
Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
16. Immunization and
Immune Assays
Text
© The McGraw−Hill
Companies, 2002
476
n expanded knowledge of immune function has yielded sig-
nificant breakthroughs in medical technology for manipulat-
ing and monitoring the immune system. One of the most prac-
tical benefits of immunology is administering vaccines and other
immune treatments against common infectious diseases such as hep-
atitis and tetanus that once caused untold sickness and death. Another
valuable application of this technology is testing the blood for signs of
infection or disease. It is routine medical practice to diagnose such in-
fections as HIV, syphilis, hepatitis B, and rubella by means of immuno-
logic analysis. Both separately and in combination, these methods
have made sweeping contributions to individual and community health
by improving diagnosis and treatment, and controlling the spread of
disease. Many hopes for the survival of humankind lie in our ability to
harness the amazing workings of the immune system.
Chapter Overview
• Discoveries in basic immune function have created powerful medical
tools to artificially induce protective immunities and to determine the
status of the immune system.
• Immunization may be administered by means of passive and active
methods.
• Passive immunity is acquired by infusing antiserum taken from other
patients’ blood that contains high levels of protective antibodies. This
form of immunotherapy is short-lived.
• Active methods involve administering a vaccine against an infectious
agent that may be encountered in the future. This form of protection
provides a longer-lived immunity.
• Vaccines contain some form of microbial antigen that has been altered
so that it stimulates a protective immune response without causing the
disease.
• Vaccines can be made from whole dead or live cells and viruses, parts
of cells or viruses, or by recombinant DNA techniques.
• Reactions between antibodies and antigens provide specific and
sensitive tests that can be used in diagnosis of disease and
identification of pathogens.
• Serology involves the testing of a patient’s blood serum for antibodies
that can indicate a current or past infection and the degree of immunity.
• Tests that produce visible interactions of antibodies and antigens
include agglutination, precipitation, and complement fixation.
A
• Assays can be used to separate antigens and antibodies and visualize
them with radioactivity or fluorescence. These include
immunoelectrophoresis, the Western blot, and direct and indirect
immunoassays.
Practical Applications of Immunologic
Function
A knowledge of the immune system and its responses to antigens
has provided extremely valuable biomedical applications in two
major areas: (1) use of antiserum and vaccination to provide artifi-
cial protection against disease and (2) diagnosis of disease through
immunologic testing.
Detail from “The Cowpock,” an 1808 etching that caricatured the worst fears
of the English public concerning Edward Jenner’s smallpox vaccine.
Immunization and
Immune Assays
CHAPTER
16

Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
16. Immunization and
Immune Assays
Text
© The McGraw−Hill
Companies, 2002
Practical Applications of Immunologic Function
477
IMMUNIZATION: METHODS OF MANIPULATING
IMMUNITY FOR THERAPEUTIC PURPOSES
The concept of artificially induced immunity was introduced in
chapters 14 and 15. Methods that actively or passively immunize
people are widely used in disease prevention and treatment. In the
case of passive immunization, a patient is given preformed anti-
bodies, which is actually a form of
immunotherapy. In the case of
active immunization, a patient is vaccinated with a microbe or its
antigens, providing a form of advance protection.
Immunotherapy: Artificial Passive Immunity
The first attempts at passive immunization involved the transfusion
of horse serum containing antitoxins to prevent tetanus and to treat
patients exposed to diphtheria. Since then, antisera from animals
have been replaced with products of human origin that function
with various degrees of specificity.
Immune serum globulin (ISG),
sometimes called
gamma globulin, contains immunoglobulin ex-
tracted from the pooled blood of at least 1,000 human donors. The
method of processing ISG concentrates the antibodies to increase
potency and eliminates potential pathogens (such as the hepatitis B
and HIV viruses). It is a treatment of choice in preventing measles
and hepatitis A and in replacing antibodies in immunodeficient pa-
tients. Most forms of ISG are injected intramuscularly to minimize
adverse reactions, and the protection it provides lasts 2 to 3 months.
A preparation called
specific immune globulin (SIG) is de-
rived from a more defined group of donors. Companies that prepare
SIG obtain serum from patients who are convalescing and in a hy-
perimmune state after such infections as pertussis, rabies, tetanus,
chickenpox, and hepatitis B. These globulins are preferable to ISG
because they contain higher titers of specific antibodies obtained
from a smaller pool of patients. Although useful for prophylaxis in
persons who have been exposed or may be exposed to infectious
agents, these sera are often limited in availability.
When a human immune globulin is not available, antisera
and antitoxins of animal origin can be used. Sera produced in
horses are available for diphtheria, botulism, and spider and snake
bites. Unfortunately, the presence of horse antigens can stimulate
allergies such as serum sickness or anaphylaxis (see chapter 17).
Although donated immunities only last a relatively short time, they
act immediately and can protect patients for whom no other useful
medication or vaccine exists.
Artificial Active Immunity: Vaccination
Active immunity can be conferred artificially by
vaccination—
exposing a person to material that is antigenic but not pathogenic.
The discovery of vaccination was one of the farthest reaching and
most important developments in medical science (Historical High-
lights 16.1). The basic principle behind vaccination is to stimulate
HISTORICAL HIGHLIGHTS
16.1
The Lively History of Active Immunization
The basic notion of immunization has existed for thousands of years. It
probably stemmed from the observation that persons who had recovered
from certain communicable diseases rarely if ever got a second case. Un-
doubtedly, the earliest crude attempts involved bringing a susceptible
person into contact with a diseased person or animal. The first recorded
attempt at immunization occurred in sixth century China. It consisted of
drying and grinding up smallpox scabs and blowing them with a straw
into the nostrils of vulnerable family members. By the tenth century, this
practice had changed to the deliberate inoculation of dried pus from the
smallpox pustules of one patient into the arm of a healthy person, a tech-
nique later called
variolation (variola is the smallpox virus). This
method was used in parts of the Far East for centuries before Lady Mary
Montagu brought it to England in 1721. Although the principles of the
technique had some merit, unfortunately, many recipients and their con-
tacts died of smallpox. This outcome vividly demonstrates a cardinal rule
for a workable vaccine: It must contain an antigen that will provide pro-
tection but not cause the disease. Variolation was so controversial that
any English practitioner caught doing it was charged with a felony.
Eventually, this human experimentation paved the way for the first
really effective vaccine, developed by the English physician Edward
Jenner in 1796 (see chapter 24). Jenner conducted the first scientifically
controlled study, one that had a tremendous impact on the advance of
medicine. His work gave rise to the words
vaccine and vaccination
(from L.,
vacca, cow), which now apply to any immunity obtained by in-
oculation with selected antigens. Jenner was inspired by the case of a
dairymaid who had been infected by a pustular infection called cowpox.
This is a related virus that afflicts cattle but causes a milder condition in
humans. She explained that she and other milkmaids had remained free
of smallpox. Other residents of the region expressed a similar confidence
in the cross-protection of cowpox. To test the effectiveness of this new
vaccine, Jenner prepared material from human cowpox lesions and inoc-
ulated a young boy. When challenged 2 months later with an injection of
crusts from a smallpox patient, the boy proved immune.
Jenner’s discovery—that a less pathogenic agent could confer
protection against a more pathogenic one—is especially remarkable in
view of the fact that microscopy was still in its infancy and the nature of
viruses was unknown. At first, the use of the vaccine was regarded with
some fear and skepticism (see chapter opening illustration). When Jen-
ner’s method proved successful and word of its significance spread, it
was eventually adopted in many other countries. Eventually, the original
virus mutated into a unique strain (
vaccina virus) that was the modern ba-
sis of the vaccine. Now that smallpox is no longer a threat, smallpox vac-
cination has been essentially discontinued in most areas.
Other historical developments in vaccination included using heat-
killed bacteria in vaccines for typhoid fever, cholera, and plague and
techniques for using neutralized toxins for diphtheria and tetanus.
Throughout the history of vaccination, there have been vocal opponents
and minimizers, but numbers do not lie. Whenever a vaccine has been in-
troduced, the prevalence of that disease has declined.
Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
16. Immunization and
Immune Assays
Text
© The McGraw−Hill
Companies, 2002
478
CHAPTER 16 Immunization and Immune Assays
a primary and secondary anamnestic response (see figure 15.18)
that primes the immune system for future exposure to a virulent
pathogen. If this pathogen enters the body, the immune response
will be immediate, powerful, and sustained.
Vaccines have profoundly reduced the prevalence and impact
of many infectious diseases that were once common and often
deadly. In this section, we survey the principles of vaccine prepara-
tion and important considerations surrounding vaccine indication
and safety. (Vaccines are also given specific consideration in later
chapters on bacterial and viral diseases.)
Principles of Vaccine Preparation
A vaccine must be con-
sidered from the standpoints of antigen selection, effectiveness,
ease in administration, safety, and cost. In natural immunity, an
infectious agent stimulates appropriate B and T lymphocytes and
creates memory clones. In artificial active immunity, the objec-
tive is to obtain this same response with a modified version of the
microbe or its components. A safe and effective vaccine should
mimic the natural protective response, not cause a serious infec-
tion or other disease, have long-lasting effects in a few doses,
and be easy to administer. Most vaccine preparations contain one
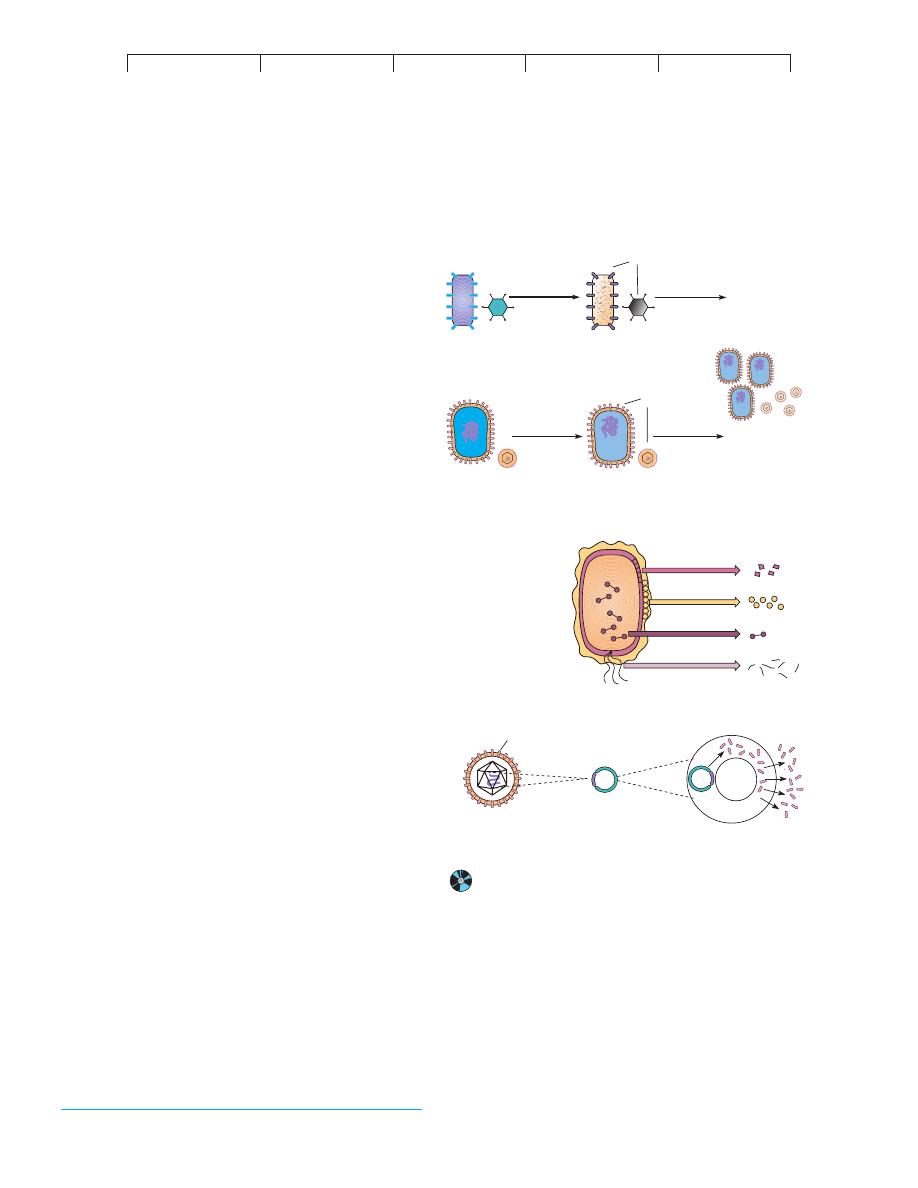
of the following antigenic stimulants (figure 16.1): (1) killed
whole cells or inactivated viruses, (2) live, attenuated cells or
viruses, (3) antigenic components of cells or viruses, or (4) ge-
netically engineered microbes or microbial antigens. A survey of
the major licensed vaccines and their indications is presented in
table 16.1.
Large, complex antigens such as whole cells or viruses are
very effective immunogens. Depending on the vaccine, these are
either killed or attenuated.
Killed or inactivated vaccines are
prepared by cultivating the desired strain or strains of a bac-
terium or virus and treating them with formalin, radiation, heat,
or some other agent that does not destroy antigenicity. One type
of vaccine for the bacterial disease typhoid fever is of this type
(see chapter 20). Salk polio vaccine and influenza vaccine con-
tain inactivated viruses. Because the microbe does not multiply,
killed vaccines often require a larger dose and more boosters to
be effective.
A number of vaccines are prepared from
live, attenuated*
microbes.
Attenuation is any process that substantially lessens or
negates the virulence of viruses or bacteria. It is usually achieved
by modifying the growth conditions or manipulating microbial
genes in a way that eliminates virulence factors. Attenuation meth-
ods include long-term cultivation, selection of mutant strains that
grow at colder temperatures (cold mutants), passage of the microbe
through unnatural hosts or tissue culture, and removal of virulence
genes. The vaccine for tuberculosis (BCG) was obtained after 13
years of subculturing the agent of bovine tuberculosis (see chapter
19). Vaccines for measles, mumps, polio (Sabin), and rubella con-
tain live, nonvirulent viruses. The advantages that favor live prepa-
rations are: (1) Viable microorganisms can multiply and produce
infection (but not disease) like the natural organism; (2) they confer
long-lasting protection; and (3) they usually require fewer doses
and boosters than other types of vaccines. Disadvantages of using
live microbes in vaccines are that they require special storage facil-
ities, can be transmitted to other people, and can mutate back to a
virulent strain (see polio, chapter 25).
If the exact antigenic determinants that stimulate immunity
are known, it is possible to produce a vaccine based on a selected
component of a microorganism. These vaccines for bacteria are
called
acellular or subcellular vaccines. For viruses, they are
(a) Whole-Cell Vaccines
Killed cell or
inactivated
virus
Dead, but
antigenicity
is retained
Heat or
chemicals
Vaccine
stimulates
immunity
but pathogen
cannot multiply.
Administer
Live, attenuated
cells or viruses
Alive, with
same antigenicity
Virulence
is eliminated
or reduced
Administer
Vaccine
can multiply and
boost immune
stimulation.
Ags
Ags
(b) Acellular or Subunit Vaccine
(c) Recombinant Vaccine
Hepatitis B virus
Surface Ag
Plasmid with gene
that codes for
surface Ag
Yeast cloning vector
Synthesis and release
of surface antigen
FIGURE 16.1
Strategies in vaccine design.
(a) Whole cells or viruses, killed
or attenuated. (b) Acellular or subunit vaccines are made by
disrupting the microbe to release various molecules or cell parts that
can be isolated and purified. (c) Recombinant vaccines are made by
isolating a gene for antigenicity from the pathogen (here a hepatitis
virus) and splicing it into a plasmid. Insertion of the recombinant
plasmid into a cloning host (yeast) results in the production of large
amounts of viral surface antigen to use in vaccine preparation.
*
attenuated
(ah-ten´-yoo-ayt-ed) L.
attenuare, to thin. Able to multiply, but nonvirulent.
Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
16. Immunization and
Immune Assays
Text
© The McGraw−Hill
Companies, 2002
Practical Applications of Immunologic Function
479
called
subunit vaccines. The antigen used in these vaccines may be
taken from cultures of the microbes, produced by rDNA technol-
ogy, or synthesized chemically.
Examples of component antigens currently in use are the
capsules of the pneumococcus and meningococcus, the protein
surface antigen of anthrax, and the surface proteins of hepatitis B
virus. A special type of vaccine is the
toxoid,* which consists of a
purified bacterial exotoxin that has been chemically denatured. By
eliciting the production of antitoxins that can neutralize the natural
toxin, toxoid vaccines provide protection against toxinoses such as
diphtheria and tetanus.
New Vaccine Strategies
Despite considerable successes, dozens of bacterial, viral, proto-
zoan, and fungal diseases still remain without a functional vaccine.
Of all of the challenges facing vaccine specialists, probably the
most difficult has been choosing a vaccine antigen that is safe and
that properly stimulates immunity. Currently, much attention is
TABLE 16.1
Currently Approved Vaccines
Disease/Preparation
Route of Administration
Recommended Usage/Comments
Contain Killed Whole Bacteria
Cholera
Subcutaneous (SQ) injection
For travelers; effect not long-term
Typhoid
Intramuscular (IM)
For travelers only; efficacy variable
Plague
SQ
For exposed individuals and animal workers; variable protection
Contain Live, Attenuated Bacteria
Tuberculosis (BCG)
Intradermal (ID) injection
For high-risk occupations only; protection variable
Acellular Vaccines (Capsular Polysaccharides)
Meningitis (meningococcal)
SQ
For protection in high-risk infants, military recruits; short duration
Meningitis (
Haemophilus influenzae) IM
For infants and children; may be administered with DTaP
Pneumococcal pneumonia
IM or SQ
Important for people at high risk: the young, elderly, and
immunocompromised; moderate protection
Pertussis (aP)
IM
For newborns and children; contains recombinant protein antigens
Toxoids (Formaldehyde-Inactivated Bacterial Exotoxins)
Diphtheria
IM
A routine childhood vaccination; highly effective in systemic protection
Tetanus
IM
A routine childhood vaccination; highly effective
Botulism
IM
Only for exposed individuals such as laboratory personnel
Contain Inactivated Whole Viruses
Poliomyelitis (Salk)
IM
Routine childhood vaccine; now used as first choice
Rabies
IM
For victims of animal bites or otherwise exposed; effective
Influenza
IM
For high-risk populations; requires constant updating for new strains;
immunity not durable
Hepatitis A
IM
Protection for travelers, institutionalized people
Contain Live, Attenuated Viruses
Adenovirus infection
Oral
For immunizing military recruits
Measles (rubeola)
SQ
Routine childhood vaccine; very effective
Mumps (parotitis)
SQ
Routine childhood vaccine; very effective
Poliomyelitis
Oral
Routine childhood vaccine; very effective, but can cause polio
Rubella
SQ
Routine childhood vaccine; very effective
Chickenpox (varicella)
SQ
Routine childhood vaccine; immunity can diminish over time
Yellow fever
SQ
Travelers, military personnel in endemic areas
Subunit Viral Vaccines
Hepatitis B
IM
Recommended for all children, starting at birth; also for health workers
and others at risk
Influenza
IM
See influenza above
Recombinant Vaccines
Hepatitis B
IM
Used more often than subunit, but for same groups
Pertussis
IM
See acellular above
Lyme Disease (LymeRIX)
IM
Made from the surface protein; used for persons with increased risk of
exposure to ticks
*
toxoid
(tawks´-oyd) Toxinlike.

Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
16. Immunization and
Immune Assays
Text
© The McGraw−Hill
Companies, 2002
480
CHAPTER 16 Immunization and Immune Assays
being focused on newer strategies for vaccine preparation that
employ antigen synthesis, recombinant DNA, and gene cloning
technology.
When the exact composition of an antigenic determinant is
known, it is possible to synthesize it. This ability permits preserva-
tion of antigenicity while greatly increasing antigen purity and con-
centration. The malaria vaccine currently being used in areas of
South America and Africa is composed of three synthetic peptides
from the parasite. Several biotechnology companies are exploring
the possibility of using plants to synthesize microbial proteins, po-
tentially leading to mass production of edible vaccine antigens.
Some of the genetic engineering concepts introduced in
chapter 10 offer novel approaches to vaccine development. These
methods are particularly effective in designing vaccines for obli-
gate parasites that are difficult or expensive to culture, such as the
syphilis spirochete or the malaria parasite. This technology pro-
vides a means of isolating the genes that encode various microbial
antigens, inserting them into plasmid vectors, and cloning them in
appropriate hosts. The outcome of recombination can be varied as
desired. For instance, the cloning host can be stimulated to synthe-
size and secrete a protein product (antigen), which is then harvested
and purified (figure 16.1
c). This is how certain vaccines for hepati-
tis B rotavirus and Lyme disease are prepared. Antigens from the
agents of syphilis,
Schistosoma, and influenza have been similarly
isolated and cloned and are currently being considered as potential
vaccine material.
Another ingenious technique using genetic recombination
has been nicknamed the
Trojan horse vaccine. The term derives
from an ancient legend in which the Greeks sneaked soldiers into
the fortress of their Trojan enemies by hiding them inside a large,
mobile wooden horse. In the microbial equivalent, genetic material
from a selected infectious agent is inserted into a live carrier
microbe that is nonpathogenic. In theory, the recombinant microbe
will multiply and express the foreign genes, and the vaccine
recipient will be immunized against the microbial antigens.
Vaccinia, the virus originally used to vaccinate for smallpox, and
adenoviruses have proved practical agents for this technique.
Vaccinia is used as the carrier in one of the experimental vaccines
for AIDS (see figure 25.19), herpes simplex 2, leprosy, and tuber-
culosis.
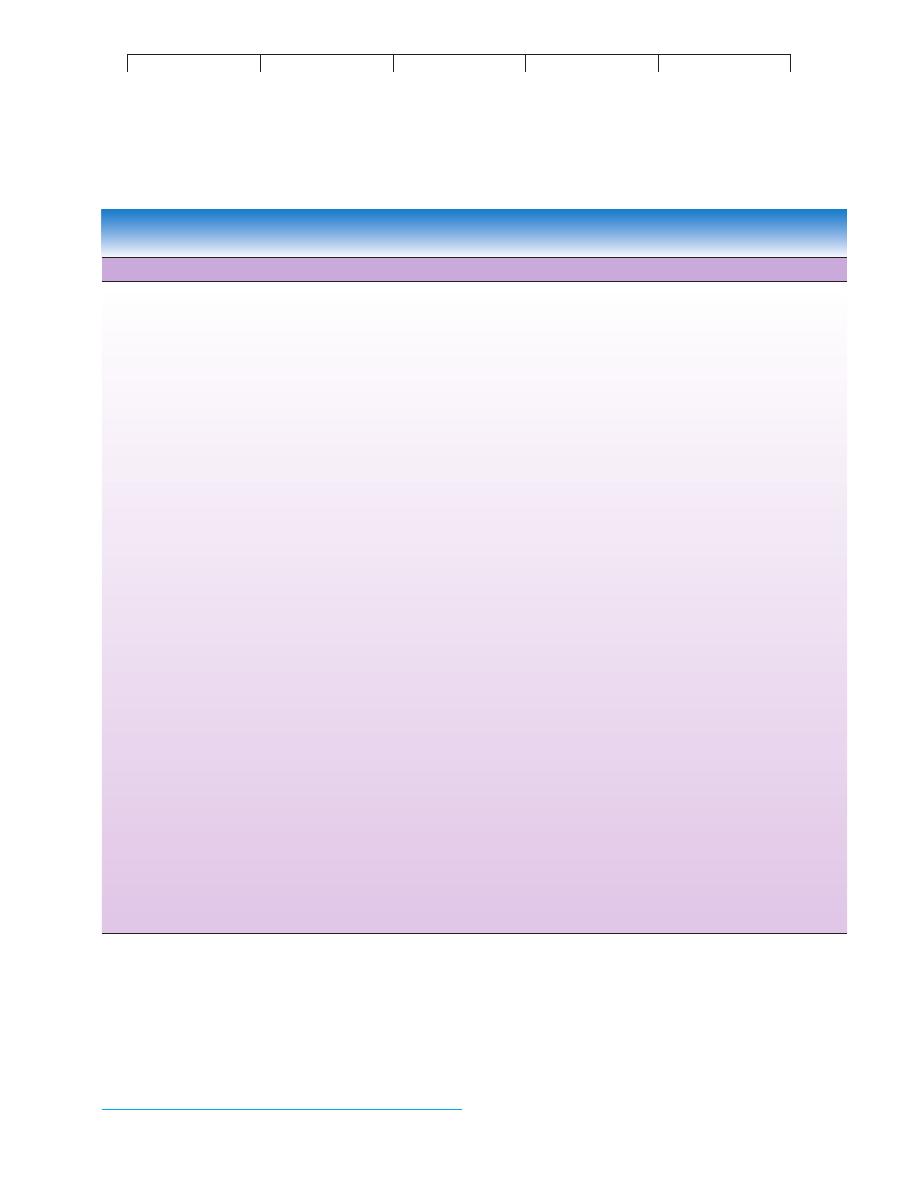
DNA vaccines are being hailed as the most promising of all
of the newer approaches to immunization. The technique in these
formulations is very similar to gene therapy as described in figure
10.13, except in this case, microbial (not human) DNA is inserted
into a plasmid vector and inoculated into a recipient (figure 16.2
a).
The expectation is that the human cells will take up some of the
plasmids and express the microbial DNA in the form of proteins.
Because these proteins are foreign, they will be recognized during
immune surveillance and cause B and T cells to be sensitized and
form memory cells.
Experiments with animals have shown that these vaccines
are very safe and that only a small amount of the foreign antigen
need be expressed to produce effective immunity. Another advan-
tage to this method is that any number of potential microbial pro-
teins can be expressed, making the antigenic stimulus more com-
plex and improving the likelihood that it will stimulate both
antibody and cell-mediated immunity. At the present time, over
30 DNA-based vaccines are being tested in animals. Vaccines for
Lyme disease, hepatitis C, herpes simplex, influenza, tuberculosis,
papillomavirus, and malaria are undergoing animal trials, most
with encouraging results.
Vaccine effectiveness relies, in part, on the production of
antibodies that closely fit the natural antigen. Realizing that such
reactions are very much like a molecular jigsaw puzzle, researchers
have proposed an entirely new concept in vaccines. The
anti-
idiotype vaccine is based on the principle that the antigen binding
(variable) region, or
idiotype,* of a given antibody (A) can be
antigenic to a genetically different recipient and can cause that
recipient’s immune system to produce antibodies (B; also called
anti-idiotypic antibodies) specific for the variable region on anti-
body A (figure 16.2
b). The purpose for making anti-idiotypic
antibodies is that they will display an identical configuration as the
desired antigen and can be used in vaccines. This method avoids
administering a microbial antigen, thus reducing the potential for
dangerous side effects. Using monoclonal antibodies, this approach
has been used to mimic the surface antigen of hepatitis B virus and
Trypanosoma with some success.
Route of Administration and Side Effects
of Vaccines
Most vaccines are injected by subcutaneous, intramuscular, or intra-
dermal routes. Oral vaccines are available for only two diseases
(table 16.1), but they have some distinct advantages. An oral dose
of a vaccine can stimulate protection (IgA) on the mucous mem-
brane of the portal of entry. Oral vaccines are also easier to give,
more readily accepted, and well tolerated. Some vaccines require
the addition of a special binding substance, or
adjuvant.* An
adjuvant is any compound that enhances immunogenicity and
prolongs antigen retention at the injection site. The adjuvant pre-
cipitates the antigen and holds it in the tissues so that it will be
released gradually. Its gradual release, presumably, facilitates con-
tact with macrophages and lymphocytes. Common adjuvants are
alum (aluminum hydroxide salts), Freund’s adjuvant (emulsion of
mineral oil, water, and extracts of mycobacteria), and beeswax.
Vaccines must go through many years of trials in experimen-
tal animals and humans before they are licensed for general use.
Even after they have been approved, like all therapeutic products,
they are not without complications. The most common of these are
local reactions at the injection site, fever, allergies, and other
adverse reactions. Relatively rare reactions (about 1 case out of
220,000 vaccinations) are panencephalitis (from measles vaccine),
back-mutation to a virulent strain (from polio vaccine), disease due
to contamination with dangerous viruses or chemicals, and neuro-
logical effects of unknown cause (from pertussis and swine flu vac-
cines). Some patients experience allergic reactions to the medium
(eggs or tissue culture) rather than to vaccine antigens. Some recent
studies have attempted to link childhood vaccinations to later
development of diabetes, asthma, and autism. After thorough
examination of records, epidemiologists have found no convincing
evidence for a connection to these diseases.
*
idiotype
(id´-ee-oh-type) Gr.
idios, own, peculiar. Another term for the antigen binding
site.
*
adjuvant
(ad´-joo-vunt) L.
adjuvare, to help.
Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
16. Immunization and
Immune Assays
Text
© The McGraw−Hill
Companies, 2002
Practical Applications of Immunologic Function
481
When known or suspected adverse effects have been de-
tected, vaccines are altered or withdrawn. Most recently, the
whole-cell pertussis vaccine was replaced by the acellular capsule
(aP) form when it was associated with adverse neurological ef-
fects. The live oral rotavirus vaccine had to be withdrawn when
children experienced intestinal blockage. Polio vaccine was
switched from live, oral to inactivated when too many cases of
paralytic disease occurred from back-mutated vaccine stocks. Vac-
cine companies are also phasing out certain preservatives
(thimerosal) that are thought to cause allergies and other potential
side effects.
Professionals involved in giving vaccinations must under-
stand their inherent risks but also realize that the risks from the
infectious disease almost always outweigh the chance of an adverse
vaccine reaction. The greatest caution must be exercised in giving
live vaccines to immunocompromised or pregnant patients, the lat-
ter because of possible risk to the fetus.
To Vaccinate: Why, Whom, and When?
Vaccination confers long-lasting, sometimes lifetime, protection
in the individual, but an equally important effect is to protect the
public health. Vaccination is an effective method of establishing
Protein
from
pathogen
Protein expresed
on surface of cell
Nucleus
Foreign protein
of pathogen is
inserted into
cell membrane,
where it will
stimulate immune
response.
Cells of subject
accept plasmid
with pathogen's
DNA. DNA is
transcribed and
translated into
various proteins.
DNA vaccine
injected into
subject.
Rabbit Ab
(b) Technology for anti-idiotypic vaccines
Mouse B-cell clone
recognizes foreign
idiotype.
Idiotype
of Ab
Rabbit Ab (antibody A)
Anti-idiotypic Ab
(antibody B)
produced.
New antibodies with
same specificity for Ag
as original Ab A.
Injected into
human as
vaccine Ag
Ab alone
injected
into mouse
Anti-idiotype has
same configuration
as Ag in (1)
Genomic DNA
inserted into
plasmid vector;
plasmid is
amplified and
prepared as
vaccine.
DNA that codes
for protein antigen
extracted from
pathogen genome.
DNA of
pathogen
Plasmid
Plasmid
with DNA
(2)
(1)
(3)
(4)
(3)
(2)
(1)
(4)
(5)
Ag
Ag
(a) Technology for DNA vaccines
FIGURE 16.2
Other technologies useful in preparing vaccines.
(a) DNA vaccines contain all or part of the pathogen’s DNA, which is used to “infect” recipient’s
cells. Processing of the DNA leads to production of an antigen protein that can stimulate a specific response against that pathogen. (b) Anti-idiotypic
vaccines use antibodies as foreign proteins to mimic the structure of the natural antigen. See text for details.
Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
16. Immunization and
Immune Assays
Text
© The McGraw−Hill
Companies, 2002
482
CHAPTER 16 Immunization and Immune Assays
herd immunity in the population. According to this concept, in-
dividuals immune to a communicable infectious disease will not
be carriers, and therefore the occurrence of that microbe will be
reduced. With a larger number of immune individuals in a popu-
lation (herd), it will be less likely that an unimmunized member
of the population will encounter the agent. In effect, collective
immunity through mass immunization confers indirect protec-
tion on the nonimmune (such as children). Herd immunity main-
tained through immunization is an important force in averting
epidemics.
Vaccination is recommended for all typical childhood dis-
eases for which a vaccine is available and for people in certain
special circumstances (health workers, travelers, military person-
nel). Table 16.2 outlines a general schedule for childhood immu-
nization and the indication for special vaccines. Not only are some
vaccines mixtures of antigens, but in certain cases several vaccines
are also administered simultaneously. Examples are military recr-
uits who receive as many as 15 injections within a few minutes and
children who receive boosters for DTaP and polio at the same time
they receive the MMR vaccine. Experts doubt that immune inter-
ference (inhibition of one immune response by another) is a signif-
icant problem in these instances, and the mixed vaccines are care-
fully balanced to prevent this eventuality. The main problem with
simultaneous administration is that side effects can be amplified.
TABLE 16.2
Recommended Regimen and Indications for Routine Vaccinations
Routine Schedules for Children and Adults
Vaccine
Birth
2
Months
4
Months
6
Months
12
Months
15
Months
18
Months
4–6
Years
11–12
Years
Adults Comments
Mixed vaccines
Td is tetanus/diphtheria; T is tetanus
alone; either one should be given as
booster every 10 years.
First dose varies with disease incidence;
booster given at either 4– 6 or 11–12 years.
Schedule depends upon source of
vaccine; given with DTaP as TriHIBit
Single vaccines
Similar schedule to DTaP; injected
vaccine
Option depends upon condition of
infant; 3 doses given
Cannot be given to children <1 year old
or
Diphtheria,
Tetanus,
Pertussis
1
(DTaP)
DTaP
one dose
Td or T
Measles,
2
Mumps,
Rubella (MMR)
MMR
MMR
MMR
Haemophilus
influenzae
type b (Hib)
Hib
Hib
Hib
Hib
IPV
IPV
Hepatitis B (HB)
HB option 1
HB option 2
HB option 3
Chickenpox
(CPV)
CPV one dose
CPV
two
doses
Shaded bars indicate that a dose can be
given once during this time frame
2
Measles vaccine (Attenuvax) can be given alone
to children during epidemics or to adults immunized
before 1970.
Used in Cases of Specific Risk Due to Occupational or Other Exposure
Group Targeted
Health care personnel; people exposed through life-style
Children 2–14 years who live in areas of high prevalence
Elderly patients, children with sickle-cell anemia
Hospital, laboratory, health care workers
People whose jobs involve contact with animals (veterinarians, forest rangers); known or
suspected exposure to rabid animal; living in areas of high incidence
Travelers to endemic regions, including military recruits (varies with geographic destination)
Vaccine
Hepatitis B (Recombivax)
Hepatitis A (Havrix)
Pneumococcus (Pneumovax)
Influenza, polio, tuberculosis (BCG)
Rabies, plague, Lyme disease
Cholera, hepatitis B, hepatitis A, measles, yellow fever,
meningococcal meningitis, polio, rabies, typhoid, plague
Pneumococcus
Vaccine (PV)
PV
PV
PV
PV
DTaP
DTaP
DTaP
DTaP
Used to protect children against otitis
media
Poliovirus (IPV)
3
IPV
one dose
IPV
1
DTaP. The diphtheria–tetanus–acellular pertussis
vaccine has replaced the DTP.
3
IPV—inactivated polio vaccine—is now indicated
as a safer alternative to the oral polio vaccine.
Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
16. Immunization and
Immune Assays
Text
© The McGraw−Hill
Companies, 2002
Practical Applications of Immunologic Function
483
SEROLOGICAL AND IMMUNE TESTS: MEASURING
THE IMMUNE RESPONSE IN VITRO
The antibodies formed during an immune reaction are important in
combating infection, but they hold additional practical value. Char-
acteristics of antibodies (such as their quantity or specificity) can
reveal the history of a patient’s contact with microorganisms or
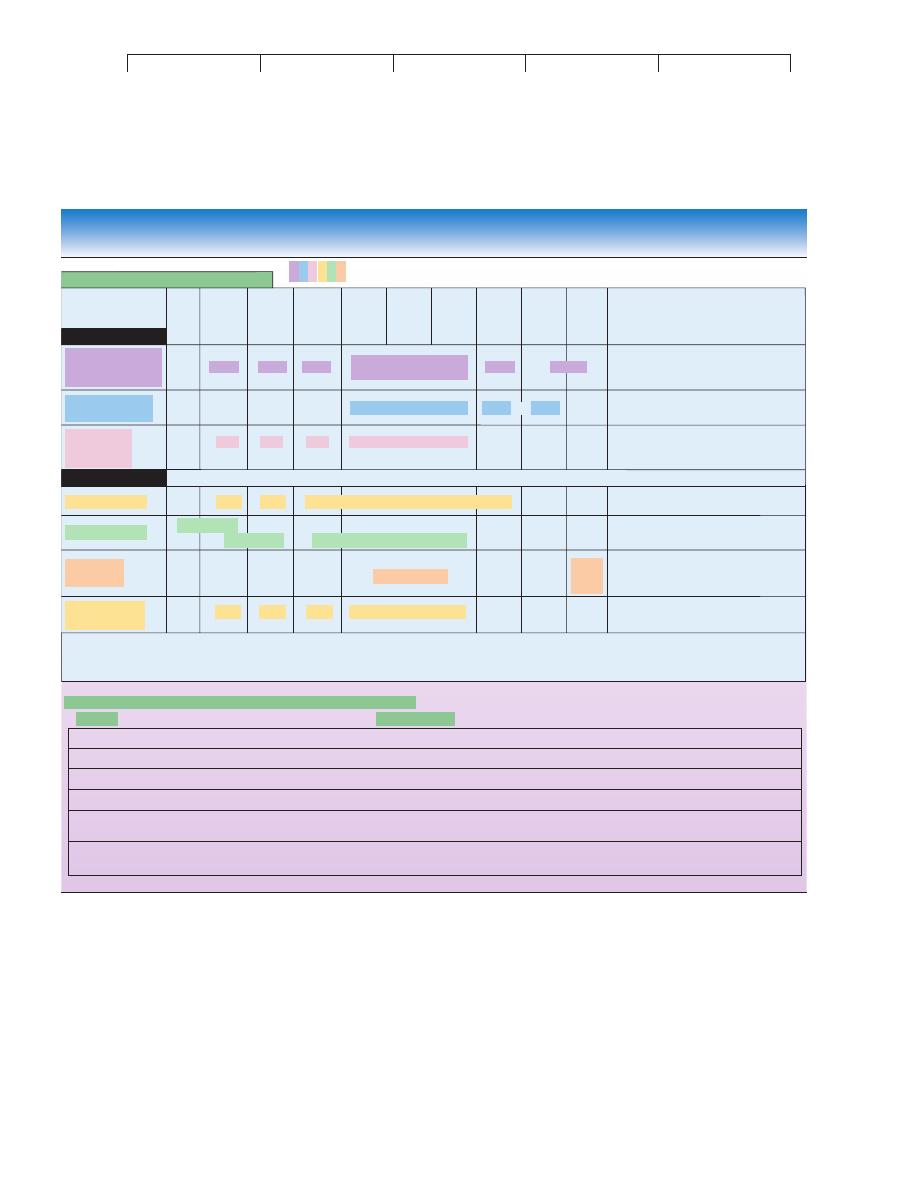
other antigens. This is the underlying basis of
serological testing.
Serology is the branch of immunology that traditionally deals with
in vitro diagnostic testing of serum. Serological testing is based on
the familiar concept that antibodies have extreme specificity for
antigens, so when a particular antigen is exposed to its specific an-
tibody, it will fit like a hand in a glove. The ability to visualize this
interaction by some means provides a powerful tool for detecting,
identifying, and quantifying antibodies—or for that matter, anti-
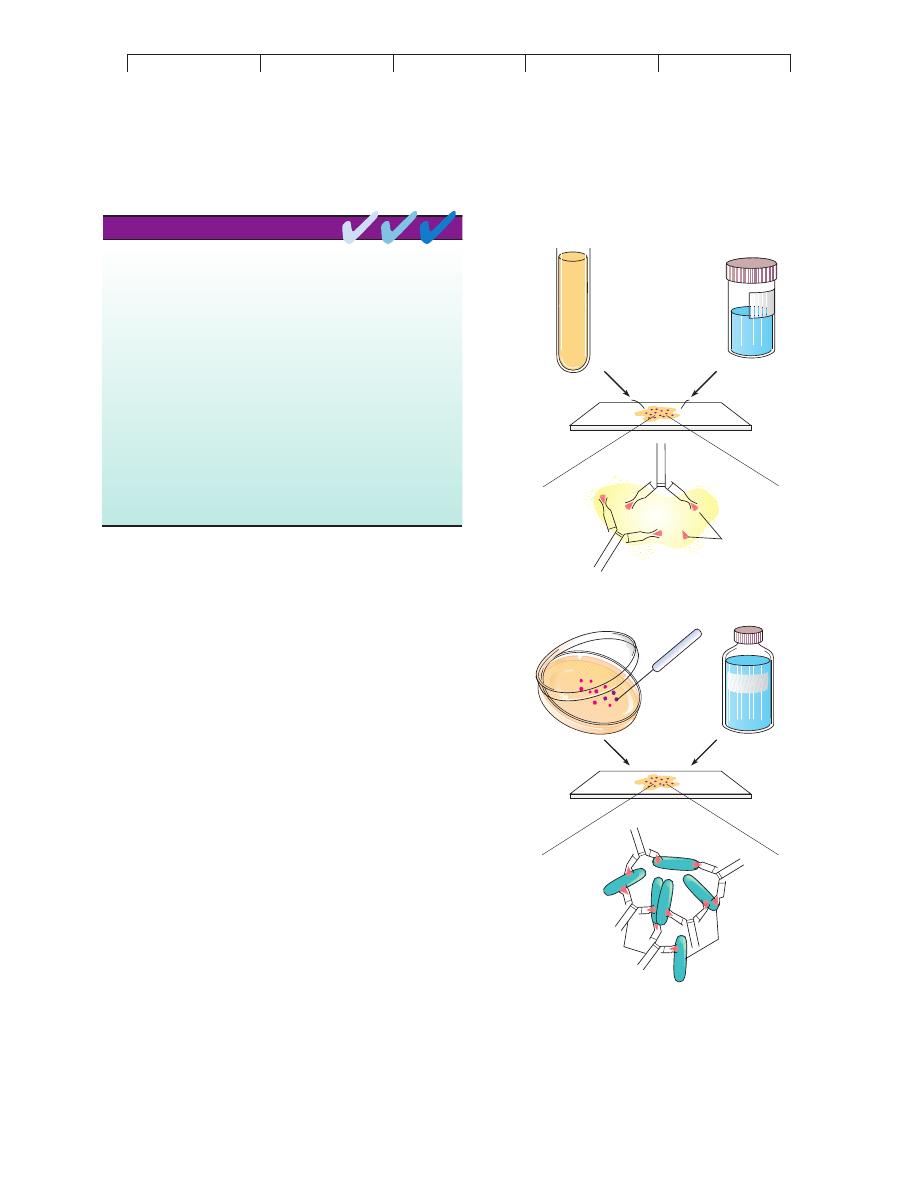
gens. The scheme works both ways, depending on the situation.
One can detect or identify an unknown antibody using a known
antigen, or one can use an antibody of known specificity to help de-
tect or identify an unknown antigen (figure 16.3). Modern serolog-
ical testing has grown into a field that tests more than just serum.
Urine, cerebrospinal fluid, whole tissues, and saliva can also be
used to determine the immunologic status of patients. These and
other immune tests are helpful in confirming a suspected diagnosis
or in screening a certain population for disease.
General Features of Immune Testing
The strategies of immunologic tests are diverse, and they underline
some of the brilliant and imaginative ways that antibodies and anti-
gens can be used as tools. We will summarize them under the head-
ings of agglutination, precipitation, immunodiffusion, complement
fixation, fluorescent antibody tests, and immunoassay tests. First
we will overview the general characteristics of immune testing, and
we will then look at each type separately.
The most effective serological tests have a high degree of
specificity and sensitivity (figure 16.4).
Specificity is the property
of a test to focus upon only a certain antibody or antigen and not to
Knowledge of the specific immune response has two practical
applications: (1) commercial production of antisera and vaccines and
(2) development of rapid, sensitive methods of disease diagnosis.
Artificial passive immunity usually involves administration of antiserum,
and occasionally B and T cells. Antibodies collected from donors
(human or otherwise) are injected into people who need protection
immediately. Examples include ISG (immune serum globulin) and SIG
(specific immune globulin).
Artificial active agents are vaccines that provoke a protective immune
response in the recipient but do not cause the actual disease.
Vaccination is the process of challenging the immune system with a
specially selected antigen. Examples are (1) killed or inactivated
microbes, (2) live, attenuated microbes, (3) subunits of microbes, and
(4) genetically engineered microbes or microbial parts.
Vaccination programs seek to protect the individual directly through
raising the antibody titer and indirectly through the development of
herd immunity.
CHAPTER CHECKPOINTS
Patient's serum
antibody content unknown
Macroscopic
reaction
Prepared
known
antigen
Isolated colony,
identity unknown
ANTISERUM
Macroscopic
reaction
Ag
Antibodies of
known
identity
Ab
Ag
(a)
(b)
FIGURE 16.3
Basic principles of testing using antibodies and antigens.
(a) In
serological diagnosis of disease, a blood sample is scanned for the
presence of antibody using an antigen of known specificity. A positive
reaction is usually evident as some visible sign, such as color change
or clumping, that indicates a specific interaction between antibody and
antigen. (The reaction at the molecular level is rarely observed.) (b) An
unknown microbe is mixed with serum containing antibodies of known
specificity, a procedure known as serotyping. Microscopically or
macroscopically observable reactions indicate a correct match between
antibody and antigen and permit identification of the microbe.
