
Antidepressant Drugs Pharma. Dr. Ali
Depression is a disease characterized by feelings of sadness and hopelessness, as well
as the inability to experience pleasure in usual activities, changes in sleep patterns and
appetite, loss of energy, and suicidal thoughts.
Biogenic Amine Hypothesis
1950: Reserpine Induce depression ( Reserpine depletes storage or amine
neurotransmitters such as serotonin and norepinephrine
Depression Amine (serotonin and norepinephrine )-dependent synaptic
transmission
(Antidepressants Amine by inhibition of their reuptake and metabolism)
mania is caused by an overproduction of these neurotransmitters.
Classification of antidepressants:
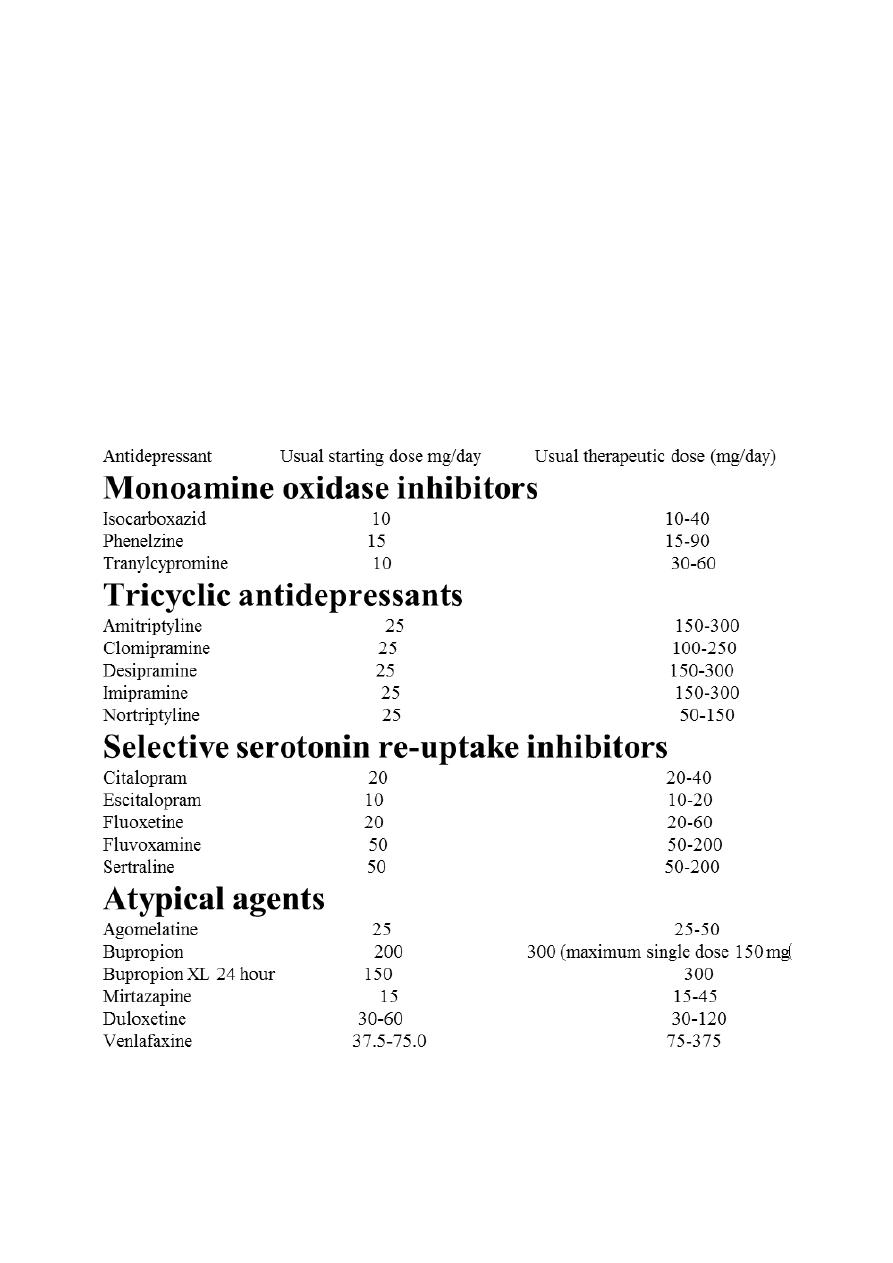
I . Monoamine oxidase inhibitors MAO-I:
Phenelzine, Tranylcypromine, Isocarboxazid and Selegiline
I I . Tricyclic antidepressants (TCAs)
A) NA + 5-HT reuptake inhibitors:
Imipramine, Amitriptyline, Trimipramine, Doxepin, Dothiepin, Clomipramine
B) Predominantly NA reuptake inhibitors:
Desipramine, Nortriptyline, Amoxapine, Reboxetine
I l l . Selective serotonin reuptake inhibitors (SSRis):
Fluoxetine, Fluvoxamine, Paroxetine, Sertraline, Citalopram, Escitalopram
IV. Atypical antidepressants
Trazodone, Mianserin, Mirtazapine, Venlafaxine, Duloxetine, Tianeptine, Amineptine,
Bupropion and others.
Monoamino oxidase inhibitors (MAOIs):
phenelzine, tranylcypromine, isocarboxazid and selegiline [ Selegiline is also used for
the treatment of Parkinson’s disease. It is the only antidepressant available in a
transdermal delivery system.]
Action:
inhibit monoamine oxidase, thus preventing the breakdown of monoamine
neurotransmitters and thereby increasing their availability. There are two isoforms of
monoamine oxidase, MAO-A and MAO-B.

MAO-A preferentially deaminates serotonin, melatonin, epinephrine,
and norepinephrine.
MAO-A preferentially deaminates other trace amines, like tyramine,
dopamine is equally deaminated by both types.
Use of MAOIs is limited due to the complicated dietary restrictions required while
taking these agents. These drugs inhibit not only MAO in the brain but also MAO in the
liver and gut that catalyzes oxidative deamination of drugs and toxic substances, such
as tyramine, which is found in certain foods. The MAOIs, therefore, show a high
incidence of drug–drug and drug–food interactions.
Selegiline administered as the transdermal patch may produce less inhibition of gut
and hepatic MAO at low doses because it avoids first-pass metabolism.
Although MAO is fully inhibited after several days of treatment, the antidepressant
action of the MAOIs, (like that of the SSRIs, SNRIs, and TCAs) is delayed several weeks.
Selegiline and tranylcypromine have an amphetamine-like stimulant effect that may
produce agitation or insomnia.
Indications:
-used in depressed patients who are unresponsive or allergic to TCA or who are
experience strong anxiety.
-Patients with low psychomotor activity may benefit from the stimulant properties of
MAOIs.
-Treatment of phobic states.
-Atypical depression.
-Because of their risk for drug–drug and drug–food interactions, the MAOIs are
considered last-line agents in many treatment settings.
Pharmacokinetics
These drugs are well absorbed after oral administration, hepatically metabolized and
excreted rapidly in urine.
Enzyme regeneration, when irreversibly inactivated, varies, but it usually occurs
several weeks after termination of the drug. Thus, when switching antidepressant
agents, a minimum of 2 weeks of delay must be allowed after termination of MAOI
therapy and the initiation of another antidepressant from any other class.

Adverse effects
Side effects include: Dry mouth, Nausea, diarrhea or constipation, Headache,
Drowsiness, Insomnia, Dizziness or light headedness, Skin reaction at the patch site,
Involuntary muscle jerks, Low blood pressure Reduced sexual desire or difficulty
reaching orgasm, Weight gain, Difficulty starting a urine flow, Muscle cramps,
Prickling or tingling sensation in the skin (paresthesia)
Severe and often unpredictable side effects, due to drug–food and drug–drug
interactions, limit the widespread use of MAOIs. For example, tyramine, which is
contained in foods, such as aged cheeses and meats, chicken liver, pickled or smoked
fish, and red wines, is normally inactivated by MAO in the gut. Individuals receiving
a MAOI are unable to degrade tyramine obtained from the diet. Tyramine causes the
release of large amounts of stored catecholamines from nerve terminals, resulting in a
hypertensive crisis. Patients must, therefore, be educated to avoid tyramine-
containing foods.
Due to the risk of serotonin syndrome, the use of MAOIs with other antidepressants is
contraindicated. For example, SSRIs should not be coadministered with MAOIs. Both
SSRIs and MAOIs require a washout period of at least 2 weeks before the other type
is administered, with the exception of fluoxetine, which should be discontinued at
least 6 weeks before a MAOI is initiated.
Tricyclic antidepressants:
1. Block NE & 5-HT reuptake, Desipramine, Nortriptyline, Amoxapine, Reboxetine
are selective NE reuptake inhibitors.
2. Block α-adrenergic, histaminic, & muscarinic receptors which results in many of
TCAs adverse effects. Amoxapine also blocks D2 receptors.
Indications:
1. depression & panic disorder.
2. Imipramine is used to control bed-wetting in children
3. Amitriptyline is used to treat migraine & chronic pain syndromes (eg. neuropathic
pain)
4. Doxepin, can be used to treat insomnia.

Pharmacokinetics
- high lipid solubility
- large volume of distribution (Vd)
- rapid absorption
- serum concentrations peak within a few hours
- significant first pass metabolism
- high protein binding
- half-life of 8-36 hours (active metabolites of imipramine and amitriptyline)
- metabolized by the liver, excreted by kidney
Adverse effects
In addition to blocking monoamine transporters, the tricyclic antidepressants produce
prominent antagonist effects at: alpha-1 adrenergic, muscarinic M1 and H-1 histamine
receptors.
- Antimuscarinic adverse effects.
-Orthostatic hypotension (most likely with imipramine & least likely with nortriptyline).
- cardiotoxicity, quinidine like life-threatening arrhythmias.
-confusion with memory dysfunction
- excessive sedation and fatigue
- weight gain.
- Sexual dysfunction ( but lower than that associated with SSRIs).
Tricyclic antidepressants are not recommended for elderly patients (65+ years)
because of their liability for inducing a toxic and confused state.
Drug interactions:
TCAs & MAOIs concomitant use result in HT, hyperpyrexia, convulsion & coma.
TCAs potentiate effects of direct-acting adrenergic drugs.
TCAs prevent indirect-acting sympathomimetic from reaching their sites of action.
TCAs used with ethanol or other CNS depressants may result in toxic sedation.
Precautions :
TCAs (like all antidepressants) should be used with caution in patients with manic-
depressive disorder (bipolar), since switch to manic behavior may occur .
TCAs have a narrow therapeutic index
Suicidal depressed patients should be given only limited quantities of TCAs with close
monitoring.
TCAs may exacerbate unstable angina, BPH, epilepsy and preexisting arrhythmias.

Selective serotonin reuptake inhibitors (SSRIs):
fluoxetine, citalopram, fluvoxamine, sertraline, paroxetine and escitalopram
The selective serotonin reuptake inhibitors (SSRIs) having 300- to 3000-fold greater
selectivity for the serotonin transporter, as compared to the norepinephrine
transporter. Moreover, the SSRIs have little blocking activity at muscarinic, α-
adrenergic, and histaminic H1 receptors. Therefore, common side effects associated
with TCAs, such as orthostatic hypotension, sedation, dry mouth, and blurred vision,
are not commonly seen with the SSRIs. Because they have different adverse effects
and are relatively safe even in overdose, the SSRIs have largely replaced TCAs and
monoamine oxidase inhibitors (MAOIs) as the drugs of choice in treating depression.
Antidepressants, including SSRIs, typically take at least 2 weeks to produce significant
improvement in mood, and maximum benefit may require up to 12 weeks or more.
Therapeutic uses
-As effective as the TCAs is depression.
-A number of other psychiatric disorders also respond favorably to SSRIs, including
obsessive–compulsive disorder, panic disorder, generalized anxiety disorder,
posttraumatic stress disorder, social anxiety disorder, premenstrual dysphoric
disorder, and bulimia nervosa (only fluoxetine is approved for bulimia).
Pharmacokinetics
All of the SSRIs are well absorbed after oral administration. Food has little effect on
absorption (except with sertraline, for which food increases its absorption).
Bioavailability - ranges from 50%- > 90%
Peak levels are seen in approximately 2 to 8 hours on average.
Protein Binding - ranges from 50% (escitalopram) to 95 % (sertraline)
The majority of SSRIs have plasma half-lives that range between 16 and 36 hours,
except for fluoxetine (1-4days) and its active metabolite S-norfluoxetine (7-15 days). It
is available as a sustained-release preparation allowing once-weekly dosing.
Metabolism by cytochrome P450 (CYP450)–dependent enzymes and glucuronide or
sulfate conjugation occur extensively.
Dosages of the SSRIs should be reduced in patients with hepatic impairment.

Adverse effects
Include: Drowsiness, Insomnia (Paroxetine and fluvoxamine are generally more
sedating), Sexual dysfunction (loss of libido, delayed ejaculation, and anorgasmia),
Nausea, Dry mouth, Diarrhea, Nervousness, agitation or restlessness, Dizziness,
Headache and Blurred vision
Use in children and teenagers: Antidepressants should be used cautiously in children
and teenagers, because about 1 out of 50 children report suicidal ideation as a result
of SSRI treatment. Pediatric patients should be observed for worsening depression and
suicidal thinking with initiation or dosage change of any antidepressant. Fluoxetine,
sertraline, and fluvoxamine are approved for use in children to treat obsessive–
compulsive disorder, and fluoxetine and escitalopram are approved to treat childhood
depression.
Overdose: Overdose with SSRIs does not usually cause cardiac arrhythmias, with the
exception of citalopram, which may cause QT prolongation.
Discontinuation syndrome: All of the SSRIs have the potential to cause a
discontinuation syndrome after their abrupt withdrawal, particularly the agents with
shorter half-lives and inactive metabolites. Fluoxetine has the lowest risk of causing an
SSRI discontinuation syndrome due to its longer half-life and active metabolite.
Atypical antidepressants:
bupropion, mirtazapine, nefazodone, trazodone, vilazodone], and vortioxetine
Bupropion
Bupropion is a weak dopamine and norepinephrine reuptake inhibitor that is used to
alleviate the symptoms of depression. Bupropion is also useful for decreasing cravings
and attenuating withdrawal symptoms of nicotine in patients trying to quit smoking.
Side effects may include dry mouth, sweating, nervousness, tremor, and a dose
dependent increased risk for seizures. It has a very low incidence of
sexual dysfunction.
Bupropion is metabolized by the CYP2B6 pathway and has a relatively low risk for
drug–drug interactions, given the few agents that inhibit/induce this enzyme.
However, bupropion may inhibit CYP2D6 and, thus, increase exposure to substrates of
this isoenzyme.
Use of bupropion should be avoided in patients at risk for seizures or those who have
eating disorders such as bulimia.

Mirtazapine
Mirtazapine enhances serotonin and norepinephrine neurotransmission by serving as
an antagonist at presynaptic α2 receptors. Additionally, some of the antidepressant
activity may be related to antagonism at 5-HT2 receptors. It is sedating because of its
potent antihistaminic activity, but it does not cause the antimuscarinic side effects of
the TCAs, or interfere with sexual function like the SSRIs. Increased appetite and
weight gain frequently occur. Mirtazapine is markedly sedating, which may be an
advantage in depressed patients having difficulty sleeping.
Nefazodone and trazodone
These drugs are weak inhibitors of serotonin reuptake. Their therapeutic benefit
appears to be related to their ability to block postsynaptic 5-HT2a receptors. Both
agents are sedating, probably because of their potent histamine H1-blocking activity.
Trazodone is commonly used off-label for the management of insomnia. Trazodone
has been associated with priapism, and nefazodone has been associated with a risk for
hepatotoxicity. Both agents also have mild to moderate α1 receptor antagonism,
contributing to orthostasis and dizziness.
Vilazodone
Vilazodone is a serotonin reuptake inhibitor and a 5-HT1a partial agonist. Although the
extent to which the 5-HT1a receptor activity contributes to its therapeutic effects is
unknown, this possible mechanism of action renders it unique from that of the SSRIs.
The adverse effect profile of vilazodone is similar to the SSRIs, including a risk for
discontinuation syndrome if abruptly stopped.
Vortioxetine
Vortioxetine utilizes a combination of serotonin reuptake inhibition, 5-HT1a agonism,
and 5-HT3 and 5-HT7 antagonism as its suggested mechanisms of action to treat
depression. It is unclear to what extent the activities other than inhibition of serotonin
reuptake influence the overall effects of vortioxetine. The common adverse effects
include nausea, vomiting, and constipation, which may be expected due to its
serotonergic mechanisms.
Side effects of atypical antidepressants
Because of the different ways atypical antidepressants work, each has unique
characteristics and varying possible side effects. For example:
Most of the atypical antidepressants caused dry mouth, dizziness or lightheadedness
Some antidepressants may help you sleep and are best taken at night, while others
may cause insomnia.
Some antidepressants may cause constipation, while others may increase the risk of
diarrhea.
Some antidepressants may increase your appetite, resulting in weight gain, while
others may cause nausea.
Some antidepressants are less likely than others to cause sexual side effects.
