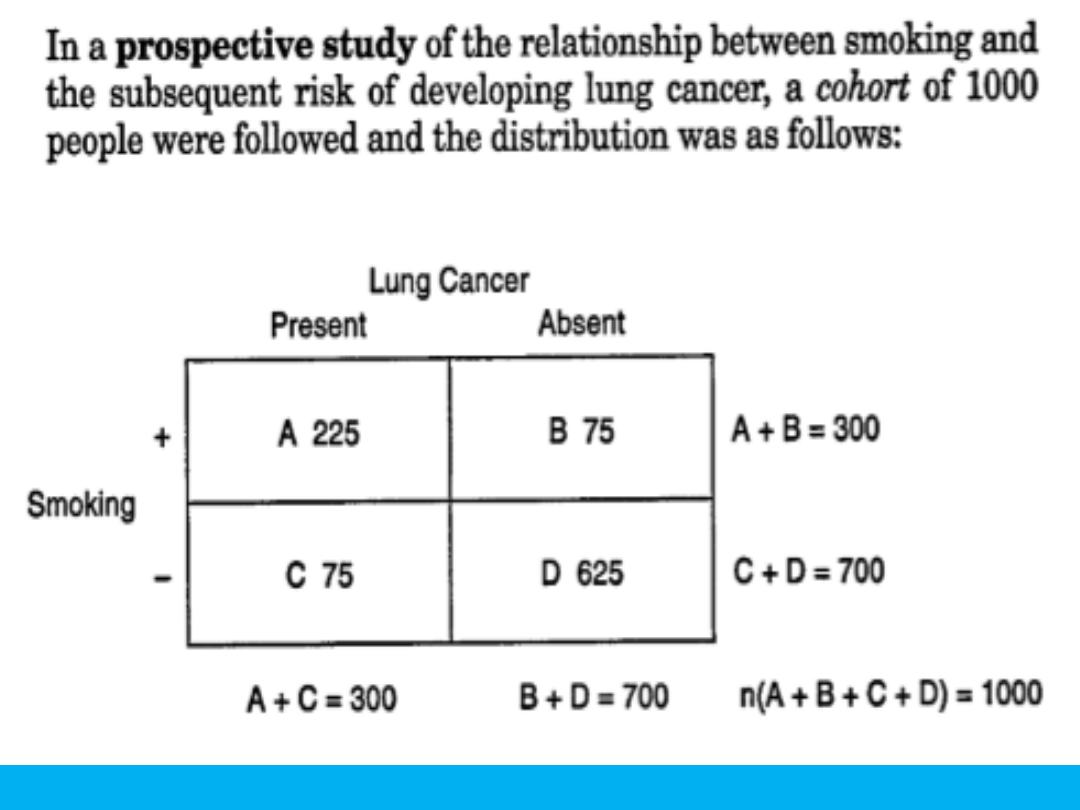
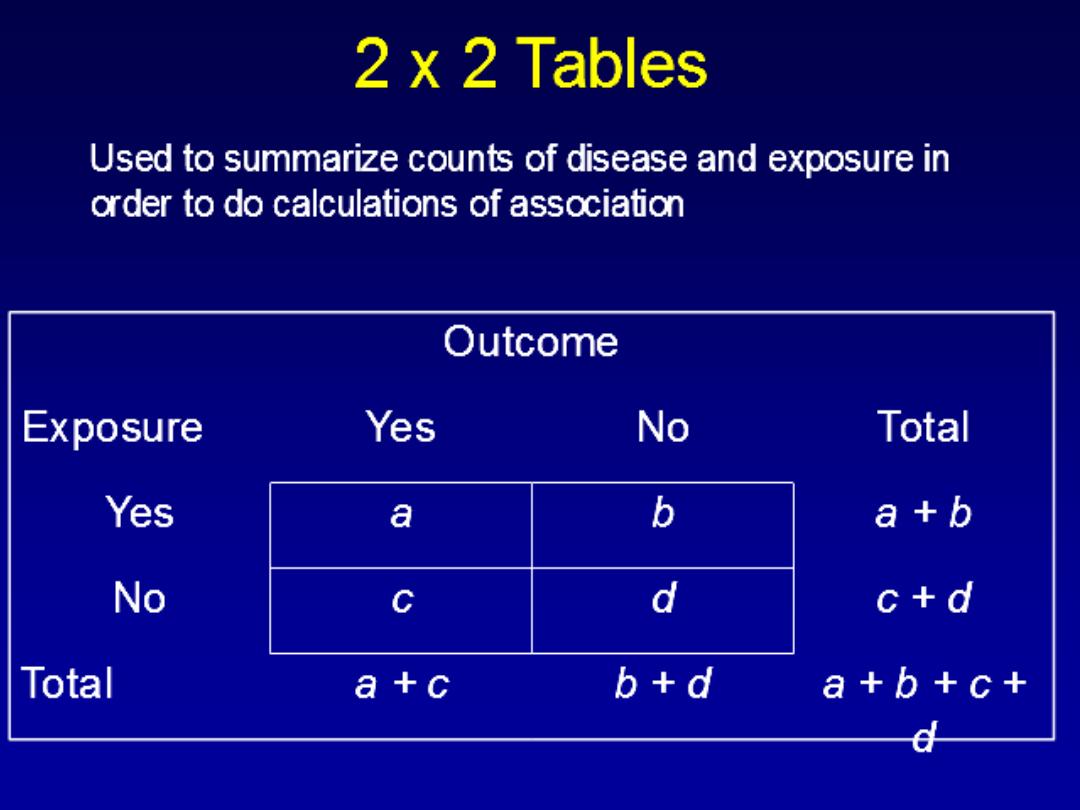
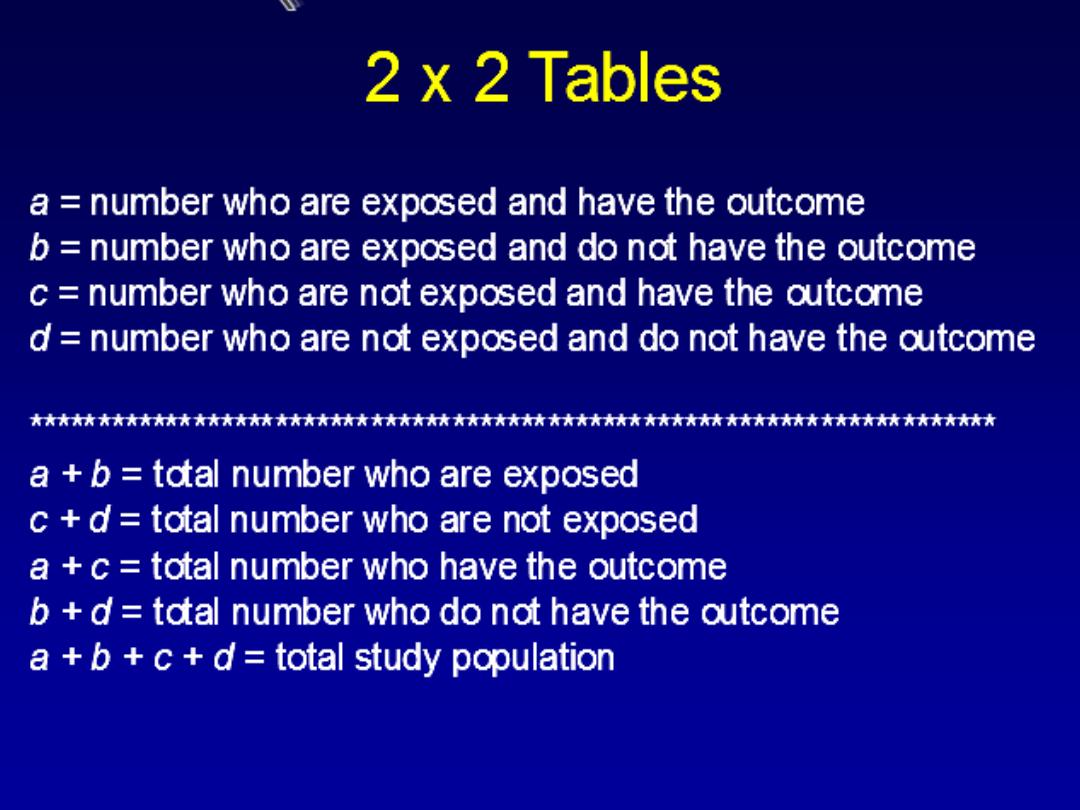
COHORT STUDY
WHAT IS a COHORT STUDY?
Dr. Sijal Fadhil Farhood AL-joborae
F.I.C.M.S (Baghdad)
M.Sc. Community (Nahrain)
M.B.Ch.B (Babylon University)

INTRODUCTION
The term cohort comes from the Latin word
cohors , meaning a group of soldiers in ancient
Rome .
Today ,we use the ward cohort to characterize
group of persons who are
any designated
followed or traced over a period of time.
In epidemiology, the term cohort is defined as a
group of people who share common
characteristics or experience within a defined
time period (e.g. age, occupation, exposure to
drug and vaccine)
COHORT STUDIES
It is an observational analytic design ,it is also
called (follow up,longtudenal,incidence and
forward looking
study
) in which a group or
groups of individual are defined on the basis
of presence or absence of a suspected risk
factor for a disease.
At the time exposure status is defined , all
potential subjects must be free from the
disease under investigation ,and illegible
participants are then followed over a period
of time to assess the occurrence of that
outcome.

THE DISTINGUISHING FEATURES OF COHORT STUDIES ARE:
A-
a cohort are identified prior to the appearance of
the disease under investigation.
B-
The study groups are observed over a period of
time to determine the frequency of disease among
them.
C-
the study proceeds forward from cause to effect.

INDICATION OF COHORT STUDY

Framework of cohort study:
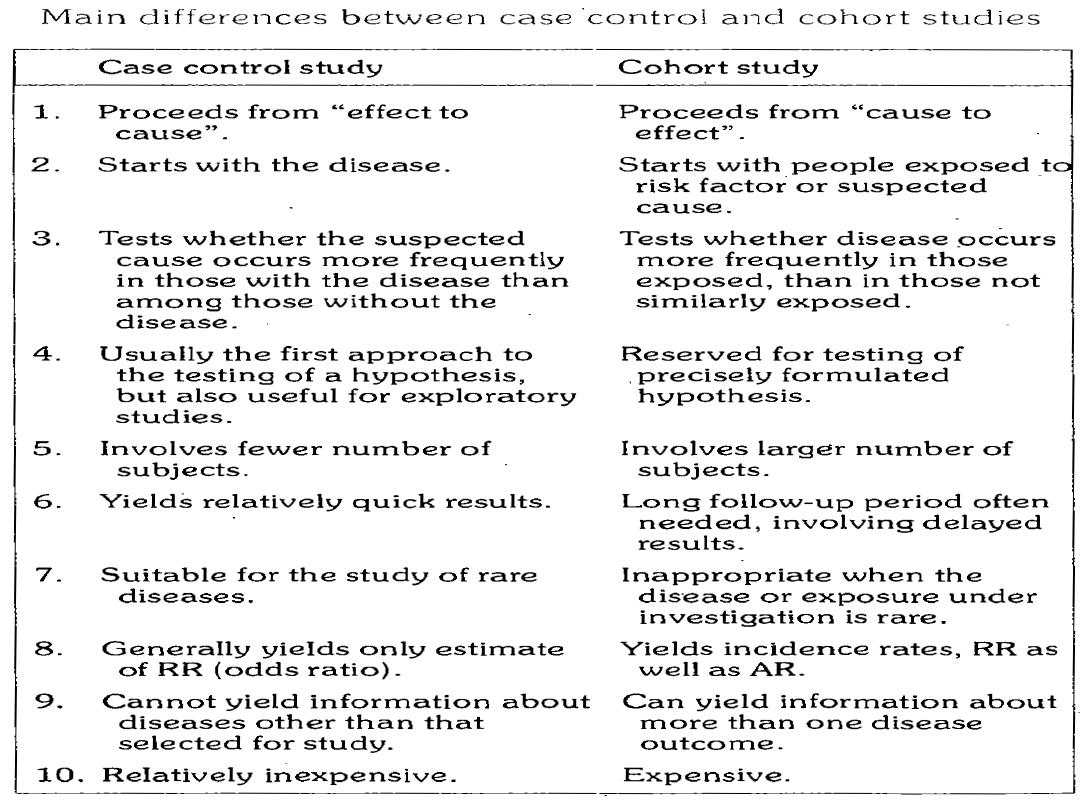
In contrast to case control studies which proceed
from ((effect to cause)),the basic approach in cohort
studies is to work from ((cause to effect)), that is in
case control study,exposure and disease have
already occurred when the study is initiated.
In cohort study,the exposure has occurred,but the
disease has not.
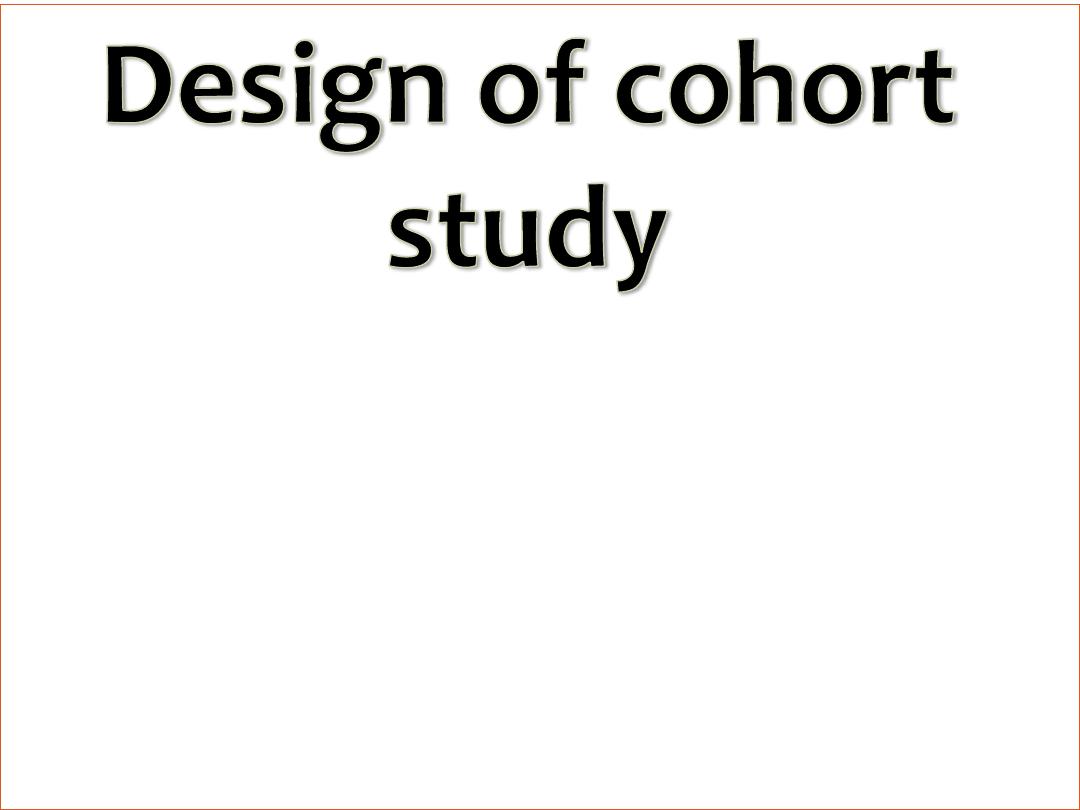
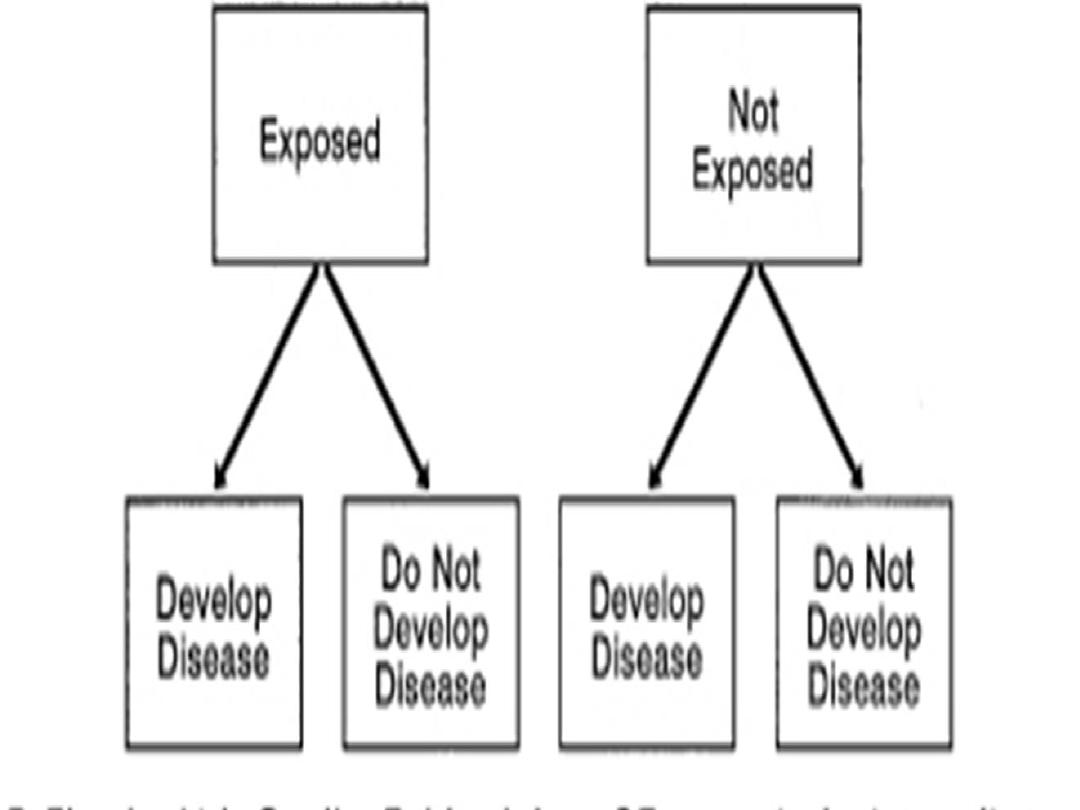

The exposed group is called index group
The unexposed group is called referent or
comparison group

A-The cohorts must be free from disease under study, if the
disease under study is coronary heart disease, the cohort
members are first examined, and those who already have
evidence of the disease under investigation are excluded.
B-In so far as the knowledge of the disease permits, both
the groups(i.e the study and control cohorts) should be
equally susceptible to the disease under study.

C-both the groups should be comparable in respect to all
the possible variables which may influence the frequency
of the disease
D-The diagnostic and eligibility criteria must be defined
beforehand ,this will depend upon the availability of
reliable methods for recognizing the disease when it
develops.

Types of
population
studied
Defined by
Follow-up
Appropriate
measure of
disease
frequency
Open or dynamic
Changeable
characteristic
Member come and
go; losses may
occur
Incidence rate
Fixed
Irrevocable event
Does not gain
members; losses
may occur
Incidence rate
Closed
Irrevocable event
Does not gain
members; no
losses occur
Cumulative
incidence
TYPES OF POPULATION STUDIED
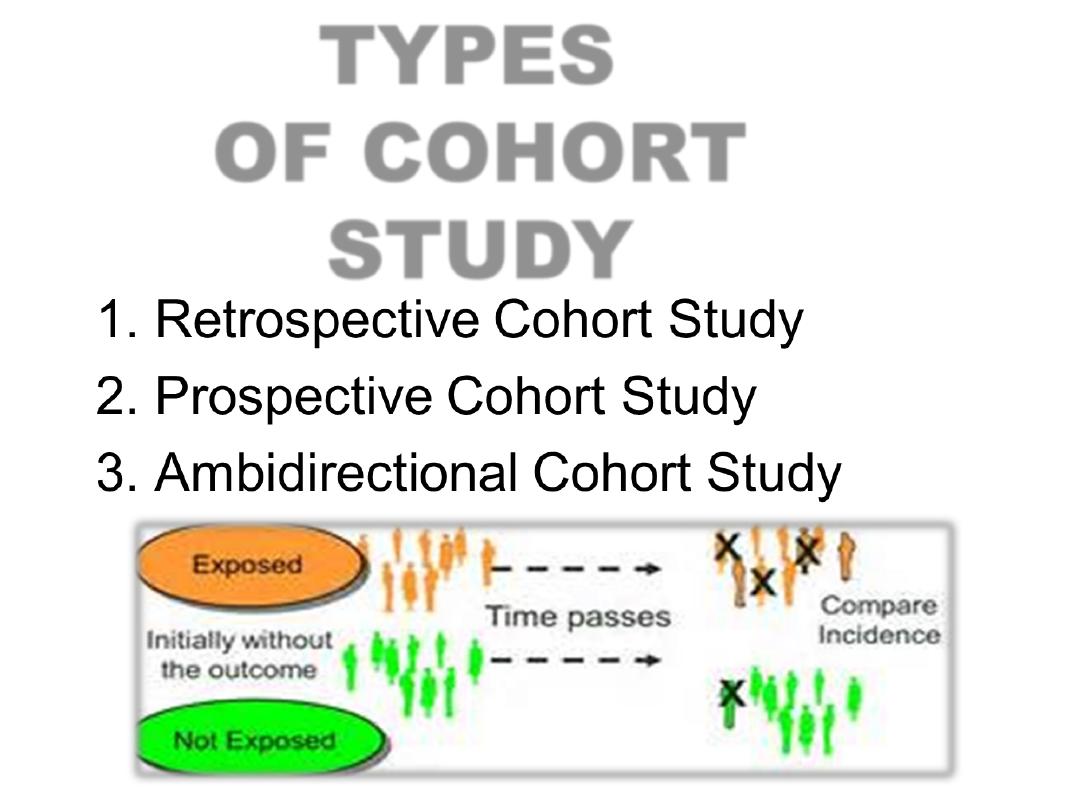
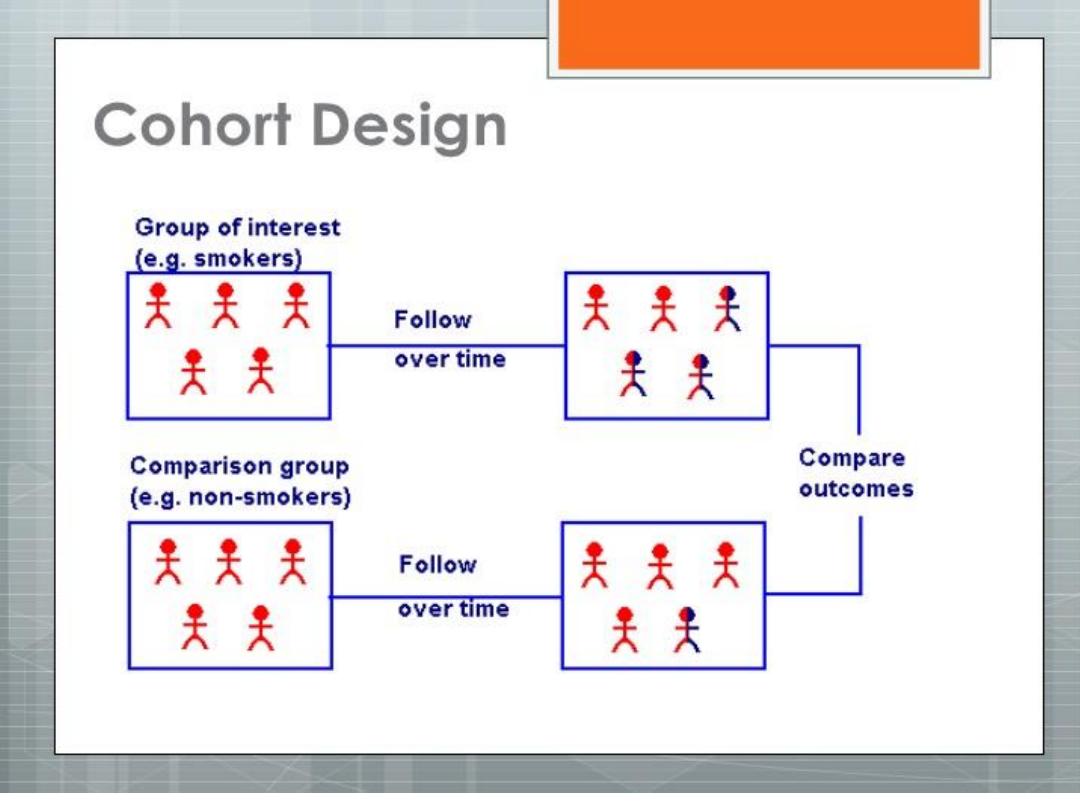
TYPES
OF COHORT
STUDY
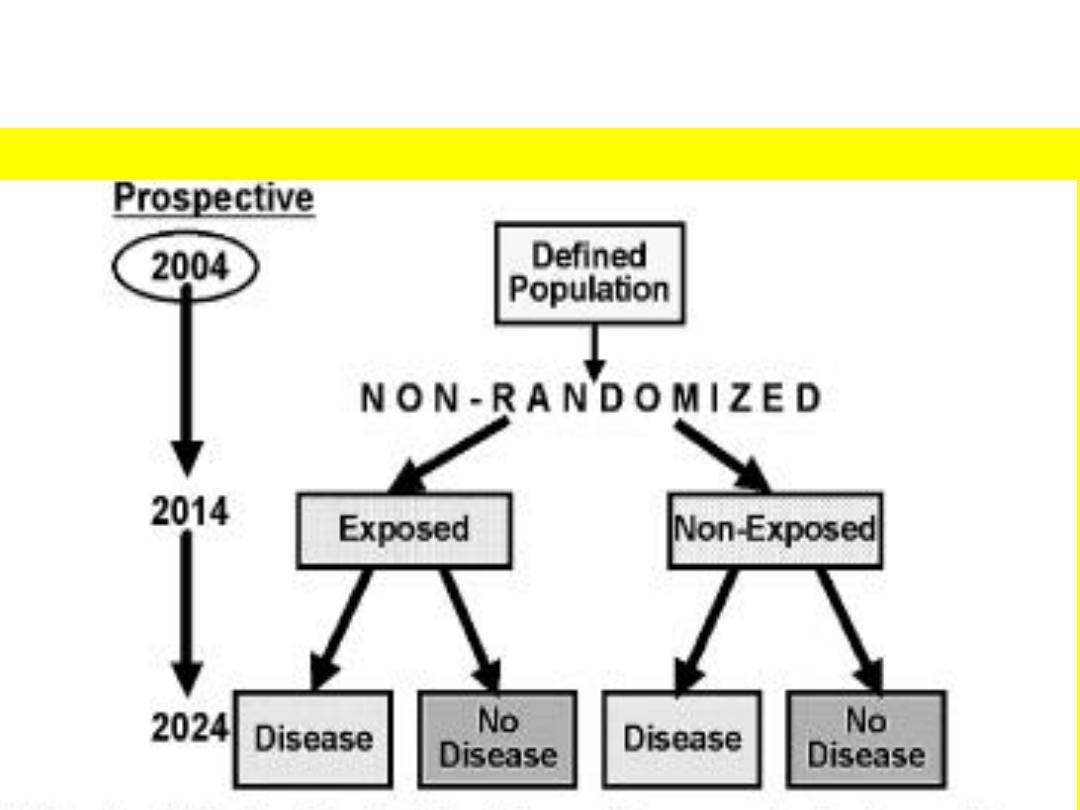
PROSPECTIVE COHORT STUDY
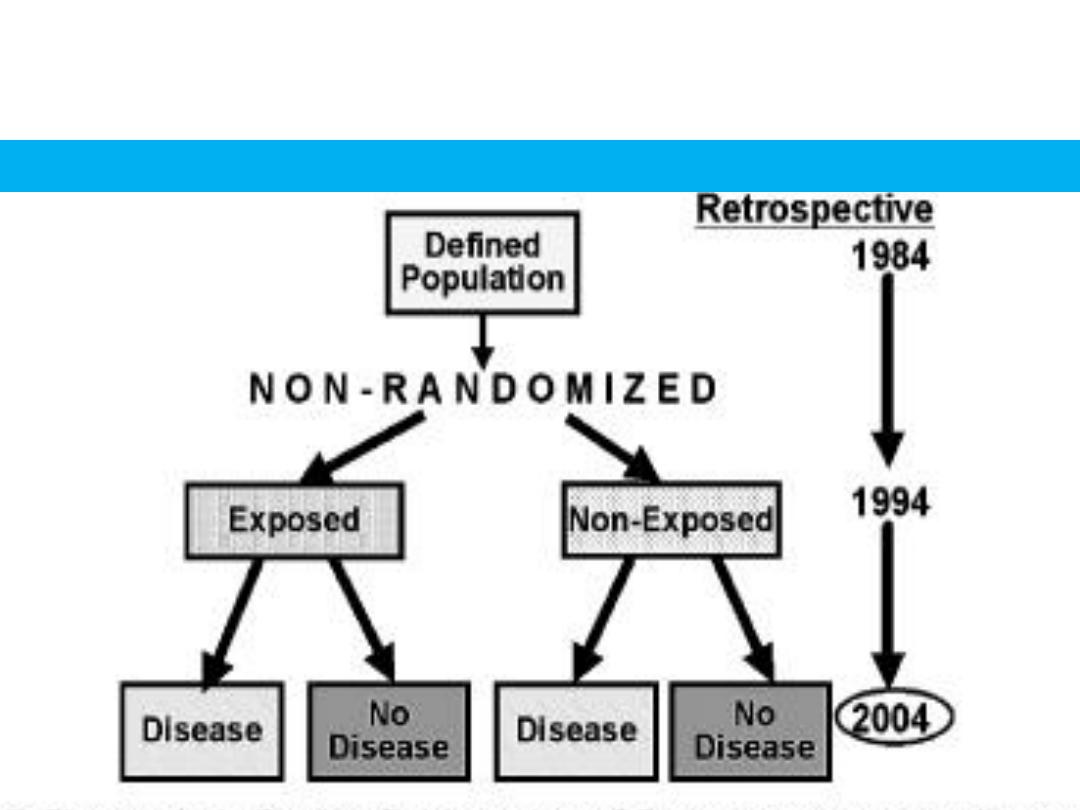
RETROSPECTIVE COHORT STUDY
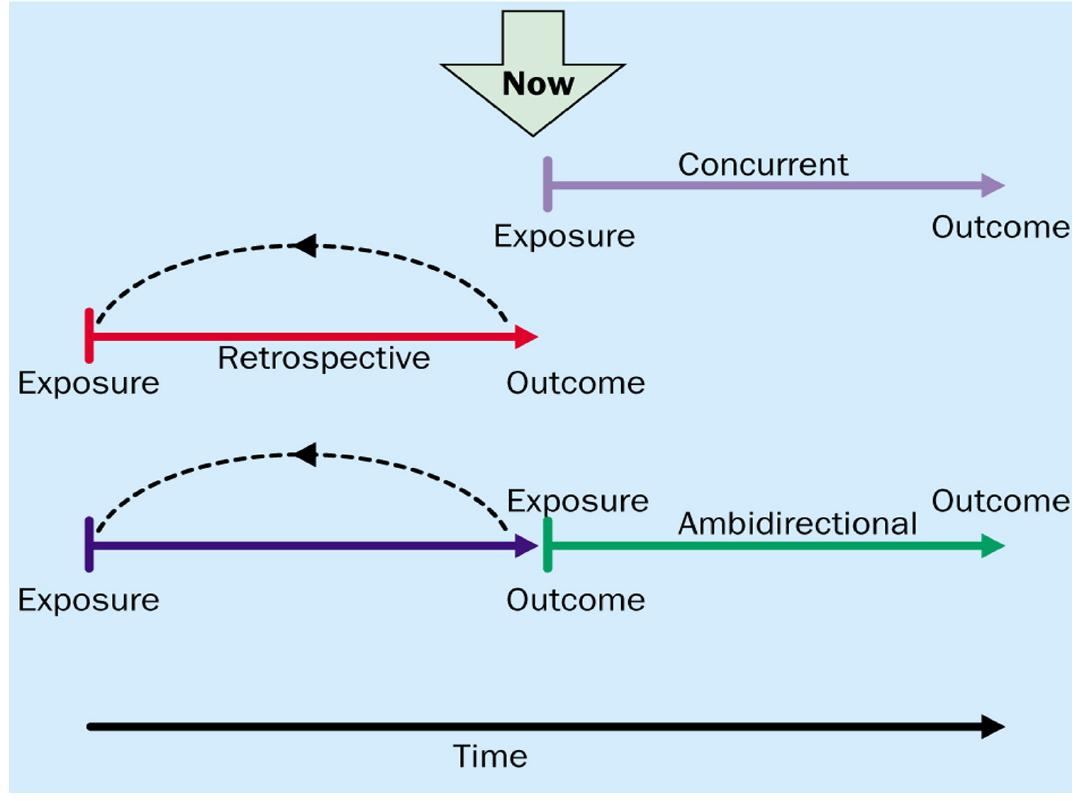

Advantages:
1- incidence can be calculated
2-several possible outcomes related to
exposure can be studied simultaneously
3-direct estimate of relative risk.
4-dose-response ratio can be calculated
5-since the comparison groups are formed
before disease develops, certain forms of bias
can be minimized like miss-classification of
individuals into exposed and unexposed
groups

3-Certain administrative problems such as loss of
experienced staff, loss of funding and extensive
record keeping are inevitable.
Disadvantages:
1-Unsuitable for investigating uncommon
disease or diseases with low incidence in the
population.
2-It takes a long time to complete the study
and obtain results(loss of follow
up)

4-Loss of substantial proportion of the original cohort,
they may migrate lose interest in the study, or simply
refuse to provide any required information
5-There may be changes in the standard methods or
diagnostic criteria of the disease over prolonged follow
up
6-Expensive
7-Ethical problems with varying importance
8-Practical considerations dictate that we must
concentrate on a limited number or factors possibly
related to disease outcome

ELEMENTS OF
COHORT STUDY
1-selection of study subjects.
2-obtaining data on exposure.
3-selection a comparison groups.
4-follow-up.
5-analysis
.

1-SELECTION OF THE EXPOSED
POPULATION
It depends on hypothesis under study,the
exposure frequency and feasibility
considerations such as availability of records
and ease of follow up.
1-Special cohorts:
2-General cohorts:

Special cohorts: are assembled to study
the health effects of rare exposures such
as uncommon occupational chemicals,
unusual diets or life styles, medical
procedures….etc
General cohorts: are typically assembled
for common exposures such as use of oral
contraceptives, dietary factors such as
vitamin use and habits such as cigarette
smoking and alcohol consumption

2-OBTAINING DATA ON EXPOSURE
Information about exposure may be obtained from:
1-cohort members: through personal interviews
and mailed questionnaire
2-review of record: certain kinds of information
(dose of radiation, kinds of surgery, or details
of medical treatments) can be obtained only
from medical records.
3-Medical examination or special
tests:
(eg.blood pressure, serum
cholesterol,ECG)
4-Enviromental surveys:
This
is the
source of obtaining information on the
best
exposure level of suspected factor in the
environment where the cohort lived or
worked


3-SELECTION OF COMPARISONS GROUPS
They should be similar to the study group in all the factors
related to the disease EXCEPT the factor under study.
1-Internal Comparison
:
strength:

2-External comparison :

the comparison with general population in
the same geographic area as the exposed
with people ,it is commonly used in
occupational studies
strength:accessible,stable data.
Limitation:lack of comparability with
exposed group,results may suffer from
healthy worker effect,data on key variable
may be missing.
3-comparison with general population:

4-FOLLOW-UP:BY
e.g. Periodic medical examination, reviewing
hospital and medical record, routine surveillance of
death records.
5-ANALYSIS:
The data are analyzed in terms of:
A-Incidence rates of outcome among exposed
and non exposed.
B-estimation of risk.
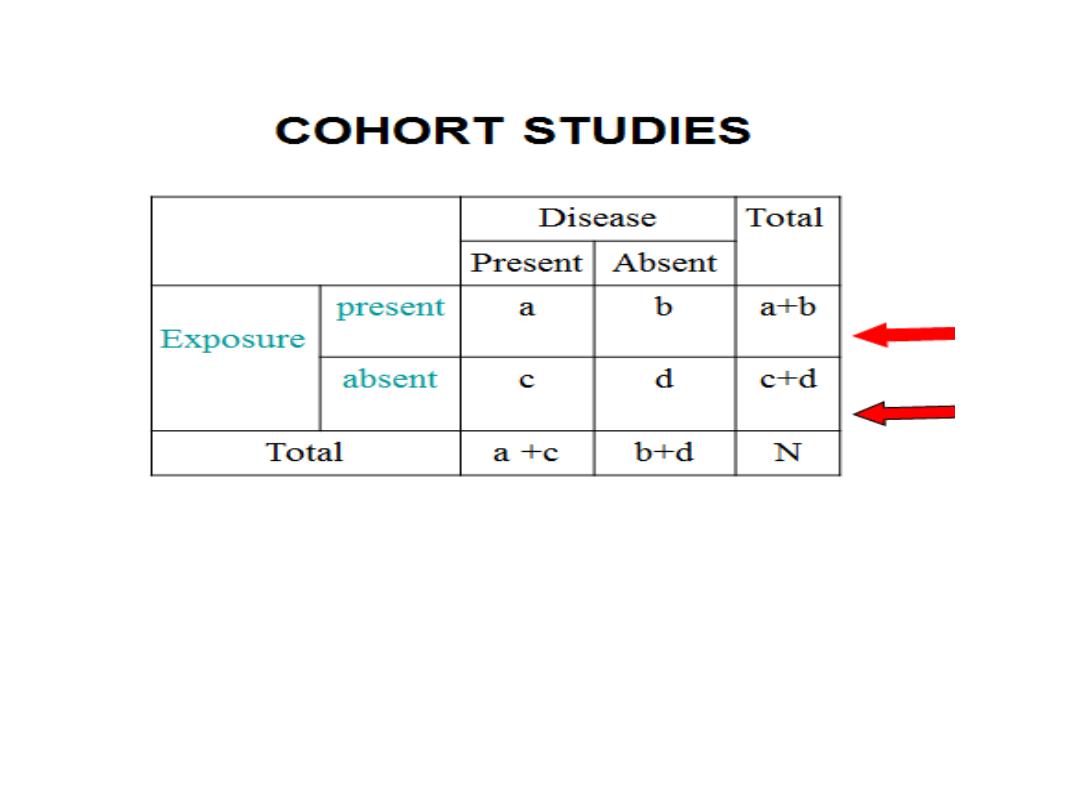
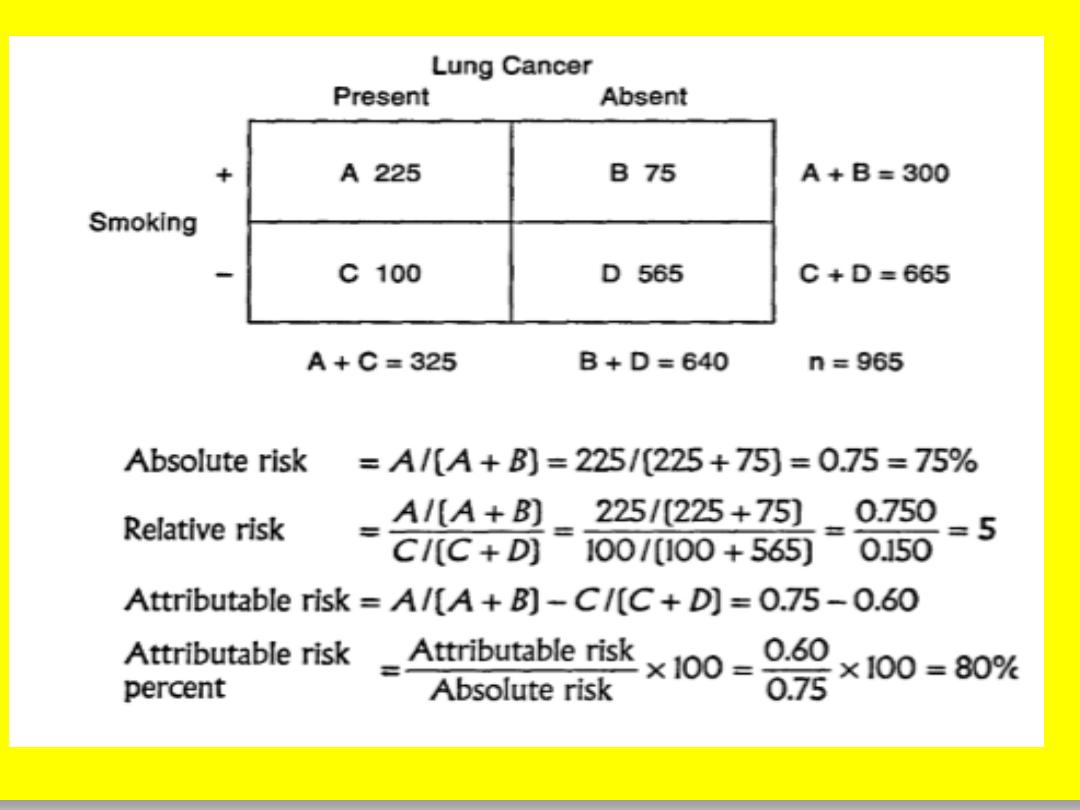
A-INCIDENCE RATES
Relative risk(RR)=incidence among exposed
---------------------------------------------------
incidence among non exposed

a / a+b
RR =-------------
c/ c+d
Where:
incidence in exposed= a/a+b
Incidence in non exposed= c/c+d

INTERPRETATION OF RR
If RR=1
risk in exposed equal to risk in non
exposed(no association).
If RR>1
risk in exposed greater than risk in non
exposed(positive association ,possibly causal).
If RR<1
risk in exposed less than risk in non
exposed(negative association, possibly protective)
2-ATTRIBUTABLE RISK(AR):
Is
the difference in the incidence rates of disease
between an exposed group and non exposed
risk
group ,some authors use the term (
) to attributable risk.
difference
AR=(incidence in exposed group)-(incidence in
non exposed group)

3-ATTRIBUTABLE RISK PERCENT(AR%)
AR%=(Incidence in exposed)-(incidence in non exposed)
----------------------------------------------------------------------------x100%
Incidence in exposed group

EXAMPLE
Bacteruria
Yes
No
total
OC
use
Yes
27
455
482
No
77
1831
1908
Total
104
2286
2390
Cohort study for bacteruria in oral contraceptive pill users:
RR = I
e
/ I
o
= [a / (a + b)]/ [c / (c + d)]
= [27/482] / [77 / 1908]
= 1.4 times bacteruria among OC users compared to non-users
Or to interpret with: those using OC are 1.4 times more to develop bacteruria
than those do not use OC
AR% = [(I
e
- I
o
) / I
e
] * 100 = [{(27/482)
– (77 / 1908)} / (27/482)] * 100
= 28.57% could be prevented if not used OC, or there is excess risk of 28.57%
to develop bacteruria on using OC.