G
LAUCOMA
IS AN OPTIC NEUROPATHY WITH CHARACTERISTIC
OPTIC DISC CHANGES AND
VF
ABNORMALITTIES
.
T
HE MAJOR RISK FACTOR IS AN INCREASE IN
IOP.

pathophysiology of glaucoma
Elevated pressure in the eye is the main factor leading to
glaucomatous damage to the eye (optic) nerve. intraocular pressure
which normally ranges between 8 millimeters (mm) and 22 mm of
mercury. The optic nerve is the most susceptible part of the eye to
high pressure because the delicate fibers in this nerve are easily
damaged either by direct pressure on the nerve or decreased blood
flow to the nerve.
The front of the eye is filled with a clear fluid called the aqueous
humor, which provides nourishment to the structures in the front of
the eye. This fluid is produced constantly by the ciliary body, which
surrounds the lens of the eye. The aqueous humor then flows
through the pupil and leaves the eye through tiny drainage
channels called the trabecular meshwork. These channels are
located at what is called the drainage angle of the eye. This angle is
where the clear cornea , which covers the front of the eye, attaches
to the base (root or periphery) of the iris , which is the colored part
of the eye.

In most people, the drainage angles are wide open, but in some
individuals, they can be narrow. For example, the usual angle is
about 45 degrees, whereas a narrow angle is about 25 degrees or
less. After exiting through the trabecular meshwork in the drainage
angle, the aqueous fluid then drains into tiny blood vessels
(capillaries) into the main bloodstream.
if the eye's trabecular meshwork becomes clogged or blocked, the
intraocular pressure may become elevated. Also, if too much fluid is
being produced within the eye, the intraocular pressure may become
too high. In either event, since the eye is a closed system, if it cannot
adequately remove the increased fluid, the pressure builds up and
optic-nerve damage may result.
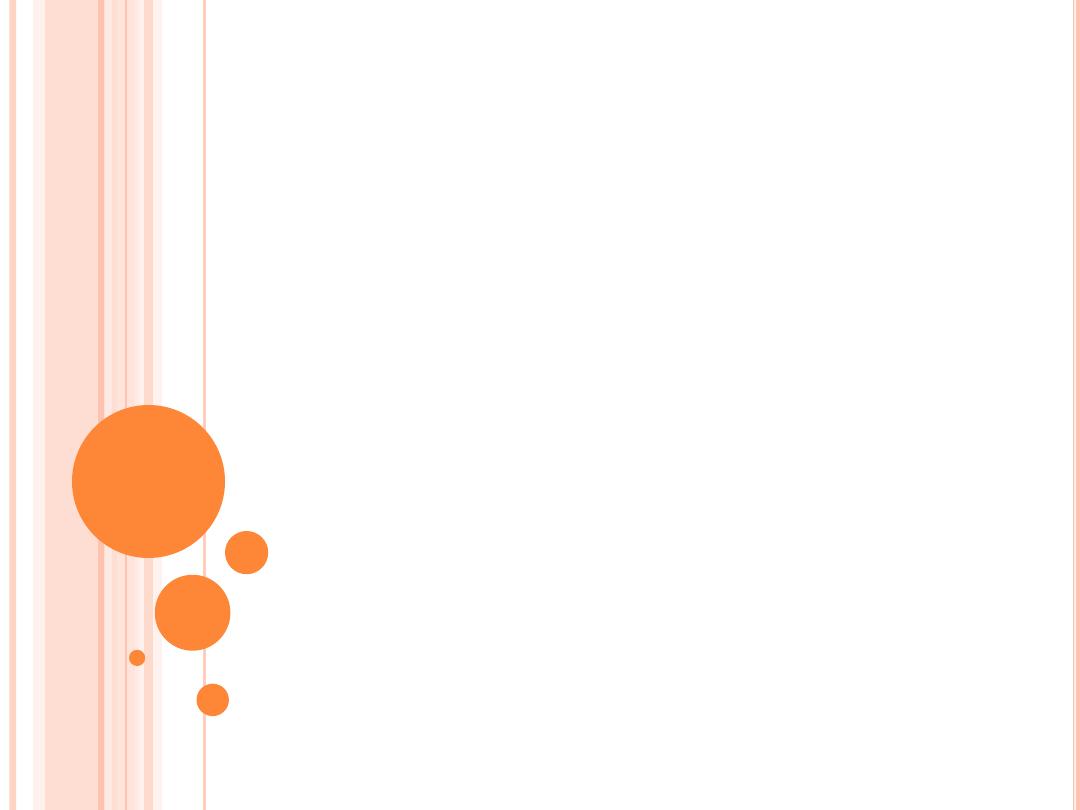
G
LAUCOMA
This diagram of the front part of the eye is in cross section to show the filtering, or
drainage, angle. This angle is between the cornea and the iris, which join each other
right where the drainage channels (trabecular meshwork) are located. The arrow
shows the flow of the aqueous fluid from the ciliary body, through the pupil, and into
the drainage channels.

CLASSIFICATION
Open angle glaucoma
Primary open angle glaucoma (POAG).
Secondary open angle glaucoma
pretrabecular (membrane).
Trabecular ( pigment dispertion, pseudoexfolation
syndrom, neovascular glaucoma).
Post trabecular (raised episcleral venous pressure)
Angle closure glaucoma
Primary angle closure glaucoma.
Secondary angle closure glaucoma.
Posterior pushing force ( posterior synchiae ,
phacomorphic glaucoma ).
anterior pulling force (peripheral anterior synchiae ,
neovascular glaucoma ).

S
TERIOD RESPONSE
Steriod response is change in IOP with steriod adminstration.
1- definition : Based on 6 wks course of topical betamethasone ,
there are 3 groups of persons
high responders ( ˃ 30 mmhg ).
5% of population
90% of POAG
25% of POAG relatives
moderate responder( 22-30 mmhg)
35% of population
Low responder (21 mmhg or less)
60% of population
2- Risk of increase IOP depend on :
Strengh of steriod e.g dexameth.betameth. And predisolone more likely to
produce IOP rise than weak steriods ( fluromethalone)
Route . Systemic steriods less likely to produce IOP rise .
Duration, frquency and dose .
3- Mechanism :
Decrease in phacocytosis, interfer with transport in TM., decrease in
prostoglandine activity

RISK FACTORS
Glaucoma is often called "the sneak thief of sight."
This is because, as already mentioned, in most
cases, the intraocular pressure can build up and
destroy sight without causing obvious symptoms.
Thus, awareness and early detection of glaucoma
are extremely important because this disease can
usually be successfully treated when diagnosed
early. While everyone is at risk for glaucoma,
certain people are at a much higher risk and need
to be checked more frequently by their eye doctor.
The major risk factors for glaucoma include the
following:
Factors that determine the mangement of open angle glaucoma
1-Severity and progression of disease
IOP level ( most important factor )
Optic nerve head changes
Visual field changes
Ocular risk factors ( CRVO,fuchs endothelial dystrophy, retinitis
pigmentosa )
2- patient factors
Age over 45 years
Family history of glaucoma
Black racial had higher rate of progression
concomitant risk factors ( DM,HT, myopia , other vascular
diseases)
History of elevated intraocular pressure
Decrease in corneal thickness and rigidity
History of injury to the eye
Use of cortisone (steroids), either in the eye or systemically (orally
or injected)
), which is seeing distant objects better
than close ones (Farsighted people may have narrow drainage
angles, which predispose them to acute [sudden] attacks of angle-
closure glaucoma.)
Compliance to follow up and medication use .

Primary open-angle glaucoma (POAG) is by far the most
common type of glaucoma. Moreover, its frequency increases
greatly with age and it is a chronic, not acute, disease. This
increase occurs because the drainage mechanism gradually
may become clogged secondary to aging, even though the
drainage angle is open. As a consequence, the aqueous fluid
does not drain from the eye properly. The pressure within the
eye, therefore, builds up painlessly and without symptoms.
since the resulting loss of vision starts on the side
(peripherally), people are usually not aware of the problem
until the loss encroaches near or into their central visual
area. This type of glaucoma is said to be primary because its
cause cannot be attributed to any discernable structural
changes within the eye.
Normal tension (pressure) glaucoma or low tension
glaucoma are variants of primary chronic open-angle
glaucoma that are being recognized more frequently than in
the past. This type of glaucoma is thought to be due to
decreased blood flow to the optic nerve. This condition is
characterized by progressive optic-nerve damage and loss of
peripheral vision (visual field) despite intraocular pressures
in the normal range or even below normal. This type of
glaucoma can be diagnosed by repeated examinations by the
eye doctor to detect the nerve damage or the visual field loss.

Congenital (infantile) glaucoma is a relatively rare, inherited type of
open-angle glaucoma. In this condition, the drainage area is not properly
developed before birth. This results in increased pressure in the eye that can
lead to the loss of vision from optic-nerve damage and also to an enlarged
eye. The eye of a young child enlarges in response to increased intraocular
pressure because it is more pliable than the eye of an adult. Early diagnosis
and treatment with medication and/or surgery are critical in these infants
and children to preserve their sight.
Secondary open-angle glaucoma is another type of open-angle glaucoma.
It can result from an eye (ocular) injury, even one that occurred many years
ago. Other causes of secondary glaucoma are inflammation in the iris of the
eye (iritis), diabetes, cataracts, or in steroid- susceptible individuals, the use
of topical (drops) or systemic (oral or injected) steroids (cortisone). It can also
be associated with a retinal detachment or retinal vein occlusion or blockage.
(The treatments for the secondary open-angle glaucomas vary, depending on
the cause.
Pigmentary glaucoma is a type of secondary glaucoma that is more
common in younger men. In this condition, for reasons not yet understood,
granules of pigment detach from the iris, which is the colored part of the eye.
These granules then may block the TM.

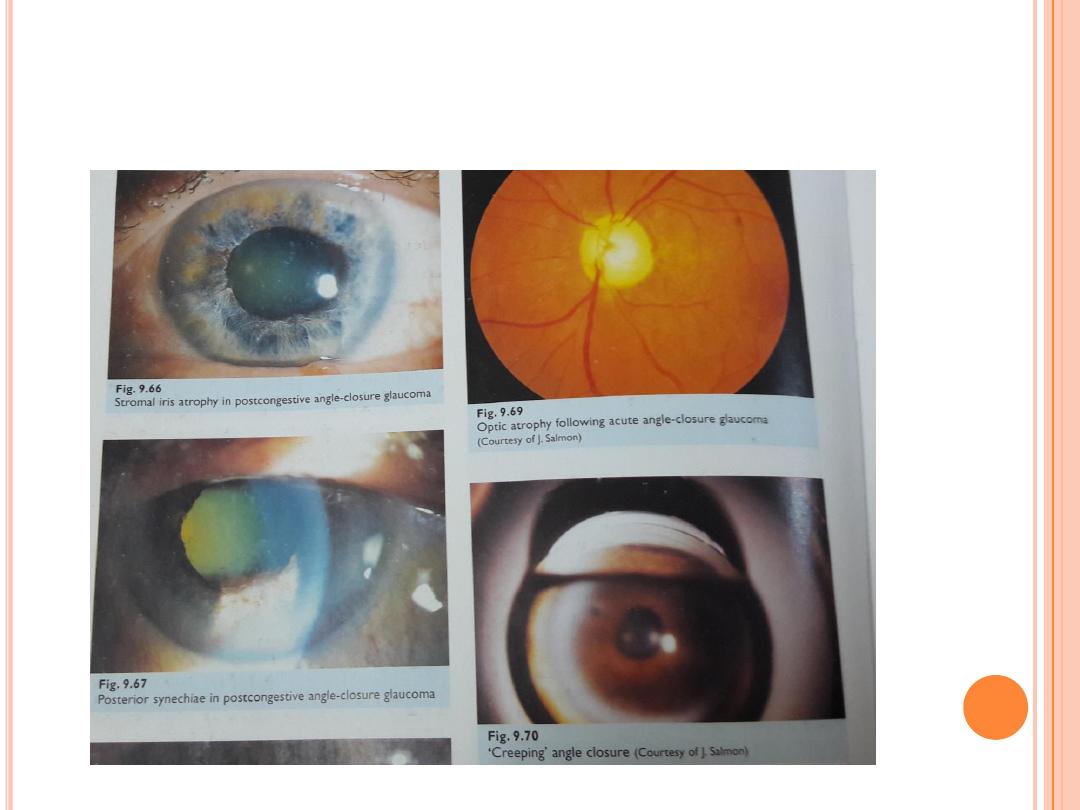
Primay Angle-closure glaucoma
Angle-closure glaucoma is a less common form of glaucoma in the Western world but
is extremely common in Asia.
Angle-closure glaucoma may be acute or chronic. The common element in both is
that a portion of or the entire drainage angle becomes anatomically closed, so that
the aqueous fluid within the eye cannot reach all or part of the trabecular
meshwork.
Acute primary angle closure glaucoma is an ophthalmic emergency
Risk factors
1-Epidemological
age : ˃ 40 years old
female sex
Race : Asians
2-Anatomical
Pupil block mechanism
Narrow angle shallow AC , relatively anterior iris –lens diaphragm, large lens
(older, cataractous) , small corneal diameter ,short axial lengh (usually
hypermetropic); risk increases with increasing lens thickness to axial length ratio.
in pupillary block apposition of the iris to the lens impedes aqous flow from PC to
AC , causing relative build up of pressure behind the iris , anterior bowing of the
peripheral iris , and subsequent angle closure
.

plateau iris mechanism
plateau iris configuration ( relatively anterior ciliary body that apposes the
peripheral iris to the trabeculum ;AC depth normal centerally ,shallow
peripherally with flat iris plane .
mild forms of plateau iris configuration are vulnerable to pupil block , but
higher plateau configuration may result in plateau iris syndrome where the
peripheral iris bunches up and blocks the trabeculum directly. This means
that angle closure can occur despite a patent PI.
3-Medication
troicamide, cyclopentolate, atropin and
citalopram and other
selective serotonin reuptake inhibitors.
Clinical features
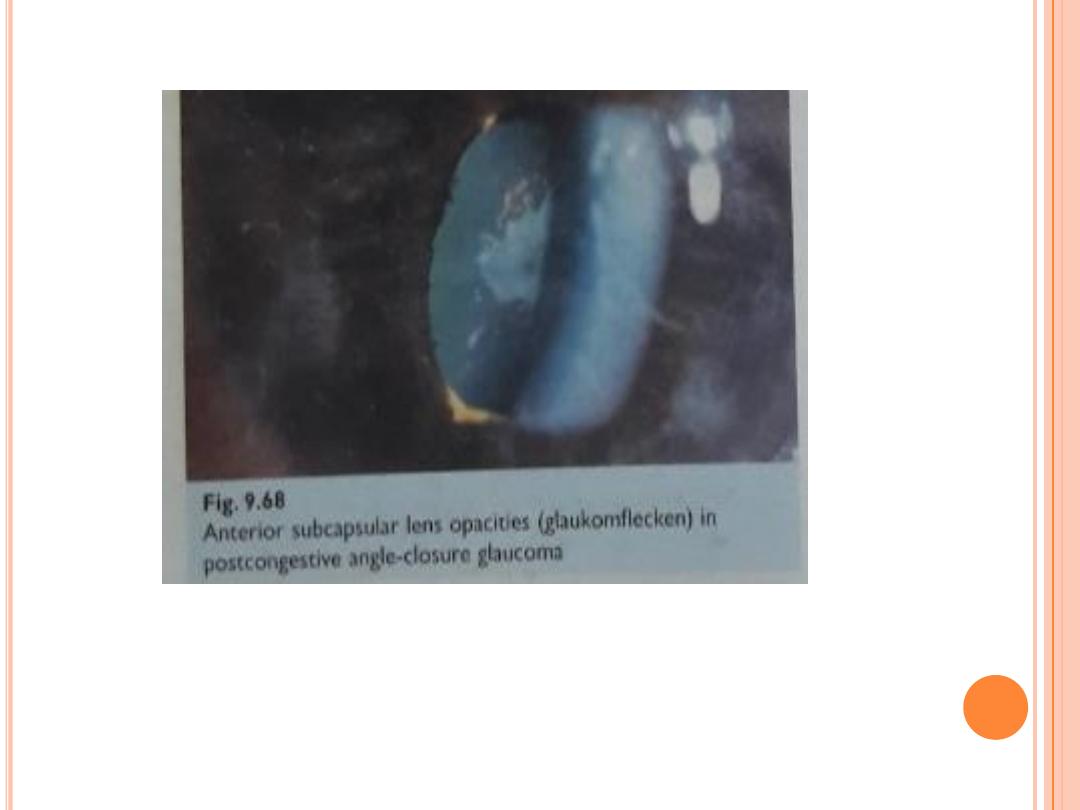
pain ( periocular ,headache, abdominal ) , blurred vision , haloes , nausea,
vomiting .
ipsilateral : red eye , raised IOP , ( usually 50 -80 mmhg) , corneal odema
, angle closed, fixed semi dilated pupil , glaucomflecken , contralateral
angle narrow; bilateral shallow AC .
DDX
consider secondary angle closure ( e.g phacomorphic , infammatory ,
neovascular )

Treatment
Immediate
systemic : acetazolamide 500mg iv stat ( then 250 mg PO 4* /d)
Beta blocker ( e.g timolol 0.5 2*/d)
sympathomimetic (e.g apraclonidine 1%)
Steriod (e.g predinsolone 1% stat then q 30-60 min)
Pilocarbine 2% ( once IOP ˂ 50 mmhg ; e.g twice in first hour then 4*/d
Admit patient and consider corneal indentation with a 4 – mirror goniolens may
help relieve pupil block, lying the patient supine may allow the lens to fall back
away from the iris ; analgesic and anti-emetics may be necessary.
Pilocarbine 1% is often given to the contralateral eye while awaiting Nd –YAG PI (
although some glaucoma specialists advice against this due to the risk of inducing
reverse pupil block ). In either case the priority is for prompt bilateral PIS.
Intermediate
Check IOP hourly until adequate control
If IOP not improving : consider systemic hyperosmotic ( e.g glycerol PO 1g/kg of 50
% solution in lemon juice or mannitol 20% solution iv 1-1.5 g /kg
If IOP still not improving : consider acute ND-YAG PI ( Can use topical glycerine to
temporarily reduce corneal odema) .

If IOP still not improving :
review the diagnosis ( e.g could this aqueous misdirection syndrome with
patent PI
Consider repeating Nd-YAG PI , or proceeding to surgical peripheral
iridectomy , argon laser iridoplasty ,paracentesis , cyclodiode
photocoagulation or emergency cataract extraction / trabeculectomy.
In contrast, remember that the problem in open-angle glaucoma is clogging
within the drainage system itself. In chronic open-angle glaucoma, portions
of the drainage angle remain closed over a long period of time and damage
the drainage system. As more and more areas become closed, the pressure
within the eye rises, often over a period of months or years.
Asian descent may have smaller eyes, narrow drainage angles, and an
increased risk of developing angle-closure glaucoma. Furthermore, this
condition may be acutely triggered by medications that can dilate the pupils.
These agents can be found in certain eyedrops, cold remedies, This condition
can also occur spontaneously in a darkened room or a movie theater, when
the pupil automatically dilates to let in more light. Sometimes, therefore,
people with narrow angles are given eyedrops to keep their pupils small
(pilocarbine).

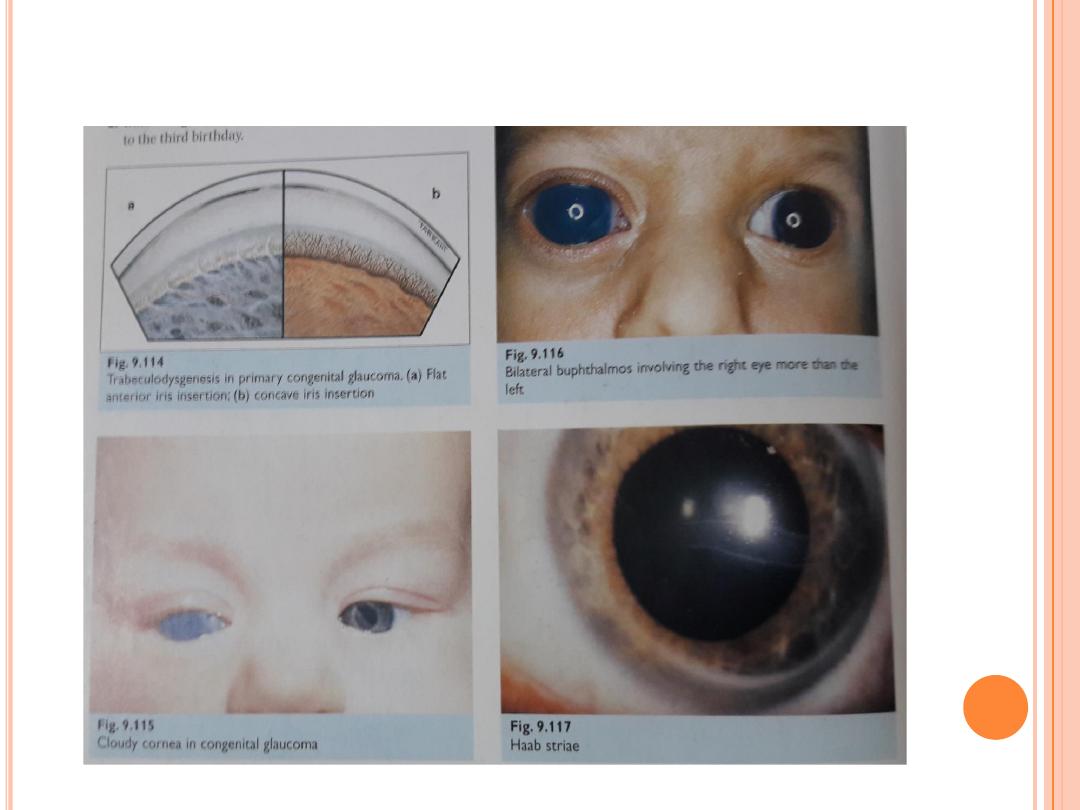
C
ONGENITAL GLAUCOMA
Symptoms of glaucoma present at birth (congenital glaucoma) and
glaucoma that develops in the first few years of life (infantile glaucoma)
may include:
Watery eyes. The baby may also appear to be sensitive to light.
Cloudy cornea . This is a sign that the clear front surface of the eye
(cornea) has been damaged.
Large eye ball. Eyes that look larger than normal because the eyeballs
have become enlarged as a result of high pressure.
Your baby may rub his or her eyes, squint, or keep the eyes closed much
of the time.

How do physicians diagnose glaucoma
An eye doctor (ophthalmologist) can usually detect those individuals
who are at risk for glaucoma (because of, for example, a narrow
drainage angle or increased intraocular pressure) before nerve
damage occurs. The doctor also can diagnose patients who already
have glaucoma by observing their nerve damage or visual field loss.
The following tests, all of which are painless, may be part of this
evaluation.
Tonometry raised IOP more than 21 mmHg
determines the pressure in the eye by measuring the tone or
firmness of its surface. Several types of tonometers are available for
this test, the most common being the applanation tonometer. After
the eye has been numbed with anesthetic eyedrops, the tonometer's
sensor is placed against the front surface of the eye. The firmer the
tone of the surface of the eye, the higher the pressure reading.
Pachymetry measures the thickness of the cornea. After the eye
has been numbed with anesthetic eyedrops, the pachymeter tip is
touched lightly to the front surface of the eye (cornea). Studies have
shown that corneal thickness can affect the measurement of
intraocular pressure. Thicker corneas may give falsely high eye
pressure readings and thinner corneas may give falsely low pressure
readings. Furthermore, thin corneas may be an additional risk
factor for glaucoma. Once a doctor knows the thickness of a patient's
cornea, he or she can more accurately interpret the patient's
tonometry.


•
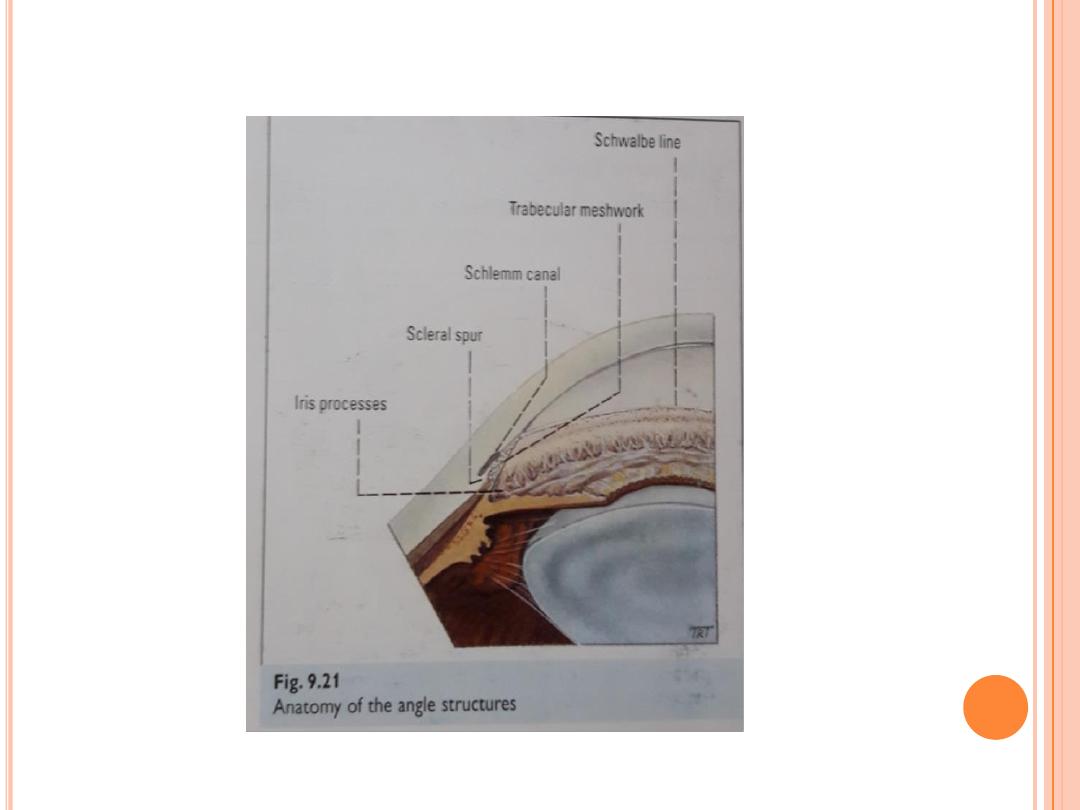
Gonioscopy is done by numbing the eye with anesthetic drops
and placing a special type of contact lens with mirrors onto the
surface of the eye. The mirrors enable the doctor to view the
interior of the eye from different directions. The purpose of
this test is to examine the drainage angle and drainage area of
the eye. In this procedure, the doctor can determine whether
the angle is open or narrow and find any other abnormalities,
such as increased pigment in the angle or long-standing
damage to the angle from prior inflammation or injury. As
indicated earlier, individuals with narrow angles have an
increased risk for a sudden closure of the angle, which can
cause an acute angle-closure glaucomatous attack. Gonioscopy
can also determine whether the eye is subject to chronic angle
closure, whether blood vessels are abnormal, or whether
hidden tumors might be blocking the drainage of the aqueous
fluid out of the eye.
•


•
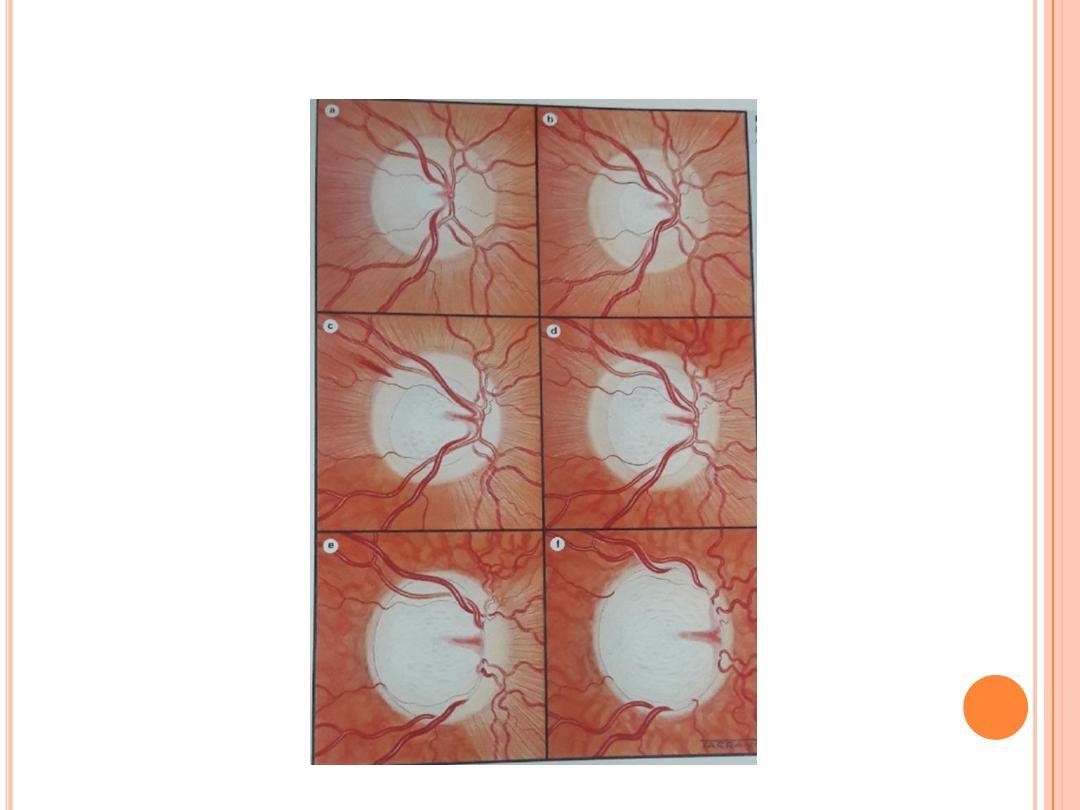
Ophthalmoscopy is an examination in which the doctor uses
a handheld device, a head-mounted device or a special lens and
the slit lamp to look directly through the pupil (the opening in
the colored iris) into the eye. This procedure is done to examine
the optic nerve (seen as the optic disc) at the back of the eye.
Damage to the optic nerve, called cupping of the disc, can be
detected in this way.
Cupping
, which is an indentation of the
optic disc, can be caused by increased intraocular pressure.
Asymmetry in the degree of optic nerve cupping between the
two eyes can be a sign of glaucoma, as can increase in optic
nerve cupping over a period of time. Additionally, a
pale color
of the nerve can suggest damage to the nerve from poor blood
flow or increased intraocular pressure. Special cameras can be
used to take photographs of the optic nerve to compare changes
over time.
Abnormal disc
•
Cup / disc ratio asymmetry
•
Large cup /disc ratio for disc size
•
Neuroretinal rim notch /thinning (ISNT rule )
•
Disc haemorrhage
•
Peripapillary atrophy

•
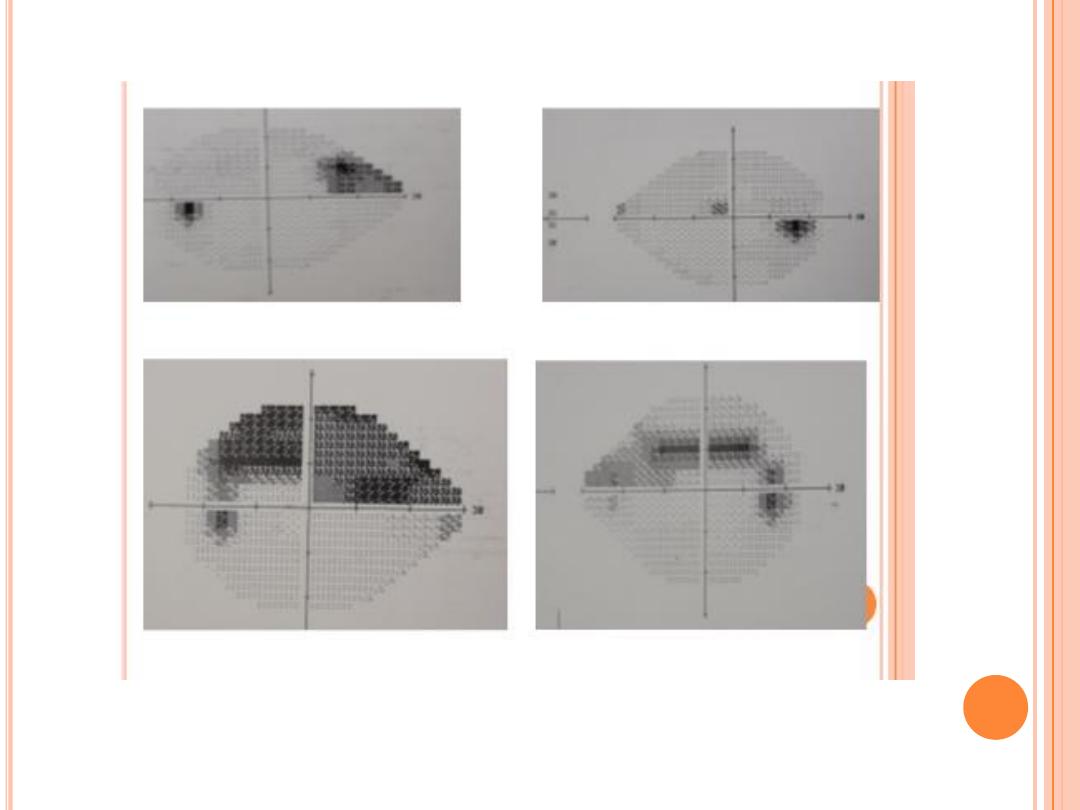
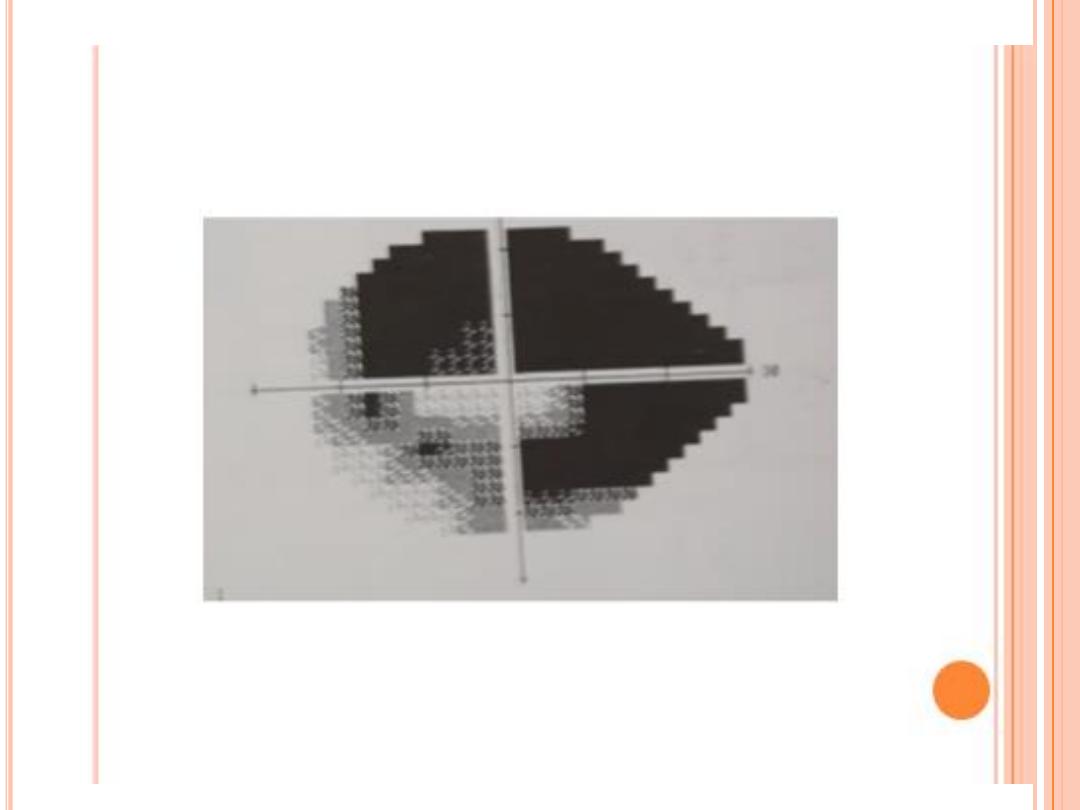
Visual field testing actually maps the visual fields to detect any early
(or late) signs of glaucomatous damage to the optic nerve. In order to
find and follow glaucoma, visual fields are measured by a computer one
eye at a time. One eye is covered and the patient places his or her chin
in a type of bowl. Lights of various intensity and size are randomly
projected around inside of the bowl. When the patient sees a light, he or
she pushes a button. This process produces a computerized map of the
visual field, outlining the areas where each eye can or cannot see. In
glaucoma, there are characteristic changes in the visual field
examination.
•
Typical localized glaucomatous scotomas
Nasal step
Paracentral scotoma within central 10˚
Arcuate scotoma and seidel ( connect to blind spot )
Altitudinal scotoma
Residual temporal or central island of vision .
VF should correlate with optic nerve appearance ; otherwise consider refractive eeror
,level of vision , media opacities, pupil size and other causes of visual field defect (
tilted ON head , ON head drusen , retinal lesions ) .

•
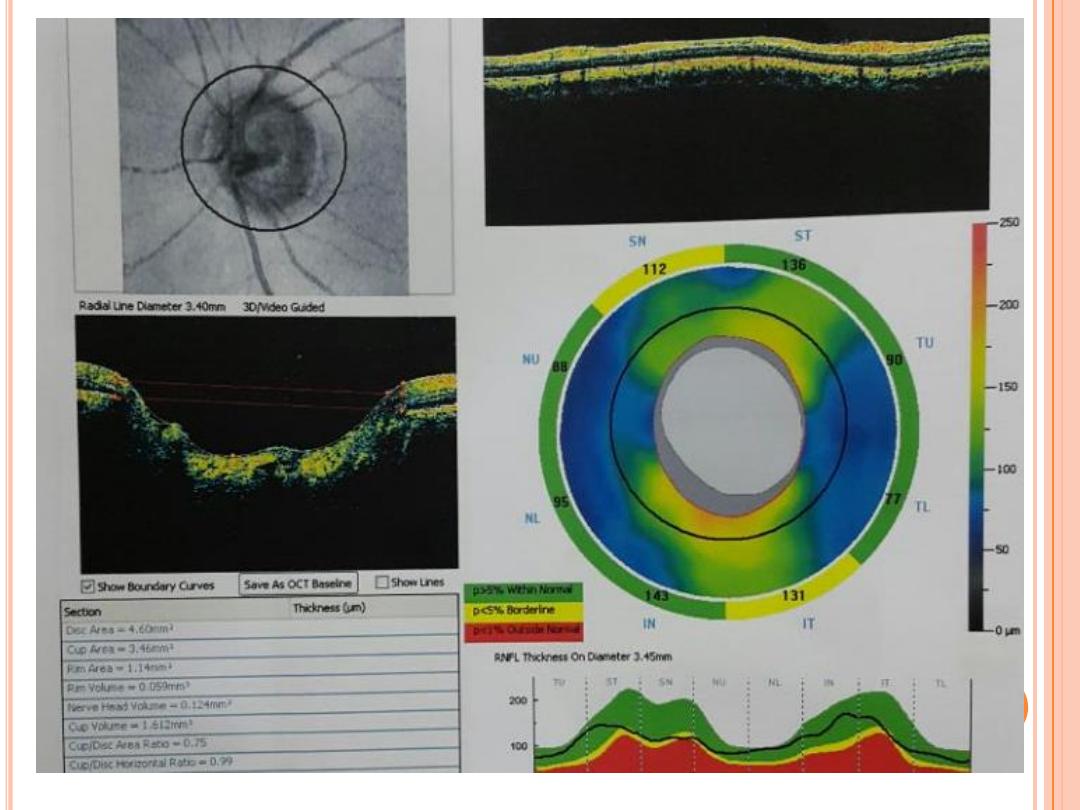
Confocal laser scanning systems and optical coherence tomography
are noninvasive imaging systems that create a three-dimensional image
of the optic nerve and retina to evaluate the degree of cupping and the
thicknesses of the retinal nerve fiber layer and ganglion cell layers to
better evaluate and quantify the presence of ocular damage from all
types of glaucoma.

W
HAT IS THE TREATMENT FOR GLAUCOMA
?
General approach
Although nerve damage and visual loss from glaucoma cannot
usually be reversed, glaucoma is a disease that can generally be
controlled. That is, treatment can make the intraocular pressure
normal and, therefore, prevent or retard further nerve damage and
visual loss. Treatment may involve the use of eyedrop medications,
pills (rarely), laser, or incisional surgery.
One or more types of eyedrops may have to be taken up to several
times a day to lower intraocular pressure. These drops work either by
reducing the production of the aqueous fluid (shutting the faucet) or
by increasing the drainage of the fluid out of the eye. Each type of
therapy has its benefits and potential complications.
It is important to remember that many patients at risk for glaucoma
or who have glaucoma also may have other eye diseases such as
cataract or macular degeneration.
An ophthalmologist can determine
whether any visual loss that one is experiencing is being caused by
glaucoma or by other eye abnormalities
.

What medications (eyedrops) treat glaucoma?
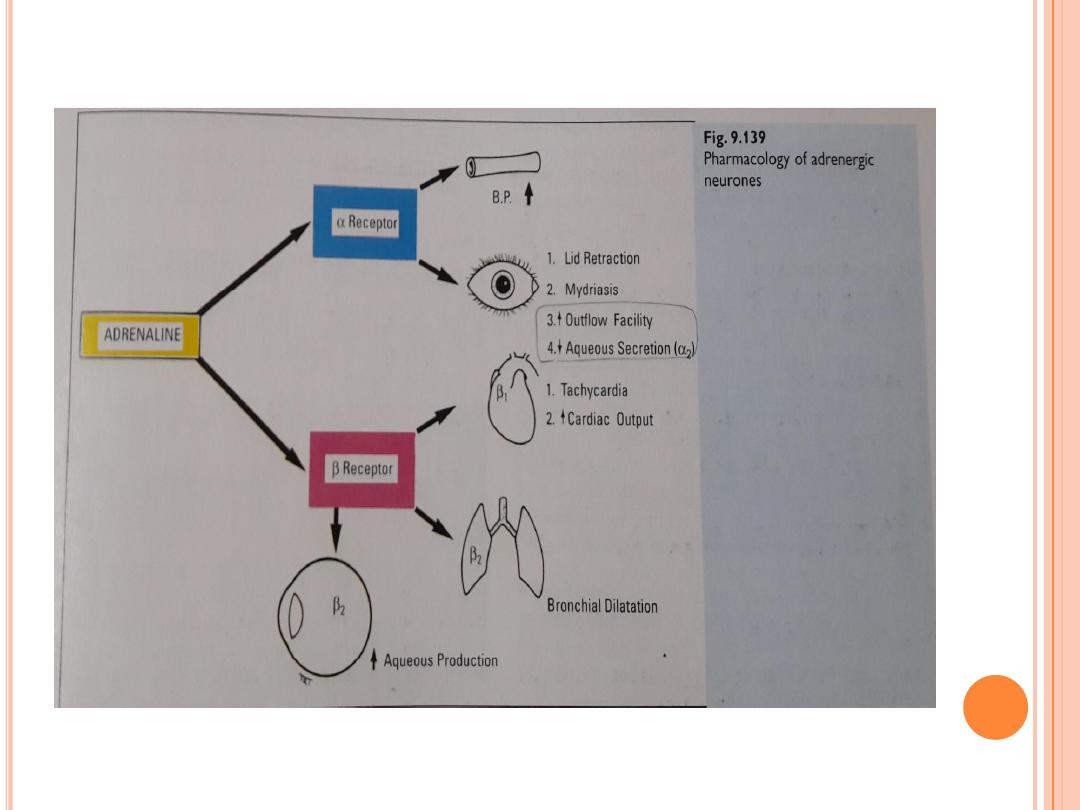
Beta-adrenergic antagonists act against, or block, adrenaline-like
substances. These drops work in the treatment of glaucoma by reducing the
production of the aqueous humor. For years, they were the gold standard (to
which other agents are compared) for treating glaucoma. A few of these
medications are timolol, levobunolol , carteolol, and metipranolol .
Used once or twice daily, these drops are very effective. However, side
effects, such as the worsening of asthma or emphysema, bradycardia (slow
heart rate), low blood pressure, fatigue, and impotence prohibit their use in
some patients. Betaxolol is a beta-adrenergic antagonist that is more
selective in working just on the eye and, therefore, carries less risk of heart
(cardiac) or lung (pulmonary) side effects than other drops of this type.
Prostaglandin analogs are similar in chemical structure to the body's
prostaglandins. Prostaglandins are hormone-like substances that are
involved in a wide range of functions throughout the body. These drops work
in glaucoma by increasing the outflow (drainage) of fluid from the eye.
The prostaglandin analogs have replaced beta-blockers as the most
commonly prescribed drops for glaucoma. They can be used just once a day.
This class of medications has fewer systemic (involving the rest of the body)
side effects than beta blockers, but can change the color of the iris as well as
thicken and darken the eyelashes. In addition, some atrophy of the fat
around the eye may occur. These drops are also more likely to cause redness
of the eyes than some other classes of eye drops. In some patients, they may
also cause inflammation inside the eye. Examples of these medications
include latanoprost (Xalatan), travoprost (Travatan), bimatoprost (Lumigan),
and tafluprost (Zioptan).

Adrenergic agonists are a type of drops that act like adrenaline. They
work in glaucoma by both reducing the production of fluid by the eye and
increasing its outflow (drainage). The most popular adrenergic agonist is
brimonidine (Alphagan). However, there is at least a 12% risk of significant
local (eye) allergic reactions. Other members of this class of drops include
epinephrine, dipivefrin (Propine), and apraclonidine (Iopidine).
Carbonic anhydrase inhibitors work in glaucoma by reducing the
production of fluid in the eye. Eyedrop forms of this type of medication
include dorzolamide (Trusopt) and brinzolamide (Azopt). They are used two
or three times daily. Carbonic anhydrase inhibitors may also be rarely taken
as pills (systemically) to remove fluid from the body, including the eye. Oral
forms of these medications used for glaucoma include acetazolamide
(Diamox) and methazolamide (Neptazane). Their use in this condition,
however, is limited due to their systemic (throughout the body) side effects,
including reduction of body potassium, kidney stones, numbness or tingling
sensations in the lips, arms, and legs, fatigue, and nausea.
.

Osmotic agents are an additional class of medications used to treat sudden
(acute) forms of glaucoma where the eye pressure remains extremely high
despite other treatments. These medications include isosorbide (Ismotic,
given by mouth) and mannitol (Osmitrol), given through the veins. These
medications must be used cautiously as they have significant side effects,
including nausea, fluid accumulation in the heart and/or lungs (congestive
heart failure and/or pulmonary edema), bleeding in the brain, and kidney
problems. Their use is prohibited in patients with uncontrolled diabetes,
heart, kidney, or liver problems.
Ophthalmologists often prescribe an eyedrop containing more than one class
of drug to patients who require more than one type of drug for control of
their glaucoma. This simplifies the taking of drops by the patient. Examples
of these include the combination of timolol and dorzolamide in the same drop
(Cosopt), the combination of timolol and brimonidine in the same drop
(Combigan), and the combination of brinzolamide and brimonidine in the
same drop (Simbrinza). Combination drops that include both beta-adrenergic
antagonists and prostaglandin analogs are available in Europe and Japan
but have not been approved by the United States Food and Drug
Administration (FDA) for use in the US.

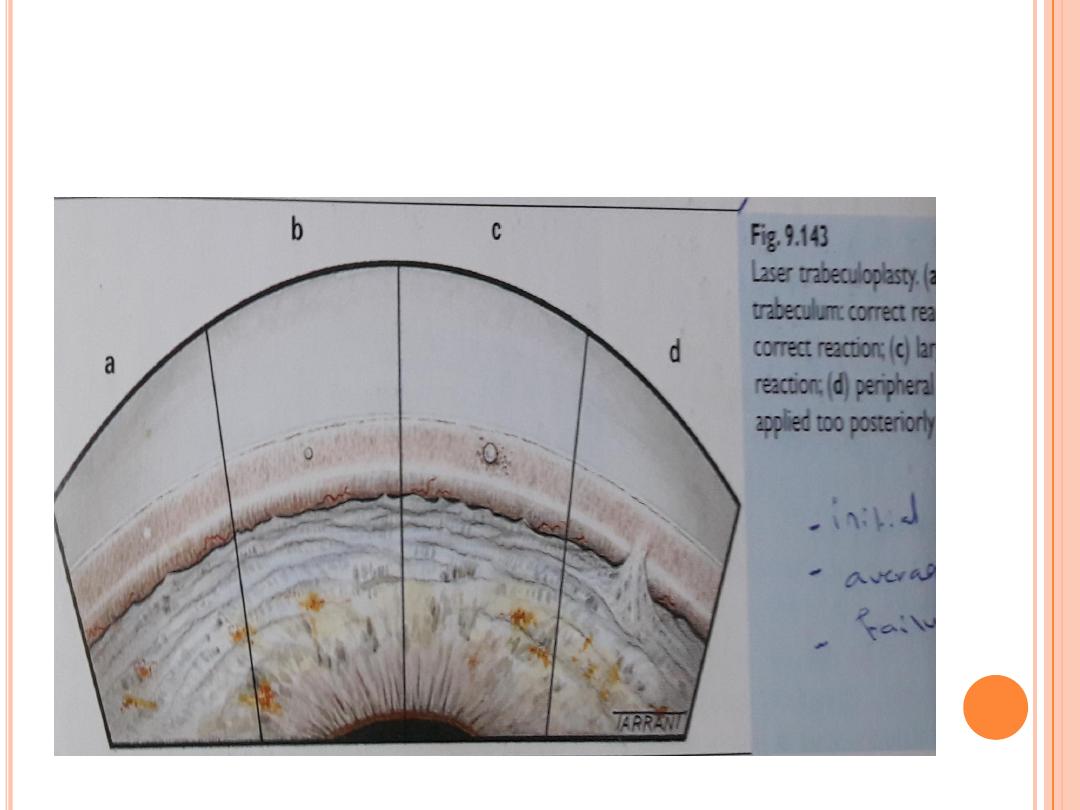
Laser therapy
There are several forms of laser therapy for glaucoma.
Laser iridotomy
involves making a hole in the colored part of the eye (iris) to allow fluid to
drain normally in eyes with narrow or closed angles.
Ar
gon laser trabeculoplasty and selective laser trabeculoplasty (SLT)
Laser trabeculoplasty does not cure glaucoma, but may be done in eyes with
open angles of increasing the number of different eyedrops, or may be
recommended when a patient is already using multiple eyedrops (maximal
medical therapy). In some cases, it is used as the initial or primary therapy for
open-angle glaucoma. This procedure is a quick, relatively painless, and safe
method of lowering the intraocular pressure. With the eye numbed by
anesthetic drops, the laser treatment is applied through a mirrored contact
lens to the angle of the eye. Microscopic laser burns to the angle allow fluid to
better exit the drainage channels.

Laser trabeculoplasty is often done in two sessions, weeks or months
apart. Unfortunately, the improved drainage as a result of the
treatment may last only about two years, by which time the drainage
channels tend to clog again. There are different types of laser
trabeculoplasty including argon laser trabeculoplasty (ALT) and
selective laser trabeculoplasty (SLT). ALT is generally not repeated
after the second session due to the formation of scar tissue in the angle.
SLT is less likely to produce scarring in the angle, so, theoretically, it
can be repeated multiple times. However, the likelihood of success with
additional treatments when prior attempts have failed is low. Thus, the
options for the patient at that time are to increase the use of eyedrops
or proceed to surgery.

Laser cyclo-ablation (also known as ciliary body destruction or
cyclophotocoagulation) is another form of laser treatment generally
reserved for patients with severe forms of glaucoma with poor visual
potential. This procedure involves applying laser burns to the part of
the eye that makes the aqueous fluid (ciliary body). This therapy
destroys the cells that make the fluid, thereby reducing the eye
pressure. This type of laser is typically performed after other more
traditional therapies have failed. Cyclocryopexy is the use of
freezing, rather than laser, to achieve a similar purpose of reducing
aqueous production.


Glaucoma surgery
Trabeculectomy is a delicate microsurgical procedure used to treat
glaucoma. In this operation, a small piece of the clogged trabecular
meshwork is removed to create an opening and a new drainage
pathway is made for the fluid to exit the eye. As part of this new
drainage system, a tiny collecting bag is created from conjunctival
tissue. (The conjunctiva is the clear covering over the white of the
eye.) This bag is called a "filtering bleb" and looks like a cystic raised
area that is at the top part of the eye under the upper lid. The new
drainage system allows fluid to leave the eye, enter the bag/bleb,
and then pass into the capillary blood circulation (thereby lowering
the eye pressure). Trabeculectomy is the most commonly performed
glaucoma surgery. If successful, it is the most effective means of
lowering the eye pressure.

Aqueous shunt devices (glaucoma implants or tubes) are
artificial drainage devices used to lower the eye pressure. They are
essentially plastic microscopic tubes attached to a plastic reservoir.
The reservoir (or plate) is placed beneath the conjunctival tissue.
The actual tube (which extends from the reservoir) is placed inside
the eye to create a new pathway for fluid to exit the eye. This fluid
collects within the reservoir beneath the conjunctiva creating a
filtering bleb. This procedure may be performed as an alternative to
trabeculectomy in patients with certain types of glaucoma. Mini
shunts without a reservoir are also used to improve safety and
reduce the post-surgery chance of pressures that are too low.


What is the prognosis of glaucoma?
The prognosis for glaucoma depends on when the
disease is detected. If the diagnosis is made before
significant optic nerve damage occurs, the
prognosis is generally good if the patient is
compliant with the treatment suggested by the
ophthalmologist. Since optic nerve damage is
permanent and previously damaged optic nerves
are more prone to additional damage, a delayed
diagnosis (one made after significant optic nerve
damage and field loss has already occurred)
requires more aggressive therapy and carries a
prognosis for future visual loss, which is guarded
over the long term
