IDIOPATHIC INFLAMMATORY BOWEL DISEASE (IBD)
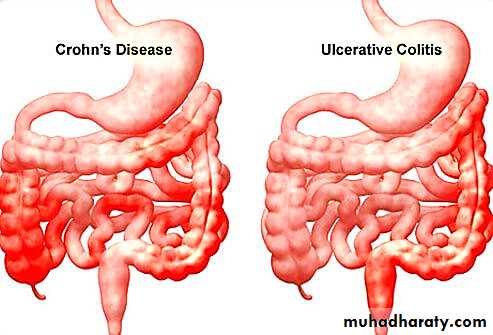
The two disorders known as inflammatory bowel disease (IBD) are Crohn's disease (CD) and ulcerative colitis (UC). These diseases have distinctly different clinical and pathological features. Both CD and UC are chronic, relapsing inflammatory disorders of obscure origin.Etiology and Pathogenesis
In the normal GIT, the mucosal immune system is always ready to respond against ingested pathogens but is unresponsive to normal intestinal microflora. In IBD, this state of homeostasis is disrupted, leading to two key pathogenic abnormalitiesStrong immune responses against normal microflora
Defects in epithelial barrier that cause microflora to reach the lymphoid tissue of the intestine
The exact cause (s) leading to the above is still not established, hence the designation idiopathic. It is postulated that IBD result from exaggerated local immune responses to microflora in the gut, in genetically susceptible individuals.
Thus, the pathogenesis of IBD involves
1. Failure of immune regulation2. Genetic susceptibility
3. Environmental triggers specifically microbial flora.
CD appears to be the result of a chronic delayed-type hypersensitivity reaction induced by IFN-γ- producing TH1 cells. This is supported by the presence of granulomas in this disease.
CROHN DISEASE (CD)
This disease may involve any level of the alimentary tract. CD occurs at any age, but the peak age of incidence is between 10 and 30 years. Smoking has been found to be a strong risk factor.Pathological features
When fully developed, Crohn disease is characterized pathologically by
1. Sharply segmental and typically transmural involvement of the bowel by an inflammatory process with mucosal damage
2. The presence of
- Small noncaseating granulomas
- Deep fissures that may eventuate in the formation of fistulae
In CD, there is involvement of the small intestine alone in about 40% of cases, of small intestine and colon in 30%, and of the colon alone in about 30%. Other portions of the GIT may also be uncommonly involved.
Gross features
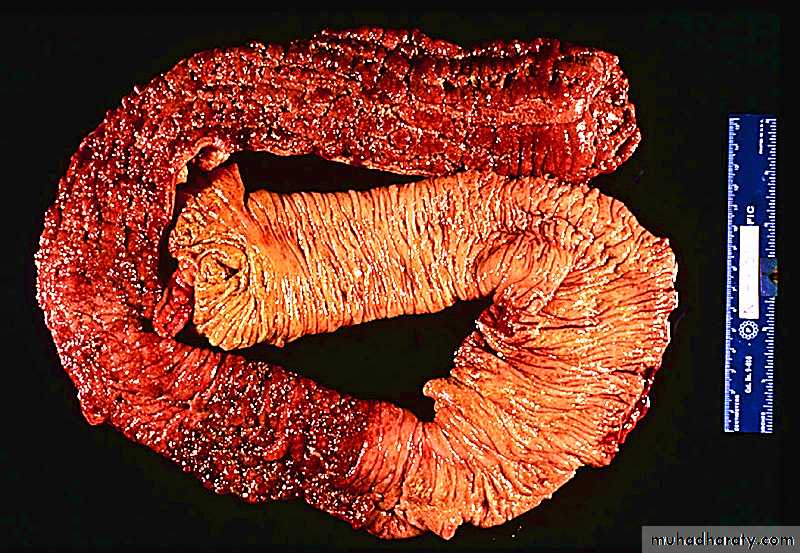
Segments of the small bowel involved by the disease show granular and dull gray serosa (normally transparent and glistening).Often the mesenteric fat wraps around the bowel (creeping fat)
The involved bowel wall is thick and rubbery (because of edema, inflammation, and fibrosis). As a result, the lumen is narrowed.
A classic feature of CD is the sharp demarcation of diseased bowel segments from adjacent uninvolved, essentially normal bowel (skip lesions).
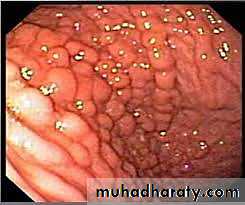
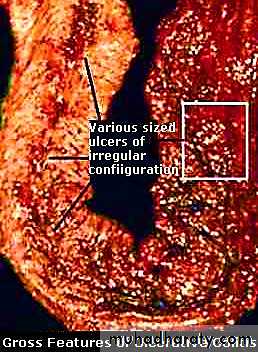
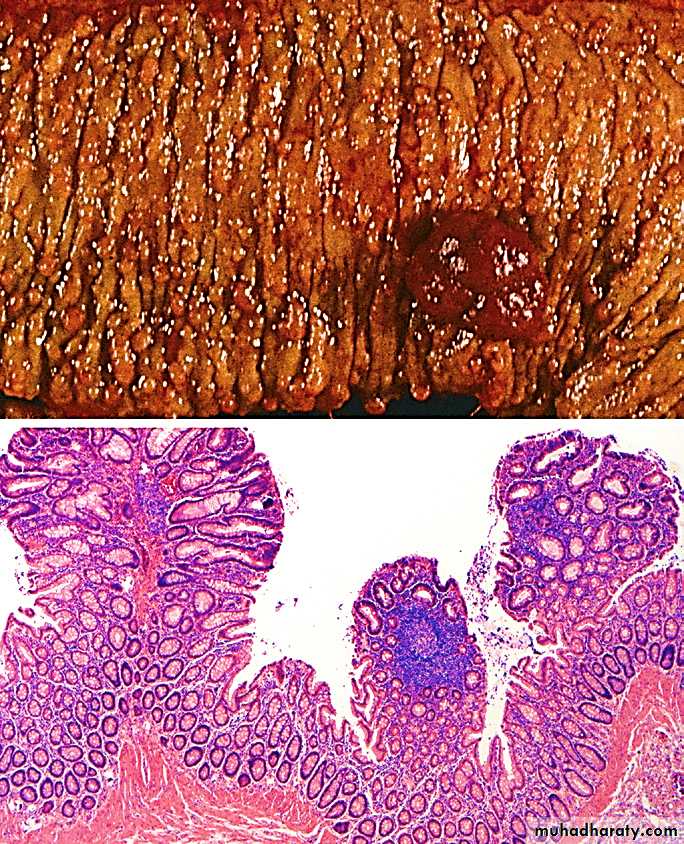
Early disease shows small mucosal ulcers that coalesce to form long, serpentine linear ulcers (i.e. long and twisted or sinuous).
As the intervening mucosa (between the ulcers) tends to be accentuated by inflammation and edema, it acquires a cobblestone appearance. (Cobble-stone, is a rounded stone, esp. of the size used for paving).
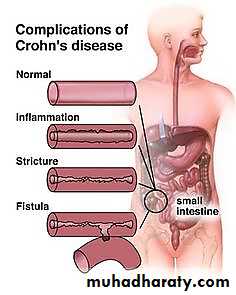
Narrow fissures develop between the mucosal folds, often penetrating deeply through the bowel wall. Further extension of these fissures leads to fistulae or sinus tracts formation, between the diseased intestinal segment and adherent structures (bowel loops, vagina, urinary bladder, skin of the abdomen) or the sinuses may end blindly within the abdominal cavity.
Free perforation or localized abscesses may develop.
Microscopic features
1. Acute mucosal inflammation: there is neutrophilic infiltration of the surface & crypt epithelium that eventually collects within the lumen of the crypts forming crypt abscesses.2. Chronic mucosal damage:
This is the hallmark of chronicity of CD (and UC). It manifests as architectural distortion (in the small intestine as villus blunting; in the colon, the crypts exhibit irregularity, and branching). Crypt destruction leads to progressive mucosal atrophy.
3. Ulcerations are the usual outcome of severe active disease; these may be superficial, or may penetrate deeply (as fissures) into underlying tissue layers.
4. Transmural chronic inflammation affecting all layers: chronic inflammatory cells (lymphocytes and plasma cells) fill the affected mucosa and, to a lesser extent, all underlying intestinal layers. Lymphoid aggregates are usually scattered throughout the bowel wall.
5. Noncaseating granulomas: in about 50% of the cases, noncaseating small granulomas may be present in all tissue layers. Because they are not always present; the absence of granulomas does not rule out the diagnosis of CD.
6. Other mural changes: in diseased segments, the muscularis mucosae usually exhibits duplication & thickening. There is also fibrosis of the submucosa, muscularis propria, and serosa that eventually leads to stricture formation.
7. Dysplastic changes of the mucosal epithelial cells are particularly important in persons with long-standing chronic disease are. These may be focal or widespread, tend to increase with time, and are thought to be related to increased risk of carcinoma, particularly of the colon.
Clinical Features
The disease usually begins with intermittent attacks of diarrhea, fever, and abdominal pain, spaced by asymptomatic periods lasting for weeks to many months. In those with colonic involvement, occult or overt fecal blood loss may lead to anemiaDuring this lengthy, chronic disease, complications may arise from
1. Fibrosing strictures, particularly of the terminal ileum (intestinal obstruction)2. Fistulas formed to other loops of bowel, urinary bladder, vagina, or perianal skin, or into a peritoneum. In the latter focal abscesses may occur.
3. Extensive involvement of the small bowel, including the terminal ileum, may cause
a. marked loss of albumin (protein-losing enteropathy)
b. generalized malabsorption
c. specific malabsorption of vitamin B12 (pernicious anemia), or malabsorption of bile salts, leading to steatorrhea.
Extra intestinal manifestations of this disease
1. Arthritis & finger clubbing2. Red nodules of the skin (Erythema nodosum)
3. Primary sclerosing cholangitis, (but the association is not as strong as in UC).
4. Renal disorders secondary to trapping of the ureters in the inflammatory process sometimes develop and leading to hydronephrosis and pyelonephritis.
5. Systemic amyloidosis (rare late consequence).
6. An increased incidence of cancer of the GIT in patients with long-standing progressive CD; however, the risk of cancer in CD is considerably less than in patients with chronic UC.
ULCERATIVE COLITIS
In contradistinction to CD, ulcerative colitis is a chronic ulcero-inflammatory disease limited to the colon and affecting only the mucosa and submucosa; it extends in a continuous fashion proximally from the rectum.Well-formed granulomas are absent. However, like CD, UC is a systemic disorder associated in some patients with arthritis, uveitis, hepatic involvement (primary sclerosing cholangitis), and skin lesions
Pathological features
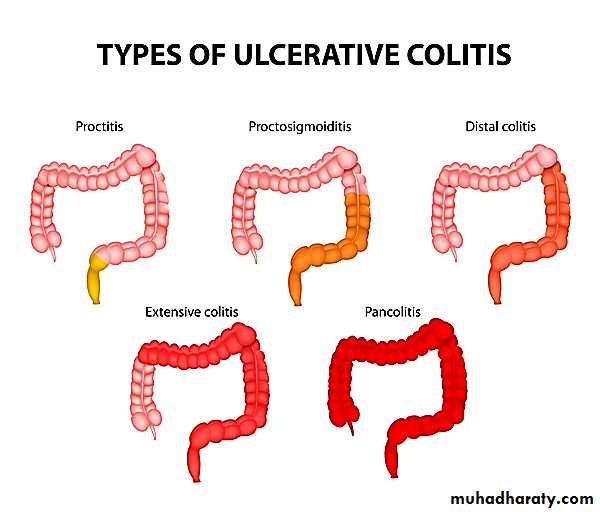
Ulcerative colitis involves the rectum and extends proximally in a retrograde fashion to involve the entire colon ("pancolitis") in the more severe cases. It is a disease of continuity, and "skip" lesions are not foundGross features
A key feature of UC is that the mucosal damage is continuous from the rectum and extending proximally.The mucosa may exhibit reddening and granularity with easy bleeding.
With fully developed severe, active inflammation, there may be extensive ulcerations of the mucosa.
Isolated islands of regenerating mucosa bulge upward to create polypoid projections (pseudopolyps).
With chronicity or healing of active disease, progressive mucosal atrophy occurs.
Thickening of the bowel wall does not occur in UC; the serosal surface is usually completely normal (cf. CD).
Only in the most severe cases of ulcerative disease (UC, CD, and other severe inflammatory diseases) does toxic damage to the muscularis propria and neural plexus lead to complete shutdown of neuromuscular function. In this instance the colon progressively swells and becomes gangrenous, a life-threatening condition called toxic megacolon.
Microscopic features
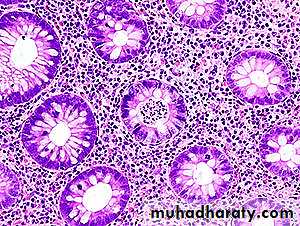
The basic mucosal alterations in UC are similar to those of colonic CD, with inflammation, chronic mucosal damage, and ulceration.There is diffuse, predominantly chronic inflammatory infiltrate in the lamina propria.
Neutrophilic infiltration of the epithelial layer may produce crypt abscesses. The latter are not specific for UC and may be observed in CD or any active inflammatory colitis.
Unlike CD, there are no granulomas.
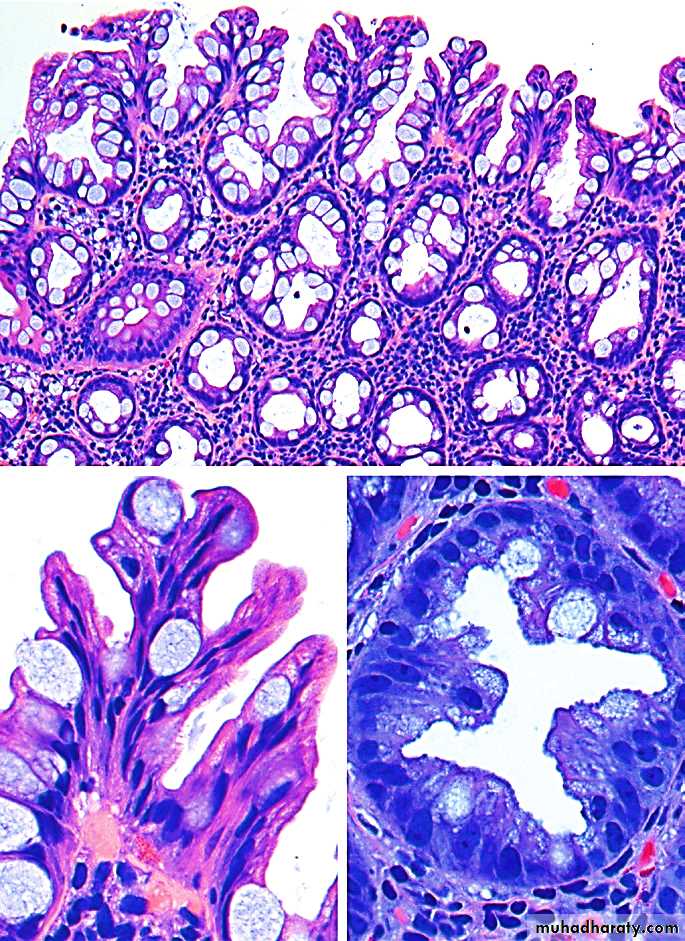
Particularly significant is the spectrum of epithelial dysplasias, which are divided into low-grade and high-grade depending on the severity. Invasive carcinoma is the ultimate lesion arising from dysplasia
To summarize UC differs pathologically from CD in the following
• 1-Well-formed granulomas are absent.• 2-There are no skip lesions.
• 3-The mucosal ulcers rarely extend below the submucosa, and
• 4-There is surprisingly little fibrosis.
• 5-Mural thickening does not occur, and the serosal surface is usually completely normal.
6-There appears to be a higher risk of carcinoma development
The outcome of UC depends on two factors:
1. The severity of active disease2. The duration of the disease
The most serious long-term complication of UC is colonic carcinoma. There is a tendency for dysplasia to arise in multiple sites. The associated carcinomas are often infiltrative without obvious exophytic masses. Historically, the risk of cancer is highest in patients with pancolitis of 10 or more years' duration.
Intestinal neoplasm
Intestinal polyps: Non-neoplastic or neoplastic.The non-neoplastic polyps can be further defined
Inflammatory, result of chronic cycles of injury and healing.
Hamartomatous polyps occur sporadically or as a part of genetic diseases e.g. Peutz-Jeghers Syndrome. In the latter case, they often are associated with increased risk of malignancy
Hyperplastic polyps are benign epithelial proliferations
Most commonly found in the left colon and rectum.Less than 0.5 cm
They are not reactive in origin, in contrast with gastric hyperplastic polyps; have no malignant potential; and must be distinguished from sessile serrated adenomas.
Hyperplastic polyp
Adenomas (Adenomatous polyps)
Adenomas are intraepithelial neoplasms that range from small, often pedunculated lesions to large neoplasms that are usually sessile. The prevalence of colonic adenomas increases progressively with age.Males and females are affected equally. Adenomatous polyps are divided into three subtypes on the basis of the epithelial architecture
1-Tubular adenomas: compose of tubular glands
2-Villous adenomas: composed of villous projections
3-Tubulovillous adenoma: composed of a mixture of the above two.
All adenomas by definition arise as the result of dysplastic epithelial proliferation.
The dysplasia ranges from low-grade to high-grade.There is strong evidence that adenomas are precursors for invasive colorectal adenocarcinomas.
The risk of cancer is high (approaching 40%) in villous adenomas more than 4 cm in diameter.
Familial adenomatous polyposis (FAP)
ADCaused by APC mutations
Patients typically have over 100 adenomas
100% get cancer before 30 yrs if untreated
Hereditary nonpolyposis colorectal cancer (HNPCC) (Lynch syndrome)
HNPCC is caused by mutations in DNA mismatch repair genes.Familial clustering of cancers at several sites including the colorectum, endometrium, stomach, ovary, ureters, brain, small bowel, hepatobiliary tract, and skin
Patients with HNPCC have far fewer polyps and develop cancer at an older age than that typical for patients with FAP but at a younger age & on right side of colon than in patients with sporadic colon cancer.
FAP and HNPCC are examples of two distinct pathways of neoplastic transformation, both of which contribute to sporadic colon cancer.
Adenocarcinoma
60 to 70 years of ageWestmore than east
Diet: are low intake of unabsorbable vegetable fiber and high intake of refined carbohydrates and fat.
Life style
Aspirin or other NSAIDs have a protective effect.
genetic and epigenetic abnormalities
APC/β-catenin pathway (classic adenomacarcinomasequence, which accounts for as much as
80% of sporadic colon tumors) + microsatellite instability pathway
Grossly:
Tumors in the proximal colon often grow as polypoid, exophytic massescarcinomas in the distal colon tend to be annular lesions that produce “napkin ring”
Right-sided colon cancers most often are called to clinical attention by the appearance of fatigue and weakness due to iron deficiency anemia.(always suspect it in elderly)
Left-sided colorectal adenocarcinomas may produce occult bleeding, changes in bowel habits, or cramping left lower quadrant discomfort.
Microscopic features
The features of right- and left-sided colonic adenocarcinomas are similar.
Differentiation (grade) may range from well-differentiated tumors to undifferentiated, frankly anaplastic masses.
Invasive tumor provokes a strong desmoplastic (fibrotic) stromal response (responsible for the characteristic firm, hard consistency of most carcinomas).
Carcinomas arising in the anal canal are mostly of squamous cell type.
The two most important prognostic factors are depth of invasion
and the presence or absence of lymph node metastases.most carcinomas arise from preexisting adenomas. This has been supported by the following observations:
1. Populations that have a high prevalence of adenomas have a high prevalence of colorectal cancer.
2. The distribution of adenomas parallel that of colorectal cancer.
3. The peak incidence of adenomas precedes that of carcinoma by some years.
4. When invasive carcinoma is identified at an early stage, a related adenoma is often present
5. The risk of cancer is directly related to the number of adenomas, that is why carcinoma complicates all those with FAP syndrome.
6. Removal of all adenomas that are suspicious reduces significantgly the incidence of cancerinoma. 98% of all cancers in the large intestine are adenocarcinomas
Tumor
Tis In situ dysplasia or intramucosal carcinomaT1 Tumor invades submucosa
T2 Tumor invades into, but not through, muscularis propria
T3 Tumor invades through muscularis propria
T4 Tumor invades adjacent organs or visceral peritoneum
Regional Lymph Nodes
NX Lymph nodes cannot be assessed
N0 No regional lymph node metastasis
N1 Metastasis in one to three regional lymph nodes
N2 Metastasis in four or more regional lymph nodes
Distant Metastasis
MX Distant metastasis cannot be assessedM0 No distant metastasis
M1 Distant metastasis or seeding of abdominal organs
Appendix
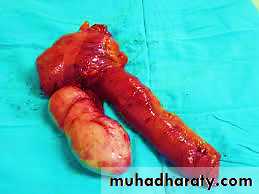
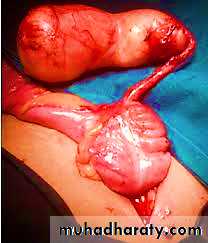
Acute appendicitis :Is most common in children and adolescents.
It is thought to be initiated by increased intraluminal pressure consequent to obstruction of the appendiceal lumencby fecalith and, less commonly, a gallstone, tumor, or ball of worms (Entrobias vermicularis) which compromises venous outflow.
Acute appendicitis without obstruction, which is of unknown pathogenesis.
Microscopy
The microscopic criterion for the diagnosis of acute appendicitis is neutrophilic infiltration of the muscularis propria (transmural inflammation)Usually, neutrophils and ulcerations are also present within the mucosa.
Tumors of appendix
Carcinoid
The most common tumor of the appendix
Most frequently involves the distal tip of the appendix, where it produces a solid bulbous swelling up to 2 to 3 cm in diameter.
Adenomas or adenocarcinomas also occur in the appendix and may cause obstruction and enlargement that mimics the changes of acute appendicitis.
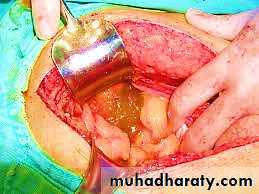
Mucocele, a dilated appendix filled with mucin:
• Obstructed appendix containing inspissated mucin or• Tumor : mucinous cystadenoma or mucinous cystadenocarcinoma.
In the latter instance, invasion through the appendiceal wall can lead to intraperitoneal seeding and spread.
In women, the resulting peritoneal implants may be mistaken for mucinous ovarian tumors.
In the most advanced cases, the abdomen fills with tenacious, semisolid mucin, a condition called pseudomyxoma peritonei.This disseminated intraperitoneal disease may be held in check for years by repeated debulking but in most instances is ultimately fatal.