Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
17. Disorders in Immunity
Text
© The McGraw−Hill
Companies, 2002
508
CHAPTER 17 Disorders in Immunity
Type II Hypersensitivities: Reactions
That Lyse Foreign Cells
The diseases termed type II hypersensitivities are a complex group
of syndromes that involve complement-assisted destruction (lysis)
of cells by antibodies (IgG and IgM) directed against those cells’
surface antigens. This category includes transfusion reactions and
some types of autoimmunities (discussed in a later section). The
cells targeted for destruction are often red blood cells, but other
cells can be involved.
HUMAN BLOOD TYPES
Chapters 14 and 15 described the functions of unique surface re-
ceptors or markers on cell membranes. Ordinarily, these receptors
play essential roles in transport, recognition, and development, but
they become medically important when the tissues of one person
are placed into the body of another person. Blood transfusions and
organ donations introduce alloantigens (molecules that differ in the
same species) on donor cells that are recognized by the lympho-
cytes of the recipient. These reactions are not really immune dys-
functions as allergy and autoimmunity are. The immune system is
in fact working normally, but it is not equipped to distinguish
between the desirable foreign cells of a transplanted tissue and the
undesirable ones of a microbe.
THE BASIS OF HUMAN ABO
ANTIGENS AND BLOOD TYPES
The existence of human blood types was first demonstrated by an
Austrian pathologist, Karl Landsteiner, in 1904. While studying in-
compatibilities in blood transfusions, he found that the serum of one
person could clump the red blood cells of another. Landsteiner iden-
tified four distinct types, subsequently called the ABO blood groups.
Like the MHC antigens on white blood cells, the ABO anti-
gen markers on red blood cells are genetically determined and com-
posed of glycoproteins. These ABO antigens are inherited as two
(one from each parent) of three alternative
alleles:* A, B, or O. A
and B alleles are dominant over O and codominant with one an-
other. As table 17.3 indicates, this mode of inheritance gives rise to
four blood types (phenotypes), depending on the particular combi-
nation of genes. Thus, a person with an
AA or AO genotype has
type A blood; genotype BB or BO gives type B; genotype AB
Type I hypersensitivity reactions result from excessive IgE production in
response to an exogenous antigen.
The two kinds of type I hypersensitivities are atopy, a chronic, local
allergy, and anaphylaxis, a systemic, potentially fatal allergic response.
The predisposition to type I hypersensitivities is inherited, but age,
geographic locale, and infection also influence allergic response.
Type I allergens include inhalants, ingestants, injectants, and contactants.
The portals of entry for type I antigens are the skin, respiratory tract,
gastrointestinal tract, and genitourinary tract.
Type I hypersensitivities are set up by a sensitizing dose of allergen and
expressed when a second provocative dose triggers the allergic
response. The time interval between the two can be many years.
The primary participants in type I hypersensitivities are IgE, basophils,
mast cells, and agents of the inflammatory response.
Allergies are diagnosed by a variety of in vitro and in vivo tests that
assay specific cells, IgE, and local reactions.
Allergies are treated by medications that interrupt the allergic response at
certain points. Allergic reactions can often be prevented by
desensitization therapy.
CHAPTER CHECKPOINTS
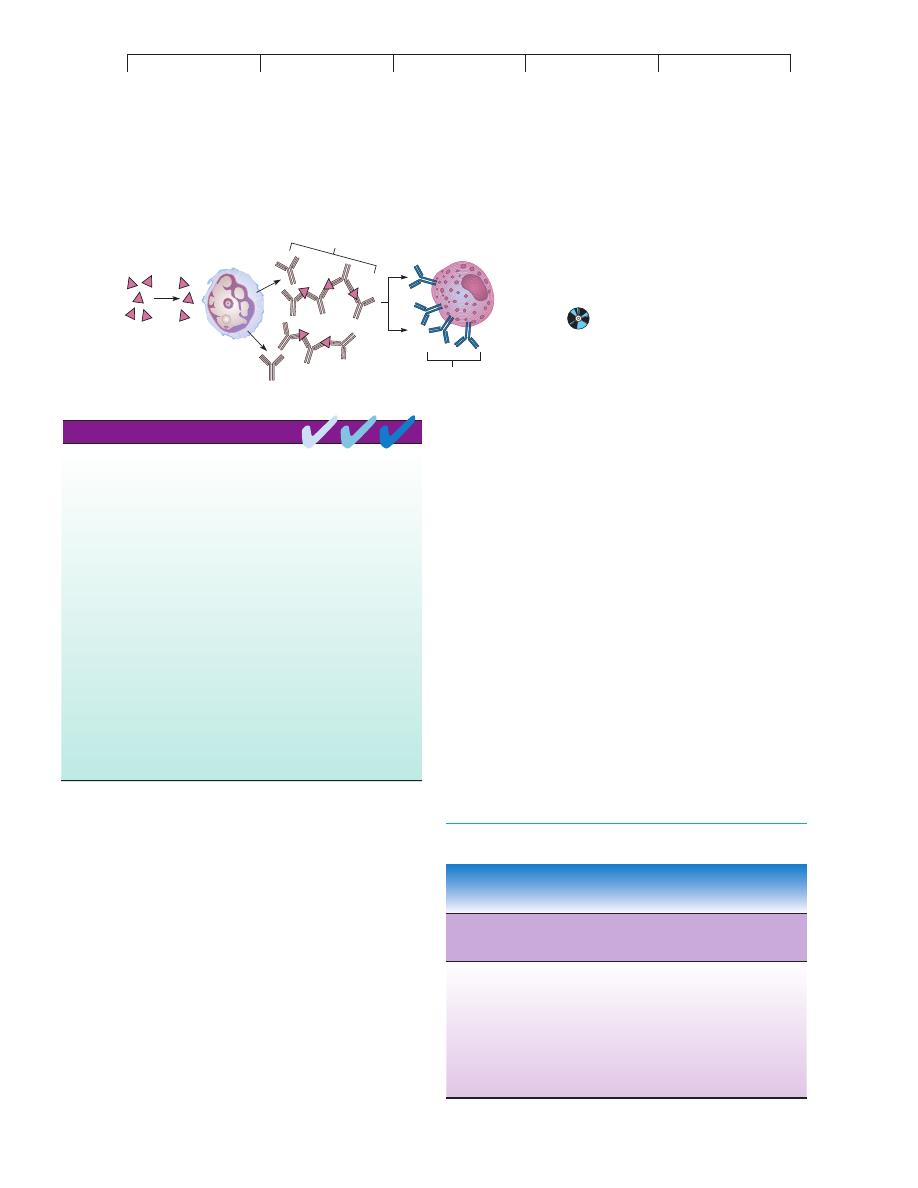
Allergen
IgG
"blocking
antibodies"
B Cell / Plasma Cell
Mast Cell
IgE
No IgE
FIGURE 17.8
The blocking antibody theory for allergic
desensitization.
An injection of allergen
causes IgG antibodies to be formed instead of
IgE; these blocking antibodies cross-link and
effectively remove the allergen before it can react
with the IgE in the mast cell.
TABLE 17.3
Characteristics of ABO Blood Groups
Phenotype A
or B* RBC
Prevalence in
Serum Content
Genotype
Antigen
Population**
of Antibodies
OO
Neither
Most common
Both anti-a
and anti-b
AA, AO
A
Second most
Anti-b
common
BB, BO
B
Third most
Anti-a
common
AB
AB
Least common
Neither
antibody
*Capital letters generally denote antigen; lowercase denotes antibody.
**True of most large populations of mixed racial and ethnic groups.
*
allele
(ah-leel
) Gr. allelon, of one another. An alternate form of a gene for a given trait.
Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
17. Disorders in Immunity
Text
© The McGraw−Hill
Companies, 2002
Type II Hypersensitivities: Reactions That Lyse Foreign Cells
509
produces
type AB; and genotype OO produces type O. Some im-
portant points about the blood types are: (1) They are named for the
dominant antigen(s); (2) the RBCs of type O persons have antigens,
but not A and B antigens; and (3) tissues other than RBCs carry A
and B antigens.
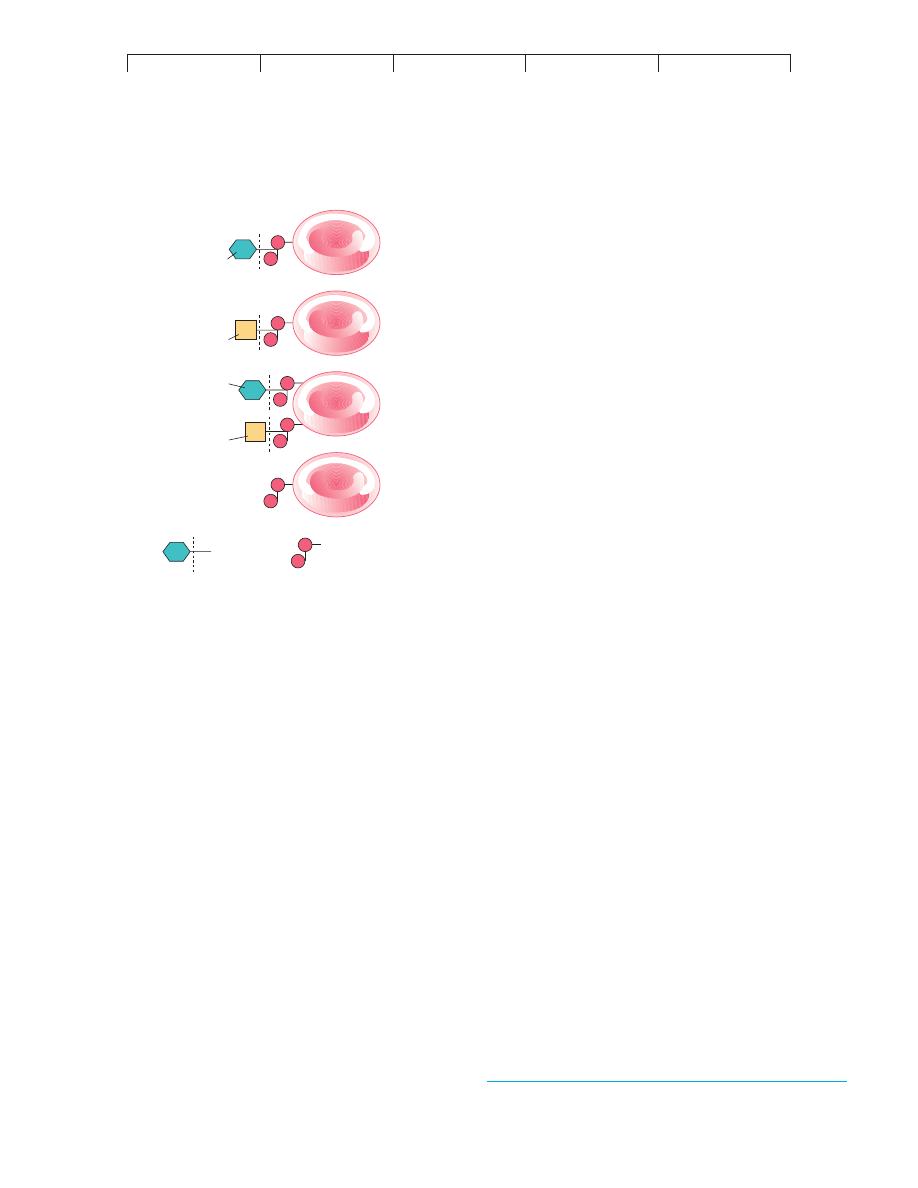
The actual origin of the AB antigens and blood types are
shown in figure 17.9. The A and B genes each code for an enzyme
that adds a terminal carbohydrate to RBC receptors during matura-
tion. RBCs of type A contain an enzyme that adds N-acetylgalac-
tosamine to the receptor; RBCs of type B have an enzyme that adds
D-galactose; RBCs of type AB contain both enzymes that add both
carbohydrates; and RBCs of type O lack the genes and enzymes to
add a terminal molecule.
ANTIBODIES AGAINST A AND B ANTIGENS
Although an individual does not normally produce antibodies in
response to his or her own RBC antigens, the serum can contain
antibodies that react with blood of another antigenic type, even
though contact with this other blood type has
never occurred.
These performed antibodies account for the immediate and in-
tense quality of transfusion reactions. As a rule, type A blood
contains antibodies (anti-b) that react against the B antigens on
type B and AB red blood cells. Type B blood contains antibodies
(anti-a) that react with A antigen on type A and AB red blood
cells. Type O blood contains antibodies against both A and B anti-
gens. Type AB blood does not contain antibodies against either
A or B antigens
1
(table 17.3). What is the source of these anti-a
and anti-b antibodies? It appears that they develop in early in-
fancy because of exposure to certain heterophile antigens that are
widely distributed in nature. These antigens are surface molecules
on bacteria and plant cells that mimic the structure of A and B
antigens. Exposure to these sources stimulates the production of
corresponding antibodies.
2
Clinical Concerns in Transfusions
The presence of ABO antigens and a, b antibodies underlie several
clinical concerns in giving blood transfusions. First, the individual
blood types of donor and recipient must be determined. By use of a
standard technique, drops of blood are mixed with antisera that
contain antibodies against the A and B antigens and are then ob-
served for the evidence of agglutination (figure 17.10).
Knowing the blood types involved makes it possible to deter-
mine which transfusions are safe to do. The general rule of compat-
ibility is that the RBC antigens of the donor must not be aggluti-
nated by antibodies in the recipient’s blood (figure 17.11). The ideal
practice is to transfuse blood that is a perfect match (A to A, B to
B). But even in this event, blood samples must be cross-matched
before the transfusion because other blood group incompatibilities
can exist. This test involves mixing the blood of the donor with the
serum of the recipient to check for agglutination.
Under certain circumstances (emergencies, the battlefield),
the concept of universal transfusions can be used. To appreciate
how this works, we must apply the rule stated in the previous para-
graph. Type O blood lacks A and B antigens and will not be agglu-
tinated by other blood types, so it could theoretically be used in any
transfusion. Hence, a person with this blood type is called a
univer-
sal donor. Because type AB blood lacks agglutinating antibodies,
an individual with this blood could conceivably receive any type of
blood. Type AB persons are consequently called
universal recipi-
ents. Although both types of transfusions involve antigen-antibody
incompatibilities, these are of less concern because of the dilution
of the donor’s blood in the body of the recipient. Additional RBC
markers that can be significant in transfusions are the Rh, MN, and
Kell antigens (see next sections).
Transfusion of the wrong blood type causes various degrees
of adverse reaction. The severest reaction is massive hemolysis
when the donated red blood cells react with recipient antibody
and trigger the complement cascade (figure 17.11). The resultant
destruction of red cells leads to systemic shock and kidney failure
brought on by the blockage of glomeruli (blood-filtering appara-
tus) by cell debris. Death is a common outcome. Other reactions
caused by RBC destruction are fever, anemia, and jaundice. A
transfusion reaction is managed by immediately halting the trans-
fusion, administering drugs to remove hemoglobin from the
blood, and beginning another transfusion with red blood cells of
the correct type.
RBC
RBC
RBC
RBC
B
A
A
B
Terminal sugar
Common receptor
Type A
Type B
Type AB
Type O
FIGURE 17.9
The genetic/molecular basis for the A and B antigens (receptors) on
red blood cells.
In general, persons with blood types A, B, and AB
inherit a gene for the enzyme that adds a certain terminal sugar to the
basic RBC receptor. Type O persons do not have such an enzyme and
lack the terminal sugar.
1. Why would this be true? The answer lies in the first sentence of the paragraph.
2. Evidence comes from germ-free chickens, which do not have antibodies against the
antigens of blood types, whereas normal chickens possess these antibodies.
Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
17. Disorders in Immunity
Text
© The McGraw−Hill
Companies, 2002
510
CHAPTER 17 Disorders in Immunity
THE RH FACTOR AND ITS
CLINICAL IMPORTANCE
Another RBC antigen of major clinical concern is the
Rh factor (or
D antigen). This factor was first discovered in experiments explor-
ing the genetic relationships among animals. Rabbits inoculated
with the RBCs of rhesus monkeys produced an antibody that also
reacted with human RBCs. Further tests showed that this monkey
antigen (termed Rh for rhesus) was present in about 85% of humans
and absent in the other 15%. The details of Rh inheritance are more
complicated than those of ABO, but in simplest terms, a person’s
Rh type results from a combination of two possible alleles—a
dominant one that codes for the factor and a recessive one that does
not. A person inheriting at least one Rh gene will be Rh
"
; only
those persons inheriting two recessive genes are Rh
#
. This factor is
denoted by a symbol above the blood type, as in O
"
or AB
#
(see
figure 17.10
c). However, unlike the ABO antigens, exposure to nor-
mal flora does not sensitize Rh
#
persons to the Rh factor. The only
ways one can develop antibodies against this factor are through pla-
cental sensitization or transfusion.
(a)
(b)
Type A Donor
Type B Recipient
Complement
Hemoglobin
being released
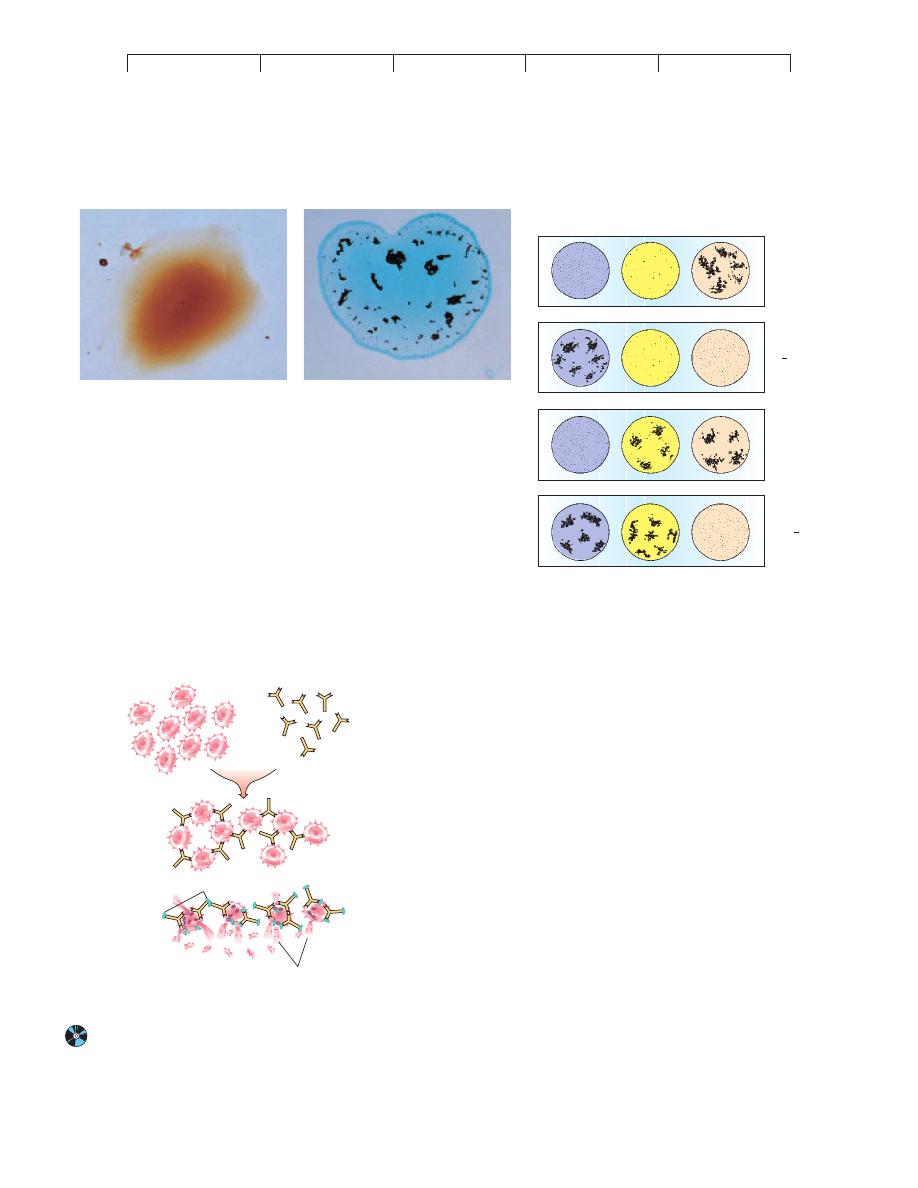
FIGURE 17.10
Interpretation of blood typing.
In this test, a drop of blood is mixed with a specially
prepared antiserum known to contain antibodies against the A, B, or Rh antigens. (a) If
that particular antigen is not present, the red blood cells in that droplet do not agglutinate
and form an even suspension. (b) If that antigen is present, agglutination occurs and the
RBCs form visible clumps. (c) Several patterns and their interpretations. Anti-a, anti-b,
and anti-Rh are shorthand for the antiserum applied to the drops. (In general, O
"
is the
most common blood type, and AB
#
is the rarest.)
FIGURE 17.11
Microscopic view of a transfusion reaction.
(a) Incompatible
blood. The red blood cells of the type A donor contain antigen A,
while the serum of the type B recipient contains anti-a antibodies that can
agglutinate donor cells. (b) Agglutination particles can block the
circulation in vital organs. (c) Activation of the complement by antibody on
the RBCs can cause hemolysis and anemia. This sort of incorrect
transfusion is very rare because of the great care taken by blood banks to
ensure a correct match.
(a)
(b)
(c)
AB
Blood
type
O+
Anti-Rh
Anti-b
Anti-a
A
B+
(c)
Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
17. Disorders in Immunity
Text
© The McGraw−Hill
Companies, 2002
Type II Hypersensitivities: Reactions That Lyse Foreign Cells
511
Hemolytic Disease of the Newborn
and Rh Incompatibility
The potential for placental sensitization occurs when a mother is
Rh
#
and her unborn child is Rh
"
. The obvious intimacy between
mother and fetus makes it possible for fetal RBCs to leak into the
mother’s circulation during childbirth, when the detachment of the
placenta creates avenues for fetal blood to enter the maternal circu-
lation. The mother’s immune system detects the foreign Rh factors
on the fetal RBCs and is sensitized to them by producing antibod-
ies and memory B cells. The first Rh
"
child is usually not affected
because the process begins so late in pregnancy that the child is
born before maternal sensitization is completed. However, the
mother’s immune system has been strongly primed for a second
contact with this factor in a subsequent pregnancy (figure 17.12
a).
In the next pregnancy with an Rh
"
fetus, fetal blood cells es-
cape into the maternal circulation late in pregnancy and elicit a
memory response. The fetus is at risk when the maternal anti-Rh
antibodies cross the placenta into the fetal circulation, where they
affix to fetal RBCs and cause complement-mediated lysis. The out-
come is a potentially fatal
hemolytic disease of the newborn
(HDN) called erythroblastosis fetalis (eh-rith
-roh-blas-toh-sis
fee-tal
-is). This term is derived from the presence of immature nu-
cleated RBCs called erythroblasts in the blood. They are released
into the infant’s circulation to compensate for the massive destruc-
tion of RBCs by maternal antibodies. Additional symptoms are se-
vere anemia, jaundice, and enlarged spleen and liver.
Maternal-fetal incompatibilities are also possible in the ABO
blood group, but adverse reactions occur less frequently than with
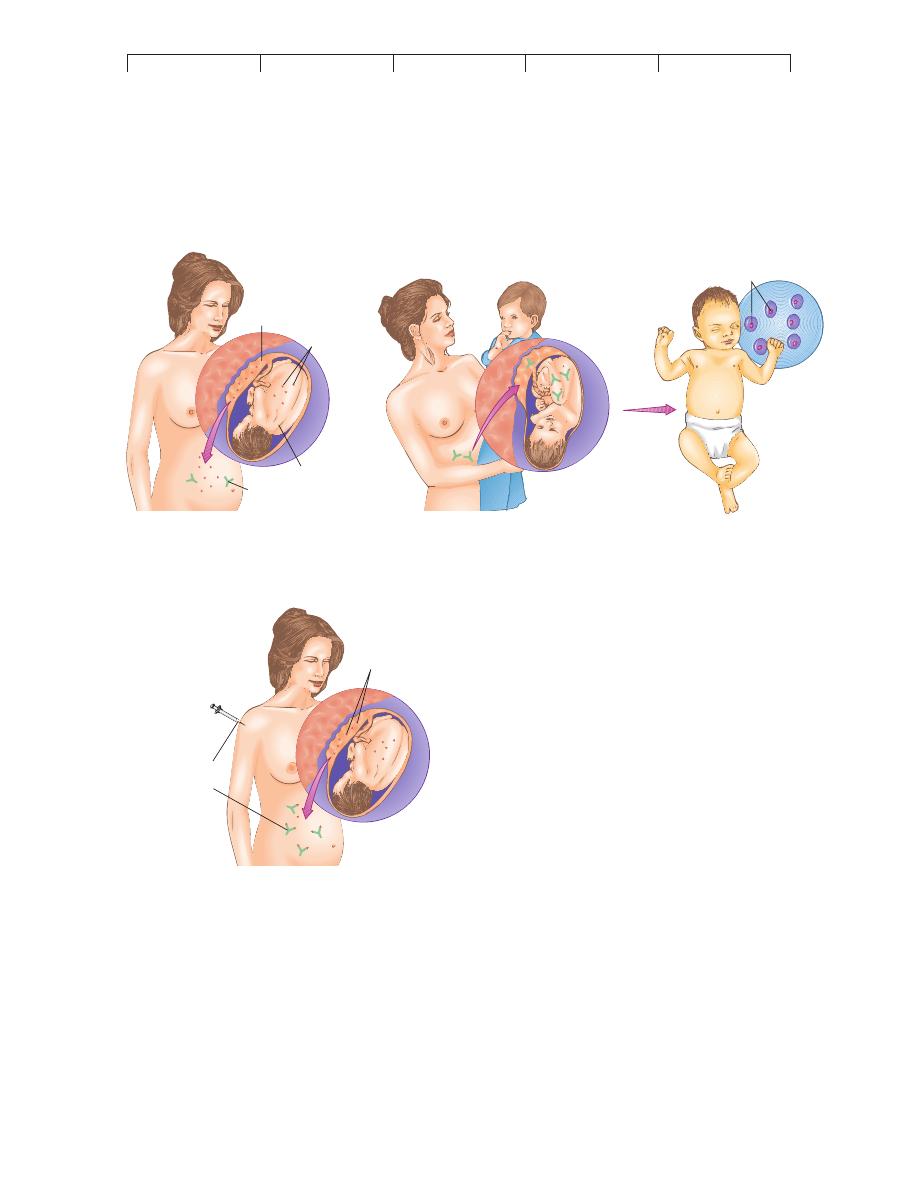
First Rh
+
fetus
Anti-Rh antibody
Rh
+
fetus
Rh factor
on RBCs
Placenta breaks
away
Rh
–
mother
Erythroblasts
in blood
Second Rh
+
fetus
Late in second pregnancy
of Rh
+
child
First Rh
+
fetus
Anti-Rh
antibodies
(RhoGAM)
Rh
+
RBCs
Rh
–
mother
(a) The development and aftermath of Rh sensitization.
(b) Prevention of erythroblastosis fetalis with anti-Rh immune globulin (RhoGAM)
FIGURE 17.12
Development and control of Rh incompatibility.
(a) Initial
sensitization of the maternal immune system to fetal Rh factor occurs
when fetal cells leak into the mother’s circulation late in pregnancy, or
during delivery, when the placenta tears away. The child will escape
hemolytic disease in most instances, but the mother, now sensitized,
will be capable of an immediate reaction to a second Rh
"
fetus and its
Rh-factor antigen. At that time, the mother’s anti-Rh antibodies pass
into the fetal circulation and elicit severe hemolysis in the fetus and
neonate. (b) Prevention of erythroblastosis fetalis with anti-Rh immune
globulin (RhoGAM). Injecting a mother who is at risk with RhoGAM
during her first Rh
"
pregnancy helps to inactivate and remove the fetal
Rh-positive cells before her immune system can react and develop
sensitivity.
Talaro−Talaro: Foundations
in Microbiology, Fourth
Edition
17. Disorders in Immunity
Text
© The McGraw−Hill
Companies, 2002
512
CHAPTER 17 Disorders in Immunity
Rh sensitization because the antibodies to these blood group anti-
gens are IgM rather than IgG and are unable to cross the placenta in
large numbers. In fact, the maternal-fetal relationship is a fascinat-
ing instance of foreign tissue not being rejected, despite the exten-
sive potential for contact (Medical Microfile 17.2).
Preventing Hemolytic Disease of the Newborn
Once sensitization of the mother to Rh factor has occurred, all other
Rh
"
fetuses will be at risk for hemolytic disease of the newborn.
Prevention requires a careful family history of an Rh
#
pregnant
woman. It can predict the likelihood that she is already sensitized or
is carrying an Rh
"
fetus. It must take into account other children
she has had, their Rh types, and the Rh status of the father. If the fa-
ther is also Rh
#
, the child will be Rh
#
and free of risk, but if the fa-
ther is Rh
"
, the probability that the child will be Rh
"
is 50% or
100%, depending on the exact genetic makeup of the father. If there
is any possibility that the fetus is Rh
"
, the mother must be pas-
sively immunized with antiserum containing antibodies against the
Rh factor (
Rh
o
[
D] immune globulin, or RhoGAM*). This anti-
serum, injected at 28 to 32 weeks and again immediately after de-
livery, reacts with any fetal RBCs that have escaped into the mater-
nal circulation, thereby preventing the sensitization of the mother’s
immune system to Rh factor (figure 17.12
b). Anti-Rh antibody
must be given with each pregnancy that involves an Rh
"
fetus. It is
ineffective if the mother has already been sensitized by a prior Rh
"
fetus or an incorrect blood transfusion, which can be determined by
a serological test. As in ABO blood types, the Rh factor should be
matched for a transfusion, although it is acceptable to transfuse
Rh
#
blood if the Rh type is not known.
OTHER RBC ANTIGENS
Although the ABO and Rh systems are of greatest medical signifi-
cance, about 20 other red blood cell antigen groups have been
discovered. Examples are the
MN, Ss, Kell, and P blood groups.
Because of incompatibilities that these blood groups present, trans-
fused blood is screened to prevent possible cross-reactions. The
study of these blood antigens (as well as ABO and Rh) has given
rise to other useful applications. For example, they can be useful in
forensic medicine (crime detection), studying ethnic ancestry, and
tracing prehistoric migrations in anthropology. Many blood cell
antigens are remarkably hardy and can be detected in dried blood
stains, semen, and saliva. Even the 2,000-year-old mummy of King
Tutankhamen has been typed A
2
MN!
MEDICAL MICROFILE
17.2
Why Doesn’t a Mother Reject Her Fetus?
what ways do fetuses avoid the surveillance of the mother’s immune sys-
tem? The answer appears to lie in the placenta and embryonic tissues.
The fetal components that contribute to these tissues are not strongly
antigenic, and they form a barrier that keeps the fetus isolated in its own
antigen-free environment. The placenta is surrounded by a dense, many-
layered envelope that prevents the passage of maternal cells, and it ac-
tively absorbs, removes, and inactivates circulating antigens.
Think of it: Even though mother and child are genetically related, the fa-
ther’s genetic contribution guarantees that the fetus will contain mole-
cules that are antigenic to the mother. In fact, with the recent practice of
implanting one woman with the fertilized egg of another woman, the sur-
rogate mother is carrying a fetus that has no genetic relationship to her.
Yet, even with this essentially foreign body inside the mother, dangerous
immunologic reactions such as Rh incompatibility are rather rare. In
*
RhoGAM
Immunoglobulin fraction of human anti-Rh serum, prepared from pooled
human sera.
Type II hypersensitivity reactions occur when preformed antibodies
react with foreign cell-bound antigens. The most common type II
reactions occur when transfused blood is mismatched to the recipient’s
ABO type. IgG or IgM antibodies attach to the foreign cells, resulting
in complement fixation. The resultant formation of membrane attack
complexes lyses the donor cells.
Type II hypersensitivities are stimulated by antibodies formed against red
blood cell (RBC) antigens or against other cell-bound antigens
following prior exposure.
Complement, IgG, and IgM antibodies are the primary mediators of
type II hypersensitivities.
The concepts of universal donor (type O) and universal recipient
(type AB) apply only under emergency circumstances. Cross-matching
donor and recipient blood is necessary to determine which
transfusions are safe to perform.
Type II hypersensitivities can also occur when Rh
#
mothers are sensitized
to Rh
"
RBCs of their unborn babies and the mother’s anti-Rh antibodies
cross the placenta, causing hemolysis of the newborn’s RBCs. This is
called hemolytic disease of the newborn, or erythroblastosis fetalis.
CHAPTER CHECKPOINTS
Type III Hypersensitivities: Immune
Complex Reactions
Type III hypersensitivity involves the reaction of soluble antigen
with antibody and the deposition of the resulting complexes in base-
ment membranes of epithelial tissue. It is similar to type II, because
it involves the production of IgG and IgM antibodies after repeated
exposure to antigens and the activation of complement. Type III
differs from type II because its antigens are not attached to the sur-
face of a cell. The interaction of these antigens with antibodies
