1
PROF.DR. MAHA SHAKIR HASSAN
Lec 2
Stomach
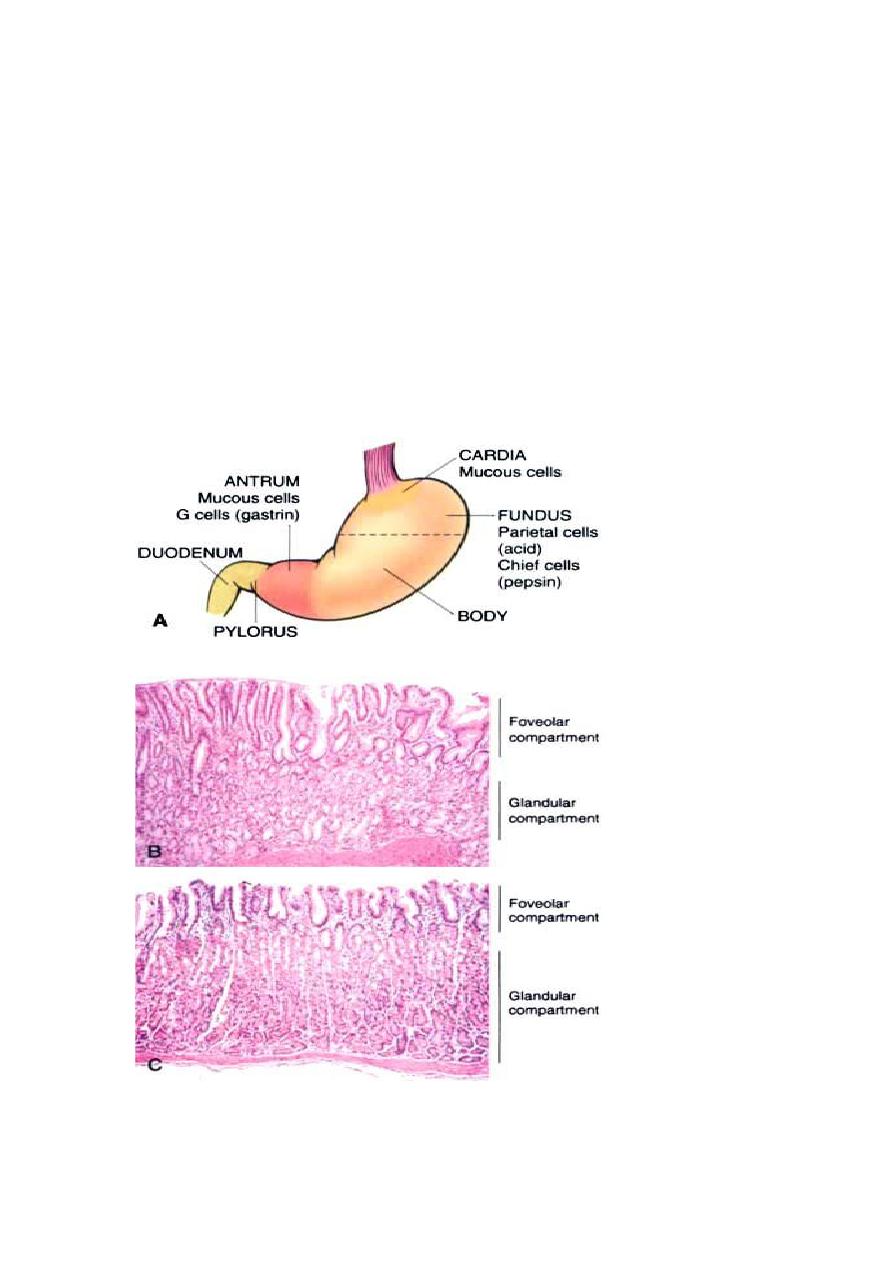
The stomach is divided into four major anatomic regions: the cardia, fundus,
body, and antrum. The cardia and antrum are lined mainly by mucin-
secreting foveolar cells that form small glands. The antral glands are similar
but also contain endocrine cells, such as G cells, that release gastrin to
stimulate luminal acid secretion by parietal cells within the gastric fundus
and body. The well-developed glands of the body and fundus also contain
chief cells that produce and secrete digestive enzymes such as pepsin.
Fig:B microscopical view of antral mucosa.
C microscopical view of funduc mucosa.

2
Congenital anomalies
Pyloric Stenosis
Congenital hypertrophic pyloric stenosis generally presents in the second or
third week of life as new-onset regurgitation and persistent, projectile
vomiting. Physical examination reveals hyperperistalsis and a firm, ovoid
abdominal mass.
Diaphragmatic Hernia.
Diaphragmatic hernia occurs when incomplete formation of the diaphragm
allows the abdominal viscera to herniate into the thoracic cavity. When
severe, the space-filling effect of the displaced viscera can cause pulmonary
hypoplasia that is incompatible with life after birth.
GASTRITIS
Acute Gastritis
Acute gastritis is an acute mucosal inflammatory process, usually of a
transient nature. The inflammation may be accompanied by hemorrhage into
the mucosa and, in more severe circumstances, by sloughing of the
superficial mucosal epithelium (erosion). This severe erosive form of the
disease is an important cause of acute gastrointestinal bleeding.
Acute gastritis is frequently associated with the following:
• Heavy use of nonsteroidal anti-inflammatory drugs (NSAIDs), particularly
aspirin.
• Excessive alcohol consumption
• Heavy smoking
• Treatment with cancer chemotherapeutic drugs
• Uremia
• Severe stress (e.g., trauma, burns, surgery)
ACUTE GASTRIC ULCERATION
Focal, acutely developing gastric mucosal defects are a well-known
complication of therapy with NSAIDs. They may also appear after severe
physiologic stress. Some of these are given specific names, based on location
and clinical associations. For example:
• Stress ulcers are most common in individuals with shock, sepsis, or

3
severe trauma.
• Ulcers occurring in the proximal duodenum and associated with severe
burns or trauma are called Curling ulcers.
• Gastric, duodenal, and esophageal ulcers arising in persons with
intracranial disease are termed Cushing ulcers and carry a high
incidence of perforation
Chronic Gastritis
In contrast to acute gastritis, the symptoms associated with chronic
gastritis are typically less severe but more persistent. Nausea and
upper abdominal discomfort may occur, sometimes with vomiting,
but hematemesis is uncommon. The most common cause of chronic
gastritis is infection with the bacillus Helicobacter pylori.
Autoimmune gastritis, the most common cause of atrophic
gastritis, represents less than 10% of cases of chronic gastritis and
is the most common form of chronic gastritis in patients without H.
pylori infection. Less common etiologies include radiation injury,
chronic bile reflux.
HELICOBACTER PYLORI GASTRITIS
These spiral-shaped or curved bacilli are present in gastric biopsy
specimens of almost all patients with duodenal ulcers and the
majority of individuals with gastric ulcers or chronic gastritis. H.
pylori organisms are present in 90% of individuals with chronic
gastritis affecting the antrum.
Pathogenesis.
H. pylori infection is the most common cause of chronic gastritis.
The disease most often presents as a predominantly antral gastritis
with high acid production, despite hypogastrinemia. The risk of
duodenal ulcer is increased in these patients and, in most, gastritis
is limited to the antrum with occasional involvement of the cardia.
In a subset of patients the gastritis progresses to involve the gastric
body and fundus. This pangastritis is associated with multifocal
mucosal atrophy, reduced acid secretion, intestinal metaplasia, and
increased risk of gastric adenocarcinoma.
Four features are linked to H. pylori virulence:
• Flagella, which allow the bacteria to be motile in viscous
mucus

4
• Urease, which generates ammonia from endogenous urea and
thereby elevates local gastric pH protect the bacteria from acid
media of stomach.
• Adhesins that enhance their bacterial adherence to surface
foveolar cells
• Toxins, that may be involved in ulcer or cancer development
by poorly defined mechanisms.
Morphology.
1. H. pylori in infected individuals. The organism is
concentrated within the superficial mucus overlying epithelial
cells.
2. The inflammatory infiltrate generally includes variable
numbers of neutrophils within the lamina propria, including
some that cross the basement membrane to assume an
intraepithelial location and accumulate in the lumen of gastric
pits to create pit abscesses. In addition, the superficial lamina
propria includes large numbers of plasma cells, and increased
numbers of lymphocytes and macrophages.
3. Lymphoid aggregates, some with germinal centers, are
frequently present that has the potential to transform into
lymphoma.
Clinical Features.
In addition to histologic identification of the organism, several
diagnostic tests have been developed including a noninvasive
serologic test for antibodies to H. pylori, fecal bacterial
detection
Effective
treatments
for
H.
pylori
infection
include
combinations of antibiotics and proton pump inhibitors.
Individuals with H. pylori gastritis usually improve after
treatment, although relapses can occur after incomplete
eradication or re-infection.
AUTOIMMUNE GASTRITIS
Autoimmune gastritis accounts for less than 10% of cases of
chronic gastritis. In contrast to that caused by H. pylori,

5
autoimmune gastritis typically spares the antrum and includes
hypergastrinemia . Autoimmune gastritis is characterized by:
• Antibodies to parietal cells and intrinsic factor that can be
detected in serum and gastric secretions
• Reduced serum pepsinogen I concentration
• Antral endocrine cell hyperplasia
• Vitamin B12 deficiency
• Defective gastric acid secretion (achlorhydria)
Pathogenesis.
Autoimmune gastritis is associated with loss of parietal cells,
which are responsible for secretion of gastric acid and intrinsic
factor. The absence of acid production stimulates gastrin release,
resulting in hypergastrinemia and hyperplasia of antral gastrin-
producing G cells. Lack of intrinsic factor disables ileal vitamin
B12 absorption, leading to B12 deficiency and a slow-onset
megaloblastic anemia (pernicious anemia). The reduced serum
pepsinogen I concentration results from chief cell destruction. In
contrast, although H. pylori can cause hypochlorhydria, it is not
associated with achlorhydria or pernicious anemia because the
parietal and chief cell damage is not as severe as in autoimmune
gastritis.
Morphology.
1. diffuse mucosal damage of the oxyntic (acid-producing)
mucosa within the body and fundus.
2. With diffuse atrophy, the oxyntic mucosa of the body and
fundus appears markedly thinned, and rugal folds are lost.
3. the inflammatory infiltrate is more often composed of
lymphocytes, macrophages, and plasma cells. Lymphoid
aggregates may be present. The superficial lamina propria
plasma cells of H. pylori gastritis are typically absent, and the
6
inflammatory reaction is most often deep and centered on the
gastric glands .
4. Loss of parietal and chief cells can be extensive. With areas
of intestinal metaplasia, characterized by the presence of
goblet cells and columnar absorptive cells .
TABLE :Characteristics of Helicobacter pylori–Associated and Autoimmune
Gastritis
H. pylori–Associated
Autoimmune
Location
Antrum
Body
Inflammatory
infiltrate
Neutrophils, subepithelial
plasma cells
Lymphocytes, macrophages
Acid production Increased to slightly decreased
Decreased
Gastrin
Normal to decreased
Increased
Serology
Antibodies to H. pylori
Antibodies to parietal cells
Sequelae
Peptic ulcer, adenocarcinoma
Atrophy, pernicious anemia,
adenocarcinoma, carcinoid tumor
Associations
Low socioeconomic status,
poverty, residence in rural areas
Autoimmune disease; thyroiditis,
diabetes mellitus, Graves disease
7
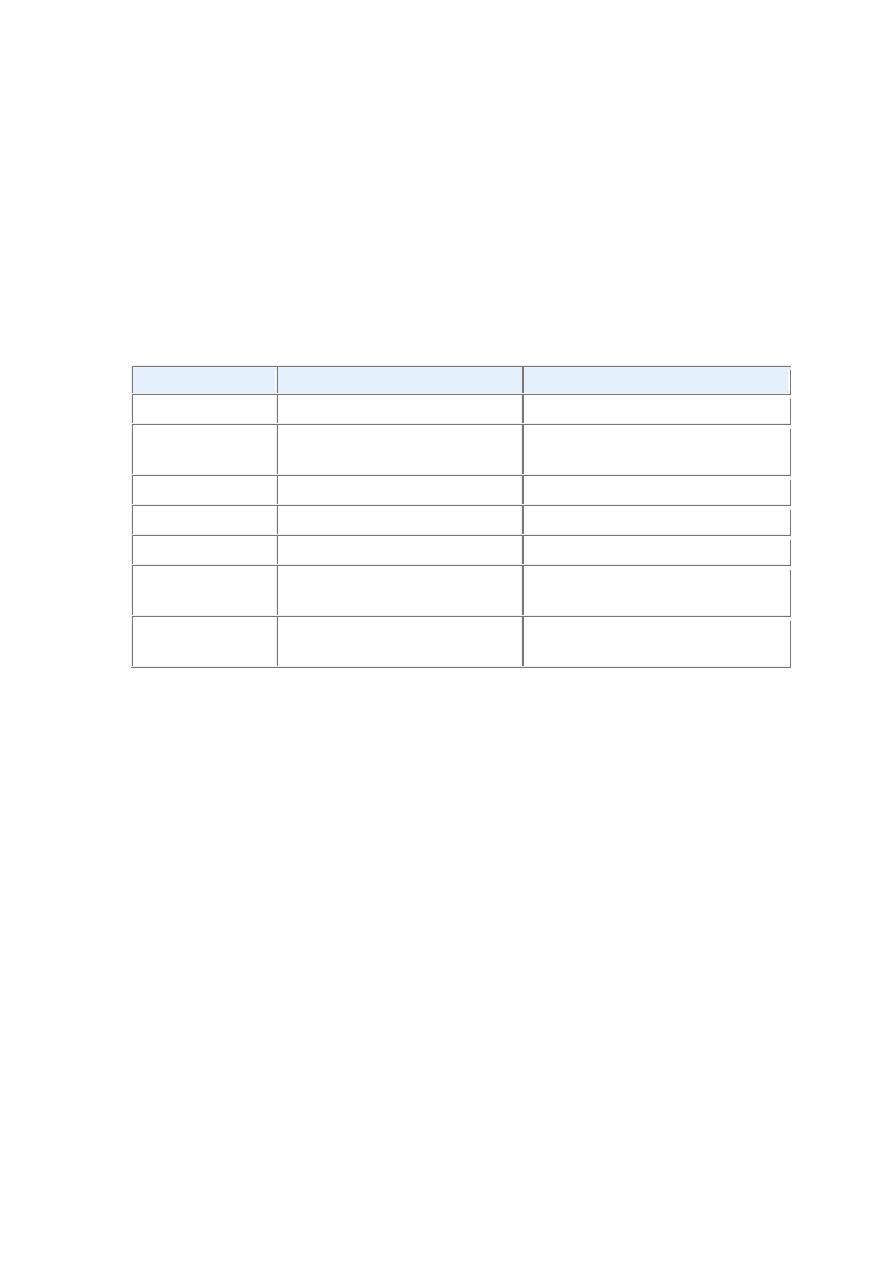
FIGURE Helicobacter pylori gastritis. A, Spiral-shaped H. pylori are highlighted in this Warthin-Starry
silver stain. Organisms are abundant within surface mucus. B, Intraepithelial and lamina propria
neutrophils are prominent. C, Lymphoid aggregates with germinal centers and abundant subepithelial
plasma cells within the superficial lamina propria are characteristic of H. pylori gastritis.
FIGURE Autoimmune gastritis. A, Low-magnification image of gastric body demonstrating deep
inflammatory infiltrates, primarily composed of lymphocytes, and glandular atrophy. B, Intestinal
metaplasia, recognizable as the presence of goblet cells admixed with gastric foveolar epithelium.

8
Peptic ulcer disease
Ulcers are defined as a breach in the mucosa of the alimentary tract that
extends through the muscularis mucosae into the submucosa or deeper. This
is to be contrasted to erosions, in which there is a breach in the epithelium of
the mucosa only.
Peptic ulcer disease (PUD) is most often associated with H. pylori–induced
hyperchlorhydric chronic gastritis, which is present in 85% to 100% of
individuals with duodenal ulcers and in 65% with gastric ulcers.
PUD may occur in any portion of the GI tract exposed to acidic gastric
juices, but is most common in the gastric antrum and first portion of the
duodenum. PUD may also occur in the esophagus as a result of GERD or
acid secretion by ectopic gastric mucosa. Gastric mucosa within a Meckel
diverticulum can result in peptic ulceration of adjacent mucosa.
Peptic Ulcers
Peptic ulcers are chronic, most often solitary, lesions that occur in any
portion of the gastrointestinal tract exposed to the aggressive action of acid-
peptic juices. At least 98% of peptic ulcers are either in the first portion of
the duodenum or in the stomach, in a ratio of about 4:1.
Pathogenesis. There are two key facts.
First, the mucosal exposure to gastric acid and pepsin.
Second, there is a very strong causal association with H. pylori infection.
It is best perhaps to consider that peptic ulcers are induced by an imbalance
between the gastroduodenal mucosal defenses and the countervailing
aggressive forces that overcome such defenses.
The array of host mechanisms that prevent the gastric mucosa from being
digested like a piece of meat include the following:
• Secretion of mucus by surface epithelial cells
• Secretion of bicarbonate into the surface mucus, to create a buffered surface
microenvironment
• Secretion of acid- and pepsin-containing fluid from the gastric pits as “jets”
through the surface mucus layer, entering the lumen directly without
contacting surface epithelial cells
• Rapid gastric epithelial regeneration
• Robust mucosal blood flow, to sweep away hydrogen ions that have back-

9
diffused into the mucosa from the lumen and to sustain the high cellular
metabolic and regenerative activity
• Mucosal elaboration of prostaglandins, which help maintain mucosal blood
flow
The imbalances of mucosal defenses and damaging forces that cause chronic
gastritis are also responsible for PUD. Thus, PUD generally develops on a
background of chronic gastritis. The reasons why some people develop only
chronic gastritis while others develop PUD are poorly understood.
H. pylori infection and NSAID use are the primary underlying causes of
PUD, and both compromise mucosal defense while causing mucosal damage.
Although more than 70% of individuals with PUD are infected by H. pylori,
fewer than 20% of H. pylori–infected individuals develop peptic ulcer. It is
probable that host factors as well as variation among H. pylori strains
determine the clinical outcomes.
The gastric hyperacidity that drives PUD may be caused by:
• H. pylori infection, parietal cell hyperplasia.
• excessive secretory responses.
• or impaired inhibition of stimulatory mechanisms such as gastrin
release. For example, Zollinger-Ellison syndrome, in which there are
multiple peptic ulcerations in the stomach, duodenum, and even
jejunum, is caused by uncontrolled release of gastrin by a tumor and
the resulting massive acid production.
More common cofactors in peptic ulcerogenesis include:
• chronic NSAID use, which causes direct chemical irritation while
suppressing prostaglandin synthesis necessary for mucosal
protection.
• cigarette smoking, which impairs mucosal blood flow and healing.
• and high-dose corticosteroids that suppress prostaglandin synthesis
and impair healing.
Duodenal ulcers are more frequent in individuals with:
• alcoholic cirrhosis.
• chronic obstructive pulmonary disease.
• chronic renal failure.
• and hyperparathyroidism.

10
In the latter two conditions, hypercalcemia stimulates gastrin production
and therefore increases acid secretion. Finally, self-imposed or exogenous
psychologic stress may increase gastric acid production.
MORPHOLOGY
Peptic ulcers are four times more common in the proximal duodenum than in
the stomach. Duodenal ulcers usually occur within a few centimeters of the
pyloric valve and involve the anterior duodenal wall. Gastric peptic ulcers
are predominantly located along the lesser curvature near the interface of the
body and antrum.
Peptic ulcers are solitary in more than 80% of patients. Lesions less than 0.3
cm in diameter tend to be shallow while those over 0.6 cm are likely to be
deeper ulcers. The classic peptic ulcer is a round to oval, sharply punched-
out defect. The mucosal margin is usually level with the surrounding
mucosa. In contrast, heaped-up margins are more characteristic of
cancers. The depth of ulcers may be limited by the thick gastric muscularis
propria or by adherent pancreas, omental fat, or the liver. Perforation into
the peritoneal cavity is a surgical emergency that may be identified by the
presence of free air under the diaphragm on upright radiographs of the
abdomen.
The base of peptic ulcers is smooth and clean as a result of peptic digestion
of exudate.
Size and location do not differentiate benign and malignant ulcers. However,
the gross appearance of chronic peptic ulcers is virtually diagnostic.
Malignant transformation of peptic ulcers is very rare.
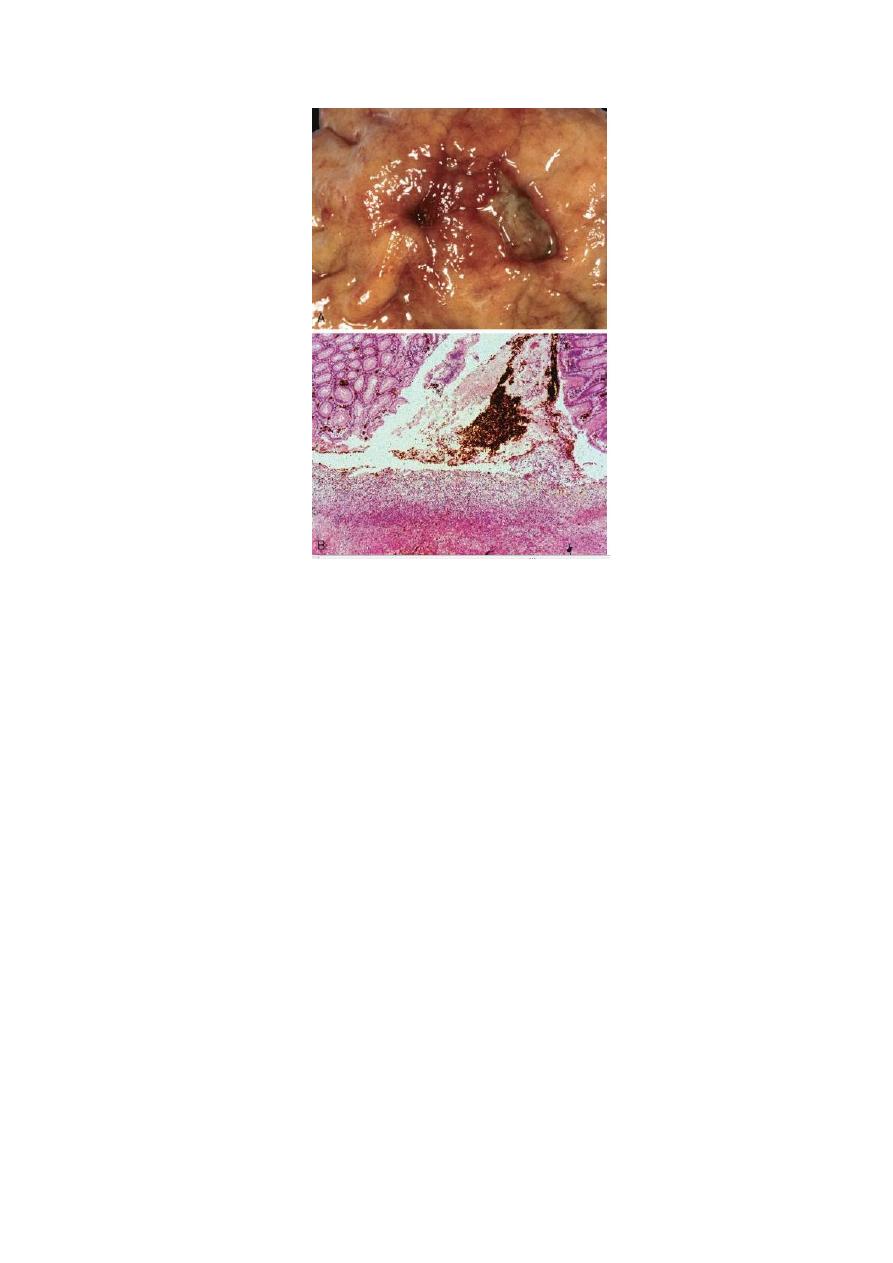
11
FIGURE Acute gastric perforation in a patient presenting with free air under the diaphragm. A, Mucosal
defect with clean edges. B, The necrotic ulcer base is composed of granulation tissue.
histologic appearance: four zones can be distinguished
1- the base and margins have a thin layer of necrotic fibrinoid debris
underlain by (2) a zone of active nonspecific inflammatory infiltration with
neutrophils predominating, underlain by (3) granulation tissue, deep to which
is (4) fibrous, collagenous scar that fans out widely from the margins of the
ulcer. Vessels trapped within the scarred area are characteristically thickened
and occasionally thrombosed.

12
Medium-power detail of the base of a nonperforated peptic ulcer, demonstraiting the layers of necrosis (N),
inflammation (I), granulation tissue (G), and scar (s) moving from the luminal surface at the top End the
muscle wall at the bottom.
