990). A retrospective analysis of the
perio‐prosthetic aspect of teeth re‐prepared
during periodontal surgery. Journal of
Clinical Periodontology 17, 313–316.
Carnevale, G., Di Febo, G., Tonelli, M.P. et al.
(1991). A retrospective analysis of the
periodontal‐prosthetic treatment of molars
with interradicular lesions. International
Journal of Periodontics and Restorative
Dentistry 11, 189–205.
Carnevale, G., Di Febo, G., and Trebbi, L. (1981).
A patient presentation: Planning a difficult
case. International Journal of Periodontics and
Restorative Dentistry 1, 50–63.
Carnevale, G., Pontoriero, R., and di Febo, G.
(1998). Long‐term effects of root‐resective
therapy in furcation‐involved molars: A
10‐ year longitudinal study. Journal of
Clinical Periodontology 25, 209–214.
Carnevale, G., Pontoriero, R., and Hurzeler, M.
(1995). Management of furcation
involvement. Periodontology 2000 9, 69–89.
Carnevale, G., Pontoriero, R., and Lindhe, J.
(1997). Treatment of furcation‐involved
teeth. In: Clinical Periodontology and
Implant Dentistry, 3rd edn (ed. J. Lindhe, T.
Karring, and N.P. Lang), 682–710.
Copenhagen: Munksgaard.
Dannewitz, B., Krieger, J.K., Husing, J., and
Eickholz, P. (2006). Loss of molars in
periodontally treated patients: A
retrospective analysis five years or more
after active periodontal treatment. Journal
of Clinical Periodontology 33, 53–61.
De Sanctis, M., and Murphey K.G. (2000). The
role of resective periodontal surgery in the
treatment of furcation defects.
Periodontology 2000 22, 154–168.
Di Febo, G., Carnevale, G., and Sterrantino,
S.F. (1985). Treatment of case of advanced
peridontitis: Clinical procedures utilizing
the ‘combined preparation’ technique.
International Journal of Periodontics and
Restorative Dentistry 1, 52–63.
Hamp, S.E., Nyman, S., and Lindhe, J. (1975).
Periodontal treatment of multirooted teeth:
Results after 5 years. Journal of Clinical
Periodontology 2, 126–135.
Horwitz, J., Machtei, E.E., Reitmeir, P. et al.
(2004). Radiographic parameters as
prognostic indicators for healing of class II
furcation defects. Journal of Clinical
Periodontology 31, 105–111.
Hou, G.L., Tsai, C.C., and Weisgold, A.S.
(1999). Treatment of molar furcation
involvement using root separation and a
crown and sleeve‐coping telescopic denture:
A longitudinal study. Journal of
Periodontology 70, 1098–1109.
Huynh‐Ba, G., Kuonen, P., Hofer, D. et al.
(2009). The effect of periodontal therapy on
the survival rate and incidence of
complications of multirooted teeth with
furcation involvement after an observation
period of at least 5 years: A systematic
review. Journal of Clinical Periodontology
36, 164–176.
Langer, B., Stein, S.D., and Wagenberg, B.
(1981). An evaluation of root resections: A
ten‐year study. Journal of Periodontology 52,
719–722.
Majzooub, Z., and Kon, S. (1992). Tooth
morphology following root resection
procedures in maxillary molars. Journal of
Periodontology 63, 290–296.
Mullally, B.H., and Linden, G.J. (1996). Molar
furcation involvement associated with
cigarette smoking in periodontal referrals.
Journal of Clinical Periodontology 23,
658–661.

Resective Approach and Restorative Options 175
Newell, D.H. (1991). The role of the
prosthodontist in restoring root‐resected
molars: A study of 70 molar root
resections. Journal of Prosthetic Dentistry
65, 7–15.
Polson, A.M., Meitner, S.W., and Zander, H.A.
(1976a). Trauma and progression of
marginal periodontitis in squirrel monkeys.
IV Reversibility of bone loss due to trauma
alone and trauma superimposed upon
periodontitis. Journal of Periodontal
Research 11, 290–298.
Polson, A.M., Meitner, S.W., and Zander, H.A.
(1976b). Trauma and progression of
marginal periodontitis in squirrel monkeys.
III Adaption of interproximal alveolar bone
to repetitive injury. Journal of Periodontal
Research 11, 279–289.
Rosenberg, M.M. (1978). Management of
osseous defects. In: Clinical Dentistry, vol. 3
(ed. J.W. Clark), 103. Philadelphia, PA:
Harper & Row.
Ross, I.F., and Thompson, R.H., Jr (1978). A
long term study of root retention in the
treatment of maxillary molars with furcation
involvement. Journal of Periodontology 49,
238–244.
Smukler, H., and Tagger, M. (1976). Vital root
amputation: A clinical and histologic study.
Journal of Periodontology 47, 324–330.
Svanberg, G., and Lindhe, J. (1973).
Experimental tooth hypermobility in the
dog: A methodological study. Odontologisk
Revy 24, 269–282.
Svardstrom, G., and Wennstrom, J.L. (2000).
Periodontal treatment decisions for molars:
An analysis of influencing factors and
long‐term outcome. Journal of
Periodontology 71, 579–585.
Tarnow, D., and Fletcher, P. (1984).
Classification of the vertical component of
furcation involvement. Journal of
Periodontology 55, 283–284.
Walter. C., Schmidt, J.C., Dula, K., and
Sculean, A. (2016). Cone beam computed
tomography (CBCT) for diagnosis and
treatment planning in periodontology:
A systematic review. Quintessence
International 47, 25–37.
Walter, C., Weiger, R., and Zitzmann, N.U.
(2011). Periodontal surgery in furcation‐
involved maxillary molars revisited: An
introduction of guidelines for
comprehensive treatment. Clinical Oral
Investigations 15, 9–20.
Wang, H.L., Burgett, F.G., Shyr, Y., and
Ramfjord, S. (1994). The influence of molar
furcation involvement and mobility on
future clinical periodontal attachment loss.
Journal of Periodontology 65, 25–29.
Zappa, U., Grosso, L., Simona, C. et al. (1993).
Clinical furcation diagnosis and
interradicular bone defects. Journal of
Periodontology 64, 219–227.

Chapter No.: 1 Title Name: <TITLENAME>
c09.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:21:25 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 177
177
Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
9.1 Introduction
Furcation involvement has been shown to be
associated with tooth loss during supportive
periodontal therapy (Graetz et al. 2015;
Dannewitz et al. 2016; see also Chapter 5).
Characterized by ridges and concavities
(Svärdström and Wennström 1988), the furca-
tion area is – for patients and dental profes-
sionals alike – particularly difficult to clean.
High plaque and consequently high bleeding
scores are generally associated with break-
down of periodontal tissues during mainte-
nance (Lang et al. 1990; Eickholz et al. 2008).
Creating an inter‐radicular space accessible
for brushing between the roots at deeply
involved furcation sites – a procedure referred
to as ‘furcation tunnelling’ – will allow for reg-
ular plaque removal from the furcation area
(Figure 9.1), thus reducing the bacterial chal-
lenge to the periodontal tissues and possibly
the risk of disease recurrence at these sites. In
this chapter, case selection, treatment proce-
dure, and the scientific background of the
long‐term prognosis of molars undergoing
tunnel preparation will be discussed.
9.2 Indication
The furcation tunnelling procedure should
be considered at stable (no more than mobil-
ity grade I) furcation‐involved molars with
advanced inter‐radicular bone loss (furcation
involvement of at least deep grade II or grade
III ‘through and through’; Hamp et al. 1975)
when accessibility to the furcation area for
plaque removal is difficult. This is particu-
larly the case if a deep lingual (at lower
molars) or mesio‐palatal (at upper molars)
furcation involvement is present. If there is
no bone loss at the lingual furcation (at lower
molars) or the mesio‐buccal furcation
entrance (at upper molars), and at the same
time a buccal furcation involvement grade II
not exceeding half the buccal‐lingual width
of the molar, there might be a possibility of
the patient accessing the furcation entrance
with an interdental brush and thus create
healthy conditions. The converse condition –
that is, no bone loss at the buccal furcation
entrance and a lingual involvement grade
II – would speak in favour of a tunnel proce-
dure to ensure lingual healing, as cleaning of
a lingual furcation entrance is difficult to
manage for the patient.
A prerequisite for the tunnelling procedure
is sufficient residual bone support at all roots.
As a rule of thumb, the alveolar bone support
should be of equal amounts at all roots, and
at least cover one‐third of the root length.
Bone loss should mainly be horizontal.
Otherwise, root resection may be considered
instead (see Chapter 8).
The accessibility of the buccal furcation
entrance should already at an early stage be
Chapter 9
Furcation Tunnelling
Stefan G. Rüdiger
Department of Periodontology, Public Dental Service/Malmö University, Malmö, Sweden
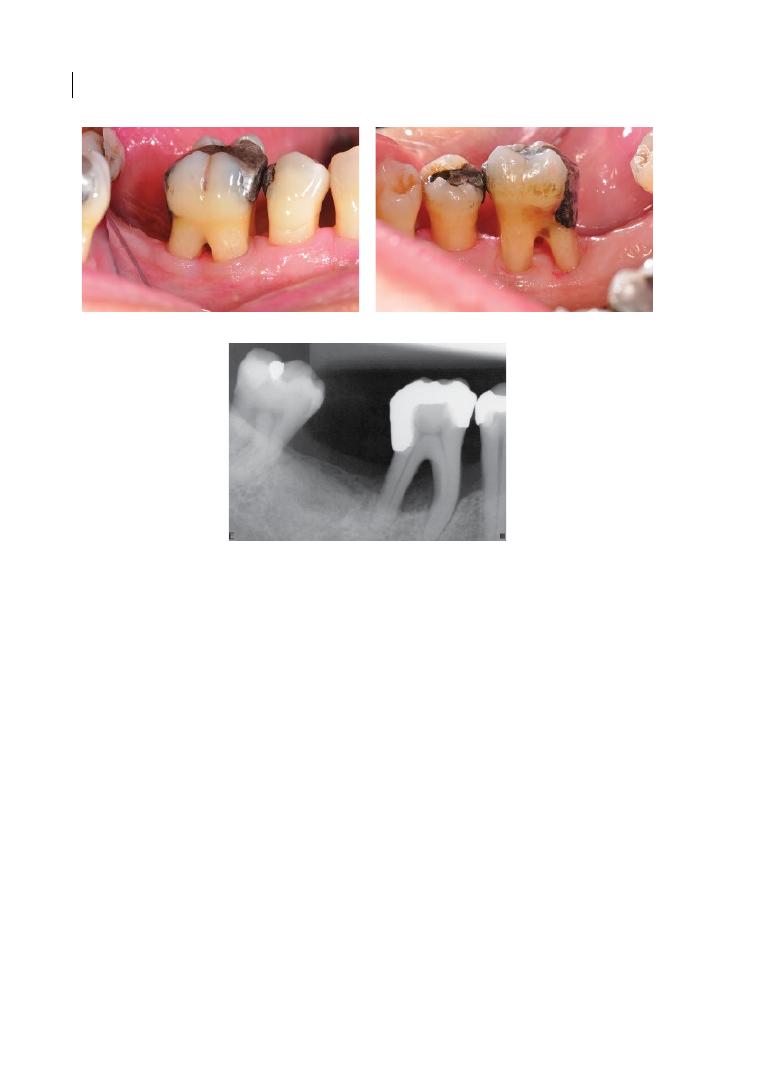
Chapter 9
178
judged clinically and radiographically. The
length of the root trunk and the diameter of
the furcation entrance are variables to con-
sider. Reasonably, the length of the root
trunk should not exceed 4 mm and the diam-
eter of the furcation entrance should be at
least 0.5 mm. Otherwise, it will be difficult
for the patient to find the furcation entrance
and insert the interdental brush into the fur-
cation. Considering anatomical measure-
ments, first mandibular molars would be the
most suitable candidates for the tunnelling
procedure, considering root trunk dimen-
sions and the divergence angle of the roots
(Chiu et al. 1991; Hou and Tsai 1997;
Paolantonio et al. 1998; Kerns et al. 1999). In
addition, the angle of the furcation area itself
should be looked at. A narrow furcation roof
would impede brushing.
Assessing the upper molars is more
difficult, as the palatal root is often superim-
posed on the furcation area on radiographs.
An eccentrically taken (especially distal
eccentric) radiograph might help to project
the furcation area in a mesio‐buccal direc-
tion. In comparison to lower molars, upper
molars more often have a longer root trunk
and a narrower divergence angle between
the two buccal roots. A further anatomical
complication in the upper jaw is the fact that
three roots create three furcation entrances.
If an interdental brush is inserted into the
buccal furcation entrance, the tooth anat-
omy would usually guide the brush to the
mesio‐palatal furcation entrance (Figure 9.2).
Thus the distal furcation entrance, which is
usually located in the middle of the distal
approximal surface, would not be cleaned
(a)
(b)
(c)
Figure 9.1
Example of a well‐functioning tunnel at a maintenance visit. The tunnel has been in function for
seven years. Healthy periodontal tissues are noted around the two roots from the buccal (a) and lingual (b)
aspects. On the intraoral radiograph (c), the inter‐radicular alveolar bone is dense, which can be taken as a sign
of stable conditions.
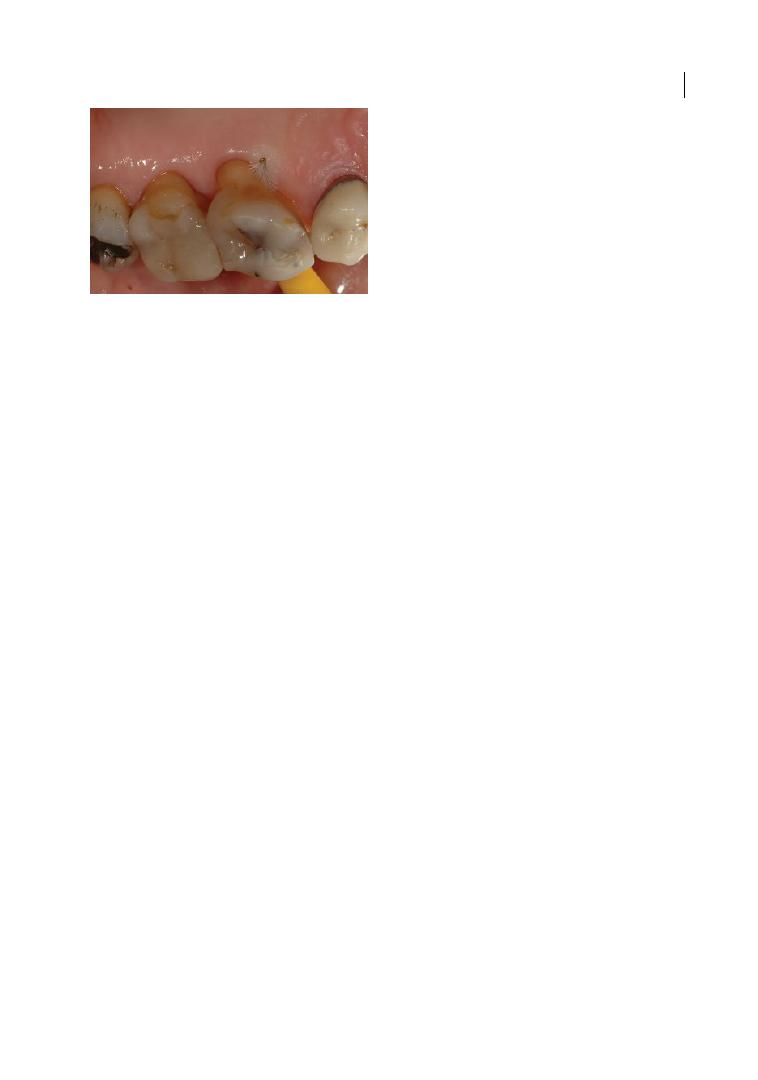
Furcation Tunnelling 179
(see Section 9.6). Provided that proper case
selection is carried out, the decision on tun-
nelling procedure can be taken intraopera-
tively. This bears the advantage of a more
accurate furcation measurement, as calculus
and in particular granulation tissue can
obstruct the path for the furcation probe,
thus underestimating the degree of furcation
involvement.
Another factor to consider when planning
for a tunnelling procedure is if prosthetic
treatment will be necessary in the area. A
tunnelling procedure might help avoid pros-
thetic treatment and thereby preserve an
intact dental arch. If prosthetic treatment is
inevitable, the prognosis of a furcation‐tun-
nelled molar should be weighed thoroughly
against the prognosis of the entire prosthetic
construction, considering a possible loss of
the furcation‐tunnelled tooth. It can be
noted that in one study 33 out of 156 tun-
nelled teeth were given a prognosis suffi-
cient to serve as an abutment for fixed
bridges (Helldén et al. 1989). Given the fact
that specific anatomical prerequisites and
the patient’s full cooperation are required
for a successful tunnelling procedure, this
type of treatment is performed only occa-
sionally. In follow‐up studies of periodontal
patients in specialist surroundings, 1–5% of
molars (Hamp et al. 1975; Kuhrau et al. 1990;
Graetz et al. 2015; Dannewitz et al. 2016)
in periodontitis patients underwent this
treatment. Though deep grade II furcation
involvements are often given as an indica-
tion for the tunnelling procedure in text-
books, in an actual retrospective cohort of
maintenance patients in a specialist unit, the
tunnelling procedure was considered only
for molars with initial furcation involvement
grade III (Dannewitz et al. 2016).
9.3 Patient Selection
Before introducing the tunnelling procedure
as a possible treatment option, the patient’s
ability and attitude to brushing between
roots should be assessed. If a surgical tunnel
preparation has been performed and the
patient is not able or willing to brush it, the
purpose of the treatment will be defied and
compliance could be further affected. Further
on, it is advisable not to introduce inter‐
radicular cleaning as the first oral hygiene
instruction procedure, but instead to con-
centrate on getting to know the patient by
their ability at ordinary interdental brushing.
As soon as the standard of oral hygiene is
acceptable, brushing of a furcation entrance
can be introduced as a preparatory step to a
furcation preparation.
9.4 The Tunnelling Procedure:
Surgical Steps
If an interdental brush can be inserted easily
into the buccal furcation entrance and with
only minor resistance pass through the entire
furcation area, it may be worth trying to create
the furcation non‐surgically. This can be an
advisable strategy if otherwise there is no indi-
cation for periodontal surgery. If the gingiva
over the lingual furcation entrance obstructs
the interdental brush from passing through an
otherwise entirely accessible through‐and‐
through furcation, lingual gingivectomy may
be performed (Figure 9.3).
Figure 9.2
Root anatomy guides the interdental
brush from the buccal to the mesio‐palatal furcation
entrance. When an interdental brush is inserted into
the buccal furcation entrance, root anatomy will
usually lead the brush to the mesial furcation
entrance.
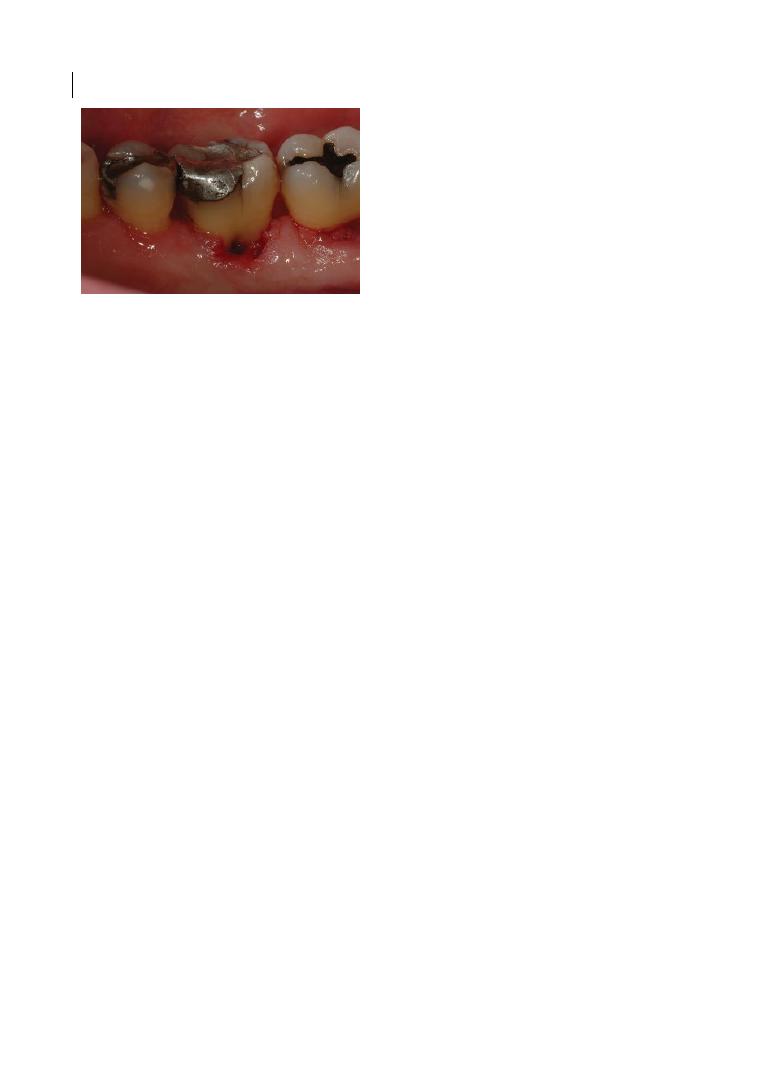
Chapter 9
180
In most cases, periodontal flap surgery
should be performed to ensure good post‐
operative access through the whole furca-
tion area. After local anaesthesia and
elevation of a full‐thickness flap, granulation
tissue is removed to judge the bone level in
the furcation involvement. Before flap
design, careful attention should be paid to
the amount of buccal ketarinized gingiva
present. In cases of large amounts of kerati-
nized gingiva, a scalloped incision can be
performed by the furcation entrance, fol-
lowed by the removal of the secondary flap
after intrasulcular incisions, to expose the
furcation area. Otherwise, in cases with a
limited amount of keratinized gingiva, intra-
sulcular incisions with no paramarginal
incisions are preferred, in order to preserve
the keratinized tissues and facilitate tissue
handling. In this case, a full split‐thickness
flap can be executed, if possible associated
with lateral buccal relieving incisions, to
then apically reposition the flap by peri-
osteal suturing (Friedman 1962) to expose
the furcation area. These two different
options are illustrated in Figure 9.4.
A straight periodontal probe, or alterna-
tively a sterile interdental brush (e.g. size yel-
low 0.7; TePe, Malmö, Sweden) can be used
to test accessibility. If bone must be removed
to ensure accessibility for the interdental
brush, a round bur or Waerhaug diamond
can be used. The bur should preferably first
be inserted from the furcation entrance that
is more involved. As soon as the operator has
an idea of the direction, further osteoplasty is
performed stepwise from both furcation
entrances. The furcation entrances should be
free of any bone ridges, which have to be
removed, thus ensuring full accessibility to
the furcation (Figure 9.5).
The ideal distance from the fornix to the
bone crest to allow for interfurcation clean-
ing should be around 5–6 mm, which is the
diameter of a size 7 interdental brush (3 mm)
plus 2–3 mm needed for the dento‐gingival
junction, and to allow for possible rebound of
gingival tissues inside the furcation. However,
no specific studies have investigated this
aspect. The advantage of the Waerhaug dia-
mond is that its torpedo‐like form allows the
bur to find its way through the furcation,
whereas such tactility is not achieved with a
round bur. Oscillating techniques (e.g. piezo-
electric instruments) can also be applied for
gentle ostectomy/osteoplasty, particularly
in the inner furcation area. After scaling
and root planing, the flaps are reposi-
tioned by sutures, ensuring bone coverage.
Interproximal single interrupted or vertical
mattress sutures may be used. Sutures
anchoring the flap to the periosteum need to
be performed in case of an apically reposi-
tioned flap. At lower molars, a suture can be
placed through the furcation. In the upper
jaw, root anatomy makes the furcation more
difficult to access for interdental cleaning,
and exposure of the furcation entrances by
trimming the periodontal flap can be advisa-
ble (Figure 9.6). Periodontal dressing can be
applied to avoid granulation tissue growing
in the furcation area. Thus, the furcation tun-
nel will be void of granulation tissue and
already accessible to an interdental brush at
suture removal. It is important to remember
that the periodontal dressing should only be
placed into the furcation entrance and not
through the whole furcation area, as it can be
painful for the patient when the dressing is
Figure 9.3
Tunnel preparation by gingivectomy.
If the interdental brush – coming from the buccal
furcation entrance – cannot pass a through‐and‐
through furcation involvement at the lingual aspect
due to soft‐tissue obstruction, gingivectomy at the
lingual aspect will create accessibility through the
whole furcation.
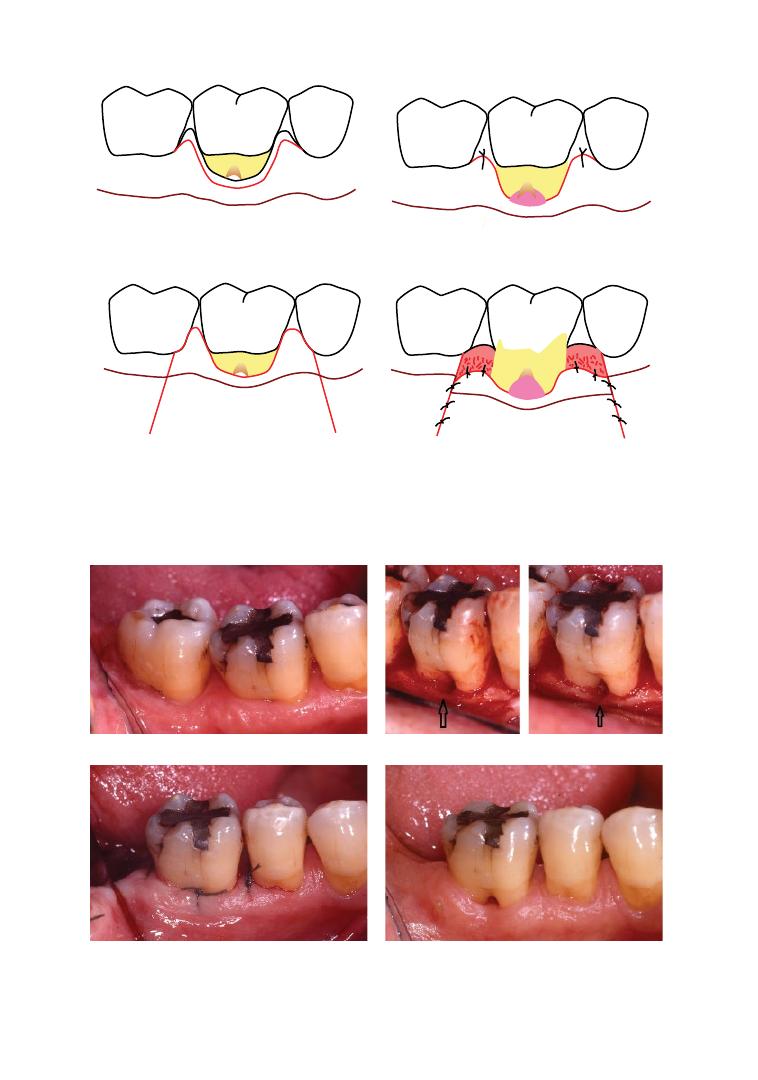
Chapter No.: 1 Title Name: <TITLENAME>
c09.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:21:25 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 181
(a)
(c)
(b)
(d)
Figure 9.4
Different approaches for tunnelling surgery needed according to the amount of keratinized gingiva
(KG). In a case of an adequate amount of KG (a), scalloped incisions can be performed to expose the furcation
area for self‐performed cleaning (b). In a case of a reduced amount of KG (c), an apically repositioned flap is
performed in order to preserve the KG while still exposing the furcation area for self‐performed cleaning (d).
(a)
(b)
(c)
(d)
Figure 9.5
Osteoplasty during tunnel operation and suturing through the furcation. A furcation entrance
can be obstructed by soft tissue (a) and bone ridges (b). A positive bone architecture has to be created by
intrasurgical osteoplasty (see arrows) to secure easy insertion of the interdental brush through the furcation.
Good soft‐tissue adaptation can be achieved in the furcation entrances by placing a suture through the
furcation (c), ensuring good healing (d).
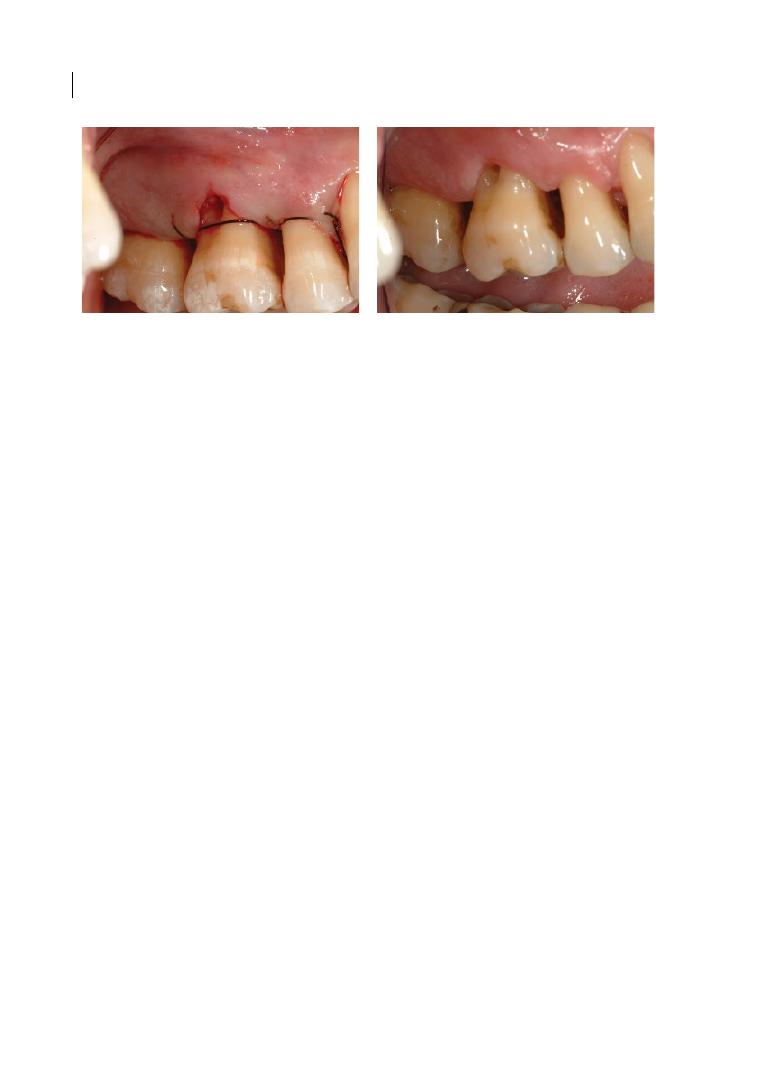
Chapter 9
182
removed from the inner part of the furcation.
In such cases, brushing in the furcation might
even not be possible at suture removal due to
tenderness in the inner part of the furcation.
To a greater extent than ordinary flap sur-
gery, periodontal surgery for tunnel prepara-
tion involves a certain ambivalence between
leaving the surgical area to heal by secondary
intention and the general intention of perio-
dontists to achieve primary healing (which
means that areas, such as the furcation
entrance, that it is crucial to clean at an early
stage should be covered by tissue). Post‐opera-
tive follow‐up of the latter needs to concentrate
on training the patient to find the furcation
entrance, whereas in the case of secondary
healing the patient has to be encouraged to
dare to brush the furcation area despite post‐
operative tenderness. This dilemma can only
be solved for each patient individually. As
already pointed out, the patient’s attitude
towards oral hygiene has to be known to the
therapist before the treatment decision for a
tunnelling procedure should be taken.
9.5 Postoperative Follow‐Up and
Oral Hygiene in the Furcation
At suture removal 7–10 days after the surgery,
the tissues in the furcation area are often still
too tender to allow for through‐and‐through
brushing. At this time point in healing, there
may not be epithelial coverage of the intraop-
eratively exposed bone, which will be the
cause of tenderness on touching. The focus
for the first post‐operative oral hygiene
instruction should be on the correct horizon-
tal insertion of the interdental brush into the
furcation entrance. The direct instruction to
the patient should be to ‘insert the brush as
far as possible’. Ideally, the patient should be
seen for post‐operative follow‐up four and
eight weeks after surgery. At four weeks, it
should be tested whether the brush can be
inserted over the middle of the furcation area,
possibly to the lingual furcation entrance. An
interdental brush should preferably be used in
its original straight shape, but if necessary it
can be bent to prevent the brush getting stuck
in the lingual gingival tissues, which tend to
grow in a coronal direction during the healing
process. The patient should be instructed to
feel the tip of the brush on the inside to ensure
that it has been inserted the whole way
through (Figure 9.7). If tissue regrowth is too
extensive, an additional gingivectomy can be
advisable. Good oral hygiene can be revealed
anatomically by good adaptation of the gin-
giva in the furcation entrances (Figure 9.8).
From a psychological point of view, it is
important that all personnel involved should
truly believe in the concept of the tunnelling
procedure. Otherwise, the patient will not
learn to brush the furcation tunnel. Especially
for a referral clinic, this aspect is important, as
(a)
(b)
Figure 9.6
At upper molars, root anatomy does often not form a straight pathway from the buccal to the
mesial furcation entrance (in contrast to the furcation at lower molars). In such a case, it is more advisable to
open the furcation by trimming the flaps, to leave the furcation entrances to heal openly (a), and not to place
sutures, which after healing ensures good accessibility for the patient (b).
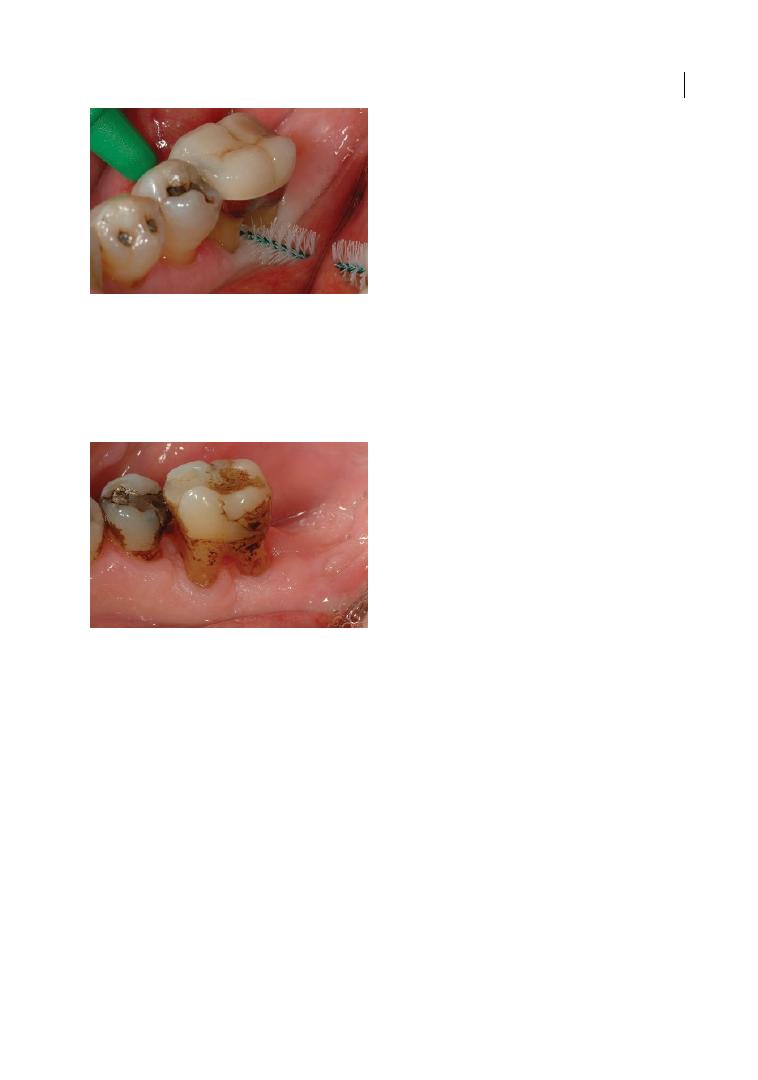
Furcation Tunnelling 183
keeping patients with a furcation tunnel for a
prolonged maintenance phase can be consid-
ered to ensure a good long‐time prognosis.
In some cases it may be necessary to
instruct the patient to brush the furcation
from the palatal or lingual aspect to get the
best brushing result. This technique requires
extensive training exercises with the patient.
In one study, patients were asked about
their experience and brushing habits as far as
the furcation tunnel was concerned (Helldén
et al. 1989). The majority (92%) did not expe-
rience discomfort in relation to the furcation
tunnel; 70% of patients said it was easy to
brush the furcation tunnel, 80% used an
interdental brush, and 27% an interspace
brush in addition to a common toothbrush
(see also Chapter 13).
9.6 Types of Teeth
For lower molars, the brushing procedure is
straightforward. There is one way in and one
way out, and usually the interdental brush can
easily be inserted through the whole furcation.
The trifurcation of upper molars makes mat-
ters more complicated. In most cases, the root
anatomy guides an interdental brush from the
buccal furcation entrance to the mesial furca-
tion entrance, which is in most cases located
in the mesio‐palatal line angle. Often the path-
way through the furcation does not allow a
straight brush to be inserted. Thus, the patient
must be instructed to bend the brush to get it
through the whole furcation. Alternatively, the
patient can be instructed to insert the brush
from the lingual aspect.
The distal furcation entrance of upper
molars is located in the middle of the approx-
imal space. The distal furcation entrance is
not accessible for brushing as long as the
neighbouring distal tooth is in place. It can
be argued that ordinary interdental brushing
would result in some cleaning of the furca-
tion entrance, since the bristles would open
as the interdental brush is moved through
the interdental space. This might be an expla-
nation for why upper molars with a function-
ing tunnel from the buccal to the mesial
furcation entrance can be kept over a pro-
longed period of time, despite the fact that
the distal furcation entrance might not
always be accessible for direct brushing.
When the neighbouring posterior tooth is
missing and the patient’s dexterity is well
developed, a functioning double tunnel may
be created (Figure 9.9). Double tunnels are
not often mentioned in the literature. Helldén
et al. (1989) reported double tunnels per-
formed on 33 maxillary molars; however,
they did not specify the treatment outcome
for this type of tunnelling. Two contralateral
double tunnels had in a case report been
Figure 9.7
Meticulous oral hygiene instruction. It is
necessary to thoroughly explain to the patient the
technique of brushing a furcation tunnel. One
crucial piece of information is to point out that the
interdental brush has to be inserted through the
whole furcation tunnel. The patient should feel the
tip of the brush with the tongue.
Figure 9.8
Gingival topography reveals the patient’s
capability to brush the tunnel. Excellent oral hygiene
after furcation tunnelling is often revealed by the
inter‐radicular gingiva showing the path of the
interdental brush through the tunnel furcation. Good
adaption of the gingiva to the root surface is seen.
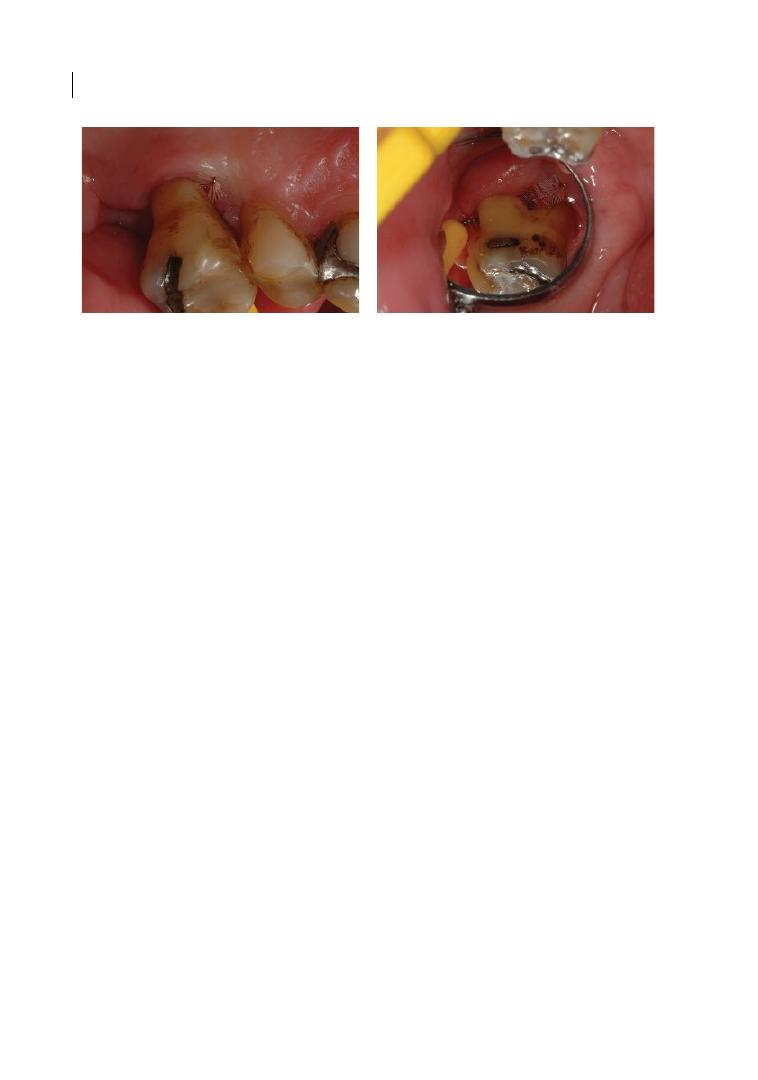
Chapter 9
184
proven to remain in stable condition over a
period of two years of maintenance therapy
(Rüdiger 2001).
Furcation tunnels in second molars are
more difficult to reach for the patient in both
the upper and lower jaws. Firstly, traction of
the corner of the mouth in combination with
the more posterior positioning of second
molars impedes correct positioning of the
interdental brush into the furcation entrance.
Secondly, the diverging angle of the roots
(buccal roots for the upper jaw) of second is
smaller than that of first molars, and the root
trunk is usually longer of second than of third
molars (Kerns et al. 1999).
In the literature, one case of a furcation
tunnel of a first upper premolar is men-
tioned. However, no details were given on
how this furcation tunnelling worked in
terms of oral hygiene, and if this tunnelled
premolar was among the tunnelled teeth
that had to be extracted (Hamp et al. 1975).
As both furcation entrances of first upper
premolars are situated in the approximal
spaces, the furcation entrances are difficult
to reach if the tooth is not rotated and no
neighbouring teeth are missing. Further
on, the majority of maxillary first premo-
lars (63%) have fused roots, and of those
having furcated roots only 10% had
the bifurcation in the cervical third of the
root lengths; that is, possibly accessible
for brushing (Joseph et al. 1996; see
also Chapter 1). Thus, the position of the
maxillary first premolar in the dental arch
and its root anatomy do not favour the pos-
sibility of using furcation tunnel proce-
dures in first upper premolars. During
15 years of clinical work at a referral clinic
for periodontology, the author has only
once come across the opportunity to intro-
duce the brushing procedure in a through‐
and‐through furcation involvement in an
upper first premolar. The second premolar
was missing and the first premolar was
rotated to such a degree that the insertion
of an interdental brush through the furca-
tion was possible. The bone loss had
reached the apical third of the roots
(Figure 9.10). Initially an improvement was
noted, but when the patient came back for
a three‐month follow‐up, the bone loss to
the apices was noted and the tooth had to
be extracted.
9.7 Pulp Reaction
The tunnelling procedure exposes consider-
able root surface areas. A pulp reaction
might be expected, as accessory root canals
are frequently found in the furcation area of
multi‐rooted teeth (Lowman et al. 1973;
Vertucci and Williams 1974; Niemann et al.
(a)
(b)
Figure 9.9
In rare cases, double tunnels can be created. In this case, the distal furcation entrance was reached
through the buccal entrance (a). The patient had learned to access both the mesial and the distal entrance
through the buccal entrance (b).
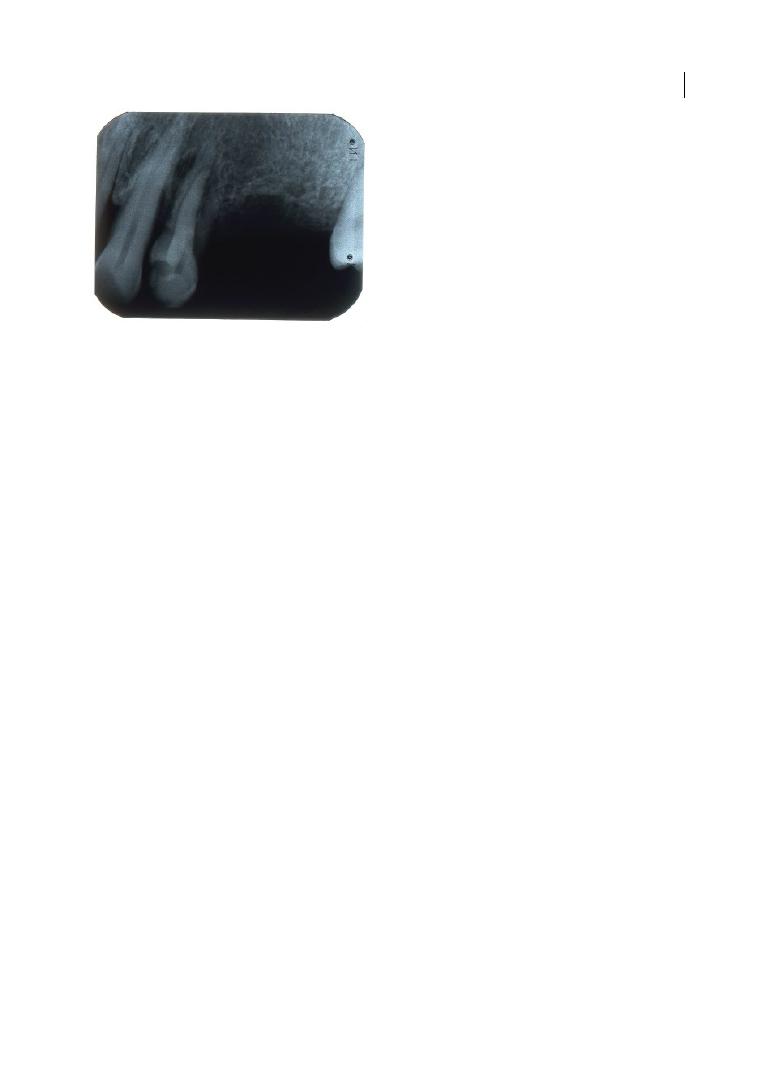
Furcation Tunnelling 185
1993; Zuza et al. 2006). However, only a
minority (10%) of the accessory canals in
the furcation are real communications
connecting the pulpal chamber with the
periodontium; the majority are blind canals
with an opening to either side and ending in
the dentine (Zuza et al. 2006). Further on,
pulpal necrosis only occurs when the main
apical foramina are involved, even if a pul-
pal inflammation can be seen at accessory
canals (Langeland et al. 1974). These ana-
tomical and histological findings corrobo-
rate clinical observation that endodontic
complications were not reported as a major
complication after furcation tunnelling
(see Table 9.1).
9.8 Caries after Furcation
Tunnelling
Molars subjected to furcation tunnelling
were reported to be at risk for root caries in
the furcation area. The prevalence of root
caries in the furcation ranges from 4.4 to
57.1% (see Table 9.1). Considering that root
caries after periodontal treatment in general
has been reported to occur with a preva-
lence of 82–90% after 10 years of mainte-
nance therapy (Ravald and Hamp 1981;
Reiker et al. 1999), caries at furcation‐tun-
nelled teeth is not to be seen as an unusual
finding. Caries in the furcation tunnel is
often difficult to detect clinically and – when
clinically manifest – is beyond restorative
dentistry’s therapeutic range, thus extrac-
tion may become inevitable (Figures 9.11
and 9.12).
9.9 Maintenance Phase
A crucial point during the maintenance
phase is the time when the patient is dis-
charged from the periodontal practice. The
introduction and instruction of the refer-
ring clinic are indicative of continuous good
prognosis of the furcation‐tunnelled tooth.
A general recommendation is periodontal
supportive therapy every third month.
Reminding the patient of the furcation tun-
nel is an important psychological aspect for
this type of patient. It has been shown that
tunnelled molars can be kept over several
years of supportive periodontal therapy (see
Table 9.1). Caries was given as a main rea-
son for tooth loss during maintenance.
There are indications of a prognostic
breaking point at 10 years of maintenance,
when the percentage of tooth loss notably
increases (Dannewitz et al. 2006, 2016). In
several studies (see Table 9.1), fluoride
prophylaxis is recommended to prevent the
development of caries lesions in the furca-
tion tunnel. In two studies (Topoll and
Lange 1987; Eickholz et al. 1991), patients
not complying with this recommendation
were over‐represented among patients los-
ing the tunnelled tooth. Large studies
are needed to draw conclusions and pro-
vide guidelines on long‐term survival of
tunnelled molars.
Figure 9.10
Upper left first premolar with mesial
bone loss reaching the apical third of the root was
chosen for furcation tunnelling. Clinically, at the
mesial aspect over the furcation entrance, there was
a pronounced swelling corresponding to the radio‐
juxtaradicular translucency. Though good
compliance with interdental brushing was achieved,
the progression of periodontitis could not be
arrested. The tooth lost stability only a few months
later and had to be extracted.

Chapter No.: 1 Title Name: <TITLENAME>
c09.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:21:25 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 186
Table 9.1
Summar
y of f
ollo
w‐up studies of multi‐r
oot
ed t
eeth subjec
ted t
o fur
ca
tion tunnel pr
epar
ation.
A
uthor/Y
ear
Sample
n
Follo
w‐up
years (mean
± SD/r
ange)
Type of study
No
. of t
eeth
Types of tunnelled
teeth
Suppor
tiv
e periodon
tal
ther
ap
y (SPT
); flouride
applica
tion
Ex
tr
ac
ted tunnelled
teeth/all r
e‐e
xamined
tunnelled t
eeth (%)
Reason f
or
ex
tr
ac
tion
H
am
p e
t al.
1975
100
5
Pr
osp
ec
tive f
ollow‐up of
m
ulti‐r
oot
ed t
ee
th
310 m
ulti‐r
oot
ed
te
et
h, 7 (2.3%) of
whic
h t
unne
lle
d
6 lower f
irst
mol
ars
, 1 upp
er
first pr
emol
ar
3–6 mon
ths
3/7 = 42.9%
C
ar
ie
s
Top
oll and
Lange 1987
28
1–8; me
an
3.4
Re
tr
osp
ec
tive f
ollow‐up
of t
unne
lle
d mol
ars
34 t
unne
lle
d mol
ars
32 lower mol
ars
,
2 upp
er mol
ars
af
ter r
es
ec
tion of
the p
al
at
al r
oot
3–4 mon
ths
; 14 p
atien
ts
com
plie
d w
ith
re
commend
ation
of
fluor
ide
pr
oph
yl
axi
s (
ge
l
applic
ation)
No e
xtrac
tions
re
po
rte
d
–
H
elldén
et al. 1989
107
0.8–8.9; me
an 3.1
Re
tr
osp
ec
tive f
ollow‐up
of t
unne
lle
d mol
ars
;
102/107 of p
atien
ts
and 149/156 of tunne
lle
d mol
ars wer
e
re
examine
d
156 t
unne
lle
d
mol
ars
52 lower mol
ars
,
91 upp
er mol
ars
,
33 of whic
h had
‘double’ t
unne
ls
3–6 mon
ths
; all p
atien
ts
wer
e adv
ise
d t
o u
se f
luor
ide
den
tr
ifr
ic
e, al
so dir
ec
tly in
the t
unne
l, and t
o r
ins
e w
ith
0.025% f
luor
ide s
olution
10/149 = 6.7%
6/10 t
ee
th b
ec
au
se
of c
ar
ie
s; ot
her
re
as
ons not
sp
ec
ifie
d
Table 9.1
Summary of follow‐up studies of multi‐rooted teeth subjected to furcation tunnel preparation.
Author/Year
Sample
n
Follow‐up
years (mean
± SD/range)
Type of study
No. of teeth
Types of tunnelled
teeth
Supportive periodontal
therapy (SPT); flouride
application
Extracted tunnelled
teeth/all re‐examined
tunnelled teeth (%)
Reason for
extraction
Hamp et al.
1975
100
5
Prospective follow‐up of
multi‐rooted teeth
310 multi‐rooted
teeth, 7 (2.3%) of
which tunnelled
6 lower first
molars, 1 upper
first premolar
3–6 months
3/7 = 42.9%
Caries
Topoll and
Lange 1987
28
1–8; mean
3.4
Retrospective follow‐up
of tunnelled molars
34 tunnelled molars 32 lower molars,
2 upper molars
after resection of
the palatal root
3–4 months; 14 patients
complied with
recommendation of fluoride
prophylaxis (gel
application)
No extractions
reported
–
Helldén
et al. 1989
107
0.8–8.9;
mean 3.1
Retrospective follow‐up
of tunnelled molars;
102/107 of patients
and 149/156 of
tunnelled molars were
reexamined
156 tunnelled
molars
52 lower molars,
91 upper molars,
33 of which had
‘double’ tunnels
3–6 months; all patients
were advised to use fluoride
dentrifrice, also directly in
the tunnel, and to rinse with
0.025% fluoride solution
10/149 = 6.7%
6/10 teeth because
of caries; other
reasons not
specified
Kuhrau
et al. 1990
59
4–8; mean
5.8
Retrospective follow‐
up of molars
under SPT
275 molars, 14
(5.1%) of which
tunnelled
14 lower molars
Regular SPT; intervals not
specified
2/14 = 14.3%
Caries
Eickholz
et al. 1991
56
1–5; mean
2.0
Retrospective follow‐up
of tunnelled lower
molars under SPT;
49/56 of patients and
68/76 of tunnelled
molars were reexamined
76 tunnelled lower
molars
76 lower molars
3 months; 39 patients
complied with
recommendation to brush a
concentrated fluoride gel
into the tunnel
5/68 = 7.4%
Not specified
Little et al.
1995
18
5.8 ± 0.83
Prospective follow‐up of
tunnelled molars
18 tunnelled molars 13 lower, 5 upper
molars
3 months;
2/18 = 11.1%
Caries
C
ha
pt
er No.: 1
Tit
le N
ame: <TITL
ENA
ME>
c09.indd
Com
p. by
: <US
ER>
D
ate: 14 Ma
y 2018
Time: 04:55:08 P
M
Stage: <S
TA
GE>
W
or
kFlow
:
<W
ORK
FL
OW>
Page N
umb
er
: 187
Feres et al.
2006
18
2–10
Retrospective follow‐up
of tunnelled molars
under SPT
30 tunnelled molars Not specified
3–6‐month interval
professional prophylaxis,
fluoride gel application
inside the tunnels, and oral
hygiene instructions
No extractions
reported
Kaltschmitt
et al. 2006
41
1–13
Retrospective follow‐up
of tunnelled molars
under SPT
56 tunnelled molars 6 upper, 50 lower
molars
SPT to varying degrees
8/56 = 14.3%; 7
lower molars, 1
upper molar
Not specified
Dannewitz
et al. 2006
71
8.9;
5.2–12.2
Retrospective follow‐up
of molars under SPT
505 molars, 14
(2.3%) of which
tunnelled
1 upper, 13 lower
molars
3‐, 6‐, 12‐month intervals
according to individual risk;
on average 1.9 ± 0.6 visits/
year
1/14 = 7.1%
Not specified
Dannewitz
et al. 2016
136*
13.2 ± 2.8
Retrospective follow‐up
of molars under SPT
1015 molars, 14
(1.4%) of which
tunnelled
1 upper, 13 lower
molars
3‐, 6‐, 12‐month intervals
according to individual risk;
on average 1.8 ± 0.5 visits/
year
5/14 = 35.7%
Not specified
SD = standard deviation.
*37 of which already were reported on by Dannewitz et al. 2006.
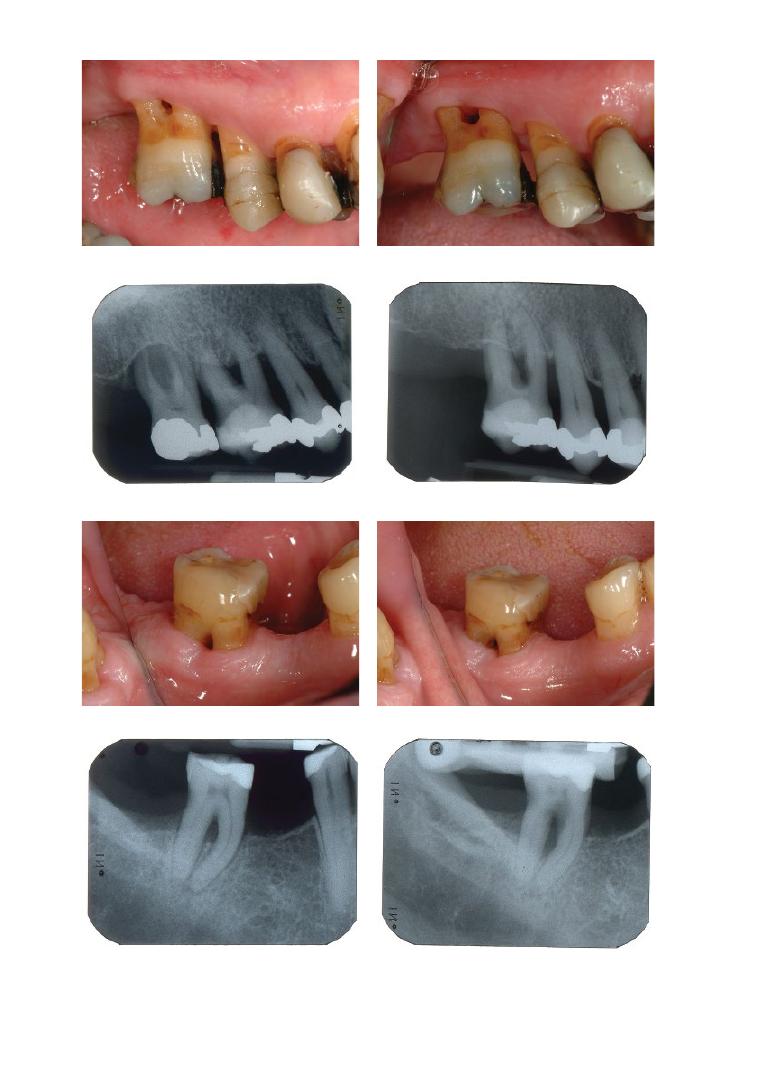
Chapter No.: 1 Title Name: <TITLENAME>
c09.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:21:25 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 188
(a)
(b)
(c)
(d)
(a)
(b)
(c)
(d)
Figures 9.11 and 9.12
Development of caries in furcation tunnels. In these two cases, caries developed within
the furcation area – before caries developed (a) and (c); with the established lesions (b) and (d). Caries
development was associated with inadequate oral hygiene during supportive periodontal therapy. The lesions
may be easily missed by mere clinical examination. Radiographs should therefore be taken during supportive
periodontal therapy to detect caries development at an early stage.

Furcation Tunnelling 189
References
Chiu, B.M., Zee, K.Y., Corbet, E.F., and
Holmgren, C.J. (1991). Periodontal
implications of furcation entrance
dimensions in Chinese first permanent
molars. Journal of Periodontology 62,
308–311.
Dannewitz, B., Krieger, J.K., Hüsing, J., and
Eickholz, P. (2006). Loss of molars in
periodontally treated patients: A
retrospective analysis five years or more
after active periodontal treatment. Journal
of Clinical Periodontolology 33, 53–61.
Dannewitz, B., Zeidler, A., Hüsing, J. et al.
(2016). Loss of molars in periodontally
treated patients: Results 10 years and more
after active periodontal therapy. Journal of
Clinical Periodontolology 43, 53–62.
Eickholz, P., Kaltschmitt, J., Berbig, J. et al.
(2008). Tooth loss after active periodontal
therapy. 1: Patient‐related factors for risk,
prognosis, and quality of outcome. Journal
of Clinical Periodontolology 43, 165–174.
Eickholz, P., Topoll, H.H., Hucke, H.P., and
Lange, D.E. (1991). Postoperative Befunde
nach Tunnelierung furkationsbeteiligter
Unterkiefermolaren (Grad III) [Postsurgical
findings after tunnel preparation in
mandibular molars with class III furcation
involvement]. Deutsche Zahnärztliche
Zeitschrift 45, 356–357.
Feres, M., Araujo, M.W., Figueiredo, L.C., and
Oppermann, R.V. (2006). Clinical evaluation
of tunnelled molars: A retrospective study.
Journal of the International Academy of
Periodontology 8, 96–103.
Friedman, N. (1962). Mucogingival surgery:
The apically repositioned flap. Journal of
Periodontolology 33, 328–340.
Graetz, C., Schützhold, S., Plaumann, A. et al.
(2015). Prognostic factors for the loss of molars:
An 18‐years retrospective cohort study.
Journal of Clinical Periodontolology 42,
943–950.
Hamp, S.‐E., Nyman, S., and Lindhe, J. (1975).
Periodontal treatment of multirooted teeth:
Results after 5 years. Journal of Clinical
Periodontology 2, 126–135.
Helldén, L.B., Elliot, A., Steffensen, B., and
Steffensen, J.E.M. (1989). Prognosis of
tunnel preparations in treatment of class III
furcations: A follow‐up study. Journal of
Periodontology 60, 182–187.
Hou, G.L., and Tsai, C.C. (1997). Types and
dimensions of root trunk correlating with
diagnosis of molar furcation involvements.
Journal of Clinical Periodontology 24,
129–135.
Joseph, I., Varma, B.R., and Bhat, K.M. (1996).
Clinical significance of furcation anatomy of
the maxillary first premolar: A biometric
study on extracted teeth. Journal of
Periodontology 67, 386–389.
Kaltschmitt, J., Radek, M., Dannewitz, B., and
Eickholz, P. (2006). Success of tunnel
preparations in molars with class III
furcation involvement. Journal of Clinical
Periodontology 33 (7), 116.
Kerns, D.G., Greenwell, H., Wittwer, J.W. et al.
(1999). Root trunk dimensions of 5 different
tooth types. International Journal of
Summary of Evidence
●
The tunnelling procedure is a treatment
method applicable in approximately
1–5% of all molar teeth in patients
referred for the treatment of periodontal
disease.
●
The best candidates for the tunnelling
procedure are lower first molars.
●
After periodontal treatment, the majority
of tunnelled molars can successfully be
kept in maintenance care over many years.
The prognosis declines after a decade.
●
Caries is the most frequent complication
leading to loss of tunnelled molars during
maintenance.

Chapter 9
190
Periodontics and Restorative Dentistry 19,
83–91.
Kuhrau, N., Kocher, T., and Plagmann, H.C.
(1990). Parodontalbehandlung
furkationsbefallener Zähne: Mit oder ohne
Radektomie? [Periodontal treatment of
furcally involved teeth: With or without root
resection?]. Deutsche Zahnärztliche
Zeitschrift 45, 455–457.
Lang, N.P., Adler, R., Joss, A., and Nyman, S.
(1990). Absence of bleeding on probing: An
indicator of periodontal stability. Journal of
Clinical Periodontology 17, 714–721.
Langeland, K., Rodrigues, H., and Dowden, W.
(1974). Periodontal disease, bacteria, and
pulpal histopathology. Oral Surgery, Oral
Medicine, Oral Pathology 37, 257–270.
Little, L.A., Beck, F.M., Bagci, B., and Horton,
J.E. (1995). Lack of furcal bone loss
following the tunnelling procedure. Journal
of Clinical Periodontology 22, 637–641.
Lowman, J.V., Burke R.S., and Pelleu G.B.
(1973). Patent accessory canals: Incidence in
molar furcation region. Oral Surgery, Oral
Medicine, Oral Pathology 36, 580–584.
Niemann, R.W., Dickinson, G.L., Jackson, C.R.
et al. (1993). Dye ingress in molars:
Furcation to chamber floor. Journal of
Endodontics 19, 293–296.
Paolantonio, M., di Placido, G., Scarano, A.,
and Piattelli, A. (1998). Molar root furcation:
Morphometric and morphologic analysis.
International Journal of Periodontics and
Restorative Dentistry 18, 489–501.
Ravald, N., and Hamp, S.‐E. (1981). Prediction
of root surface caries in patients treated for
advanced periodontal disease. Journal of
Clinical Periodontology 8, 400–414.
Reiker, J., van der Velden, U., Barendregt, D.S.,
and Loos, B.G. (1999). A cross‐sectional
study into the prevalence of root caries
in periodontal maintenance patients.
Journal of Clinical Periodontology 26,
26–32.
Rüdiger, S.G. (2001). Mandibular and
maxillary furcation tunnel preparations:
Literature review and a case report. Journal
of Clinical Periodontology 28, 1–8.
Svärdström, G., and Wennström, J.L. (1988).
Furcation topography of the maxillary and
the mandibular first molars. Journal of
Clinical Periodontology 15, 271–275.
Topoll, H.H., and Lange, D.E. (1987). Die
Tunnelierung mehrwurzliger Zähne:
Ergebnisse 8 Jahre post operationem.
[Tunnel preparation of multirooted
teeth: Results 8 years after surgery].
Deutsche Zahnärztliche Zeitschrift 42,
445–449.
Vertucci, F.J., and Williams, R.G. (1974).
Furcation canals in the human mandibular
first molar. Oral Surgery, Oral Medicine,
Oral Pathology 38, 308–314.
Zuza, E.P., Toledo, B.E., Hetem, S. et al. (2006).
Prevalence of different types of accessory
canals in the furcation area of third molars.
Journal of Clinical Periodontology 77,
1755–1761.

Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
Chapter No.: 1 Title Name: <TITLENAME>
c10.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:21:49 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 191
191
10.1 Introduction
This book’s journey has taken the reader
through the anatomy of molars with furcation
involvement (FI) and the challenges facing the
clinician engaging in periodontal treatment of
such molars. Recent technological advances
have led to the birth of several techniques and
devices which can help in the treatment of
molars with FI. The aim of this chapter is to
review what has changed in the treatment of
furcation‐involved teeth in recent years, to
present the evidence for the efficacy of these
new therapeutic modalities, and to predict
possible future treatment avenues. A major
improvement in periodontal non‐surgical
treatment, including the treatment of furcation
defects, has been achieved with the introduc-
tion of mini‐curettes and slim‐line ultrasonic
inserts, which have been covered in Chapter 3.
Other treatments, which we could consider
‘alternative’ to the ‘traditional’ treatments
described so far, are described in what follows.
10.2 Periodontal Endoscope
In an attempt to overcome the limitations of
traditional closed‐root instrumentation and
to give the clinician the possibility of visu-
ally debriding root surfaces, periodontal
endoscopy was proposed nearly 20 years ago
(Ozawa et al. 1999). The periodontal endo-
scope has been specifically designed to explore
and visualize periodontal pockets in patients
with periodontitis. The advantage of this
instrument is the subgingival visualization of
the root surface at high magnification (24× to
48×; Kwan 2005). When combined with the
use of micro ultrasonic instruments, endo-
scopic debridement can be accomplished in a
more accurate, conservative, and minimally
invasive way (Geisinger et al. 2007), thus
potentially reducing the need for surgical
intervention. Molars with FI, due to their dif-
ficult access for debridement, may represent
optimal candidates for this technology.
However, when comparing 35 pairs of multi‐
rooted teeth that received either endoscopy‐
aided scaling and root planing (SRP) or
traditional SRP, Michaud and co‐workers
(2007) did not find a significant difference in
calculus deposit removal between the two
groups, as assessed on digital images taken
with a stereomicroscope. The same conclu-
sions were reported by a more recent rand-
omized split‐mouth study, where one quadrant
underwent traditional SRP and another quad-
rant underwent SRP with the help of the peri-
odontal endoscope (Blue et al. 2013).
Nevertheless, a significant decrease in perio-
dontal outcomes of inflammation (namely
Chapter 10
Innovative and Adjunctive Furcation Therapy: Evidence
of Success and Future Perspective
Luigi Nibali and Elena Calciolari
Centre for Immunobiology and Regenerative Medicine, Centre for Oral Clinical Research, Institute of Dentistry, Barts and the London
School of Medicine and Dentistry, Queen Mary University of London (QMUL), London, UK

Chapter 10
192
bleeding on probing, BOP, and gingival index,
GI) was observed in the endoscopy‐aided SRP
group compared to the SRP group.
Although endoscopy may be an attractive
option, especially for sites that have not
responded to traditional non‐surgical treat-
ment and for patients where surgery is con-
traindicated, the use of the periodontal
endoscope may be difficult in narrow furca-
tions, or in curved roots, root proximity, and
in the presence of overhanging restorations,
as that may hinder the access for the endo-
scope and the instruments (Kwan 2005).
Furthermore, we should not forget that, apart
from the high cost, the use of endoscopic
instrumentation can be difficult to master, it
requires dedicated training, and it is associ-
ated with a steep learning curve.
10.3 Laser Therapy
In the past two decades, the use of lasers in
the treatment of periodontal disease has
attracted increasing interest. The word ‘laser’
is an acronym for ‘light amplification by
stimulated emission of radiation’ and it
broadly refers to any device that emits light,
is spatially coherent, and is collimated. Lasers
can be classified according to their active
medium (gas lasers and solid lasers), tissue
applicability (hard‐tissue and soft‐tissue
lasers), range of wavelength, and risk associ-
ated with their application (Verma et al.
2012). Dental laser wavelengths are typically
located within the near, mid, and far infrared
portions of the electromagnetic spectrum
(EMS; Lomke, 2009).
In periodontal therapy, different lasers
have been proposed for removal of pocket
epithelium (Borrajo et al. 2004; Saglam et al.
2014; Ustun et al. 2014), removal of subgingi-
val calculus deposits (Eberhard et al. 2003;
Schwarz et al., 2003; Lopes et al. 2010),
reduction of bacterial load (Moritz et al.
1998; Yaneva et al. 2014), root surface decon-
tamination (Barone et al. 2002), and enhance-
ment of periodontal regeneration (Dogan
et al. 2016; Taniguchi et al. 2016).
The current evidence supporting the
adjunctive use of dental lasers (mainly the
diode or the Nd:YAG) is of poor quality and
insufficient to warrant their use in the treat-
ment of chronic or aggressive periodontitis
or in periodontal maintenance therapy (Cobb
2016). In fact, since the 2011 statement from
the American Academy of Periodontology
that the use of dental lasers as monotherapy
or in addition to non‐surgical periodontal
instrumentation did not provide any tangible
advantage in terms of subgingival debride-
ment, reduction of subgingival bacterial
levels, and root debridement (American
Academy of Periodontology 2011), very little
has changed. Recent meta‐analyses have
claimed short‐term (six months) benefits of
the use of the Nd:YAG or diode lasers in con-
junction with non‐surgical periodontal ther-
apy in terms of probing pocket depth (PPD)
and BOP reductions compared with mechan-
ical debridement alone (Roncati and Gariffo
2014; Sgolastra et al. 2014). However, they
also highlighted the poor quality of the
available studies and the need for long‐term,
well‐designed, parallel, independent rand-
omized controlled trials (RCTs) with suffi-
cient statistical power and appropriate laser
settings in order to be able to draw more
robust conclusions.
Besides the limited scientific evidence,
current limitations on the use of lasers to
treat molars with FI include the often high
cost of the devices (which makes it difficult
to justify their use to the patient), the need
for additional education/training of the prac-
titioner (this includes education on the dif-
ferent properties associated with the different
wavelengths, as well as some fundamentals
of physics), and the need to implement safety
measures. In addition, and probably most
importantly, there is a lack of evidence on the
best setting to use for each different laser in
terms of wavelength, energy density, power
output, frequency/duration of irradiation,
distance between the cells, and the laser
spot/probe.
To the best of our knowledge, there is only
a limited number of studies that have

Innovative and Adjunctive Furcation Therapy 193
specifically considered laser treatment in
molars with FI, and the results are in line
with those reported for other periodontally
involved teeth. In a double‐blind RCT, de
Andrade et al. (2008) compared clinical and
microbiological parameters of 17 patients
with class II furcation lesions treated either
with SRP, or with SRP followed by pulsed
Nd:YAG laser treatment. The results showed
that the adjunctive laser promoted a signifi-
cant higher reduction in the total bacteria
colony‐forming units (CFU) immediately
after the treatment, but after six weeks no
significant differences were detected between
the two groups. Furthermore, no significant
differences in terms of periodontal clinical
parameters between baseline and six weeks
after treatment were found between the two
groups. In a more recent split‐mouth study,
an erbium, chromium: yttrium‐scandium‐
gallium‐garnet (Er,Cr:YSGG) laser was used
in association with SRP in molars with degree
II or III furcation involvement (Ge et al.
2017). A significantly higher reduction of
PPD and BOP was observed at six and twelve
weeks in the laser‐treated group compared
to the control group (SRP only). The visual
analogue scale (VAS) pain score was also sig-
nificantly lower when the laser was applied.
An attractive additional laser‐based treat-
ment modality is the so‐called phototherapy,
photomodulation, or low‐level laser therapy
(LLLT), which employs lasers at a low dose
with the aims of alleviating pain or inflam-
mation, inducing immunomodulation, and
promoting wound healing and tissue regen-
eration (Anders et al. 2015). Recent evidence
suggests that LLLT is able to enhance osteo-
blastic proliferation and differentiation
(Amid et al. 2014), increase gene expression
of collagen and vascular endothelial growth
factor in fibroblastic cells (Martignago et al.
2015), promote the production of nucleic
acid (Saperia et al. 1986), and increase mito-
chondrial respiratory chain and adenosine
triphosphate (ATP) synthesis (Agrawal et al.
2014). Future studies are needed to explore
the potential benefits of adding LLLT to, for
instance, periodontal regenerative treatment,
also in furcation‐involved molars. Another
application of phototherapy in periodontol-
ogy is antimicrobial photodynamic therapy,
which combines LLLT and a photosensitizer
with the aim of destroying pathogens in the
pocket with reactive oxygen species, and this
will be discussed in more detail in the next
section.
10.4 Photodynamic Therapy
The rationale behind photodynamic therapy
(PDT) comes from the need to obtain anti-
microbial effects without the risk of causing
the onset of microbial resistance. With PDT,
bacteria are sensitized using a dye (photo-
sensitizer) coming into contact with their
membrane, and are then destroyed following
irradiation with light of the correct energy
and wavelength. More specifically, a laser
light is used to activate the dye molecules to
reach a high energy triplet state, which reacts
with oxygen to create singlet oxygen, which
in turn destroys the bacterial membrane.
Toluidine blue (TBO) and methylene blue
(MB) are used as dyes, due to their ideal
properties of low toxicity, cationic charge for
attachment to Gram‐ membranes, red light
absorption, rapid transition from ‘singlet’ to
‘triplet’ state, long maintenance of ‘triplet’
state, and high production of photoproduct
(Atieh 2010; Soukos and Goodson 2011;
Gursoy et al. 2013).
Although the original concept of PDT is
already over 100 years old, only in recent
years has it reached clinical applications in
various fields of medicine, including oncol-
ogy, dermatology, and dentistry (Konopka
and Goslinski 2007). Its characteristics make
PDT potentially suitable for the treatment of
periodontitis, either in the non‐surgical or
surgical phase, with the aim of boosting the
antimicrobial effects of mechanical plaque
removal without the risk of causing micro-
bial resistance (Andrade et al. 2013; Sgolastra
et al. 2013a, b; Souza et al. 2016). Studies
have shown that photo‐inactivation of path-
ogenic bacteria harvested from periodontal
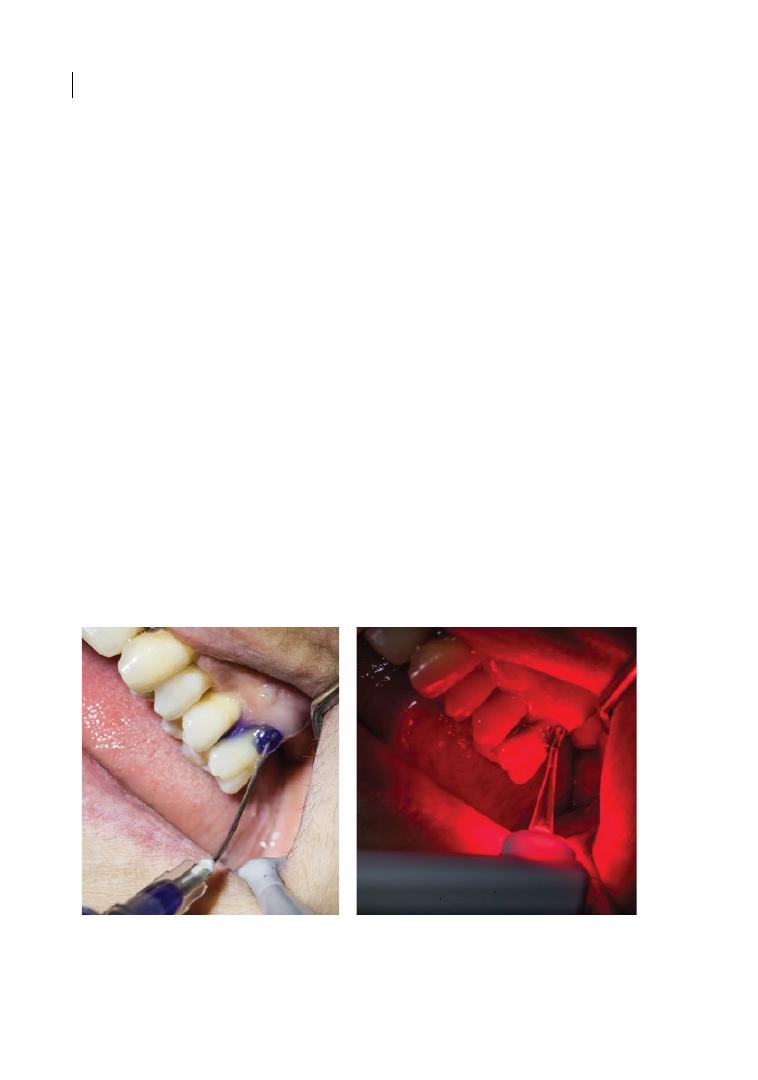
Chapter 10
194
pockets is possible in vitro by using TBO and
a 635 nm laser activation (Qin et al. 2008).
Despite some promising animal models and
clinical studies, a systematic review and
meta‐analysis of seven RCTs using PDT as an
adjunct or alternative to subgingival debride-
ment showed no significant clinical benefits,
with reduction in some bacteria in some
studies (Porphyromonas gingivalis) but not
in others, and no consistent adverse events
reported (Sgolastra et al. 2013a). The same
group performed a new systematic review
that included both RCTs and parallel‐design
studies (Sgolastra et al. 2013b). After remov-
ing outlier studies, the meta‐analysis indi-
cated a significant positive effect in terms of
PPD reduction (0.19, 95% confidence inter-
val [CI] 0.007–0.31, p = 0.002) and CAL gain
(0.37, 95% CI 0.26–0.47, p < 0.0001) at three
months. However, the clinical significance of
these data is limited and no differences were
observed at six months. Remarkably, sub-
group analysis revealed that studies adopting
a time of application of 60 seconds showed a
higher and significant PPD reduction and
CAL gain.
Owing to the difficult access for mechan-
ical debridement, furcation defects repre-
sent an ideal scenario where PDT could be
helpful during initial therapy, surgical ther-
apy, or maintenance in order to reduce
bacterial load and promote healing. An
advantage of PDT includes the possibility
to apply it topically into a periodontal
pocket, thus avoiding overdose and reduc-
ing the probability of side effects and
microflora disturbance in other sites of the
oral cavity (which are instead associated
with systemic antimicrobials; Wainwright
1998; Hamblin and Hasan 2004). Figure 10.1
shows a case of furcation involvement
treated with PDT.
De Almeida and co‐workers (2008) histo-
metrically assessed the influence of PDT on
bone loss in furcation areas of rats with experi-
mentally induced periodontitis. The PDT
group demonstrated less bone loss compared
to the control group, the group treated only
with topical MB, and the group treated only
with LLLT at 7 days (1.986 ± 0.417 mm
2
). At 15
days, the PDT (1.641 ± 0.115 mm
2
) and MB
groups (1.991 ± 0.294 mm
2
) demonstrated
(a)
(b)
Figure 10.1
Upper left first molar (UL6) of patient with chronic periodontitis, affected by degree II FI and
treated with photodynamic therapy, following non‐surgical furcation debridement with ultrasonic devices.
(a) Application of dye (methylene blue) inside the furcation area; (b) activation of the dye by photodynamic
therapy light.

Innovative and Adjunctive Furcation Therapy 195
less bone loss compared to the control
(4.062 ± 0.416 mm
2
) and LLLT (2.641 ± 0.849 mm
2
)
groups.
To the best of our knowledge, only a few
RCTs have evaluated PDT specifically in
molars with FI. A double‐blind RCT evalu-
ated PDT for the treatment of class II furca-
tions in patients with chronic periodontitis
(Luchesi et al. 2013); 21 patients who under-
went SRP with the adjunct of the photosen-
sitizer only (control) and 16 patients who
underwent SRP followed by PDT (test)
completed the six‐month follow‐up. While
PDT was able to reduce gingival crevicular
fluid (GCF) levels of inflammatory media-
tors and the concentration of P. gingivalis
and Tannerella forsythia at six months, no
significant differences in terms of CAL and
PPD were detected between the two groups
at either three or six months. Nevertheless,
when interpreting these results we need to
consider that the study lacked a true nega-
tive control group, as the presence of the
photosensitizer dye may have optimized the
results of the control group. Moreover, this
study used a single PDT application as an
adjunct to SRP, while it is plausible that
repeated PDT applications would have
resulted in more positive outcomes (Lulic
et al. 2009). In a split‐mouth RCT, Andrade
et al. (2013) collected data on 14 patients
with bilateral lower molars with class III
furcation lesions scheduled for extraction.
In the control side traditional SRP was per-
formed, while in the test side SRP was fol-
lowed by a session of PDT. At 45 days post
initial therapy, the class III furcation lesions
were surgically accessed, and flap surgery
with SRP and flap surgery with SRP + PDT
was performed in the control and test group,
respectively. At 21 days post surgery, the
newly formed granulation tissue was col-
lected, and real‐time polymerase chain
reaction (PCR) showed a significant up‐
regulation of the TIMP‐, 2/MMP‐2, and
OPG/RANKL mRNA ratios in the test
group, thus suggesting a role for PDT in
positively modulating extracellular matrix
and bone remodelling.
10.5 Air‐polishing Devices
Since in periodontally compromised teeth
root surfaces are subjected to continuous
abrasive instrumentation during lifelong per-
iodontal maintenance therapy, debridement
techniques that are at the same time effective
and minimally invasive should be aimed at.
Keeping this in mind, air‐polishing devices
might represent a valid alternative to
mechanical instrumentation. Air‐abrasive
technology has been applied in the dental
field for more than 60 years (reviewed by
Petersilka 2011). The idea is to use an abra-
sive powder that is introduced into a stream
of compressed air to clean and polish the
tooth surface by removing the deposits
attached to it or by smoothing their texture.
This abrasive process depends on the prop-
erties of the particles applied (shape, geo-
metrical form, hardness) and on the pressure
of the air and water used (Petersilka 2011).
Sodium bicarbonate–based air polishing
has been successfully applied for supragingi-
val plaque and stain removal since the 1980s
(Berkstein et al. 1987; Barnes et al. 1990).
However, the use of sodium bicarbonate on
cement and dentine is not advisable, as sig-
nificant tissue removal may occur (Atkinson
et al. 1984; Horning et al. 1987; Petersilka
et al. 2003a). Furthermore, this type of air‐
polishing device may cause reversible soft‐
tissue irritation and damage, such as
epithelial erosions with exposure of the
underlying connective tissue (Hunter et al.
1989; Kontturi‐Narhi et al. 1989; Kozlovsky
et al. 2005). To overcome these limitations
and minimize the hard‐ and soft‐tissue
trauma, glycine powder air‐polishing devices
have been introduced. Several clinical trials
have evaluated the efficacy of this air‐polish-
ing system for subgingival biofilm removal
with positive results, but no study distin-
guished between single‐ and multi‐rooted
teeth. In a randomized split‐mouth con-
trolled study in patients receiving supportive
periodontal therapy, the use of glycine
powder was more effective in reducing the
number of CFU in comparison to hand

Chapter 10
196
instrumentation with curettes in pockets of
3–5 mm (Petersilka et al. 2003b, c). Another
split‐mouth controlled study did not find
significant differences at a microbiological
level between SRP and subgingival air polish-
ing; however, the use of glycine‐based air
polishing was perceived as less painful/
uncomfortable by the patients and less time
consuming by the operator (Moene et al.
2010). While only shallow pockets (up to
5 mm) were included in these studies, a more
recent study from Flemmig and co‐workers
(2012) considered pockets from 4 to 9 mm.
Their results showed that glycine air polish is
more effective in removing the subgingival
biofilm, and may induce a beneficial shift of
the oral microbiota (lower total viable bacte-
rial count) compared to traditional SRP.
In conclusion, air‐polishing devices might
represent a valid alternative to mechanical
instrumentation, for both periodontal treat-
ment and periodontal maintenance therapy.
The use of glycine powder air polishing
seems well tolerated by the patient and no
severe adverse events have been reported.
However, a few cases of air emphysema (all
successfully healed) have been reported
(Finlayson and Stevens 1988; Fruhauf et al.
2005) and the patient needs to be informed
about this rare complication. New air‐polishing
powders, such as erythritol‐based powders
(Hagi et al. 2013, 2015), are now under inves-
tigations and future randomized trials will
have to confirm their efficacy. Further studies
on molars with FI are advisable to test the
efficacy of this technique specifically for
multi‐rooted teeth.
10.6 Local Antimicrobials
Given the microbial aetiology of periodonti-
tis, antimicrobial adjuncts to mechanical
debridement could be considered a valid
treatment option for periodontal treatment.
Bearing in mind that mechanical subgingival
biofilm disruption with ultrasonic inserts
and curettes is essential for the healing of
periodontal pockets (Badersten et al. 1984),
the additional use of antimicrobial agents
directly in the site could lead to a further
reduction in microbial load and a better heal-
ing of the lesion. Local antimicrobials could
be used as topical applications, for sustained
release (drug delivered in effective concen-
trations for less than 24 hours) or controlled
delivery (drug delivered in effective concen-
trations for more than 24 hours), within
different delivery systems (Herrera et al.
2012; Jepsen and Jepsen 2016). The use of
antiseptics (including chlorhexidine, sodium
hypochlorite, and povidone‐iodine) and anti-
biotics (including tetracyclines and metroni-
dazole) has been reported in the periodontal
literature and several agents are available on
the market. Systematic reviews show that
when local antibiotics are used as adjuncts
to subgingival debridement, short‐term
improvement in clinical parameters meas-
ured as PPD reductions and CAL gain can be
achieved (Hanes and Purvis 2003; Bonito
et al. 2005; Matesanz‐Perez et al. 2013). No
studies with long‐term data on periodontal
stability or tooth loss are available.
As repeatedly observed before, difficulty in
accessing the furcation area for debridement
and the potentially high microbial load inside
the furcation lesions make FI potentially
amenable to the adjunctive use of local anti-
microbials. However, what is the evidence for
the role of local antimicrobials specifically
for the healing and maintenance of FI molars?
Tonetti and co‐workers (1998) included 127
patients with class II mandibular furcation
with BOP in SPT in a randomized multi‐
centre controlled trial. All subjects received
SRP and oral hygiene instructions, while
tests also had tetracycline fibres applied
inside the furcation defects. Subjects were
followed up to six months, when periodontal
clinical measurements were taken for the last
time before the end of the study. The authors
observed that, despite increased reductions
in BOP and PPD in the test group at three
months (compared to controls), no differ-
ences between groups were observed at six
months. Consistent results were observed in
a separate study on 32 patients with chronic
periodontitis, who received initial pocket/
root debridement by ultrasonic instrumentation,

Innovative and Adjunctive Furcation Therapy 197
followed by random assignment to further
treatment by ultrasonic instrumentation
with or without adjunctive local application
of an 8.8% doxycycline gel in residual defects
(Tomasi and Wennstrom 2011). Clinical
examinations were repeated three and nine
months after retreatment. The retreatment
including the local antibiotic resulted in
‘closure’ of 50% of degree I furcation sites,
compared to 29% for sites treated with
mechanical debridement only (p > 0.05), and
in a reduction in depth of degree II furcation
sites of 17% in the test and 11% in the control
group (p > 0.05). No differences for the out-
come variable ‘furcation improvement’ were
detected between the two groups, suggesting
that improvement in molar FI after non‐sur-
gical periodontal therapy was not enhanced
by adjunctive locally applied doxycycline
(Tomasi and Wennstrom 2011).
The use of povidone‐iodine as an adjunct to
SRP in the treatment of class II furcations has
been investigated in two RCTs, but only lim-
ited (Ribeiro Edel et al. 2010) or no additional
clinical benefits (Del Peloso Ribeiro et al.
2006) resulted from the use of this antiseptic.
In a randomized parallel‐arm controlled
study, the clinical efficacy of subgingival
ultrasonic instrumentation irrigated with
essential oils (EOs) was compared with chlo-
rhexidine (CHX) or distilled water (control)
in 45 patients with class II FI (Yilmaz and
Bayindir 2012). When comparing the test
groups (EOs and CHX) to the control group,
no significant differences in the improvement
of periodontal clinical parameters were
reported at one and three months after treat-
ment, with the exception of BOP, which was
significantly reduced in the EOs group com-
pared to the CHX and control groups at both
one and three months. Figure 10.2 shows a
case of a maxillary molar furcation lesion
treated with local antibiotics.
10.7 Systemic Antimicrobials
Systemic antibiotics have been proposed since
the 1970s as adjuncts for the treatment of
periodontitis, initially mainly for early‐onset
forms, thanks to their effect on the subgingi-
val microbiota. Baer and Socransky (1979)
followed up patients with ‘periodontosis’
(what we would now classify as aggressive
periodontitis, AgP), treated with oral hygiene
instructions, non‐surgical, and surgical
approaches associated with adjunctive sys-
temic antibiotics, and concluded that antibiot-
ics such as tetracyclines and penicillin could
be a helpful adjunct to patient management,
including full‐thickness flaps and curettage.
Later, metronidazole was introduced in peri-
odontal therapy for its effect on Aggregatibacter
actinomyecetemcomitans (then known as
Actinobacillus actinomycetemcomitans; Saxen
and Asikainen 1993). Interest in the use of
adjunctive systemic antibiotics increased with
the evidence from laboratory studies, showing
that the rate of metronidazole uptake by
A. actinomyecetemcomitans bacterial cells
simultaneously incubated with amoxicillin
was higher than the uptake in cells incubated
with metronidazole alone (Pavicic et al. 1995).
Hence, several papers were published suggest-
ing improved clinical outcomes (PPD
reductions and CAL gains) when the amoxi-
cillin–metronidazole ‘cocktail’ was used as an
adjunct to SRP (van Winkelhoff et al. 1992;
Winkel et al. 2001). While several randomized
placebo‐controlled trials on the adjunctive use
of amoxicillin and metronidazole flourished,
several other antibiotics or combinations of
them were introduced as adjuncts to perio-
dontal therapy, for both chronic and aggres-
sive periodontitis.
Most original papers and systematic
reviews only report short‐term data (e.g. six
months or twelve months), making it difficult
to understand possible long‐term benefits.
Systematic reviews tend to agree that sys-
temic antibiotics used as adjuncts to SRP
provide clinical improvements (PPD reduc-
tions, CAL gain) compared with SRP alone
or SRP and placebo. These clinical improve-
ments range from 0.3 to 0.5 additional PPD
reductions and 0.2 to 0.4 mm additional CAL
gain (as full‐mouth average), and seem to be
more pronounced in AgP cases (Herrera
et al. 2002, 2012; Sgolastra et al. 2012a, b;
Buset et al. 2015). Studies venturing into
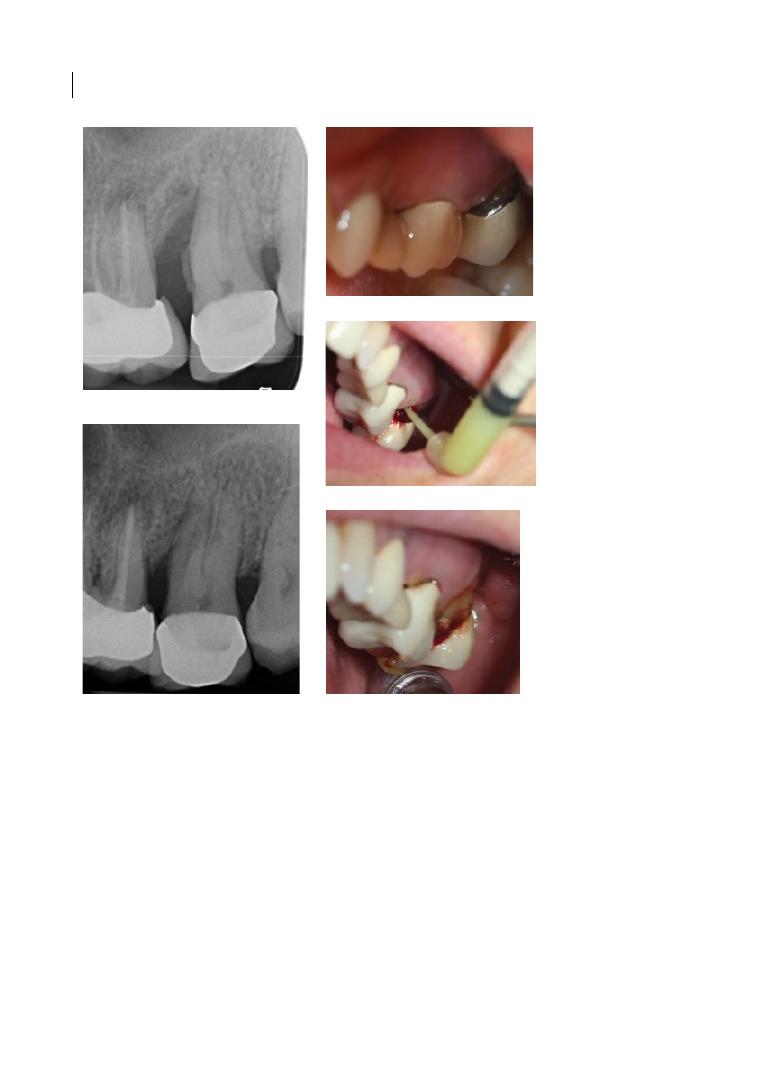
Chapter 10
198
longer‐term follow‐ups show conflicting
evidence. A study on 506 patients with mod-
erate to severe periodontitis observed reduc-
tions in attachment loss favouring the test
group (adjunctive amoxicillin and metroni-
dazole) for up to 27 months of follow‐up
(Harks et al. 2015). A prospective study with
13‐year follow‐up using 250 mg tetracycline
hydrochloride (HCl) four times a day for
three weeks adjunctive to SRP found that
short‐term benefits seemed to disappear
with time (Ramberg et al. 2001). A recent
systematic review highlighted that the clini-
cal benefits of adjunctive systemic antibiotics
seem to diminish over time, from the three‐
month follow‐up to the one‐year follow‐up
(a)
(e)
(b)
(c)
(d)
Figure 10.2
Application of local antibiotic after non‐surgical debridement in degree II mesial furcation lesion
of a maxillary second molar, associated with mesial intrabony defect (10 mm probing pocket depth) and
extensive presence of subgingival deposits. (a) Periapical radiograph; (b) clinical photograph; (c) antibiotic
application from the mesio‐buccal aspect of the pocket associated with the furcation defect; (d) antibiotic
overflowing from the pocket; (e) radiographic re‐evaluation six months after treatment, showing bone fill with
reduction in the intrabony defect depth (now 6 mm probing pocket depth and degree I furcation
involvement).

Innovative and Adjunctive Furcation Therapy 199
(Keestra et al. 2015). Hence, doubts persist
about the long‐term effect of antibiotics used
as adjunctive therapy.
Furthermore, adverse events and risk of
developing antibiotic resistance should be an
important concern when deciding whether
to use adjunctive antibiotic therapy.
Generally, deep pockets seem to benefit
more from an adjunctive antibiotic regime,
as the antibiotic could be of more help in
sites where the effectiveness of mechanical
debridement is more limited (Guerrero et al.
2005). For the same reasons, it can be sup-
posed that teeth affected by FI could benefit
from adjunctive systemic antibiotics.
However, no study seems to have specifically
tested this hypothesis. To the best of our
knowledge, only a subanalysis of data from a
large clinical trial has tried to answer the
question of whether systemic amoxicillin and
metronidazole adjunctively to mechanical
debridement might significantly improve
periodontal clinical parameters at molar and
premolar furcation sites (Eickholz et al.
2016). Although PPD reduction and CAL
gain at furcation sites were noticeably
improved after antibiotic therapy compared
with placebo, no difference in the change of
furcation degrees between the treatments
could be detected.
10.8 Probiotics
When considering the pathogenesis of peri-
odontitis, it is well accepted that this disease
requires the presence of a susceptible host
together with the presence of pathogenic
bacteria (Socransky and Haffajee 1992).
Periodontitis is thought to be the result of a
dysbiotic process, characterized by a shift in
the composition of the normal subgingival
biofilm towards a more pathogenic one
(Hajishengallis and Lamont 2012). Therefore,
there has been growing interest in the possi-
bility of using probiotics with the aim of
shifting the oral microbiota equilibrium back
to a condition of oral health.
Probiotics are defined as ‘living microor-
ganisms which, when administered in ade-
quate amounts, confer a health benefit for
the host’ (FAO/WHO 2001). The most com-
mon probiotics belong to two main genera,
Lactobacillus and Bifidobacterium. The
suggested mechanisms of action of probiot-
ics in the oral cavity include a modulation of
the host immune‐inflammatory response, a
direct inhibition of periodontopathogenic
bacteria via the production of antimicrobial
substances (such as lactic acid, hydrogen per-
oxide, and bacteriocin‐like substances), and
an indirect effect originating from competi-
tive exclusion systems, so that by competing
for the same niches and nutrients, ‘good’ bac-
teria can reduce the chances of pathogens
replicating and adhering to tooth surfaces
(Laleman and Teughels 2015). However,
three systematic reviews have been recently
published to evaluate the overall efficacy of
probiotic therapy in the treatment of perio-
dontally compromised teeth. Matsubara and
co‐workers (2016) included 12 RCTs
(both split mouth and parallel designed) and
concluded that the use of oral probiotics
alone or associated with SRP is well tolerated
(no adverse effects reported) and is associ-
ated with an overall tendency for improved
clinical parameters and reduced levels of
periodontal pathogens. Martin‐Cabezas and
co‐workers (2016) included only RCTs com-
paring SRP alone (or associated with pla-
cebo) to SRP associated with assumption of
Lactobacillus reuteri in the quality assess-
ment. Meta‐analysis showed a statistically
significant CAL gain (‐0.42 mm, p = 0.002)
and BOP reduction (‐14.66, p = 0.003) for
SRP associated with the probiotic treatment
versus SRP alone in the short term.
Furthermore, when stratifying for pocket
depth, the use of probiotics was significantly
beneficial in moderate (‐0.18, p = 0.001) and
deep (‐0.67, p
<
0.001) pockets. Finally,
Gruner and co‐workers (2016) included
RCTs broadly evaluating the efficacy of
any form of probiotic therapy for the
management of caries and periodontitis.

Chapter 10
200
The meta‐analysis reported that probiotics
significantly reduced BOP (standardized
mean difference, SMD: ‐1.15; 95% CI
‐1.68/‐0.62), PPD (SMD: ‐0.86, 95% CI
‐1.55/‐0.17), and gingival index (SMD: ‐0.86;
95% CI ‐1.52/‐0.20), but did not affect plaque
index or CAL. Unfortunately, specific studies
investigating this treatment modality in fur-
cation lesions are missing.
Although clinical data look promising, the
heterogeneity of the available studies in
terms of population included (experimental
gingivitis patients, healthy patients, patients
with chronic periodontitis, patients with
aggressive periodontitis), parameters evalu-
ated (microbiological parameters in plaque
or saliva, plaque index, PPD, CAL, etc.), pro-
tocol adopted (probiotics as monotherapy
vs probiotics after SRP), and probiotics
employed do not allow robust conclusions to
be drawn. Further research is needed to dem-
onstrate the efficacy of certain probiotic
strains in oral health, as well as their desired
concentration and vehicle.
10.9 Surgical Innovations
Periodontal surgical techniques have gradu-
ally striven to become less and less invasive,
in order to reduce morbidity and patient
discomfort. The development of minimally
invasive surgical periodontal techniques
includes the use of microsurgical instru-
ments and magnification, and it is based on
the principle of preserving as much of the
soft tissue as possible (Harrel 1999). Incisions,
flap elevation, and suturing techniques have
been modified by a series of papilla‐preser-
vation techniques (Takei et al. 1985; Cortellini
et al. 1995, 1999) and, more recently, by mini-
mally invasive surgical therapy (Cortellini
and Tonetti 2007, 2009; Trombelli et al.
2009), moving from double flaps to single
flaps. These techniques are aimed mainly at
the treatment of intrabony defects, and have
been shown to yield favourable clinical
results with reduced tissue trauma compared
with traditional surgical techniques.
Remarkably, recent RCTs show that, when a
good stabilization of the blood clot in the
surgical area is achieved, the use of grafting
materials may not add any additional bene-
fits in intrabony defects (Trombelli et al.
2010; Cortellini and Tonetti 2011; Ribeiro
et al. 2011; Mishra et al. 2013). The same
principles of reduction of surgical flap,
minimizing trauma, and stimulating the for-
mation of a stable blood clot could be imple-
mented for surgical approaches to furcations.
However, these techniques are explicitly not
indicated for furcation lesions (Cortellini and
Tonetti 2007), and there seems to be a lack of
specific innovative surgical techniques for
the treatment of furcation‐involved molars.
10.10 Furcation ‘Filling’
Some researchers have attempted closure of
the furcation lesion with the use of restora-
tive materials (e.g. ionomeric cement or cal-
cium hydroxiapatite cement). This defies the
principles of periodontal regeneration and of
maintenance of reduced microbial load in
the furcation region discussed in this book.
Not surprisingly, such therapy has encoun-
tered failure, with worsening in periodontal
clinical measurements and high risk of tooth
loss (Anderegg and Metzler 2000; Fowler and
Breault 2001; Rupprecht et al. 2001). This
stresses once more the importance of allow-
ing plaque removal, either self‐performed or
professional or both, inside the furcation
lesion when regeneration of the furcation
lesion is not feasible.
Conclusion
Researchers and clinicians are striving to
identify more efficient ways for the successful
treatment of periodontitis, and specifically
of molars with furcation involvement.
Keeping in mind the uncontested impor-
tance of oral hygiene instructions and

Innovative and Adjunctive Furcation Therapy 201
subgingival debridement, new technologies
could soon provide a helping hand for the
treatment of complex cases. Tools to improve
the efficacy of intrafurcation biofilm removal,
to reduce the treatment time, and to improve
the patient’s perceived comfort, such as peri-
odontal endoscope, lasers and air‐powder
devices, or antimicrobial agents (local or
systemic antibiotics, photodynamic therapy)
and probiotics have been tested as adjuncts
for the treatment and long‐term mainte-
nance of furcation lesions. Sadly, the evi-
dence for their efficacy in the clinical
outcomes of furcation treatment is still lack-
ing, despite some initial promising results.
Future well‐designed studies are warranted
to shed light on the additional benefits asso-
ciated with these new treatment modalities.
References
Agrawal, T., Gupta, G.K., Rai, V. et al. (2014).
Pre‐conditioning with low‐level laser (light)
therapy: Light before the storm. Dose
Response 12, 619–649. doi:10.2203/dose‐
response.14‐032.Agrawal.
American Academy of Periodontology (2011).
American Academy of Periodontology
statement on the efficacy of lasers in the
non‐surgical treatment of inflammatory
periodontal disease. Journal of
Periodontology 82, 513–514. doi:10.1902/
jop.2011.114001.
Amid, R., Kadkhodazadeh, M., Ahsaie, M.G.,
and Hakakzadeh, A. (2014). Effect of low
level laser therapy on proliferation and
differentiation of the cells contributing in
bone regeneration. Journal of Lasers in
Medical Science 5, 163–170.
Anderegg, C.R., and Metzler, D.G. (2000).
Retention of multi‐rooted teeth with class
III furcation lesions utilizing resins: Report
of 17 cases. Journal of Periodontology 71,
1043–1047. doi:10.1902/jop.2000.71.6.1043.
Anders, J.J., Lanzafame, R.J., and Arany, P.R.
(2015). Low‐level light/laser therapy versus
photobiomodulation therapy. Photomedicine
and Laser Surgery 33, 183–184. doi:10.1089/
pho.2015.9848.
Andrade, P.F., Garlet, G.P., Silva, J.S. et al.
(2013). Adjunct effect of the antimicrobial
photodynamic therapy to an association of
non‐surgical and surgical periodontal
treatment in modulation of gene expression:
A human study. Journal of Photochemistry
and Photobiology B 126, 119–125.
doi:10.1016/j.jphotobiol.2013.06.012.
Atieh, M.A. (2010). Photodynamic therapy as
an adjunctive treatment for chronic
periodontitis: A meta‐analysis. Lasers in
Medical Science 25, 605–613. doi:10.1007/
s10103‐009‐0744‐6.
Atkinson, D.R., Cobb, C.M., and Killoy, W.J.
(1984). The effect of an air‐powder abrasive
system on in vitro root surfaces. Journal of
Periodontology 55, 13–18. doi:10.1902/
jop.1984.55.1.13.
Summary of Evidence
●
The treatment of molars with furcation
involvement desperately needs new meth-
ods to improve clinical efficacy, patient
comfort, and long‐term outcomes.
●
Antimicrobial adjuncts (local antibiotics,
photodynamic therapy) and probiotics
and technology for improved biofilm
removal (periodontal endoscope, lasers,
and air‐powder devices) are being tested,
with the potential of being used for the
treatment of specific furcation cases.
●
Clinical efficacy, costs, and learning
curves for these potential adjuncts still
need to be systematically assessed before
they can potentially be routinely imple-
mented in the treatment of molars with
furcation involvement.

Chapter 10
202
Badersten, A., Nilveus, R., and Egelberg, J.
(1984) Effect of nonsurgical periodontal
therapy. II. Severely advanced periodontitis.
Journal of Clinical Periodontology 11, 63°76.
Baer, P.N., and Socransky, S.S. (1979).
Periodontosis: Case report with long‐
term follow‐up. Periodontal Case Reports 1,
1–6.
Barnes, C.M., Russell, C.M., Gerbo, L.R. et al.
(1990). Effects of an air‐powder polishing
system on orthodontically bracketed and
banded teeth. American Journal of
Orthodontics and Dentofacial Orthopedics
97, 74–81. doi:10.1016/
S0889‐5406(05)81712‐3.
Barone, A., Covani, U., Crespi, R., and
Romanos, G.E. (2002). Root surface
morphological changes after focused versus
defocused CO2 laser irradiation: A scanning
electron microscopy analysis. Journal of
Periodontology 73, 370–373. doi:10.1902/
jop.2002.73.4.370.
Berkstein, S., Reiff, R.L., McKinney, J.F., and
Killoy, W.J. (1987). Supragingival root
surface removal during maintenance
procedures utilizing an air‐powder abrasive
system or hand scaling: An in vitro study.
Journal of Periodontology 58, 327–330.
doi:10.1902/jop.1987.58.5.327.
Blue, C.M., Lenton, P., Lunos, S. et al. (2013).
A pilot study comparing the outcome of
scaling/root planing with and without
Perioscope technology. Journal of Dental
Hygiene 87, 152–157.
Bonito, A.J., Lux, L., and Lohr, K.N. (2005).
Impact of local adjuncts to scaling and root
planing in periodontal disease therapy: A
systematic review. Journal of Periodontology
76, 1227–1236. doi:10.1902/
jop.2005.76.8.1227.
Borrajo, J.L., Varela, L.G., Castro, G.L. et al.
(2004). Diode laser (980 nm) as adjunct to
scaling and root planing. Photomedicine and
Laser Surgery 22, 509–512. doi:10.1089/
pho.2004.22.509.
Buset, S.L., Zitzmann, N.U., Weiger, R., and
Walter, C. (2015). Non‐surgical periodontal
therapy supplemented with systemically
administered azithromycin: A systematic
review of RCTs. Clinical Oral Investigations 19,
1763–1775. doi:10.1007/s00784‐015‐1499‐z.
Cobb, C.M. (2016). Is there clinical benefit
from using a diode or Nd:YAG laser in the
treatment of periodontitis? Journal of
Periodontology 87, 1117–1131. doi:10.1902/
jop.2016.160134.
Cortellini, P., Prato, G.P., and Tonetti, M.S.
(1995). The modified papilla preservation
technique: A new surgical approach for
interproximal regenerative procedures.
Journal of Periodontology 66, 261–266.
doi:10.1902/jop.1995.66.4.261.
Cortellini, P., Prato, G.P., and Tonetti, M.S.
(1999). The simplified papilla preservation
flap: A novel surgical approach for the
management of soft tissues in regenerative
procedures. International Journal of
Periodontics and Restorative Dentistry 19,
589–599.
Cortellini, P., and Tonetti, M.S. (2007). A
minimally invasive surgical technique with
an enamel matrix derivative in the
regenerative treatment of intra‐bony
defects: A novel approach to limit morbidity.
Journal of Clinical Periodontology 34, 87–93.
doi:10.1111/j.1600‐051X.2006.01020.x.
Cortellini, P., and Tonetti, M.S. (2009).
Improved wound stability with a modified
minimally invasive surgical technique in the
regenerative treatment of isolated
interdental intrabony defects. Journal of
Clinical Periodontology 36, 157–163.
doi:10.1111/j.1600‐051X.2008.01352.x.
Cortellini, P., and Tonetti, M.S. (2011). Clinical
and radiographic outcomes of the modified
minimally invasive surgical technique with
and without regenerative materials: A
randomized‐controlled trial in intra‐bony
defects. Journal of Clinical Periodontology
38, 365–373.
doi:10.1111/j.1600‐051X.2011.01705.x.
de Almeida, J.M., Theodoro, L.H., Bosco, A.F.
et al. (2008). In vivo effect of photodynamic
therapy on periodontal bone loss in dental
furcations. Journal of Periodontology 79,
1081–1088. doi:10.1902/jop.2008.070456.
de Andrade, A.K., Feist, I.S., Pannuti, C.M.
et al. (2008). Nd:YAG laser clinical assisted

Innovative and Adjunctive Furcation Therapy 203
in class II furcation treatment. Lasers in
Medical Science 23, 341–347. doi:10.1007/
s10103‐007‐0482‐6.
Del Peloso Ribeiro, E., Bittencourt, S.,
Ambrosano, G.M. et al. (2006). Povidone‐
iodine used as an adjunct to non‐surgical
treatment of furcation involvements. Journal
of Periodontology 77, 211–217. doi:10.1902/
jop.2006.050095.
Dogan, G.E., Aksoy, H., Demir, T. et al. (2016).
Clinical and biochemical comparison of
guided tissue regeneration versus guided
tissue regeneration plus low‐level laser
therapy in the treatment of class II furcation
defects: A clinical study. Journal of Cosmetic
Laser Therapy 18, 98–104. doi:10.3109/1476
4172.2015.1114637.
Eberhard, J., Ehlers, H., Falk, W. et al.
(2003). Efficacy of subgingival calculus
removal with Er:YAG laser compared to
mechanical debridement: An in situ study.
Journal of Clinical Periodontology 30,
511–518.
Eickholz, P., Nickles, K., Koch, R. et al. (2016).
Is furcation involvement affected by
adjunctive systemic amoxicillin plus
metronidazole? A clinical trials exploratory
subanalysis. Journal of Clinical
Periodontology 43, 839–848. doi:10.1111/
jcpe.12594.
FAO/WHO (2001). Report of joint FAO/WHO
expert consultation on evaluation of health
and nutritional properties of probiotics in
food including powder milk with live lactic
acid bacteria. Cordoba: Food and
Agriculture Organization/World Health
Organization.
Finlayson, R.S., and Stevens, F.D. (1988).
Subcutaneous facial emphysema secondary
to use of the Cavi‐Jet. Journal of
Periodontology 59, 315–317. doi:10.1902/
jop.1988.59.5.315.
Flemmig, T.F., Arushanov, D., Daubert, D. et al.
(2012). Randomized controlled trial
assessing efficacy and safety of glycine
powder air polishing in moderate‐to‐deep
periodontal pockets. Journal of
Periodontology 83, 444–452. doi:10.1902/
jop.2011.110367.
Fowler, E.B., and Breault, L.G. (2001). Failure
of resin ionomers in the retention of
multi‐rooted teeth with Class III furcation
involvement: A rebuttal case report. Journal
of Periodontology 72, 1084–1091.
doi:10.1902/jop.2001.72.8.1084.
Fruhauf, J., Weinke, R., Pilger, U. et al. (2005).
Soft tissue cervicofacial emphysema after
dental treatment: Report of 2 cases with
emphasis on the differential diagnosis of
angioedema. Archives of Dermatology 141,
1437–1440. doi:10.1001/
archderm.141.11.1437.
Ge, L., Zhang, Y., and Shu, R. (2017).
Er,Cr:YSGG laser application for the
treatment of periodontal furcation
involvements. Photomedicine and Laser
Surgery 35, 92–97. doi:10.1089/
pho.2016.4145.
Geisinger, M.L., Mealey, B.L., Schoolfield, J.,
and Mellonig, J.T. (2007). The effectiveness
of subgingival scaling and root planing: An
evaluation of therapy with and without the
use of the periodontal endoscope. Journal of
Periodontology 78, 22–28. doi:10.1902/
jop.2007.060186.
Gruner, D., Paris, S., and Schwendicke, F.
(2016). Probiotics for managing caries and
periodontitis: Systematic review and
meta‐analysis. Journal of Dentistry 48,
16–25. doi:10.1016/j.jdent.2016.03.002.
Guerrero, A., Griffiths, G.S., Nibali, L. et al.
(2005). Adjunctive benefits of systemic
amoxicillin and metronidazole in non‐
surgical treatment of generalized aggressive
periodontitis: A randomized placebo‐
controlled clinical trial. Journal of Clinical
Periodontology 32, 1096–1107.
doi:10.1111/j.1600‐051X.2005.00814.x.
Gursoy, H., Ozcakir‐Tomruk, C., Tanalp, J.,
and Yilmaz, S. (2013). Photodynamic
therapy in dentistry: A literature review.
Clinical Oral Investigations 17, 1113–1125.
doi:10.1007/s00784‐012‐0845‐7.
Hagi, T.T., Hofmanner, P., Eick, S. et al. (2015).
The effects of erythritol air‐polishing powder
on microbiologic and clinical outcomes
during supportive periodontal therapy: Six‐
month results of a randomized controlled

Chapter 10
204
clinical trial. Quintessence International 46,
31–41. doi:10.3290/j.qi.a32817.
Hagi, T.T., Hofmanner, P., Salvi, G.E. et al.
(2013). Clinical outcomes following
subgingival application of a novel erythritol
powder by means of air polishing in
supportive periodontal therapy: A
randomized, controlled clinical study.
Quintessence International 44, 753°761.
doi:10.3290/j.qi.a30606.
Hajishengallis, G., and Lamont, R.J. (2012).
Beyond the red complex and into more
complexity: The polymicrobial synergy and
dysbiosis (PSD) model of periodontal
disease etiology. Molecular Oral
Microbiology 27, 409–419.
doi:10.1111/j.2041‐1014.2012.00663.x.
Hamblin, M.R., and Hasan, T. (2004).
Photodynamic therapy: A new antimicrobial
approach to infectious disease?
Photochemical and Photobiological Sciences
3, 436–450. doi:10.1039/b311900a.
Hanes, P.J., and Purvis, J.P. (2003). Local
anti‐infective therapy: Pharmacological
agents. A systematic review. Annals of
Periodontology 8, 79–98. doi:10.1902/
annals.2003.8.1.79.
Harks, I., Koch, R., Eickholz, P. et al. (2015). Is
progression of periodontitis relevantly
influenced by systemic antibiotics? A
clinical randomized trial. Journal of Clinical
Periodontology 42, 832–842. doi:10.1111/
jcpe.12441.
Harrel, S.K. (1999). A minimally invasive
surgical approach for periodontal
regeneration: Surgical technique and
observations. Journal of Periodontology 70,
1547–1557. doi:10.1902/
jop.1999.70.12.1547.
Herrera, D., Matesanz, P., Bascones‐Martinez,
A., and Sanz, M. (2012). Local and systemic
antimicrobial therapy in periodontics.
Journal of Evidence Based Dental Practice
12, 50–60. doi:10.1016/
S1532‐3382(12)70013‐1.
Herrera, D., Sanz, M., Jepsen, S. et al. (2002). A
systematic review on the effect of systemic
antimicrobials as an adjunct to scaling and
root planing in periodontitis patients.
Journal of Clinical Periodontology 29 (Suppl.
3), 136–159; discussion 160–162.
Horning, G.M., Cobb, C.M., and Killoy, W.J.
(1987). Effect of an air‐powder abrasive
system on root surfaces in periodontal
surgery. Journal of Clinical Periodontology
14, 213–220.
Hunter, K.M., Holborow, D.W., Kardos, T.B.
et al. (1989). Bacteraemia and tissue damage
resulting from air polishing. British Dental
Journal 167, 275–278.
Jepsen, K., and Jepsen, S. (2016). Antibiotics/
antimicrobials: Systemic and local
administration in the therapy of mild to
moderately advanced periodontitis.
Periodontology 2000 71, 82–112.
doi:10.1111/prd.12121.
Keestra, J.A., Grosjean, I., Coucke, W. et al.
(2015). Non‐surgical periodontal therapy
with systemic antibiotics in patients with
untreated chronic periodontitis:
A systematic review and meta‐analysis.
Journal of Periodontal Research 50,
294–314. doi:10.1111/jre.12221.
Konopka, K., and Goslinski, T. (2007).
Photodynamic therapy in dentistry. Journal
of Dental Research 86, 694–707.
Kontturi‐Narhi, V., Markkanen, S., and
Markkanen, H. (1989). The gingival effects
of dental airpolishing as evaluated by
scanning electron microscopy. Journal of
Periodontology 60, 19–22. doi:10.1902/
jop.1989.60.1.19.
Kozlovsky, A., Artzi, Z., Nemcovsky, C.E., and
Hirshberg, A. (2005). Effect of air‐polishing
devices on the gingiva: Histologic study in
the canine. Journal of Clinical
Periodontology 32, 329–334.
doi:10.1111/j.1600‐051X.2005.00678.x.
Kwan, J.Y. (2005). Enhanced periodontal
debridement with the use of micro ultrasonic,
periodontal endoscopy. Journal of the
California Dental Association 33, 241–248.
Laleman, I., and Teughels, W. (2015).
Probiotics in the dental practice: A review.
Quintessence International 46, 255–264.
doi:10.3290/j.qi.a33182.
Lomke, M.A. (2009). Clinical applications of
dental lasers. General Dentistry 57, 47–59.

Innovative and Adjunctive Furcation Therapy 205
Lopes, B.M., Theodoro, L.H., Melo, R.F. et al.
(2010). Clinical and microbiologic follow‐up
evaluations after non‐surgical periodontal
treatment with erbium:YAG laser and
scaling and root planing. Journal of
Periodontology 81, 682–691. doi:10.1902/
jop.2010.090300.
Luchesi, V.H., Pimentel, S.P., Kolbe, M.F. et al.
(2013). Photodynamic therapy in the
treatment of class II furcation: A
randomized controlled clinical trial. Journal
of Clinical Periodontology 40, 781–788.
doi:10.1111/jcpe.12121.
Lulic, M., Leiggener Gorog, I., Salvi, G.E. et al.
(2009). One‐year outcomes of repeated
adjunctive photodynamic therapy during
periodontal maintenance: A proof‐of‐
principle randomized‐controlled clinical
trial. Journal of Clinical Periodontology 36,
661–666.
doi:10.1111/j.1600‐051X.2009.01432.x.
Martignago, C.C., Oliveira, R.F., Pires‐Oliveira,
D.A. et al. (2015). Effect of low‐level laser
therapy on the gene expression of collagen
and vascular endothelial growth factor in a
culture of fibroblast cells in mice. Lasers in
Medical Science 30, 203–208. doi:10.1007/
s10103‐014‐1644‐y.
Martin‐Cabezas, R., Davideau, J.L.,
Tenenbaum, H., and Huck, O. (2016).
Clinical efficacy of probiotics as an
adjunctive therapy to non‐surgical
periodontal treatment of chronic
periodontitis: A systematic review and
meta‐analysis. Journal of Clinical
Periodontology 43, 520–530. doi:10.1111/
jcpe.12545.
Matesanz‐Perez, P., Garcia‐Gargallo, M.,
Figuero, E. et al. (2013). A systematic review
on the effects of local antimicrobials as
adjuncts to subgingival debridement, compared
with subgingival debridement alone, in the
treatment of chronic periodontitis. Journal
of Clinical Periodontology 40, 227–241.
doi:10.1111/jcpe.12026.
Matsubara, V.H., Bandara, H.M., Ishikawa,
K.H. et al. (2016). The role of probiotic
bacteria in managing periodontal disease:
A systematic review. Expert Revies of Anti
Infection Therapy 14, 643–655. doi:10.1080/
14787210.2016.1194198.
Michaud, R.M., Schoolfield, J., Mellonig, J.T.,
and Mealey, B.L. (2007). The efficacy of
subgingival calculus removal with
endoscopy‐aided scaling and root planing: A
study on multirooted teeth. Journal of
Periodontology 78, 2238–2245. doi:10.1902/
jop.2007.070251.
Mishra, A., Avula, H., Pathakota, K.R., and
Avula, J. (2013). Efficacy of modified
minimally invasive surgical technique in the
treatment of human intrabony defects with
or without use of rhPDGF‐BB gel: A
randomized controlled trial. Journal of
Clinical Periodontology 40, 172–179.
doi:10.1111/jcpe.12030.
Moene, R., Decaillet, F., Andersen, E., and
Mombelli, A. (2010). Subgingival plaque
removal using a new air‐polishing device.
Journal of Periodontology 81, 79–88.
doi:10.1902/jop.2009.090394.
Moritz, A., Schoop, U., Goharkhay, K. et al.
(1998). Treatment of periodontal pockets
with a diode laser. Lasers in Surgical
Medicine 22, 302–311.
Ozawa, T., Tsuchida, M., Yamazaki, Y. et al.
(1999). Clinical application of a fiberscope
for periodontal lesions: Case reports.
Quintessence International 30, 615–622.
Pavicic, M.J., van Winkelhoff, A.J.,
Pavivic‐Temming, Y.A., and de Graaff, J.
(1995). Metronidazole susceptibility factors
in Actinobacillus actinomycetemcomitans.
Journal of Antimicrobial Chemotherapy
35, 263–269.
Petersilka, G.J. (2011). Subgingival air‐
polishing in the treatment of periodontal
biofilm infections. Periodontology 2000 55,
124–142.
doi:10.1111/j.1600‐0757.2010.00342.x.
Petersilka, G.J., Bell, M., Mehl, A. et al. (2003a).
Root defects following air polishing. Journal
of Clinical Periodontology 30, 165–170.
Petersilka, G.J., Steinmann, D., Haberlein, I.
et al. (2003b). Subgingival plaque removal in
buccal and lingual sites using a novel low
abrasive air‐polishing powder. Journal of
Clinical Periodontology 30, 328–333.

Chapter 10
206
Qin, Y., Luan, X., Bi, L. et al. (2008). Toluidine
blue‐mediated photoinactivation of
periodontal pathogens from supragingival
plaques. Lasers in Medical Science 23,
49–54. doi:10.1007/s10103‐007‐0454‐x.
Ramberg, P., Rosling, B., Serino, G. et al.
(2001). The long‐term effect of systemic
tetracycline used as an adjunct to non‐
surgical treatment of advanced
periodontitis. Journal of Clinical
Periodontology 28, 446–452.
Ribeiro, F.V., Casarin, R.C., Junior, F.H. et al.
(2011). The role of enamel matrix derivative
protein in minimally invasive surgery in
treating intrabony defects in single‐rooted
teeth: A randomized clinical trial. Journal of
Periodontology 82, 522–532. doi:10.1902/
jop.2010.100454.
Ribeiro Edel, P., Bittencourt, S., Sallum, E.A.
et al. (2010). Non‐surgical instrumentation
associated with povidone‐iodine in the
treatment of interproximal furcation
involvements. Journal of Applied Oral
Science 18, 599–606.
Roncati, M., and Gariffo, A. (2014). Systematic
review of the adjunctive use of diode and
Nd:YAG lasers for nonsurgical periodontal
instrumentation. Photomedicine and Laser
Surgery 32, 186–197. doi:10.1089/
pho.2013.3695.
Rupprecht, R.D., Horning, G.M., and Towle,
H.J., III (2001). A clinical evaluation of
hydroxyapatite cement in the treatment of
Class III furcation defects. Journal of
Periodontology 72, 1443–1450. doi:10.1902/
jop.2001.72.10.1443.
Saglam, M., Kantarci, A., Dundar, N., and
Hakki, S.S. (2014). Clinical and biochemical
effects of diode laser as an adjunct to
nonsurgical treatment of chronic
periodontitis: A randomized, controlled
clinical trial. Lasers in Medical Science 29,
37–46. doi:10.1007/s10103‐012‐1230‐0.
Saperia, D., Glassberg, E., Lyons, R.F. et al.
(1986). Demonstration of elevated type I
and type III procollagen mRNA levels in
cutaneous wounds treated with helium‐
neon laser: Proposed mechanism for
enhanced wound healing. Biochemistry and
Biophysics Reserch Communications 138,
1123–1128.
Saxen, L., and Asikainen, S. (1993).
Metronidazole in the treatment of localized
juvenile periodontitis. Journal of Clinical
Periodontology 20, 166–171.
Schwarz, F., Sculean, A., Berakdar, M. et al.
(2003). In vivo and in vitro effects of an
Er:YAG laser, a GaAlAs diode laser, and
scaling and root planing on periodontally
diseased root surfaces: A comparative
histologic study. Lasers in Surgery and
Medicine 32, 359–366. doi:10.1002/
lsm.10179.
Sgolastra, F., Gatto, R., Petrucci, A., and
Monaco, A. (2012a). Effectiveness of
systemic amoxicillin/metronidazole as
adjunctive therapy to scaling and root
planing in the treatment of chronic
periodontitis: A systematic review and
meta‐analysis. Journal of Periodontology 83,
1257–1269. doi:10.1902/jop.2012.110625.
Sgolastra, F., Petrucci, A., Gatto, R., and
Monaco, A. (2012b). Effectiveness of
systemic amoxicillin/metronidazole as an
adjunctive therapy to full‐mouth scaling and
root planing in the treatment of aggressive
periodontitis: A systematic review and
meta‐analysis. Journal of Periodontology 83,
731–743. doi:10.1902/jop.2011.110432.
Sgolastra, F., Petrucci, A., Gatto, R. et al.
(2013a). Photodynamic therapy in the
treatment of chronic periodontitis: A
systematic review and meta‐analysis. Lasers
in Medical Science 28, 669–682. doi:10.1007/
s10103‐011‐1002‐2.
Sgolastra, F., Petrucci, A., Severino, M. et al.
(2013b). Adjunctive photodynamic therapy
to non‐surgical treatment of chronic
periodontitis: A systematic review and
meta‐analysis. Journal of Clinical
Periodontology 40, 514–526. doi:10.1111/
jcpe.12094.
Sgolastra, F., Severino, M., Petrucci, A. et al.
(2014). Nd:YAG laser as an adjunctive
treatment to nonsurgical periodontal
therapy: A meta‐analysis. Lasers in Medical
Science 29, 887–895. doi:10.1007/
s10103‐013‐1293‐6.

Innovative and Adjunctive Furcation Therapy 207
Socransky, S.S., and Haffajee, A.D. (1992). The
bacterial etiology of destructive periodontal
disease: Current concepts. Journal of
Periodontology 63, 322–331. doi:10.1902/
jop.1992.63.4s.322.
Soukos, N.S., and Goodson, J.M. (2011).
Photodynamic therapy in the control of oral
biofilms. Periodontology 2000 55, 143–166.
doi:10.1111/j.1600‐0757.2010.00346.x.
Souza, E., Medeiros, A.C., Gurgel, B.C., and
Sarmento, C. (2016). Antimicrobial
photodynamic therapy in the treatment of
aggressive periodontitis: A systematic
review and meta‐analysis. Lasers in Medical
Science 31, 187–196. doi:10.1007/
s10103‐015‐1836‐0.
Takei, H.H., Han, T.J., Carranza, F.A., Jr et al.
(1985). Flap technique for periodontal bone
implants: Papilla preservation technique.
Journal of Periodontology 56, 204–210.
doi:10.1902/jop.1985.56.4.204.
Taniguchi, Y., Aoki, A., Sakai, K. et al. (2016).
A novel surgical procedure for Er:YAG
laser‐assisted periodontal regenerative
therapy: Case series. International Journal of
Periodontics and Restorative Dentistry 36,
507–515. doi:10.11607/prd.2515.
Tomasi, C., and Wennstrom, J.L. (2011).
Locally delivered doxycycline as an adjunct
to mechanical debridement at retreatment
of periodontal pockets: Outcome at
furcation sites. Journal of Periodontology 82,
210–218. doi:10.1902/jop.2010.100308.
Tonetti, M.S., Cortellini, P., Carnevale, G. et al.
(1998). A controlled multicenter study of
adjunctive use of tetracycline periodontal
fibers in mandibular class II furcations with
persistent bleeding. Journal of Clinical
Periodontology 25, 728–736.
Trombelli, L., Farina, R., Franceschetti, G.,
and Calura, G. (2009). Single‐flap approach
with buccal access in periodontal
reconstructive procedures. Journal of
Periodontology 80, 353–360. doi:10.1902/
jop.2009.080420.
Trombelli, L., Simonelli, A., Pramstraller, M.
et al. (2010). Single flap approach with and
without guided tissue regeneration and a
hydroxyapatite biomaterial in the
management of intraosseous periodontal
defects. Journal of Periodontology 81,
1256–1263. doi:10.1902/jop.2010.100113.
Ustun, K., Erciyas, K., Sezer, U. et al. (2014).
Clinical and biochemical effects of 810 nm
diode laser as an adjunct to periodontal
therapy: A randomized split‐mouth clinical
trial. Photomedicine and Laser Surgery 32,
61–66. doi:10.1089/pho.2013.3506.
van Winkelhoff, A.J., Tijhof, C.J., and de
Graaff, J. (1992). Microbiological and
clinical results of metronidazole plus
amoxicillin therapy in Actinobacillus
actinomycetemcomitans‐associated
periodontitis. Journal of Periodontology 63,
52–57. doi:10.1902/jop.1992.63.1.52.
Verma, S.K., Maheshwari, S., Singh, R.K., and
Chaudhari, P.K. (2012). Laser in dentistry:
An innovative tool in modern dental
practice. National Journal of Maxillofacial
Surgery 3, 124–132.
doi:10.4103/0975‐5950.111342.
Wainwright, M. (1998). Photodynamic
antimicrobial chemotherapy (PACT).
Journal of Antimicrobial Chemotherapy 42,
13–28.
Winkel, E.G., Van Winkelhoff, A.J.,
Timmerman, M.F. et al. (2001). Amoxicillin
plus metronidazole in the treatment of adult
periodontitis patients: A double‐blind
placebo‐controlled study. Journal of Clinical
Periodontology 28, 296–305.
Yaneva, B., Firkova, E., Karaslavova, E., and
Romanos, G.E. (2014). Bactericidal effects of
using a fiber‐less Er:YAG laser system for
treatment of moderate chronic
periodontitis: Preliminary results.
Quintessence International 45, 489–497.
doi:10.3290/j.qi.a31803.
Yilmaz, H.G., and Bayindir, H. (2012).
Clinical evaluation of chlorhexidine and
essential oils for adjunctive effects in
ultrasonic instrumentation of furcation
involvements: A randomized controlled
clinical trial. International Journal of
Dental Hygiene 10, 113–117.
doi:10.1111/j.1601‐5037.2011.00538.x.

Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
Chapter No.: 1 Title Name: <TITLENAME>
c11.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:21:58 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 209
209
11.1 Implants vs Periodontal
Multi‐rooted Teeth: What is
the Clinical Problem?
Previous chapters have unequivocally shown
that in periodontal patients, the posterior
maxilla and mandible are often those areas
that are the worst affected in terms of severity
of periodontal disease and ultimately tooth
loss (Hirschfeld and Wasserman 1978; McFall,
1982; McGuire and Nunn 1996), and that fur-
cation defects are a well‐established local risk
factor for both attachment and tooth loss.
The predictability of either regenerative
(Avila‐Ortiz 2015) or resective (Langer et al.
1981; Carnevale et al. 1991; Blomlöf et al.
1997) surgical management of teeth with fur-
cation involvement (FI) is variable, and is
dependent on a number of local (related to
furcation anatomy) and systemic factors.
Besides the therapeutic advances in the man-
agement of furcation defects, periodontal
treatment success rates are higher in single‐
rooted teeth (Wang et al. 1994), making it
easier to predict their prognosis in compari-
son to multi‐rooted teeth (McGuire 1991).
Therefore, patient‐related factors, treat-
ment cost, and the dentist’s clinical experi-
ence and training often influence the decision
on whether to treat or extract a multi‐rooted
tooth with a furcation defect (Zitzmann et al.
2011; Donos et al. 2012).
The difficulty in assessing the prognosis of
teeth with bone loss beyond the root furca-
tion following periodontal treatment and the
increased popularity of dental implants have
shifted the decision from treating these teeth
towards replacing them with implants. In
other words, why bother with treating diffi-
cult‐to‐treat molars with FI, when we could
extract them and replace them with implants?
The concept of extracting teeth with FI with
questionable prognosis and substituting
them with dental implants is mainly based on
the following clinical assumptions:
●
The lower predictability of furcation treat-
ment in relation to the high morbidity,
time, and cost of such treatments, which
usually involve complex periodontal sur-
gery, endodontic and restorative compo-
nents, and the need for lengthy supportive
periodontal therapy.
●
The higher long‐term survival rates of
implant‐supported restorations (Moraschini
et al. 2015), which, besides their higher cost,
can make them a better restorative solution
in terms of cost versus benefit (Brägger et al.
2005; Bouchard et al. 2009).
●
The potentially superior functional and
aesthetic outcomes of implant‐supported
Chapter 11
Furcation: Why Bother? Treat the Tooth or Extract
and Place an Implant?
Nikos Mardas
1
and Stephen Barter
2
1
Centre for Immunobiology and Regenerative Medicine, Centre for Oral Clinical Research, Institute of Dentistry, Barts and the London
School of Medicine and Dentistry, Queen Mary University of London (QMUL), London, UK
2
Private practice, Eastbourne, UK

Chapter 11
210
restorations over the surgical management
of teeth with FI, which may result in
increased tooth mobility, root hypersensi-
tivity, and gingival recession.
●
An early, ‘strategic’ extraction will prevent
further bone loss and thereby facilitate
implant treatment that may otherwise be
difficult considering the anatomical limita-
tions that are usually present in the poste-
rior maxilla and mandible (Kao 2008).
All these assumptions could be strongly
debated in the light of clinical evidence.
Although reported implant survival rates are
high, they may not surpass the longevity of
periodontally compromised teeth (Donos
et al. 2012), especially in the posterior max-
illa, where a variety of local factors (quality
and quantity of bone, proximity to anatomi-
cal structures, and need for grafting) may
result in reduced implant survival rates
(Drago 1992; Becker et al. 1999; Graziani
et al. 2004, Pjetursson et al. 2008). When
implants are placed in patients with a history
of periodontal disease, they are associated
with a higher incidence of biological compli-
cations (peri‐implantitis), characterized by a
similar pathogenesis and systemic risk
factors (e.g. smoking, diabetes) to periodon-
tal disease.
Consequently, implants in ‘periodontal
patients’ present lower success and probably
lower survival rates than implants placed in
periodontally healthy patients (Donos et al
2012; Sousa et al. 2016a). Severe forms of
periodontal disease, which commonly result
in posterior teeth with advanced furcation
defects, are associated with higher rates of
implant loss (Sousa et al. 2016a) and
increased peri‐implant bone loss around
implants placed to substitute teeth with FI
(Hardt et al. 2002). In the only comparative
study available to date, Fugazzotto (2001)
reported similar success rates of implants
(97%) and root‐resected molars (96.8%) after
0–13 years of function. Furthermore, more
recent cost‐effectiveness studies have shown
that the cost of periodontal therapy is rela-
tively lower than the cost and maintenance of
implants or bridgework (Pretzl et al. 2009;
Fardal et al. 2013). This is of course related to
the additional consideration of the higher
rate of technical complications associated
with implant‐borne prostheses (Brägger
et al., 2005; Albrektsson et al. 2012). This will
be discussed in more detail in Chapter 12.
Finally, we cannot base our decision on
patient preferences or aesthetic outcomes,
since studies comparing aesthetic or patient‐
based outcomes following periodontal treat-
ment for retention of teeth with FI or dental
implants do not exist (Lang et al. 2012).
Based on this evidence, we could claim that
dental implants are not a substitute for furca-
tion‐involved teeth, but rather a solution for
restoring a lost molar when the treatment to
maintain teeth has failed or is not indicated.
Therefore, different considerations should be
involved in the decision‐making process for
each clinical approach, and it cannot simply
be about ‘keeping bone for an implant’ by
removing the tooth early. These considera-
tions include the following:
●
The strategic role of the furcation‐involved
tooth in the overall restorative treatment
plan.
●
The predictability of the periodontal furca-
tion treatment following an estimation of:
– Local factors such as the extent of FI,
the residual attachment levels, presence
of caries, endodontic complications,
and restorative problems.
– Systemic factors such as smoking, dia-
betes, or specific medication that may
influence the longevity of periodontal
teeth (but potentially also of implants).
– The patient’s compliance in maintaining
a high level of oral hygiene and in follow-
ing an intensive supportive periodontal
therapy programme.
●
The predictability of an implant‐supported
restoration following an estimation of:
– Residual bone quantity in relation to
anatomical limitations and the com-
plexity of any bone augmentation pro-
cedures necessary to overcome these
limitations.

Furcation: Why Bother? 211
– Systemic factors that may influence the
longevity of implants or compromise
the results of bone augmentation.
– Patient compliance and their ability to
undergo the necessary surgical and
restorative procedures.
●
Patient’s expectations in terms of aesthetics,
function, duration, and type of treatment.
●
A detailed cost–benefit analysis that
should be presented to the patient and
include initial and maintenance therapies,
as well as the cost for the management of
complications for each clinical approach.
When the decision to extract a tooth with FI
is taken, the clinical issues with placing an
implant are largely centred on whether suf-
ficient residual alveolar bone remains for
the placement of an implant of adequate
length without the need for bone augmenta-
tion. There are different anatomical consid-
erations in each arch and conditions may
have significant variance, even between dif-
ferent sites in the same patient. These are
described later in the chapter, as are the
subquestions regarding what constitutes
‘sufficient’ alveolar bone height and implant
length.
11.2 Anatomical Considerations
for implant Placement in the
Posterior Maxilla and Mandible
11.2.1 Bone ‘Quality’
Leckholm and Zarb (1985) classified the
concept of bone ‘quality’ into four subtypes
depending on the ratio of cortical to cancel-
lous bone, and since then other classifica-
tions of bone quality have been proposed.
However, bone quality is not only deter-
mined by the density of the cortical and
cancellous components. It is characterized
by a combination of factors, such as the
degree of vascularity and cellular vitality, the
quality of the collagen content and mineral
crystal size, plus accumulated microscopic
damage and rate of bone turnover.
It is often assumed that the posterior max-
illa has ‘poor’ bone quality, based on thinner
cortical plates and a less dense trabecular
structure, with increasing adipose content
towards the maxillary tuberosity region
giving a lower bone ‘density’. Some authors
suggest that implant placement in type 4
bone is associated with an increased failure
rate (Goiato et al. 2014). However, higher
rates of implant failure have also been
reported in dense mandibular bone (van
Steenberghe et al. 2003).
Bone density in the posterior mandible can
also be variable. When alveolar ridge width
reduces following tooth loss, the cortical
plates may become closer together, leaving a
smaller trabecular space between them.
Conversely, a medullary compartment with
sparse trabeculation may be found, even in the
presence of thick cortical plates, and achiev-
ing primary implant stability may be difficult.
The lower vascularity of bone with a
smaller trabecular compartment may result
in decreased oxygen tension in the bone and
a reduction in vital osteocytes, with a conse-
quent effect on bone healing and osseo‐inte-
gration (van Steenberghe et al. 2003).
However, there are publications that show
little variation in implant survival rates in
‘poor’‐ or ‘good’‐quality bone, particularly if
the implants have a micro‐roughened surface
(Stanford 2010).
It is possible that a significant confounding
factor is the operator, in that it may be more
difficult to achieve adequate primary implant
stability in soft bone. On the contrary, oste-
otomy preparation may be more challenging
in dense bone, and without careful tech-
nique, sharp drills, and adequate cooling, the
bone may be overheated, causing local necro-
sis and rapid loss of initial mechanical
implant stability, before the biological stabil-
ity achieved by new bone formation
has reached a sufficient state (Bashutski
et al. 2009).
Excessive insertion torque of an implant
placed in dense bone may also cause ‘com-
pression necrosis’ (Chrcanovic and Custódio
2009), with damage to the microvascular
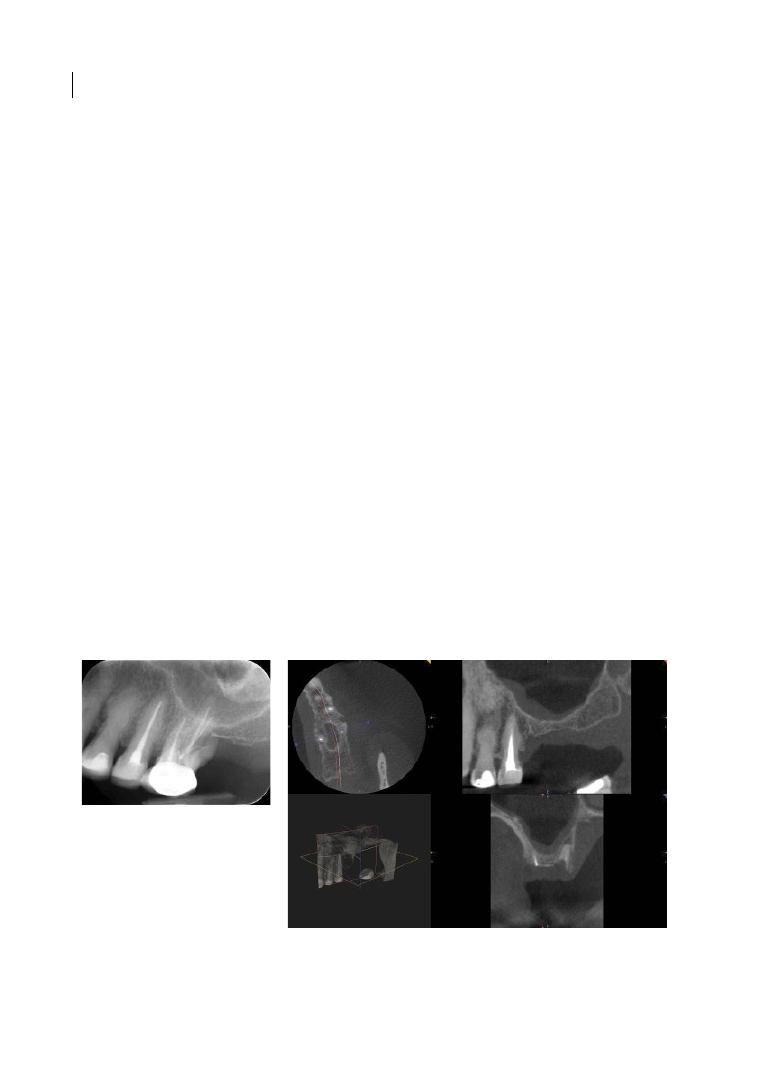
Chapter 11
212
system and trabecular structure beyond the
physiological capacity of bone repair. Similar
mechanisms have been described in ortho-
paedic surgery (Winwood et al. 2006).
Similar criticism has been applied to the
use of osteotomes in the posterior maxilla to
‘improve’ bone density by condensation
rather than drilling in soft bone (Blanco et al.
2008). Compressing bone in this way does
not increase bone density, but may lead to a
need for increased bone remodelling due to
trabecular damage that cannot be fully real-
ized due to microvascular damage.
In conclusion, besides bone quality there
are considerable site‐ and patient‐specific
variables that, together with operator experi-
ence and skill, can have a significant impact
on the outcome of implant placement.
11.2.2 Bone Loss and Implant
Positioning
It is an accepted fact that resorption and
remodelling of the alveolar process takes
place following the extraction of a tooth. The
relative volumes of alveolar and basal bone
loss will be subject to variation between
different individuals and even between
different sites in the same individual, and will
affect the possibility of implant placement
with or without bone augmentation. When a
molar tooth with periodontal disease‐related
bone loss to or beyond the root furcations is
extracted, a greater degree of pre‐extraction
alveolar bone loss will have occurred, result-
ing in an even bigger osseous defect
(Figure 11.1).
Alveolar ridge preservation is a treatment
concept that could potentially reduce the
post‐extraction ridge dimensional changes
(MacBeth et al. 2017), decrease the clinical
need for additional ridge augmentation dur-
ing implant placement, and consequently
facilitate implant placement (Mardas et al.
2015). These potential advantages, however,
may not directly apply to the molar regions,
and the clinician should base their decision
on the accurate diagnosis of all local and
patient‐related factors (i.e. tooth location,
reason for extraction, treatment duration,
healing time, cost–benefit, and patient
expectations and preferences).
Post‐extraction alveolar resorption occurs
in an apico‐lingual direction, moving the
crest of the ridge medially and reducing the
available vertical height of bone (Cawood
(a)
(b)
Figure 11.1
(a) Left maxillary first molar affected by endodontic‐periodontal pathology. (b) Computed
tomography (CT) scan taken after extraction, showing reduction of vertical height, as well as root residuals.

Furcation: Why Bother? 213
and Howell 1988). The former may lead to a
tendency to place the implant too medially in
the bony ridge; the latter may create a
tendency to place the implant at a deeper
vertical position than adjacent teeth in
bounded saddles, or complicate implant
placement due to the proximity of important
anatomical structures.
It is no longer acceptable merely to place
the implant into the available bone volume
and attempt to restore this ‘as well as possi-
ble’ once the implant is integrated. According
to the principle of ‘restoration‐driven implant
placement’, the implant should be installed in
the correct position for the intended restora-
tion. Therefore, whatever the intended
restorative outcome is, the optimal implant
position should be planned at the outset.
Furthermore, the ongoing maintenance of
peri‐implant tissue health should be an
essential part of the pre‐operative planning,
especially in patients who are clearly suscep-
tible to periodontal disease and therefore at a
greater risk of biological complications. The
pre‐operative implant restorative plan should
be based on the following factors:
●
The restoration being in the appropriate
position for a balanced occlusion.
●
The possibility of placing the implant such
that it emerges through attached mucosa.
●
The avoidance of lateral overhangs of the
restoration such as ridge‐lap, either of the
implant‐supported crown or caused by
pink‐coloured gingivae imitating exten-
sions of veneering material. These create
stagnation areas, and may impede effective
oral hygiene by the patient and access for
peri‐implant probing for the clinician.
●
The avoidance of a significant vertical dif-
ference between the bone crest of an adja-
cent tooth and the implant/abutment
interface, which may otherwise result in a
deep soft‐tissue pseudo‐pocket around the
implant, again creating a stagnation area.
●
Even in the posterior region, aesthetics may
still be an issue. Maxillary molar‐to‐molar
smiles are common, particularly in females,
and orthodontic treatment with premolar
extractions may result in mesial movement
of molar teeth, making the region more vis-
ible. While it may not be possible to avoid a
longer implant crown than adjacent teeth,
‘filling’ the buccal corridor with the correct
crown contour may be of aesthetic and
functional importance.
Bone loss renders certain anatomical struc-
tures more superficial and this may create
problems for implant placement. In the max-
illary molar regions, the main issue is the
degree of pneumatization of the maxillary
antra; in the mandible, the position of the
inferior alveolar nerve and submandibular
fossa are the relevant considerations. In clini-
cal practice these anatomical limitations are
usually managed with bone‐augmentation
procedures or reduced‐length implants.
11.3 Implant Placement in the
Mandibular Molar Region
Loss of vertical height of bone in the poste-
rior mandible may occur following extrac-
tion of mandibular multi‐rooted teeth. As
this process occurs, the residual crest of bone
becomes closer to the mandibular canal.
Depending on individual site anatomy, this
can commonly render the inferior alveolar
nerve so superficial that implant placement
can be difficult, or inadvisable.
Damage to the inferior alveolar nerve in
the mandibular canal may occur by compres-
sion, penetration, or transection of the canal,
with either surgical drills or the implant
itself. As the nerve exits the canal, there is
also a risk of iatrogenic damage during flap
preparation, elevation, or retraction.
Careful pre‐surgical investigation is essen-
tial, taking into consideration that there is a
considerable degree of variation in the course
of the inferior alveolar nerve, including bifid
canals, multiple canals, and multiple mental
foramina (Carter and Keen 1971; Naitoh
et al. 2009).

Chapter 11
214
Damage to the inferior alveolar nerve can
take many forms, with all but the most minor
carrying a significant risk of irreversible neu-
ropathy (Seddon 1942). Consequent altered
sensation, often accompanied by neuropathic
pain, can cause a lifelong reduction in quality
of life and significant psychological difficul-
ties (Lam et al. 2003).
It is important to realize that nerve dam-
age can occur even without actual penetra-
tion of the canal, via a compression injury
(neurapraxia) being compounded by
inflammatory oedema within the canal, or
bleeding that, as a consequence of the neu-
rotoxicity of haemoglobin, causes further
neural damage (Regan and Rogers 2003).
Prompt and appropriate action must be
taken in the event of actual or suspected
damage; early intervention can reduce
the risk of lifetime impact (Renton and
Yilmaz 2012).
In cases of multiple tooth loss and extended
edentulous spaces, more advanced vertical
bone loss will also render the submandibular
space more superficial. Within this space are
branches of blood vessels, which, if damaged
by inadvertent perforation of the lingual
mandibular cortex, can cause significant
haemorrhage. In rare cases the airway may
be compromised to a life‐threatening degree
(Niamtu 2001; Dubois et al. 2010).
Accurate pre‐surgical clinical and radio-
logical investigation is therefore para-
mount, and an adequate safety margin must
be maintained between the osteotomy
preparation and important anatomical
features.
11.3.1 Bone Augmentation
for Implant Placement in the
Mandibular Molar Region
When there is inadequate vertical height of
bone over the inferior dental canal for the
safe placement of dental implants, short‐arch
or non‐implant‐supported restoration is
undoubtedly the most predictable option,
and should always be considered rather than
risking iatrogenic nerve damage.
The options available to the clinician for
implant placement in resorbed mandibular
molar sites are:
●
Horizontal augmentation (where vertical
height is not an issue).
●
Vertical augmentation.
●
Use of shorter implants.
The technique of inferior alveolar nerve lat-
eralization will not be discussed, as this is
neither a technique that is commonly used
due to a significant complication rate, nor for
which there is a large body of clinical and
scientific documentation.
Vertical bone‐augmentation techniques
have been described for many years, includ-
ing onlay grafting and distraction osteogen-
esis. However, the ability of these techniques
to predictably regenerate the desired vertical
bone volume is limited, both by anatomical
and biological demands as well as the due to
practical difficulties often encountered by
the operator (Rocchietta et al. 2008).
It is difficult to determine the efficacy of
different techniques due to the usual factors:
few studies, wide variation in techniques and
materials, and a lack of appropriate measure-
ments, even in such basic matters as pre‐ and
post‐augmentation bone levels (Keestra et al.
2016).
A similar criticism can be applied to lateral
bone augmentation (Esposito et al. 2009).
Consequently, it is valid considering the
alternative of short or reduced‐diameter
implants.
11.4 Bone Augmentation
for Implant Placement in the
Maxillary Molar Region
The options available to the clinician for
implant placement in resorbed maxillary
molar sites are:
●
Horizontal augmentation (where vertical
height is not an issue).
●
Subantral augmentation via a lateral
antrostomy (classic ‘sinus lift’).

Furcation: Why Bother? 215
●
Subantral augmentation via implant oste-
otomy (variously called ‘osteotome tech-
nique’, ‘sinus tap’, and other monikers).
●
Use of shorter implants.
11.4.1 Horizontal Bone Augmentation
The concept of horizontal (lateral) bone aug-
mentation is well documented (Donos et al.
2008; Chappuis et al. 2017), with predictable
outcomes provided that the basic tenets of
guided bone regeneration are observed:
●
Space maintenance and form shaping.
●
Blood clot stabilization.
●
Effective compartmentalization of the
graft (prevention of soft‐tissue invasion).
●
Adequate vascular and cell supply.
For the first and second requirements to be
satisfied, the graft material and the mem-
brane have to possess sufficient rigidity to
preserve the form of the graft and eliminate
micro‐motion. This could be achieved in
three‐walled defects that can adequately
contain a particulate graft, or when a block of
augmentation material or a semirigid and
reinforced membrane is used in cases of
non‐space‐containing defects.
It is widely recognized that barrier mem-
brane function is an essential component in
the achievement of a predictable outcome,
particularly when using particulate grafts.
Any free graft has to have an adequate cell
and vascular supply to be viable. This is
largely derived from adequate adjacent
healthy bone. One therefore has to exercise
caution when considering the simultaneous
placement of an implant, which has the
potential to form a barrier between the host
bone and the graft material, depending on
defect morphology.
11.4.2 Sinus Lift
The sinus lift technique was first described
using a lateral antrostomy in 1980 by Boyne
and James, and has since proved to be a safe
and predictable procedure, with no lasting
effect on maxillary sinus health and function
(Timmenga et al. 2003). The general princi-
ple is that access to the antrum is created via
an osseous window and the Schneiderian
membrane is elevated intact, creating a
subantral space into which a biocompatible
scaffold can be inserted to conduct new bone
growth, providing an adequate bed for
implant placement. As with any surgical pro-
cedure there is the potential for complica-
tions; consequently, any surgeon performing
the procedure should be properly trained
and experienced, and able to deal with intra‐
and post‐operative complications.
The main complication leading to chronic
rhino‐sinus symptoms is the perforation of
the Schneiderian membrane, with the poten-
tial escape of graft materials into the sinus.
Loss of graft compartmentalization may
result in an inflammatory reaction, the loss
of patency of the ostium (van den Bergh et al.
2000; Wiltfang et al. 2000; Doud Galli et al.
2001), a compromised mucociliary transport
system, and ultimately a potential need for
graft removal with endoscopic sinus surgery.
The reported incidence of membrane perfo-
ration is from 10% to over 50% of cases
(Timmenga et al. 1997; Block et al. 1998;
Schwartz‐Arad et al. 2004; Pikos 2008;
Pjetursson et al. 2008). There are many
authors who suggest that, up to a certain size,
repair of the ruptured sinus lining with a col-
lagen membrane is possible (Becker et al.
2008); others question the efficacy of this
technique (Aimetti et al. 2001).
Even without membrane perforation, there
is a certain incidence of post‐operative
chronic sinusitis in patients predisposed to
rhino‐sinus disease (Timmenga et al. 1997).
An accurate medical history with relevant
questions is therefore important in patient
assessment.
There is evidence that certain osteocon-
ductive bone grafts, like deproteinized
bovine bone mineral, can provide a bone‐
formation outcome as reliable as autogenous
bone, which removes the need for a donor
site and simplifies the overall procedure con-
siderably (Handschel et al. 2009; Kim et al.
2009). It seems that covering the antrostomy

Chapter 11
216
with a barrier membrane after graft place-
ment tends to result in a better implant prog-
nosis (Jensen and Terheyden 2009), perhaps
because to achieve the optimal restorative
position, the implant tends to be placed as
buccally as possible and the use of mem-
branes appears to have a significant impact
on the amount of soft‐tissue invasion into the
lateral aspect of the graft (Choi et al. 2009).
However, subantral augmentation does not
replace the alveolar bone; it provides a differ-
ent volume of bone to facilitate implant
placement. Depending on the degree of
lateral bone loss, implants placed into this
bone bed may not be closely related to the
positions of the missing teeth. Some clini-
cians suggest that for implant placement to
be appropriate for a fixed reconstruction,
lateral augmentation will also be required
(Chiapasco and Zaniboni 2009). Lateral bone
grafting in such a situation will not always be
practical, particularly if there are adjacent
teeth with bone loss: reconstruction of any
vertical component of bone loss will be tech-
nically difficult and unpredictable.
Implant survival rates are variously quoted
as being equal to (61.7–100%, average 91.8%;
Wallace and Froum 2003) or slightly more var-
iable (73–100% for non‐ augmented sinuses,
36–100% for augmented sinuses on a patient
basis; 75–100% for both non‐ augmented and
augmented sites on an implant basis; Graziani
et al. 2004) than those reported in the unaug-
mented posterior maxilla, suggesting that
variables such as implant type and operator
experience are important factors.
11.4.3 Osteotome‐mediated
Sinus Floor Elevation
This technique was first described by
Summers (1998) and has since seen several
iterations of modifications, in a desire to
simplify the process of subantral augmenta-
tion. The basic theory is that the floor of
the antrum is elevated blindly, via the
implant osteotomy, without perforating the
Schneiderian membrane. Most of the reports
detailing this technique were performed in
situations of significant residual alveolar
ridge height (rAH). When a perforation of
the Schneiderian membrane was suspected,
the implant was placed without any bone
grafts to reduce the risk of sinusitis presented
by graft particles escaping into the sinus;
something that did not influence the implant‐
related outcomes.
A significant determinant of implant suc-
cess using the osteotome technique is the
height of the rAH (Toffler 2004). As rAH
decreases, the need for greater graft height
increases. This means a greater degree of
membrane elevation, which may result in an
increased rate of Schneiderian membrane
perforation (Nkenke et al. 2002; Velloso
2006). However, with this ‘blind’ approach it
is not possible to reliably detect membrane
perforations (Ferrigno et al. 2006; Ardekian
et al. 2006). Other risks associated with the
osteotome technique include benign parox-
ysmal positional vertigo (Iida et al. 2000;
Kaplan et al. 2003; Di Girolamo et al. 2005;
Chiarella et al. 2008; Kim et al. 2010) and loss
of the implant into the sinus where simulta-
neous implant placement is attempted
(Galindo et al. 2005; Chiapasco et al. 2009),
or even several years after implant placement
(Udea and Kaneda 1992; Iida et al. 2000).
Even if a reasonable degree of augmentation
material can be inserted via the implant oste-
otomy without membrane perforation, bone
growth is known to be incremental from the
bony walls, since the vascular and osteoblastic
cell supply derives primarily from there (Jensen
et al. 1998). It has been shown that there is sig-
nificant remodelling of grafts placed using the
osteotome technique, and that actual bone
gain is limited to small amounts of additional
bone on the walls of the implant, with little
bone at the apex after 12 months (Brägger et al.
2004; Leblebicioglu et al. 2005). Furthermore,
occlusal load in the posterior maxilla is pri-
marily borne by the cortical plates and dissi-
pated to the palatal bone and zygomatic
process (Gross and Nissan 2001; Gross et al.
2001; Yacoub et al. 2002). The degree of bone
contact on the implant is of course determined
by available rAH, and also by the amount of
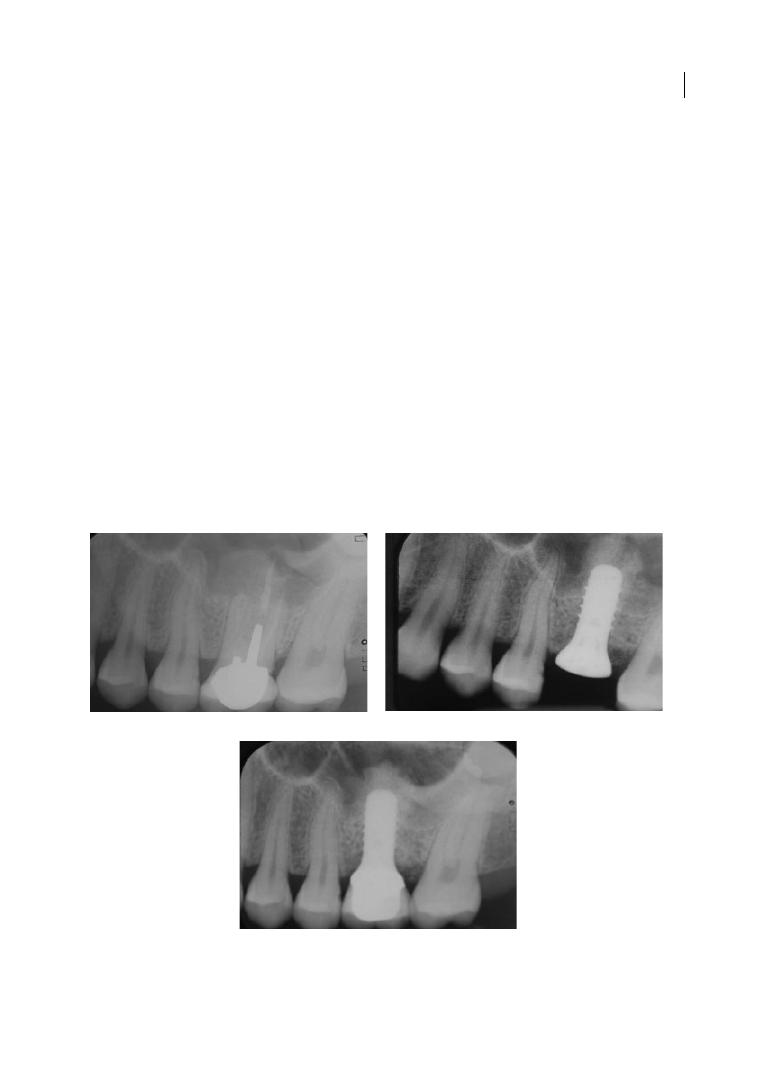
Furcation: Why Bother? 217
graft; however, if that graft is not in contact
with the walls of the sinus, contribution to
load‐bearing capacity is insignificant (Tepper
et al. 2002). The quality of regenerated bone is
also important in reducing the stress in the
native bone, which could otherwise lead to
crestal bone resorption (Fanascu et al. 2003).
This has raised questions of whether there is
a critical height of bone at which there is an
advantage of the osteotome technique over the
lateral window approach in terms of implant
survival. In a meta‐regression analysis of the
two approaches, it was noted that there was a
correlation between bone height and implant
survival, with an rAH of 4 mm, in the lateral
window approach. There was no correlation
with the osteotome approach, but the rAH and
implant survival rate were highly variable in
the included studies, with many different tech-
niques being used (Chao et al. 2010).
Besides the technique’s extensive use, the
‘evidence’ supporting the osteotome‐mediated
sinus floor elevation has a significant degree
of heterogeneity and drawing meaningful
conclusions is difficult (Tan et al. 2008).
Similar outcomes in terms of implant sur-
vival can be achieved with short implants,
and some authors have suggested that there
may not be any harm in placing an implant
that penetrates the antral floor (Brånemark
et al. 1984; Pierreisnard et al. 2003).
Given the increased morbidity and overall
treatment time scale associated with the lat-
eral window approach to sinus floor augmen-
tation, and the doubt surrounding the value
of the osteotome technique, it is highly rele-
vant to question the need for such additional
surgery if it can be avoided. An example of
osteotome‐mediated sinus elevation and
implant placement is provided in Figure 11.2.
(a)
(b)
(c)
Figure 11.2
(a) Left maxillary first molar affected by root fracture. (b) Implant placement was carried out three
months after extraction, with the use of osteotome‐mediated sinus elevation associated with bone grafts.
(c) An implant‐supported crown was placed three months later.

Chapter 11
218
11.5 Short Implants
Dental implants with an infrabony length
of
≤
8
mm have been defined as short
(Renouard and Nisand 2006), although
‘ultra‐short’ implants are considered to be
those with lengths ≤ 6 mm (Deporter 2013).
Short implants have been suggested as an
alternative to bone augmentation procedures
in the maxillary and mandibular posterior
segments, where the residual post‐extraction
bone volume is limited by anatomical struc-
tures (maxillary sinus, mandibular canal),
but there is sufficient alveolar ridge width to
allow the use of standard implant diameters
of ≥ 3.75 mm. Short implants offer a less inva-
sive treatment approach for patients who are
not able to undergo more complex bone‐
augmentation procedures, thereby minimiz-
ing complication rates, morbidity, cost, and
duration of treatment (Nisand and Renouard
2014; Thoma et al. 2015). On the other hand,
short implants will usually present a higher
crown‐to‐implant ratio, especially in cases
with increased interarch distance that may
lead to unfavourable loading conditions and
more technical complications (Quaranta
et al. 2014). It remains uncertain whether a
high crown‐to‐implant ratio may lead to
excessive crestal bone loss and implant fail-
ure (Garaicoa‐Pazmino et al. 2014). Various
modifications in implant design, surface
technology, and different implant insertion
methods have been suggested to address
these issues (Deporter 2013). Finally, the
clinical implications of peri‐implantitis on
implant prognosis may be more pronounced
in the case of shorter implants compared to
longer implants.
Systematic reviews present comparable
mid‐term survival rates between short and
longer implants with moderate rough sur-
faces (Annibali et al. 2012; Atieh et al. 2012).
Most of the reported failures were predomi-
nantly early, with superior survival rates in
the mandible. However, short implants with
a reduced diameter (<3.75 mm) may have
higher failure rates (up to 10%) after 3–5
years in function (das Neves et al. 2006). For
ultra‐short implants the available data are
limited. One study showed that 6 mm long
implants had an average survival rate of
93.7% following an observation period of at
least one year after placement (Srinivasan
et al. 2014).
Short implants could be considered as a
valid treatment alternative to sinus floor aug-
mentation for the restoration of maxillary
molars, provided that the residual height of
the alveolar ridge is ≥ 5 mm. The 16–18‐month
survival rate for short implants was similar to
that of long implants placed in augmented
sinuses (99% vs 99.5%; Thoma et al. 2015). The
complication rate was significantly higher in
patients receiving sinus
augmentation.
Membrane perforation (Figure 11.3) was
the most common complication, although
this did not seem to compromise implant
survival.
Short implants may also be considered as
an alternative to simultaneous or staged ver-
tical bone augmentation in the posterior
mandible when the residual height of the
alveolar ridge is ≥ 5 mm. Felice et al. (2014)
reported similar survival rates but fewer
complications and peri‐implant crestal bone
loss with 6.6 mm short implants placed in
posterior maxilla in comparison to vertical
augmentation after five years of loading. A
recent systematic review based on only four
randomized controlled trials, mainly by the
same group, concluded that similar survival
rates (95.1% vs 96.2%) and maintenance of
crestal bone level should be expected after
both procedures in the short term (Nisand
et al. 2015). The complication rate was again
higher with vertical augmentation, where
temporary nerve paraesthesia was observed
in 56% of cases, in contrast to only 17% when
short implants were used. Graft fracture,
inability to place long implants, and soft‐
tissue dehiscence were other observed
complications.
Besides the encouraging short‐term results
in terms of survival rates, long‐term data on
short implants as an alternative to sinus or
vertical bone augmentation are still lacking,
and it remains unclear to what extent the
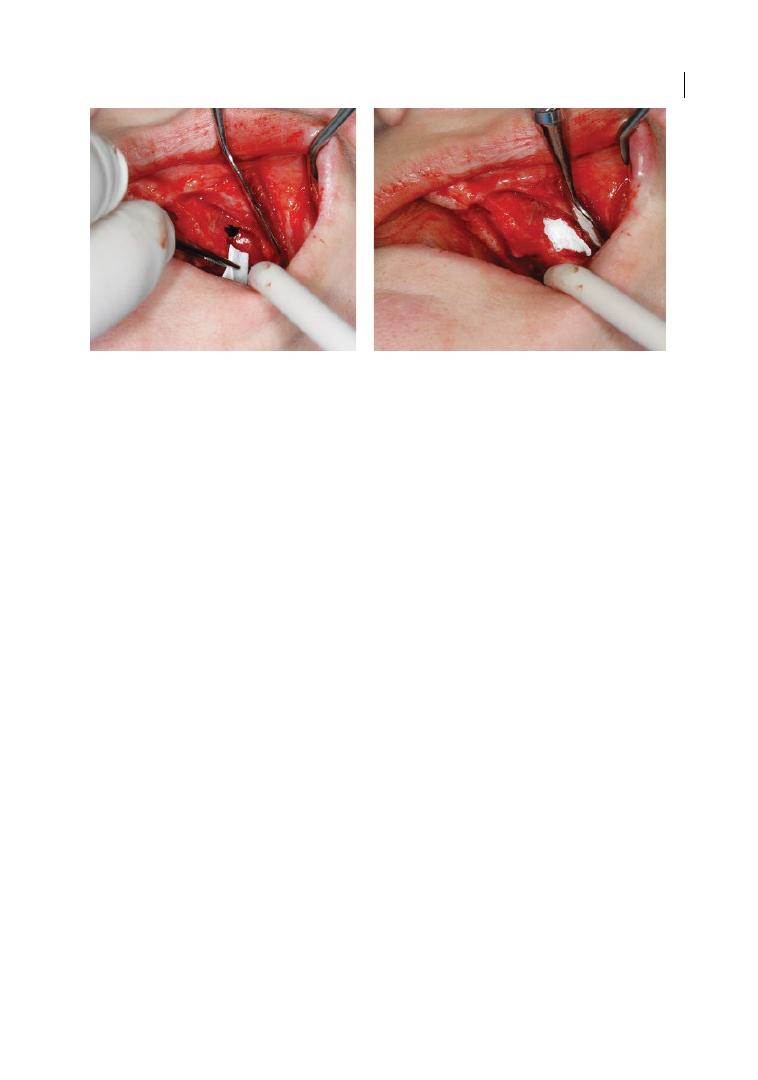
Furcation: Why Bother? 219
type of prosthetic restoration (single or
splinted crowns, cantilevers) may influence
bone levels or implant survival. For this rea-
son, it has been recommended that short
implants should be used only if bone quality
is favourable, and that immediate loading
and non‐working side occlusal interferences
are avoided.
11.6 Implant Biological
Complications
Several longitudinal studies report implant
survival rates ranging from 92.8 to 97.1%
over a period up to 10 years (Albrektsson and
Donos 2012; Srinivasan et al. 2014), support-
ing the use of dental implants as a valid treat-
ment option for the replacement of missing
teeth. However, implant failures remain a
possibility. Failures can be divided into bio-
logical (early or primary, and late or second-
ary), mechanical, technical, iatrogenic, and
those related to inadequate patient adapta-
tion (Heitz‐Mayfield et al. 2014).
The biological complications (and specifi-
cally peri‐implantitis) are usually the most
difficult to manage. Peri‐implantitis is a site‐
and patient‐specific, chronic infection that is
initiated by polymicrobial dysbiotic biofilms
(Edmiston et al. 2015; Hajishengallis 2015).
The disease affects both soft and hard tissues
around osseo‐integrated implants, leading to
bone loss and the formation of a peri‐implant
pocket (Zitzmann and Berglundh 2008). The
prevalence of peri‐implantitis was reported
to be of the order of 10% of implants and 20%
of patients (circa 5–10‐year follow‐up after
implant placement; Mombelli et al. 2012),
and from 1 to 47% (estimated weighted mean
[EWM] 22%, 95% confidence interval [CI]
14–30%; Derks and Tomasi 2015). However,
the prevalence of peri‐implantitis was 39.3%
at a patient level in patients with a history of
periodontitis (Marrone et al. 2013), and
lower survival and success rates were
observed in these patients when compared to
periodontally healthy individuals (Sousa
et al. 2016). In addition, the severity and type
of periodontal disease appear to exert a nega-
tive effect on the rate of biological complica-
tions with dental implants (Mengel et al.
2007; Gatti et al. 2008; De Boever et al. 2009;
Levin et al. 2011; Roccuzzo et al. 2014).
Other risk indicators strongly associated
with peri‐implantitis are smoking, excess
cement, poor oral hygiene, and lack of sup-
portive periodontal therapy (Renvert and
Quirynen 2015), whereas there is limited
evidence regarding the association between
diabetes, alcohol consumption, and peri‐
implant diseases, with conflicting and limited
Figure 11.3
Sinus membrane perforation occurred during lateral window sinus elevation approach and
repaired with the use of a resorbable membrane.

Chapter 11
220
evidence for the association with peri‐
implant diseases and absence of keratinized
mucosa, genetic traits, implant surface char-
acteristics, time of loading, and the position
of the implant within the arch (Heitz‐
Mayfield 2008; Dereka et al. 2012; Renvert
and Polyzois 2015).
The therapy of peri‐implantitis is mainly
directed at the disruption and removal of the
biofilm, the resolution of inflammation, and
ultimately the arrest of disease progression
(Heitz‐Mayfield and Mombelli 2014).
Suggested therapies are based on well‐estab-
lished clinical protocols used for the treat-
ment of periodontitis, including various
combinations of mechanical and non‐
mechanical debridement methods with or
without surgical access, and the use of adjunc-
tive therapies such as antibiotics, antiseptics,
and laser treatments (Lindhe and Meyle 2008).
While the elimination of the bacterial patho-
gens and their remnants is vital for clinically
stable outcomes, implant surface topography,
the initial severity of disease, and defect mor-
phology may influence the outcome of non‐
surgical and surgical therapies (Schwarz et al.
2006, 2010; Sousa et al. 2016). Although there
is evidence that some non‐surgical methods
for implant surface decontamination may be
effective at reducing the bacterial load, non‐
surgical treatment is insufficient to treat peri‐
implantitis and does not lead to satisfactory
clinical outcomes (Renvert et al. 2008, 2009).
A meta‐analysis of treatment outcomes has
identified the main surgical procedures that
are predominantly performed: access‐flap
debridement; surgical resection; regeneration
with bone grafts; and guided tissue regenera-
tion (Chan et al. 2014). In the latter case, the
outcomes of regenerative therapy are reported
to vary the most. For this reason, several
clinicians often suggest implant explantation
as the only predictable approach for managing
advanced peri‐implantitis, and advocate that
prevention is the most effective way to man-
age biological complications around implants.
References
Aimetti, M., Romagnoli, R., Ricci, G., and Massei G.
(2001). Maxillary sinus elevation: The effect of
macrolacerations and microlacerations of the
sinus membrane as determined by endoscopy.
International Journal of Periodontics and
Restorative Dentistry 21, 581–589.
Summary of Evidence
●
Dental implants are not an alternative to
periodontal treatment of furcation‐
involved teeth, but rather a restorative
solution when any other treatment to
maintain them in the dentition has failed
or is not indicated.
●
Loss or extraction of furcation‐involved
teeth usually results in extensive bone loss
that may complicate implant placement:
in the maxilla due to sinus pneumatiza-
tion and in the mandible due to the posi-
tion of the inferior alveolar nerve and
submandibular fossa. In clinical practice
these anatomical limitations are usually
managed with extensive bone augmenta-
tion procedures that increase the com-
plexity, cost, and sometimes the treatment
time and risk of complications.
●
Short implants could be an alternative to
extensive vertical bone‐augmentation pro-
cedures; however, their use has not been
adequately evaluated in the long term.
●
Biological complications and especially
peri‐implantitis are quite common in
patients with a history of periodontal
disease. This risk should be extensively
presented and discussed with the patient
before the extraction of teeth with furca-
tion involvement and their replacement
with dental implants, acknowledging at
the same time the limitations of current
peri‐implantitis treatment modalities.

Furcation: Why Bother? 221
Albrektsson, T., Donos, N., and Working
Group 1 (2012). Implant survival and
complications: The third EAO consensus
conference 2012. Clinical Oral Implants
Research 23 (Suppl. 6), 63–65.
Annibali, S., Cristalli, M.P., Dell’Aquila, D. et al.
(2012). Short dental implants: A systematic
review. Journal of Dental Research 91,
25–32.
Ardekian, L., Oved‐Peleg, E., Mactei, E.E., and
Peled, M. (2006). The clinical significance of
sinus membrane perforation during
augmentation of the maxillary sinus. Journal
of Oral and Maxillofacial Surgery 64,
277–282.
Atieh, M.A., Zadeh, H., Stanford, C.M., and
Cooper, L.F. (2012). Survival of short dental
implants for treatment of posterior partial
edentulism: A systematic review.
International Journal of Oral and
Maxillofacial Implants 27, 1323–1331.
Avila‐Ortiz, G., De Buitrago, J.G., and Reddy,
M.S. (2015). Periodontal
regeneration – furcation defects:
A systematic review from the AAP
Regeneration Workshop. Journal of
Periodontology 86 (Suppl. 2), S108–S130.
doi: 10.1902/jop.2015.130677.
Bashutski, J.D., D’Silva, N.J., Wang, H.L.
(2009). Implant compression necrosis:
Current understanding and case report.
Journal of Periodontology 80, 700–704.
doi:10.1902/jop.2009.080581.
Becker, S.T., Terheyden, H., Steinriede, A. et al.
(2008). Prospective observation of 41
peforations of the Schneiderian membrane
during sinus floor elevation. Clinical Oral
Implants Research 19, 1285–1289.
Becker, W., Becker, B.E., Alsuwyed, A., and
Al‐Mubarak, S. (1999). Long‐term
evaluation of 282 implants in maxillary and
mandibular molar positions: A prospective
study. Journal of Periodontology 70,
896–901.
Blanco, J., Suárez, J., Novio, S. et al. (2008).
Histomorphometric assessment in human
cadavers of the peri‐implant bone density in
maxillary tuberosity following implant
placement using osteotome and
conventional techniques. Clinical Oral
Implants Research 19, 505–510.
Block, M., Kent, J., Kallukaran, F. et al. (1998).
Bone maintenance 5 to 10 years after sinus
grafting. Journal of Oral and Maxillofacial
Surgery 56, 706–714.
Blomlöf, L., Jansson, L., Appelgren, R. et al.
(1997). Prognosis and mortality of root‐
resected molars. International Journal of
Periodontics and Restorative Dentistry 17,
190–201.
Bouchard, P., Renouard, F., Bourgeois, D. et al.
(2009). Cost‐effectiveness modeling of
dental implant vs. bridge. Clinical Oral
Implants Research 20, 583–587.
Boyne, P.J., and James, R.A. (1980). Grafting of
the maxillary sinus floor with autogenous
marrow and bone. Journal of Oral Surgery
38, 613–616.
Brägger, U., Gerber, C., Joss, A. et al. (2004).
Patterns of tissue remodelling after
placement of ITI dental implants using an
osteotome technique: A longitudinal
radiographic case cohort study. Clinical
Oral Implants Research 15, 158–166.
Brägger, U., Krenander, P., and Lang, N.P.
(2005). Economic aspects of single‐tooth
replacement. Clinical Oral Implants
Research 16, 335–341.
Brånemark, P.‐I., Adell, R., Albrektsson, T.
et al. (1984). An experimental and clinical
study of osseointegrated implants
penetrating the nasal cavity and maxillary
sinus. Journal of Oral and Maxillofacial
Surgery 42, 497–505.
Carnevale, G., Di Febo, G., Tonelli, M.P. et al.
(1991). A retrospective analysis of the
periodontal‐prosthetic treatment of molars
with interradicular lesions. International
Journal of Periodontics and Restorative
Dentistry 11, 189–205.
Carter, R.B., and Keen, E.N. (1971). The
intramandibular course of the inferior
alveolar nerve. Journal of Anatomy 108,
433–440.
Cawood, J.I., and Howell, R.A. (1988).
A classification of the edentulous jaws.
International Journal of Oral and
Maxillofacial Surgery 17, 232–236.

Chapter 11
222
Chan, H.‐L., Lin, G.‐H., Suarez, F. et al. (2014).
Surgical management of peri‐implantitis:
A systematic review and meta‐analysis of
treatment outcomes. Journal of
Periodontology 85, 1027–1041.
Chao, Y.L., Chen, H.H., Mei, C.C. et al. (2010).
Meta‐regression analysis of the initial bone
height for predicting implant survival rates
of two sinus elevation procedures. Journal of
Clinical Periodontology 37, 456–465.
Chappuis, V., Cavusoglu, Y., Buser, D., and von
Arx, T. (2017). Lateral ridge augmentation
using autogenous block grafts and guided
bone regeneration: A 10‐year prospective
case series study. Clinical Implant Dentistry
and Related Research 19, 85–96.
Chiapasco, M., Felisati, G., Maccari, A. et al.
(2009). The management of complications
following displacement of oral implants in
the paranasal sinuses: A multicenter clinical
report and proposed treatment protocols.
International Journal of Oral and
Maxillofacial Surgery 38, 1273–1278.
Chiapasco, M., and Zaniboni, M. (2009).
Methods to treat the edentulous posterior
maxilla: Implants with sinus grafting.
Journal of Oral and Maxillofacial Surgery,
67, 867–871.
Chiarella, G., Leopardi, G., De Fazio, L. et al.
(2008). Benign paroxysmal positional
vertigo after dental surgery. European
Archives of Oto‐Rhino‐Laryngology 265,
119–122.
Choi, K., Kan, J.Y.K., Boyne, P.J. et al. (2009).
The effects of resorbable membrane on
human maxillary sinus graft: A pilot study.
International Journal of Oral and
Maxillofacial Implants 24, 73–80.
Chrcanovic, B.R., and Custódio, A.L. (2009).
Mandibular fractures associated with
endosteal implants. Oral and Maxillofacial
Surgery 13, 231–238. doi:10.1007/
s10006‐009‐0171‐7.
das Neves, F.D., Fones, D., Bernardes, S.R.
et al. (2006). Short implants: An analysis of
longitudinal studies. International Journal of
Oral and Maxillofacial Implants 21, 86–93.
De Boever, A.L., Quirynen, M., Coucke, W.
et al. (2009). Clinical and radiographic study
of implant treatment outcome in
periodontally susceptible and non‐
susceptible patients: A prospective long‐
term study. Clinical Oral Implants Research
20, 1341–1350.
Deporter D. (2013). Short dental implants:
What works and what doesn’t? A literature
interpretation. International Journal of
Periodontics and Restorative Dentistry 33,
457–464.
Dereka, X., Mardas, N., Chin, S. et al. (2012). A
systematic review on the association
between genetic predisposition and dental
implant biological complications. Clinical
Oral Implants Research 23, 775–788.
Derks, J., and Tomasi, C. (2015). Peri‐implant
health and disease: A systematic review of
current epidemiology. Journal of Clinical
Periodontology 42 (Suppl. 16), S158–S171.
Di Girolamo, M., Napolitano, B., Arullani, C.A.
et al. (2005). Paroxysmal positional vertigo
as a complication of osteotome sinus floor
elevation. European Archives of Oto‐Rhino‐
Laryngology 262, 631–633.
Donos, N., Laurell, L., and Mardas, N. (2012).
Hierarchical decisions on teeth vs. implants
in the periodontitis‐susceptible patient: The
modern dilemma. Periodontology 2000 59,
89–110.
Donos, N., Mardas, N., and Chadha, V. (2008).
Clinical outcomes of implants following lateral
bone augmentation: Systematic assessment of
available options (barrier membranes, bone
grafts, split osteotomy). Journal of Clinical
Periodontology 35, 173–120.
Doud Galli, S.K., Lebowitz, R.A., Giacchi, R.J.
et al. (2001). Chronic sinusitis complicating
sinus lift surgery. American Journal of
Rhinology 15, 181–186.
Drago, C.J. (1992). Rates of osseointegration of
dental implants with regard to anatomical
location. Journal of Prosthodontics 1,
29–31.
Dubois, L., de Lange, J., Baas, E., and Van
Ingen, J. (2010). Excessive bleeding in the
floor of the mouth after endosseous implant
placement: A report of two cases.
International Journal of Oral and
Maxillofacial Surgery 39, 412–415.

Furcation: Why Bother? 223
Edmiston, C.E., McBain, A.J., Roberts, C., and
Leaper, D. (2015). Clinical and
microbiological aspects of biofilm‐
associated surgical site infections. Advances
in Experimental Medicine and Biology 830,
47–67.
Esposito, M., Grusovin, M.G., Felice, P. et al.
(2009). The efficacy of horizontal and
vertical bone augmentation procedures for
dental implants: A Cochrane systematic
review. European Journal of Oral
Implantology 2, 167–184.
Fanuscu, M.I., Iida, K., Caputo, A.A., and
Nishimura, R.D. (2003). Load transfer by an
implant in a sinus‐grafted maxillary model.
International Journal of Oral and
Maxillofacial Implants 18, 667–674.
Fardal, Ø., and Grytten, J. (2013). A
comparison of teeth and implants during
maintenance therapy in terms of the number
of disease‐free years and costs: An in vivo
internal control study. Journal of Clinical
Periodontology 40, 645–651.
Felice, P., Cannizzaro, G., Barausse, C. et al.
(2014). Short implants versus longer
implants in vertically augmented posterior
mandibles: A randomised controlled trial
with 5‐year after loading follow‐up.
European Journal of Oral Implantology 7,
359–369.
Fugazzotto, P.A. (2001). A comparison of the
success of root resected molars and molar
position implants in function in a private
practice: Results of up to 15‐plus years.
Journal of Periodontology 72, 1113–1123.
Galindo, P., Sánchez‐Fernández, E., Avila, G.
et al. (2005). Migration of implants into the
maxillary sinus: Two clinical cases.
International Journal of Oral and
Maxillofacial Implants 20, 291–285.
Garaicoa‐Pazmino, C., Suarez‐Lopez del Amo,
F., Monje, A. et al. (2014). Influenceof
crown/implant ratio on marginal bone loss:
A systematic review. Journal of
Periodontology 85, 1214–1221.
Gatti, C., Gatti, F., Chiapasco, M., and
Esposito, M. (2008). Outcome of dental
implants in partially edentulous patients
with and without a history of periodontitis:
A 5‐year interim analysis of a cohort study.
European Journal of Oral Implantation 1,
45–51.
Goiato, M.C., dos Santos, D.M., Santiago, J.F. Jr
et al. (2014). Longevity of dental implants in
type IV bone: A systematic review.
International Journal of Oral and
Maxillofacial Surgery 43, 1108–1116.
doi:10.1016/j.ijom.2014.02.016.
Graziani, F., Donos, N., Needleman, I. et al.
(2004). Comparison of implant survival
following sinus floor augmentation
procedures with implants placed in pristine
posterior maxillary bone: A systematic
review. Clinical and Oral Implants 15,
677–682.
Gross, M.D., and Nissan, J. (2001). Stress
distribution around maxillary implants in
anatomic photoelastic models of varying
geometry. Part II. Journal of Prosthetic
Dentistry 85, 450–454.
Gross, M.D., Nissan, J., and Samuel, R. (2001).
Stress distribution around maxillary
implants in anatomic photoelastic models of
varying geometry. Part I. Journal of
Prosthetic Dentistry 85, 442–449.
Hajishengallis, G. (2015). Periodontitis: From
microbial immune subversion to systemic
inflammation. Nature Reviews: Immunology
15, 30–44.
Handschel, J., Simonowska, M., Naujoks, C. et al.
(2009). A histomorphometric meta‐analysis of
sinus elevation with various grafting materials.
Head & Face Medicine 5, 12.
Hardt, C.R.E., Gröndahl, K., Lekholm, U., and
Wennström, J.L. (2002). Outcome of
implant therapy in relation to experienced
loss of periodontal bone support. Clinical
Oral Implants Research 13, 488–494.
Heitz‐Mayfield, L.J. (2008). Peri‐implant
diseases: Diagnosis and risk indicators.
Journal of Clinical Periodontology 35 (Suppl.
8), 292–304.
Heitz‐Mayfield, L.J.A., and Mombelli, A. (2014).
The therapy of peri‐implantitis: A systematic
review. International Journal of Oral and
Maxillofacial Implants 29, 325–345.
Heitz‐Mayfield, L.J., Needleman, I., Salvi, G.E.,
and Pjetursson, B.E. (2014). Consensus

Chapter 11
224
statements and clinical recommendations
for prevention and management of biologic
and technical implant complications.
International Journal of Oral and
Maxillofacial Implants 29 (Suppl.),
346–350.
Hirschfeld, A., and Wasserman, B. (1978).
A long‐term survey of tooth loss in 600
treated periodontal patients. Journal of
Periodontology 49, 225–237.
Iida, S., Tanaka, N., Kogo, M., and Matsuya, T.
(2000). Migration of a dental implant into
the maxillary sinus: A case report.
International Journal of Oral and
Maxillofacial Surgery 29, 358–359.
Jensen, O.T., Shulman, L.B., Block, M.S., and
Iacono, V.J. (1998). Report of the Sinus
Consensus Conference of 1996.
International Journal of Oral and
Maxillofacial Implants 13 (Suppl.), 11–45.
Jensen, S.S., and Terheyden, H. (2009). Bone
augmentation procedures in localized
defects in the alveolar ridge: Clinical results
with different bone grafts and bone‐
substitute materials. International Journal of
Oral and Maxillofacial Implants 24,
218–236.
Kao, R.T. (2008). Strategic extraction:
A paradigm shift that is changing our
profession. Journal of Periodontology 79,
971–977.
Kaplan, D.M., Attal, U., and Kraus, M. (2003).
Bilateral benign paroxysmal positional
vertigo following a tooth implantation.
Journal of Laryngology and Otology 117,
312–313.
Keestra, J.A.J., Barry, O., de Jong, L., and Wahl,
G. (2016). Long‐term effects of vertical bone
augmentation: A systematic review. Journal
of Applied Oral Science 24, 3–17.
Kim, M.S., Lee, J.K., Chang, B.S., and Um, H.S.
(2010). Benign paroxysmal positional
vertigo as a complication of sinus floor
elevation. Journal of Periodontology and
Implant Science 40, 86–89.
Kim, Y.K., Yun, P.Y., Kim, S.G., and Lim, S.C.
(2009). Analysis of the healing process in
sinus bone grafting using various grafting
materials. Oral Surgery, Oral Medicine, Oral
Pathology, Oral Radiology, Endodontology
107, 204–211.
Lam, N.P., Donoff, R.B., Kaban, L.B., and
Dodson, T.B. (2003). Patient satisfaction
after trigeminal nerve repair. Oral Surgery,
Oral Medicine, Oral Pathology, Oral
Radiology, Endodontology 95, 538–543.
Lang, N.P., Zitzmann, N.U.; Working Group 3
of the VIII European Workshop on
Periodontology (2012). Clinical research in
implant dentistry: Evaluation of implant‐
supported restorations, aesthetic and
patient‐reported outcomes. Journal of
Clinical Periodontology 39, 133–138.
Langer, B., Stein, S.D., and Wagenberg, B.
(1981). An evaluation of root resections:
A ten‐year study. Journal of Periodontology
52, 719–722.
Leblebicioglu, B., Ersanli, S., Karabuda, C. et al.
(2005). Radiographic evaluation of dental
implants placed using an osteotome
technique. Journal of Periodontology 76,
385–390.
Lekholm, U., and Zarb, G.A. (1985). Patient
selection and preparation. In: Tissue
Integrated Prostheses: Osseointegration in
Clinical Dentistry (ed. P.I. Branemark, G.A.
Zarb, and T. Albrektsson), 199–209.
Chicago, IL: Quintessence.
Levin, L., Ofec, R., Grossmann, Y., and Anner,
R. (2011). Periodontal disease as a risk for
dental implant failure over time: A long‐
term historical cohort study. Journal of
Clinical Periodontology 38, 732–737.
Lindhe, J., and Meyle, J.; Group D of European
Workshop on Periodontology (2008).
Peri‐implant diseases: Consensus Report of
the Sixth European Workshop on
Periodontology. Journal of Clinical
Periodontology 35 (Suppl. 8), 282–285.
doi:10.1111/j.1600‐051X.2008.01283.x.
MacBeth, N., Trullenque‐Eriksson, A., Donos,
N., and Mardas, N. (2017). Hard and soft
tissue changes following alveolar ridge
preservation: A systematic review. Clinical
Oral Implants Research 28, 982–1004.
Mardas, N., Trullenque‐Eriksson, A., MacBeth,
N. et al. (2015). Does ridge preservation
following tooth extraction improve implant

Furcation: Why Bother? 225
treatment outcomes: A systematic review.
Group 4: Therapeutic concepts & methods.
Clinical Oral Implants Research 26 (Suppl.
11), 180–201.
Marrone, A., Lasserre, J., Bercy, P., and Brecx,
M.C. (2013). Prevalence and risk factors for
peri‐implant disease in Belgian adults.
Clinical Oral Implants Research 24,
934–940.
McFall, W.T., Jr (1982). Tooth loss in 100
treated patients with periodontal disease:
A long‐term study. Journal of Periodontology
53: 539–549.
McGuire, M.K. (1991). Prognosis versus
actual outcome: A long‐term survey of 100
treated periodontal patients under
maintenance care. Journal of Periodontology
62, 51–58.
McGuire, M.K., and Nunn, M.E. (1996).
Prognosis versus actual outcome. III. The
effectiveness of clinical parameters in
accurately predicting tooth survival. Journal
of Periodontology 67, 66–74.
Mengel, R., Behle, M., and Flores‐de‐Jacoby, L.
(2007). Osseointegrated implants in subjects
treated for generalized aggressive
periodontitis: 10‐year results of a
prospective, long‐term cohort study. Journal
of Periodontology 78, 2229–2237.
Mombelli, A., Müller, N., and Cionca, N.
(2012). The epidemiology of peri‐
implantitis. Clinical Oral Implants Research
23 (Suppl. 6), 67–76.
Moraschini, V., Poubel, L.A., Ferreira, V.F., and
Barboza Edos, S. (2015). Evaluation of
survival and success rates of dental implants
reported in longitudinal studies with a
follow‐up period of at least 10 years: A
systematic review. International Journal of
Oral and Maxillofacial Surgery 44, 377–388.
Naitoh, M., Hiraiwa, Y., Aimiya, H. et al.
(2009). Accessory mental foramen
assessment using cone‐beam computed
tomography. Oral Surgery, Oral Medicine,
Oral Pathology, Oral Radiology,
Endodontology 107, 289–294.
Niamtu, J., III (2001). Near‐fatal airway
obstruction after routine implant
placement. Oral Surgery, Oral Medicine,
Oral Pathology, Oral Radiology,
Endodontology 92, 597–600.
Nisand, D., Picard, N., and Rocchietta, I.
(2015). Short implants compared to
implants in vertically augmented bone:
A systematic review. Clinical Oral Implants
Research 26 (Suppl. 11), 170–179.
doi:10.1111/clr.12632.
Nisand, D., and Renouard, F. (2014). Short
implant in limited bone volume.
Periodontology 2000 66, 72–96.
Nkenke, E., Schlegel, A., Schultze‐Mosgau, S.
et al. (2002). The endoscopically controlled
osteotome sinus floor elevation: A preliminary
prospective study. International Journal of
Oral and Maxillofacial Implants 17, 557–566.
Pierrisnard, L., Renouard, F., Renault, P., and
Barquins, M. (2003). Influence of implant
length and bicortical anchorage on implant
stress distribution. Clinical Implant
Dentistry and Related Research 5, 254–262.
Pikos, M.A. (2008). Maxillary sinus membrane
repair: Update on technique for large and
complete perforations. Implant Dentistry
17, 24–31.
Pjetursson, B.E., Tan, W.C., Zwahlen, M., and
Lang, N.P. (2008). A systematic review of the
success of sinus floor elevation and survival
of implants inserted in combination with
sinus floor elevation. Journal of Clinical
Periodontology 35, 216–240.
Pretzl, B., Wiedemann, D., Cosgarea, R. et al.
(2009). Effort and costs of tooth
preservation in supportive periodontal
treatment in a German population.
Journal of Clinical Periodontology 36,
669–676.
Quaranta, A., Piemontese, M., Rappelli, G.
et al. (2014). Technical and biological
complications related to crown to implant
ratio: A systematic review. Implant Dentistry
23, 180–187.
Regan, R., and Rogers, B. (2003). Delayed
treatment of haemoglobin neurotoxicity.
Journal of Neurotrauma 20, 111–120.
Renouard, F., and Nisand, D. (2006). Impact of
implant length and diameter on survival
rates. Clinical Oral Implants Research 17
(Suppl. 2), 35–51.

Chapter 11
226
Renton, T., and Yilmaz, Z. (2012). Managing
iatrogenic trigeminal nerve injury: A case
series and review of the literature.
International Journal of Oral and
Maxillofacial Surgery 41, 629–637.
Renvert, S., and Polyzois, I. (2015). Risk
indicators for peri‐implant mucositis:
A systematic literature review. Journal of
Clinical Periodontology 42, S172–S186.
Renvert, S., and Quirynen, M. (2015). Risk
indicators for peri‐implantitis: A narrative
review. Clinical Oral Implants Research 26,
15–44.
Renvert, S., Roos‐Jansåker, A.M., and Claffey,
N. (2008). Non‐surgical treatment of peri‐
implant mucositis and peri‐implantitis:
A literature review. Journal of Clinical
Periodontology 35 (Suppl. 8), 305–315.
doi:10.1111/j.1600‐051X.2008.01276.x.
Renvert, S., Samuelsson, E., Lindahl, C., and
Persson, G.R. (2009). Mechanical non‐
surgical treatment of peri‐implantitis:
A double‐blind randomized longitudinal
clinical study. I: Clinical results. Journal of
Clinical Periodontology 36, 604–609.
doi:10.1111/j.1600‐051X.2009.01421.x.
Rocchietta, I., Fontana, F., and Simion, M.
(2008). Clinical outcomes of vertical bone
augmentation to enable dental implant
placement: A systematic review. Journal of
Clinical Periodontology 35, 203–215.
Roccuzzo, M., Bonino, L., Dalmasso, P., and
Aglietta, M. (2014). Long‐term results of a
three arms prospective cohort study on
implants in periodontally compromised
patients: 10‐year data around sandblasted
and acid‐etched (SLA) surface. Clinical Oral
Implants Research 25, 1105–1112.
Schwartz‐Arad, D., Herzberg, R., and Dolev, E.
(2004). The prevalence of surgical
complications of the sinus graft procedure
and their impact on implant survival.
Journal of Periodontology 75, 511–516.
Schwarz, F., Papanicolau, P., Rothamel, D. et al.
(2006). Influence of plaque biofilm removal
on reestablishment of the biocompatibility
of contaminated titanium surfaces. Journal
of Biomedical Material Research 77,
437–444.
Schwarz, F., Sahm, N., Schwarz, K., and
Becker, J. (2010). Impact of defect
configuration on the clinical outcome
following surgical regenerative therapy of
peri‐implantitis. Journal of Clinical
Periodontology 37, 449–455.
Seddon, H.J. (1942). A classification of nerve
injuries. British Medical Journal 2,
237–239.
Sousa, V., Mardas, N., Farias, B. et al. (2016a).
A systematic review of implant outcomes in
treated periodontitis patients. Clinical Oral
Implants Research 27: 787–844.
Sousa, V., Mardas, N., Spratt, D. et al. (2016b).
Experimental models for contamination of
titanium surfaces and disinfection protocols.
Clinical Oral Implants Research 27,
1233–1242.
Srinivasan, M., Vazquez, L., Rieder, P. et al.
Survival rates of short (6 mm) micro‐rough
surface implants: A review of literature and
meta‐analysis. Clinical Oral Implants
Research 25, 539–545.
Stanford, C.M. (2010). Surface modification of
biomedical and dental implants and the
processes of inflammation, wound healing
and bone formation. International Journal of
Molecular Sciences 11, 354–369.
doi:10.3390/ijms11010354.
Summers, R. (1998). Sinus floor elevation with
osteotomes. Journal of Esthetic Dentistry 10,
164–171.
Tan, W.C., Lang, N.P., Zwahlen, M., and
Pjetursson, B.E. (2008). A systematic review
of the success of sinus floor elevation and
survival of implants inserted in combination
with sinus floor elevation. Part II:
Transalveolar technique. Journal of Clinical
Periodontology 35(Suppl. 8), 241–254.
Tepper, G., Haas, R., Zechner, W. et al. (2002)
Three‐dimensional finite element analysis of
implant stability in the atrophic posterior
maxilla: A mathematical study of the sinus
floor augmentation. Clinical Oral Implants
Research 13, 657–665.
Thoma, D.S., Zeltner, M., Hüsler, J. et al.; EAO
Supplement Working Group 4 (2015). Short
implants versus sinus lifting with longer
implants to restore the posterior maxilla:

Furcation: Why Bother? 227
A systematic review. Clinical Oral Implants
Research 26 (Suppl. 11), 154–169.
Timmenga, N.M., Raghoebar, G.M., Boering,
G., and van Weissenbruch, R. (1997).
Maxillary sinus function after sinus lifts for
the insertion of dental implants. Journal of
Oral and Maxillofacial Surgery 55, 936–939.
Timmenga, N.M., Raghoebar, G.M., Boering,
G. et al. (2003). Maxillary sinus floor
elevation surgery: A clinical, radiographic
and endoscopic evaluation. Clinical Oral
Implants Research 14, 322–328.
Toffler, M. (2004). Osteotome‐mediated sinus
floor elevation: A clinical report.
International Journal of Oral and
Maxillofacial Implants 19, 266–273.
Ueda, M., and Kaneda, T. (1992). Maxillary
sinusitis caused by dental implants: Report
of two cases. Journal of Oral and
Maxillofacial Surgery 50, 285–287.
van den Bergh, J.P., Bruggenkate ten, C.M.,
Disch, F.J., and Tuinzing, D.B. (2000).
Anatomical aspects of sinus floor elevations.
Clinical Oral Implants Research 11,
256–265.
van Steenberghe, D., Quirynen, M., Molly, L.,
and Jacobs, R. (2003). Impact of systemic
diseases and medication on
osseointegration. Periodontology 2000 33,
163–171.
Velloso, G.R., Vidigal, G.M., de Freitas, M.M.
et al. (2006). Tridimensional analysis of
maxillary sinus anatomy related to sinus
lift procedure. Implant Dentistry 15,
192–196.
Wallace, S.S., and Froum, S.J. (2003). Effect of
maxillary sinus augmentation on the
survival of endosseous dental implants: A
systematic review. Annals of Periodontology
8, 328–343.
Wang, H.‐L., Burgett, F.G., Shyr, Y., and
Ramfjord, S. (1994). The influence of molar
furcation involvement and mobility on
future clinical periodontal attachment loss.
Journal of Periodontology 65, 25–29.
Wiltfang, J., Schultze‐Mosgau, S., Merten,
H.A. et al. (2000). Endoscopic and
ultrasonographic evaluation of the
maxillary sinus after combined sinus floor
augmentation and implant insertion. Oral
Surgery, Oral Medicine, Oral Pathology,
Oral Radiology, Endodontology 89,
288–291.
Winwood, K., Zioupos, P., Currey, J.D. et al.
(2006). The importance of the elastic and
plastic components of strain in tensile and
compressive fatigue of human cortical bone
in relation to orthopaedic biomechanics.
Journal of Musculoskeletal and Neuronal
Interactions 6, 134–141.
Yacoub, N., Ismail, Y.H., and Mao, J.J. (2002).
Transmission of bone strain in the
craniofacial bones of edentulous human
skulls upon dental implant loading. Journal
of Prosthetic Dentistry 88, 192–199.
Zitzmann, N.U., and Berglundh, T. (2008).
Definition and prevalence of peri‐implant
diseases. Journal of Clinical Periodontology
35, 286–291.
Zitzmann, N.U., Scherrer, S.S., Weiger, R. et al.
(2011). Preferences of dental care providers
in maintaining compromised teeth in
relation to their professional status:
Implants instead of periodontally involved
maxillary molars? Clinical Oral Implants
Research 22, 143–150.

Chapter No.: 1 Title Name: <TITLENAME>
c12.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:22:08 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 229
229
Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
12.1 Health Economic
Relevance of Furcation
Involvement
A growing number of patients keep the
majority of their teeth throughout their life,
with even multi‐rooted teeth being retained.
In Germany, for example, around one‐third
of adults aged 65 years or older retain all
their first or second molars (Jordan and
Micheelis 2016). This high number of
retained teeth generates periodontal treat-
ment needs (Jordan and Micheelis 2016;
Holtfreter et al. 2010; Kassebaum et al. 2014).
On the other hand, those who loose teeth are
more likely to have them replaced nowadays
than in the past, mainly because the demand
for replacements is growing while replace-
ment via implant‐supported crowns is widely
available (Micheelis and Schiffer 2006;
Roos‐Jansaker et al. 2006).
Both tooth retention and replacement
generate costs, which are relevant not only
for patients (when they bear them on their
own) but also for public or private insurers
(who need to weigh incurring costs against
the resulting health benefit and the demand
from patients for such costly procedures;
that is, their justifiability in both the public
and private domain). Treatment costs are
also relevant from an equity perspective,
since they might determine the utilization
of services, with those who cannot afford
treatment avoiding it, increasing existing
health disparities (Zhong 2010).
In general, systematic periodontal treat-
ment seems to allow long‐term retention of
most periodontally compromised teeth
(Hatch et al. 2001; Loesche et al. 2002; Fardal
et al. 2004; Chambrone and Chambrone
2006; Eickholz et al. 2008; Graetz et al.
2017a,b, 2011, 2013, 2015; Johansson et al.
2013; Salvi et al. 2014). In many cases, such
retention of teeth in subjects with periodon-
titis seems affordable, with costs for support-
ive periodontal therapy (SPT) being limited
(Pretzl et al. 2009; Fardal and Grytten 2013;
Schwendicke et al. 2016b).
In contrast, for multi‐rooted teeth with
furcation involvement (FI) – that is, mainly
compromised molars – long‐term retention
might be more difficult to achieve, with
survival times being possibly correlated with
the degree of FI (Checchi et al. 2002; König
et al. 2002; Dannewitz et al. 2006a; Johansson
et al. 2013; Graetz et al. 2015), as discussed in
Chapter 5. As a result, more complex and
expensive treatments and more frequent SPT
visits are often needed for retaining such
teeth, which might have an impact on the
costs of tooth retention (Pretzl et al., 2009;
Lee et al. 2012; Schwendicke et al. 2014).
Chapter 12
Is it Worth it? Health Economics of Furcation Involvement
Falk Schwendicke
1
and Christian Graetz
2
1
Department of Operative and Preventive Dentistry, Charité‐Universitätsmedizin Berlin, Berlin, Germany
2
Clinic for Conservative Dentistry and Periodontology, Christian‐Albrechts‐University, Kiel, Germany

Chapter 12
230
Given the growing demand for retaining or
replacing molars and the wide availability of
both options in most developed dental set-
tings, costs come into focus. In rationalized
healthcare settings, where funding is limited
and competition for it between sectors is
high, it is important to quantify both the
costs of a disease and the cost‐effectiveness
of different treatment options. For molars
with FI, this means estimating the annual
retention costs, and comparing them with
the alternative costs for tooth replacement
via implant‐supported crowns, or fixed or
removal dental prostheses. Such cost estima-
tion should best account not only for perio-
dontal but also endodontic, restorative, and
prosthetic treatments. Moreover, the func-
tionality (or other kind of utility) of retained
versus replaced or even missing furcation‐
involved molars needs to be known to weigh
it against the expected costs. Last, the evalu-
ation of costs and utilities (or any other
kind of health benefit) should be performed
in time horizons relevant to payers and con-
sumers; that is, long term.
12.2 Health Economic
Analyses
Health economic analyses are typically
defined according to the evaluated outcome
(Vernazza et al. 2012):
●
Cost‐of‐disease studies investigate the
required treatment efforts for managing or
resolving a disease or its symptoms. Such
costs are usually termed direct costs. Cost‐
of‐disease studies further measure indirect
costs (e.g. those for getting to the doctor or
dentist) and opportunity costs (e.g. those for
not being able to work during this time).
●
Cost‐effectiveness studies measure costs
against the effectiveness of a treatment.
Effectiveness usually means a clinical out-
come (survival time of a tooth, retention
time of a restoration), as determined in a
real‐life setting or in rather artificial
(
randomized controlled) settings. Note
that while the latter is termed ‘efficacy’, no
such strict separation is done for health
economic studies.
●
Cost‐utility analyses measure costs against
the resulting utility, like quality‐ or disabil-
ity‐adjusted life years. They involve the
subjective value placed by someone (usu-
ally patients) on a certain health state. One
needs to elicit these utilities, which is not
always easy and has not been widely done
so far with regard to tooth values. (What is
the utility of a filled tooth against a sound
one? What that of a periodontally impaired
but asymptomatic molar against a non‐
compromised molar?)
●
Cost–benefit analyses transform effective-
ness or utility – that is, the health out-
come – into a monetary value. They
theoretically allow comparison of effort
and outcome on the same scale, but their
methodology is not fully accepted and has
only very sparsely been applied in dentistry
so far.
All these types of analyses can be performed
in one of two ways. The first involves the
(re‐)use of primary data, for example from
cohort or controlled studies. For example,
cohort studies allow estimation of the exact
costs incurred for staff (via recording staff
hours and factorizing them with the costs
per hour for different staff) or materials (fac-
torizing unit price and used units). This
micro‐costing allows very detailed and real-
istic cost estimation. In addition, these stud-
ies can estimate the effectiveness of the
performed treatments (how long a tooth was
retained etc.). Randomized controlled trials
also often collect cost data, allowing com-
parison of different treatment strategies (like
scaling and root planing versus open‐flap
debridement).
The second way also involves re‐use of
data, although not in the original framework
of a clinical study, but rather in a mathemati-
cal model. Modelling studies construct a
hypothetical path of a tooth or patients (‘the
model’) reflecting the clinical (natural) path-
way. Teeth or patients can translate from one
to another health status (e.g. molar with FI
without furcation caries → molar with FI

Health Economics of Furcation Involvement 231
with furcation caries), with the chance of
such translation depending on transition
probabilities. For each translation, a treat-
ment is assumed (e.g. application of fluoride
varnish to arrest a lesion, or restoration),
generating costs. Many of these models are
analysed via Monte Carlo micro‐simulations,
which allow parameter uncertainty to be
introduced. This is done via simulating a
number of patients (e.g. 1000), with transi-
tion probabilities (or other uncertain param-
eters) being randomly sampled from a certain
range of the parameter. The sampling of this
population is then simulated again for a
series of times (e.g. 1000), allowing estima-
tion of both the per‐patient and per‐popula-
tion variance. In general, such models allow
investigation of several groups over a longer
time than most clinical trials (randomized
trials rarely follow up patients for decades),
with high validity given that most data are
from reviews and meta‐analyses. They have,
however, limited applicability to other set-
tings than the one analysed, and are only as
valid as the assumptions made. They need
validation via sensitivity analyses, assessing
the impact the uncertainties have on the
finding of a study.
The available health economic studies on
molars with FI are mainly cost‐of‐disease
studies, with data collection in prospectively
or retrospectively assessed cohorts, or cost‐
effectiveness studies, using models to dem-
onstrate the costs and cost‐effectiveness of
different strategies of retaining or replacing
furcation‐involved molars.
12.3 The Costs of
Furcation Involvement
Several clinical studies have attempted to
measure the costs of retaining furcation‐
involved molars. Most of these have followed
molars after successful active periodontal
treatment (APT), which were regularly
retreated as part of the SPT.
For example, a German study retrospec-
tively assessed molars in patients who all had
received initial or follow‐up periodontal
treatments, including subgingival debride-
ment (Graetz et al. 2015). The therapeutic
strategy for these molars was aimed at
improving access to furcations, allowing
regular individual and professional oral
hygiene in these areas (Figure 12.1).
Conservative scaling and root planing
(SRP) had been performed for molars with
probing pocket depths (PPDs) < 5 mm with-
out bleeding on probing, and open‐flap
surgery including furcation debridement if
PPD ≥ 5 mm plus bleeding or PPD ≥ 6 mm
regardless of bleeding was present (Kocher
and Plagmann 1999). Root‐resection therapy
or tunnelling was mainly provided in molars
with advanced bone loss which had received
endodontic treatment, as well as for molars
with furcation caries (Figure 12.2).
Tunnelling was only performed for lower
molars with both lingual and buccal FI degree
II, or FI degree III with limited access for oral
hygiene, often combined with persistent
inflammation, if resection was not possible.
Different retrospective studies demonstrated
long‐term stability for molars with such a
treatment strategy during regular SPT
(Graetz et al. 2017b, 2013, 2015; Figure 12.3).
Costs were assessed in the context of
German healthcare. As such, any costs
occurring to payers (regardless if this was the
statutory insurance, the patient, or their pri-
vate insurer) were estimated using fee items
of German item catalogues. The authors
quantified the resources provided based on
the number of periodontal, restorative,
endodontic, or prosthetic treatments, which
had been ascertained via case records.
Resources and fee item–based costs were
calculated per tooth. Services provided to
more than one tooth (e.g. examination, anti-
biotics) were distributed among the teeth
present. Items charged per tooth (like SRP)
were not distributed.
The study (Schwendicke et al. 2016a)
assessed 2306 molars in 379 patients (mean
initial age 45.7, standard deviation [SD]
10.0 years). The majority of molars (72.8%)
had no PPD > 4 mm after APT. Molars were
followed up for 16.5 (SD 6.8) years. Over this
period of SPT, a mean of 0.07 (SD 0.12) SRP
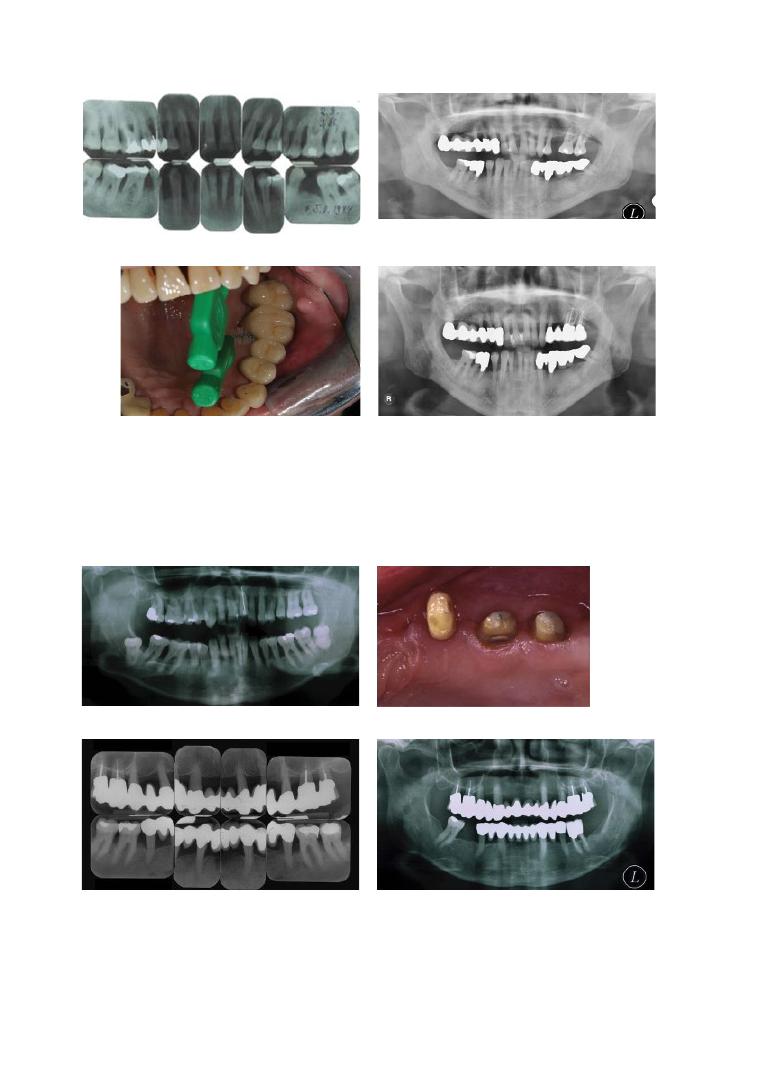
Chapter No.: 1 Title Name: <TITLENAME>
c12.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:22:08 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 232
(a)
(b)
(c)
(d)
Figure 12.2
Woman, aged 36 years and non‐smoker, diagnosed with aggressive periodontitis and horizontal
bone loss in both jaws and furcation involvement degree III (Hamp et al. 1975) of all upper molars and tooth
14 (UR4), as well as degree II (Hamp et al. 1975) of all lower molars (a: initial status). Initial periodontal therapy
with scaling and root planing was followed by open‐flap debridement of all premolars and molars. After re‐
evaluation, supportive periodontal therapy was commenced and after 1.5 years the buccal roots of all upper
molars were resected and fixed dental prostheses provided (b). Two (c) and 17 years later (d), no further
attachment loss was noted. Tooth 46 (LR6) had to be extracted due to a fracture.
(a)
(b)
(c)
(d)
Figure 12.1
Man, aged 42 years and non‐smoker, diagnosed with chronic periodontitis and horizontal bone
loss in both jaws with furcation involvement degree II up to III (Hamp et al. 1975) on all first and second molars
(a: initial status). He received scaling and root planing and open‐flap debridement of all posterior teeth. After
22 years of regular supportive periodontal therapy (b), endodontic treatment was required, with trisection of
the upper right molars and prosthetic reconstruction being performed. Afterwards, furcation cleaning with
interdental brushes (c) was possible. The situation remained stable for a further seven years (d, last observation).
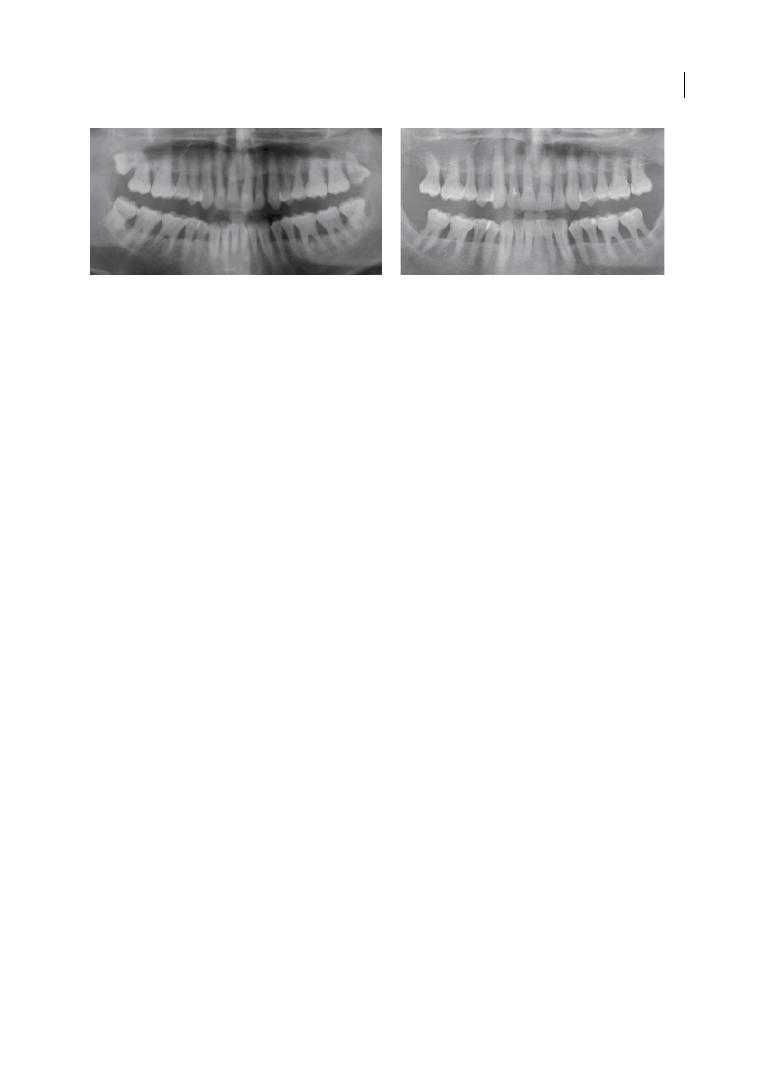
Health Economics of Furcation Involvement 233
had been provided per year (Table 12.1).
Similarly, a mean of 0.04 (SD 0.11) flap
debridements (FD) had been provided per
year. This number was increased in older
subjects, molars with PPD ≥ 5 mm, mobile
molars, and those with prosthetic treatment
initially present. Resections had been mainly
performed in upper molars, molars with
mobility grade 3, FI degree III, bone loss, and
those with endodontic treatment, periapical
lesions, or previous prosthetic treatment.
Few molars received endodontic, restorative,
or prosthetic treatments, with prosthetically
restored molars being more likely to receive
endodontic therapy or prosthetic retreat-
ment. The last component assessed was SPT,
with a mean of 2.49 (SD 0.12) SPT visits per
year and per patient.
Based on these estimates for the resources
used, annual costs per molar were €18.28 (SD
16.91) for all treatments and €13.04 (SD 9.58)
for periodontal treatments only (Table 12.2).
The robustness of these estimates was dem-
onstrated by calculating costs for patients
with different health insurances (which
allows for different charges being claimed by
dentists). Total treatment costs increased
significantly for molars with FI, PPD ≥ 5 mm,
bone loss, endodontic or prosthetic treat-
ment, and periapical lesions. If analysed on a
patient level, mean costs per year were
€137.86 (SD 370.03). There was a significant
association between these costs and smoking
status (costs being higher in current smokers
than non‐smokers).
Another study assessed the tooth‐ retention
efforts in periodontally compromised but
successfully treated subjects over 10 years of
SPT, again within German healthcare (Pretzl
et al. 2009). This study found that 0.34 SRP
had been provided over the 10 years (includ-
ing the first SRP). What can be seen from
both studies is the low number of treatments
needed to retain compromised molars. Total
periodontal treatment costs for tooth reten-
tion ranged between €6 and €13, which is low
given the costs of alternative options (like
implants or fixed dental prostheses). What
was further shown was that periodontal
treatment costs made up around two‐thirds
of the total long‐term costs; that is, most
molars did not generate significant costs for
endodontic or restorative treatment (furca-
tion caries, for example, was found in only
2% of teeth over the whole observation
period). In both studies, the periodontal
treatment efforts were higher in teeth with
bone loss, severe FI, prosthetic abutment sta-
tus, and maxillary molars, but not patients
with aggressive versus chronic periodontitis.
Practitioners should be aware of these
predictors, as they determine not only the
(a)
(b)
Figure 12.3
Man, aged 47 years and smoker; chronic periodontitis and horizontal bone loss in both jaws
with furcation involvement degree II (Hamp et al. 1975) on all upper molars and right lower molars, as well as
degree III (Hamp et al. 1975) on all left lower molars (a: initial status). Open‐flap debridement of all molars,
including tunnelling of the left lower molars, was provided. The patient stopped smoking after the 8th year
during SPT. After 28 years of regular supportive periodontal therapy (b), the periodontal situation remained
stable.

Chapter No.: 1 Title Name: <TITLENAME>
c12.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:22:08 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 234
Table 12.1
Mean ( SD ) number of treatments provided per year of retention. Differences of number of treatments between groups are indicated in bold
(p < 0.05, ANOVA ). For more than two groups, different superscript letters indicate a significantly different number of treatments according to the Bonferroni
post‐hoc test (p < 0.05).
Parameter
N
Deep scaling/
root planing
Surgical flap
debridement
Root resection
Supportive
therapy
Endodontic
treatment
Restorative
treatment
Prosthetic
treatment
Patients’ age at T0
<50
738
0.09 (0.17)
0.04 (0.09)
0.01 (0.04)
2.48 (0.12)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
≥50
1568
0.06 (0.08)
0.05 (0.12)
0.01 (0.04)
2.51 (0.12)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
Gender
Male
950
0.07 (0.10)
0.04 (0.12)
0.01 (0.05)
2.48 (0.12)
0.01 (0.05)
0.00 (0.01)
0.01 (0.03)
Female
1356
0.07 (0.10)
0.04 (0.11)
0.01 (0.03)
2.49 (0.13)
0.01 (0.03)
0.00 (0.01)
0.01 (0.03)
Diagnosis
Aggressive periodontitis
453
0.07 (0.13)
0.04 (0.12)
0.01 (0.03)
2.49 (0.10)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
Chronic periodontitis
1853
0.06 (0.09)
0.05 (0.09)
0.01 (0.04)
2.49 (0.10)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
Number of teeth at T0
≥24
1760
0.06 (0.12)
0.04 (0.10)
0.01 (0.03)
2.49 (0.10)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
<24
546
0.08 (0.12)
0.05 (0.11)
0.01 (0.06)
2.50 (0.10)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
Smoking status
Non‐smoker
1458
0.07 (0.09)
0.04 (0.10)
0.00 (0.04)
2.48 (0.11)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
Former smoker
547
0.07 (0.13)
0.05 (0.15)
0.00 (0.06)
2.49 (0.15)
0.01 (0.04
0.00 (0.01)
0.01 (0.03)
Smoker
301
0.07 (0.10)
0.05 (0.11)
0.00 (0.02)
2.50 (0.11)
0.01 (0.02
0.00 (0.01)
0.01 (0.03)
Jaw
Maxilla
1108
0.07 (0.14)
0.05 (0.11)
0.01 (0.06)
2.49 (0.13)
0.01 (0.05)
0.00 (0.01)
0.01 (0.03)
Mandible
1198
0.07 (0.09)
0.03 (0.11)
0.00 (0.02)
2.48 (0.12)
0.01 (0.03)
0.00 (0.01)
0.01 (0.03)
Maximum PPD at T1
<5 mm
1678
0.06 (0.08)
0.03 (0.108)
0.01 (0.03)
2.47 (0.09)
0.01 (0.03)
0.00 (0.01)
0.01 (0.03)
≥5 mm
628
0.09 (0.19)
0.08 (0.16)
0.01 (0.06)
2.52 (0.17)
0.01 (0.05)
0.00 (0.01)
0.01 (0.03)

Chapter No.: 1 Title Name: <TITLENAME>
c12.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:22:08 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 235
Table 12.1
Mean ( SD ) number of treatments provided per year of retention. Differences of number of treatments between groups are indicated in bold
(p < 0.05, ANOVA ). For more than two groups, different superscript letters indicate a significantly different number of treatments according to the Bonferroni
post‐hoc test (p < 0.05).
Parameter
N
Deep scaling/
root planing
Surgical flap
debridement
Root resection
Supportive
therapy
Endodontic
treatment
Restorative
treatment
Prosthetic
treatment
Patients’ age at T0
<50
738
0.09 (0.17)
0.04 (0.09)
0.01 (0.04)
2.48 (0.12)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
≥50
1568
0.06 (0.08)
0.05 (0.12)
0.01 (0.04)
2.51 (0.12)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
Gender
Male
950
0.07 (0.10)
0.04 (0.12)
0.01 (0.05)
2.48 (0.12)
0.01 (0.05)
0.00 (0.01)
0.01 (0.03)
Female
1356
0.07 (0.10)
0.04 (0.11)
0.01 (0.03)
2.49 (0.13)
0.01 (0.03)
0.00 (0.01)
0.01 (0.03)
Diagnosis
Aggressive periodontitis
453
0.07 (0.13)
0.04 (0.12)
0.01 (0.03)
2.49 (0.10)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
Chronic periodontitis
1853
0.06 (0.09)
0.05 (0.09)
0.01 (0.04)
2.49 (0.10)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
Number of teeth at T0
≥24
1760
0.06 (0.12)
0.04 (0.10)
0.01 (0.03)
2.49 (0.10)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
<24
546
0.08 (0.12)
0.05 (0.11)
0.01 (0.06)
2.50 (0.10)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
Smoking status
Non‐smoker
1458
0.07 (0.09)
0.04 (0.10)
0.00 (0.04)
2.48 (0.11)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
Former smoker
547
0.07 (0.13)
0.05 (0.15)
0.00 (0.06)
2.49 (0.15)
0.01 (0.04
0.00 (0.01)
0.01 (0.03)
Smoker
301
0.07 (0.10)
0.05 (0.11)
0.00 (0.02)
2.50 (0.11)
0.01 (0.02
0.00 (0.01)
0.01 (0.03)
Jaw
Maxilla
1108
0.07 (0.14)
0.05 (0.11)
0.01 (0.06)
2.49 (0.13)
0.01 (0.05)
0.00 (0.01)
0.01 (0.03)
Mandible
1198
0.07 (0.09)
0.03 (0.11)
0.00 (0.02)
2.48 (0.12)
0.01 (0.03)
0.00 (0.01)
0.01 (0.03)
Maximum PPD at T1
<5 mm
1678
0.06 (0.08)
0.03 (0.108)
0.01 (0.03)
2.47 (0.09)
0.01 (0.03)
0.00 (0.01)
0.01 (0.03)
≥5 mm
628
0.09 (0.19)
0.08 (0.16)
0.01 (0.06)
2.52 (0.17)
0.01 (0.05)
0.00 (0.01)
0.01 (0.03)
Mobility at T0
0
1833
0.07 (0.13)
0.03 (0.07)
a
0.00 (0.02)
a
2.48 (0.09)
a
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
1
332
0.06 (0.09)
0.07 (0.16)
b
0.01 (0.05)
b
2.50 (0.15)
b
0.01 (0.05)
0.00 (0.01)
0.02 (0.03)
2
77
0.05 (0.07)
0.11 (0.27)
c
0.01 (0.03)
b
2.52 (0.26)
b
0.01 (0.04)
0.00 (0.00)
0.01 (0.03)
3
64
0.08 (0.12)
0.15 (0.27)
c
0.04 (0.19)
c
2.61 (0.27)
c
0.01 (0.04)
0.00 (0.02)
0.01 (0.03)
FI at T1
0
1105
0.07 (0.13)
0.03 (0.09)
a
0.00 (0.04)
a
2.47 (0.11)
a
0.01 (0.03)
a
0.00 (0.01)
a
0.01 (0.02)
a
1
652
0.07 (0.10)
0.04 (0.06)
a
0.01 (0.03)
a
2.48 (0.07)
a
0.01 (0.04)
a
0.00 (0.01)
a
0.01 (0.03)
a
2
356
0.07 (0.11)
0.07 (0.13)
b
0.01 (0.03)
a
2.51 (0.14)
b
0.01 (0.03)
a
0.00 (0.01)
a
0.01 (0.03)
a
3
193
0.06 (0.12)
0.11 (0.23)
b
0.03 (0.11)
b
2.54 (0.22)
b
0.03 (0.08)
b
0.01 (0.02)
b
0.02 (0.05)
b
Bone loss at T0
>50%
980
0.07 (0.15)
0.06 (0.14)
0.01 (0.06)
a
2.50 (0.15)
a
0.01 (0.05)
a
0.00 (0.01)
0.01 (0.03)
25‐50%
882
0.07 (0.10)
0.04 (0.10)
0.00 (0.02)
b
2.48 (0.11)
b
0.01 (0.03)
b
0.00 (0.01)
0.01 (0.03)
<25%
444
0.07 (0.06)
0.02 (0.04)
0.00 (0.02)
b
2.46 (0.06)
b
0.01 (0.03)
b
0.00 (0.01)
0.01 (0.03)
Endodontic treatment
Not present
2163
0.07 (0.12)
0.04 (0.11)
0.01 (0.02)
2.48 (0.12)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
Present
143
0.07 (0.10)
0.04 (0.16)
0.06 (0.14)
2.52 (0.16)
0.01 (0.02)
0.00 (0.01)
0.02 (0.04)
Periapical lesion
Not present
2243
0.07 (0.12)
0.05 (0.11)
0.01 (0.04)
2.49 (0.12)
0.01 (0.04)
0.00 (0.01)
0.01 (0.03)
Present
63
0.05 (0.10)
0.03 (0.18)
0.04 (0.10)
2.53 (0.13)
0.01 (0.04)
0.00 (0.01)
0.03 (0.07)
Prosthetic treatment
Not present
1460
0.07 (0.13)
0.04 (0.12)
0.00 (0.02)
2.49 (0.13)
0.01 (0.04)
0.00 (0.01)
0.00 (0.00)
Present
846
0.07 (0.10)
0.09 (0.09)
0.01 (0.06)
2.48 (0.10)
0.01 (0.05)
0.00 (0.01)
0.03 (0.04)
ANOVA = analysis of variance; FI = furcation involvement; N = number of molars; PPD = probing pocket depth; SD = standard deviation; T = time.

Chapter 12
236
Table 12.2
Mean (SD) periodontal and total treatment costs per retention year. Base case (privately insured
patient) and sensitivity (publically insured patient) analyses are shown. Differences of costs between groups
are indicated in bold (p < 0.05, ANOVA). For more than two groups, different superscript letters indicate
significantly different costs according to the Bonferroni post‐hoc test (p < 0.05).
Base case analysis
Sensitivity analysis
Parameter
N
Total
treatment
costs per year
Periodontal
treatment costs
per year
Total treatment
costs per year
Periodontal
treatment
costs per year
Patients‘ age at T0
<50
738
19.45 (17.71)
13.74 (11.98)
21.08 (21.77)
15.99 (16.66)
≥50
1568
17.70 (16.52)
12.62 (8.22)
18.44 (17.22)
14.05 (10.23)
Gender
Male
950
17.44 (16.83)
12.61 (7.25)
18.48 (16.91)
14.18 (8.66)
Female
1356
18.82 (16.90)
13.26 (10.90)
19.83 (19.99)
15.01 (14.84)
Diagnosis
Aggressive periodontitis
453
17.11 (13.64)
18.51 (17.59)
13.25 (6.41)
18.15 (13.99)
14.81 (7.99)
Chronic periodontitis
1853
12.93 (10.25)
19.55 (19.79)
14.64 (13.52)
Number of teeth at T0
≥24
1760
17.33 (16.20)
12.61 (8.99)
18.23 (17.99)
14.10 (14.66)
<24
546
21.19 (18.74)
14.20 (11.23)
22.82 (20.91)
16.55 (14.97)
Smoking status
Non‐smoker
1458
18.33 (17.51)
12.91 (10.39)
19.32 (19.82)
14.58 (14.14)
Former smoker
547
17.64 (16.51)
13.28 (8.97)
19.10 (18.29)
15.07 (10.82)
Smoker
301
18.73 (14.49)
12.90 (5.89)
19.40 (14.02)
14.41 (6.55)
Jaw
Maxilla
1108
19.04 (18.49)
13.60 (11.12)
20.51 (21.19)
15.56 (15.00)
Mandible
1198
17.50 (15.33)
12.50 (7.86)
18.15 (16.27)
13.88 (9.91)
Maximum PPD at T1
<5 mm
1678
17.34 (15.69)
12.38 (9.26)
17.96 (17.12)
13.82 (12.49)
≥5 mm
628
20.62 (19.50)
14.71 (10.32)
22.92 (22.33)
16.96 (12.98)
Mobility at T0
0
1833
17.33 (14.27)
a
12.38 (6.49)
a
17.96 (14.60)
a
13.82 (8.60)
a
1
332
21.12 (21.17)
a
14.41 (12.23)
b
22.82 (23.39)
b
16.61 (15.78)
b
2
77
21.00 (22.45)
a
15.59 (13.01)
b
23.27 (26.46)
b
17.29 (14.03)
b
3
64
26.98 (37.77)
b
20.12 (32.67)
c
37.01 (54.486
c
27.77 (47.66)
c
FI at T1
0
1105
16.50 (13.27)
a
11.95 (6.75)
a
17.04 (14.69)
a
13.19 (8.91)
a
1
652
16.72 (11.72)
a
12.49 (5.12)
a
17.64 (11.36)
a
14.00 (6.45)
b
2
356
20.60 (16.99)
b
14.20 (9.07)
b
22.06 (18.29)
b
16.04 (11.14)
b
3
193
29.07 (35.69)
c
18.64 (23.59)
c
33.69 (42.37)
c
23.35 (32.62)
c
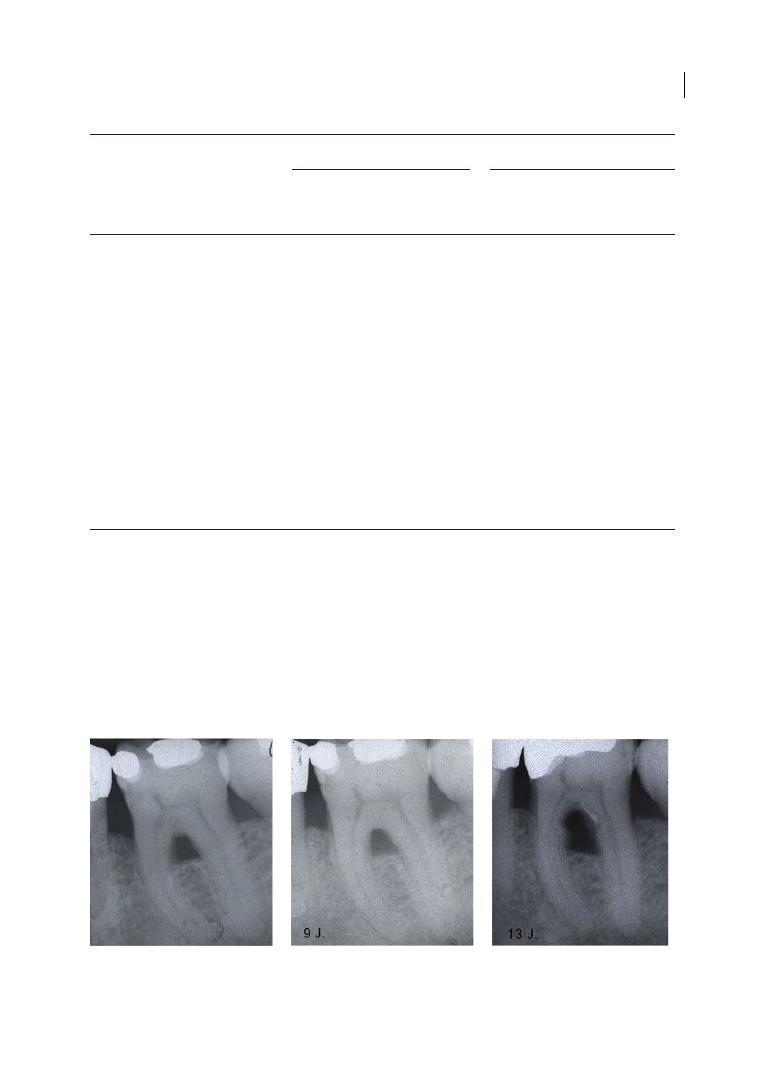
Health Economics of Furcation Involvement 237
chance of clinical success, but also the efforts
needed to ensure this success, and should
guide decision making towards retaining
or replacing teeth. It should be noted that
prosthetically involved molars were generally
found to be more expensive to retain, which
indicates not only periodontal treatment
needs, but also the necessity of retreating
prosthetically (due to caries, fractures,
porcelain chippings) or endodontically
(Goodacre et al. 2003; Walton 2013; see also
Figure 12.4).
Table 12.2
(Continued)
Base case analysis
Sensitivity analysis
Parameter
N
Total
treatment
costs per year
Periodontal
treatment costs
per year
Total treatment
costs per year
Periodontal
treatment
costs per year
Bone loss at T0
>50%
980
19.71 (19.25)
a
14.28 (13.02)
a
21.74 (23.54)
a
16.55 (17.51)
25‐50%
882
17.68 (16.47)
b
12.51 (6.49)
b
18.15 (16.14)
b
13.81 (8.12)
<25%
444
16.15 (11.00)
b
11.16 (3.23)
b
16.25 (9.13)
b
12.34 (3.55)
Endodontic treatment
Not present
2163
17.52 (14.84)
12.50 (6.23)
18.25 (15.09)
13.83 (7.42)
Present
143
29.28 (33.97)
21.20 (28.77)
35.84 (45.82)
27.95 (40.89)
Peri‐apical lesion
Not present
2243
17.70(14.66)
12.74 (7.68)
18.67 (15.92)
14.28 (9.78)
Present
63
36.19 (49.92)
22.15 (34.36)
42.75 (60.09)
29.50 (49.65)
Prosthetic treatment
Not present
1460
13.80 (12.71)
12.99 (10.05)
15.90 (17.53)
14.58 (13.30)
Present
846
25.91 (20.25)
13.04 (8.89)
25.14 (19.43)
14.81 (11.41)
ANOVA = analysis of variance; FI = furcation involvement; N = number of molars; PPD = probing pocket depth;
SD = standard deviation; T = time.
(a)
(b)
(c)
Figure 12.4
First lower molar with furcation involvement degree III (Hamp et al. 1975) after treatment with
tunnelling for cleaning at home (a). The situation remained stable for over 9 years (b), while after 13 years (c) a
carious lesion in the furcation developed, leading to endodontic involvement and, eventually, extraction.
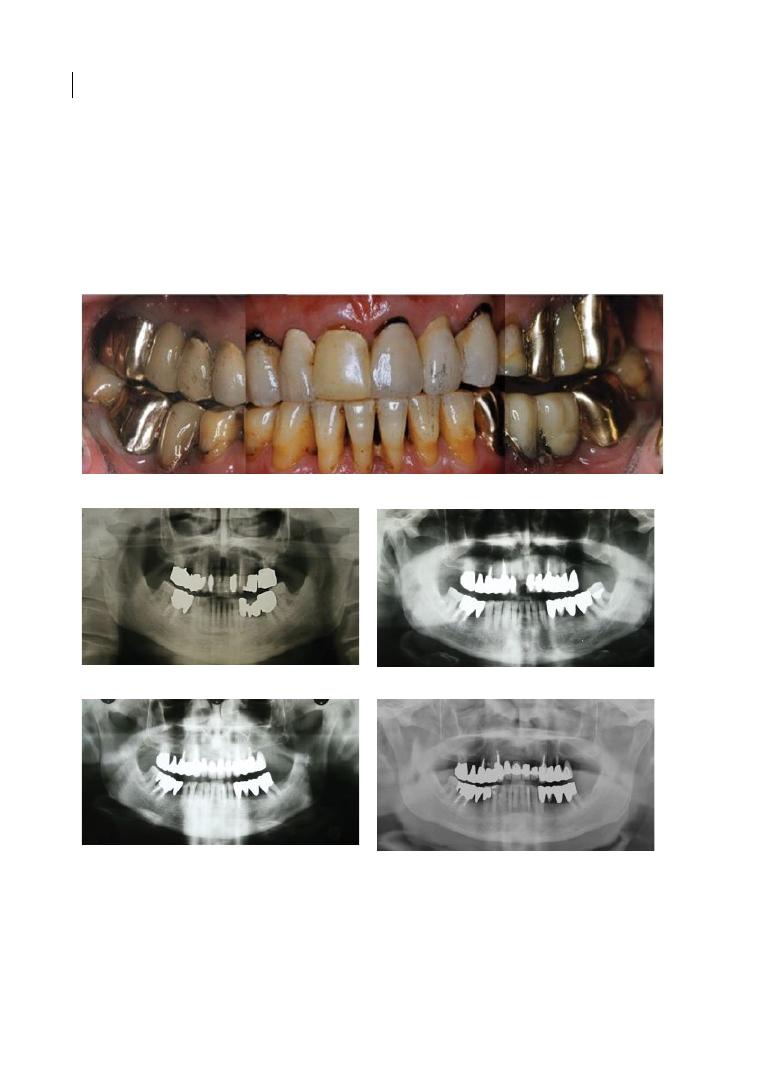
Chapter 12
238
Such increased risks of reinterventions for
prosthetically restored teeth was shown
mainly if prosthetics had been placed prior to
periodontitis treatment (Pretzl et al. 2008;
Graetz et al. 2013), while prosthetics inserted
after successful initial therapy and during
systematic SPT were not necessarily found
to generate more treatment efforts and
costs (Yi et al. 1995; Lulic et al. 2007; Fardal
and Linden 2010; Graetz et al, 2013; see also
Figure 12.5).
Overall, the very limited available evi-
dence finds retention of FI molars to require
more effort than non‐molar or non‐FI teeth,
which has impacts on the costs required for
retention. This is truer in molars with severe
(a)
(b)
(c)
(d)
(e)
Figure 12.5
Man, aged 59 years and non‐smoker, diagnosed with generalized chronic periodontitis and
horizontal bone loss up to one‐quarter of the root length in both jaws and furcation involvement degree I
(lower molars) and II (upper molars) (Hamp et al. 1975) (a and b). Initial periodontal therapy using scaling and
root planing followed by open‐flap debridement of all pre/molars with extraction of tooth 17 (UR7) (as a result
of a root carious lesion) was provided. After re‐evaluation, a regular supportive periodontal therapy was started
and after one year a fixed dental prosthesis was fitted. Seven years later, tooth 11 fractured (c) and a fixed
dental prosthesis was provided in the front (d). This situation remained stable for 29 years (e, last observation).

Health Economics of Furcation Involvement 239
furcation involvement (FI degree III),
but not necessarily for FI degree I. More
important, however, is the fact that overall
retention costs were very limited, with only
a few euros per year being required to retain
such molars. Based on this finding, it is now
relevant to compare these costs with those
generated by other, alternative treatments.
12.4 Cost‐effectiveness of
Retaining Furcation‐involved
Molars
There are only very few data comparing the
cost‐effectiveness of different strategies to
retain molars with FI. One recent study
(Schwendicke et al. 2014) used a mathemati-
cal model to assess the cost‐effectiveness of
treatment alternatives for periodontally
affected, vital molars with furcation FI, com-
paring tooth‐retaining strategies with tooth
removal and replacement via implant‐
supported crowns (ISCs). Categories of
tooth‐retaining strategies were
●
Conservative, non‐surgical furcation ther-
apy involving SRP.
●
SRP with surgical access (i.e. FD).
●
For teeth with FI degree II or III, root
resection (RR; i.e. hemisection, trisection,
or amputation), as well as
●
Guided tissue regeneration (GTR, including
insertion of bone‐substitute material and
placement of a resorbable membrane), and
●
Tunnelling (TU, for mandibular molars
only).
Tooth‐retaining strategies were compared
with the removal and replacement of teeth
via ISCs.
This study assessed cost‐effectiveness as
lifetime treatment costs (initial plus follow‐
up treatments) per retention time of the
tooth or implant (in years). All analyses were
performed separately for molars with FI
degree I, lower molars with F degree II/III,
and upper molars with FI degree II/III, again
in the context of German healthcare.
The study simulated an initially 50‐year‐
old male patient with an average remaining
life expectancy of 29.7 years. The model used
consisted of the initial and various follow‐up
health states (Figure 12.6), simulating the
natural history of a periodontally affected
tooth or an ISC.
As to the costs assessed, the following
assumptions had been made:
●
All initial therapies comprised full case
assessment including oral hygiene assess-
ment, advice and motivation, radiographs,
scale and polish, re‐evaluation, and the
necessary treatment as already outlined,
including anaesthesia, possible endodon-
tic, surgical, or prosthetic procedures, and
short‐term post‐operative care.
●
Supportive periodontal or implant treat-
ment involved biannual re‐revaluation,
scale and polish, subgingival retreatment,
and antiseptic irrigation, as well as radio-
graphic reassessment every two years. For
teeth but not implants, fluoridation of root
surfaces was assumed to be additionally
performed.
●
Modelling involved fatal complica-
tions – that is, those leading to loss of the
tooth or implant (for example periodontal
complications or untreatable root caries,
or untreatable peri‐implantitis or implant
fracture, respectively) – and non‐fatal
complications – for instance treatable
caries at restoration margins, treatment‐
responsive peri‐implantitis, or loss of
crowns or abutments. Mending of compli-
cations was assumed to generate costs, and
involved repair or renewal of restorations,
recementation or refixing of crowns or
abutments, and peri‐implant treatment
(Mombelli and Lang, 1998).
For molars with FI degree I, SRP was both less
costly and more effective than ISC. Compared
with FD, ISC was always more costly, but also
more effective (i.e. implants were retained
longer). Regardless of the dental arch, treat-
ing molars with FI degree II/III via tooth‐
retaining options was found to be more
Chapter 12
240
effective and less costly than tooth removal
and replacement via ISC (Figure 12.7).
This cost‐effectiveness ranking – with
implants being more costly than tooth‐
retaining strategies – did hold even under
the worst‐case assumptions modelled, and
was also stable regardless of how costs were
estimated.
Retaining teeth was significantly less costly
than removing and replacing them, mainly as
ISCs are so costly initially, but also as retreat-
ments on ISCs (which are not needed very
frequently) are relatively costly. For example,
treating peri‐implantitis is not only challeng-
ing but costly, as is mending non‐biological
complications (ceramic chippings, crown de‐
cementations, fractures), which usually
involve costly materials and often generate
further staff costs for dental technicians.
This finding is in line with a number of
observational studies from another health-
care setting as well (Fardal and Grytten 2013;
Martin et al. 2014).
More specifically, it is unlikely that remov-
ing and replacing molars with FI degree I will
be cost‐effective (the 10‐year survival rate for
molars treated via SRP and SPT was 97% in
this study). This is in line with FI degree
I being found to have only limited impact on
tooth success when compared with FI degree
0 (Salvi et al. 2014; Graetz et al., 2015), but
also to only limited impact on required treat-
ment efforts (as has been shown already and
in Tables 12.1 and 12.2). FD seems less effec-
tive than ISCs, while being more costly than
SRP. It is therefore doubtful if such a strategy
can be more cost‐effective than SRP in molars
with FI degree I (Heitz‐Mayfield et al. 2002).
Molars with Fl I
SRP
Tooth loss
Implant loss
Refill/repair/
crown
Refill/repair/
crown
Recement/
-crown
Periodontal/Endodontic/Restorative
Complications
Periodontal/Endodontic/
Restorative Complications
Technical/Biological
Complications
RCT
RCT
CIST
FD
ISC
RR
GTR
TU
Molars with Fl II or Ill
Replace with ISC
Replace with ISC
Tooth loss
Replace with ISC
Figure 12.6
State transition diagram. Molars with furcation involvement (FI) degrees I and II/III were analysed
separately, with different treatments being compared. All periodontal treatments were compared with
implant‐supported crowns (ISC). For all teeth, periodontal, endodontic, and restorative complications were
modelled, with fatal (leading to tooth loss) and non‐fatal (mendable) complications being simulated
separately. For implants, we modelled technical (loss of crowns, abutment fracture, implant fracture) and
biological complications (peri‐implantitis), again with separate simulation of fatal and non‐fatal failures. If
complications were mendable, teeth and implants were allocated to follow‐up treatments, which generated
costs. In case these treatments were not final, i.e. another retreatment was possible (for example re‐restoration
after repair), this was modelled as well. Eventually, lost teeth or implants were assumed to be replaced using
implant‐supported crowns. Note that within base case analysis, all failed teeth or implants were assumed to be
(re‐)replaced. To explore the effects of this assumption, sensitivity analyses were performed. CIST = cumulative
supportive interceptive therapy; FD = flap debridement; GTR = guided tissue regeneration; RCT = root canal
treatment; RR = root resection; SRP = scaling and root planing; TU = tunnelling.
Health Economics of Furcation Involvement 241
In general, there is debate around the cost‐
effectiveness of FD versus SRP, as the effec-
tiveness gain seems limited (also in non‐FI
degree I teeth) while the additional costs are
substantial (Antczak‐Bouckoms and
Weinstein 1987). There is, however, some
indication that the need for maintenance
visits seems reduced after FD compared with
SRP, which could offset initially higher treat-
ment costs (Miremadi et al. 2015).
There is greater uncertainty as to how best
to treat molars with FI degree II or III (or
whether to replace them). Treatment options
have been discussed extensively in this book
(mainly in Chapters 7, 8, and 9). RR espe-
cially was relatively costly, as costs occur not
only for the periodontal procedure, but also
root canal treatment (note that – as discussed
already – RR might be mainly applied to
molars which had received endodontic
therapy earlier) and crown placement
(Carnevale et al. 1991; Huynh‐Ba et al. 2009;
Schwendicke et al. 2013). When considering
the range of estimated survival rates for
RR – 91% after 10 years in Schwendicke et al.
(2013); 93% after 3.5
years in Helldén
et al. (1989); 68% after 10 years in Blomlöf
et al. (1997); and 83% after 7 years in
Little et al. (1995; reviewed in Chapter 8), it
remains uncertain whether the high costs
for RR are truly justified.
What is clear from the existing studies is
that one cannot really attempt to compare
most periodontal treatments with one
another, since their indications differ (RR will
not be applied to the same teeth as SRP etc.).
(a)
2500
2100
1700
1300
Cost in Euro
Cost in Euro
900
2500
2100
1700
1300
900
Cost in Euro
2500
2100
1700
1300
900
ISC
ISC
ISC
ISC
ISC
RR
RR
RR
RR
GTR
GTR
GTR
TU
TU
GTR
ISC
SRP
SRP
FD
22
24
26
Effectiveness in years
Effectiveness in years
28
30
22
24
26
28
30
Effectiveness in years
22
24
26
28
30
FD
(b)
(c)
Figure 12.7
Cost‐effectiveness of different strategies to treat molars with furcation involvement (FI). For FI
degree I, we compared conservative scaling and root planing (SRP) and flap debridement and SRP (FD) with
implant‐supported crowns (ISC). For molars with FI degrees II or III, root resection (RR), guided tissue
regeneration (GTR), and tunnelling (TU, for lower molars) were compared with ISC. The presented
cost‐effectiveness planes (a, b, c) demonstrate the discounted lifetime costs (y‐axis) per effectiveness (in years
of tooth or implant retention). In the case of FI degree I (a), SRP was more effective and less costly than ISC,
while FD was both less effective and less costly than ISC. For upper (b) or lower (c) molars with FI degree II or III,
ISC was dominated by all tooth‐retaining strategies.
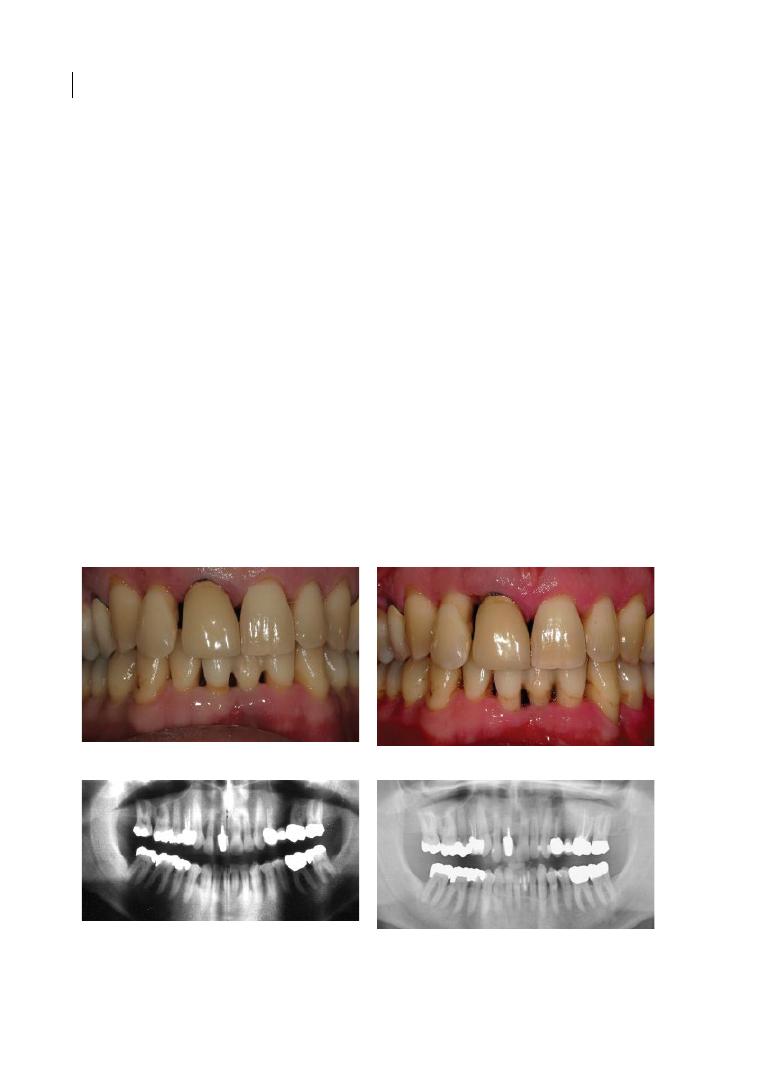
Chapter 12
242
What should be further borne in mind is
that different periodontal treatments
require different degrees of patient motiva-
tion and compliance. For example, tunnel-
ling might require highly motivated patients
to maintain the tunnel and prevent root
caries (Hamp et al. 1975). That said, doubts
remain regarding the postulated high risk
of caries within tunnels (Little et al. 1995;
Dannewitz et al. 2006b; Feres et al. 2006), as
was seen in the German cohort study dis-
cussed, which reported such caries in only
2% of molars over 16.5 years (Schwendicke
et al. 2016a). Lastly, retention and replace-
ment are not the only viable options for
treating molars with FI; shortened dental
arches might also yield sufficient function-
ality and subjective oral health (Wolfart
et al. 2014), while generating limited initial
and long‐term costs (Faggion et al. 2011;
Wolfart et al. 2012). Here again, the availa-
ble data are insufficient to assign monetary
or utility values to missing, replaced, or
retained teeth, which would allow different
strategies to be properly compared.
12.5 Research Gaps
Adhesive dentistry increases the number of
options for dealing with furcation‐involved
teeth. Splinting of different teeth or (resected)
roots, or even using extracted teeth as
adhesive bridge pontic, has been performed
not only in the anterior region, but also
posteriorly (Figures 12.8 and 12.9). Clinical
experience allows the hypotheses that using
glass fibre–enforced ribbons could allow
similar retention periods to be achieved to
conventional adhesive (Maryland) bridges
used normally in the front, with similar
complications of debonding and fracture
(Miettinen and Millar 2013). However,
reliable efficacy data and any data on cost‐
effectiveness or the value patients place on
such treatments are missing at present.
(a)
(b)
(c)
(d)
Figure 12.8
Man, aged 46 years, with generalized chronic periodontitis after extraction with immediately
re‐fixation of tooth 31 (LL1) (a, b: before extraction) and long‐term stability of the situation during supportive
periodontal therapy over 10 years (c, d).
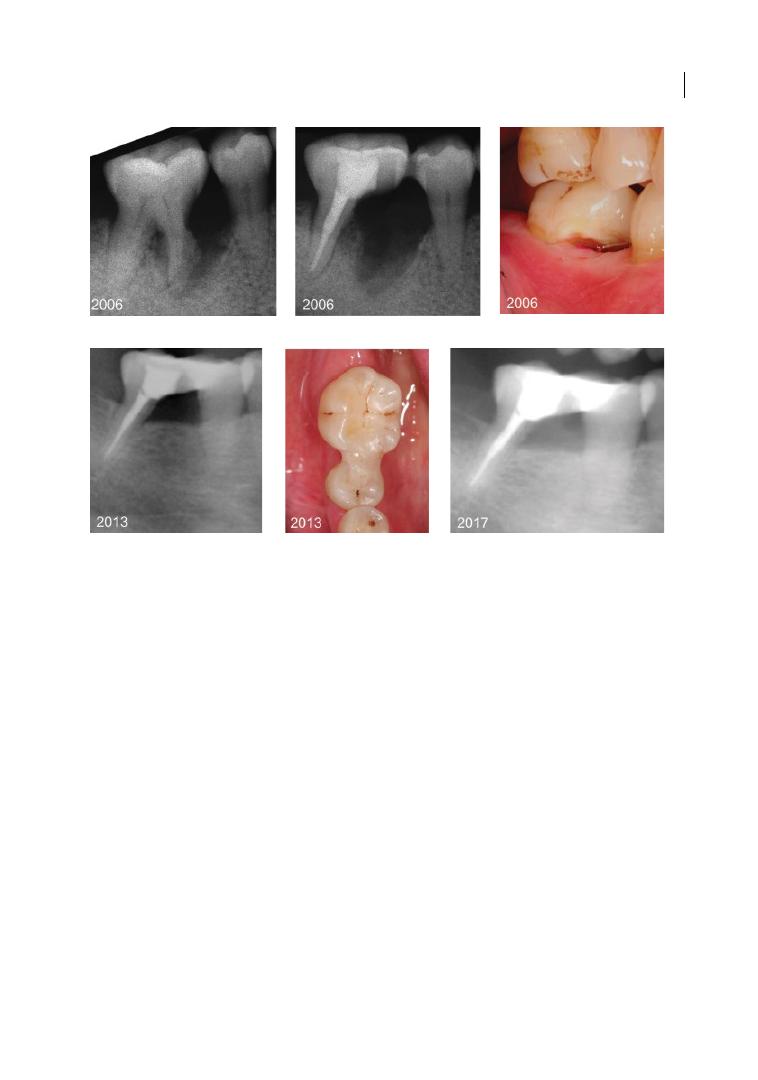
Health Economics of Furcation Involvement 243
In general, there are growing data on cost‐
effectiveness, largely stemming from either
cohort or modelling studies. Recording of
efficiency data alongside randomized trials is
not common at present and can be recom-
mended. Cost–utility or cost–benefit analy-
ses are even less common, mainly since the
subjective value that patients place on single
retained, replaced, or missing teeth is not
known at present.
12.6 Conclusions
Given both the demographic changes in
many rich countries as well as the epidemio-
logical shift in older populations – who retain
more teeth than ever before – retaining
molars with FI is highly relevant from a pub-
lic health and health economic perspective.
A range of study types has been employed in
this field, mainly to describe the costs of
retaining these molars, but also to compare
the cost‐effectiveness of different retention
and replacement strategies. Based on these
studies, retaining molars with FI might
require more effort than retaining non‐FI
teeth. However, the resulting annual treat-
ment costs are nevertheless very limited.
The larger proportion of these costs is for
periodontal, not other (restorative, endodon-
tic, prosthetic) treatments. There are a
number of factors which are associated with
greater effort being needed, resulting in
higher costs, like bone loss, severe FI, mobil-
ity, or status as prosthetic abutment. Dentists
should consider these factors in treatment
decisions. If comparing different strategies
for managing molars with FI, tooth retention
seems probably less costly than tooth
(a)
(c)
(b)
(d)
(f)
(e)
Figure 12.9
Woman, aged 47 years, with generalized chronic periodontitis and root resorption of the mesial
root of tooth 46 (LR6) (a). After successful endodontic treatment and resection of the mesial root (b), a fibre‐
enforced pontic was directly placed (c), allowing splinting of teeth 46 and 45 (LR5). Long‐term stability was
achieved during supportive periodontal therapy over 11 years (d–f).

Chapter 12
244
replacement via ISC. This is mainly because
implant placement but also maintenance is
relatively costly compared to costs for tooth
retention.
In conclusion, dentists should not focus only
on the reported success or survival rates of cer-
tain treatments (which are, for example, very
high for implants). Instead, they should con-
sider the long‐term consequences and extent
of possible retreatments, as well as their feasi-
bility and costs. Retaining molars with FI is
likely to be both achievable and cost‐effective.
References
Antczak‐Bouckoms, A.A., and Weinstein,
M.C. (1987). Cost‐effectiveness analysis of
periodontal disease control. Journal of
Dental Research 66, 1630–1635.
Blomlof, L., Jansson, L., Appelgren, R. et al.
(1997). Prognosis and mortality of root‐
resected molars. International Journal of
Periodontics and Restorative Dentistry
17, 190–201.
Carnevale, G., Di Febo, G., Tonelli, M.P.
et al. (1991). A retrospective analysis of the
periodontal‐prosthetic treatment of molars
with interradicular lesions. International
Journal of Periodontics and Restorative
Dentistry 11, 189–205.
Chambrone, L.A., and Chambrone, L. (2006).
Tooth loss in well‐maintained patients with
chronic periodontitis during long‐term
supportive therapy in Brazil. Journal of
Clinical Periodontolpgy 33, 759–764.
doi:10.1111/j.1600‐051X.2006.00972.x.
Checchi, L., Montevecchi, M., Gatto, M.R., and
Trombelli, L. (2002). Retrospective study of
tooth loss in 92 treated periodontal patients.
Journal of Clinical Periodontology
29, 651–656.
Dannewitz, B., Krieger, J.K., Husing, J., and
Eickholz, P. (2006a) Loss of molars in
periodontally treated patients: A
retrospective analysis five years or more
after active periodontal treatment. Journal
of Clinical Periodontology 33, 53–61.
doi:10.1111/j.1600‐051X.2005.00858.x.
Dannewitz, B., Krieger, J.K., Hüsing, J., and
Eickholz, P. (2006b). Loss of molars in
periodontally treated patients:
A retrospective analysis five years or more
after active periodontal treatment. Journal
of Clinical Periodontology 33, 53–61.
doi:10.1111/j.1600‐051X.2005.00858.x.
Eickholz, P., Kaltschmitt, J., Berbig, J. et al.
(2008). Tooth loss after active periodontal
therapy. 1: Patient‐related factors for risk,
prognosis, and quality of outcome. Journal
of Clinical Periodontology 35, 165–174.
doi:10.1111/j.1600‐051X.2007.01184.x.
Faggion, C.M., Jr, Giannakopoulos, N.N., and
Listl, S. (2011). How strong is the evidence
for the need to restore posterior bounded
edentulous spaces in adults? Grading the
quality of evidence and the strength of
recommendations. Journal of Dentistry 39,
108–116. doi:10.1016/j.jdent.2010.11.002.
Fardal, O., and Grytten, J. (2013). A
comparison of teeth and implants during
maintenance therapy in terms of the number
of disease‐free years and costs: An in vivo
internal control study. Journal of Clinical
Periodontology 40, 645–651. doi:10.1111/
jcpe.12101.
Summary of Evidence
●
Teeth with furcation involvement (FI) can
be retained in the long term, but at higher
costs than teeth without FI.
●
These costs increase with higher degrees
of FI, bone loss, and mobility.
●
However, removing and replacing teeth
using implant‐supported crowns does not
seem to be less costly.
●
Dentists should consider these risk factors
and the required treatment needs for plan-
ning the retention or removal of teeth.

Health Economics of Furcation Involvement 245
Fardal, O., Johannessen, A.C., and Linden, G.J.
(2004). Tooth loss during maintenance
following periodontal treatment in a
periodontal practice in Norway. Journal
of Clinical Periodontology 31,
550–555.
doi:10.1111/j.1600‐051X.2004.00519.x.
Fardal, O., and Linden, G.J. (2010). Long‐term
outcomes for cross‐arch stabilizing bridges
in periodontal maintenance patients:
A retrospective study. Journal of Clinical
Periodontology 37, 299–304.
doi:10.1111/j.1600‐051X.2009.01528.x.
Feres, M., Araujo, M.W., Figueiredo, L.C., and
Oppermann, R.V. (2006). Clinical evaluation
of tunneled molars: A retrospective study.
Journal of the International Academy of
Periodontology 8, 96–103.
Goodacre, C J., Bernal, G., Rungcharassaeng,
K., and Kan, J.Y. (2003). Clinical
complications in fixed prosthodontics.
Journal of Prosthetic Dentistry 90, 31–41.
doi:10.1016/s0022391303002142.
Graetz, C., Dörfer, C.E., Kahl, M. et al. (2011).
Retention of questionable and hopeless
teeth in compliant patients treated for
aggressive periodontitis. Journal of Clinical
Periodontology 38, 707°714.
doi:10.1111/j.1600‐051X.2011.01743.x.
Graetz, C., Schutzhold, S., Plaumann, A. et al.
(2015). Prognostic factors for the loss of
molars: An 18‐years retrospective cohort
study. Journal of Clinical Periodontology
42, 943–950. doi:10.1111/jcpe.12460.
Graetz, C., Schwendicke, F., Kahl, M. (2013).
Prosthetic rehabilitation of patients with
history of moderate to severe periodontitis:
A long‐term evaluation. Journal of Clinical
Periodontology 40, 799–806. doi:10.1111/
jcpe.12124.
Graetz, C., Sälzer, S., Plaumann, A.,
Schlattmann, P., Kahl, M., Springer, C.,
Dörfer, C., and Schwendicke, F. (2017a).
Tooth loss in generalized aggressive
periodontitis: Prognostic factors after 17
years of supportive periodontal treatment. J
Clin Periodontol 44, 612–619. doi:10.1111/
jcpe.12725.
Graetz, C., Plaumann, A., Schlattmann, P.,
Kahl, M., Springer, C., Sälzer, S., Gomer, K.,
Dörfer, C., and Schwendicke, F. (2017b).
Long-term tooth retention in chronic
periodontitis – results after 18 years of a
conservative periodontal treatment regimen
in a university setting. J Clin Periodontol 44,
169–177. doi:10.1111/jcpe.12680.
Hamp, S.E., Nyman, S., and Lindhe, J. (1975).
Periodontal treatment of multirooted teeth:
Results after 5 years. Journal of Clinical
Periodontology 2, 126–135.
Hatch, J.P., Shinkai, R.S., Sakai, S. et al. (2001).
Determinants of masticatory performance
in dentate adults. Archives of Oral Biology
46, 641–648.
Heitz‐Mayfield, L.J., Trombelli, L., Heitz, F.
et al. (2002). A systematic review of the
effect of surgical debridement vs
non‐surgical debridement for the treatment
of chronic periodontitis. Journal of Clinical
Periodontology 29 (Suppl. 3), 92–102;
discussion 160–162.
Helldén, L.B., Elliot, A., Steffensen, B., and
Steffensen, J.E. (1989). The prognosis of
tunnel preparations in treatment of class III
furcations: A follow‐up study. Journal of
Periodontology 60, 182–187. doi:10.1902/
jop.1989.60.4.182.
Holtfreter, B., Kocher, T., Hoffmann, T. et al.
(2010). Prevalence of periodontal disease
and treatment demands based on a German
dental survey (DMS IV). Journal of Clinical
Periodontology 37, 211–219.
doi:10.1111/j.1600‐051X.2009.01517.x.
Huynh‐Ba, G., Kuonen, P., Hofer, D. et al.
(2009). The effect of periodontal therapy on
the survival rate and incidence of
complications of multirooted teeth with
furcation involvement after an observation
period of at least 5 years: A systematic
review. Journal of Clinical Periodontology
36, 164–176.
doi:10.1111/j.1600‐051X.2008.01358.x.
Johansson, K.J., Johansson, C.S., and Ravald, N.
(2013). The prevalence and alterations of
furcation involvements 13 to 16 years after
periodontal treatment. Swedish Dental
Journal 37, 87–95.
Jordan, A.R., and Micheelis, W. (2016). Fünfte
Deutsche Mundgesundheitsstudie (DMS V).
Köln: Deutscher Ärzte-Verlag.

Chapter 12
246
Kassebaum, N.J., Bernabe, E., Dahiya, M. et al.
(2014). Global burden of severe
periodontitis in 1990–2010: A systematic
review and meta‐regression. Journal of
Dental Research 93, 1045–1053.
doi:10.1177/0022034514552491.
Kocher, T., and Plagmann, H.C. (1999).
Root debridement of molars with furcation
involvement using diamond‐coated sonic
scaler inserts during flap surgery: A pilot
study. Journal of Clinical Periodontology
26, 525–530.
König, J., Plagmann, H.C., Rühling, A., and
Kocher, T. (2002). Tooth loss and pocket
probing depths in compliant periodontally
treated patients: A retrospective analysis.
Journal of Clinical Periodontology 29,
1092–1100. doi:cpe291208 [pii].
Lee, K.L., Corbet, E.F., and Leung, W.K.
(2012). Survival of molar teeth after
resective periodontal therapy: A
retrospective study. Journal of Clinical
Periodontology 39, 850–860.
doi:10.1111/j.1600‐051X.2012.01918.x.
Little, L.A., Beck, F.M., Bagci, B., and Horton,
J.E. (1995). Lack of furcal bone loss
following the tunnelling procedure. Journal
of Clinical Periodontology 22, 637–641.
Loesche, W.J., Giordano, J.R., Soehren, S., and
Kaciroti, N. (2002). The nonsurgical
treatment of patients with periodontal
disease: Results after five years. Journal of the
American Dental Association 133, 311–320.
Lulic, M., Bragger, U., Lang, N.P. et al. (2007).
Ante’s (1926) law revisited: A systematic
review on survival rates and complications
of fixed dental prostheses (FDPs) on severely
reduced periodontal tissue support. Clinical
Oral Implants Research 18 (Suppl. 3), 63–72.
doi:10.1111/j.1600‐0501.2007.01438.x.
Martin, J.A., Fardal, O., Page, R.C. et al. (2014).
Incorporating severity and risk as factors to
the Fardal cost‐effectiveness model to create
a cost‐benefit model for periodontal
treatment. Journal of Periodontology 85,
e31–e39. doi:10.1902/jop.2013.130237.
Micheelis, W., and Schiffer, U. (2006). Vierte
Deutsche Mundgesundheitsstudie (DMS‐IV).
Köln: Deutscher Zahnärzte.
Miettinen, M., and Millar, B.J. (2013). A review
of the success and failure characteristics of
resin‐bonded bridges. British Dental Journal
215, E3. doi:10.1038/sj.bdj.2013.686.
Miremadi, S.R., De Bruyn, H., Steyaert, H.
et al. (2015). A randomized controlled trial
comparing surgical and non‐surgical
periodontal therapy: A 3‐year clinical and
cost‐effectiveness analysis. Journal of
Clinical Periodontology 42, 740–747.
doi:10.1111/jcpe.12434.
Mombelli, A., and Lang, N.P. (1998). The
diagnosis and treatment of peri‐implantitis.
Periodontology 2000 17, 63–76.
Pretzl, B., Kaltschmitt, J., Kim, T.S. et al.
(2008). Tooth loss after active periodontal
therapy. 2: Tooth‐related factors. Journal of
Clinical Periodontology 35, 175–182.
doi:10.1111/j.1600‐051X.2007.01182.x.
Pretzl, B., Wiedemann, D., Cosgarea, R. et al.
(2009). Effort and costs of tooth
preservation in supportive periodontal
treatment in a German population. Journal
of Clinical Periodontology 36, 669–676.
doi:10.1111/j.1600‐051X.2009.01409.x.
Roos‐Jansaker, A.M., Lindahl, C., Renvert, H.,
and Renvert, S. (2006). Nine‐ to fourteen‐
year follow‐up of implant treatment. Part II:
Presence of peri‐implant lesions. Journal of
Clinical Periodontology 33, 290–295.
doi:10.1111/j.1600‐051X.2006.00906.x.
Salvi, G.E., Mischler, D.C., Schmidlin, K. et al.
(2014). Risk factors associated with the
longevity of multi‐rooted teeth: Long‐term
outcomes after active and supportive
periodontal therapy. Journal of Clinical
Periodontology 41, 701–707. doi:10.1111/
jcpe.12266.
Schwendicke, F., Graetz, C., Stolpe, M., and
Dorfer, C.E. (2014). Retaining or replacing
molars with furcation involvement: A
cost‐effectiveness comparison of different
strategies. Journal of Clinical Periodontology
41, 1090–1097. doi:10.1111/jcpe.12315.
Schwendicke, F., Plaumann, A., Stolpe, M. et al.
(2016a). Retention costs of periodontally
compromised molars in a German
population. Journal of Clinical Periodontology
43, 261–270. doi:10.1111/jcpe.12509.

Health Economics of Furcation Involvement 247
Schwendicke, F., Stolpe, M., Meyer‐Lueckel, H.
et al. (2013). Cost‐effectiveness of one‐ and
two‐step incomplete and complete excavations.
Journal of Dental Research 90, 880–887.
Schwendicke, F., Stolpe, M., Plaumann, A., and
Graetz, C. (2016b). Cost‐effectiveness of
regular versus irregular supportive
periodontal therapy or tooth removal.
Journal of Clinical Periodontology 43,
940–947. doi:10.1111/jcpe.12595.
Vernazza, C., Heasman, P., Gaunt, F., and
Pennington, M. (2012). How to measure the
cost‐effectiveness of periodontal treatments.
Periodontology 2000 60, 138–146.
doi:10.1111/j.1600‐0757.2011.00406.x.
Walton, T.R. (2013). The up to 25‐year survival
and clinical performance of 2,340 high
gold‐based metal‐ceramic single crowns.
International Journal of Prosthodontics
26, 151–160. doi:10.11607/ijp.3136.
Wolfart, S., Marre, B., Wostmann, B.
et al. (2012). The randomized
shortened dental arch study: 5‐year
maintenance. Journal of Dental Research
91, 65 s–71 s. doi:10.1177/0022034
512447950.
Wolfart, S., Muller, F., Gerss, J. et al. (2014).
The randomized shortened dental
arch study: Oral health‐related quality
of life. Clinical Oral Investigations 18,
525–533. doi:10.1007/s00784‐013‐0991‐6.
Yi, S.W., Ericsson, I., Carlsson, G.E., and
Wennstrom, J.L. (1995). Long‐term follow‐
up of cross‐arch fixed partial dentures in
patients with advanced periodontal
destruction: Evaluation of the supporting
tissues. Acta Odontologica Scandinavica
53, 242–248.
Zhong, H. (2010). On decomposing the
inequality and inequity change in health care
utilization: Change in means, or change in
the distributions? International Journal of
Health Care Finance and Economics 10,
369–386. doi:10.1007/s10754‐010‐9085‐z.

Chapter No.: 1 Title Name: <TITLENAME>
c13.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:22:26 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 249
249
Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
13.1 Introduction
Dentistry is moving towards a more patient‐
centred approach, with more attention paid
to the patient’s point of view and striving
to improve the patient’s quality of life.
Ultimately, as treating clinicians we should
realize that reduction of probing pocket
depths and bleeding on probing are just sur
rogate measures of disease progression and
they do not necessarily mirror the patient’s
aims and needs. Furthermore, every patient is
different and what works for one patient may
not work for another. However, it is interest
ing to note how most studies in the periodon
tal literature have focused so much on clinical
measurements, leaving aside aspects such as
costs and effects on the patient’s quality of
life. Equally, previous chapters of this book
have focused on clinical parameters, bone
levels, and ‘success’ as defined by the treating
periodontist, but not based on the patient’s
perceptions. This chapter aims to review
studies focusing on patient quality‐of‐life
measures relative to furcation involvement.
Given the paucity of data on this, anecdotally
the feedback from some patients treated for
furcation involvement will be presented, in
order to give the reader a perspective from
people who are on the receiving end of the
treatment discussed in this book.
13.2 Patient‐reported
Outcome Measures
in Periodontology
Socio‐environmental measures such as func
tion and psychological well‐being have been
applied to dentistry in the last few decades
(Locker 1988). These measures aim to assess
parameters related to the impact of oral
health which are not objectively measurable
by the treating clinician. The World Health
Organization (WHO) defines quality of life
as individuals’ perception of their position in
life in the context of the culture and value
systems in which they live and in relation to
their goals, expectations, standards, and con
cerns (WHOQOL Group 1994). It is a broad‐
ranging concept affected in a complex way by
the person’s physical health, psychological
state, level of independence, social relation
ships, personal beliefs, and relationship to
salient features of their environment. The
American Dental Association emphasized
the importance of quality‐of‐life measures by
stating: ‘Oral health is a functional, struc
tural, aesthetic, physiologic and psychosocial
state of well‐being and is essential to an
individual’s general health and quality of life’
(Glick and Meyer 2014; Glick et al. 2017).
Over the last decade, patient‐reported out
come measures (PROMs) have increasingly
Chapter 13
Deep Gaps between the Roots of the Molars:
A Patient’s Point of View
Luigi Nibali
Centre for Immunobiology and Regenerative Medicine, Centre for Oral Clinical Research, Institute of Dentistry, Barts and the London
School of Medicine and Dentistry, Queen Mary University of London (QMUL), London, UK

Chapter 13
250
been recognized as a research priority and
incorporated in periodontal research studies
(Aslund et al. 2008; Buset et al. 2016). PROMs
are defined as standardized measures used to
capture the subjective effect of a disease or
treatment on a patient’s life, including daily
activities and well‐being (US Department of
Health and Human Services et al. 2009).
Health‐related quality of life (HRQoL) and
oral health–related quality of life (OHRQoL)
are often used as measures of PROMs in
medicine and more specifically dentistry.
While the related questionnaires provide
data about health or disease status (struc
ture/function/activity/participation), other
questionnaires such as the Oral Impacts on
Daily Performance (OIDP) and the Oral
Health Impact Profile (OHIP‐14) assess
the impacts of oral diseases on daily life
( disease prevention/dysfunction/failure) in a
pre‐determined period (Adulyanon et al.
1996; Slade 1997). These are generic
OHRQoL measures, so they are not specifi
cally designed for patients with periodontitis.
In other words, they measure the oral
impacts of oral conditions in general, not
attributing them to particular diseases/
conditions. However, they have been used
in studies of periodontal patients with
the underlying – but largely untested –
assumption that in such a patient sample
most of the reported oral impacts would be
due to that specific oral condition; that is, a
periodontal condition in this case. Among
the generic OHRQoL measures, the OIDP
allows also for a condition‐specific version,
whereby patients attribute the reported oral
impacts to specific ‘causes’, in other words
conditions. Furthermore, while most of these
measures assess the frequency of oral impacts
(i.e. how often they were experienced), the
OIDP measures both the frequency and the
severity of oral impacts. Despite their differ
ences, all these questionnaires tend to focus
on covering the physical, psychological, and
social aspects of the oral impacts on daily life.
The relevant literature goes beyond the
oral health field and extends to generic
HRQoL measures. The EuroQol Question
naire (EQ‐5D‐5 L) is a measure of HRQoL
including self‐assessments of mobility, pain/
discomfort, self‐care, anxiety/depression,
and usual activities, recorded by patients on
an ordinary scale with five levels (Herdman
et al. 2011). It is unclear whether the valua
tions would refer to periodontal disease or to
any other condition coexisting in the same
person (or their combination, for that mat
ter). Using the EuroQol Questionnaire, a
cross‐sectional study on a random sample of
709 45‐ to 54‐year‐old Australians was able
to differentiate the impacts of varying degrees
of periodontal diseases (from gingivitis to
periodontitis; Brennan et al. 2007). For exam
ple, having a pocket depth of ≥ 6 mm was
associated with a prevalence of pain/discom
fort in 25.8% of cases, compared with 6.1%
pain/discomfort for patients with gingivitis.
In a separate study, when OHIP‐14 and OIDP
structured interviewer‐administered ques
tionnaires, global self‐report, and perceived
need for dental treatment questions were
administered to 264 patients, the majority
(61.0%) rated their oral health status poorly
and 203 (76.9%) perceived a need for treat
ment, highlighting the importance of patient‐
driven treatment needs (Lawal et al. 2015).
A systematic review on the impact of
periodontitis on OHRQoL suggested that,
although most studies showed a negative
impact of periodontitis, it is difficult to draw
definitive conclusions due to the heterogene
ity of methods and reporting and confound
ing by other oral conditions (Al‐Harthi
et al. 2013). A more recent systematic review
found a relationship between clinical perio
dontal disease extent and severity and
OHRQoL (Buset et al. 2016). No specific
studies on furcation treatment were detected
among those included in these reviews.
Some studies have attempted to investi
gate the effects of periodontal treatment on
patient‐centred outcomes. In a study on per
iodontitis patients in the UK, the OIDP
index was administered to 45 patients at
baseline and one month after treatment
(Tsakos et al. 2010); 17 of the patients
received intensive and 28 received

A Patient’s Point of View 251
‘ conservative’ periodontal care. Both the
generic and condition‐specific versions of
the OIDP for periodontal conditions were
used, and one of the aims of the study was to
estimate the minimally important difference
for this measure among periodontal patients.
The mean OIDP score after treatment was
significantly lower than at baseline, indicat
ing improvements in quality of life, with no
differences between treatment groups. In
general, the generic and condition‐specific
versions of the OIDP performed similarly,
but the differences were more distinct, with
a higher effect size, when the condition‐spe
cific version was used. This provided evi
dence in favour of the condition‐specific
version, even in a population of patients with
severe periodontitis where no such differ
ence would be expected, as almost all oral
impacts would be due to the periodontal
condition (rather than any other oral condi
tion). A difference of five points in the OIDP
was estimated to correspond to clinically
meaningful differences, thereby providing
context for changes in OHRQoL when using
this measure (Tsakos et al. 2010).
In a randomized controlled clinical trial,
both the OIDP and OHRQoL questionnaires
were given to 90 patients divided into two
groups: scaling and root planing (SRP, n = 45)
and one‐stage ‘full‐mouth disinfection’
(FMD, n = 45). All patients were then reas
sessed at two time points: 30 days and 180
days after treatment. Patients treated by
both SRP and FMD showed improvement
in all periodontal clinical parameters and
OHRQoL after treatment, with no significant
differences between treatment groups
(Santuchi et al. 2016). In a study by Makino‐
Oi and co‐workers (2016), improvements in
OHRQoL (in the domains pain and eating/
chewing function) mirrored clinical improve
ments after non‐surgical and then surgical
treatment of moderate to severe chronic
periodontitis. A greater improvement was
noted following surgical therapy, with no fur
ther improvements during maintenance care.
A randomized controlled trial evaluating
two educational programmes including
87 patients with chronic periodontitis assessed
OHRQoL with two different generic meas
ures: the General Oral Health Assessment
Index (GOHAI), which assesses the presence
of symptoms, and the UK oral health‐related
quality‐of‐life measure (OHQoL‐UK), which
assesses the impact of oral health using
a conceptualization of health beyond
the absence of disease. Improvements in
OHRQoL for both the GOHAI and the
OHQoL‐UK were detected after non‐
surgical periodontal therapy in both study
groups, without any significant difference
between the two groups. This research also
assessed the minimally important difference
for these measures, again providing some
context for the differences observed (Jonsson
and Öhrn 2014). An earlier systematic review
investigated the effect of surgical periodontal
therapy on OHRQoL. At that time, only three
studies qualified following full‐text screening
and the results were conflicting. Again, no
specific assessment of furcation intervention
was tested (Shanbhag et al. 2012).
13.3 Patient‐reported
Outcome Measures
in Furcation Involvement
Helldén and co‐workers (1989) presented
clinical and patient‐reported outcomes
from a retrospective study on molars with
furcation involvement treated with tunnel
preparations. A total of 156 teeth among
107 patients had been treated surgically with
tunnel preparations from 1977 to 1985.
In 1986, all patients were asked to return for
a re‐evaluation, and 102 attended. All teeth
involved were affected by degree III furcation
involvement and were treated with tunnel
ling surgery. In particular, following eleva
tion of full‐thickness flaps, the furcations
were widened by round burs at the entrance
and then by bone files to create space for
post‐surgical inter‐radicular plaque control,
after which the flaps were apically positioned,
sutured, and covered with surgical dressing.

Chapter 13
252
After removal of the dressing, the patients
were shown how to use interdental brushes
inside the tunnelled area. The majority of the
patients rinsed with 0.1% chlorhexidine for
4–6 weeks and at each post‐surgical visit a
fluoride varnish was applied to the teeth.
Following three‐ to six‐monthly maintenance
visits for two years, the patients returned to
their referring dentists for continued follow‐
up. On average, patients were re‐examined
37.5 months after surgery. Before the clinical
re‐evaluation, the following five questions
were asked of patients about their experience
with the furcation‐involved teeth (Helldén
et al. 1989):
1)
Do you have any discomfort from the tun
nel area?
2)
Does the gingiva bleed in the tunnel area?
3)
Is the tooth sensitive to cold or warm
temperatures?
4)
Do you easily get access to the tunnel area
for cleaning?
5)
What kind of oral hygiene aids do you use
in the tunnel areas?
At the end of the follow‐up period, 10 of 102
teeth had been extracted and 7 had been
hemisected or root resected, while 11 had
developed incipient root caries and 12 teeth
showed established caries lesions. Based on
patient feedback, most cases were not asso
ciated with any discomfort (92%), gingival
bleeding (72%), or sensitivity to cold or
warm temperatures (95%). Most patients
used a common toothbrush (98%) for the
outer part of the tooth and an interdental
brush for the tunnel areas (80%). Although
plaque removal presented some difficulties,
most such areas (70%) were found to be eas
ily accessible for cleaning procedures by
patients. The study by Helldén and co‐work
ers represented a pioneering attempt to
obtain information on subjective percep
tions in patients affected by furcation
involvement, although without a validated
questionnaire. A systematic review on peri
odontal regeneration of furcation defects by
the American Academy of Periodontology
recently reported that none of the reviewed
studies had included any patient‐reported
outcomes. The authors highlighted the need
to introduce this aspect in furcation research
(Avila‐Ortiz et al. 2015).
13.4 Patient Feedback
Given the paucity of data on PROMs relative
to furcation involvement, some patients
treated by the author were asked to provide
feedback on their experience with furcation
treatment. These are provided in this section.
Patient Feedback 1 (70‐year‐old
Female, 10 Years After Tunnelling
Surgery)
‘I was made aware of the gap between the
roots of my bottom right last tooth
10 years ago when I had a surgical proce-
dure on it. Now I feel that, as I go through
the process of cleaning my teeth, I auto-
matically clean inside the roots and it
doesn’t feel any different than cleaning the
other teeth. Occasionally I get some food
stuck on that tooth. I come regularly to see
the hygienist and periodontist and I am
used to it now.’
Patient Feedback 2 (65‐year‐old
Male, 12 Years After Tunnelling
Surgeries)
‘When I first saw a periodontist my mouth
was in a poor state. Whenever I brushed
my teeth my gums bled, and I was afraid
of losing my teeth but I did not know how
to make them better. At that stage I did
not realize that bone loss caused by gum
disease meant that the gaps between the
furcations in the roots were hard or impos-
sible to clean, and were providing a gath-
ering place for bacteria causing the disease
to continue and worsen. The periodontist
performed a couple of operations to open
up the gums to enable me to clean into

A Patient’s Point of View 253
these gaps, and showed me how to do the
cleaning using little interdental brushes.
From that time on I have been able to
clean my teeth fully without any bleeding,
and the disease has gone. I still lost some
of my teeth; three of them have been
replaced by implants, and these together
with what remains of my natural teeth are
sufficient for me to eat effectively. I have
always dreaded the idea of having den-
tures, which seemed inevitable, but more
than 12 years later I am keeping the dis-
ease and decay at bay and I remain hope-
ful of avoiding dentures for the foreseeable
future.’
Patient Feedback 3 (60‐year‐old
Male, 10 Years After Tunnelling
Surgery)
‘Having undergone surgery to my right
upper teeth to combat gum disease some
years ago I am very fortunate to say that
after several years I am being able to
maintain a reasonable level of health in
this area. Following the surgery I took the
view that I would work hard to maintain
the situation and by diligence have, it
seems, managed to do so. I have a cleans-
ing regime that I adhere to rigidly and
includes both morning and night a pro-
cess which takes me approximately
15 minutes each time. And includes
cleansing with an interdental brush, floss-
ing, cleansing with ‘sensitive’ toothpaste
and good quality electric brush and more
work with the interdental brush to finish
off. At times when the mouth is sensitive I
brush with a specific gel. Although this
takes some time and I have to exercise
some personal discipline I believe it is
time well spent and will do anything to
prevent the loss of further teeth. The
health of my mouth in general has also
improved greatly. I also believe that my
general health has improved as a result of
this improvement in my oral health. I am
very grateful that through this regular
care and this regime I have managed to
extend the life of these teeth. May this
continue!’
Patient Feedback 4 (50‐year‐old
Male, 6 Years After Tunnelling
Surgery on One Molar and
Extraction and Implant Placement
on the Contralateral Molar)
‘Following treatment by my dentist
whereby I had a tooth extraction on my
right lower jaw and surgery to keep a tooth
on my left lower jaw. With reference to my
right jaw and tooth extraction I allowed
the bone in that area of extraction to grow
back before having an implant to secure
and strengthen the teeth in that area. The
additional surgery I had on my left jaw
meant that my tooth was saved. My dentist
inserted a small piece of tube between the
two roots to enable the cleaning of the tooth
and root area. All I do now on a daily basis
is clean in between my teeth and roots
using interdental brushes which is totally
pain free and easy to do.’
Patient Feedback 5 (45‐year‐old
Female, 10 Years After Root‐
resection Surgery)
‘Soon after I was referred to the periodon-
tist for treatment for my gums, a very fact‐
facing appointment, back tooth removed
and three operations to clean the roots and
I started to realize that there was a serious
chance that I would lose some if not all my
teeth, if I did not make a change. I then
started on an ongoing treatment plan of
seeing the periodontist every six months
and the hygienist every alternative six
months, but at this point I was still smok-
ing. I was eventually able to give up smok-
ing. Treatment is ongoing, I still have most
of my teeth and I plan to keep them. I wish
there was a once and for all cure, but am
resigned to having ongoing maintenance
treatment.’

Chapter 13
254
Patient Feedback 6 (63‐year‐old
Male, 5 Years After Molar
Extraction and Implant Placement)
‘Given my experience as a dental patient,
I consider myself an expert. I’ve had caries,
gum disease and extractions, followed by
crowns and bridges. I work very hard at
my dental hygiene, but I recognize I could
probably do better. Over the years I’ve had
problems around my crowns and bridges on
my back teeth, both aesthetic concerns and
several flare‐ups with inflammation and
discomfort. I think that this is because plaque
gets stuck under the crown and bridgework.
Some years ago, one of my molars was finally
extracted and replaced with an implant. I’ve
had to wait a few months after the extraction
to finally have a crown on the implant, but
I understand that is normal procedure. I feel
the implant like a normal tooth, like it’s
always been there and I’ve no longer had any
discomfort or inflammation.’
13.5 Reflections on
Patient Feedback
While accepting that this is by no means a
representative patient sample and that
reporting feedback for the treating clinician
may be seen as a biased exercise, several
important items emerged from this feed
back. It is clear that these patients were
highly motivated and made a long‐term
commitment to the survival of their teeth.
They effectively decided to change their
dental health behaviour, including giving up
smoking (‘I eventually gave up smoking’) and
working hard on their oral hygiene (‘I have a
cleansing regime that I adhere to rigidly and
includes both morning and night a process
which takes me approximately 15 minutes
each time’). In some cases this did not appear
to be a burden on the patient (‘All I do now
on a daily basis is clean in between my teeth
and roots using interdental brushes which is
totally pain free and easy to do’), while
in other cases it did (‘am resigned to
having ongoing maintenance treatment’).
The specific act of cleaning inside a tunnelled
furcation area was described as a relatively
easy task by these patients (‘I automatically
clean inside the roots and it doesn’t feel any
different than cleaning the other teeth’).
The patients’ perception of the treatment
carried out is also very interesting, ranging
from what seems like a good understanding
of the surgical tunnelling procedure (‘the gaps
between the furcations in the roots were hard
or impossible to clean, and were providing a
gathering place for bacteria causing the dis
ease to continue and worsen. The periodon
tist performed a couple of operations to open
up the gums to enable me to clean into these
gaps’) to a rather more imaginative interpre
tation (‘My dentist inserted a small piece of
tube between the two roots to enable the
cleaning of the tooth and root area’).
The experience of having a dental implant to
replace a molar previously affected by severe
periodontal disease was also seen as very pos
itive (‘I’ve had problems around my crowns
and bridges on my back teeth, both aesthetic
concerns and several flare‐ups with inflam
mation and discomfort. … one of my molars
was finally extracted and replaced with an
implant. … I feel the implant like a normal
tooth, like it’s always been there and I’ve no
longer had any discomfort or inflammation’).
13.6 Implementation of
PROMs in Furcation
Treatment
Given the increased recognition of the impor
tance of patient‐reported outcomes in medi
cine, the future should see the use of OHRQoL
measures in studies investigating furcation
treatment. The pioneering effort by Helldén
and co‐workers (1989, see earlier discussion)
to investigate patient perceptions should be
extended by using validated OHRQoL
measures, and potentially by developing a
condition‐specific validated questionnaire
which also takes into account items such as
sensitivity, ease of cleaning inside the furca
tion area, and the alternatives of extraction

A Patient’s Point of View 255
with or without replacement. The develop
ment of such a condition‐specific measure
would need to be driven by qualitative
research highlighting the main concerns of
patients with furcation involvement.
More importantly, it would be ideal to use
PROMs as outcomes in long‐term randomized
controlled trials testing different modalities of
furcation treatment (for example conservative
treatment vs root resection vs extraction and
implant therapy). This could provide answers
on the effects of these procedures on patients’
quality of life and, together with clinical and
financial considerations, could help design fur
cation treatment guidelines. In the everyday
clinical reality, an assessment of patient per
ceptions related to periodontal treatment
needs to accompany purely clinical and finan
cial considerations. This could be considered
in the treatment planning stages as well as
being an outcome measure to assess the effec
tiveness of interventions.
Acknowledgements
The invaluable help and advice of Dr George
Tsakos, University College London, in the
preparation and revision of this chapter is
gratefully acknowledged.
References
Adulyanon, S., Vourapukjaru, J., and
Sheiham, A. (1996). Oral impacts
affecting daily performance in a low dental
disease Thai population. Community
Dentistry and Oral Epidemiology 24,
385–389.
Al‐Harthi, L.S., Cullinan, M.P., Leichter, J.W.,
and Thomson, W.M. (2013). The impact of
periodontitis on oral health‐related quality
of life: A review of the evidence from
observational studies. Australian Dental
Journal 58, 274–277.
Aslund, M., Suvan, J., Moles, D.R. et al. (2008).
Effects of two different methods of non‐
surgical periodontal therapy on patient
perception of pain and quality of life: A
randomized controlled clinical trial. Journal
of Periodontology 79,1031–1040.
Avila‐Ortiz, G., De Buitrago, J.G., and
Reddy, M.S. (2015). Periodontal
regeneration – furcation defects:
A systematic review from the AAP
Regeneration Workshop. Journal of
Periodontology 86 (Suppl. 2), S108–S130.
Brennan, D.S., Spencer, A.J., and Roberts‐
Thomson, K.F. (2007). Quality of life and
disability weights associated with
periodontal disease. Journal of Dental
Research 86, 713–717.
Buset, S.L., Walter, C., Friedmann, A. et al.
(2016). Are periodontal diseases really
silent? A systematic review of their effect on
quality of life. Journal of Clinical
Periodontology 43, 333–344.
Glick, M., and Meyer, D.M. (2014). Defining
oral health: A prerequisite for any health
policy. Journal of the American Dental
Association 145, 519–520.
Glick, M., Williams, D.M., Kleinman, D.V. et al.
(2017). Reprint of: A new definition for oral
Summary of Evidence
●
The attention to patients’ preferences and
points of view needs to take centre stage
in treatment planning. As such, patient‐
reported outcome measures (PROMs) are
fast becoming essential outcomes of any
periodontal research study.
●
There is a paucity of data on PROMs in
patients with furcation involvement.
●
Investigation of PROMs relative to furca
tion involvement should, together with
clinical and financial considerations, form
the basis of furcation treatment planning.

Chapter 13
256
health supported by FDI opens the door to a
universal definition of oral health. Journal of
Dentistry 57, 1–3.
Helldén, L.B., Elliot, A., Steffensen, B., and
Steffensen, J.E.M. (1989). Prognosis of
tunnel preparations in treatment of class III
furcations: A follow‐up study. Journal of
Periodontology 60, 182–187.
Herdman, M., Gudex, C., Lloyd, A. et al.
(2011). Development and preliminary
testing of the new five‐level version of
EQ‐5D (EQ‐5D‐5L). Quality of Life Research
20, 1727–1736.
Jönsson, B., and Öhrn, K. (2014). Evaluation of
the effect of non‐surgical periodontal
treatment on oral health‐related quality of
life: Estimation of minimal important
differences 1 year after treatment. Journal of
Clinical Periodontology 41, 275–282.
Lawal, F.B., Taiwo, J.O., and Arowojolu, M.O.
(2015). Comparison of two oral health‐
related quality of life measures among adult
dental patients. Oral Health and
Preventative Dentistry 13, 65–74.
Locker, D. (1988). Measuring oral health:
A conceptual framework. Community
Dental Health 5, 3–18.
Makino‐Oi, A., Ishii, Y., Hoshino, T. et al.
(2016). Effect of periodontal surgery on oral
health‐related quality of life in patients who
have completed initial periodontal therapy.
Journal of Periodontal Research 51,
212–220.
Santuchi, C.C., Cortelli, J.R., Cortelli, S.C.
et al. (2016). Scaling and root planing per
quadrant versus one‐stage full‐mouth
disinfection: Assessment of the impact of
chronic periodontitis treatment on quality
of life – a clinical randomized, controlled
trial. Journal of Periodontology 87, 114–123.
Shanbhag, S., Dahiya, M., and Croucher, R.
(2012). The impact of periodontal therapy
on oral health‐related quality of life in
adults: A systematic review. Journal of
Clinical Periodontology 39, 725–735.
Slade, G.D. (1997) Derivation and validation of
a short‐form oral health impact profile.
Community Dentistry and Oral
Epidemiology 25, 284–290.
Tsakos, G., Bernabé, E., D’Aiuto, F. et al.
(2010). Assessing the minimally important
difference in the oral impact on daily
performances index in patients treated for
periodontitis. Journal of Clinical
Periodontology 37, 903–909.
U.S. Department of Health and Human
Services, Food and Drug Administration,
Center for Drug Evaluation and Research
(CDER) et al. (2009). Guidance for industry:
Patient‐reported outcome measures: Use in
medical product development to support
label claims. Rockville, MD: FDA.
WHOQOL Group (1994). Development of the
WHOQOL: Rationale and current status.
International Journal of Mental Health
23, 24–56.

Chapter No.: 1 Title Name: <TITLENAME>
c14.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:22:33 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 257
257
Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
In an attempt to apply the knowledge gained
throughout this book to the treatment of
cases with furcation involvement, two
example cases are presented. They will be
discussed in light of the evidence gained in
each of the previous 13 chapters.
14.1 Case 1 (Maxillary)
A 50‐year‐old male patient presented with a
complaint of bleeding on brushing and gum
recessions. He was medically healthy, never
smoked, and was not aware of any family
history of periodontal disease. His oral
hygiene was good, with full mouth plaque
scores < 10%; he had generalized gingival
recessions and localized probing pocket
depths (PPD) > 4 mm only on upper molars.
The case will be dissected based on the
evidence provided in each of the previous
chapters before reaching a treatment plan-
ning decision.
14.1.1 Anatomy (Chapter 1)
The maxillary first and second right and left
molars in Figure 14.1 present a relatively
‘normal’ anatomy, with three roots each, all
apparently distally curved to varying degrees.
The root trunks appear reasonably small,
in favour of longer root cones. High root
divergence appears on teeth 16 (UR6) and
26 (UL6), while the roots of 17 (UR7) and
27 (UL7) are less divergent. Among predis-
posing factors described in Chapter 1,
bifurcation ridges, enamel projections, and
enamel pearls do not seem to be present.
14.1.2 Diagnosis (Chapter 2)
Although two‐dimensional radiographic
examination is not completely reliable for fur-
cation diagnosis, the areas of radiolucency
between roots, coupled with interproximal
bone levels apical to the furcation entrances,
indicate a likely triple through‐and‐through
furcation involvement (FI) for teeth 16, 17, and
27. Doubts exist about possible FI on tooth 26.
Clinical examination with a curved Nabers
probe confirms this, with a diagnosis of triple
(buccal, mesial, and distal) degree III FI on
teeth 16, 17, and 27 (Hamp et al. 1975). The
same diagnosis would be given when using the
Glickman (1953) classification and the classi-
fication modified by Ammons and Harrington
(2006; see Table 2.4). Degree I FI was recorded
only distal of 26. Vertical subclassification
(Tarnow and Fletcher 1984) is reported in
Table 14.1. The comprehensive diagnosis
system in the table summarizes clinical and
radiographic findings on example tooth 16.
Chapter 14
Assessment of Two Example Cases Based
on a Review of the Literature
Luigi Nibali
Centre for Immunobiology and Regenerative Medicine, Centre for Oral Clinical Research, Institute of Dentistry, Barts and the London
School of Medicine and Dentistry, Queen Mary University of London (QMUL), London, UK
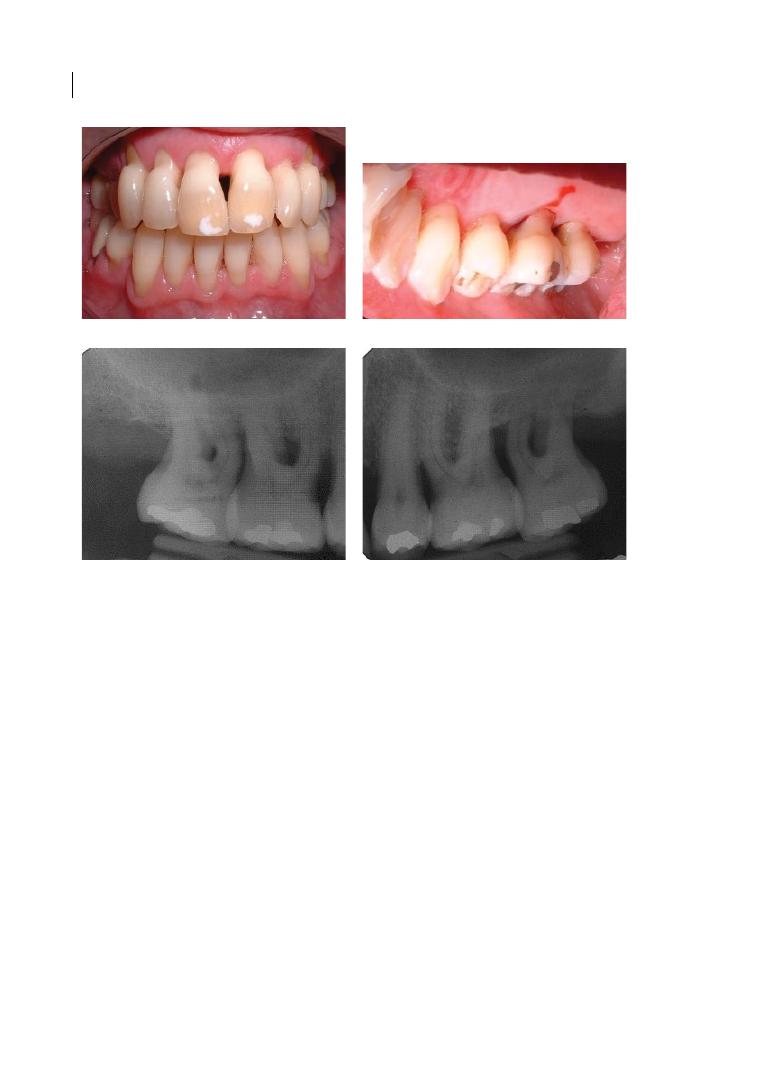
Chapter 14
258
14.1.3 Initial Therapy (Chapter 3)
Most furcation areas, including probably
those in this case, have narrow entrances
(<0.75 mm). Ultrasonic scalers, especially
with slimline tips, have been shown to be
better suited than hand instruments for the
debridement of narrow furcation areas
(Matia et al. 1986; Sugaya et al. 2002), par-
ticularly in degree II and III FI (Leon and
Vogel 1987). Micro Mini Five® Gracey curettes
could also be helpful in narrow furcation
entrances (see Chapter 3 for details).
However, interproximal FI is likely to respond
less favourably to mechanical debridement
compared to buccal furcations (Del Peloso
Ribeiro et al. 2007) and more residual calcu-
lus will be left with a closed (non‐surgical)
approach compared to an open approach
(Matia et al. 1986; Fleischer et al. 1989).
Although this patient exhibits very good oral
hygiene levels, it is crucial that further oral
hygiene reinforcements are given as part of
initial periodontal therapy. Research shows
that in interproximal sites, bristled or rubber
interdental brushes remove more plaque
than flossing or brushing alone (Christou
et al. 1998; Abouassi et al. 2014). Therefore,
the use of interdental brushes of the correct
size and shape in the molar region should be
encouraged and discussed with the patient.
14.1.4 Endodontics (Chapter 4)
Although all four maxillary molars have res-
torations, they are not large enough to
endanger the endodontic status of the teeth.
However, accessory canals in the furcation
region are frequent and might represent a
communication pathway between endodon-
tic and periodontal pathologies through
(a)
(b)
(c)
(d)
Figure 14.1
Case 1, clinical photograph of frontal (a) and upper right palatal (b), followed by baseline
periapical radiographs of upper molars (c and d), the only teeth with probing pocket depth > 4 mm at first visit.

Assessment of Two Example Cases 259
the induction of inflammatory responses.
Furthermore, a small radiolucency area
seems to be present on 17 (palatal root).
Therefore, it is always advisable to test tooth
vitality. Vitality testing was positive for all
tested molars (16, 17, 26, and 27). Hence no
endodontic treatment was required, but
retesting during treatment and maintenance
therapy was recommended.
14.1.5 Long‐term Prognosis
(Chapter 5)
Molars with FI have a higher risk of tooth
loss than molars with no FI (twice as likely to
be lost up to 15 years of follow‐up), following
comprehensive periodontal treatment (non‐
surgical and surgical). For degree III FI, the
relative risk of tooth loss is approximately
three times that of degree I FI molars and
twice compared with degree II FI molars
(Nibali et al. 2016). In a follow‐up mainte-
nance programme varying from 5 to 53 years,
30% of degree III FI molars are lost (Nibali
et al. 2016). Meta‐analyses providing specific
data for maxillary degree III FI molars, or
specifically for first or second molars, are
lacking. Based on these data, it may be con-
sidered worth trying to maintain this tooth.
Treatment options need to be assessed in the
following sections. A strict post‐treatment
maintenance programme including three‐ to
Table 14.1
Case 1, diagnosis of furcation on 16 based on Muller and Eger (1999).
Patient PL (age 50)
Tooth 16
Mobility (0, 1, 2, 3)
0
Elongation (0, 1)
0
Sensibility testing (1: positive, 2: negative)
1
Endodontic dx (0: OK, 1: revision necessary)
0
Caries/restorations (0: caries free, 1: small caries or filling,
2: extended caries, large filling, 3: artificial crown)
1
Rx diagnosis
Mesial root
Distal root
Palatal root
M
D
M
D
M
D
Bone loss
2
2
2
2
1
1
m/d roots m/p roots
d/p roots
Separation degree
1
1
1
Degree of divergence 1
1
1
Clinical diagnosis
mb
b
db
mp
p
dp
BOP (0,1)
1
0
1
1
0
0
Plaque (0,1)
0
0
0
0
0
1
PPD
6
6
6
6
3
7
vCAL
7
7
8
9
6
9
hCAL
–
6
–
6
–
6
Degree
–
III B
–
III B
–
III C
Bone loss: 0 (≤ ⅓ root length), 1 (⅓–⅔ root length), 2 (≥ ⅔ root length); separation degree:
0 (< ⅓), 1 (> ⅓); degree of divergence: 0 (≤30°), 1(>30°).
B = buccal; BOP = bleeding on probing; CAL = clinical attachment loss; D = distal;
h = horizontal; M = mesial; P = palatal; PPD = probing pocket depth; v = vertical.

Chapter 14
260
four‐monthly periodontal charting, supra‐
and subgingival debridement, and oral
hygiene reinforcement and motivation is
recommended based on the available litera-
ture (Nibali et al. 2016).
14.1.6 Regeneration
(Chapters 6 and 7)
The first aim of periodontal treatment, where
possible, should be to regenerate the lost per-
iodontal support. However, despite some
successful reports of regeneration of degree
III FI molars in animal models (Chapter 6),
human studies do not support the use of
regeneration in maxillary degree III FI molars
(Chapter 7). In particular, one randomized
controlled trial with split‐mouth design with
11 patients compared guided tissue regener-
ation (GTR) and open‐flap debridement
(OFD) in the treatment of maxillary inter-
proximal degree III furcation defects
(Pontoriero and Lindhe 1995). Baseline and
six‐month examinations were performed by
re‐entry after flap elevation. Neither GTR
nor OFD led to even partial closure of the
22 degree III furcations. Based on these data,
these teeth are not suitable for regenerative
treatment of their FI.
14.1.7 Resective Therapy
(Chapter 8)
Common sense would suggest that if you
cannot close the furcation by regeneration, at
least you should eliminate it surgically or
make it cleansable. The options of root sepa-
ration, root amputation, and root resection
could be considered in a case of advanced FI,
as for the molars in this case. However, these
options are more suitable when, for example,
one root is affected to a greater extent than
the others. Furthermore, particular caution
should be taken when the teeth are intact,
because this is an invasive procedure involv-
ing a considerable biological cost that must
always be carefully evaluated. For this rea-
son, in this case, inadequate residual attach-
ment on the remaining roots and tooth vitality
do not make root resection the preferred
treatment option for teeth 16, 17, and 27.
14.1.8 Tunnelling (Chapter 9)
The furcation tunnelling procedure should
be considered at stable (no more than mobil-
ity grade I) furcation‐involved molars with
advanced inter‐radicular bone loss (prefera-
bly degree III FI) when accessibility to the
furcation area for plaque removal is difficult.
As a rule of thumb, the alveolar bone sup-
port should be of equal amounts at all roots
and at least cover one‐third of the root
length, with mainly horizontal bone loss.
The length of the root trunk should not
exceed 4 mm and the diameter of the furca-
tion entrance should be at least 0.5 mm.
Fulfilment of these criteria for teeth 17, 16,
and 27, this patient’s full cooperation, good
oral hygiene dexterity and attitude, and a
relatively low caries risk make tunnelling
surgery an attractive treatment option in this
case. However, we should bear in mind that
very few reports of long‐term success of tri-
ple maxillary tunnels exist in the literature
(Helldén et al. 1989).
14.1.9 Innovative and Adjunctive
Therapy (Chapter 10)
In order to maximize the efficacy of non‐
surgical therapy of FI on the molars pre-
sented here, adjunctive systems such as an
endoscope, lasers, photodynamic therapy,
air‐polishing devices, antimicrobials, or
probiotics could be considered. However,
this should be carefully weighed in the light
of costs and the so far poor evidence for their
efficacy specific to furcations (de Andrade
et al. 2008; Ribeiro Edel et al. 2010; Tomasi
and Wennstrom 2011; Eickholz et al. 2016).
14.1.10 Extraction and Implant
Placement (Chapter 11)
Given the extensive loss of periodontal sup-
port and FI and the perceived low predicta-
bility of furcation treatment, teeth 17, 16, and
Assessment of Two Example Cases 261
27 could be considered hopeless by some
treating clinicians based on published prog-
nostic systems (Becker et al. 1984; Machtei
et al. 1989). If a decision was made to extract
them, the options of a shortened dental arch,
partial dentures, or implants could be con-
sidered. Bearing in mind the high long‐term
survival rates of implant‐supported restora-
tions (Moraschini et al. 2015) and the
r elatively young age and motivation of the
patient, implant placement was discussed as
his main alternative option to tooth reten-
tion. However, as discussed in Chapter 11,
reduced quantity of bone, proximity to maxil-
lary sinus, need for grafting, and previous his-
tory of periodontitis may result in reduced
implant‐survival rates in this case (Becker
et al. 1999; Drago 1992; Graziani et al. 2004;
Pjetursson et al. 2008). The option of extrac-
tion, socket preservation, and short implants
could also be considered, provided enough
residual bone was available to allow implant
placement (Thoma et al. 2015).
14.1.11 Health Economics
(Chapter 12)
A handful of studies have now shown that
treating molars with degree III FI via tooth‐
retaining options was more effective and less
costly than tooth removal and replacement
via implant‐supported restorations (Fardal
and Grytten 2013; Martin et al. 2014;
Schwendicke et al. 2014). Among possible
treatment options, considering that all
molars are still vital, it remains uncertain
whether the high costs for root‐resective
therapy could be truly justified (Little et al.
1995; Blomlof et al. 1997; see Chapter 12
for more details).
14.1.12 Patient’s Point of View
(Chapter 13)
In the absence of data on patient‐reported
outcomes on treatment of degree III FI, some
suggestions could be drawn from the paper
on tunnelling discussed in Chapter 13
(Helldén et al. 1989). Most cases treated
with tunnelling were not associated with any
discomfort, gingival bleeding, or sensitivity.
Although plaque removal presented some
difficulties, most furcation areas were found
to be easily accessible for cleaning proce-
dures by patients with interdental brushes. In
this specific case, the patient expressed his
wish to maintain these teeth for as long as
possible, and he showed very good oral hygiene
and commitment.
14.1.13 Treatment Decision
Based on all these considerations, backed
when possible by data from the literature,
it was decided to maintain the molars
affected by FI and to carry out non‐surgical
debridement and oral hygiene reinforce-
ment. Following re‐evaluation two months
later, residual pockets and FI were detected
on the affected molars and it was decided
to proceed with tunnelling surgeries for 16,
17, and 27, followed by strict supportive
periodontal therapy (see Chapter 9 for a
step‐by‐step guide on the furcation tunnel-
ling surgical technique). Post‐operative
photos and radiographs are presented in
Chapter 15 (Figure 15.8). The main reasons
for this choice are summarised in
Table 14.2).
Table 14.2
Case 1, main reasons for treatment
choice (tunnelling surgery).
Factors
TOOTH
Good root divergence
Short root trunk
Prevalently horizontal bone loss
affecting all roots
Tooth vitality and no restorative
concerns
Importance in masticatory function
PATIENT
Clear medical history
Non‐smoker
Motivation to keep teeth
No financial or other concerns for
surgical treatment
Good oral hygiene dexterity
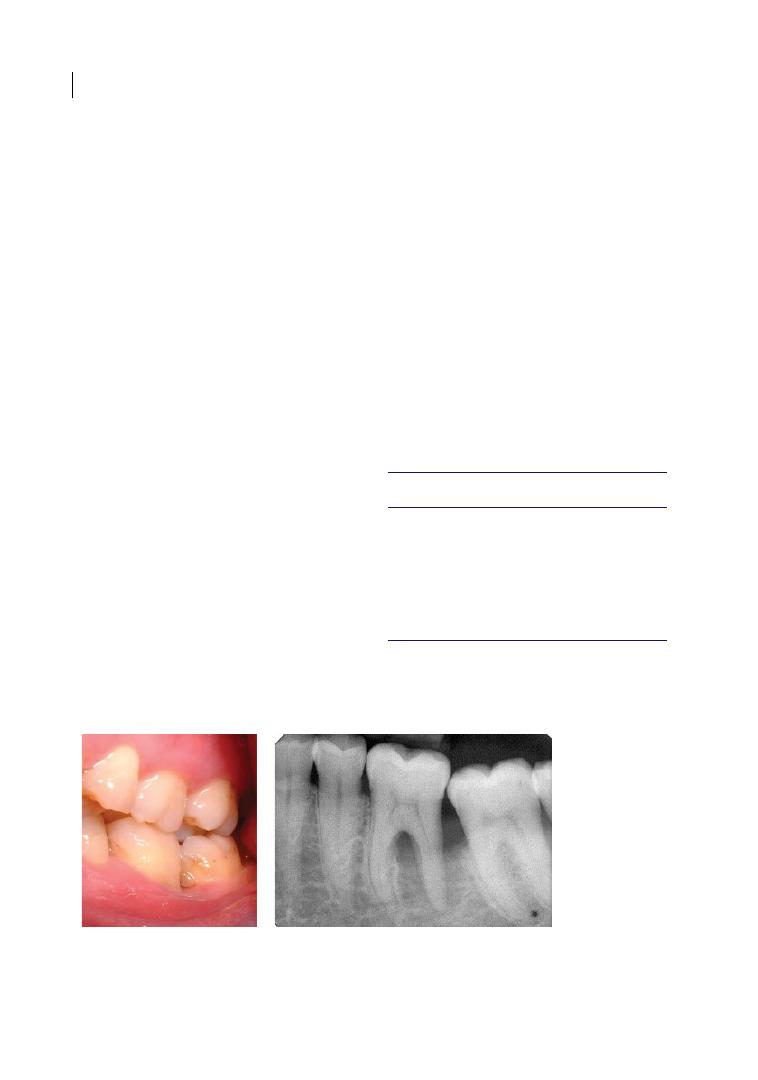
Chapter 14
262
14.2 Case 2 (Mandibular)
A 47‐year‐old male patient presented with a
complaint of occasional bleeding on brush-
ing and discomfort from the lower left gingi-
vae. He was medically healthy, he used to
smoke (10 a day for 20 years, and gave up 10
years before the first examination), and was
not aware of any family history of periodon-
tal disease. His oral hygiene was fair, with full
mouth plaque scores of 40% (generalized
interproximal plaque), localized gingival
recessions, and localized probing pocket
depths > 4 mm only on the lower left first
molar (LL6). The case will be dissected based
on the evidence provided in each of the pre-
vious chapters before reaching a treatment
planning decision.
14.2.1 Anatomy (Chapter 1)
The mandibular left first molar (LL6) in
Figure 14.2 presents a relatively ‘normal’
anatomy, with two roots (mesial and dis-
tal). The mesial root is slightly distally
tilted towards the apex. The root trunks
and root cones appear to be of average
length and root divergence seems normal.
Among the predisposing factors described
in Chapter 1, bifurcation ridges, enamel
projections, and enamel pearls do not seem
to be present.
14.2.2 Diagnosis (Chapter 2)
Although two‐dimensional radiographic
examination is not completely reliable for
furcation diagnosis, the area of radiolu-
cency between mesial and distal roots,
coupled with distal interproximal bone
levels apical to the furcation entrances,
suggests a likely through‐and‐through fur-
cation involvement (FI). However, clinical
examination with a curved Nabers probe
only resulted in degree II buccal and lin-
gual FI (Hamp et al. 1975) and a possible
degree III when using the Glickman (1953)
classification and the classification modi-
fied by Ammons and Harrington (2006;
see Table 2.4). Probing pocket depths are
reported in Table 14.3.
(a)
(b)
Figure 14.2
Case 2, clinical photograph (a) of left mandibular first molar, followed by baseline periapical
radiograph (b).
Table 14.3
Case 2, evaluation.
Tooth 36 (LL6)
v‐PPD
CAL
h‐PPD
Mesio‐buccal
2
2
–
Buccal
8
10
5
Disto‐buccal
12
15
–
Mesio‐lingual
3
3
–
Lingual
7
7
4
Disto‐lingual
10
11
–
CAL = clinical attachment loss; h‐PPD = horizontal
probing pocket depth; v‐PPD = vertical probing
pocket depth.

Assessment of Two Example Cases 263
On vertical probing, degree C FI was diag-
nosed (exceeding 6 mm in both buccal and
lingual furcations; Tarnow and Fletcher
1984). Therefore, a degree II C diagnosis was
given to both furcations, but with doubt on
possible degree III through‐and‐through. It
is important to note that the furcation defect
is combined with a very deep distal intra-
bony defect, reaching the apex of the distal
root, with some reduced bone support also
on the mesial aspect of neighbouring tooth
37 (LL7).
In this case, due to the difficulty in probing
the furcation, it may be worth considering a
three‐dimensional radiograph to ascertain
the presence of residual alveolar bone by the
furcation fornex for treatment planning
purposes.
14.2.3 Initial Therapy (Chapter 3)
As discussed for Case 1, due to narrow furca-
tion entrances ultrasonic scalers with slim‐
line tips, possibly complemented by Micro
Mini Five® Gracey curettes, are particularly
suited for the debridement of FI. It is likely
that residual calculus might be left with a
closed (non‐surgical) approach compared to
the open approach (Matia et al. 1986;
Fleischer et al. 1989), although probably not
as much as in interproximal furcations.
Particular attention needs to be paid to the
difficult‐to‐reach concavity around the
furcation fornix. An improvement in oral
hygiene levels is necessary for this patient,
with the introduction of large interdental
brushes for cleaning the interproximal area
between LL6 and LL7.
14.2.4 Endodontics (Chapter 4)
Although the tooth is non‐restored, due to
extensive FI and a distal intrabony defect
reaching the apex, a neuro‐vascular bundle
damage may have occurred (Langeland et al.
1974). Therefore, it is advisable to test tooth
vitality. Vitality testing was positive for 36.
Some authors have even advocated that since
pulp necrosis might occur during periodontal
healing, root canal treatment could be pre-
ventively performed to avoid any interference
with the regeneration process in case of peri-
odontal bone loss to the apex (Cortellini and
Tonetti 2001). However, this was not consid-
ered necessary in this case. Instead, retesting
of vitality after treatment was planned.
14.2.5 Long‐term Prognosis
(Chapter 5)
According to the evidence discussed in
Chapter 5, following comprehensive perio-
dontal treatment, as a furcation‐involved
molar this tooth has a higher risk of tooth loss
than a molar with no FI (twice as likely to be
lost up to 15 years of follow‐up). According to
a recent systematic review (Nibali et al. 2016),
18% of degree II FI molars are lost in a follow‐
up maintenance programme varying from
5 to 53 years, and this tooth’s relative risk of
needing extraction is 1.67 compared with
degree I FI molars. The risk could actually be
higher for this tooth, affected at least by dou-
ble degree II FI and possibly by degree III FI.
Based on these data, it may be worth trying to
maintain this tooth. Treatment options need
to be assessed, as in the following sections.
A strict post‐treatment maintenance pro-
gramme including three‐ to four‐monthly
periodontal charting, supra‐ and subgingival
debridement, and oral hygiene reinforcement
and motivation are recommended based on
the available literature (Nibali et al. 2016).
14.2.6 Regeneration
(Chapters 6 and 7)
Tooth 36 seems to be affected by degree II FI,
although a degree III FI cannot be ruled out.
Despite some successful reports of regenera-
tion of degree III furcation‐involved molars
in animal models, according to the literature
reviewed in Chapter 7, human studies do not
support the use of regeneration in maxillary
degree III FI molars. However, in case of
mandibular FI II, the chances are good of

Chapter 14
264
achieving at least a partial fill of the furcation
defect, converting the degree II to a degree I
FI. Treatment with either guided tissue
regeneration (GTR) or enamel matrix deriva-
tive (EMD) has produced histological evi-
dence of regeneration (Stoller et al. 2001;
Nevins et al. 2003) and consistently more
favourable clinical outcomes (reduction to
degree I or closure) compared with access
flaps in degree II mandibular furcations
(Jepsen et al. 2002, 2004). The high bone sup-
port on the mesial root of 36 (above the fur-
cation fornix) as well as on the mesial root of
the adjacent 37 (next to the distal intrabony
defect of 36) would have a beneficial impact
on the regeneration potential, provided that
good soft‐tissue closure of the defect could
be achieved post‐operatively. Therefore, if
degree II FI was confirmed intra‐surgically,
according to the evidence reviewed in
Chapter 7, this molar appears to be suitable
to benefit from regenerative furcation ther-
apy, although, due to the bilateral degree II FI
(in the best‐case scenario), the results of
regenerative therapy might be less predicta-
ble than in a single degree II FI.
14.2.7 Resective Therapy
(Chapter 8)
The options of root separation, root amputa-
tion, and root resection could be considered
in cases of advanced FI, like this one. The
options of root amputation (removing the
distal root and leaving the crown intact) or
root resection (removing the distal root and
the relative section of the crown) are particu-
larly suitable when one root is affected to a
greater extent than the others, which is the
case for the tooth in question. Although the
distal root has a significantly smaller root
surface area than the mesial one (Dunlap and
Gher 1985), the mesial root has a deep con-
cavity, which makes it more difficult to endo-
dontically treat and properly prepare and
restore. The reported long‐term survival of
root‐resected molars is in the 60–90% range
for studies up to 10 years (Langer et al. 1981;
Carnevale et al. 1998; reviewed in Chapter 8).
However, caution should be employed, as the
tooth presented here is not restored, hence a
root‐resection procedure would involve a
considerable biological cost.
14.2.8 Tunnelling (Chapter 9)
As seen for Case 1, the furcation tunnelling
procedure should be considered for furca-
tion‐involved molars with advanced inter‐
radicular bone loss (preferably FI degree III)
when accessibility to the furcation area for
plaque removal is difficult. Indications for
tunnelling are mainly good oral hygiene dex-
terity and motivation, and horizontal bone
loss covering at least one‐third of the root
length. Therefore, this case does not seem
suitable for tunnelling surgery.
14.2.9 Innovative and Adjunctive
Therapy (Chapter 10)
As discussed for Case 1, adjunctive therapy
to non‐surgical periodontal treatment could
be considered, but should be carefully bal-
anced with costs and the so far poor evidence
for their efficacy specific to furcations (de
Andrade et al. 2008; Ribeiro Edel et al. 2010;
Tomasi and Wennstrom 2011; Eickholz
et al. 2016).
14.2.10 Extraction and Implant
Placement (Chapter 11)
Given the extensive loss of periodontal sup-
port to the apex and FI, tooth 36 might be
considered hopeless based on published
prognostic systems (Becker et al. 1984;
Machtei et al. 1989; Cortellini et al. 2011).
Reduced bone quantity on the distal aspect of
the tooth may mean that there might be need
for bone grafting after extraction; a previous
history of periodontitis might result in a
reduced implant‐survival rate in this case
(Drago 1992; Becker et al. 1999; Graziani
et al. 2004; Pjetursson et al. 2008). However, it
is not inconceivable to think that, instead of
distal root resection, extraction of the tooth
and replacement could be an option here.

Assessment of Two Example Cases 265
14.2.11 Health Economics
(Chapter 12)
As discussed earlier, studies have now shown
that treating molars with degree II and III FI
via tooth‐retaining options was more effective
and less costly than tooth removal and replace-
ment via implant‐supported restorations
(Fardal and Grytten 2013; Martin et al. 2014;
Schwendicke et al. 2014). However, this may
not apply if the more invasive and expensive
options of root resection, endodontic therapy,
and restoration are chosen for tooth retention
(Little et al. 1995; Blomlof et al. 1997).
14.2.12 Patient’s Point of View
(Chapter 13)
In the absence of data on patient‐reported
outcomes on treatment of furcation‐involved
molars (in particular degree II, as in this
case), patient preferences are very important.
In this specific case, the patient was very keen
to maintain 36 for as long as possible.
14.2.13 Treatment Decision
Based on all these considerations, backed
when possible by data from the literature, it
was decided to maintain 36 and to carry out
non‐surgical debridement and oral hygiene
instructions. Following re‐evaluation two
months later, residual pockets, bleeding on
probing, and FI were detected on this tooth
(see Table 14.4).
Given the residual FI and residual deep
pockets, associated with a high risk of future
tooth loss (Matuliene et al. 2008; Nibali et al.
2016), a decision to attempt surgical explora-
tion and if possible regenerative surgery was
made. No through‐and through FI could be
detected intra‐surgically, although probably
only a very limited layer of bone may have
been present. Therefore, regenerative therapy
with EMD was provided (see Chapter 7 for a
step‐by‐step guide to the furcation regener-
ative surgical technique). Post‐
operative
photos and radiographs are presented in
Chapter 15 (Figure 15.5). The main reasons
for this choice are summarized in Table 14.5.
References
Abouassi, T., Woelber, J.P., Holst, K. et al.
(2014). Clinical efficacy and patients’
acceptance of a rubber interdental bristle: A
randomized controlled trial. Clinical Oral
Investigations 18, 1873–1880. doi:10.1007/
s00784‐013‐1164‐3.
Table 14.4
Case 2, re‐evaluation.
Tooth 36 (LL6)
v‐PPD
CAL
h‐PPD
Mesio‐buccal
2
2
–
Buccal
6
8
4
Disto‐buccal
10
13
–
Mesio‐lingual
3
3
–
Lingual
6
6
4
Disto‐lingual
9
11
–
CAL = clinical attachment loss; h‐PPD = horizontal
probing pocket depth; v‐PPD = vertical probing
pocket depth.
Table 14.5
Case 2, main reasons for treatment
choice (regenerative therapy with enamel matrix
derivative).
Factors
TOOTH
Good mesial bone support
Good bone support on neighbouring
tooth 37
Reduced tooth divergence
Intrabony defect affecting distal root
and furcation
Tooth vitality and no restorative
concerns
Importance in masticatory function
PATIENT Clear medical history
Non‐smoker
Motivation to keep the tooth
No financial or other concerns for
surgical treatment

Chapter 14
266
Ammons, W.F., and Harrington G.W. (2006).
Furcation: Involvement and treatment. In:
Carranza’s Clinical Periodontology (ed.
M.G. Newman, H.H. Takei, P.R. Klokkevold,
and F.A. Carranza), 991–1004. St. Louis,
MO: Saunders Elsevier.
Becker, W., Becker, B.E., Alsuwyed, A., and
Al‐Mubarak, S. (1999). Long‐term
evaluation of 282 implants in maxillary and
mandibular molar positions: A prospective
study. Journal of Periodontology
70, 896–901.
Becker, W., Becker, B.E., and Berg, L.E. (1984).
Periodontal treatment without maintenance:
A retrospective study in 44 patients. Journal
of Periodontology 55, 505–509.
Blomlöf, L., Jansson, L., Appelgren, R. et al.
(1997). Prognosis and mortality of root‐
resected molars. International Journal of
Periodontics and Restorative Dentistry
17, 190–201.
Carnevale, G., Pontoriero, R., and di Febo, G.
(1998). Long‐term effects of root‐resective
therapy in furcation‐involved molars: A
10‐year longitudinal study. Journal of
Clinical Periodontology 25, 209–214.
Christou, V., Timmerman, M.F., Van der
Velden, U., and Van der Weijden, F.A.
(1998). Comparison of different approaches
of interdental oral hygiene: Interdental
brushes versus dental floss. Journal of
Periodontology 69, 759–764. doi:10.1902/
jop.1998.69.7.759.
Cortellini, P., Stalpers, G., Mollo, A., and
Tonetti, M.S. (2011). Periodontal
regeneration versus extraction and
prosthetic replacement of teeth severely
compromised by attachment loss to the
apex: 5‐year results of an ongoing
randomized clinical trial. Journal of Clinical
Periodontology 38, 915–924.
Cortellini, P., and Tonetti, M.S. (2001).
Evaluation of the effect of tooth vitality on
regenerative outcomes in infrabony defects.
Journal of Clinical Periodontology
28, 672–679.
de Andrade, A.K., Feist, I.S., Pannuti, C.M.
et al. (2008). Nd:YAG laser clinical assisted
in class II furcation treatment. Lasers in
Medical Science 23, 341–347. doi:10.1007/
s10103‐007‐0482‐6.
Del Peloso Ribeiro, E., Bittencourt, S., Nociti,
F.H., Jr et al. (2007). Comparative study of
ultrasonic instrumentation for the non‐
surgical treatment of interproximal and
non‐interproximal furcation involvements.
Journal of Periodontology 78, 224–230.
doi:10.1902/jop.2007.060312.
Drago, C.J. (1992). Rates of osseointegration of
dental implants with regard to anatomical
location. Journal of Prosthodontics 1, 29–31.
Dunlap, R.M., and Gher, M.E. (1985). Root
surface measurements of the mandibular
first molar. Journal of Periodontology
56, 234–248.
Eickholz, P., Nickles, K., Koch, R. et al. (2016).
Is furcation class involvement affected by
adjunctive systemic amoxicillin plus
metronidazole? A clinical trial’s exploratory
subanalysis. Journal of Clinical
Periodontology 43, 839–848.
Fardal, O., and Grytten, J. (2013).
A comparison of teeth and implants during
maintenance therapy in terms of the number
of disease‐free years and costs: An in vivo
internal control study. Journal of
Clinical Periodontology 40, 645–651.
doi:10.1111/jcpe.12101.
Fleischer, H.C., Mellonig, J.T., Brayer, W.K.
et al. (1989). Scaling and root planing
efficacy in multirooted teeth. Journal of
Periodontology 60, 402–409. doi:10.1902/
jop.1989.60.7.402.
Glickmann, I. (1953). Clinical Periodontology.
Pennsylvania, PA: Saunders.
Graziani, F., Donos, N., Needleman, I. et al.
(2004). Comparison of implant survival
following sinus floor augmentation
procedures with implants placed in pristine
posterior maxillary bone: A systematic
review. Clinical Oral Implants 15, 677–682.
Hamp, S.E., Nyman, S., and Lindhe, J. (1975).
Periodontal treatment of multirooted teeth:
Results after 5 years. Journal of Clinical
Periodontology 2, 126–135.
Helldén, L.B., Elliot, A., Steffensen, B., and
Steffensen, J.E.M. (1989). Prognosis of
tunnel preparations in treatment of class III

Assessment of Two Example Cases 267
furcations: A follow‐up study. Journal of
Periodontology 60, 182–187.
Jepsen, S., Eberhard, J., Herrera, D., and
Needleman, I. (2002). A systematic review of
guided tissue regeneration for periodontal
furcation defects: What is the effect of
guided tissue regeneration compared with
surgical debridement in the treatment of
furcation defects? Journal of Clinical
Periodontology 29 (Suppl. 3), 103–116.
Jepsen, S., Heinz, B., Jepsen, K. et al. (2004).
A randomized clinical trial comparing enamel
matrix derivative and membrane treatment of
buccal Class II furcation involvement in
mandibular molars. Part I: Study design and
results for primary outcomes. Journal of
Periodontology 75, 1150–1160.
Joseph, I., Varma, B.R., and Bhat, K.M. (1996).
Clinical significance of furcation anatomy of
the maxillary first premolar: A biometric
study on extracted teeth. Journal of
Periodontology 67, 386–389.
Langeland, K., Rodrigues, H., and Dowden, W.
(1974). Periodontal disease, bacteria, and
pulpal histopathology. Oral Surgery, Oral
Medicine, Oral Pathology 37, 257–270.
Langer, B., Stein, S.D., and Wagenberg, B.
(1981). An evaluation of root resections:
A ten‐year study. Journal of Periodontology
52, 719–722.
Leon, L.E., and Vogel, R.I. (1987). A
comparison of the effectiveness of hand
scaling and ultrasonic debridement in
furcations as evaluated by differential dark‐
field microscopy. Journal of Periodontology
58, 86–94. doi:10.1902/jop.1987.58.2.86.
Little, L.A., Beck, F.M., Bagci, B., and Horton,
J.E. (1995). Lack of furcal bone loss
following the tunnelling procedure. Journal
of Clinical Periodontology 22, 637–641.
Machtei, E.E., Zubrey, Y., Yehuda, A.B., and
Soskolne, W.A. (1989). Proximal bone loss
adjacent to periodontally ‘hopeless’ teeth
with and without extraction. Journal of
Periodontology 60, 512–515.
Martin, J.A., Fardal, O., Page, R.C. et al. (2014).
Incorporating severity and risk as factors to
the Fardal cost‐effectiveness model to create
a cost–benefit model for periodontal
treatment. Journal of Periodontology 85,
e31–e39. doi:10.1902/jop.2013.130237.
Matia, J.I., Bissada, N.F., Maybury, J.E., and
Ricchetti, P. (1986). Efficiency of scaling of
the molar furcation area with and without
surgical access. International Journal of
Periodontics and Restorative Dentistry
6, 24–35.
Matuliene, G., Pjetursson, B.E., Salvi, G.E. et al.
(2008). Influence of residual pockets on
progression of periodontitis and tooth loss:
Results after 11 years of maintenance.
Journal of Clinical Periodontology 35,
685–695.
Moraschini, V., Poubel, L.A., Ferreira, V.F., and
Barboza Edos, S. (2015). Evaluation of
survival and success rates of dental implants
reported in longitudinal studies with a
follow‐up period of at least 10 years: A
systematic review. International Journal of
Oral and Maxillofacial Surgery 44, 377–388.
Muller, H.P., and Eger, T. (1999). Furcation
diagnosis. Journal of Clinical Periodontology
26, 485–498.
Nevins, M., Camelo, M., Nevins, M.L. et al.
(2003). Periodontal regeneration in humans
using recombinant human platelet‐derived
growth factor‐bb (rhPDGF‐BB) and
allogenic bone. Journal of Periodontology
74, 1282–1292.
Nibali, L., Zavattini, A., Nagata, K. et al.
(2016). Tooth loss in molars with and
without furcation involvement: A systematic
review and meta‐analysis. Journal of Clinical
Periodontology 43, 156–166.
Pjetursson, B.E., Tan, W.C., Zwahlen, M., and
Lang, N.P. (2008). A systematic review of the
success of sinus floor elevation and survival
of implants inserted in combination with
sinus floor elevation. Journal of Clinical
Periodontology 35, 216–240.
Pontoriero, R., and Lindhe, J. (1995). Guided
tissue regeneration in the treatment of
degree III furcation defects in maxillary
molars. Journal of Clinical Periodontology
22, 810–812.
Ribeiro Edel, P., Bittencourt, S., Sallum, E.A.
et al. (2010). Non‐surgical instrumentation
associated with povidone‐iodine in the

Chapter 14
268
treatment of interproximal furcation
involvements. Journal of Applied Oral
Sciences 18, 599–606.
Schwendicke, F., Graetz, C., Stolpe, M., and
Dorfer, C.E. (2014). Retaining or replacing
molars with furcation involvement: A
cost‐effectiveness comparison of different
strategies. Journal of Clinical Periodontology
41, 1090–1097.
Stoller, N.H., Johnson, L.R., and Garrett, S.
(2001). Periodontal regeneration of a class II
furcation defect utilizing a bioabsorbable
barrier in a human: A case study with
histology. Journal of Periodontology 72,
238–242.
Sugaya, T., Kawanami, M., and Kato, H. (2002).
Effects of debridement with an ultrasonic
furcation tip in degree II furcation
involvement of mandibular molars. Journal
of the International Academy of
Periodontology 4, 138–142.
Tarnow, D., and Fletcher, P. (1984).
Classification of the vertical component of
furcation involvement. Journal of
Periodontology 55, 283–284.
Thoma, D.S., Zeltner, M., Hüsler, J. et al.
(2015). EAO Supplement Working Group
4 – EAO CC 2015. Short implants versus
sinus lifting with longer implants to restore
the posterior maxilla: A systematic review.
Clinical Oral Implant Research 26 (Suppl.
11), 154–169.
Tomasi, C., and Wennstrom, J.L. (2011).
Locally delivered doxycycline as an adjunct
to mechanical debridement at retreatment
of periodontal pockets: Outcome at
furcation sites. Journal of Periodontology 82,
210–218. doi:10.1902/jop.2010.100308.

Chapter No.: 1 Title Name: <TITLENAME>
c15.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:22:44 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 269
269
Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
15.1 Introduction
What do you do when facing a molar with
furcation involvement (FI)? Extract?
Ignore? Treat? And how? Regeneration?
Root resection? Several papers in the peri-
odontal literature have covered the treat-
ment of molars with FI. Previous chapters
of this book have reviewed and carefully
scrutinized the evidence for no treatment,
and for conservative, regenerative, and
resective therapy. But how do we approach
decision‐making? How can we merge the
patient’s preferences and needs with the
financial considerations and the clinical
criteria discussed in this book to achieve a
favourable outcome? We embark on this
chapter with a scientific evidence‐based
approach to try to answer these questions.
However, in the absence of randomized
controlled trials comparing different man-
agement options for molars with varying
degrees of FI, pragmatic considerations and
experience will complement the evidence
in order to obtain treatment guidelines.
The main points to consider are highlighted
in what follows.
15.2 First Things First:
Proper Diagnosis
Diagnosis is the first step towards treatment.
As in any other field of medicine, every effort
needs to be expended for a correct diagnosis
of the problem (Khullar et al. 2015). Most of
the mistakes I have made in my professional
career or have seen made by students or col-
leagues were due to incorrect diagnosis. This
is particularly important for furcations, since
diagnosis is not straightforward. Therefore,
spending more time and effort for diagnostic
purposes before rushing to pick up blade or
forceps is recommended.
Furcation diagnosis has been covered by
Eickholz and Walter in Chapter 2. Their clear
message is that clinical and radiographic
diagnoses need to be combined to obtain a
correct measure of the involvement of the
furcation area. A curved Nabers probe is vital
for measuring the bone loss in the furcation
area, although the difference between degrees
II and III might be difficult to ascertain, espe-
cially in maxillary molars. Three‐dimensional
radiography may also be needed for treat-
ment planning purposes in some cases.
Chapter 15
Furcations: A Treatment Algorithm
Luigi Nibali
Centre for Immunobiology and Regenerative Medicine, Centre for Oral Clinical Research, Institute of Dentistry, Barts and the London
School of Medicine and Dentistry, Queen Mary University of London (QMUL), London, UK
Chapter 15
270
Figure 15.1 shows a furcation diagnosis
algorithm, essential for treatment planning.
It is suggested that a Nabers probe is used in
all cases of probing pocket depths > 4 mm in
molars, to establish a furcation diagnosis. In
the case of FI degree II or III, further diag-
nostic tests, including periapical radiographs
and endodontic and occlusal assessments,
may be necessary for treatment planning.
When the diagnosis and extent of FI are not
clear, posing doubts about the best treatment
plan, cone‐beam computed tomography
(CBCT) may be justifiable as a useful
diagnostic adjunct, especially for maxillary
molars (Walter et al. 2016; see Chapter 2 for
more details).
The main differential diagnostic elements
to consider for complex treatment planning
are briefly highlighted in Table 15.1.
Bearing in mind all the factors discussed,
treatment guidelines for different degrees of
FI are proposed in the following sections.
These cannot be applied as ‘blanket’
treatment guidelines, but need to be adapted
to every different patient and every different
molar.
15.3 Degree I Furcation
Involvement
The evidence from the literature, summa-
rized in a recent systematic review and dis-
cussed in Chapter 5, suggests that increasing
FI degree is associated with an increased
risk of tooth loss (Nibali et al. 2016). The
long‐term risk of tooth loss appears minimal
in degree I FI molars undergoing regular care
compared with molars with no FI. In fact,
re‐examining data from that systematic
review, it appears that molars with degree I FI
undergoing regular supportive periodontal
therapy (SPT) have an identical tooth loss
rate to molars with no FI (0.01 teeth/patient/
year; Nibali et al. 2016). For this reason, the
consensus opinion at present is that degree I
FI is not suitable for complex treatment such
as periodontal regeneration. Authors of a
previous systematic review had also con-
cluded that degree I FI could be success-
fully managed by non‐surgical mechanical
debridement (Huynh‐Ba et al. 2009).
Less is known about FI degree I molars not
undergoing regular periodontal care. Data
Periodontal probe: pockets
on molars
Probing degree I
Probing degree II or III
Periapical radiograph, assessment
of endodontic status, and occlusion
Nabers probe
Complex treatment planning
Consider cone-beam CT
No probing in furcation
Periodontal
treatment
Maintenance/treatment
Figure 15.1
Proposed algorithm for furcation diagnosis.

Furcations: A Treatment Algorithm 271
from SHIP (Study for Health In Pomerania)
show an incidence rate ratio (IRR) for molar
loss of 1.73 (95% confidence interval [CI]
1.34–2.23, p < 0.001) for degree I FI versus no
FI after 11 years of follow‐up, suggesting that
‘no treatment’ worsens the prognosis of molars
with FI degree I (Nibali et al. 2017a).
Therefore, it could be suggested that oral
hygiene instructions and non‐surgical therapy
represent the treatment of choice for degree I
FI, irrespective of location or other factors.
Odontoplasty might complement this treat-
ment in cases where the furcation entrance
anatomy might contribute to the presence of a
degree I FI and might interfere with oral
hygiene manoeuvres (see Figure 15.2).
15.4 Degree II Furcation
Involvement
The real complexity starts when considering
degree II FI, as this seems to be the threshold
at which the risk of tooth loss sharply increases
(Nibali et al. 2016, 2017a). Therefore, treat-
ment is needed to reduce the impact of such
FI in determining tooth loss. The main treat-
ment goal should be the reduction of degree
Table 15.1
Main differential diagnostic elements to consider for complex furcation treatment planning.
Factors
Thresholds/grades
ANATOMY
Endodontic status
Degree 1–5 (Ørstavik et al. 1986)
Restorability
Class I–III (Esteves et al. 2011)
Degree of furcation involvement
I–III (Hamp et al. 1975)
Number of furcation
involvements
Single, double, or triple
Vertical probing
A–C (Tarnow and Fletcher 1984)
Degree of separation (root
divergence)
30° (Muller and Eger 1999)
Separation degree
⅓ (Muller and Eger 1999)
Bone loss
⅓, ⅔ of root length (Muller and Eger 1999)
Position of furcation fornix
relative to bone crest and
interproximal bone levels
Coronal or apical (Bowers et al. 2003)
Furcation width
Narrow or wide (Horwitz et al. 2004)
Other anatomical features
Length of root cone, root trunk etc.
PATIENT
Medical history
Healthy vs medically compromised
Smoking
Current/former/never
Preferences/motivation
Refusal to undergo surgery
Financial
Inability/unwillingness to pay for complex
treatment
Oral hygiene dexterity
Inability to access furcation entrance
OPERATOR
Ability/experience
STRATEGY
Abutment
Functional
RISK
Anatomical risks, surgical risks
ALTERNATIVES
Replacement with implant, prosthetic bridge, removable denture
Chapter 15
272
II FI to degree I FI or to no FI (ideal). It is
implied that oral hygiene instructions and
non‐surgical therapy (with or without
adjuncts) are a prerequisite for this treatment
algorithm, and they should always represent
the starting point. In some occasions, oral
hygiene instructions and non‐surgical
therapy can already lead to the reduction of
degree II FI to degree I FI through mecha-
nisms including reduction of gingival
oedema, epithelial reattachment, reduced
probe penetration, and potentially radio-
graphic bone fill (see the case in Figure 15.3).
However, residual degree II FI after causal
therapy needs to be further addressed.
15.4.1 Mandibular Degree II
Furcations
Except for cases of degree II FI after initial
periodontal therapy where odontoplasty
and/or surgical osteoplasty could lead to a
reduction to degree I FI, reduction of degree
II FI could be achieved via regenerative ther-
apy. Chapters 6 and 7 have presented the evi-
dence for the efficacy of regeneration in
furcations. Based on the discussion in these
chapters, an important differentiation needs
to be made between maxillary and mandibu-
lar degree II furcations. Figure 15.4 shows a
Furcation degree I
Oral hygiene and non-surgical
maintenance
(consider odontoplasty)
Supportive periodontal therapy
Figure 15.2
Proposed algorithm for furcation
treatment (degree I furcation involvement).
(a)
(b)
Figure 15.3
(a) Periapical radiographs of molars of a female 32‐year‐old aggressive periodontitis patient at
periodontal diagnosis. Radiolucency inside the furcation areas is visible, particularly for UR6 and 7 (both
degree II clinical furcation involvement [FI] diagnosis), UL6 and 7 (degree I FI), LL6 (degree I FI),
and LR6 (degree II FI), often associated with intrabony defects. (b) Periapical radiographs of the same molars
one year after initial periodontal therapy (oral hygiene instructions and supra‐ and subgingival debridement
with adjunctive systemic antibiotics and extraction of UL8), showing radiographic bone fill in furcation defects
and intrabony defects, associated with clinical reduction of FI degrees (now only degree I for UR6 and 7, UL6
and 7, and LR6).
Furcations: A Treatment Algorithm 273
proposed algorithm for the treatment of
mandibular degree II FI. A hierarchy is pre-
sented starting from the preferred choice at
the top, although this is rather arbitrary and
not strictly evidence based.
Another important differentiation to be
drawn is based on whether the furcation is
single (e.g. only buccal or only lingual) or
double (both buccal and lingual). In a case of
single degree II mandibular FI following
initial therapy, regeneration seems to be the
preferred choice. The evidence reviewed by
Jepsen and Jepsen in Chapter 7 shows that,
although complete furcation closure in degree
II FI is not a predictable outcome, treatment
with either guided tissue regeneration (GTR)
or enamel matrix derivative (EMD) has pro-
duced histological evidence of regeneration
(Stoller et al. 2001; Nevins et al. 2003) and
consistently more favourable clinical out-
comes (reduction to degree I or closure)
compared with access flaps, especially in
mandibular furcations (Jepsen et al. 2002,
2004). Alternatives to regenerative therapy
are non‐surgical maintenance/SPT, access
flap with or without osteoplasty, or apically
positioned flap (APF), to improve access for
professional cleaning of the furcation region.
Similar treatment options are recommended
for combined degree II and I mandibular
furcations.
However, when double degree II mandibu-
lar furcations are present, regeneration
becomes much less predictable, albeit not
impossible (see Figure 15.5, in relation to
Case 2 described in Chapter 14).
In contrast, tunnelling comes into play as
probably the preferred option. Tunnelling
surgery can improve access to self‐performed,
as well as professional, cleaning of the furca-
tion area, so it could be indicated too in cases
of double degree II mandibular FI. Studies
with 5–10 years follow‐up show success rates
varying between 51 and 93% (Hamp et al.
1975; Dannewitz et al. 2006). Patient selec-
tion (good oral hygiene and motivation, low
caries risk) and tooth selection (short root
trunk, favourable root divergence; Muller
Mandibular degree II
Non-surgical
maintenance,
odontoplasty
Access flap/apically
positioned flap/
osteplasty
Regeneration
Non-surgical maintenance,
odontoplasty
Access flap/apically positioned flap/
osteoplasty
Single
Combined
Resection/hemisection
Tunnelling
Regeneration
II + II
II + I
Non-surgical
maintenance,
odontoplasty
Access flap/apically
positioned flap/
osteoplasty
Regeneration
Supportive periodontal therapy
Figure 15.4
Proposed algorithm for furcation treatment (degree II mandibular furcation involvement).
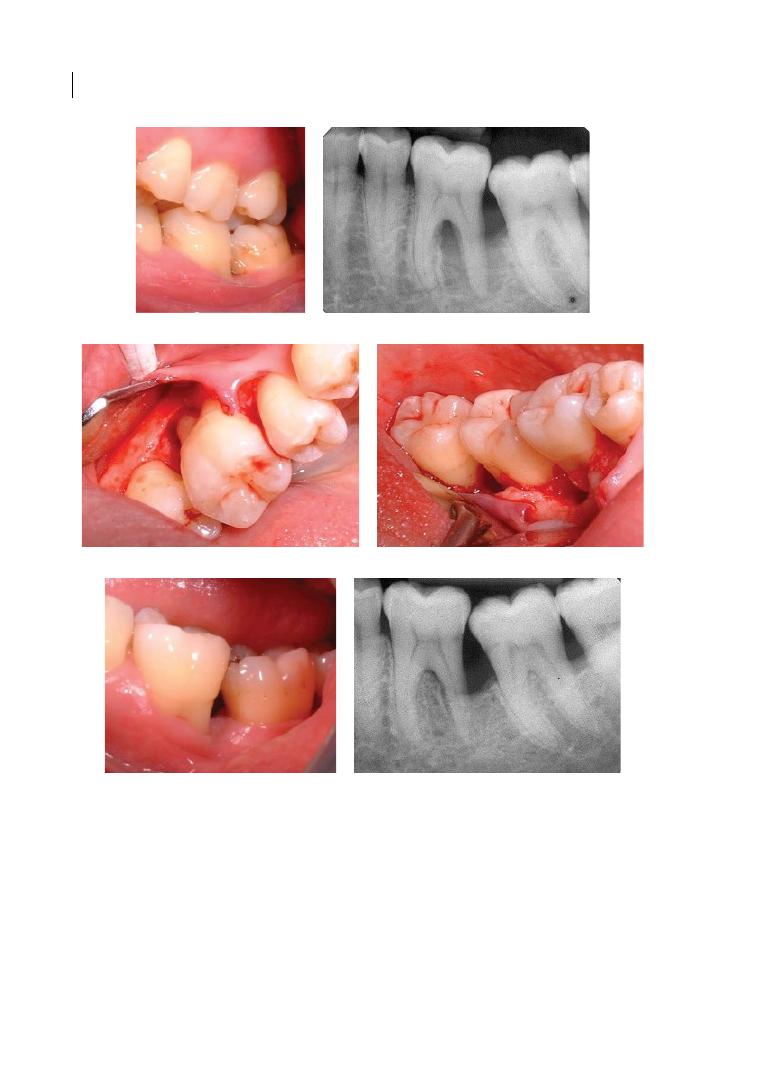
Chapter 15
274
and Eger 1999) are vital for tunnelling, and
for access reasons mandibular furcations
are clearly more suitable.
Other options such as non‐surgical
maintenance/odontoplasty, root resection/
hemisection, or access flap (or APF) are also
realistic. We should bear in mind that root‐
resection studies with follow‐ups at 5–10
years showed success rates of 62–100%
(Carnevale et al. 1998; Dannewitz et al. 2006)
(c)
(d)
(e)
(f)
(a)
(b)
Figure 15.5
Clinical photograph (a) and periapical radiograph (b) of 47‐year‐old patient diagnosed with
chronic periodontitis and affected by double degree II furcation involvement and distal intrabony defect
(disto‐buccal and disto‐lingual probing pocket depth [PPD] 12 mm) on tooth 36 (LL6). Following non‐surgical
periodontal therapy, regenerative surgery with enamel matrix derivative was carried out (c and d, buccal and
lingual intra‐operative views after full‐thickness flaps elevation), with favourable outcomes at five years after
surgery, with reduction of PPD to 4 mm and only degree I furcation lingual (e) and fill of intrabony and
furcation defect (f).
Furcations: A Treatment Algorithm 275
and, with the exception of three studies, the
average survival rate of the molars treated
with root separation/resection was close to
90% (see Chapter 8). Access flap (or APF) with
or without ostectomy could improve access
for professional cleaning of the furcation area
and lead to reduction of probing pocket
depths and inflammation (Wang et al. 1994).
15.4.2 Single Maxillary Degree II
Buccal Furcations
Maxillary molars with degree II FI after
initial periodontal therapy present probably
the most challenging scenario in terms of
potential treatment choices and expected
outcome. Again, in the absence of rand-
omized controlled trials comparing all
options, we need to draw guidelines mainly
based on low‐evidence studies, experience,
and common sense. Figure 15.6 shows
options for degree II maxillary furcations.
Favourable results could be achieved with
GTR therapy and EMD in maxillary degree II
furcations (Yukna and Yukna 1996; Casarin
et al. 2010), but the evidence suggests that
such results are more predictable in buccal
sites (Pontoriero and Lindhe 1995a).
Therefore, regeneration could be a good
alternative to non‐surgical maintenance,
odontoplasty, and access flap surgery in
order to reduce the buccal furcation to
degree I or to achieve complete closure. The
decision for regenerative therapy would
depend on factors mentioned in the previous
section (see Table 15.1), such as smoking and
financial considerations, as well as good
interproximal bone levels, reduced vertical
furcation component, and reduced furcation
width, all shown to favour regeneration in
animal and human studies (Pontoriero et al.
1992; Bowers et al. 2003; Horwitz et al. 2004).
15.4.3 Single Maxillary Degree II
Interproximal Furcations
Overall, the application of GTR, EMD, or
combination therapy (EMD/bone grafts) to
proximal furcations in maxillary molars
achieved some furcation closures, but was
not as favourable as that in mandibular
furcations or buccal maxillary furcations
Interproximal
Maxillary degree II
Buccal
Non-surgical
maintenance,
odontoplasty
Access flap/apically
positioned flap/
osteoplasty
Resection
Non-surgical
maintenance,
odontoplasty
Access flap/osteoplasty
Regeneration
Single
Combined
Interproximal
Buccal/interproximal
Access flap/apically
positioned flap/
osteoplasty
Access flap/apically
positioned flap/
osteoplasty
Resection/
root separation
Resection/root
separation
Tunnelling
Tunnelling
Non-surgical
maintenance,
odontoplasty
Non-surgical
maintenance,
odontoplasty
Regeneration
Supportive periodontal therapy
Figure 15.6
Proposed algorithm for furcation treatment (degree II maxillary furcation involvement).

Chapter 15
276
(Pontoriero and Lindhe 1995a; Avera
et al. 1998; Casarin et al. 2010; Peres et al.
2013). Therefore, non‐surgical maintenance/
odontoplasty, access flap/osteoplasty (or
APF), and potentially also root resection
could be valid alternatives. The latter could
be particularly suitable for previously endo-
dontically treated molars.
15.4.4 Combined Maxillary
Degree II Furcations
Although regenerative periodontal therapy
may be a viable option for degree II maxillary
molars, this would be probably only limited
to single degree II maxillary defects, as the
unpredictability would certainly increase
several‐fold in multiple degree II maxillary FI
(which often could hide a true degree III FI).
Therefore, root resection, access flap, and
tunnelling procedures are probably the best
alternatives to choose from in these cases.
As previously discussed for mandibular
degree II furcations, root‐resective surgery
and access flap/APF with or without ostec-
tomy could be good potential alternatives
(Wang et al. 1994; Carnevale et al. 1998).
Root resection/separation is mainly recom-
mended for previously endodontically
treated maxillary molars with combined
furcations degree II, particularly when the
worst‐affected root is the disto‐buccal.
Tunnelling, given the correct patient and
molar selection, could be another appropri-
ate treatment choice in selected cases.
When for patient or tooth reasons these
options are not feasible or not worth pursu-
ing, non‐surgical maintenance with frequent
SPT recalls based on a patient risk profile
(Lang and Tonetti 2003) remains a valid
option, with the clear limitation of difficul-
ties in achieving proper debridement of the
furcation area (Fleischer et al. 1989).
Root resection and tunnelling options may
be more indicated in cases of combined
buccal/interproximal degree II furcations,
rather than when both are interproximal.
The reasons for this are that resecting the
palatal root (in cases of mesial and distal
degree II FI) is less favourable owing to ana-
tomical features (see Chapter 8), and that
access to the furcation tunnel for self‐per-
formed hygiene is easier from a buccal access
(see Chapter 9). Therefore, a slightly different
hierarchy of choice is presented in Figure 15.6,
with root resection and tunnelling consid-
ered to have more ‘worth’ for combined
buccal–interproximal FI, and more conserva-
tive options probably indicated for combined
interproximal furcations.
The unlikely case of triple non‐through‐
and‐through degree II furcation involvement
could be considered within the degree III FI
treatment options.
15.5 Degree III Furcation
Involvement
When bone loss in the furcation area goes
through and through, from one root‐separation
area to another on the same tooth, we are
faced with degree III FI. The main treatment
challenge here derives from the limitations of
regenerative therapy in such cases. No clini-
cal study in humans has so far reported any
degree III furcation closures with GTR, EMD,
or both combined, but only clinical reduc-
tions in furcation degree in some very lim-
ited cases (Pontoriero et al. 1989; Pontoriero
and Lindhe 1995b; Eickholz et al. 1998, 1999;
Jepsen et al. 2002; Donos et al. 2004). Jepsen
and Jepsen in Chapter 7 reviewed the availa-
ble evidence to conclude that degree III FI
cannot be improved predictably by regenera-
tive therapy, until new developments in
regenerative material and techniques are
available (discussed in Chapter 6). Therefore,
it would be difficult to justify the use of
regenerative surgery on degree III FI at pre-
sent. The persistence of a degree III furcation
defect which is not regenerable and is diffi-
cult to clean (see Chapter 3) equals a higher
risk of future tooth loss than for degree I and
degree II FI molars (Nibali et al. 2016). Hence
the need to find a solution to manage these
cases and to reduce the risk of tooth loss.
The treatment options are presented in
Furcations: A Treatment Algorithm 277
Figure 15.7, divided between maxillary and
mandibular molars.
The difference, clearly, is that maxillary
molars, with three furcation entrances, could
potentially have three through‐and‐through
furcation lesions, making the access for clean-
ing (both professional and self‐performed)
very challenging. On the other hand,
mandibular degree III FI molars can only
have one through‐and‐through furcation
lesion, with access both buccally and
lingually. While bearing in mind the evidence
of success for tunnelling and root‐resection
procedures in long‐term clinical trials
(Helldén et al. 1989; Carnevale et al. 1998),
we need to stress the importance of proper
diagnosis and patient and molar selection.
The factors highlighted in Table 15.1 need
again to be kept in mind in order to ensure
long‐term success.
15.5.1 Mandibular Degree III
Furcations
Tunnelling could be considered the treat-
ment of choice for mandibular degree III FI.
As already mentioned, tunnelling should be
avoided in cases with high caries risk, high
tooth sensitivity, poor compliance, or poor
manual dexterity, as lack of proper oral
hygiene defies the purpose of this therapy.
Molars with a short root trunk, high degree
of separation (root divergence), and a large
band of keratinized gingiva are particularly
suitable for this procedure (see Chapter 9).
When tunnelling is not indicated, root sepa-
ration (premolarization) could be considered
a valid alternative, especially in cases of good
residual bone support in the distal aspect of
the distal root and in the mesial aspect of the
mesial root. This would again aim to improve
cleaning (by removal of the furcation region),
but would entail extensive restorative work.
Long‐term non‐surgical maintenance
( preceded or not by surgical access) and root
resection represent possible alternatives if
tooth survival is chosen.
15.5.2 Maxillary Degree III
Furcations
Root resection or non‐surgical maintenance
could be considered the treatment of choice
for degree III FI maxillary molars. These two
Root resection/separation
Maxillary degree III
Mandibular degree III
Tunnelling
Non-surgical maintenance
Extraction
Non-surgical maintenance
Root resection
Tunnelling
Extraction
Root separation
Root separation
Supportive periodontal therapy
Figure 15.7
Proposed algorithm for furcation treatment (degree III furcation involvement).

Chapter 15
278
procedures have different indications, as root
resection is generally more advisable in the
case of a double degree III FI, mainly affecting
one root. In this case, the resection of a root
(preferably if it is the smallest disto‐ buccal
root) could leave two easily maintainable
roots with no FI. As Rotundo and Fonzar dis-
cussed in Chapter 6, root resection in a case
of a triple degree III FI is less advisable, as it
would anyway result in a residual difficult‐to‐
clean furcation. In such a case (triple degree
III FI), regular subgingival debridement
seems the most reasonable option in cases
where tooth survival is preferred. This could
be preceded or not by surgical access for
furcation debridement, shown to improve
professional cleaning efficacy (see Chapter 3).
Other factors mentioned earlier, such as
patient preferences and financial considera-
tions, play an important role in the decision
of whether to maintain such teeth or not.
Under exceptional circumstances, tunnel-
ling could be considered an option for triple
degree III FI, mainly in cases of very compli-
ant patients with good manual dexterity and
low caries risk. Tunnelling can occur either
naturally, by virtue of gingival recession, as a
result of oral hygiene and non‐surgical ther-
apy, or can be created surgically. The main
reason for the failure of tunnelling is not
periodontal but restorative, linked with root
caries following the exposure of the root sur-
face (Helldén et al. 1989). Figure 15.8 shows a
case of surgically created triple degree III FI
in maxillary molars maintained for over
10 years with no clinical and radiographic
signs of disease progression or caries (Case 1
described in Chapter 14).
Root separation is also a possible option
for degree III maxillary molars, associated or
not with root resection. Careful endodontic
and prosthetic considerations are needed
before deciding on this treatment option
(Carnevale et al. 1998).
However, in making the decision on
whether to extract or not, one should take
into account that, despite a higher tooth loss
risk, the majority of maxillary degree III FI
molars could be maintained over at least a
10–15‐year period of periodontal supportive
care (Nibali et al. 2016), meaning that extrac-
tion of molars affected by degree III FI should
not be a given.
15.6 Upper Premolars
Upper first premolars are normally two‐
rooted, while second maxillary premolars
and mandibular canines are occasionally
two‐rooted. However, very little data exist in
the literature about the treatment of non‐
molar teeth with FI (Hamp et al. 1975).
As discussed in Chapter 7, the majority of
maxillary first premolars have fused roots. In
upper premolars with separated roots, the
furcation entrance is on average 8 mm apical
to the cemento‐enamel junction and only
7–10% of furcation entrances are in the coro-
nal third of the root (Joseph et al. 1996;
Dababneh and Rodan 2013). Furthermore,
root grooves and concavities are the norm in
upper premolars. Owing to these anatomical
features, root resection and tunnelling are
not usually viable options for upper premo-
lars with FI, and access flap and/or non‐
surgical maintenance should be preferred.
15.7 Innovative Treatment
Novel treatments for furcation‐involved
teeth were discussed in Chapter 10. Some of
these, such as adjunctive photodynamic
therapy, lasers, or air‐polishing devices,
could be added to the options discussed in
this chapter, although more evidence needs
to be gathered before routinely recommend-
ing these therapies.
15.8 So, When Should
We Extract?
The emphasis of this chapter, and perhaps of
the whole book, has been on tooth retention.
However, there are cases where even the most
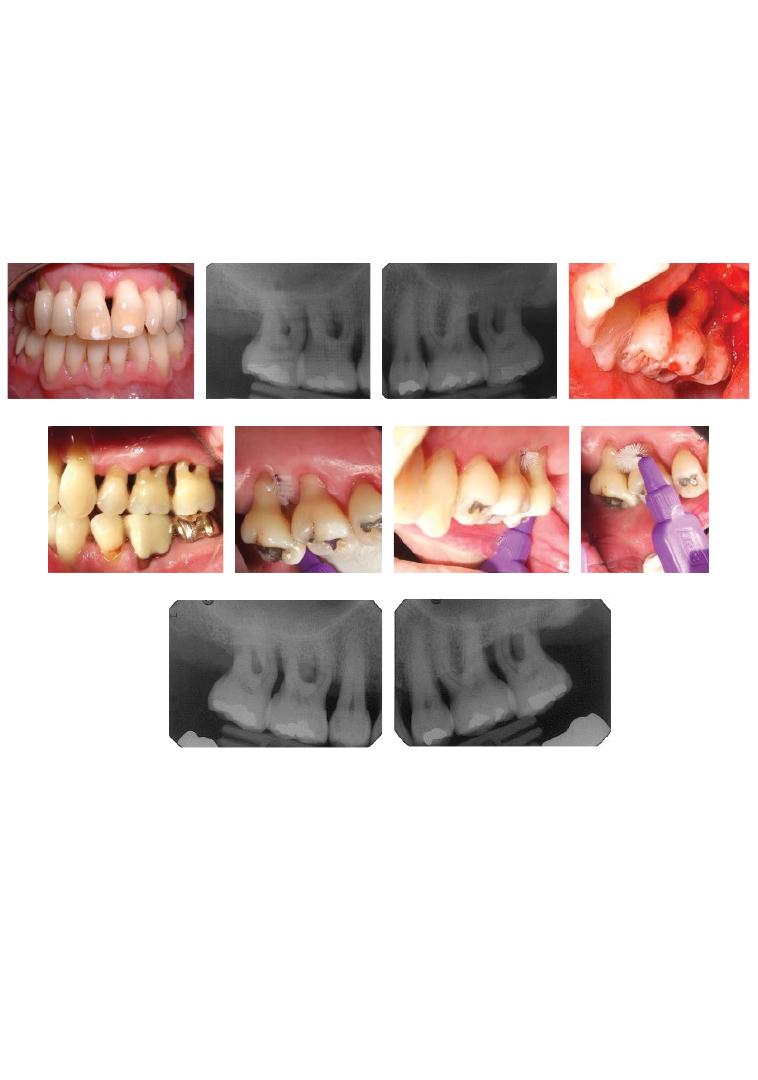
Chapter No.: 1 Title Name: <TITLENAME>
c15.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:22:44 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 279
(a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
(i)
(j)
Figure 15.8
Clinical photograph (a) and periapical radiographs (b and c) of 50‐year‐old patient diagnosed with chronic periodontitis and
affected by triple degree III furcation involvement on 17 (UR7), 16 (UR6), and 27 (UL7). Following non‐surgical periodontal therapy,
tunnelling surgery was carried out (d, intra‐operative view after full‐thickness flaps elevation and osteoplasty), with favourable outcomes at
12 years after surgery with probing pocket depths < 5 mm (e), continued good oral hygiene access to furcations (f, g, and h), and stable
radiographic bone levels (i and j).

Chapter 15
280
daring periodontist has to admit that a tooth
is hopeless or irrational to treat, and extraction
might be the best option. Several definitions
of ‘hopeless’ have been proposed in the peri-
odontal literature. Becker and
colleagues
(1984) reported a range of criteria for hopeless
teeth, including degree III FI or bone
loss > 75%. Machtei and co‐workers (1989)
defined as hopeless teeth with bone loss ≥ 50%,
or radiographic evidence of total bone loss in
the furcation area (grade III FI). In their land-
mark prognosis paper, McGuire and Nunn
(1996) more vaguely identified as hopeless
teeth with ‘inadequate attachment’. Hopeless
has also been defined as having loss of ≥ 70%
bone height (Graetz et al. 2011) or as having
bone loss to the apex (Cortellini et al. 2011).
We recently assigned an ‘unfavourable’
prognosis to teeth with ≥ 70% bone loss which
were also either unrestorable (Esteves et al.
2011), with an endodontic periapical index
(PAI) score of 4 (Ørstavik et al. 1986), or with
mobility grade III or FI degree III (Nibali et al.
2017b). This new prognostic system aims to
be more conservative than previous propos-
als, based on the data discussed earlier show-
ing that degree III FI alone should not qualify
a tooth as hopeless (Nibali et al. 2016). In the
reality of everyday practice, patient‐related
factors (e.g. risk of caries, compliance, and
smoking), patient preferences, financial con-
siderations, and overall strategic value need
to accompany clinical and radiographic crite-
ria in reaching a decision about maintaining
or extracting. Further considerations on
possible extraction and implant replacement
can be found in Chapter 11.
Finally, it is important to remember that
community‐wide oral hygiene instruction
and primary prevention programmes are the
best measures for reducing oral diseases and
tooth loss.
References
Avera, J.B., Camargo, P.M., Klokkevold, P.R.
et al. (1998). Guided tissue regeneration
in class II furcation involved maxillary
molars: A controlled study of 8 split‐mouth
cases. Journal of Periodontology 69,
1020–1026.
Becker, W., Becker, B.E., and Berg, L.E. (1984).
Periodontal treatment without maintenance:
Summary of Evidence
●
Furcation diagnosis is crucial for treat-
ment planning purposes.
●
Degree I, II, and III maxillary and man-
dibular molars have very different treat-
ment indications.
●
Degree I furcation involvement (FI) has a
good long‐term prognosis and does not
represent a higher risk of tooth loss if
under periodontal maintenance care.
●
Degree II FI could benefit from periodon-
tal regeneration or other conservative
therapy (or root resection).
●
Degree II interproximal maxillary FI is
less suitable for regeneration.
●
Degree III FI is linked with a higher risk of
tooth loss and treatment options need to
be carefully considered before embarking
on treatment or extraction.
●
A holistic approach, including clinical and
radiographic parameters but also overall
strategic tooth value, financial considera-
tions, and patient preferences, needs to be
pursued in order to obtain the most satis-
factory outcome of furcation therapy.
●
General dentists should bear in mind that
referral to a periodontist is advised for
molars with severe FI, and that most
molars with FI can be maintained func-
tionally in the long term.
●
Adherence to patient‐tailored supportive
periodontal therapy is crucial to reduce
the long‐term tooth loss risk.

Furcations: A Treatment Algorithm 281
A retrospective study in 44 patients. Journal
of Periodontology 55, 505–509.
Bowers, G.M., Schallhorn, R.G., McClain, P.K.
et al. (2003). Factors influencing the
outcome of regenerative therapy in
mandibular class II furcations: Part I.
Journal of Periodontology 74, 1255–1268.
Carnevale, G., Pontoriero, R., and di Febo, G.
(1998). Long‐term effects of root‐resective
therapy in furcation‐involved molars: A
10‐year longitudinal study. Journal of
Clinical Periodontology 25, 209–214.
Casarin, R.C., Ribeiro Edel, P., Nociti, F.H., Jr
et al. (2010). Enamel matrix derivative
proteins for the treatment of proximal class
II furcation involvements: A prospective
24‐month randomized clinical trial.
Journal of Clinical Periodontology
37, 1100–1109.
Cortellini, P., Stalpers, G., Mollo, A., and
Tonetti, M.S. (2011). Periodontal
regeneration versus extraction and
prosthetic replacement of teeth severely
compromised by attachment loss to the
apex: 5‐year results of an ongoing
randomized clinical trial. Journal of Clinical
Periodontology 38, 915–924.
Dababneh, R., and Rodan, R. (2013).
Anatomical landmarks of maxillary
bifurcated first premolars and their
influence on periodontal diagnosis and
treatment. Journal of the International
Academy of Periodontology 15, 8–15.
Dannewitz, B., Krieger, J.K., Husing, J., and
Eickholz, P. (2006). Loss of molars in
periodontally treated patients:
A retrospective analysis five years or more
after active periodontal treatment. Journal
of Clinical Periodontology 33, 53–61.
Donos, N., Glavind, L., Karring, T., and
Sculean, A. (2004). Clinical evaluation of an
enamel matrix derivative and a
bioresorbable membrane in the treatment of
degree III mandibular furcation
involvement: A series of nine patients.
International Journal of Periodontics and
Restorative Dentistry 24, 362–369.
Eickholz, P., and Hausmann, E. (1999).
Evidence for healing of class II and III
furcations 24 months after GTR therapy:
Digital subtraction and clinical
measurements. Journal of Periodontology
70, 1490–1500.
Eickholz, P., Kim, T.‐S., and Holle, R. (1998).
Regenerative periodontal surgery with
non‐resorbable and biodegradable barriers:
Results after 24 months. Journal of Clinical
Periodontology 25, 666–676.
Esteves, H., Correia, A., and Araújo, F. (2011)
Classification of extensively damaged teeth
to evaluate prognosis. Journal of the
Canadian Dental Association 77, 105.
Fleischer, H.C., Mellonig, J.T., Brayer, W.K.
et al. (1989). Scaling and root planing
efficacy in multirooted teeth. Journal of
Periodontology 60, 402–409.
Graetz, C., Dorfer, C.E., Kahl, M. et al. (2011).
Retention of questionable and hopeless
teeth in compliant patients treated for
aggressive periodontitis. Journal of Clinical
Periodontology 38, 707–714.
Hamp, S.E., Nyman, S., and Lindhe, J. (1975).
Periodontal treatment of multirooted teeth:
Results after 5 years. Journal of Clinical
Periodontology 2, 126–135.
Helldén, L.B., Elliot, A., Steffensen, B., and
Steffensen, J.E.M. (1989). Prognosis of
tunnel preparations in treatment of class III
furcations: A follow‐up study. Journal of
Periodontology 60, 182–187.
Horwitz, J., Machtei, E.E., Reitmeir, P. et al.
(2004). Radiographic parameters as
prognostic indicators for healing of class II
furcation defects. Journal of Clinical
Periodontology 31, 105–111.
Huynh‐Ba, G., Kuonen, P., Hofer, D. et al.
(2009). The effect of periodontal therapy on
the survival rate and incidence of
complications of multirooted teeth with
furcation involvement after an observation
period of at least 5 years: A systematic
review. Journal of Clinical Periodontology
36, 164–176.
Jepsen, S., Eberhard, J., Herrera, D., and
Needleman, I. (2002). A systematic review
of guided tissue regeneration for
periodontal furcation defects: What is the
effect of guided tissue regeneration

Chapter 15
282
compared with surgical debridement in the
treatment of furcation defects? Journal of
Clinical Periodontology 29 (Suppl. 3),
103–116.
Jepsen, S., Heinz, B., Jepsen, K. et al. (2004).
A randomized clinical trial comparing
enamel matrix derivative and membrane
treatment of buccal class II furcation
involvement in mandibular molars. Part I:
Study design and results for primary
outcomes. Journal of Periodontology 75,
1150–1160.
Joseph, I., Varma, B.R., and Bhat, K.M. (1996).
Clinical significance of furcation anatomy of
the maxillary first premolar: A biometric
study on extracted teeth. Journal of
Periodontology 67, 386–389.
Khullar, D., Jha, A.K., and Jena, A.B. (2015).
Reducing diagnostic errors: Why now.
New England Journal of Medicine 373,
2491–2493.
Lang, N.P., and Tonetti, M.S. (2003).
Periodontal risk assessment for patients in
supportive periodontal therapy (SPT). Oral
Health & Preventive Dentistry 1, 7–16.
Machtei, E.E., Zubrey, Y., Yehuda, A.B., and
Soskolne, W.A. (1989). Proximal bone loss
adjacent to periodontally ‘hopeless’ teeth
with and without extraction. Journal of
Periodontology 60, 512–515.
McGuire, M.K., and Nunn, M.E. (1996).
Prognosis versus actual outcome. III. The
effectiveness of clinical parameters in
accurately predicting tooth survival. Journal
of Periodontology 67, 666–674.
Muller, H.P., and Eger, T. (1999). Furcation
diagnosis. Journal of Clinical Periodontology
26, 485–498.
Nevins, M., Camelo, M., Nevins, M.L. et al.
(2003). Periodontal regeneration in humans
using recombinant human platelet‐derived
growth factor‐BB (rhPDGF‐BB) and
allogenic bone. Journal of Periodontology
74, 1282–1292.
Nibali, L., Krajewski, A., Donos, N. et al.
(2017a). The effect of furcation involvement
on tooth loss in a population without
regular periodontal therapy. Journal of
Clinical Periodontology 44, 813–821.
Nibali, L., Sun, C., Akcalı, A. et al. (2017b).
A retrospective study on periodontal disease
progression in private practice. Journal of
Clinical Periodontology 44, 290–297.
Nibali, L., Zavattini, A., Nagata, K. et al.
(2016). Tooth loss in molars with and
without furcation involvement: A systematic
review and meta‐analysis. Journal of Clinical
Periodontology 43, 156–166.
Ørstavik, D., Kerekes, K., and Eriksen, H.M.
(1986). The periapical index: A scoring
system for radiographic assessment of apical
periodontitis. Endodontics & Dental
Traumatology 2, 20–34.
Peres, M.F.S., Ribeiro, E.D.P., Casarin, R.C.V.
et al. (2013). Hydroxyapatite/β‐tricalcium
phosphate and enamel matrix derivative for
treatment of proximal class II furcation
defects: A randomized clinical trial.
Journal of Clinical Periodontology
40, 252–259.
Pontoriero, R., and Lindhe, J. (1995a). Guided
tissue regeneration in the treatment of
degree II furcations in maxillary molars.
Journal of Clinical Periodontology
22, 756–763.
Pontoriero, R., and Lindhe, J. (1995b). Guided
tissue regeneration in the treatment of
degree III furcation defects in maxillary
molars. Journal of Clinical Periodontology
22, 810–812.
Pontoriero, R., Lindhe, J., Nyman, S. et al.
(1989). Guided tissue regeneration in the
treatment of defects in mandibular molars:
A clinical study of degree III involvements.
Journal of Clinical Periodontology
16, 170–174.
Pontoriero, R., Nyman, S., Ericsson, I., and
Lindhe, J. (1992). Guided tissue regeneration
in surgically‐produced furcation defects: An
experimental study in the beagle dog.
Journal of Clinical Periodontology
19, 159–163.
Stoller, N.H., Johnson, L.R., and Garrett, S.
(2001). Periodontal regeneration of a class II
furcation defect utilizing a bioabsorbable
barrier in a human: A case study with
histology. Journal of Periodontology
72, 238–242.

Furcations: A Treatment Algorithm 283
Tarnow, D., and Fletcher, P. (1984). Classification
of the vertical component of furcation
involvement. Journal of Periodontology 55,
283–284.
Walter, C., Schmidt, J.C., Dula, K. et al.
(2016). Cone beam computed tomography
(CBCT) for diagnosis and treatment
planning in periodontology: A systematic
review. Quintessence International 47,
25–37.
Wang, H.L., Burgett, F.G., Shyr, Y., and
Ramfjord, S. (1994). The influence of
molar furcation involvement and mobility on
future clinical periodontal attachment loss.
Journal of Periodontology 65, 25–29.
Yukna, C.N., and Yukna, R.A. (1996). Multi‐
center evaluation of bioabsorbable collagen
membrane for guided tissue regeneration in
human class II furcations. Journal of
Periodontology 67, 650–657.

Chapter No.: 1 Title Name: <TITLENAME>
bindex.indd
Comp. by: <USER> Date: 14 May 2018 Time: 04:23:02 PM Stage: <STAGE> WorkFlow:
<WORKFLOW>
Page Number: 285
285
Diagnosis and Treatment of Furcation-Involved Teeth, First Edition. Edited by Luigi Nibali.
© 2018 John Wiley & Sons Ltd. Published 2018 by John Wiley & Sons Ltd.
Companion website: www.wiley.com/go/nibali/diagnosis
Page numbers in italics refer to illustrations; those in bold refer to tables
a
accessory canals 56–57, 57
Acute Defect Model 107, 111
Acute/Chronic Defect Model 107–108,
111–112, 115–116
air‐polishing devices 195–196
allograft 48–49
alveolar ridge preservation 212–213
amoxicillin 197–199
animal models of regenerative furcation
therapy 105–128
available models 105–106
dog 106
miniature pig 106
primates 106
class II defects 110–117
bone grafts 110–113
cell therapy 114–117
enamel matrix proteins 13–114
growth factors 114, 115–116
guided tissue regeneration 110–113
class III defects 117–124
bone grafts 117–119
cell therapy 124
enamel matrix proteins 119–120, 121,
122, 123
growth factors 120–124
guided tissue regeneration 117,
118–119
defect types 106–108
critical size defect concept 108–110
experimentally induced defects
107–108
naturally occurring periodontitis 107,
111–112
ethical codes 127
limitations 125–127
antimicrobial treatment
local 196–197,
198
systemic 197–199
antiseptic treatment 196
apical foramina 57–58
b
barrier membranes see guided tissue
regeneration (GTR)
bifurcation ridges 4–5, 4
bite test 72
bone grafts 48–49
animal models 110–113, 117–119
combined with enamel matrix
derivative 147–148
combined with guided tissue
regeneration 139–144,
145
bone loss 232–233, 238
implant positioning and 212–213
bone morphogenetic proteins (BMPs), animal
models 114, 121
bone quality 211
bone resorption 64
c
caries, after furcation tunnelling 185,
188, 237
cavity test 76
cell therapy, animal models 114–116, 124, 125
Index

Index
286
cemento‐enamel junction (CEJ) 5
cervical enamel projections (CEPs) 6–8, 7, 8,
48, 50
classification 6
Chronic Defect Model 107, 112
clinical diagnosis 15–22, 23
examination 72
reproducibility and validity 22
see also diagnosis
cone‐beam computed tomography
(CBCT) 26–28,
26, 270
costs
cost‐effectiveness studies 230,
239–242, 241
cost‐of‐disease studies 230
cost‐utility analyses 230
cost–benefit analysis 230
furcation involvement costs 231–239,
234–237
see also health economic aspects
cracked tooth syndrome 58
cracked tooth testing 73
critical size defect (CSD) 108–110
curettes 39, 45, 46
d
debridement 34, 39–45, 46
effectiveness studies 40–45, 41–44
healing after 33–34
imperfect 34
longitudinal studies 34–38, 35–37
open flap 48–49, 50
versus guided tissue regeneration 139,
140–142
versus non‐surgical closed
debridement 40
dental floss 47
dentine tubules 55–56
diagnosis 71–77, 269–271
algorithm 270,
270
case examples 257, 262–263, 262
clinical 15–22,
23, 72
pre‐surgical 161–162
radiographic 22–28,
25, 26, 73, 74,
162, 269
reproducibility and validity 22
differential diagnosis 271
digital subtraction radiography (DSR)
25, 25
disease progression 91–92
documentation 22,
24
e
economic aspects see health economic
aspects
enamel matrix derivative (EMD)
animal models 113–114, 119–120, 121,
122, 123, 125
degree II furcation defects 144–148,
273, 274
combination therapy 147–148
mandibular molars 144–146, 147
maxillary molars 146–147, 148
versus guided tissue regeneration
144–146, 147
degree III furcation defects 149
regenerative periodontal surgery 150
enamel pearls 8–9, 8, 9
endodontic‐periodontal disease
bacteria involved 59
classification 63–71
primary endodontic lesion 64, 65, 65, 66
primary endodontic lesion with
secondary periodontal
involvement 66–68,
68
primary periodontal lesion 64–66, 67
primary periodontal lesion with
secondary endodontic
involvement 68–70,
69
true combined lesion 70–71, 71, 72, 78
diagnosis of see diagnosis
effect on pulp tissue 60–63, 61–62
endodontically treated teeth 77–78
management 77
see also periodontitis
EuroQol Questionnaire 250
f
fibroblast growth factor (FGF), animal
models 114, 120–121
fistula tracking 73
fistulous sinus track 73, 73
furcation probes 16–17, 16, 269
furcation ridges 4–5, 4
furcation tunnelling 177–185, 178
caries after 185, 188, 237
case examples 260, 261, 261, 264
degree II furcation defects 273–274, 276

Index 287
degree III furcation defects 277, 278, 279
follow‐up studies 186–187
indications 177–179
maintenance phase 185
oral hygiene in the furcation 182–184, 183
double tunnels 183–184, 184
patient selection 179
postoperative follow‐up 182–183
pulp reaction 184–185
tunnelling procedure 179–182, 180,
181, 182
upper first premolar 184, 185
g
gingivectomy 179,
180
gingivitis 10, 33
Gracey curettes 39, 45, 46
case examples 258, 263
growth factors, animal models 114, 115–116,
120–124
guided tissue regeneration (GTR) 79,
109, 273
animal models 109, 110–113, 117,
124–125
bone grafts and GTR 110–113
degree II furcation defects 138–144
animal models 110–113
combination therapy 139–144, 145
long‐term results 144
non‐resorbable versus biodegradable
barrier membranes 139, 143
versus enamel matrix derivative
144–146, 147
versus open‐flap debridement 139,
140–142
degree III furcation defects 148–149
animal models 117
mandibular molars 149
maxillary molars 149
regenerative periodontal surgery 150
h
healing after debridement 33–34
health economic aspects
case examples 261, 265
cost‐effectiveness studies 239–242, 241
furcation involvement costs 231–239,
234–237
health economic analysis 230–231
relevance of furcation
involvement 229–230
research gaps 242–243
health‐related quality of life (HRQoL) 250
home care 45–47
horizontal bone augmentation 215
horizontal probing attachment loss
(PAL‐H) 15,
23
horizontal probing bone level (PBL‐H) 15
i
iatrogenic lesions 11, 78
inferior alveolar nerve damage 213–214
root canal perforations 58
implant placement 209–211
anatomical considerations 211–213
bone loss and implant positioning
212–213, 212
bone quality 211–212
bone augmentation in maxillary molar
region 214–217
horizontal augmentation 215
osteome‐mediated sinus floor
elevation 216–217,
217
sinus lift 215–216
case examples 260–261, 264
complications 219–220,
219
mandibular molar region 213–214
bone augmentation 214
short implants 218–219
inferior alveolar nerve damage 213–214
insulin growth factor (IGF‐1), animal
models 114
interdental brushes 46–47
case examples 258, 263
tunnelling procedure and 179, 179,
180, 180
intermediate bifurcation ridge (IBR) 4
l
laser therapy 192–193
lateral canals 56–57
local antibiotic therapy 196–197, 198
low‐level laser therapy (LLLT) 193
m
magnetostrictive scalers 39
mesenchymal stem cells (MSCs) 114–117
metronidazole 197–199

Index
288
microbial plaque see plaque
minimally invasive surgical therapy 200
mobility test 72
molars 1
anatomical aetiological factors 6–9
cervical enamel projections 6–8,
6, 7, 8
enamel pearls 8–9, 8, 9
anatomical factors in furcation
involvement 2–6,
3
bifurcation ridges 4–5, 4
furcation entrance area 3–4
root surface area (RSA) 5
root trunk length 5–6
anatomy 1–2
development 2
periodontal aetiological factors 9–11
iatrogenic factors 11
occlusal trauma 10
plaque‐associated inflammation 10
pulpal pathology 10–11
vertical root fractures 10, 58, 58, 59
root resection 27
multi‐rooted teeth 15–16, 16
o
occlusal trauma 10, 73, 74
open flap debridement 48–49, 50
versus guided tissue regeneration 139,
140–142
versus non‐surgical closed debridement 40
Oral Health Impact Profile (OHIP‐14) 250
oral health‐related quality of life
(OHRQoL) 250, 251
implementation of in furcation treatment
254–255
oral hygiene
after furcation tunnelling 182–184, 183
degree I furcation involvement 271
patient home care 45–47
Oral Impacts on Daily Performance (OIDP)
questionnaire 250, 251
Osteogain 120
osteogenic protein‐1 (OP‐1) 121–124
osteoplasty 180,
181
outcome measures 137–138
clinical outcomes 138
degree II furcation defects 137–138
degree III furcation defects 138
histology 137
patient‐reported outcome measures
(PROMS)
furcation involvement 251–252
periodontology 249–251
overhanging dental restorations 11
p
palpation 72
patient feedback 252–254
patient selection
furcation tunnelling 179
regenerative furcation therapy 149
resective therapy 170
patient‐reported outcome measures
(PROMS) 249–252
furcation involvement 251–252
implementation of in furcation
treatment 254–255
periodontology 249–251
percussion 72
peri‐implantitis 219–220
risk factors 219–220
therapy 220
periodontal chart 24
periodontal endoscope 191–192
periodontal ligament (PDL) 114
periodontal therapy 92, 92
periodontitis 10, 33, 40, 64–66, 101, 199
disease progression 91–92
model 239,
240
see also tooth loss
furcation involvement frequency 28–29
health economics studies 232, 233, 238,
242, 243
see also endodontic‐periodontal disease
photodynamic therapy (PDT) 193–195, 194
phototherapy 193
piezoelectric scalers 39, 46
plaque 33–34
removal 33–34
effectiveness evaluation 38
see also debridement
role in gingivitis and periodontitis 33
plaque‐associated inflammation 10
platelet‐derived growth factor (PDGF), animal
models 114, 121
platelet‐rich fibrin (PRF) 148
platelet‐rich plasma (PRP) 114–117, 148
pocket probing 73–74
povidone‐iodine 197

Index 289
powered scalers 39
powered toothbrushes 45–46
probes 16–17,
16, 269
probing 73–74
probiotics 199–200
pulp vitality tests 74–76, 75
pulpal pathology 10–11
necrosis 63, 69, 70, 76
periodontal disease relationship 60–63,
61–62
periodontal furcation therapy
relationship 78–82
non‐surgical therapy 78–79
regenerative therapy 79–80
resective therapy 80–82
pulpitis 65, 76
reaction to tunnelling procedure 184–185
r
radiographic diagnosis 22–28, 73, 74, 269
digital subtraction radiography (DSR)
25, 25
pre‐surgical 162
radiation exposure issues 28
three‐dimensional radiography 26–28, 26
regenerative furcation therapy 79–80, 105,
137–152
case examples 260, 263–264
challenges 150–151
degree II furcation defects 138–148,
273, 275
enamel matrix derivative 144–148
guided tissue regeneration 138–144
platelet concentrates 148
degree III furcation defects 148–149
enamel matrix derivative 149
guided tissue regeneration 148–149
outcome measures 137–138
clinical outcomes 138
degree II furcation defects 137–138
degree III furcation defects 138
histology 137
see also patient‐reported outcome
measures (PROMS)
patient selection 149
pulp relationship 79–80
recommendations 80
regenerative periodontal surgery 150,
151, 152
tooth selection 149–150
see also animal models of regenerative
furcation therapy
resective therapy 80–82, 81, 161–173
anatomical considerations 161
case examples 260, 264
contraindications 169–170
degree II furcation defects 276
degree III furcation defects 277–278
indications 163–167
root resection 164–167, 165
root separation 163–164, 164, 166–167,
166, 167
furcation tunnelling 177–179
patient selection 170
pre‐surgical diagnosis 161–162
procedure 170–173
crown build‐up 171
endodontic treatment 170
final prosthesis 173
periodontal surgery 172–173
provisional restoration 172
root separation/resection 171–172
pulp relationship 80–82
recommendations 82
scientific studies 167, 168, 169
tooth selection 170
restorations, overhanging 11
rizectomy 59, 78, 165, 166
rizotomy 164
root canal
accessory canals 56–57, 57
iatrogenic perforations 58
root complex 5
root cone length 6
root dentine hypersensitivity 79
root fractures 10, 58, 58, 59
root resection 27
root resorption 58–59, 60
root separation 163–164, 164
root surface area (RSA) 5
mandible 5
maxilla 5
root trunk length 5–6
s
scaling and root planing 33–34, 39
case examples 258, 263
endoscopy‐aided 191–192
healing after 33–34
see also debridement

Index
290
selective anaesthesia test 77
short implants 218–219
sinus lift 215–216
osteome‐mediated sinus floor elevation
216–217, 217
sonic scalers 39
split‐mouth design 34
stellate reticulum 2
surgical treatment
furcation regeneration 149–150
patient selection 149
regenerative periodontal surgery 150
tooth selection 149–150, 151
innovations 200
minimally invasive surgery 200
root separation/resection 170–173
crown build‐up 171
during preliminary prosthetic
preparation 171–172
endodontic treatment 170
final prosthesis 173
patient selection 170
periodontal surgery 172–173
provisional restoration 172
tooth selection 170
tunnelling procedure 179–182, 180,
181, 182
see also open flap debridement; resective
therapy
systemic antimicrobial treatment 197–199
t
tetracyclines 197–199
three‐dimensional radiography 26–28, 26
tooth extraction
versus treatment 209–211
when to extract 278–280
see also implant placement
tooth loss 92–101
by different furcation degree 99
by vertical furcation component 99
risk of 98, 99–101, 100
study procedures 95–97
treated teeth 93–99
untreated furcation‐involved teeth 93
tooth mobility 72
tooth selection
regenerative furcation therapy 149–150
resective therapy 170
toothbrushes 45–46
transforming growth factor‐beta (TGF‐β),
animal models 114
treatment
degree I furcation involvement
270–271, 272
degree II furcation involvement 271–276,
273, 275
mandibular 272–275,
273
maxillary, combined 276
maxillary, single buccal 275, 275
maxillary, single interproximal 275–276
degree III furcation involvement
276–278, 277
mandibular 277
maxillary 277–278,
279
innovative treatment 278
options 162–163
treatment versus extraction and
implants 209–211
upper premolars 184, 185, 278
when to extract 278–280
see also specific treatments
tunnelling see furcation tunnelling
u
ultrasonic scalers 39, 45, 47, 258, 263
upper premolar treatment 278
furcation tunnelling 184, 185
v
vertical root fractures 10, 58, 58, 59
w
WaterPik 47
