

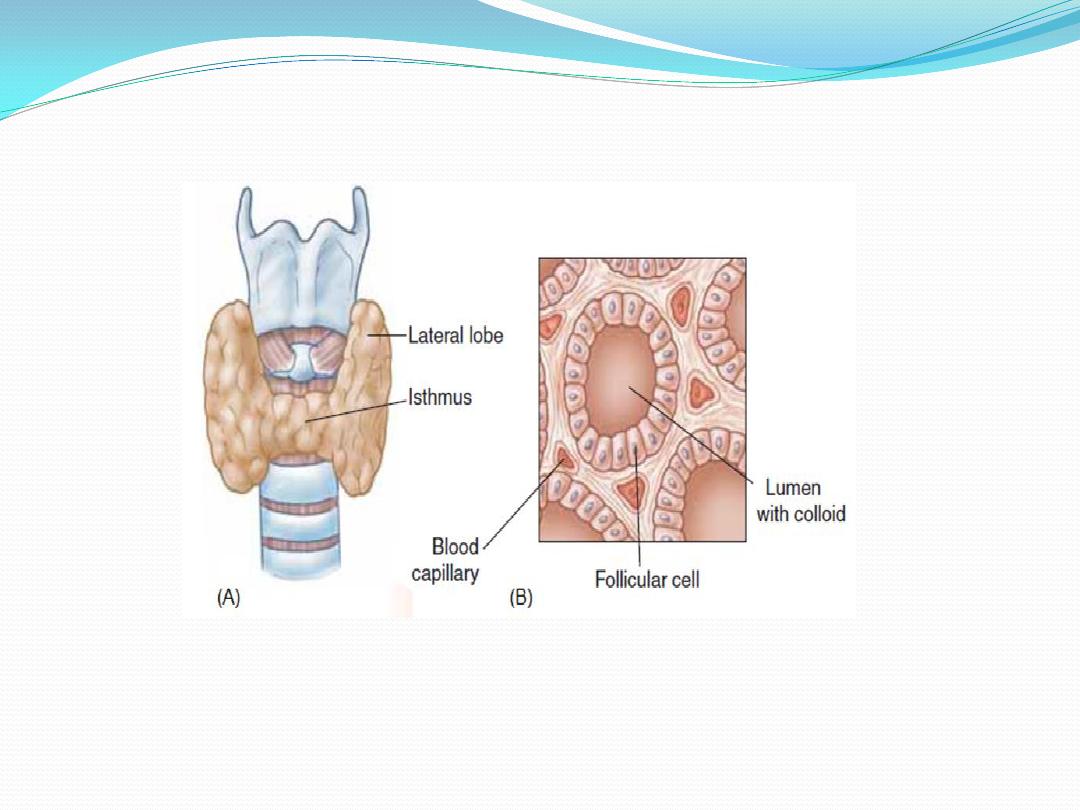
The thyroid gland
is a butterfly-shaped structure lying
over the ventral surface of the trachea
just below the larynx. This gland
produces two classes of hormones
synthesized by two distinct cell types:
• Thyroid hormones (T3 and T4)
synthesized by follicular cells
• Calcitonin synthesized by
parafollicular cells

Thyroid Follicle
The thyroid gland is composed of a large number
(3million) of tiny, saclike structures called follicles. These
are the functional units of the thyroid. Each follicle is
formed by a single layer of epithelial (follicular) cells and
is filled with a secretory substance called colloid, which
consists largely of a glycoprotein-tyrosine complex called
. Each follicle is surrounded by a dense
thyroglobulin
capillary network separated from epithelial cells by a well
. The apical membranes of
basement membrane
defined
the follicular cells, which face the lumen, are covered with
apical
microvilli and pseudopods formed from the
extend into the lumen. The amount of thyroid
membrane
hormones stored within the colloid is enough to supply
the body for 2 to 3 months
.

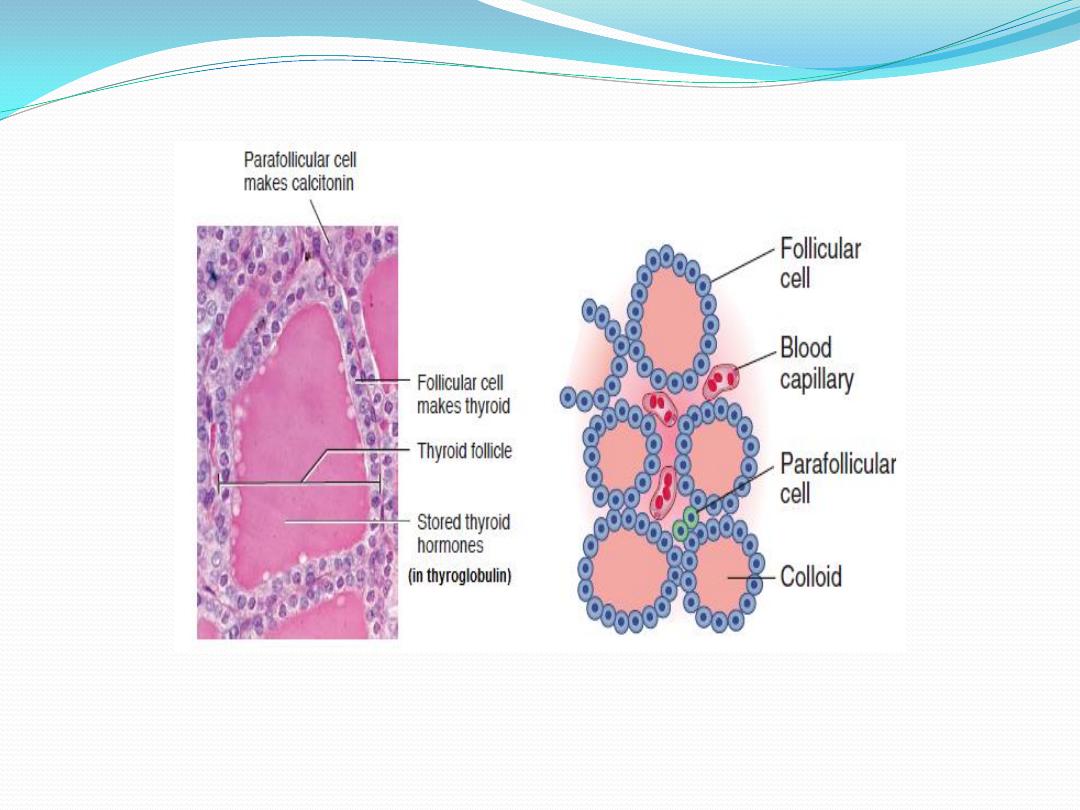
Parafollicular Cells
In addition to the epithelial cells that
secrete T4 and T3, the wall of the thyroid
follicle contains small numbers of
parafollicular cells. The parafollicular cell is
usually embedded in the wall of the follicle,
inside the basal lamina surrounding the
follicle. Parafollicular cells produce and
.
calcitonin
secrete the hormone

Synthesis of thyroid hormone
The steps involved in the synthesis of thyroid hormone are:
1. Synthesis and Secretion of the Thyroglobulin.
Thyroglobulin is synthesized in the follicular cells, then it enters
the lumen via exocytosis. This large protein contains tyrosyl
groups.
2. Iodide Uptake
Iodide uptake is via a sodium/iodide symporter on the basal
membrane (NIS). This pump can raise the concentration of I
within the cell to as much as 250 times that of plasma. The pump
, which
thiocyanate
perchlorate and
can be blocked by anions like
compete with Iodine. The NIS derives its energy from Na
+
/ K
+
-
ATPase, which drives the process.
3. Oxidation of Iodide
Once Iodide is pumped into the cell, it traverses the cell to the
apical membrane, where it is oxidized into iodine
atoms, by
. Thyroid peroxidase is inhibited by
Thyroid peroxidase
(PTU), which blocks the synthesis of thyroid
polythiouracil
hormones by blocking all of the steps catalyzed by thyroid
peroxidase
.
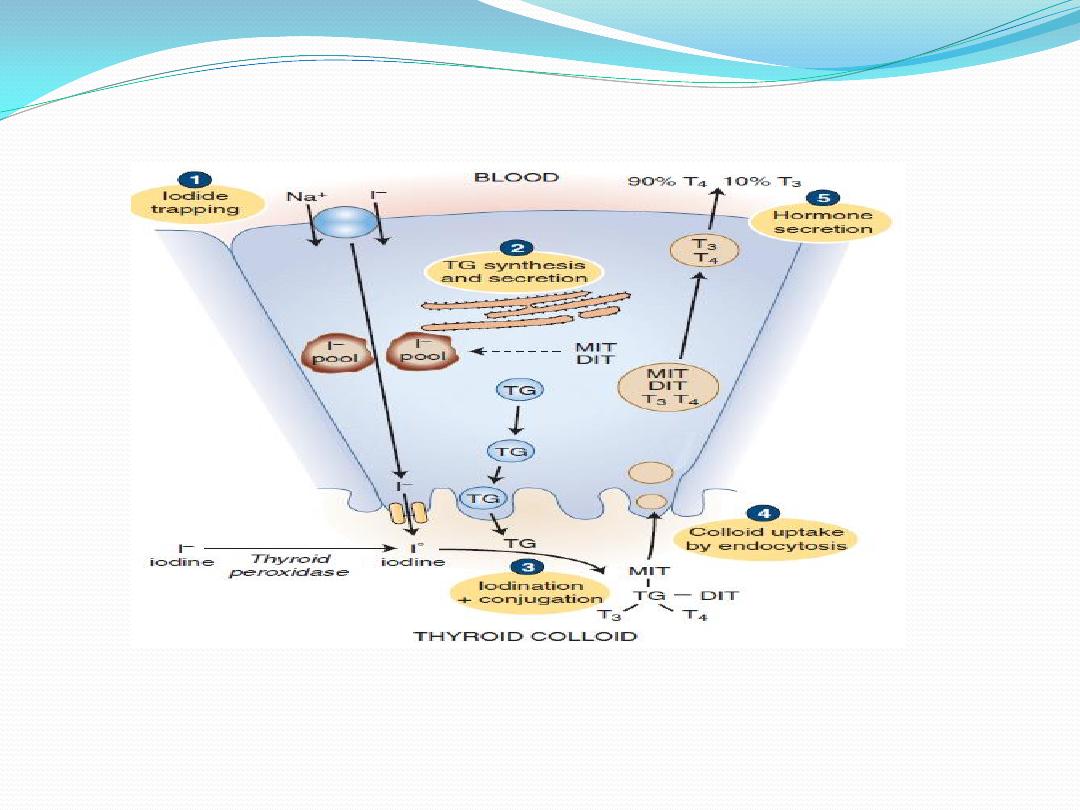

4. Iodination
At the apical membrane, just inside the lumen of the follicle, iodine
atoms combines with the tyrosine moieties of thyroglobulin, to form
monoiodotyrosine (MIT). The iodination of thyroglobulin is
. A second iodine atom
thyroid peroxidase
catalyzed by the enzyme
may be added to a MIT residue by this same enzymatic process,
forming a diiodotyrosine (DIT).
5. Coupling
Peroxidase enzyme promotes the coupling of iodinated tyrosine in
the thyroglobulin molecule. When two DITs couple,
tetraiodothyronine (T4) is formed. When one DIT and one MIT
combine, triiodothyronine (T3) is formed. The hormones stored in
the follicular lumen as colloid until the thyroid gland is stimulated to
secrete its hormones. The thyroid is unique among endocrine glands
in that it stores its product extracellularly in follicular lumens as large
precursor molecules.

Secretion of T3 and T4 from the Thyroid Gland
1.Pinocytosis: Pieces of the follicular colloid are taken
back into the follicle by endocytosis.
2.Fusion: The endocytosed material fuses with
lysosomes, which transport it toward the basal surface of
the cell.
3.Proteolysis of thyroglobulin: Within the lysosomes, the
thyroglobulin is broken into T4, T3, DIT, and MIT.
4.Secretion: T4 and T3 are secreted into the blood, with
the T4:T3 ratio being as high as 20:1 .
removes the
deiodinase
.Deiodination: A microsomal
5
iodine from iodinated tyrosines (DIT and MIT) but not
from the iodinated thyronines (T3 and T4). The iodine is
then available for resynthesis of hormone. (Individuals
with a deficiency of this enzyme are more likely to
develop symptoms of iodine deficiency
).

Conversion of T4 to T3
T4 is the major secretory product of the thyroid
gland and is the predominant thyroid hormone in
the blood. However, about 40% of the T4 secreted
by the thyroid gland is converted to T3 by
enzymatic removal of the iodine atom at position
5
-
of the thyronine ring structure. This reaction is
catalyzed by a 5
-
-deiodinase located in the liver,
kidneys, and thyroid gland. The T3 formed by this
deiodination and that secreted by the thyroid react
with thyroid hormone receptors in target cells;
of
is the physiologically active form
3
T
therefore,
the thyroid hormones
.

Transport of Thyroxine and Triiodothyronine to Tissues
About 70% of the circulating thyroid is bound to thyroid-binding
binding
-
thyroxine
). The remainder is attached to
TBG
globulin (
has the higher
4
. T
albumin
) and
transthyretin
pre albumin (
affinity for binding proteins; therefore, it binds more tightly to
protein than does T3, and consequently has a greater half-life
thanT3.
• T4 half-life = 7 days • T3 half-life = 1 day
Metabolism of thyroid hormones
, particularly in the
deiodinations
undergo enzymatic
3
, T
4
Both T
liver and kidneys, which inactivate them. T4 and, to a lesser
glucuronic
with
conjugation
are also metabolized by
3
extent, T
acid in the liver. The conjugated hormones are secreted into the
bile and eliminated in the feces.

Physiologic actions of thyroid hormones
1. Metabolic Rate, thyroid hormones increase metabolic rate, as evidenced
by increased O
2
consumption and heat production.it also increase the
activity of the membrane-bound Na/K- ATPase in many tissues.
2. Thyroid hormones are essential for normal menstrual cycles.
3. Growth and Maturation (T 4 and T 3 are anabolic hormones), thyroid
hormones are absolutely necessary for normal brain maturation, without
adequate thyroid hormones during the prenatal period, abnormalities
rapidly develop in nervous system maturation. These abnormalities lead to
unless replacement therapy is
cretinism
mental retardation and lead to
started soon after birth.
4. Lipid Metabolism, thyroid hormone accelerates cholesterol clearance
from the plasma.
5. CHO Metabolism, thyroid hormone increases the rate of glucose
absorption from the small intestine.
6. Cardiovascular Effects
Thyroid hormones increase cardiac output by increasing heart rate and
stroke volume. It increase contractility by increasing the number and
affinity of β-adrenergic receptors in the heart to catecholamines (positive
inotropic), it act on the SA node, and directly increase heart rate (positive
chronotropic effects).

Hypothyroidism
is a common endocrine disorder that affects about
1% of the adult population at some times.
Inadequate thyroid hormone production can result
from failure at the level of thyroid gland itself
(primary hypothyroidism), or it can be due to a
lack of stimulation from TSH. Low TSH levels can
result from pituitary dysfunction (secondary
hypothyroidism) or from lack of pituitary
stimulation by hypothalamic TRH (tertiary
hypothyroidism)
.


Primary Hypothyroidism
L5
Most common cause is an autoimmune destruction of
the thyroid with lymphocytic infiltration like
and
3
; TSH increased while, T
Hashimoto's thyroiditis
T4 decreased. The condition characterized by:
• Decreased basal metabolic rate and oxygen
consumption
• Plasma cholesterol and other blood lipids tend to be
elevated.
• Anemia, constipation, horseness in speech, the skin is
dry and cool
• Accumulation of subcutaneous mucopolysaccharides
that give rise to a nonpitting edema (myxedema)
.

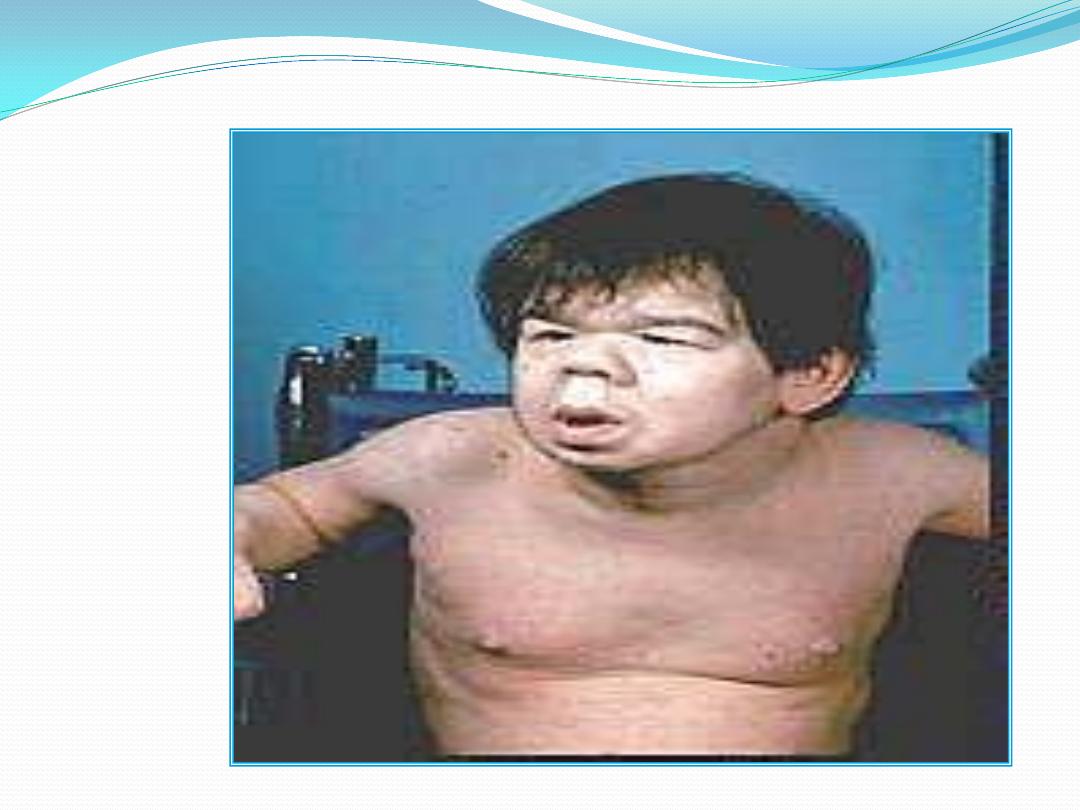
Cretinism
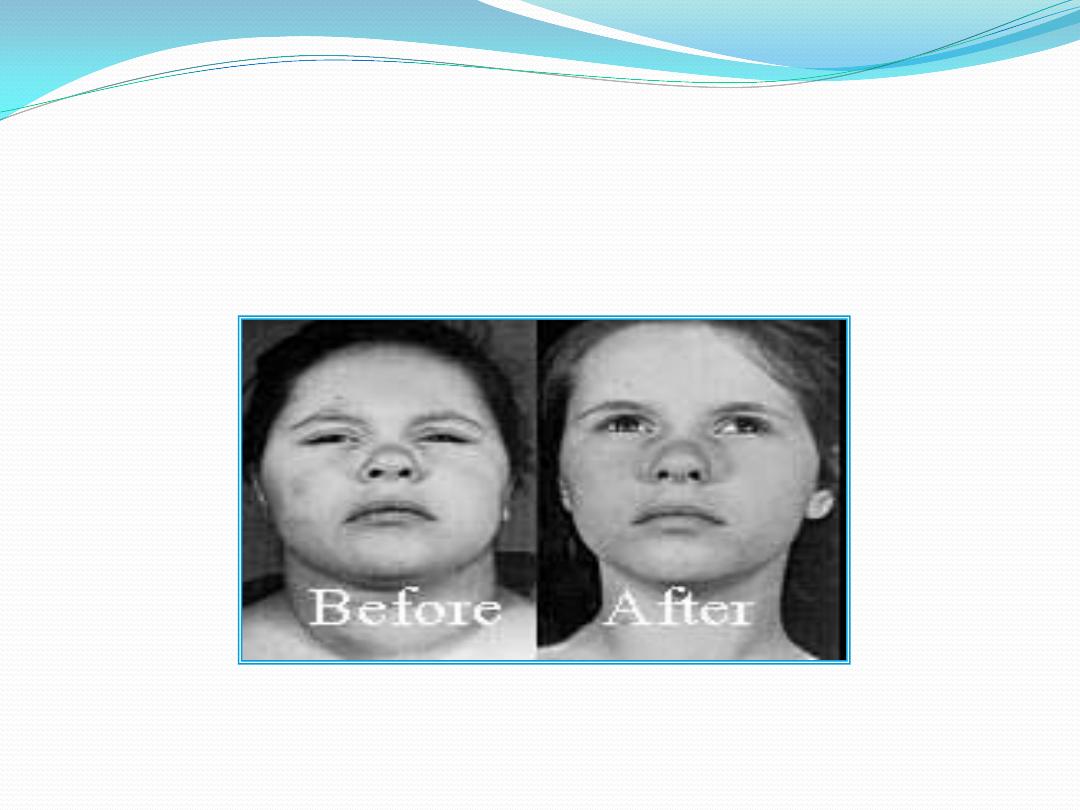
Untreated postnatal hypothyroidism results in
cretinism, a form of dwarfism with mental retardation.
T4 supplementation begun in the first 6 weeks of life
results in normal intelligence.
Acquired hypothyroidism during childhood results in
dwarfism but there is no mental retardation. A major
way thyroid hormones promote normal body growth is
by stimulating the expression of the gene for growth
hormone (GH) in the somatotrophs of the anterior
pituitary gland. In a thyroid hormone-deficient
individual, GH synthesis by the somatotrophs is greatly
is impaired; therefore, a thyroid hormone-deficient
individual will also be GH-deficientreduced and
consequently GH secretion

Primary Hyperthyroidism (Graves' Disease)
Thyrotoxicosis by definition is the clinical syndrome whereby tissues
are exposed to high levels of thyroid hormone (hyperthyroidism).The
, an
Graves' disease
most common cause of thyrotoxicosis is
autoimmune problem due to antibody formation against the TSH
receptor in the plasma membranes of thyroid follicular cells. These
antibodies bind to the TSH receptor, and produces effects similar to
those caused by the action of TSH. In Graves' disease the thyroid is
symmetrically enlarged. Cardiac output, contractility, and heart rate
are increased with possibly palpitations and arrhythmias. Weight loss
with increased food intake, protein wasting and muscle weakness.
Tremor, nervousness, and excessive sweating.
The wide-eyed stare (exophthalmos) in patients with Graves' is caused
by an infiltration of orbital soft tissues and extraocular muscles and
the resulting edema.
A goiter is simply an enlarged thyroid and does not designate
functional status. A goiter can be present in hypo-, hyper-, and
euthyroid states.
There is no correlation between thyroid size and function.

A generalized enlargement of the thyroid is
considered a "diffuse goiter. Diffuse enlargement
often results from prolonged stimulation by TSH or
TSH-like factor; e.g., Hashimoto's thyroiditis,
Graves' disease, diet deficient in iodine
.
