hemodynamic 2 dr.lameia phathology
congestion
Morphological changes in venous congestion
***Pulmonary congestion:
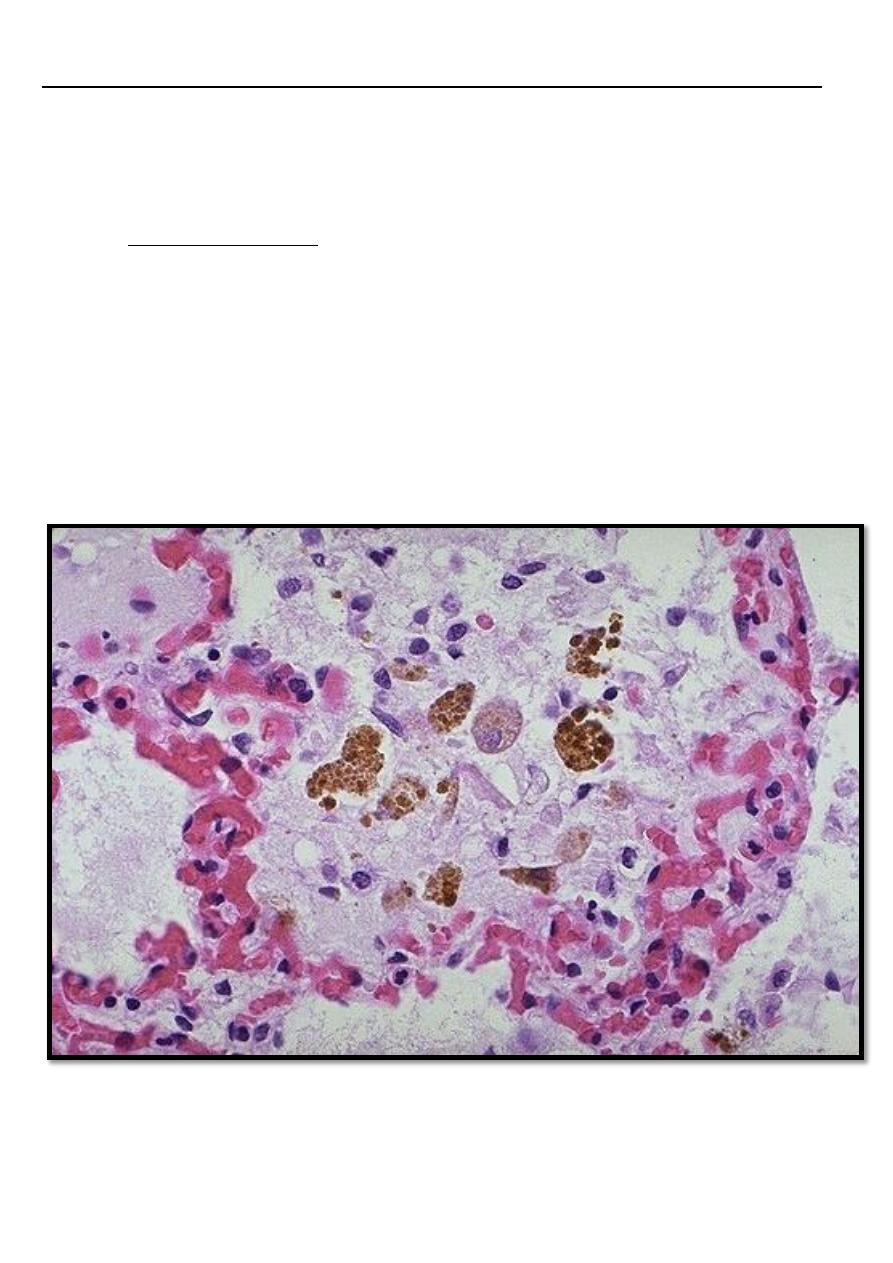
In case of left sided heart failure → raised pressure in the pulmonary veins → alveolar
capillaries become distended, engorged with blood, alveolar septal edema and minute intra
alveolar hemorrhage → break down of RBCs & phagocytosis of intra-alveolar red cell debris by
macrophages leading to accumulation of (hemosidrin –laden macrophages) (heart failure
cells) with a transudate in the alveolar spaces.
In chronic pulmonary congestion, the septa become thickened and fibrotic.
Pulmonary congestion with dilated capillaries and leakage of blood into alveolar spaces
leads to an increase in hemosiderin-laden macrophages,
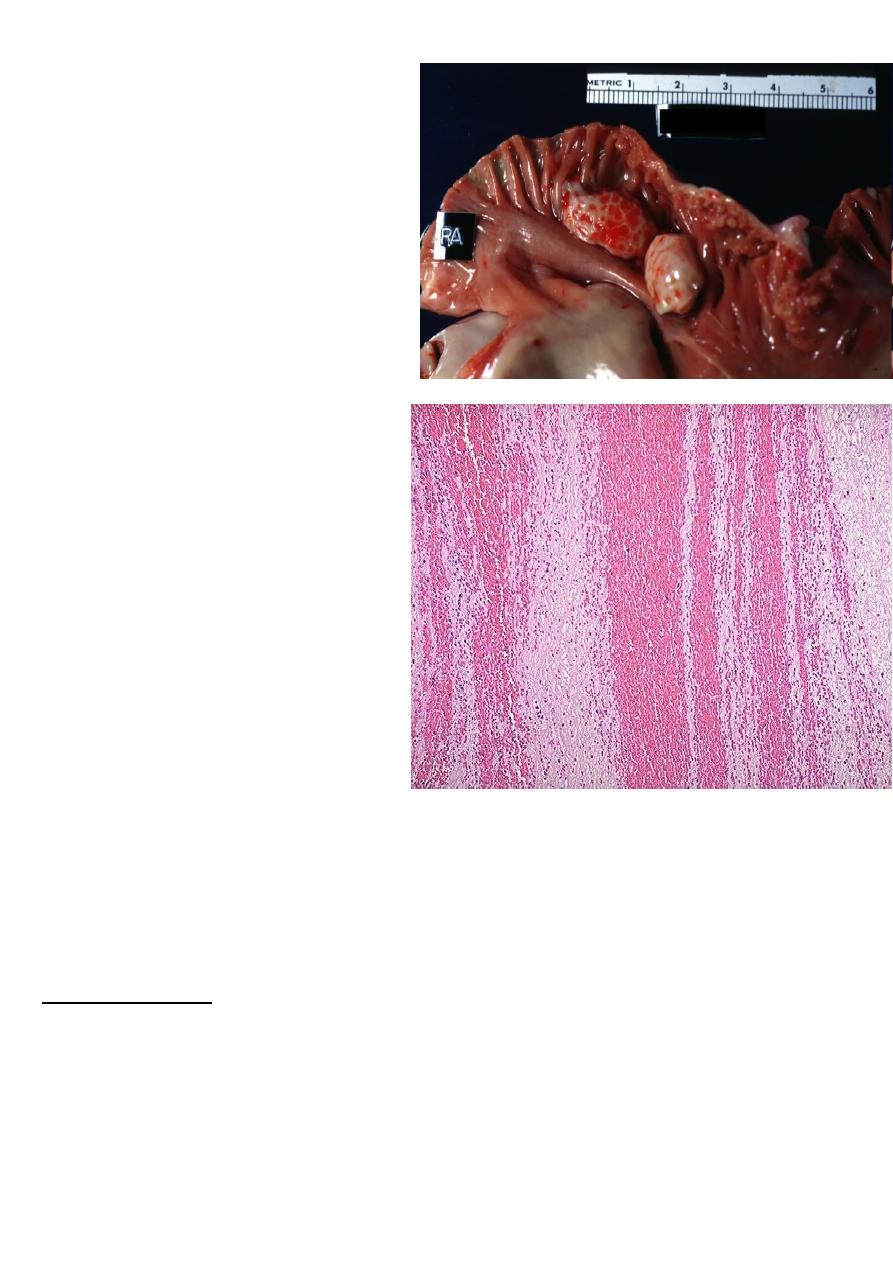
Congestion of the liver
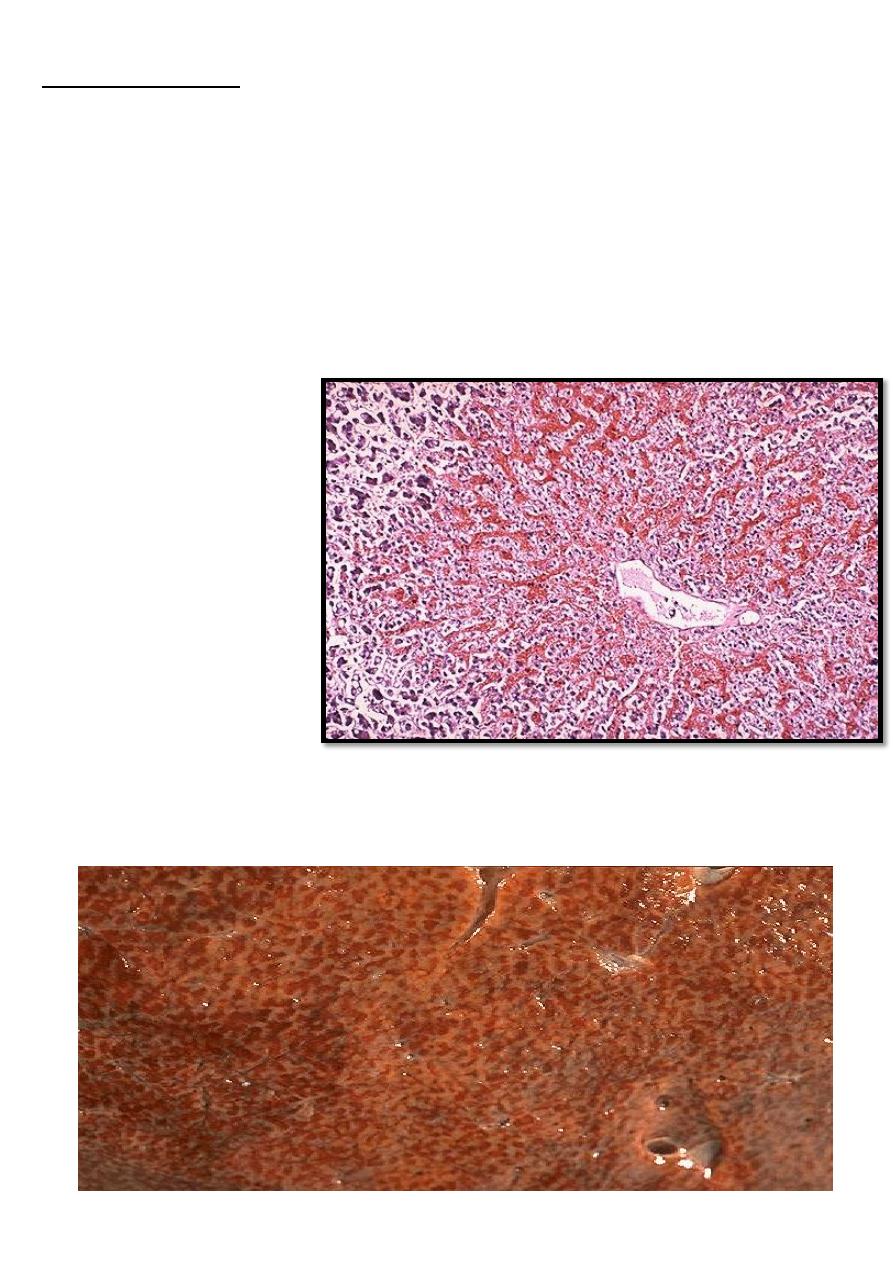
Usually follows right sided heart failure →liver moderately enlarged & tender.
Micro:- the central vein and sinusoids are distended with blood and may be central
hepatocyte degeneration; the periportal hepatocytes are better oxygenated because of their
proximity to hepatic arterioles, so they are less hypoxic & may develop only fatty changes.
Grossly:- central regions of hepatic lobules red-brown surrounding by uncongested tan, some
time fatty liver giving it an appearance called "nut meg liver".
In sever long standing hepatic congestion, hepatic fibrosis can develop, termed ”cardiac
cirrhosis”
Microscopically, the nutmeg
pattern results from
congestion around the
central veins, as seen here.
This is usually due to a "right
sided" heart failure
venous congestion of the liver ”nutmeg liver”.
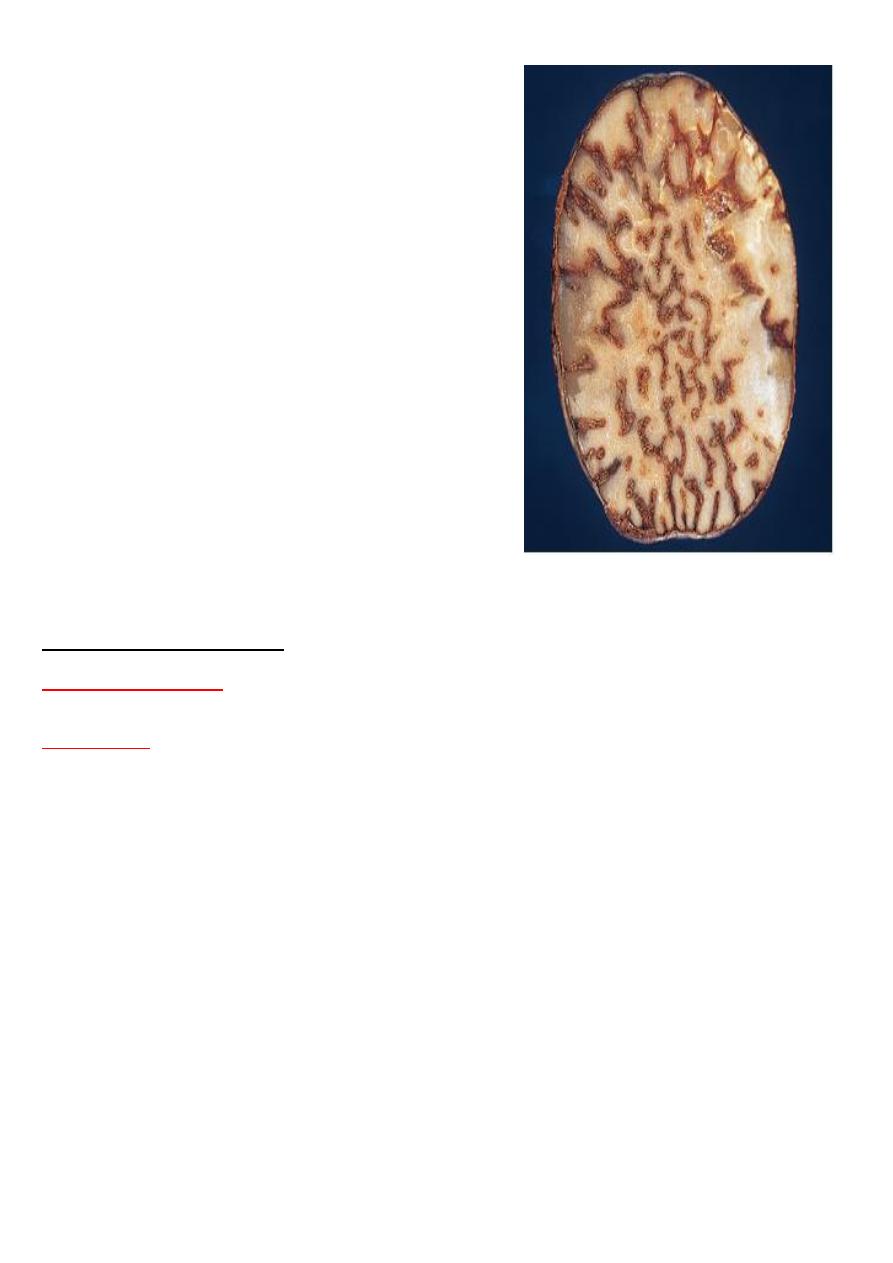
the natural nutmeg
Hemostasis and thrombosis
Normal hemostasis:
is the Maintaining blood in a fluid, clot-free state in normal vessels while
inducing the rapid formation of a localized plug at a site of vascular injury.
Thrombosis:
is the pathologic form of hemostasis , it means the formation of blood clot
(thrombus) in uninjured vessels or thrombotic occlusion of vessel after relatively minor injury.
Hemostasis depends on three general components:
• a) Vascular wall
• b) Platelets
• c) Coagulation pathways
Whenever a vessel is ruptured or severed, hemostasis is achieved .
The sequence of events in hemostasis at a sit of vascular injury include:
Transient period of arteriolar vasoconstriction.
Endothelial injury expose highly thrombogenic
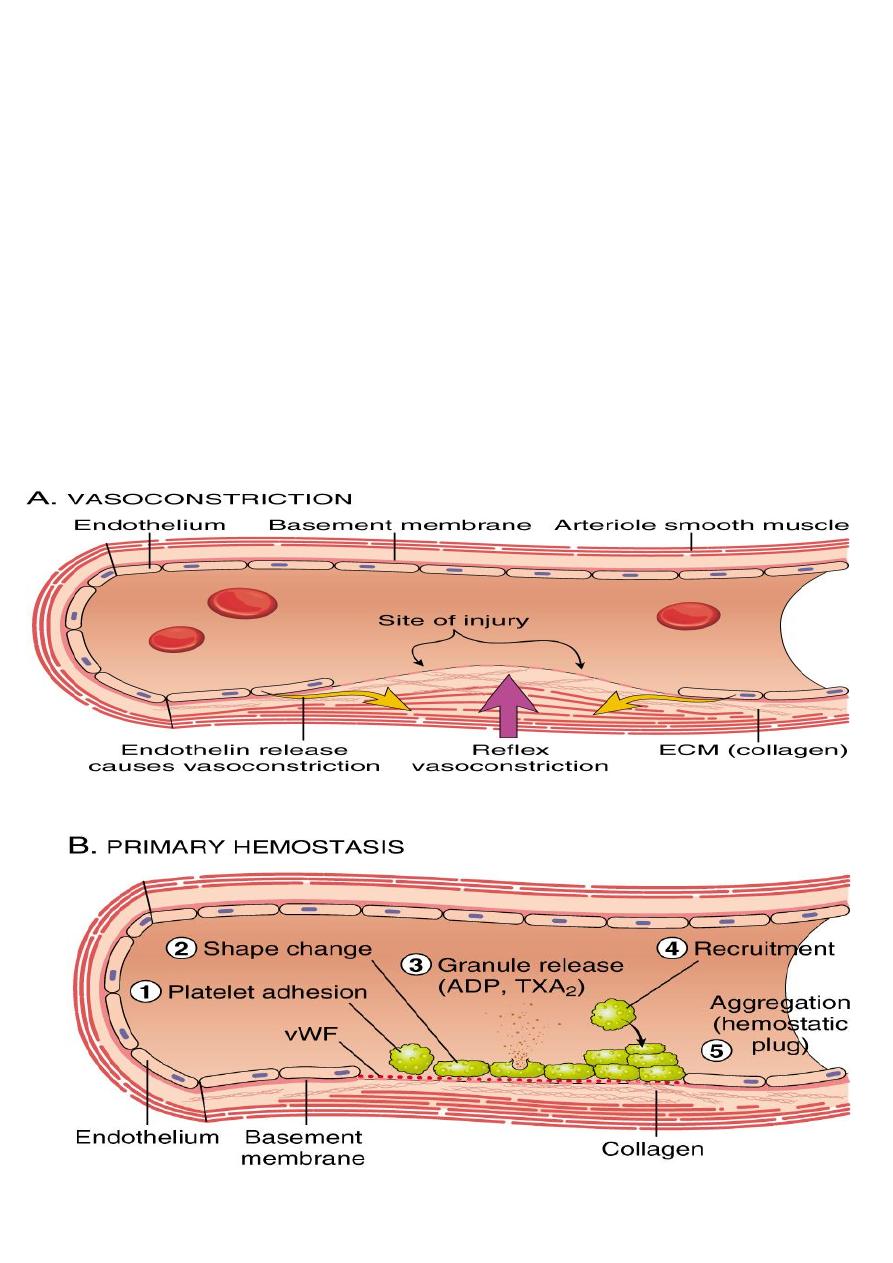
subendothelial extracellular matrix (ECM), then platelets adhere to ECM by VonWillebrand
factor and become activated. Activation of platelets results in change of its shape (increase
surface expression of phospholipid complex) and release secretory granules (adenosine
diphosphate (ADP) and thromboxane A2 (TXA2)) which will lead to the further platelets
aggregation, and to form primary hemostatic plug.
Tissue factor (
factor III or thromboplastin
) which also exposed at the site of injury, this
factor activates the coagulation cascade which creating a fibrin meshwork deposition
(
secondary plug
).
Polymerized fibrin and platelet aggregates form
a solid permanent plug
.
At this stage counter-regulatory mechanisms (tissue plasminogen activator(t-PA) &
thrombomodulin) are set into motion to limit the hemostatic plug to the site of injury.
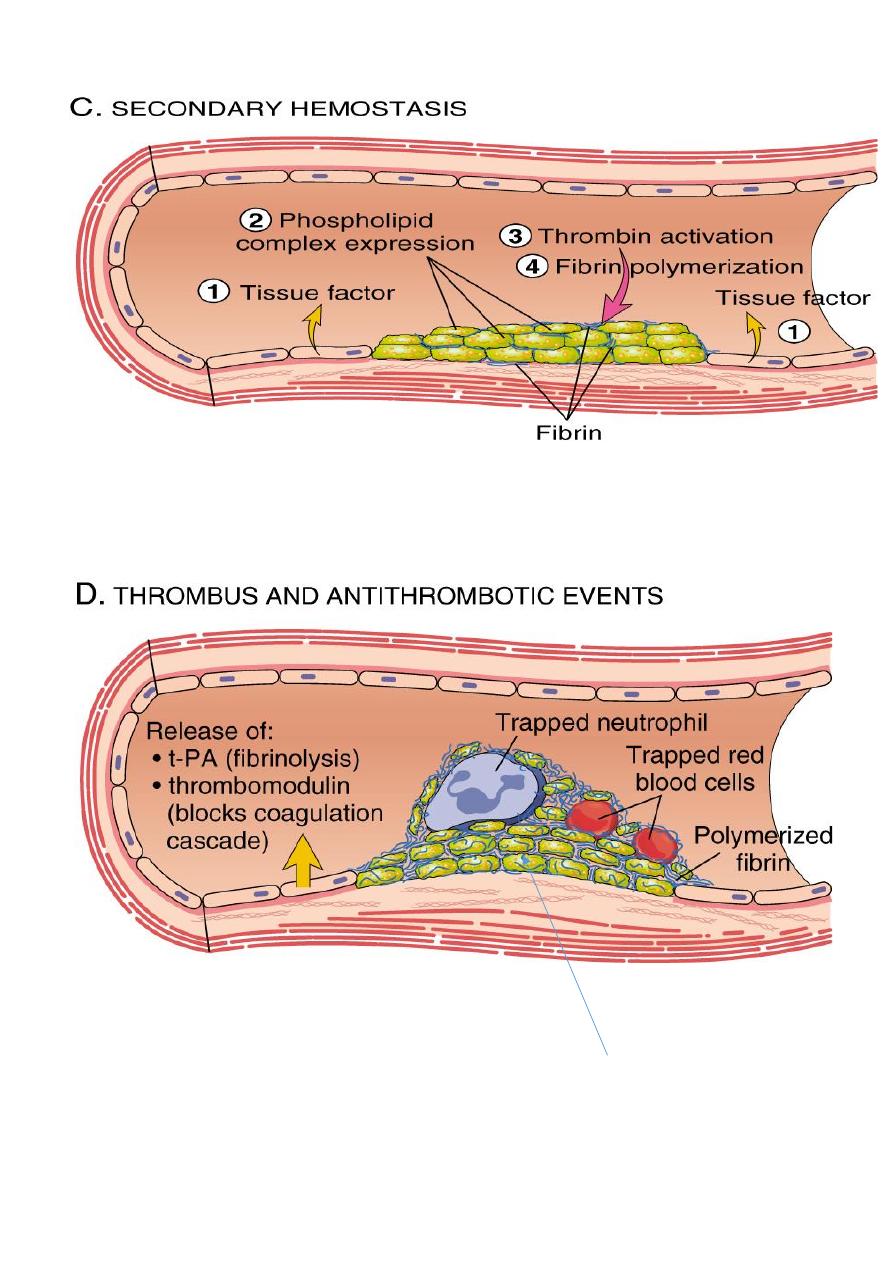
Permanent plug
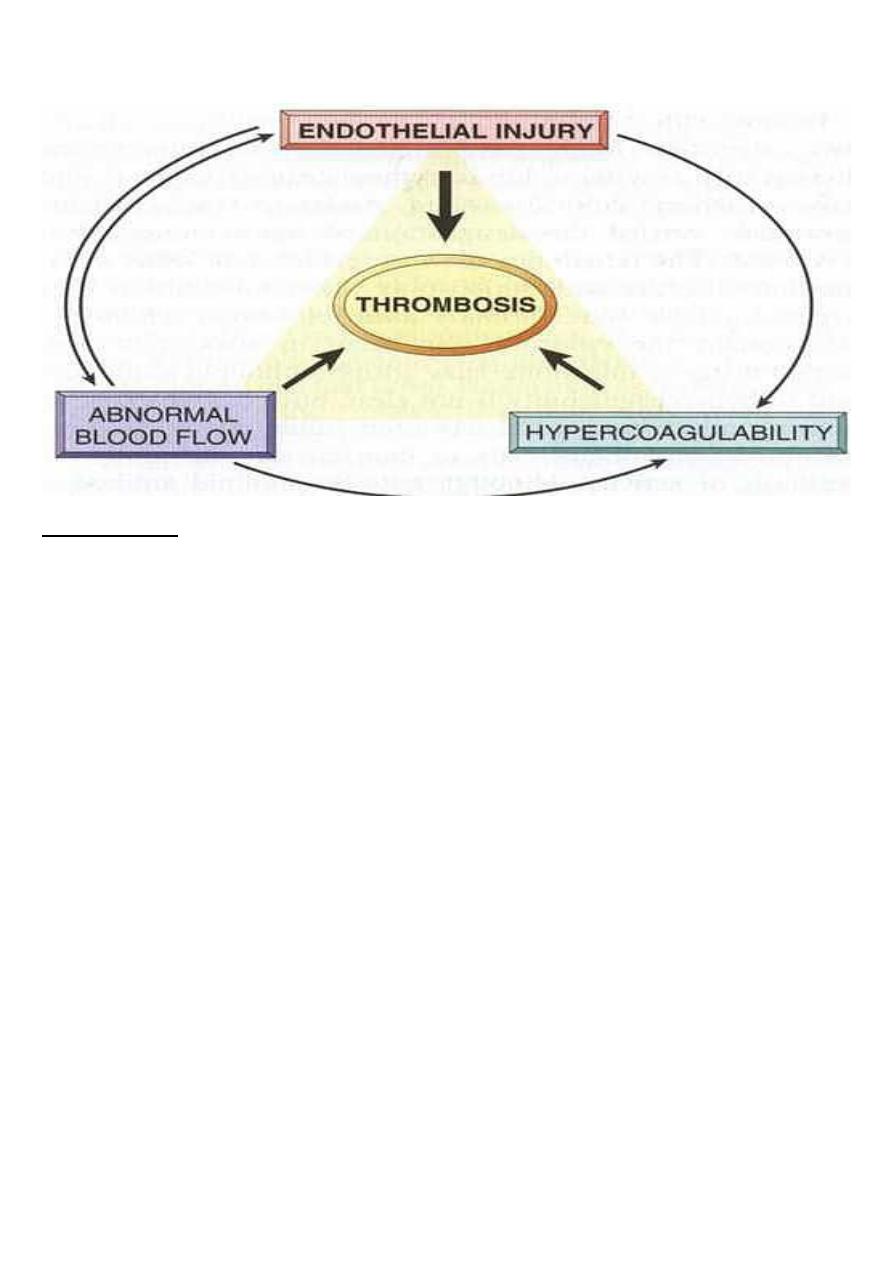
Three primary influences predispose to thrombus formation (Virchow triad);
Virchow triad
:
1. Endothelial injury : (by it self can lead to thrombosis) e.g. in MI, valvulitis, ulcerative
atherosclerosis plaques, vasculitis.
Endothelial dysfunction in the absence of endothelial cell loss can influence clotting events
(may elaborate greater amounts of procoagulant factors or may synthesize fewer
anticoagulant effectors). Endothelial dysfunction may occur with hypertension, bacterial
endotoxins, radiation and products absorbed from cigarette smoke .
2. Stasis or turbulence of blood flow, causing;
A. Disruption of laminar flow and bring platelets into contact with endothelium
B. Preventing dilution of activated clotting factors by fresh-flowing blood.
C. Retarding the inflow of clotting factor inhibitors.
D. Promote endothelial cell activation.
e.g. aortic aneurysms, acute MI, atrial fibrillation and hyperviscosity syndromes as in
polycythemia.
3. Blood hypercoagulability:
can be divided into:
Primary as in mutation of factor V gene and prothrombin gene (most common),
antithrombin III dificiency or protein C or S deficiency
Secondary as in prolonged bedrest, MI, tissue damage( surgery, burn, trauma, fracture),
cancer, late pregnancy, ocp use, polycythemia, Nephrotic syndrome, smoking ……ect.

Morphology:
Thrombi can develop anywhere in the cardiovascular system (e.g., in cardiac
chambers, on valves, or in arteries, veins, or capillaries).
The size and shape of thrombi depend on the site of origin and the cause.
Arterial or cardiac thrombi usually begin at sites of
turbulence or endothelial injury
; venous
thrombi characteristically occur at sites of
stasis.
Thrombi are focally attached to the underlying vascular surface; arterial thrombi tend to grow
retrograde
from the point of attachment, while venous thrombi extend
in the direction of
blood flow
(thus both propagate toward the heart). The propagating portion of a thrombus is
often poorly attached and therefore prone to fragmentation and embolization
• Thrombi often have grossly and microscopically apparent laminations
called lines of
Zahn
; these represent pale platelet and fibrin deposits alternating with darker red cell–
rich layers. Such laminations signify that a thrombus has formed in
flowing blood
; their
presence can therefore distinguish antemortem thrombosis from the bland
nonlaminated clots that occur postmortem
•
Arterial thrombi
are frequently o
c
clusive; the most common sites in decreasing order of
frequency are the coronary, cerebral, and femoral arteries.
• They typically consistof a friable meshwork of platelets, fibrin, red cells, and
degenerating leukocytes
:(pale thrombus)
are gray white in color, .
•
Venous thrombosis (phlebothrombosis)
is almost invariably occlusive, with the
thrombus forming a long cast of the lumen. Because these thrombi form in the
sluggish venous circulation.
• They tend to contain more enmeshed red cells (and relatively few platelets) and
are therefore
known as red, or stasis, thrombi
.
• The veins of the lower extremities are most commonly involved (90% of cases);
however, upper extremities, periprostatic plexus, or the ovarian and periuterine
veins can also develop venous thrombi
Postmortem clots
can sometimes be mistaken for antemortem venous thrombi. However,
postmortem clots are gelatinous with a dark red dependent portion where red cells have
settled by gravity and a yellow “
chicken fat
” upper portion; they are usually
not attached
to
the underlying wall. In comparison, red thrombi are firmer and are focally attached, and
sectioning typically reveals gross and/or microscopic
lines of Zahn.
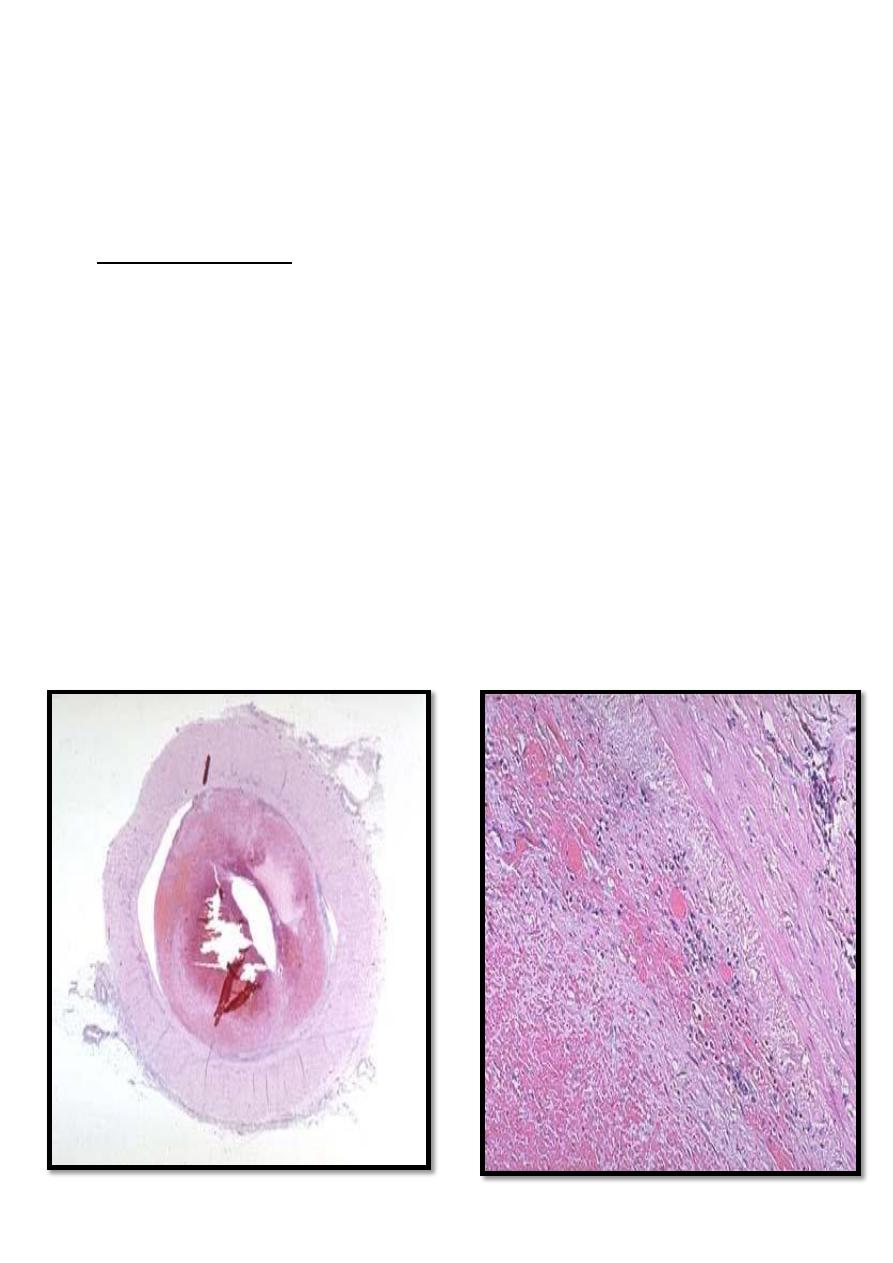
Right atrial mural thrombus with
lines of Zahn
Microscopic appearance of thrombus'
the line of Zahn’.
Mural thrombi
: are thrombi arise in heart chambers or aortic lumen.
Vegetations
: are thrombi formed on heart valves as in infective endocarditis
Venous thrombosis
Occur in 90% in the veins of lower extremities
Superficial venous thromboses
_occur in saphenous veins ( with Varicosities)
_
symptomatic
thrombi can cause local congestion, swelling, pain, and tenderness the
local edema and impaired venous drainage do predispose the overlying skin to infections
from slight trauma and to the development of varicose ulcers
_
rarely embolize
Deep vein thromboses
_rapidly offset by collateral channels (50%
asymptomatic)
_
embolize to lungs
and give rise to pulmonary infarction
Fate of the thrombus:
1. Propagation to obstruct a critical vessel or branch.
2. Embolization in part or in whole.
3. Dissolution (removal by fibrinolytic action).
4. Organization and re-canalization.
Thrombi are significant because:
1. They cause obstruction of vessels.
Artery → infarction, vein → congestion
2. They are possible sources of emboli.
Fates of thrombus
recanalization organization
